Abstract
Physalis Calyx seu Fructus is the dry calyx or the calyx with fruit of the Solanaceae plant Physalis alkekengi L. var. franchetii (Mast.) Makino, with a long history of use in medicine and food. However, despite its many potential therapeutic and culinary applications, P. alkekengi is not being exploited for these applications on a large scale. This study analysed various research related to the different chemical components of P. alkekengi, including steroids, flavonoids, alkaloids, phenylpropanoids, sucrose esters, piperazines, volatile oils, polysaccharides, amino acids, and trace elements. In addition, research related to the pharmacological activities of P. alkekengi, including its anti-inflammatory, anti microbial, antioxidative, hypoglycaemic, analgesic, anti-tumour, and immunomodulatory effects were investigated. Research articles from 1974 to 2023 were obtained from websites such as Google Scholar, Baidu Scholar, and China National Knowledge Infrastructure, and journal databases such as Scopus and PubMed, with the keywords such as Physalis alkekengi, components, effects, and activities. This study aims to provide a comprehensive understanding of the progress of phytochemical and pharmacological research on the phytochemical and pharmacological aspects of P. alkekengi and a reference for the better exploitation of P. alkekengi in the food and pharmaceutical industries.
Keywords: Physalis alkekengi, Chemical components, Pharmacological effects, Mechanisms, Physalins, Botanical origin
Graphical abstract
1. Introduction
Physalis Calyx seu Fructus, also known as Jin-Deng-Long, is the dried calyx or calyx with fruit of Physalis alkekengi L. var. franchetii (Mast.) Makino, which is a perennial herb in the Solanaceae family. It is widely distributed in Europe and Asia, including Korea and Japan, and is also cultivated in North America. In China, it is cultivated mainly in the northeast, northwest, and Inner Mongolia, with more widespread cultivation and wild growth in the northeast [1,2].
Physalis alkekengi has been used in medicine for nearly two thousand years and has been recorded in many herbal books throughout the ages, with a wide range of functions and applications. It was first recorded in Erya [3], one of the earliest dictionaries in China, and was annotated by Guo Pu. The earliest publication on traditional Chinese medicine, Shen Nong Ben Cao Jing [4] of the Han Dynasty, records that P. alkekengi has flat nature and sour flavour, and is used to treat fever and fullness, calm the mind and invigorate the vital energy, facilitate the flow of water, and alleviate pain during childbirth. Li Shizhen of the Ming dynasty recorded in Compendium of Materia Medica [5] that its seedlings, leaves, roots, and stems have bitter flavour and cold nature, and are non-toxic, and are used to relieve heat and fullness, calm the mind, improve vitality, and aid diuresis. According to Shen Nong Ben Cao Jing, the juice of P. alkekengi is effective in treating jaundice. Additionally, P. alkekengi has been used to treat sore throat, hoarse voice, cough with phlegm, aspergillosis, and eczema [6].
At present, more than 530 compounds have been isolated from P. alkekengi, mainly including steroids, flavonoids, alkaloids, phenylpropanoids, sucrose esters, piperazines, volatile oils, polysaccharides, various amino acids, and trace elements [[7], [8], [9], [10]]. Modern pharmacological studies have shown that P. alkekengi has anti-inflammatory, anti microbial, antioxidative, hypoglycaemic, analgesic, anti-tumour, and immune regulating effects and is of great nutritional and medicinal value. However, despite its many potential therapeutic and culinary applications, P. alkekengi is not being exploited for these applications on a large scale. In order to further exploit and utilize this natural resource, data relating to the chemical composition and pharmacological research of P. alkekengi from 1974 to 2023 were obtained using websites, such as Google Scholar, Baidu Scholar, and China National Knowledge Infrastructure, and journal databases, such as Scopus and PubMed, with the keywords such as Physalis alkekengi, components, effects, and activities, in an attempt to comprehensively review the chemical composition and pharmacological research progress of P. alkekengi.
2. Botanical origin
A botanical map of P. alkekengi L. var. franchetii (Mast.) Makino and pictures of dried calyxes and fruits are shown in Fig. 1(a–d). The stems are sparsely branched or unbranched, and the nodes are sometimes dilated and often pubescent, especially the younger parts. The leaves vary in shape from long-ovate to broadly ovate and sometimes rhombic-ovate, are between 5 and 15 cm long and 2–8 cm wide, apically acuminate, and have bases that are asymmetrically and narrowly cuneate. Their margins are either complete and undulate or are coarsely toothed, and both surfaces are pilose, with greater density along the nerves. Petioles are between 1 and 3 cm long. Pedicels are between 0.6 and 1.6 cm long, erect when flowering, but curve downwards in maturity, and are densely pilose but not deciduous. Calyxes are broadly campanulate, approximately 0.6 cm in length, densely pilose, with triangular teeth and hirsute margins. It has a white, rotate corolla, between 1.5 and 2 cm in diameter, with broad and short lobes spreading apically, which abruptly narrows into a triangular spike; the exterior is pubescent, and the margin is ciliate. The stamens and style are both shorter than the corolla. The fruiting pedicel is 2–3 cm long and persistently pilose. The fruiting calyx is ovate, 2.5–4 cm long and 2–3.5 cm wide, thinly leathery, and conspicuously reticulate, with 10 longitudinal ribs. It is orange or fiery red in colour, persistently pilose, apically closed, and the base is depressed. The soft, juicy berries are globose, orange-red in colour, and 1–1.5 cm in diameter. Finally, the seeds are reniform, yellowish in colour, and approximately 0.2 cm in length [2,11,12].
Fig. 1.
Images of P. alkekengi. (a) The whole plant; (b) Dried calyxes with fruits; (c) Dried calyxes; (d) Dried fruits.
3. Chemical components
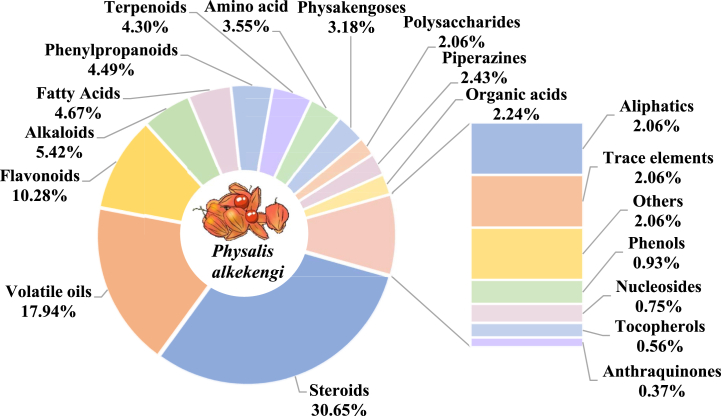
Among the 530 chemical constituents isolated from P. alkekengi, steroids and flavonoids are the main active ingredients. The composition percentages of each compound type in P. alkekengi are shown in Fig. 2, and the compound information is summarised in Table 1.
Fig. 2.
Proportion of compound types isolated from P. alkekengi.
Table 1.
Compounds in Physalis alkekengi.
| NO. | Compound | Molecular Formula | Origin Parts | Reference |
|---|---|---|---|---|
| Steroids | ||||
| 1 | Physalin A | C28H30O10 | Stems, Leaves | [7,8,10,15,32] |
| 2 | Physalin B | C28H30O9 | Stems, Leaves | [7,8,10,15,32] |
| 3 | Physalin C | C28H30O9 | Calyxes | [7,8,10,33] |
| 4 | Physalin D | C28H32O11 | Fruits | [7,8,10,34] |
| 5 | Physalin D1 | C28H32O11 | Fruits | [35] |
| 6 | Physalin E | C28H32O11 | Calyxes | [7,10,36] |
| 7 | Physalin F | C28H30O10 | Calyxes | [7,10,26,37] |
| 8 | Physalin G | C28H30O10 | Calyxes | [7,8,10,26] |
| 9 | Physalin H | C28H31ClO10 | Calyxes | [7,10,26] |
| 10 | Physalin I | C29H34O11 | Whole plants | [7,10,38] |
| 11 | Physalin J | C28H32O11 | Stems, Leaves | [7,10,15] |
| 12 | Physalin J1 | C28H32O11 | Stems, Leaves | [15] |
| 13 | Physalin K | C28H30O11 | Leaves | [8,10] |
| 14 | Physalin L | C28H32O10 | Whole plants | [7,8,10,39] |
| 15 | Physalin M | C28H32O9 | Whole plants | [7,8,10,39] |
| 16 | Physalin N | C28H30O10 | Fruits, Calyxes | [7,8,10,40] |
| 17 | Physalin O | C28H32O10 | Fruits, Calyxes | [7,8,10,40] |
| 18 | Physalin P | C28H30O10 | Fruits | [41] |
| 19 | Physalin Q | C28H30O12 | Leaves | [7] |
| 20 | Physalin QQ | C29H34O10 | Roots, Stems | [42] |
| 21 | Physalin R | C28H30O9 | Epigeal parts | [7,8,10,32] |
| 22 | Physalin S | C28H32O10 | Epigeal parts | [7,8,10] |
| 23 | Physalin T | C28H34O11 | Calyxes | [7,8,43] |
| 24 | Physalin U | C29H34O11 | Whole plants | [7] |
| 25 | Physalin V | C30H34O10 | Whole plants | [7] |
| 26 | Physalin W | C30H36O11 | Whole plants | [7,8,38] |
| 27 | Physalin W′ | C28H30O10 | Aerial parts | [7,8] |
| 28 | Physalin X | C28H30O10 | Roots, Stems | [7,8,42] |
| 29 | Physalin X′ | C28H30O10 | Calyxes | [33] |
| 30 | Physalin Y | C28H32O10 | Calyxes | [7,33] |
| 31 | Physalin Z | C28H30O10 | Calyxes | [7,33] |
| 32 | Physalin Ⅰ | C29H34O10 | Calyxes | [7,33] |
| 33 | Physalin Ⅱ | C29H34O10 | Calyxes | [7,33] |
| 34 | Physalin III | C28H32O12 | Calyxes | [44] |
| 35 | Physalin IV | C28H32O12 | Calyxes | [44] |
| 36 | Physalin V | C28H32O10 | Calyxes | [45] |
| 37 | Physalin VI | C28H32O11 | Calyxes | [45] |
| 38 | Physalin VII | C29H34O11 | Calyxes | [45] |
| 39 | Physalin XIII | C29H32O11 | Whole plants | [46] |
| 40 | Isophysalin I | C29H34O11 | Calyxes | [45] |
| 41 | Isophysalin A | C28H29O10 | Calyxes | [45] |
| 42 | Isophysalin B | C28H30O9 | Stems, Leaves | [7,8,15,42] |
| 43 | Isophysalin G | C28H30O10 | Calyxes | [7,8,47] |
| 44 | Alkekengilin A | C28H28O9 | Calyxes | [7,8,48] |
| 45 | Alkekengilin B | C28H28O9 | Calyxes | [7,8,48] |
| 46 | 2,3,25,27-Tetrahydrophysalin A | C28H34O10 | Calyxes | [33] |
| 47 | 3-Hydroxyphysalin A | C28H32O11 | Calyxes | [33] |
| 48 | 3-Methoxyphysalin A | C29H34O11 | Calyxes | [33] |
| 49 | 3-Methoxy-7-hydroxy-6-deoxyphysalin D | C29H36O12 | Calyxes | [33] |
| 50 | 3-Methoxy-6,7,9,10-tetradehydrophysalin B | C29H32O10 | Calyxes | [33] |
| 51 | 3-O-Methylphysalin X | C29H32O10 | Calyxes | [44] |
| 52 | 3β-Hydroxy-2-hydrophysalin A | C28H32O11 | Calyxes | [7,49] |
| 53 | 3β-ethoxyl-2,3-dihydro-4,7-didehydrophysalin B | C30H34O10 | Calyxes, Fruits | [50] |
| 54 | 7α-Hydroxy-5-deoxy-4-dehydrophysalin IX | C28H30O11 | Fruits, Calyxes | [51] |
| 55 | 7β-Hydroxyphysalin A | C28H30O10 | Fruits, Calyxes | [33,40] |
| 56 | 7β-Hydroxyphysalin L | C28H32O10 | Calyxes | [26,33,41] |
| 57 | 7β-Hydroxy-25,27-didehydrophysalin L | C28H30O10 | Calyxes | [33] |
| 58 | 7β-Hydroxyphysalin O | C28H32O10 | Calyxes | [33] |
| 59 | 7β-Methoxylisophysalin B | C29H32O10 | Calyxes | [45] |
| 60 | 7β-Methoxylisophysalin C | C29H32O10 | Calyxes | [45] |
| 61 | 7β-Ethoxyl-isophysalin C | C30H34O10 | Calyxes, Fruits | [50] |
| 62 | 4,7-Dehydrophysalin B | C28H29O9 | Calyxes | [45] |
| 63 | 4,7-Didehydrophysalin B | C28H28O9 | Calyxes, Roots, Stems | [33,36,41,42] |
| 64 | 4,7-Didehydroneophysalin B | C28H28O9 | Calyxes, Fruits | [41] |
| 65 | 4,7-Didehydro-7-deoxyphysalin A | C28H28O9 | Calyxes | [33] |
| 66 | 4,7-Didehydro-7-deoxyneophysalin A | C28H28O9 | Calyxes | [26,33] |
| 67 | 4,7-Didehydro-7-deoxyneophysalin L | C28H30O9 | Calyxes | [7] |
| 68 | 4-Hydroxy-25,27-dihydroneophysalin A | C28H32O11 | Calyxes | [33] |
| 69 | 25,27-Didehydrophysalin L | C28H30O10 | Calyxes | [26,52] |
| 70 | 25,27-Dihydro-4,7-didehydro-7-dehydroneophysalin A | C28H30O9 | Calyxes | [7,8,10] |
| 71 | 25,27-Dihydro-4,7-didehydro-7-deoxyphysalin A | C28H30O9 | Calyxes | [33] |
| 72 | 25,27-Dihydro-4,7-didehydro-7-deoxyneophysalin A | C28H30O9 | Calyxes | [7,30,36] |
| 73 | 3α-Methoxy-2,3-dihydro-4,7-didehydrophysalin B | C29H32O10 | Stems, Leaves | [53] |
| 74 | 3β-Methoxy-2,3-dihydro-4,7-didehydrophysalin B | C29H32O10 | Stems, Leaves | [53] |
| 75 | 5,6α-Epoxy-physalin C | C28H30O10 | Calyxes | [33] |
| 76 | 5,6β-Epoxy-physalin C | C28H30O10 | Calyxes | [33] |
| 77 | 5-Deoxy-4-dehydrophysalin IX | C28H30O10 | Fruits, Calyxes | [51] |
| 78 | 5α-Ethoxy-6β- hydroxy-5,6-dihydrophysalin B | C30H36O11 | Calyxes, Fruits | [54] |
| 79 | 5α-hydroxy-7-dehydro-25,27-dihydro-7-deoxyneophysalin A | C28H32O10 | Calyxes | [49] |
| 80 | 5α-Hydroxy-25,27-dihydro-4,7-didehydro-7-deoxyneophysalin A | C28H32O10 | Calyxes | [30,55] |
| 81 | 5α-Hydroxy-25,27-dihydro-7-dehydro-7-deoxyneophysalin A | C28H32O10 | Fruits, Calyxes | [56] |
| 82 | 5α,7α-Dihydroxy-25,27-dihydrophysalin A | C28H34O11 | Calyxes | [33] |
| 83 | 5α,7β-Dihydroxy-25,27-dihydrophysalin A | C28H34O11 | Calyxes | [33] |
| 84 | 5α,6β-dihydroxy-25,27-dihydro-7-deoxyphysalin A | C28H34O11 | Calyxes | [49] |
| 85 | 5α,6β-Dihydroxyphysalin C | C28H32O11 | Fruits | [29] |
| 86 | 5α,6β-Dihydroxyphysalin R | C28H32O11 | Calyxes | [7,49] |
| 87 | 5β,6β-Dihydroxyphysalin D | C28H32O11 | Calyxes | [33] |
| 88 | 6-Hydroxy-4,5-didehydro-7-deoxyphysalin A | C28H30O10 | Calyxes | [33] |
| 89 | 6-Hydroxy-25,27-dihydro-4,5-didehydro-7-deoxyphysalin A | C28H32O10 | Calyxes | [33] |
| 90 | 16,24-cyclo-13,14-secoergosta-2-ene-18,26-dioic acid-14:17,14:27-diepoxy-11β,13,20,22-tetrahydroxy-5α-methoxy-1,15-dioxo-γ-lactone-δ-lactone | C28H30O12 | Calyxes | [45] |
| 91 | Physagulin A | C30H38O7 | Whole plants | [7,10] |
| 92 | Physagulin B | C30H39ClO7 | Whole plants | [7,10,38] |
| 93 | Physagulin D | C34H52O10 | Whole plants | [7,10] |
| 94 | Physagulin J | C30H42O8 | Whole plants | [38] |
| 95 | Withaphysalin B | C28H36O6 | Calyxes | [57] |
| 96 | Withaphysalin E | C28H34O7 | Calyxes | [7,10] |
| 97 | Withaphysalin F | C28H36O7 | Calyxes | [7,10] |
| 98 | Withaphysalin G | C28H36O6 | Calyxes | [7,10] |
| 99 | Withaphysalin N | C28H36O7 | Calyxes | [57] |
| 100 | Withaphysalin U | C30H41ClO7 | Calyxes | [57] |
| 101 | Withagulatin A | C28H38O6 | Roots, Stems | [42] |
| 102 | Withanolide A | C28H38O6 | Roots, Stems | [7,58] |
| 103 | Withangulatin A | C30H38O8 | Whole plants | [38] |
| 104 | Withaminimin | C30H40O8 | Whole plants | [38] |
| 105 | Withalkekengin | C30H41ClO7 | Whole plants | [38] |
| 106 | Physapubescin | C30H42O8 | Stems, Leaves | [15] |
| 107 | Physapubescin G | C30H42O8 | Stems, Leaves | [15] |
| 108 | Physapubescin I | C32H44O10 | Stems, Leaves | [15] |
| 109 | Physapubescin K | C31H44O8 | Stems, Leaves | [15] |
| 110 | Physapubescin M | C27H38O7 | Stems, Leaves | [15] |
| 111 | Physapubescin N | C28H42O8 | Stems, Leaves | [15] |
| 112 | Alkekenginin A | C30H42O8 | Fruits | [35] |
| 113 | Alkekenginin B | C33H54O9 | Fruits | [35] |
| 114 | Alkekenginin C | C32H46O11 | Stems, Leaves | [15] |
| 115 | Alkekenginin D | C31H46O9 | Stems, Leaves | [15] |
| 116 | Alkekenginin E | C30H44O9 | Stems, Leaves | [15] |
| 117 | Alkekenginin F | C31H46O9 | Stems, Leaves | [15] |
| 118 | 26-carbonyl-physapubescin A | C30H48O8 | Stems, Leaves | [15] |
| 119 | 26-ethoxy-physapubescin B | C32H46O8 | Stems, Leaves | [15] |
| 120 | 5-hydroxyl-6-chloro-physapubescin B | C30H43ClO8 | Stems, Leaves | [15] |
| 121 | Philadelphicalactone A | C28H40O7 | Fruits | [59] |
| 122 | 15-hydroxy-withaphysalin B | C28H36O7 | Calyxes | [57] |
| 123 | 15-hydroxy-withphysalin U | C28H34O6 | Calyxes | [57] |
| 124 | (17S,20R,22R)-5β,6β-epoxy-18,20-dihydroxy-1-oxowitha-2,24-dienolide | C28H38O6 | Calyxes | [57] |
| 125 | (17S, 20R,22R)-5β,6β:18,20-diepoxy-15α,18β-dihydroxy-1-oxowitha-24-enolide (18R and 18S) | C28H38O7 | Calyxes | [57] |
| 126 | (17S,20R,22R)-5β,6β:18,20-diepoxy-18β-hydroxy-1-oxowitha-24-enolide (18R and 18S) | C28H38O6 | Calyxes | [57] |
| 127 | (20S.22R)-15α-acetoxy-5α-chloro-6β,14β-dihydroxy-1-oxowitha-2,24-dienolide | C30H41ClO7 | Whole plants | [60] |
| 128 | (22R)-5β,6β:14α,17:14β,26-triepoxy-2α-ethoxy-13,20,22-trihydroxy-1,15-dioxo-16α,24-cyclo-13,14-secoergosta-18,27-dioic acid 18 → 20,27 → 22-dilactone | C30H36O11 | Whole plants | [60] |
| 129 | 23-hydroxy-jitosapogenin-3-O-β-d-glucose-(1 → 4)-β-d-galacto side | C39H64O15 | Fruits | [61] |
| 130 | 26-O-β-d-glucopyranosyl-3β,20α,26-triol-25(R)-Δ5,22-diene-furosta-3-O-α-l-rhamnopyranosyl(1 → 2)-[α-l-rhamnopyranosyl (1 → 4)]-β-d-glucopyranosyl | C51H82O22 | Fruits | [61] |
| 131 | 2α,3β-dihydroxy-5α-pregn-16-en-20-one-3-O-β-D-glucopyranos yl-(1 → 4)-β-d-galactopyranoside | C33H52O13 | Fruits | [61] |
| 132 | Physanol A | C36H50O4 | Fruits, Seeds | [7,8,10,14,62] |
| 133 | Physanol B | C36H52O4 | Fruits, Seeds | [7,8,10,14,62] |
| 134 | Physalindicanol B | C28H46O2 | Calyxes, Fruits | [54] |
| 135 | Gramisterol | C29H48O | Calyxes, Fruits | [7,10,14,54] |
| 136 | Obtusifoliol | C30H50O | Calyxes, Fruits | [7,10,14] |
| 137 | Saringosterol | C29H48O2 | Calyxes | [8] |
| 138 | β-Sitosterol | C29H50O | Fruits, Calyxes | [30] |
| 139 | 7-Oxo-β-sitosterol | C29H48O2 | Whole plants | [46] |
| 140 | 7β-Hydroxysitosterol | C29H50O2 | Whole plants | [46] |
| 141 | Sargassuol A | C27H43O3 | Whole plants | [46] |
| 142 | Stigmasterol | C29H48O | Calyxes | [32] |
| 143 | 14α-Methyl-5α-β(11) cholesterol | C28H48O | Calyxes | [7,8] |
| 144 | Cholesterol | C27H46O | Calyxes | [7,10] |
| 145 | 24-Methyl-cholesterol | C28H48O | Calyxes | [7] |
| 146 | 24-Ethyl-cholesterol | C29H50O | Calyxes | [10] |
| 147 | Cycloartanol | C30H52O | Seeds | [7,10] |
| 148 | Cycloartenol | C30H50O | Seeds | [7,10] |
| 149 | Lanost-8-en-3β-ol | C30H52O | Seeds | [10] |
| 150 | Daucosterol | C35H60O6 | Calyxes | [58,63] |
| 151 | Isofucosterol | C29H48O | Roots, Stems | [7,58] |
| 152 | 3β,24ξ-Dihydroxy-ergosta-5, 25-dienolide | C28H46O2 | Whole plants | [38] |
| 153 | Ergosta-5,25-diene-3β, 24ξ-diol | C28H46O2 | Calyxes, Fruits | [54] |
| 154 | (22E)-5α,8α-Epidioxyergosta-6,22-dien-3β-ol | C28H44O3 | Calyxes, Fruits | [54] |
| 155 | (3β)-3-Hydroxy-26,27-dinorcholest-5-en-24-one | C25H40O2 | Calyxes, Fruits | [54] |
| 156 | 26,27-Dinorcholest-4-ene-3,24-dione | C25H38O2 | Calyxes, Fruits | [54] |
| 157 | (3β, 22E)-3-Hydroxy-26,27-dinorcholesta-5,22-dien-24-one | C25H38O2 | Calyxes, Fruits | [54] |
| 158 | 3β-Hydroxy-(22E,24R)-ergosta-5,8,22-trien-7-one | C28H42O2 | Calyxes, Fruits | [54] |
| 159 | 3β-Hydroxystigmasta-5,22-dien-7-one | C29H46O2 | Whole plants | [46] |
| 160 | Stigmasta-5,22-dien-3β,7β-diol | C29H48O2 | Whole plants | [46] |
| 161 | 3β-hydroxy-cholest-5-en-7-one | C27H44O2 | Whole plants | [46] |
| 162 | (24R)-5,28-stigmastadiene-3β,24-diol-7-one | C29H47O10 | Whole plants | [46] |
| 163 | (24S)-5,28-stigmastadiene-3β,24-diol-7-one | C29H47O10 | Whole plants | [46] |
| 164 | Gitogenin | C27H44O4 | Calyxes, Fruits | [54] |
| Flavonoids | ||||
| 165 | Physaflavonol | C17H14O8 | Calyxes, Aerial parts | [52] |
| 166 | Ombuine | C17H14O7 | Calyxes | [7,8,14,20] |
| 167 | Luteolin | C15H10O6 | Calyxes | [26,63] |
| 168 | Cynaroside | C21H20O11 | Calyxes | [26] |
| 169 | Catechin | C15H14O | Calyxes | [26] |
| 170 | L-Epicatechin | C15H14O6 | Calyxes | [26] |
| 171 | Rutin | C27H30O16 | Calyxes | [26] |
| 172 | Quercetin | C15H10O7 | Fruits, Calyxes | [26,40,63] |
| 173 | Kaempferide | C16H12O6 | Fruits, Calyxes | [40] |
| 174 | Kaempferol | C15H10O6 | Calyxes | [7,30] |
| 175 | Myricetin | C15H10O8 | Calyxes | [7,30] |
| 176 | Diosmetin | C16H12O6 | Calyxes | [26] |
| 177 | Apigenin | C15H10O5 | Calyxes | [7,26] |
| 178 | Chrysoeriol | C16H12O6 | Calyxes, Fruits | [7,8,54] |
| 179 | Eriodictyol | C15H10O5 | Calyxes, Fruits | [54] |
| 180 | Phytolaccin | C17H14O7 | Roots, Stems | [42] |
| 181 | Rhamnazin | C17H14O7 | Calyxes, Fruits | [54] |
| 182 | Wogonin | C16H12O5 | Fruits, Calyxes | [56] |
| 183 | Nobiletin | C21H22O8 | Fruits, Calyxes | [56] |
| 184 | Liquiritigenin | C15H12O4 | Fruits, Calyxes | [56] |
| 185 | Luteolin-4′-O-glucoside | C21H20O11 | Calyxes, Fruits | [64] |
| 186 | Luteolin-7-O-glucoside | C21H20O11 | Calyxes, Fruits | [64] |
| 187 | Luteolin-7-β-D-glucoside | C21H20O11 | Calyxes | [10] |
| 188 | Luteolin-4-O-β-D-glucoside | C21H20O11 | Calyxes, Fruits | [40] |
| 189 | Luteolin-7-O-α-D-glucoside | C21H20O11 | Calyxes | [36] |
| 190 | Luteolin-7-O-β-D-glucoside | C21H20O11 | Roots, Stems | [7,58,65,66] |
| 191 | Luteolin-7-O-α-d-glucopyranoside | C21H20O11 | Calyxes | [14] |
| 192 | Luteolin-7-O-β-d-glucopyranoside | C21H20O11 | Calyxes | [30] |
| 193 | Luteolin-4′-O-β-d-glucopyranoside | C21H20O11 | Calyxes | [30,66] |
| 194 | Luteolin-7,4′-di-O-β-d-glucopyranoside | C27H30O16 | Calyxes | [7,8,14,20] |
| 195 | Luteolin-7,3′-di-O-β-d-glucopyranoside | C27H30O16 | Calyxes | [66] |
| 196 | Quercetin-3-O-β-d-glucopyranoside | C21H20O12 | Calyxes | [66] |
| 197 | Quercetin-3,7-di-O-β-d-glucopyranoside | C27H30O17 | Calyxes | [66] |
| 198 | 3′,4′-O-demethyl quercetin | C17H14O7 | Calyxes | [37] |
| 199 | 3′,4′-Dimethoxymyricetin | C17H14O8 | Calyxes | [8,67] |
| 200 | 5,7-Dimethoxycoumarin | C11H10O4 | Calyxes | [26] |
| 201 | 5,4′,5′-Trihydroxy-7,3′-dimethoxyflavonol | C17H14O8 | Fruits, Calyxes | [7,8] |
| 202 | 5,6,7-Trimethoxy-flavone | C18H16O5 | Calyxes | [7] |
| 203 | Isoquercitrin | C21H20O12 | Fruits, Calyxes | [7,26,30,40] |
| 204 | Kaemperide-3-O-glucoside | C22H22O11 | Fruits, Calyxes | [40] |
| 205 | 4′-Methoxy kaempferol | C16H12O6 | Calyxes | [36] |
| 206 | Kaempferol-4′-methoxy-7-O-β-d-glucopyranoside | C22H20O11 | Calyxes | [7,30] |
| 207 | Kaempferol-4′-methoxy-3-O-β-d-glucopyranoside | C22H20O11 | Calyxes | [7,30] |
| 208 | Kaempferol-3-O-β-d-Glucose | C21H10O11 | Calyxes | [37] |
| 209 | Dihydrokaempferol-7-O-glucoside | C21H22O11 | Calyxes, Fruits | [40] |
| 210 | 3,7-di-O-α-L-rhamnopyransoyl kaempferol | C27H22O14? | Calyxes | [37] |
| 211 | Apigenin-7-glucoside | C21H20O10 | Calyxes | [26] |
| 212 | Apigenin-7-O-β-D-glucoside | C21H20O10 | Calyxes | [68] |
| 213 | Apigenin-7-O-β-d-glucopyranoside | C21H20O10 | Calyxes | [14] |
| 214 | Chrysoeriol-7-O-β-glucopyranoside | C22H22O11 | Calyxes, Fruits | [41] |
| 215 | Chrysoeriol-7-O-β-D-glucoside | C22H22O11 | Calyxes | [68] |
| 216 | Diosmetin-O-β-d-glucopyranoside | C22H22O11 | Calyxes | [16] |
| 217 | Diosmetin-7-O-β-D-glucoside | C22H22O11 | Calyxes | [68] |
| 218 | Malvidin-3-O-glucoside | C23H24O12 | Calyxes, Fruits | [40] |
| 219 | Rhamnazin-3-O-glucopyranoside | C23H26O12 | Calyxes, Fruits | [64] |
| Alkaloids | ||||
| 220 | 3α-Tigloyloxytropane | C13H21NO2 | Roots | [7,8,10,14] |
| 221 | Tigloidine | C13H21NO2 | Roots | [8,14] |
| 222 | Tropine | C8H15NO | Roots | [7,8,10,14] |
| 223 | Hygrine | C8H15NO | Roots | [7,8] |
| 224 | Cuscohygrine | C13H24N2O | Roots | [7,8,14] |
| 225 | Pseudotropine | C8H15NO | Roots | [7,8,10] |
| 226 | 3α-Tigloyloxy tropane N-oxide | C13H21NO3 | Roots | [7,8,10] |
| 227 | Phygrine | C6H28N2O2 | Roots | [8,14,69] |
| 228 | Calystegin A3 | C7H13NO3 | Roots | [8,70] |
| 229 | Calystegin A5 | C7H13NO3 | Roots | [8,70] |
| 230 | Calystegin B1 | C7H13NO4 | Roots | [8,70] |
| 231 | Calystegin B2 | C7H13NO4 | Roots | [8,70] |
| 232 | Calystegin B3 | C7H13NO4 | Roots | [8,70] |
| 233 | Calystegin C1 | C7H13NO5 | Roots | [8,70] |
| 234 | 1β-Amino-2α,3β,5β-trihydroxycycloheptane | C7H16NO3 | Roots | [8,14,70] |
| 235 | Anaferine | C13H24N2O | Roots | [8] |
| 236 | Anahygrine | C13H24N2O | Roots | [8] |
| 237 | Trans-N-feruloyl-3-O-methyldopamine | C19H21NO5 | Calyxes, Fruits | [41] |
| 238 | 5-Hydroxy-2-pyridinemethanol | C6H7NO2 | Calyxes, Fruits | [41] |
| 239 | Feruloyltyramine | C18H19NO4 | Calyxes, Fruits | [64] |
| 240 | N-trans-feruloyltyramine | C18H19NO4 | Calyxes | [31] |
| 241 | N-p-coumaroyltyramine | C17H17NO3 | Calyxes | [31] |
| 242 | Neoechinulin A | C19H21N3O2 | Calyxes, Fruits | [64] |
| 243 | 3-(4-hydroxy-3-methoxyphenyl)-N-(4-methylphenyl)-2-propenamide | C17H17NO3 | Calyxes, Fruits | [64] |
| 244 | Aurantiamide | C25H26N2O3 | Calyxes, Fruits | [54] |
| 245 | Isoechinulin A | C24H29N3O3 | Calyxes, Fruits | [54] |
| 246 | N-benzoyl-L-phenylalaninol | C16H17NO2 | Calyxes, Fruits | [54] |
| 247 | Aurantiamide acetate | C27H28N2O4 | Calyxes, Fruits | [54] |
| 248 | Ginsenine | C13H14N2O2 | Fruits | [61] |
| Phenylpropanoids | ||||
| 249 | Ferulic acid | C10H10O4 | Calyxes | [16,26] |
| 250 | Trans-ferulic acid | C10H10O4 | Whole plants | [39] |
| 251 | Chlorogenic acid | C16H18O9 | Fruits, Calyxes | [16,31,41] |
| 252 | Syringalide B | C24H28O10 | Fruits, Calyxes | [16,41,68] |
| 253 | Syringaresinol | C22H26O8 | Fruits, Calyxes | [41] |
| 254 | 3-Caffeoylquinic acid methyl ester | C18H22O9 | Fruits, Calyxes | [16,31,41] |
| 255 | (+)-Medioresinol-O-β-D-di-glucopyranoside | C33H44O17 | Fruits, Calyxes | [16,68] |
| 256 | (+) -Syringaresinol-O-β-D-di-glucopyranoside | C34H46O18 | Fruits, Calyxes | [16,41,68] |
| 257 | (+)-Pinoresinol-O-β-D-di-glucopyranoside | C32H42O16 | Fruits, Calyxes | [16,41,68] |
| 258 | Scopoletin-7-O-β-D-di-glucopyranoside | C22H28O14 | Fruits, Calyxes | [68] |
| 259 | Syringaresinol-4′-O-β-d-glucopyranoside | C28H36O13 | Calyxes | [31] |
| 260 | p-Coumaric acid | C9H8O3 | Calyxes | [26,36,41] |
| 261 | 6,6′,7,7′-Tetrahydroxy-5,5′-dicoumarol | C18H10O8 | Calyxes | [36] |
| 262 | Caffeic acid | C9H8O4 | Fruits, Calyxes | [30,40,41] |
| 263 | Esculetin | C9H6O4 | Calyxes | [26,30] |
| 264 | 8-Hydroxy-7-methoxycoumarin | C10H8O4 | Fruits, Calyxes | [41] |
| 265 | 3,4-Dimethoxy-5-hydroxy-cinnamyi alcohol-9-O-β-d-glucopyranoside | C17H24O9 | Fruits, Calyxes | [41] |
| 266 | Sachaliside 1 | C15H20O7 | Fruits, Calyxes | [41] |
| 267 | 3-caffeoyl quinic acid | C16H18O9 | Fruits, Calyxes | [40] |
| 268 | 4,5,3′,4′-Tetrahydroxy-2,7′-cycloligna-7,7′-dien-9,9′-olide | C18H12O6 | Calyxes | [30] |
| 269 | Syringaresinol-4,4′-O-di-β-D-glucoside | C34H46O18 | Calyxes | [30] |
| 270 | Cinnamic acid | C9H8O2 | Fruits | [71] |
| 271 | p-Hydroxy-cinnamic acid | C9H8O3 | Fruits | [71] |
| 272 | Schizandrin | C24H32O7 | Fruits, Calyxes | [56] |
| Terpenoids | ||||
| 273 | Physalisitin A | C15H24O3 | Calyxes | [16] |
| 274 | Physalisitin B | C15H24O2 | Calyxes | [16] |
| 275 | Physalisitin C | C15H22O2 | Calyxes | [16] |
| 276 | Citroside A | C19H30O8 | Fruits, Calyxes | [16,41] |
| 277 | (6S,9R)-Roseoside | C19H30O8 | Fruits, Calyxes | [16,41] |
| 278 | (6S,9S)-Roseoside | C19H30O8 | Fruits, Calyxes | [16,41] |
| 279 | (6R,9S)-3-Oxo-α-ionol-β-d-glucopyranoside | C19H30O7 | Fruits, Calyxes | [16,41] |
| 280 | Oleanolic acid | C30H48O3 | Calyxes | [37,58] |
| 281 | Physanoside A | C25H40O12 | Leaves, Stems | [16,72] |
| 282 | Physanoside B | C25H40O12 | Leaves, Stems | [16,72] |
| 283 | Neryl-1-O-β-d-glucopyranosyl-(1 → 2)-O-[α-L-arabinopy-ranosyl-(1 → 6)]-O-β-d-glucopyranoside | C27H46O15 | Calyxes | [16,31] |
| 284 | Ursolic acid | C30H48O | Calyxes | [16] |
| 285 | Blumenol A | C13H20O3 | Fruits, Calyxes | [56,64] |
| 286 | Dehydrovomifoliol | C13H18O3 | Fruits, Calyxes, Roots, Stems | [41,42] |
| 287 | 3β-Hydroxy-5,6-epoxy-7-megastigmen-9-one | C13H20O3 | Fruits, Calyxes | [41] |
| 288 | Rel-(3E)-4-[(1R,2R,4S)-1,2,4-trihydroxy-2,6,6-trimethylcyclohexyl]-3-buten-2-one | C13H22O4 | Fruits, Calyxes | [41] |
| 289 | 4aβ-Decahydro-8aα-methyl-4-methylene-6β-(1-methylethenyl)-1α,3α-naphthalenediol | C15H24O2 | Calyxes, Fruits | [54] |
| 290 | Capsidiol | C15H24O2 | Calyxes, Fruits | [54] |
| 291 | (+)-Anhydro-β-rotunol | C15H20O2 | Calyxes, Fruits | [54] |
| 292 | Pubinernoid A | C11H16O3 | Fruits, Calyxes | [41,54] |
| 293 | 4-(3,4-dihydroxy-4-methylpentyl)-3-(hydroxymethyl)-2,4-dimethylcyclohexa-2,5-dien-1-one | C15H24O4 | Roots, Stems | [42] |
| 294 | 7-(3-hydroxyprop-1-en-2-yl)-1,4a-dimethyl-5,6,7,8-tetrahydronaphthalen-2(4aH)-one | C15H20O2 | Roots, Stems | [42] |
| 295 | 3-O-α-l-Arabinopyranose-Hedera sapogenin-28-O-(4-O-acetyl)-α-l-rhamnopyranose-(1 → 4)-β-d-glucopyranose-(1 → 6)-β-d-glucopyranosyl | C49H78O19 | Fruits | [61] |
| Physakengoses | ||||
| 296 | Physakengose A | C29H50O13 | Aerial parts | [18] |
| 297 | Physakengose B | C33H56O14 | Aerial parts | [18] |
| 298 | Physakengose C | C31H52O14 | Aerial parts | [18] |
| 299 | Physakengose D | C29H50O13 | Aerial parts | [18] |
| 300 | Physakengose E | C34H58O14 | Aerial parts | [18] |
| 301 | Physakengose F | C34H56O14 | Aerial parts | [18] |
| 302 | Physakengose G | C36H60O15 | Aerial parts | [18] |
| 303 | Physakengose H | C36H58O15 | Aerial parts | [18] |
| 304 | Physakengose I | C36H62O14 | Aerial parts | [18] |
| 305 | Physakengose J | C36H60O14 | Aerial parts | [18] |
| 306 | Physakengose K | C38H64O15 | Aerial parts | [17] |
| 307 | Physakengose L | C35H58O15 | Aerial parts | [17] |
| 308 | Physakengose M | C35H58O15 | Aerial parts | [17] |
| 309 | Physakengose N | C35H58O14 | Aerial parts | [17] |
| 310 | Physakengose O | C34H58O14 | Aerial parts | [17] |
| 311 | Physakengose P | C22H34O13 | Aerial parts | [17] |
| 312 | Physakengose Q | C22H36O13 | Aerial parts | [17] |
| Piperazines | ||||
| 313 | (3S,6R)-3-isopropyl-6-(2-methyl propyl)-2,5-piperazine diketone | C11H20N2O2 | Calyxes | [19] |
| 314 | (3S, 6S)-3-isobutyl-6-isopropyl-2,5-piperazine diketone | C11H20N2O2 | Calyxes | [19] |
| 315 | (3S,6S)-3,6-di-(2-methyl propyl)-2,5-piperazine diketone | C12H22N2O2 | Calyxes | [19] |
| 316 | (3S,6S)-3,6-di-isopropyl-2,5-piperazine diketone | C10H18N2O2 | Calyxes | [19] |
| 317 | (3S,6R)-3-(2-methyl propyl)- 6-benzyl-2,5-piperazine diketone | C15H20N2O2 | Calyxes | [19] |
| 318 | (3S,6S)-3-isobutyl-6-benzyl-2,5-piperazine diketone | C15H20N2O2 | Calyxes | [19] |
| 319 | (3S,6S)-3-isopropyl-6-(p-hydroxy benzyl)-2,5-piperazine diketone | C14H18N2O3 | Calyxes | [19] |
| 320 | (3S,6R)-3-isopropyl-6-(p-hydroxy benzyl)-2,5-piperazine diketone | C14H18N2O3 | Calyxes | [19] |
| 321 | (3S,6R)-3-(2-methyl propyl)-6-(p-hydroxy benzyl)-2,5-piperazine diketone | C15H20N2O3 | Calyxes | [19] |
| 322 | (3S,6S)-3-isobutyl-6-(p-hydroxy benzyl)-2,5-piperazine diketone | C15H20N2O3 | Calyxes | [19] |
| 323 | (3S,6S)-3-isopropyl-6-benzyl-2,5-piperazine diketone | C14H18N2O2 | Calyxes | [19] |
| 324 | (3S,6R)-3-isobutyl-6-(2-methyl propyl)-2,5-piperazine diketone | C12H22N2O2 | Calyxes | [19] |
| 325 | (3S,6S)-3-benzyl-6-(p-hydroxy benzyl)-2, 5-piperazine diketone | C18H18N2O3 | Calyxes | [19] |
| Volatile oils | ||||
| 326 | 3,4-Dihydroxyphenethyl alcohol | C8H10O3 | Calyxes | [37] |
| 327 | Octanoic acid | C8H16O2 | Calyxes with fruit stalk | [20,21] |
| 328 | 3,7-dimethyl- (E)-2,6-Octadien-1-ol | C10H18O | Calyxes | [20] |
| 329 | 2,4-decadienal | C10H16O | Calyxes | [20] |
| 330 | 6,10-dimethyl-(Z)-5,9-undecadien-2-one | C13H22O | Calyxes | [20] |
| 331 | 4-(2,6,6-trimehyl-cyclohexen-1-yl)-(E)-3-buten-2-one | C13H20O | Calyxes | [20] |
| 332 | 6,11-dimethyl-2,6,10-dodecatrien-1-ol | C14H24O | Calyxes | [20] |
| 333 | Tetradecanoic acid | C14H28O2 | Calyxes | [20] |
| 334 | 2,3,5,8-tetramethyl-decane | C14H30 | Calyxes | [20] |
| 335 | 6,10,14-trimethyl-2-pentadecanone | C18H36O | Calyxes | [20] |
| 336 | 6,10,14- trimethyl-5,9,13-petadecatrien-2-one | C18H30O | Calyxes | [20] |
| 337 | 1-chloro-octadecane | C18H37Cl | Calyxes | [20] |
| 338 | 14-methyl-pentadecanoic acid-methyl ester | C17H34O2 | Calyxes | [20] |
| 339 | 1,(E)-11,(Z)-13-octadecatriene | C18H32 | Calyxes | [20] |
| 340 | (Z)-9-octadecenal | C18H34O | Calyxes | [20] |
| 341 | n Decanoic acid | C10H20O2 | Calyxes with fruit stalk | [21] |
| 342 | (E)-6,10-Dimethyl-5,9-undecadien-2-one | C13H22O | Calyxes with fruit stalk | [21] |
| 343 | (E)-4-(2,6,6-Trimethyl-1-cyclohexane-1alkenyl)-3-butene-2-one | C13H20O | Calyxes with fruit stalk | [21] |
| 344 | 3,3,7,7-Tetramethyl-5-(2-methyl-1-allyl)-tricyclic[4.1.0.0.2.4]-heptane | C15H24 | Calyxes with fruit stalk | [21] |
| 345 | 3,7,11- Trimethyl-1,6,10-dodecatrien-3-ol | C15H26O | Calyxes with fruit stalk | [21] |
| 346 | α-Bisabolol | C15H26O | Calyxes with fruit stalk | [21] |
| 347 | (−)-Spatula eucalyptol | C15H24O | Calyxes with fruit stalk | [21] |
| 348 | Isoaromadendrene oxide | C15H24O | Calyxes with fruit stalk | [21] |
| 349 | Decalin-1,1,4,7-tetramethyl-1H-cyclopropyl[e] azulene-4-ol | C15H26O | Calyxes with fruit stalk | [21] |
| 350 | Cubenol | C15H26O | Calyxes with fruit stalk | [21] |
| 351 | Cadinol | C15H26O | Calyxes with fruit stalk | [21] |
| 352 | Epiglobulol | C15H26O | Calyxes with fruit stalk | [21] |
| 353 | Oleyl alcohol | C18H34O2 | Calyxes with fruit stalk | [21] |
| 354 | 2-Nonadecanone | C19H38O | Calyxes with fruit stalk | [21] |
| 355 | 1,5-Dimethyl-3-hydroxy-8-(1-methylene-2-hydroxyethyl)-di-cyclo[4.4.0]decane-5-ene | C15H24O2 | Calyxes with fruit stalk | [21] |
| 356 | Trans-Longipino carvenol | C15H24O | Calyxes with fruit stalk | [21] |
| 357 | Myristic acid | C14H28O2 | Calyxes with fruit stalk | [21] |
| 358 | Solavetivone | C15H22O | Calyxes with fruit stalk | [21] |
| 359 | Pentadecanoic acid | C15H30O2 | Calyxes with fruit stalk | [21] |
| 360 | Hexahydrofarnesylacetone | C18H36O | Calyxes with fruit stalk | [21] |
| 361 | Farnesylacetone | C18H30O | Calyxes with fruit stalk | [21] |
| 362 | (Z)-7-Methyl hexadecenoate | C17H32O2 | Calyxes with fruit stalk | [21] |
| 363 | Butyl octyl phthalate | C20H30O4 | Calyxes with fruit stalk | [21] |
| 364 | n-Palmitic Acid | C16H32O2 | Calyxes, Fruits | [21,23,54] |
| 365 | 9,12-Octadecadienoic acid methyl ester | C19H34O2 | Calyxes with fruit stalk | [21] |
| 366 | 5- Dodecyl-2(3H)-furan | C16H30O2 | Calyxes with fruit stalk | [21] |
| 367 | 9,12-Linoleic acid | C18H32O2 | Calyxes with fruit stalk | [21] |
| 368 | Heptacosane | C27H56 | Calyxes with fruit stalk | [21] |
| 369 | Octacosane | C28H68 | Calyxes with fruit stalk | [21] |
| 370 | Methyl palmitate | C17H34O2 | Roots, Stems | [23] |
| 371 | Ethyl palmitate | C16H32O2 | Roots, Stems | [23] |
| 372 | (Z)9-Octadecenamide | C13H35NO | Roots, Stems | [23] |
| 373 | 6,9-Methyl octadecadienoate | C19H34O2 | Roots, Stems | [23] |
| 374 | 8,9-Didehydro-9-formylisolongifolene | C15H18O2 | Roots, Stems | [23] |
| 375 | Solavetivone | C15H22O | Roots, Stems | [23] |
| 376 | (E)11-Hexadecenoic acid | C16H30O2 | Roots, Stems | [23] |
| 377 | 5-Dodecyl-2-furanone | C16H30O2 | Roots, Stems | [23] |
| 378 | Aromadendrene-2-oxide | C15H24O | Roots, Stems | [23] |
| 379 | 1-Cyclohexyl heptene | C13H24 | Roots, Stems | [23] |
| 380 | (E)4-(2,6,6-trimethyl-2-cyclohexenyl)-3-butene-2ketone | C13H20O | Roots, Stems | [23] |
| 381 | 1-Pentadecene | C15H30 | Roots, Stems | [23] |
| 382 | Pentadecane | C15H32 | Roots, Stems | [23] |
| 383 | Hexadecane | C16H34 | Roots, Stems | [23] |
| 384 | Heptadecane | C17H36 | Roots, Stems | [23] |
| 385 | Octadecane | C18H38 | Roots, Stems | [23] |
| 386 | Nonadecane | C19H40 | Roots, Stems | [23] |
| 387 | Pentacosane | C25H52 | Roots, Stems | [23] |
| 388 | Tetratetracontane | C44H90 | Roots, Stems | [23] |
| 389 | (E)2,4-Diphenyl-4-methyl amylene | C18H20 | Roots, Stems | [23] |
| 390 | (Z,Z,Z)9,12,15-Octadecatrienoicacid, methyl ester | C19H32O2 | Roots, Stems | [23] |
| 391 | α-Pinene | C10H16 | Fruits | [28] |
| 392 | Camphene | C10H16 | Fruits | [28] |
| 393 | Sabinene | C10H16 | Fruits | [28] |
| 394 | β-Pinene | C10H16 | Fruits | [28] |
| 395 | Myrcene | C10H16 | Fruits | [28] |
| 396 | p-Cymene | C10H14 | Fruits | [28] |
| 397 | Limonene | C10H16 | Fruits | [28] |
| 398 | γ-Terpinene | C10H16 | Fruits | [28] |
| 399 | Camphenilone | C9H14O | Fruits | [28] |
| 400 | β-Linalool | C10H18O | Fruits | [28] |
| 401 | Nonanal | C9H18O | Fruits | [28] |
| 402 | Camphor | C10H16O | Fruits | [28] |
| 403 | 1-Terpinen-4-ol | C10H18O | Fruits | [28] |
| 404 | α-Terpineol | C10H18O | Fruits | [28] |
| 405 | Nerol | C10H18O | Fruits | [28] |
| 406 | n-Tridecane | C13H28 | Fruits | [28] |
| 407 | Isoamyl benzyl ether | C12H18O | Fruits | [28] |
| 408 | Neryl acetate | C12H20O2 | Fruits | [28] |
| 409 | Sibirene | C15H24 | Fruits | [28] |
| 410 | β-Caryophyllene | C15H24 | Fruits | [28] |
| 411 | Germacrene D | C15H24 | Fruits | [28] |
| 412 | β-Selinene | C15H24 | Fruits | [28] |
| 413 | α-Zingiberene | C15H24 | Fruits | [28] |
| 414 | Bicyclogermacrene | C15H24 | Fruits | [28] |
| 415 | δ-Cadinene | C15H24 | Fruits | [28] |
| 416 | α-Cadinene | C15H24 | Fruits | [28] |
| 417 | 1-epi-Cubenol | C15H26O | Fruits | [28] |
| 418 | (2E,6E)-Methyl farnesoate | C16H26O2 | Fruits | [28] |
| 419 | (2Z,6E)-Farnesyl acetate | C17H28O2 | Fruits | [28] |
| 420 | (5Z,9E)-Farnesyl acetone | C18H30O | Fruits | [28] |
| 421 | Phytol | C20H40O | Fruits | [28] |
| Polysaccharides | ||||
| 422 | Mannose | C6H12O6 | Roots, Stems | [23] |
| 423 | GlcUA | C6H10O7 | Roots, Stems | [23] |
| 424 | Galactose | C6H12O6 | Roots, Stems | [23] |
| 425 | Xylose | C5H10O5 | Roots, Stems | [23] |
| 426 | Arabinose | C5H10O5 | Roots, Stems | [23] |
| 427 | Rhamnose | C6H12O5 | Roots, Stems | [23] |
| 428 | α-d-glucose | C6H12O6 | Calyxes | [65] |
| 429 | GalA | C6H10O7 | Fruits | [73] |
| 430 | Fucose | C6H12O5 | Roots | [22] |
| 431 | Sucrose | C12H22O11 | Fruits | [61] |
| 432 | Maltose | C12H22O11 | Fruits | [61] |
| Amino acids | ||||
| 433 | Arginine | C6H14N4O2 | Calyxes | [26] |
| 434 | l-Phenylalanine | C9H11NO2 | Calyxes | [26] |
| 435 | Glutamic acid | C5H9NO4 | Calyxes | [26] |
| 436 | Valine | C5H11NO2 | Calyxes | [26] |
| 437 | l-Proline | C5H9NO2 | Calyxes | [71] |
| 438 | M-Phenylalanine | C9H11NO2 | Calyxes | [71] |
| 439 | l-Leucine | C6H13NO2 | Fruits | [71] |
| 440 | L-Tryptophan | C11H19N2O2 | Fruits | [71] |
| 441 | Aspartic acid | C4H7NO4 | Seeds | [28] |
| 442 | Serine | C3H7NO3 | Seeds | [28] |
| 443 | Glycine | C2H5NO2 | Seeds | [28] |
| 444 | Histidine | C6H9N3O2 | Seeds | [28] |
| 445 | Threonine | C4H9NO3 | Seeds | [28] |
| 446 | Alanine | C3H7NO2 | Seeds | [28] |
| 447 | Cysteine | C3H7NO2S | Seeds | [28] |
| 448 | Tyrosine | C9H11NO3 | Seeds | [28] |
| 449 | Methionine | C5H11NO2S | Seeds | [28] |
| 450 | Lysine | C6H14N2O2 | Seeds | [28] |
| 451 | Isoleucine | C6H13NO2 | Seeds | [28] |
| Fatty Acids | ||||
| 452 | Capric | C20H40O2 | Seeds, Peels | [28] |
| 453 | Undecylic | C11H22O2 | Seeds, Peels | [28] |
| 454 | Lauric | C12H24O2 | Seeds, Peels | [28] |
| 455 | Tridecylic | C13H26O2 | Seeds, Peels | [28] |
| 456 | Myristoleic | C14H26O2 | Seeds, Peels | [28] |
| 457 | Palmitoleic | C16H30O2 | Seeds, Peels | [28] |
| 458 | Margaric | C17H34O2 | Seeds, Peels | [28] |
| 459 | Heptadecenoic | C17H32O2 | Seeds, Peels | [28] |
| 460 | Stearic | C18H36O2 | Seeds, Peels | [28] |
| 461 | Oleic | C18H34O2 | Seeds, Peels | [28] |
| 462 | Linoleic | C18H32O2 | Seeds, Peels | [28] |
| 463 | Linolenic | C18H30O2 | Seeds, Peels | [28] |
| 464 | Eicosadienoic | C20H36O2 | Seeds, Peels | [28] |
| 465 | Eicosatrienoic | C20H34O2 | Seeds, Peels | [28] |
| 466 | Eicosatetraenoic | C20H32O2 | Seeds, Peels | [28] |
| 467 | Eicosapentaenoic | C20H30O2 | Seeds, Peels | [28] |
| 468 | n-Hexacosanoic acid | C26H52O2 | Fruits, Calyxes | [41] |
| 469 | Hendecanoic acid | C11H22O2 | Calyxes, Fruits | [54] |
| 470 | Tetra-cosanic acid | C24H48O2 | Calyxes | [37] |
| 471 | (Z)-9,10,11-trihydroxy-12-octadecenoic acid | C18H34O5 | Calyxes | [37,58] |
| 472 | Tricosanoic Acid | C23H46O2 | Aerial parts | [52] |
| 473 | Glyceryl monostearate | C21H42O4 | Roots, Stems | [58] |
| 474 | Glyceryl ester of Behenic Acid | C25H50O4 | Calyxes | [52] |
| 475 | Succinct acid | C4H6O4 | Fruits | [29] |
| 476 | (8,11)-Dienoic acid | C16H28O2 | Fruits | [29] |
| Organic acids | ||||
| 477 | Nicotinic acid | C6H5NO2 | Fruits | [71] |
| 478 | Vanillic acid | C8H8O4 | Fruits, Calyxes | [41] |
| 479 | Citric acid | C6H8O7 | Calyxes | [26,30,40] |
| 480 | Succinic acid | C4H6O4 | Fruits, Calyxes | [40] |
| 481 | Cumaric acid | C9H8O3 | Fruits, Calyxes | [40] |
| 482 | Quinic acid | C7H12O6 | Calyxes | [26] |
| 483 | Gallic acid | C7H6O5 | Calyxes | [26] |
| 484 | Gentisic Acid | C7H6O4 | Calyxes | [26] |
| 485 | 3-Indoleacrylic acid | C11H9NO2 | Calyxes | [26] |
| 486 | 5-Methyl-3-pyridinecarboxylicacid | C7H7NO2 | Fruits | [34] |
| 487 | 5-Hydroxymethylfuroic acid | C6H6O4 | Fruits | [34,64,74] |
| 488 | 2-((2-Ethylhexyloxy)carbonyl)benzoic acid | C16H22O4 | Fruits | [29] |
| Aliphatics | ||||
| 489 | N-tetracosane | C24H50 | Calyxes, Fruits | [64] |
| 490 | Dibutyl phthalate | C16H22O4 | Calyxes, Fruits | [64] |
| 491 | Bis(2-ethylhexyl)phthalate | C6H6O4 | Calyxes, Fruits | [64] |
| 492 | 1-O-(9Z,12Z-octadecadienoyl)glycerol | C21H38O4 | Calyxes, Fruits | [54] |
| 493 | Methyl(10E,12Z)-9-hydroxy-octadecadienoate | C19H34O3 | Calyxes, Fruits | [54] |
| 494 | 1,5-Dimethyl citrate | C8H12O7 | Fruits | [34,74] |
| 495 | 5-Hydroxymethylfurfural | C6H6O3 | Fruits, Calyxes | [56] |
| 496 | 5-(hydroxymethyl)-2-(dimethoxymethyl)furan | C8H12O4 | Fruits, Calyxes | [56] |
| 497 | 1-Citric acid ethyl ester | C8H12O7 | Fruits | [29] |
| 498 | 1-Citric acid methyl ester | C7H10O7 | Fruits | [29] |
| 499 | 9,12-Ethyl octadeca-9,12-dienoate | C20H36O2 | Fruits | [29] |
| Nucleosides | ||||
| 500 | Adenine | C5H5N5 | Calyxes | [31] |
| 501 | Adenosine | C10H13N5O4 | Calyxes | [31] |
| 502 | Guanosine | C10H13N5O5 | Calyxes | [26] |
| 503 | Uridine | C9H12N2O6 | Calyxes | [26] |
| Anthraquinones | ||||
| 504 | Emodin | C15H10O5 | Calyxes | [26] |
| 505 | Aurantio-obtusin-6-O-β-D-glucoside | C23H24O12 | Calyxes | [26] |
| Phenols | ||||
| 506 | Ethyl caffeate | C11H12O4 | Calyxes | [30] |
| 507 | Ethyl ferulate | C12H14O4 | Calyxes | [30] |
| 508 | Syringic acid | C9H10O5 | Calyxes, Fruits | [30,71] |
| 509 | Hydroxytyrosol | C8H10O3 | Fruits | [71] |
| 510 | Hydroquinone | C6H6O2 | Calyxes | [52] |
| Tocopherols | ||||
| 511 | α-Tocopherol | C29H50O2 | Seeds, Peels | [28] |
| 512 | β-Tocopherol | C28H48O2 | Seeds, Peels | [28] |
| 513 | γ-Tocopherol | C28H48O2 | Seeds, Peels | [28] |
| Trace elements | ||||
| 514 | Potassium (K) | K | Seeds | [28] |
| 515 | Sodium (Na) | Na | Seeds | [28] |
| 516 | Calcium (Ca) | Ca | Seeds | [28] |
| 517 | Magnesium (Mg) | Mg | Seeds | [28] |
| 518 | Iron (Fe) | Fe | Seeds | [28] |
| 519 | Manganese (Mn) | Mn | Seeds | [28] |
| 520 | Copper (Cu) | Cu | Seeds | [28] |
| 521 | Zinc (Zn) | Zn | Seeds | [28] |
| 522 | Lead (Pb) | Pb | Seeds | [28] |
| 523 | Cadmium (Cd) | Cd | Seeds | [28] |
| 524 | Chromium (Cr) | Cr | Seeds | [28] |
| Others | ||||
| 525 | 7-Epiloliolide | C11H16O3 | Fruits, Calyxes | [41] |
| 526 | Tetillapyrone | C11H14O6 | Fruits, Calyxes | [41] |
| 527 | 3,5-dimethoxy-4-hydroxybenzaldehyde | C9H10O4 | Roots, Stems | [42] |
| 528 | 1-O-β-D-glucopyra-n-osyl-2-N-(2′-hydroxypalmitoyl)octadeca sphi-nga-4,8-dienine | C40H75NO9 | Fruits | [29] |
| 529 | Dihydrofuran-2,5-dione | C4H4O3 | Fruits | [29] |
| 530 | Cyclo-(L-leucyl-L-isoleucyl) | C12H22N2O2 | Fruits | [29] |
| 531 | Cyclo(tyrosine-amidocaproic) -bipeptid | C15H20N2O3 | Calyxes | [74] |
| 532 | Cuneataside E | C24H40O11 | Calyxes | [52] |
| 533 | 1-O-[3-O-2-methyl-5-(2,3,4-trimethyl)phenyl-2,3-pentanediol]-β-d-xylopyranosyl-(1 → 6)-β-d-galactopyranoside | C26H42O11 | Fruits, Calyxes | [56] |
| 534 | (Z)-Hex-3-en-1-ol O-β-d-xylcopyranosyl-(1–6)-β-D-glucopyran-osyl-(1–2)-β-d-glucopyranoside | C23H40O15 | Calyxes | [75] |
| 535 | (E)-Hex-3-en-1-ol O-β-d-xylcopyranosyl-(1–6)-β-D-glucopyran-osyl-(1–2)-β-d-glucopyranoside | C23H40O15 | Calyxes | [75] |
3.1. Steroids
Steroids are the main components of P. alkekengi. A total of 164 steroids have been isolated and identified from the calyx, fruit, and above-ground parts of P. alkekengi, accounting for 30.65% of the total compound types. These include physalins, neophysalins, sterols, and withanolides, among which physalins are the most abundant. The study of physalins in P. alkekengi began in 1969 with the isolation and identification of physalin A by Japanese scholars, and since then several physalins compounds have been identified. Phylasins are a class of steroidal compounds with a bitter taste [8]. The basic structure of physalins consists of a 13,14-seco-16,24-cycloergostane skeleton. Neophysalins were first discovered by Japanese scholars in 1991 [13]. The difference between neophysalins and physalins is that the C-15 of physalins is directly linked to C-16, and C-14 forms a lactone ring with C-17, whereas the C-14 of neophysalins is directly linked to C-16, and C-15 forms a lactone ring with C-17 [8].
Sterols are mainly found in the fruit, seeds, and calyx of P. alkekengi [14]. At present, physanol A and physanol B have been isolated from the fruits of P. alkekengi, and a variety of 4α-methyl sterols, mainly gramisterol and obtusifoliol, have been isolated from the unsaponifiables of the seed oil, in addition to a variety of 4-desmethyl sterols [10]. Withanolides are a class of ergostane lactones containing 28 carbon atoms derived from the ergostane backbone and characterised by the formation of δ- or γ-lactones by linking the C-22 to the C-26, or the C-23 to the C-26 in the side chain [15]. Specific information on the steroids in P. alkekengi is given in Table 1.
3.2. Flavonoids
Flavonoids are a class of compounds characterised by the parent nucleus of 2-phenylchromogenic ketones. Fifty-five flavonoids have been isolated from P. alkekengi, accounting for 10.28% of the total compound types, which is one of the important active ingredients in P. alkekengi. Flavonoids in P. alkekengi are mostly isolated from the fruit, calyx, and calyx-fruit combination [7], mainly including flavonoids and flavonoid glycosides. Specific information on the flavonoids in P. alkekengi is given in Table 1.
3.3. Alkaloids
Alkaloids are a class of naturally-occurring nitrogen-containing organic compounds. Twenty-nine alkaloids have been isolated from P. alkekengi, accounting for 5.42% of the total compound types. Alkaloids in P. alkekengi are predominantly concentrated in the roots and lower portions of the primary stem [7]. These mainly include tigloidine, tropine, hygrine, cuscohygrine, pseudotropine, and phygrine. Specific information on the alkaloid constituents in P. alkekengi is given in Table 1.
3.4. Phenylpropanoids
Phenylpropanoids have a benzo-alpha-pyrone structure as their parent nucleus. Twenty-four phenylpropanoids have been isolated from the calyxes of P. alkekengi, accounting for 4.49% of the total compound types. These mainly include a variety of phenylpropionic acids such as ferulic acid, chlorogenic acid, and caffeic acid [16]. Specific information on the phenylpropanoids in P. alkekengi is given in Table 1.
3.5. Physakengoses
Physakengoses are primarily composed of sucrose and long-chain fatty acid esters [16]. Zhang et al. [17,18] isolated 17 new physakengoses from P. alkekengi, including physakengoses A-Q. Specific information on the physakengoses in P. alkekengi is given in Table 1.
3.6. Piperazines
Piperazines are a class of compounds featuring the piperazine structure. Shu et al. [19] isolated thirteen piperazines from P. alkekengi, marking the first ever discovery of these compounds in Physalis L. Specific information on the piperazines in P. alkekengi is given in Table 1.
3.7. Volatile oils
Volatile oils refer to a cluster of fragrant substances that exhibit volatility. Ninety-six volatile oils have been isolated from P. alkekengi, accounting for 17.94% of the total compound types. These mainly include fatty acids and sesquiterpenoids [8], among which fatty acids such as octanoic, decanoic, pentadecanoic, n-palmitic, and myristic acids are the main components. In addition, the volatile components are mainly in the calyx, with less in the fruit [20,21]. Specific information on the volatile oils in P. alkekengi is given in Table 1.
3.8. Polysaccharides
Polysaccharides play an important role in various life processes and possess multiple health benefits. The polysaccharides are mainly extracted from the fruit and calyx parts of P. alkekengi [22], among which 8.9% polysaccharides content in the fruits [7], such as mannose, glucose, galactose, xylose, arabinose, rhamnose, fucose, sucrose, and maltose [23]. Specific information on the polysaccharide analogues in P. alkekengi is given in Table 1.
3.9. Amino acids
Amino acids are a class of organic compounds containing basic amino and acidic carboxyl groups. Physalis alkekengi contains a variety of amino acids, with nineteen amino acids having been isolated and identified, accounting for 3.55% of the total compound types. The fruits of P. alkekengi contain 18 essential amino acids, accounting for 30.66% of the total amino acids [24], among which arginine, glutamic acid, and aspartic acid are the mainly components [25]. In addition, the calyxes contain 16 amino acids, mainly including phenylalanine, glutamic acid, proline, valine, and tryptophan [26], of which the essential amino acids account for 29.13% of the total amino acids [27]. Specific information on the amino acids in P. alkekengi is given in Table 1.
3.10. Other chemical components
In addition, P. alkekengi also contains trace elements such as potassium, sodium, calcium, magnesium, iron, manganese, copper, zinc, lead, cadmium, and chromium [28]. It also contains aliphatics such as n-tetracosane, dibutyl phthalate, 1-citric acid ethyl ester, 1-citric acid methyl ester, ethyl linoleate, and 5-hydroxymethyl furfural [29], organic acids such as citric, succinic, cumaric, quinic, gallic, and gentisic acids [26], terpenoids such as citroside A, (6S,9R)-roseoside, (6S,9S)-roseoside, and ursolic acid [16], phenols such as ethyl caffeate, ethyl ferulate, and syringic acid [30], tocopherols α, β, and γ [28], anthraquinones such as emodin and aurantio-obtusin-6-o-β-d-glucoside [26], nucleosides such as adenosine, guanosine, and uridine [31], and many other compounds.
4. Pharmacological effects
Modern pharmacological studies have shown that P. alkekengi has various pharmacological effects such as anti-inflammatory, anti microbial, antioxidative, hypoglycaemic, analgesic, immunomodulatory, and anti-tumour activities. A schematic diagram of the main pharmacological mechanism of effects of P. alkekengi is shown in Fig. 3.
Fig. 3.
Main pharmacological mechanism of effects of P. alkekengi.
Abbreviations: SOD, Superoxide dismutase; CAT, Catalase; GSH-Px, Glutathione peroxidase; COX-2, Cyclooxygenase; 5-LOX, 5-Lipoxygenase; PLA2, Phospholipase A2; PGE2, Prostaglandin E2; LTB4, Leukotriene B4; IL, Interleukin; Akt, also known as PKB, Protein kinase B; MAPK, Mitogen-activated protein kinase; NF-κB, Nuclear factor kappa-B; iNOS, Inducible nitric oxide synthase; NO, Nitric oxide; TNF-α, Tumour necrosis factor-α; MDA, Malondialdehyde; DPPH, 2,2-Diphenyl-1-picrylhydrazyl; 'OH, Hydroxyl radical; ABTS, 2,2-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); O2-, Superoxide anion; NaNO2, Sodium nitrite; KEAP1, Kelch-like ECH-associated protein 1; NRF2, Nuclear factor-erythroid 2-related factor 2; GLUT4, Glucose transporter 4; PI3K, Phosphatidylinositol-3-kinase; InsR, Insulin receptor; GK, Glucokinase; GLUT2, Glucose transporter 2; PK, Pyruvate kinase; PEPCK, Phosphoenolpyruvate carboxykinase; LTA4H, Leukotriene A-4 hydrolase; IgG, Immunoglobulin G; IgG1, Immunoglobulin G1; IgG2b, Immunoglobulin G2b; STAT3, Signal transducers and activators of transcription 3; ROS, Reactive oxygen species; JAK2, Janus kinase 2; CDK1, Cycle protein-dependent kinase 1; PARP, Poly ADP-ribose polymerase; mTOR, Mammalian target of rapamycin; CDK2, Cyclin-dependent kinase 2; IFN-γ, Interferon γ; PKC, Protein kinase C.
4.1. Anti-inflammatory effects
The inflammatory response is a defensive reaction of the body to cellular damage and is the basis for the pathogenesis of multiple diseases. The crude extract of P. alkekengi by water extraction and alcohol precipitation could significantly inhibit xylene-induced acute oedema and exudative inflammation and reduce the number of inflammatory cells in rats with acute pharyngitis [76]. The aqueous extract of P. alkekengi alleviated symptoms of dextran sulphate sodium-induced ulcerative colitis in mice. It can reduce the secretion of the inflammatory factors interleukin (IL)-6 and IL-1β by increasing the antioxidant activity of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) and significantly inhibit their mRNA expression in mouse colonic tissues, thus alleviating colitis [77]. The aqueous extract of the calyx of P. alkekengi may exert anti-inflammatory effects by inhibiting cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) expression and activities and inhibiting phospholipase A2 (PLA2), thereby doubly inhibiting the arachidonic acid metabolic pathway and reducing the production of prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) [78]. The alcohol extracts of the fruits of P. alkekengi were found to inhibit the secretion of the hyperglycaemia-induced pro-inflammatory factors IL-31 and IL-33, while upregulating the anti-inflammatory factor IL-10, thereby suppressing inflammation [79]. In addition, the ethanol, petroleum ether, and ethyl acetate extracts of P. alkekengi can alleviate lipopolysaccharide (LPS)-induced inflammatory responses, mainly by blocking protein kinase B (Akt, also known as PKB) and p38 mitogen-activated protein kinase (MAPK) signalling pathways, inhibiting nuclear factor kappa-B (NF-κB) transcription and inducible nitric oxide synthase (iNOS) and COX-2 expression, and reducing the production of nitric oxide (NO), PGE2, tumour necrosis factor-α (TNF-α), IL-1, IL-6, and reactive oxygen species [54,64,80].
The total steroidal saponins from the calyx of P. alkekengi can dose-dependently inhibit the production of the inflammatory factors NO, IL-6, IL-1β, and monocyte chemotactic protein-1 (MCP-1), thereby suppressing the expression of COX-2 to alleviate the inflammatory response, and have a significant inhibitory effect on LPS-induced inflammatory response in RAW264.7 macrophages [81]. Wang et al. [82] showed that physalin A reduced the overproduction of PGE2, TNF-α, and NO, inhibited the expression of COX-2 and iNOS, significantly inhibited nuclear translocation of NF-κB p65 and phosphorylation of inhibitor of NF-κB (IκB-α), and exerted anti-inflammatory effects by blocking LPS-induced activation of the NF-κB signalling pathway in RAW 264.7 cells.
Yao et al. [83] found galuteolin in P. alkekengi reduced the secretion of TNF-α, IL-6, and NO as well as gene copy number of TNF-α, IL-6, and iNOS in LPS-induced RAW264.7 cells, resulting in effective anti-inflammatory effects. Chen et al. [84] found that the combination of physalin A, luteolin, and cynaroside had significant synergistic inhibitory effects on LPS-induced NO and TNF-α release from macrophages, and the combination significantly reduced LPS-induced expression of iNOS protein. In addition, Zhang et al. [85] found a dose-dependent inhibition of COX-2 enzymes by sesquiterpenoids in P. alkekengi, and Xu [15] showed that withanolides also have strong anti-inflammatory activity. In conclusion, numerous studies have shown that all extracted parts of P. alkekengi show significant anti-inflammatory activity and can be widely studied and applied as anti-inflammatory agents. Among the active components, steroids and flavonoids are most studied for their anti-inflammatory effects. The possible main components are physalins and luteolin, which mainly exert anti-inflammatory effects by inhibiting the expression of COX-2, iNOS, and NF-κB p65, reducing various pro-inflammatory factors, and thereby increasing antioxidant capacity.
4.2. Anti microbial effects
Pathogenic microorganisms can cause infections and many diseases when they invade the body. The ethanol extract of the calyx of P. alkekengi has an inhibitory effect on alpha and beta Streptococcus, Staphylococcus aureus, Bacillus subtilis, and Bacillus cereus, with the strongest inhibitory effect on beta Streptococcus and Bacillus cereus [65,86]. In addition, the ethanol extract and the polysaccharide of P. alkekengi promote the growth of probiotics such as Bacteroides, Clostridium, and Lactobacillus, inhibit the growth of pathogenic bacteria such as Escherichia coli, and improve the balance of intestinal microecology [49,87,88]. It has been determined that the methanol and dichloromethane extracts and physalin D of P. alkekengi had antibacterial effects against gram-positive bacterial species, gram-negative bacterial species, and Candida species by broth microdilution and disk diffusion methods, with the best antibacterial effect against gram-positive bacteria [89]. And the ethyl acetate-extracted parts of P. alkekengi also showed antibacterial activity against Helicobacter pylori [90].
Meng et al. [91] found that the total saponin content of P. alkekengi had an inhibitory effect on four common food spoilage bacteria, including E. coli, Salmonella typhimurine, Shigella fowlerii, and Listeria monocytogenes. Yang et al. [92] found that physalin B and physalin E have good antibacterial effects on alpha and beta-haemolytic streptococcus, S. pneumoniae, S. aureus, and Moraxella catarrhalis, and the minimum inhibitory concentration of physalin B was lower than the minimum inhibitory concentration of physalin E and has stronger bacteriostatic activity. Chlorogenic acid has also been found to have significant antimicrobial activity against S. aureus, S. pneumoniae, B. subtilis, E. coli, Shigella dysenteriae, and S. typhimurium [93]. Furthermore, physakengoses B, E-H and K-Q, new compounds discovered in P. alkekengi by Zhang et al. [17,18], have strong bacteriostatic activity against S. aureus, B. subtilis, Pseudomonas aeruginosa, and E. coli.
Meira et al. [94] showed that physalins B, D, F, and G have anti-Trypanosoma cruzi activity, of which physalins B and F are the most effective compounds for trypanosomes and epithelial cell forms. Treatment with physalins can reduce its invasion and development, which may be related to the inhibition of T. cruzi protease activity, leading to alterations in its Golgi apparatus. Guimarães et al. [95] found that physalins B and F can reduce the percentage of Leishmania infection macrophages and the number of intracellular parasites in vitro at macrophages at non-cytotoxic concentrations, with potent antileishmanic activity. Using physalin D to treat mice infected with Plasmodium burgdorferi can reduce parasitaemia and delay death, demonstrating its antimalarial activity against P. falciparum [96]. Among the five fractions (P1, P2, P3, P4, and P5) obtained by Yao [83], P2, P3, and P4 had inhibitory effects on the growth of Mycoplasma toxin, among which P2 and P3 had strong inhibitory effects. In conclusion, many experiments have shown that the multiple extracts of P. alkekengi have significant anti microbial effects, and the main active ingredients are physalins, physakengoses, and chlorogenic acid. Physalis alkekengi extracts have inhibitory effect on a variety of pathogenic bacteria and parasites and have a regulatory effect by inhibiting harmful bacteria while promoting beneficial bacteria. However, despite their potential, the specific mechanism of action of these extracts is rarely studied, and further research is needed.
4.3. Antioxidative effects
Oxidative stress damage is a common stress injury that predisposes an organism to ageing and various chronic diseases if excess oxygen free radicals are present. The aqueous extracts of the calyx of P. alkekengi can enhance the resistance of nematodes to oxidative stress by up-regulating the expression levels of the antioxidant genes gst-4, gst-7, sod-3, and hsp16.2 in nematodes, thereby delaying aging. In addition, the aqueous extract from P. alkekengi was proven to have significant antioxidative effects [97]. Furthermore, the aqueous extract of P. alkekengi can also prevent nonalcoholic fatty liver disease (NAFLD) in mice by reducing the malondialdehyde (MDA) content in the liver tissue of NAFLD model mice [98]. Pei et al. [99] found that n-hexane-acetone extracted from P. alkekengi can reduce the MDA content and increase the SOD and GSH-Px enzyme activities in aging rats induced by d-galactose, which can effectively enhance the antioxidant capacity of rats. It has been shown that both the leaf and fruit extracts of P. alkekengi showed inhibition of xanthine oxidase, which mainly contains total phenols, flavonoids, and carotenoids [100]. Additionally, Wu et al. [71] examined the scavenging ability of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals and found that the antioxidant activity of the petroleum ether part of P. alkekengi fruit was superior to that of the calyx.
P. alkekengi polysaccharides have a strong scavenging ability against 2, 2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+), hydroxyl radical ('OH), superoxide anion (O2−), and DPPH, and have significant antioxidant activity [[101], [102], [103]]. Li et al. [104] found that the calyx, stems, and leaves of P. alkekengi have stronger antioxidant activity by fluorescence recovery after photobleaching method, in which the total flavonoids of stems and calyx had stronger scavenging ability of against DPPH, and the total flavonoids of stems and fruits had stronger scavenging activity of against ABTS+. It has been shown that the total flavonoids from the calyx of P. alkekengi have the ability to scavenge oxygen radicals such as ABTS+, 'OH, O2−, DPPH, and sodium nitrite (NaNO2), and the scavenging ability is enhanced with increasing mass [105]. Zhang et al. [106] showed that physalin B could ameliorate oxidative stress by activating the P62–kelch-like ECH-associated protein 1 (KEAP1)–nuclear factor-erythroid 2-related factor 2 (NRF2) antioxidant pathway to improve NAFLD. Huang et al. [107] used supercritical carbon dioxide extraction to recover carotenoids from the calyx of P. alkekengi and confirmed their antioxidant capacity using free radical scavenging activity tests. In conclusion, numerous studies have shown that P. alkekengi extracts have significant antioxidative effects, with the main active ingredients being polysaccharides and flavonoids. The mechanism of action is related to enhancing antioxidant gene expression, increasing antioxidant enzyme activity, enhancing resistance to oxidative stress, scavenging oxygen free radicals, thereby reducing oxidative stress damage. Therefore, the use of P. alkekengi extract as a natural antioxidant in health and care products and cosmetics has broad prospects for development and application.
4.4. Hypoglycaemic effects
Diabetes is a metabolic disease characterised by hyperglycaemia, and chronic damage to tissues and organs is easily caused by long-term hyperglycaemia. Both the aqueous and ethanol extracts of the calyx of P. alkekengi lowered blood glucose levels and increased glucose tolerance in streptozotocin (STZ)-induced diabetic rats, with the ethanol extract of the calyx of P. alkekengi having a more significant hypoglycaemic effect [108]. Zhang et al. [109] found that ethyl acetate extracted from P. alkekengi can improve glucolipid metabolism in high-fat diet combined with STZ-induced diabetic rats by stimulating glucose uptake and utilization. Hu et al. [110] found that ethyl acetate extracts of the above-ground parts and fruits of P. alkekengi could reduce cytochrome P450-2E1 expression, inhibit α-glucosidase, reduce oxidative stress, and enhance glucose transporter 4 (GLUT4) expression and insulin sensitivity, which showed antidiabetic activity both in vitro and in vivo.
Li et al. [111] elucidated the hypoglycaemic mechanism of P. alkekengi polysaccharides by establishing a mouse model of tetraoxonin-induced diabetes. The study demonstrated that P. alkekengi polysaccharides can repair and protect the pancreas and pancreatic islet cells to stimulate insulin secretion and lower blood glucose levels. They can also regulate liver glucose metabolism by increasing the synthesis of hepatic glycogen and the content of glucokinase and improve the disorder of glucose metabolism in diabetic mice, thus lowering blood glucose concentration. The molecular mechanism of action involves activation of the phosphatidylinositol-3-kinase (PI3K)/Akt insulin signalling pathway and upregulation of GLUT4, Akt, PI3K, and insulin receptor (InsR) mRNA, which are key molecules of the insulin signalling pathway. This further enhances the effect of insulin signalling, improving the sensitivity of the body to insulin, stimulating glucose transport, promoting the utilization and metabolism of sugar in peripheral tissues and target organs, thus lowering blood glucose. In addition, the steroidal saponins in the calyx of P. alkekengi can also reduce blood glucose concentrations and alleviate hyperglycaemic symptoms in tetraoxypyrimidine-induced diabetic mice [112]. Li et al. [113] found that the total steroidal saponins of P. alkekengi inhibited α-amylase in a dose-related manner and speculated that competitive reversible inhibition was involved. In addition, physalins in sterols inhibit both α-glucosidase and α-amylase, with more significant inhibition of α-amylase [114]. Wang [115] showed that the polyphenols in the fruits of P. alkekengi can also lower blood glucose by promoting the expression of glucokinase (GK), glucose transporter 4 (GLUT2), and pyruvate kinase (PK), inhibiting the expression of glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK), and stimulating key enzymes of glucose metabolism to regulate glucose metabolism in the livers of II-diabetic mice. Mezhlumyan et al. [116] showed that protein fractions in P. alkekengi also had high hypoglycaemic activity. In conclusion, a variety of compounds in P. alkekengi have hypoglycaemic effects, and their main active ingredients are polysaccharides, sterols, and polyphenols. The glucose-lowering mechanism of P. alkekengi extract is mainly related to the activation of the insulin signalling pathway, thereby enhancing insulin level and sensitivity, and hepatic glucose metabolising enzyme activity, and inhibiting glycoside and starch hydrolases, thus lowering blood glucose level. In recent years, the hypoglycaemic effect of P. alkekengi has been gradually emphasised, among which the hypoglycaemic effect of the calyx polysaccharides has been studied in greater detail. The significant hypoglycaemic activity of these polysaccharides demonstrated their potential for the development of a natural hypoglycaemic agent for application in pharmaceuticals and health products. Furthermore, the hypoglycaemic effect of the polyphenolic components of the fruit also provides scope for the development and application of P. alkekengi.
4.5. Analgesic effects
Pain is a subjective feeling of discomfort caused by damage to tissues in the body. Gong et al. [117] used various pain measurement methods to measure the pain response of mice after gavage with aqueous extract of P. alkekengi, indicating that the extract has an analgesic effect. In addition, studies have also shown that aqueous extracts and ethyl acetate sites from the calyx of P. alkekengi can also inhibit inflammation-induced pain [78,80]. After measuring the analgesic effect of physalin A using hot plate and torsion methods, Zhao et al. [118] used molecular docking techniques and found that physalin A might exert the analgesic effect by regulating leukotriene A-4 hydrolase (LTA4H) enzyme activity in the arachidonic acid metabolic pathway. Therefore, physalins have been proposed as the active ingredient that generates this effect. Few studies on the analgesic effects of P. alkekengi have been conducted to date. However, the analgesic activity of natural medicine is characterised by few side effects, so P. alkekengi shows great clinical potential and warrants further research.
4.6. Immunomodulatory effects
Immunomodulation is a physiological function of the body that relies on the immune system to recognise and eliminate antigenic foreign substances and maintain its own physiological dynamic balance and relative stability. It has been shown that the soluble polysaccharides and calyx saponins of P. alkekengi significantly increased the antibody titres of anti-OVA-specific antibodies immunoglobulin G (IgG), immunoglobulin G1 (IgG1), and immunoglobulin G2b (IgG2b) in mouse anti-serum, significantly induced and promoted Th1 and Th2 cell-mediated humoral immune responses, thereby enhancing the cellular and humoral immune responses [119,120]. In addition, P. alkekengi fruit polysaccharides could bind to toll-like receptor 4, a surface receptor on mouse bone marrow dendritic cells to affect the immune function of bone marrow dendritic cells and promote initial T cell differentiation to Th1 and Th2 [121]. Yang et al. [122] co-immunised mice with water-soluble polysaccharides of P. alkekengi as a nucleic acid vaccine adjuvant, which significantly enhanced their immune response and laid the foundation for the development of P. alkekengi polysaccharides in vaccine adjuvants. In conclusion, the immunomodulatory effects of P. alkekengi are mainly attributable to its polysaccharides and saponins, and the mechanism of action may be related to promoting T cell differentiation and stimulating Th1 and Th2 immune responses.
4.7. Anti-tumour effects
Cancer is a serious threat to human life and health and causes millions of deaths worldwide every year. Most malignant tumours are treated clinically by surgical resection combined with radiotherapy, but both methods can cause serious irreversible damage to the body. Therefore, there is an urgent need for the research of natural drugs with anti-tumour activity and broad application prospects. It has been shown that the alcohol extract of P. alkekengi can effectively inhibit the proliferation and promote apoptosis of colon cancer cells [123]. The trichloromethane extract of P. alkekengi showed antiproliferative effects on HeLa, MCF-7, and A431 cell lines, with the fraction containing physalin D being the most active [124].
Steroids are the main active ingredients in the anti-tumour activity of P. alkekengi. Li et al. [35] found that steroids in P. alkekengi exhibited strong cytotoxicity against HeLa human cervical cancer, SMMC-7721 human hepatocellular carcinoma, and HL-60 human hepatocellular carcinoma tumour cell lines, with physalin B exhibiting the strongest cytotoxicity. Fu et al. [125] showed that physalins in sterols could enhance apoptosis in multiple myeloma cells by inhibiting signal transducers and activators of transcription 3 (STAT3) signalling pathway-induced expression of downstream target genes. He et al. [126] found that physalin A could selectively induce apoptosis in human fibrosarcoma HT1080 cells by activating the death receptor-related exogenous apoptotic pathway and upregulating the expression of caspase-3 and caspase-8, and physalin A had no growth inhibitory effect on normal cells. Physalin A can also inhibit cancer cell proliferation by participating in the p38 MAPK/reactive oxygen species (ROS) pathway to induce G2/M cell cycle block in human non-small cell lung cancer A549 cells, as well as inhibit tumour cell xenograft growth and promote apoptosis by inhibiting the janus kinase 2 (JAK2)/3-STAT3 signalling pathway [127,128]. Shin et al. [129] found that physalin A could also increase the expression of detoxifying enzymes by activating NRF2 and its target genes through the regulation of extracellular regulated protein kinases and p38 kinases in Hepa-1c1c7 and HepG2 hepatocellular carcinoma cells, thereby inhibiting cancer progression at the initial stages of carcinogenesis. Hao et al. [130] found that physalin A also induced iNOS expression and NO production in human melanoma A375-S2 cells, thereby inducing apoptosis and autophagy in A375-S2 cells. In addition, physalin A can also treat breast cancer through various pathways by increasing the mRNA expression level of the apoptosis-specific gene Bax, inducing autophagy in EGFR2 cancer cells, and inhibiting the Hedgehog and Hippo signalling pathways, cancer stem cell-specific genes, and mammosphere formation [[131], [132], [133]]. Wang et al. [134] showed that physalin B significantly reduced the activity of three human breast cancer cell lines: MCF-7, MDA-MB-231, and T-47D. The mechanism of action may be to induce cell cycle arrest in the G2/M phase in a p53-dependent manner and to promote the cleavage of poly ADP-ribose polymerase (PARP), caspase-3, caspase-7, and caspase-9 to stimulate apoptosis. In addition, it has been shown that physalin B induced G2/M block and inhibited proliferation of human non-small cell lung cancer A549 cells by altering mitochondrial function through upregulating p21, and downregulating cyclin B1, cell division control protein cell cycle protein-dependent kinase 1 (CDK1) and oxidative phosphorylation multi-subunit activity [135]. Sun et al. [57] isolated withanolides from the calyx of P. alkekengi, in which withaphysalin B and a new withanolide compound exhibited strong cytotoxicity against A549 and K562 cell lines and induced apoptosis, with a possible mechanism of action through inhibition of the PI3K–Akt–mammalian target of rapamycin (mTOR) signalling pathway to exert anti-tumour effects.
Moreover, Ji [136] showed that luteoloside could block gastric cancer cells in S-phase by inhibiting the protein expression levels of the S-phase-related proteins cyclin-dependent kinase 2 (CDK2) and cyclin E1, inhibit the migration and invasion ability of gastric cancer cells, and up-regulate the protein expression of the apoptotic substrate PARP and the shedder of apoptotic core protein caspase-3, thereby regulating the apoptotic signalling pathway to promote apoptosis. This has the effect of promoting the ubiquitous degradation of mesenchymal-epithelial transition protein (MET) to inhibit the PI3K/Akt/mTOR pathway, which ultimately inhibits the proliferation, migration, and invasion of gastric cancer cells. In addition to this, Zhang et al. [85] also found that sesquiterpenoids have some cytotoxic properties. In conclusion, P. alkekengi has certain inhibitory effects on a variety of tumours, and its mechanism may be related to inducing G2/M phase arrest of cancer cells through various pathways to promote apoptosis and inhibit the proliferation of cancer cells. The main active ingredients of P. alkekengi with anti-tumour effects are steroidal compounds, especially the physalins, while the flavonoids and terpenoids in P. alkekengi also have anti-tumour activities. Therefore, the inhibitory effect of many types of cancer by various components of P. alkekengi demonstrates its potential as a prospective natural, anti-tumour medicine and warrants further research.
4.8. Anti-asthma effects
Asthma is a common multifactorial respiratory disease that is usually characterised by airway inflammation, immune cell aggregation, reversible airflow obstruction, and bronchial hyperresponsiveness. It has been shown that the methanol extract of P. alkekengi can inhibit airway hyperresponsiveness in ovalbumin (OVA)-induced asthmatic mice [137]. Bao [138] found that the aqueous extract of P. alkekengi can effectively reduce the total leukocyte count and eosinophil count in the blood of sensitised asthmatic mice, decrease the expression of interleukin-5 (IL-5) and interferon γ (IFN-γ) in lung tissue, selectively reduce the intensity of Th1 and Th2 expression in lung tissue, and reverse the imbalanced Th1/Th2 ratio. Liu et al. [139] found that different concentrations of P. alkekengi could significantly inhibit the release of histamine in the lung tissues of OVA-sensitised asthmatic mice, alleviate the inflammation of lung tissues of asthmatic mice, and reduce lung tissue damage, thus improving the symptoms of asthmatic mice and prolonging the latency period of asthma induction. Furthermore, Wu [140] showed that flavonols in P. alkekengi could activate the Nrf2-regulated defence system and are effective components in the treatment of respiratory diseases. In conclusion, the extracts of P. alkekengi have an anti-asthma effect, with flavonoids being the likely active ingredients contributing to this effect, and the mechanism of action may be related to reducing the expression of IL-5 and IFN-γ. Since the symptoms associated with asthma are related to inflammation and the immune system, the mechanisms of anti-asthmatic effect of P. alkekengi are also related to its anti-inflammatory and immunomodulatory effects.
4.9. Other effects
Furthermore, P. alkekengi has been shown have hypolipidemic, diuretic, vasodilatory, nephroprotective, and antifertility effects. Dong et al. [141] investigated the hypolipidemic effects of the aqueous and alcohol extracts of the calyx and fruit of P. alkekengi by establishing a hyperlipidaemic rat model and noted that the extracts of P. alkekengi were all effective in preventing hyperlipidaemia, with the aqueous extract of the calyx having the best therapeutic effect. Experimental results by Yang [27] showed that the diuretic effect of the calyx of P. alkekengi is not only related to its glycolic acid content, but also to the high potassium and magnesium content of P. alkekengi. Liu et al. [142] found that the aqueous extracts of P. alkekengi inhibited calcium inflow and the protein kinase C (PKC) signalling pathway, thereby relaxing phenylephrine and potassium chloride-induced vasoconstriction in a concentration- and non-endothelium-dependent manner in rat thoracic aorta. Ashtiyani et al. [143] showed that alcohol extracts from P. alkekengi could reduce urea nitrogen, serum creatinine, and sodium/potassium levels elevated due to cisplatin, thereby improving cisplatin-induced nephrotoxicity. Vessal et al. [144] found that aqueous extracts of the fruit and calyx of P. alkekengi can reduce progesterone levels and time-dependently inhibit the activity of the uterine creatine kinase BB-isoenzyme in mothers, thereby reducing the birth rate of pups and producing a antifertility effect.
5. Conclusions and future perspectives
This paper reviews the phylogenetic origin, chemical composition, pharmacological effects, and mechanism of action of P. alkekengi. More than 530 chemical components have been isolated and identified in P. alkekengi, mainly including steroids, flavonoids, alkaloids, volatile oils, polysaccharides, and other components. Among these, steroids, flavonoids, and volatile oils account for the largest proportion of components. However, volatile oils evaporate easily and are not suitable medicinal components; therefore, little research has been conducted on these oils. Among the sterols, the most researched components are the physalins, which are unique to Physalis L. A large number of pharmacological studies have demonstrated the anti-inflammatory, anti microbial, antioxidative, hypoglycaemic, analgesic, immunomodulatory, anti-tumour, and anti-asthma effects and their mechanisms of action in P. alkekengi. In addition, its diuretic, hypolipidemic, vasodilatory, nephroprotective, and antifertility activities have also been demonstrated, providing a theoretical basis for its use in clinical settings and as a health food. Among them, the main focus is on its anti-inflammatory, anti microbial, antioxidative, hypoglycaemic, and anti-tumour effects. Combined with phytochemical and pharmacological analysis, steroids, flavonoids, and polysaccharides are the main active ingredients of P. alkekengi. Among these, physalins are the main active ingredients contributing to the anti-inflammatory, anti microbial, analgesic, anti-tumour, and antioxidative effects of P. alkekengi. The flavonoids mainly contribute to the anti-inflammatory, antioxidative, anti-tumour, and anti-asthma effects, and the polysaccharides mainly contribute to the antioxidative, hypoglycaemic, and immunomodulatory effects of P. alkekengi. As a natural medicine unique to the northeast region of China, P. alkekengi is cheap, widely cultivated, rich in resources, and has a variety and high content of pharmacologically active ingredients, most notably physalins. Therefore, P. alkekengi is highly valuable and warrants further in-depth research.
Although P. alkekengi and its active ingredients have been used in the treatment of several diseases and have been extensively studied pharmacologically in animal studies, the conclusions of these studies are still limited.
Although scholars at home and abroad have done a lot of research on P. alkekengi, there are still some problems to be solved. First, the pharmacological mechanisms of action of the anti microbial, analgesic, diuretic, and hypolipidemic effects are still unclear, and the conformational relationship of many pharmacological activities at the level of animal models, metabolomics, and macro-genomics of intestinal microflora is also less studied and warrants further in-depth research. Second, there are more studies on fruits and calyx, but few studies on roots, stems and leaves. These organs also contain medicinal components and therefore warrant further research. Third, because P. alkekengi appears mostly in the north-eastern region of China and is rare in other regions, it is not widely used as a food and health product, and research progress is slow. Fourth, although P. alkekengi showed good activity in both in vivo and ex vivo models, further confirmation of its effective use and possible clinical application is needed. In summary, as a natural medicinal and dual-use plant with a variety of functional properties, the development of related products has great potential and development prospects.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Funding
This research was supported by the Central Support for Local Universities Reform and Development Funds for Talent Training Projects (Key Technology and Product Creation for the Discovery of Quality Markers of Ganoderma Lucidum , an Authentic Medicinal Herb of Longjiang); the University Nursing Program for Young Scholars With Creative Talents in Heilongjiang Province (grant number UNPYSCT-2020219); the Science and Technology Innovation Talent Project of the Harbin Science and Technology Bureau (grant number CXRC20221106324); and the Harbin University of Commerce Research Support Program for doctor (grant number 22BQ02).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Contributor Information
Bing Tian, Email: 280808589@qq.com.
Wenlan Li, Email: lwldzd@163.com.
Zhiwei Sun, Email: sunzhiwei@hrbcu.edu.cn.
References
- 1.Wang L.J., Jing Y.R., Chen L.X., et al. Research progress on pharmacological effects and development and application of Physalis alkekengi L.var. franchetii (Mast.) Makino[J] Heilongjiang Agricultural Sciences. 2018;(12):163–165. doi: 10.11942/j.issn1002-2767.2018.12.0163. [DOI] [Google Scholar]
- 2.Editorial committee of flora of China . vol. 67. Science Press; Beijing: 1978. (Chinese Academy of Sciences. Flora of China). part 1. [Google Scholar]
- 3.(Jin) P. Guo Er ya zhushu, Peking University Press, Beijing, 1999.
- 4.Wu (Wei) P., Qing, Sun X.Y., Sun F.J., et al. Guangxi Science and Technology Press; Nanning: 2016. Shennong Herbal Scripture. [Google Scholar]
- 5.S Z Li (Ming) Beijing Yanshan Press; Beijing: 2010. Original. Compendium of Materia Medica. [Google Scholar]
- 6.Chinese Pharmacopoeia Commission . China Medical Science Press; Beijing: 2020. Chinese Pharmacopoeia (Part I) [Google Scholar]
- 7.Gao P.Y., Jin M., Du C.L., et al. Research progress of Chinese medicine Physalis alkekengi L.var. franchetii [J] J. Shenyang Pharm. Univ. 2014;31(9):732–737. doi: 10.14066/j.cnki.cn21-1349/r.2014.09.008. [DOI] [Google Scholar]
- 8.Guo Y., Liu J., Nie L.X., et al. Research progress of Physalis alkekengi L.var. franchetii [J] J. Chin. Med. Mater. 2012;35(12):2039–2045. doi: 10.13863/j.issn1001-4454.2012.12.043. [DOI] [Google Scholar]
- 9.Wu S., Ni L., Zhang Y.J., et al. Research progress on Physalis alkekengi L.var. franchetii in recent ten years[J] J. Chin. Med. Mater. 2019;42(10):2462–2467. doi: 10.13863/j.issn1001-4454.2019.10.049. [DOI] [Google Scholar]
- 10.Wang M.D., Yang S.S. Review of chemical constituents and pharmacological effects of Physalis alkekengi L.var. franchetii [J] Journal of Liaoning University of Traditional Chinese Medicine. 2005;7(4):341–342. doi: 10.13194/j.jlunivtcm.2005.04.39.wangmd.023. [DOI] [Google Scholar]
- 11.Jing Y., Yan P.S., Feng C., et al. Natural products from Physalis Alkekengi L. Var. Franchetii (mast.) Makino: a review on their structural analysis, quality control, pharmacology, and pharmacokinetics[J] Molecules. 2022;27(3):695. doi: 10.3390/molecules27030695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li A.L., Chen B.J., Li G.H., Zhou M.X., Li Y.R., Ren D.M., Lou H.X., Wang X.N., Shen T. Physalis alkekengi l. var. franchetii (Mast.) Makino: an ethnomedical, phytochemical and pharmacological review[J] J. Ethnopharmacol. 2018;210:260–274. doi: 10.1016/j.jep.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Kawai M., Ogura T., Butsugan Y., et al. Hayashi M. Benzilic acid rearrangement-type reaction of physalins to neophysalins. structural revision of one of the dehydration products of physalin A[J] Tetrahedron. 1991;47(12):2103–2110. doi: 10.1016/s0040-4020(01)96121-6. [DOI] [Google Scholar]
- 14.Wen J., Sheng J.J. Research progress on chemical constituents of Chinese medicine Physalis alkekengi L.var. franchetii [J] Scientific and Technological Innovation. 2017;(18):121. doi: 10.3969/j.issn.1673-1328.2017.18.117. [DOI] [Google Scholar]
- 15.Huang M., He J.X., Hu H.X., et al. Withanolides from the genus Physalis: a review on their phytochemical and pharmacological aspects [J] J. Pharm. Pharmacol. 2020;72(5):649–669. doi: 10.1111/jphp.13209. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Bai L.L., Yi K., et al. Research progress on chemical constituents and pharmacological effectsof Physalis alkekengi L. var. franchetii (Mast.) Makino[J] Natural Product Research and Development. 2022;34(2):324–343. doi: 10.16333/j.1001-6880.2022.2.018. [DOI] [Google Scholar]
- 17.Zhang C., Luo J., Liu R., et al. Physakengoses K-Q, seven new sucrose esters from Physalis alkekengi var. franchetii[J] Carbohydr. Res. 2017;449:120–124. doi: 10.1016/j.carres.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C.Y., Luo J.G., Liu R.H., et al. 1H NMR spectroscopy-guided isolation of new sucrose esters from Physalis alkekengi var. franchetii and their antibacterial activity[J] Fitoterapia. 2016;114:138–143. doi: 10.1016/j.fitote.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Shu Z.P., Li X.L., Xu B.Q., et al. Piperazine constituents in fruits of Physalis alkekengi var. francheti[J] Chin. Tradit. Herb. Drugs. 2014;45(4):471–475. doi: 10.7501/j.issn.0253-2670.2014.04.004. [DOI] [Google Scholar]
- 20.Zhao Q. Shenyang Pharmaceutical University; 2005. Study on Chemical Constituents of Physalis Alkekengi L.Var. franchetii[D] [Google Scholar]
- 21.Xu L., Wang B., Jia T.Z. Study on volatile oil components of two medicinal materials of Physalis alkekengi and Cynanchum komarovii[J] Chin. Tradit. Pat. Med. 2007;29(12):1840–1843. doi: 10.3969/j.issn.1001-1528.2007.12.045. [DOI] [Google Scholar]
- 22.Liu X.G., Bian J., Gao P.Y., et al. Research progress on polysaccharides from Physalis alkekengi[J] Agriculture of Jilin. 2019;445(4):56–57. doi: 10.14025/j.cnki.jlny.2019.04.018. [DOI] [Google Scholar]
- 23.Zhou Z.H. Jilin Agricultural University; 2012. Studies on Chemical Composition and Biological Activity of Rhizome of Physalis Alkekengi L. Var. franchetii(Mast.) Makino[D] [Google Scholar]
- 24.Wang Y.H., Zhang Z.H., Jin H., et al. Determination of amino acids in different parts of Physalis alkekengi[J] Biotic Resources. 2009;31(1):81–83. doi: 10.3969/j.issn.1006-8376.2009.01.021. [DOI] [Google Scholar]
- 25.Yu H.Y., Yan J.Z., Guo M., et al. Analysis of proteins in the extracts of Physalis alkekengi L. var. franchetii (Mast.) Makino using nanoflow reversed-phase liquid chromatographytandem mass spectrometry[J] Chin. J. Chromatogr. 2013;31(4):362–366. doi: 10.3724/SP.J.1123.2012.11023. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C.Y., Zhang Z.X., Wang D., et al. Analysis of chemical constituents from calyxes of Physalis alkekengi var. franchetii by UPLC-Q-Orbitrap HRMS[J] J. Chin. Med. Mater. 2021;44(11):2590–2598. doi: 10.13863/j.issn1001-4454.2021.11.017. [DOI] [Google Scholar]
- 27.Yang X.H., Tian D.D., Zhou X.P., et al. Determination of inorganic elements and amino acid content in calyx of Physalis alkekengi[J] Journal of Jilin University(Medicine Edition) 2001;27(1):17–18. doi: 10.3969/j.issn.1671-587X.2001.01.006. [DOI] [Google Scholar]
- 28.Popova V., Petkova Z., Mazova N., et al. Chemical composition assessment of structural parts (seeds, peel, pulp) of Physalis alkekengi L. fruits[J] Molecules. 2022;27(18):5787. doi: 10.3390/molecules27185787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y. Harbin Institute of Technology; 2011. The Research on Chemical Composition of the Fresh Fruit of Physalis Alkekengi L.Var. Francheti and harmonia axyridis[D] [Google Scholar]
- 30.Zhang C.H. Shenyang Pharmaceutical University; 2009. Bioactive Constituents and Quality Evaluation of Physalis Alkekengi L.Var. franchetii[D] [Google Scholar]
- 31.Chen L., Xia G., Liu Q., et al. Chemical constituents from the calyces of Physalis alkekengi var. franchetii[J] Biochem. Systemat. Ecol. 2014;54:31–35. doi: 10.1016/j.bse.2013.12.030. [DOI] [Google Scholar]
- 32.Li J., Li J., Li D.K. Studies on the chemical constituents of Physalis alkekengi (I)[J] Chin. Tradit. Herb. Drugs. 2002;33(8):692–693. doi: 10.3321/j.issn:0253-2670.2002.08.011. [DOI] [Google Scholar]
- 33.Huang C., Xu Q.M., Chen C., et al. The rapid discovery and identification of physalins in the calyx of Physalis alkekengi L.var.franchetii (Mast.) Makino using ultra-high performance liquid chromatography-quadrupole time of flight tandem mass spectrometry together with a novel three-step data mining strategy. [J]. J Chromatogr A. 2014;1361:139–152. doi: 10.1016/j.chroma.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Yu T. Liaoning University Of Traditional Chinese Medicine; 2008. Study on Chemical Composition and Purification Process of Physalis Alkekengi fruit[D] [Google Scholar]
- 35.Li X., Zhao J.P., Yang M., et al. Physalins and withanolides from the fruits of Physalis alkekengi L. var. franchetii (Mast.) Makino and the inhibitory activities against human tumor cells[J] Phytochem. Lett. 2014;10:95–100. doi: 10.1016/j.phytol.2014.08.004. [DOI] [Google Scholar]
- 36.Zhang N., Chu X.Q., Jiang J.Q. Chemical constituents in ethyl acetate extract from Physalis alkekengi var. franchetii[J] Chin. Tradit. Herb. Drugs. 2015;46(8):1120–1124. doi: 10.7501/j.issn.0253-2670.2015.08.004. [DOI] [Google Scholar]
- 37.Xing F., Jiang J.Q. Chemical constituents in the calyx of Physalis alkekengi var. franchetii[J] Pharmaceutical and Clinical Research. 2013;21(4):344–346. doi: 10.13664/j.cnki.pcr.2013.04.008. [DOI] [Google Scholar]
- 38.Li Y.Z. Guangxi Normal University; 2007. Study on the Chemical Constituents of the Seeds of O. Fragrans and the Whole Plants of P. Alkekengi Var. francheti[D] [Google Scholar]
- 39.Li J., Li J., Li D.K. Studies on the chemical constituents of Physalis alkekengi (Ⅱ)[J] Chin. Tradit. Herb. Drugs. 2002;33(9):23–24. doi: 10.3321/j.issn:0253-2670.2002.09.010. [DOI] [Google Scholar]
- 40.Yang D., Li F.T., Liu M., et al. Analysis of chemical constituents in Physalis Calyx seu Fructus using UPLC-Q-Orbitrap MS/MS[J] J. Chin. Mass Spectrom. Soc. 2021;42(3):253–260. doi: 10.7538/zpxb.2020.0077. [DOI] [Google Scholar]
- 41.Lv H. Shandong University of Traditional Chinese Medicine; 2019. Chemical Constituents from Physalis Alkekengi L.Var. Franchetii and Their Biological Activities[D] [Google Scholar]
- 42.Zhang C. Guizhou University; 2019. Study on Chemical Constituents in Roots and Stems of Physalis alkekengi[D] [Google Scholar]
- 43.Kawai M., Yamamoto T., Makino B., et al. The structure of physalin T from Physalis alkekengi var. franchetti.[J] J. Asian Nat. Prod. Res. 2001;3(3):199–205. doi: 10.1080/10286020108041391. [DOI] [PubMed] [Google Scholar]
- 44.Xu W.X., Chen J.Q., Liu J.Q., et al. Three new physalins from Physalis alkekengi var. franchetii[J] Natural Products and Bioprospecting. 2013;3:103–106. doi: 10.1007/s13659-013-0021-z. [DOI] [Google Scholar]
- 45.Yang Y.K., Xie S.D., Xu W.X., et al. Six new physalins from Physalis alkekengi var. franchetii and their cytotoxicity and antibacterial activity. [J]. Fitoterapia. 2016;112:52–144. doi: 10.1016/j.fitote.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Tan M.Q., Niu Z., Zhang M., et al. Studies on the steroids from the whole herb of Physalis alkekengi var. franchetii[J] Chin. Tradit. Herb. Drugs. 2021;52(17):5203–5209. doi: 10.7501/j.issn.0253-2670.2021.17.011. [DOI] [Google Scholar]
- 47.Qiu L., Zhao F., Jiang Z.H., et al. Steroids and flavonoids from Physalis alkekengi var. franchetii and their inhibitory effects on nitric oxide production. [J]. J Nat Prod. 2008;71(4):643–646. doi: 10.1021/np700713r. [DOI] [PubMed] [Google Scholar]
- 48.Li K., Diao Y.P., Wang M.D., et al. Chemical constituents of the fruits of Physalis alkekengi L. var. franchetii (Mast.) Makino[J] Chin. J. Org. Chem. 2010;30(1):128–131. [Google Scholar]
- 49.Li X.L., Zhang C.L., Wu D.C., et al. In vitro effects on intestinal bacterium of physalins from Physalis alkekengi var. Francheti.[J] Fitoterapia. 2012;83(8):1460–1465. doi: 10.1016/j.fitote.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 50.Sun J.L., Jiang Y.J., Lin C. Two new physalin derivatives from Physalis alkekengi L. var. franchetii (Mast.) Makino.[J] Nat. Prod. Res. 2019;35(2):203–206. doi: 10.1080/14786419.2019.1619724. [DOI] [PubMed] [Google Scholar]
- 51.Sun J.M., He J.X., Huang M., et al. Two new physalins from Physalis alkekengi L. var. franchetii (Mast.) Makino.[J] Nat. Prod. Res. 2021;(20):5206–5212. doi: 10.1080/14786419.2021.1924713. [DOI] [PubMed] [Google Scholar]
- 52.Yuan Y. Liaoning University Of Traditional Chinese Medicine; 2010. Study on Chemical Composition and Quality Control of Physalis Alkekengi L.Var. franchetii[D] [Google Scholar]
- 53.Qiu L., Jiang Z.H., Liu H.X., et al. A pair of 3-epimeric physalins from Physalis alkekengi L. var. franchetii[J] J. Asian Nat. Prod. Res. 2008;10(9):881–885. doi: 10.1080/10286020802144735. [DOI] [PubMed] [Google Scholar]
- 54.Hu H.X. Shandong University; 2020. Chemical Constituents of Physalis Calyx Seu Fructus and Their Biological activities[D] [Google Scholar]
- 55.Zhang C.H., Wang Z.T., Yang Y.P., et al. A novel cytotoxic neophysalin from Physalis alkekengi var. francheti[J] Chin. Chem. Lett. 2009;20(11):1327–1330. doi: 10.1016/j.cclet.2009.06.005. [DOI] [Google Scholar]
- 56.Peng X.F., Wu Q., Gao C., et al. Chemical constituents from the fruit calyx of Physalis alkekengi var. francheti[J] J. Chin. Pharmaceut. Sci. 2015;24(9):600–606. doi: 10.5246/jcps.2015.09.076. [DOI] [Google Scholar]
- 57.Sun Y., Guo T., Zhang F.B., et al. Isolation and characterization of cytotoxic withanolides from the calyx of Physalis alkekengi L. var franchetii[J] Bioorg. Chem. 2020;96 doi: 10.1016/j.bioorg.2020.103614. [DOI] [PubMed] [Google Scholar]
- 58.Li Z.C., Chen Z., Li X.R., et al. Chemical constituents in roots and stems of Physalis alkekengi var. franchetii[J] Chin. Tradit. Herb. Drugs. 2012;43(10):1910–1912. [Google Scholar]
- 59.Li X., Zhao J.P., Yang M., et al. Physalins and withanolides from the fruits of Physalis alkekengi L. var. franchetii (Mast.) Makino and the inhibitory activities against human tumor cells[J] Phytochem. Lett. 2014;10:95–100. doi: 10.1016/j.phytol.2014.08.004. [DOI] [Google Scholar]
- 60.Li Y.Z., Pan Y.M., Huang X.Y., et al. Withanolides from Physalis alkekengi var. francheti[J] Helv. Chim. Acta. 2008;91(12):2284–2291. doi: 10.1002/hlca.200890248. [DOI] [Google Scholar]
- 61.Li G.Y., Guan T., Liang H.D., et al. A new spirostanol steroid saponin in fruit of Physalis alkekengi L. [J] Acta Chinese Medicine and Pharmacology. 2022;50(4):37–40. doi: 10.19664/j.cnki.1002-2392.220081. [DOI] [Google Scholar]
- 62.Sharma N.K., Kulshreshtha D.K., Tandon J.S., et al. Two new sterols from Physalis franchetii fruit[J] Phytochemistry. 1974;13(10):2239–2245. doi: 10.1016/0031-9422(74)85035-1. [DOI] [Google Scholar]
- 63.Liang H. Liaoning University Of Traditional Chinese Medicine; 2007. Study on Chemical Constituents of Fruits of Physalis alkekengi[D] [Google Scholar]
- 64.Sun J.M. Shandong University of Traditional Chinese Medicine; 2021. Study on Chemical Constituents of Physalis Alkekengi Var. Franchetii and Their Antiinflammatory and Antioxidant activities[D] [Google Scholar]
- 65.Zhao H.D. Liaoning University Of Traditional Chinese Medicine; 2008. Studies on the Components and Active Part of Physalis[D] [Google Scholar]
- 66.Qiu L., Jiang Z.H., Liu H.X., et al. Flavonoid glycosides chemical constituents of Physalis alkekengi calyx[J] J. Shenyang Pharm. Univ. 2007;143(12):744–747. doi: 10.3969/j.issn.1006-2858.2007.12.005. [DOI] [Google Scholar]
- 67.Ma Z. Liaoning University Of Traditional Chinese Medicine; 2007. Studies on the Component and Identification of Physalis alkekengi[D] [Google Scholar]
- 68.Shu Z.P., Xu B.Q., Xing N., et al. Chemical constituents of physalis calyx seu fructus[J] Chin. J. Exp. Tradit. Med. Formulae. 2014;20(21):99–102. doi: 10.13422/j.cnki.syfjx.2014210099. [DOI] [Google Scholar]
- 69.Basey K., Mcgaw B.A., Phygrine J. G. Woolley. An alkaloid from Physalis species[J] Phytochemistry. 1992;31(12):4173–4176. doi: 10.1016/0031-9422(92)80437-J. [DOI] [Google Scholar]
- 70.Asano N., Kato A., Kizu H., et al. 1β-amino-2α,3β,5β-trihydroxycycloheptane from Physalis alkekengi var. francheti[J]. Phytochemistry. 1996;42(3):719–721. doi: 10.1016/0031-9422(96)00034-9. [DOI] [Google Scholar]
- 71.Wu S., Wu L.L., Zhao J., et al. Study on component analysis and antioxidant activity of petroleum ether extract from calyx and fruit in Physalis alkekengi L.var. francheti(Mast.) Makino[J] Journal of Jiamusi University(Natural Science Edition) 2020;38(1):99–102. doi: 10.3969/j.issn.1008-1402.2020.01.025. [DOI] [Google Scholar]
- 72.Qiu L., Zhao F., Liu H., et al. Two new megastigmane glycosides, physanosides A and B, from Physalis alkekengi L. var. franchetii, and their effect on no release in macrophages. [J]. Chemistry & Biodiversity. 2008;5(5):758–763. doi: 10.1002/cbdv.200890072. [DOI] [PubMed] [Google Scholar]
- 73.Tong H.B., Zhu M., Feng K., et al. Purification, characterization and in vitro antioxidant activities of polysaccharide fractions isolated from the fruits of Physalis alkekengi L. [J] J. Food Biochem. 2011;35(2):524–541. doi: 10.1111/j.1745-4514.2010.00400.x. [DOI] [Google Scholar]
- 74.Cai Q., Liu Y.Q., Ma Z., et al. Isolation and identification of chemical constituents from the fruit and calyx of Physalis alkekengi L.var.franchetii(Mast.)Makino[J] J. Shenyang Pharm. Univ. 2009;26(10):807–810. doi: 10.14066/j.cnki.cn21-1349/r.2009.10.010. 817. [DOI] [Google Scholar]
- 75.Shu Z.P., Tang Y., Yang Y.N., et al. Two new 3-hexenol glycosides from the calyces of Physalis alkekengi var. franchetii[J] Nat. Prod. Res. 2019;35(8):1274–1280. doi: 10.1080/14786419.2019.1645657. [DOI] [PubMed] [Google Scholar]
- 76.Shu Z.P., Xing N., Wang Q.H., et al. Antibacterial and anti-inflammatory activities of Physalis alkekengi var. franchetii and its main constituents. [J]. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/4359394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Q., Xu N.N., Hu X.J., et al. Anti-colitic effects of physalin B on dextran sodium sulfate-induced BALB/c mice by suppressing multiple inflammatory signaling pathways[J] J. Ethnopharmacol. 2020;259 doi: 10.1016/j.jep.2020.112956. [DOI] [PubMed] [Google Scholar]
- 78.Li Y., Feng H.H., Jiang Y.W., et al. The effects of Physalis Alkekengi on inflammatory and arachidonic acid metabolic pathway[J] Trans. Beijing Inst. Technol. 2015;35(7):762–766. doi: 10.15918/j.tbit1001-0645.2015.07.020. [DOI] [Google Scholar]
- 79.Vicas L.G., Jurca T., Baldea I., et al. Physalis alkekengi L. Extract reduces the oxidative stress, inflammation and apoptosis in endothelial vascular cells exposed to hyperglycemia. [J]. Molecules. 2020;25(16):3747. doi: 10.3390/molecules25163747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moniruzzaman M., Bose S., Kim Y.M., et al. The ethyl acetate fraction from Physalis alkekengi inhibits LPS-induced pro-inflammatory mediators in BV2 cells and inflammatory pain in mice.[J] J. Ethnopharmacol. 2016;181:26–36. doi: 10.1016/j.jep.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 81.Kang H., Kwon S.R., Choi H.Y. Inhibitory effect of Physalis alkekengi L. var. franchetii extract and its chloroform fraction on LPS or LPS/IFN-γ-stimulated inflammatory response in peritoneal macrophages[J] J. Ethnopharmacol. 2011;135(1):95–101. doi: 10.1016/j.jep.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 82.Wang L.Y., Gu J.P., Zong M.Y., et al. Anti-inflammatory action of physalin A by blocking the activation of NF-κB signaling pathway. [J]. J Ethnopharmacol. 2020;267 doi: 10.1016/j.jep.2020.113490. [DOI] [PubMed] [Google Scholar]
- 83.Yi Y.Y., Yao M.M., Sun P.P., et al. Effect of ethanol extract and fractions of physalis calyx seu fructus on inflammation and Mycoplasma Gallisepticum. [J]. Pakistan Veterinary Journal. 2020;40(3):283–288. doi: 10.29261/pakvetj/2020.036. [DOI] [Google Scholar]
- 84.Li J.X., Han D.W., Li L., et al. Research progress of the pharmacological effect of Physalis. alkekengi L. var. franchetii.[J] Jilin Journal of Chinese Medicine. 2019;39(4):555–560. doi: 10.13463/j.cnki.jlzyy.2019.04.037. [DOI] [Google Scholar]
- 85.Zhang J.L., Zhou F.F., Li Y.Z., et al. New sesquiterpenoids with COX-2 inhibitory activity from the medical plant Physalis. alkekengi L. var. franchetii.[J]. Fitoterapia. 2020;141 doi: 10.1016/j.fitote.2020.104470. [DOI] [PubMed] [Google Scholar]
- 86.Wang Z.Y., Su T.T., Li N., et al. Study on extraction and antimicrobial activity of extracts from Physalis alkekengil L. var. francheti(Mast.) Makino[J] Food Science and Technology. 2009;34(8):142–145. doi: 10.13684/j.cnki.spkj.2009.08.073. [DOI] [Google Scholar]
- 87.Zhao X., Chen Z.Y., Yin Y.L., et al. Effects of polysaccharide from Physalis alkekengi var. francheti on liver injury and intestinal microflora in type-2 diabetic mice. [J]. Pharmaceutical Biology. 2017;55(1):2020–2025. doi: 10.1080/13880209.2017.1345953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X.L., Zhang C.L., Li W.L., et al. In vivo effects on the intestinal microflora of Physalis alkekengi var. francheti extracts. [J]. Fitoterapia. 2013;87:43–48. doi: 10.1016/j.fitote.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 89.Helvaci S., Kökdil G., Kawai M., et al. Antimicrobial activity of the extracts and physalin D from Physalis alkekengi and evaluation of antioxidant potential of physalin D.[J] Pharmaceut. Biol. 2010;48(2):142–150. doi: 10.3109/13880200903062606. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y., Wang S.L., Zhang J.Y., et al. Anti-ulcer and anti-helicobacter pylori potentials of the ethyl acetate fraction of Physalis alkekengi L. var. franchetii (Solanaceae) in rodent.[J] J. Ethnopharmacol. 2017;211:197–206. doi: 10.1016/j.jep.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 91.Meng Q.R., Li L.B., Wang X.W. Antimicrobial effect of total saponins from Physalis alkekengi calyx[J] Food Sci. (N. Y.) 2013;34(19):84–87. doi: 10.7506/spkx1002-6630-201319019. [DOI] [Google Scholar]
- 92.Yang Y.J., Sha C.W., Nie Y., et al. Experimental study on anti-inflammatory and antibacterial effects of Physalin B, E[J] Pharm. Today. 2015;25(3):167–168. 171. [Google Scholar]
- 93.Lou Z., Wang H., Zhu S., et al. Antibacterial activity and mechanism of action of chlorogenic acid[J] J. Food Sci. 2011;76(6):398–403. doi: 10.1111/j.1750-3841.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- 94.Meira C.S., Guimarães E.T., Bastos T.M., et al. Physalins B., F Seco-steroids isolated from Physalis angulata L., strongly inhibit proliferation. ultrastructure and infectivity of trypanosoma cruzi[J] 2013;140(14):1811–1821. doi: 10.1017/S0031182013001297. [DOI] [PubMed] [Google Scholar]
- 95.Guimarães E.T., Lima M.S., Santos L.A., et al. Activity of physalins purified from Physalis angulata in vitro and in vivo models of cutaneous leishmaniasis[J] J. Antimicrob. Chemother. 2009;64(1):84–87. doi: 10.1093/jac/dkp170. [DOI] [PubMed] [Google Scholar]
- 96.Sá M.S., Menezes M.N., Krettli A.U., et al. Antimalarial activity of physalins B, D, F, and G[J] J. Nat. Prod. 2011;74(10):2269–2272. doi: 10.1021/np200260f. [DOI] [PubMed] [Google Scholar]
- 97.Guo J., Li Y.F., Li S.J., et al. Anti-aging activity of Physalis calyx and its mechanism[J] Food Ferment. Ind. 2021;47(8):140–144. doi: 10.13995/j.cnki.11-1802/ts.025674. [DOI] [Google Scholar]
- 98.Wang C.H., Sun X.F., Wang S.M., et al. Preventive effect of Physalis alkekengi L. var. franchetii extract on NAFLD mice[J] Chinese Journal of Veterinary Medicine. 2018;54(5):59–62. 135. [Google Scholar]
- 99.Pei L.P., Dong F.H., Kong H.Y., et al. Improving effect of ground cherry's extraction on antioxidant ability in agng rats[J] Journal of Beijing University of Traditional Chinese Medicine. 2007;30(10):688–690. doi: 10.3321/j.issn:1006-2157.2007.10.012. 694. [DOI] [Google Scholar]
- 100.Hoshani M., Mianabadi M., Aghdasi M., et al. Inhibition effects of Physalis alkekengi extract on xanthine oxidase activity in different phenological stages[J] Clin. Biochem. 2011;44(13):343. doi: 10.1016/j.clinbiochem.2011.08.854. [DOI] [Google Scholar]
- 101.Cheng Y.K., Li L., Meng Z.K., et al. Component analysis and free radicals scavenging activity of Physalis alkekengi L. polysaccharide[J] Chem. Res. Chin. Univ. 2008;24(2):167–170. doi: 10.1016/S1005-9040(08)60034-3. [DOI] [Google Scholar]
- 102.Liu X.G., Bian J., Li D.Q., et al. Structural features, antioxidant and acetylcholinesterase inhibitory activities of polysaccharides from stem of Physalis alkekengi L.[J] Ind. Crop. Prod. 2019;129:654–661. doi: 10.1016/j.indcrop.2018.12.047. [DOI] [Google Scholar]
- 103.Ge Y., Duan Y.F., Fang G.Z., et al. Polysaccharides from fruit calyx of Physalis alkekengi var. francheti: isolation, purification, structural features and antioxidant activities[J] Carbohydrate Polymers. 2009;77(2):188–193. doi: 10.1016/j.carbpol.2008.12.020. [DOI] [Google Scholar]
- 104.Li C.H., Xue C.S., Li L. Optimized extraction and antioxidant activity of flavonoid from Physalis alkekengi L. J]. Northern Horticulture. 2018;422(23):124–131. doi: 10.11937/bfyy.20180722. [DOI] [Google Scholar]
- 105.Zhong F.L., Wang W.J., Wang X.L., et al. Antioxidant abilities of total flavonoids in vitro in persistent calyx of Clayx seu Fructus Physalis[J] Journal of Dalian Polytechnic University. 2017;36(6):397–401. doi: 10.3969/j.issn.1674-1404.2017.06.003. [DOI] [Google Scholar]
- 106.Zhang M.H., Li J., Zhu X.Y., et al. Physalin B ameliorates nonalcoholic steatohepatitis by stimulating autophagy and NRF2 activation mediated improvement in oxidative stress. J. Free Radic. Biol. Med. 2021;164:1–12. doi: 10.1016/j.freeradbiomed.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 107.Huang Z., Ma Q., Liu S., et al. Benign recovery of carotenoids from Physalis alkekengi L.var. francheti through supercritical CO2 extraction: Yield, antioxidant activity and economic evaluation[J] J. CO2 Util. 2020;36:9–17. doi: 10.1016/j.jcou.2019.10.015. [DOI] [Google Scholar]
- 108.Wu H.J., Chen D.Z. Effects of different extracts of Physalis alkekengi L. var. franchetii (Mast.)Makino on blood glucose tolerance in mice and blood glucose level in diabetic nephropathy rats[J] Anhui Medical and Pharmaceutical Journal. 2018;22(7):1245–1248. doi: 10.3969/j.issn.1009-6469.2018.07.007. [DOI] [Google Scholar]
- 109.Zhang Q., Hu X.F., Xin M.M., et al. Antidiabetic potential of the ethyl acetate extract of Physalis alkekengi and chemical constituents identified by HPLC-ESI-QTOF-MS. [J]. J Ethnopharmacol. 2018;225:202–210. doi: 10.1016/j.jep.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 110.Hu X.F., Zhang Q., Zhang P.P., et al. Evaluation of in vitro/in vivo anti-diabetic effects and identification of compounds from Physalis alkekengi. [J]. Fitoterapia. 2018;127:129–137. doi: 10.1016/j.fitote.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 111.Guo Y., Li S.J., Li J.X., et al. Anti-hyperglycemic activity of polysaccharides from calyx of Physalis alkekengi var. franchetii Makino on alloxan-induced mice [J] Int. J. Biol. Macromol. 2017;99:249–257. doi: 10.1016/j.ijbiomac.2017.02.086. [DOI] [PubMed] [Google Scholar]
- 112.Liu Y.L., Han S.Y., Zhao H., et al. Hypoglycemic activity of a saponin isolated from fruit calyx of Physalis alkekengi L. var. francheti(Mast.) Makino[J] J. Northeast Normal Univ. 2010;42(2):105–109. doi: 10.16163/j.cnki.22-1123/n.2010.02.023. [DOI] [Google Scholar]
- 113.Li X.D., Zhai J.X., Wang Y.J., et al. Study on the extraction process and inhibitory effect on α-amylase of total steroids from Physalis alkekengi L.[J] Cereals & Oils. 2022;35(11):129–133. doi: 10.3969/j.issn.1008-9578.2022.11.027. [DOI] [Google Scholar]
- 114.Li C.H., Fu Y.Y., Xue C.S., et al. Optimization of extraction and in vitro sugar-lowering activity of physalin from the calyx of Physalis alkekengi L.[J] Food Res. Dev. 2023;44(5):127–134. doi: 10.12161/j.issn.1005-6521.2023.05.019. [DOI] [Google Scholar]
- 115.Wang Y.L. Shanxi Agricultural University; 2018. Effect of Polyphenols from Physalis Fruit on Glucose Metabolism in Type Ⅱ Diabetic mice[D] [Google Scholar]
- 116.Mezhlumyan L.G., Khikmatullaev I.L., Rakhimova S.K., et al. Amino-acid composition and hypoglycemic properties of proteins from Physalis alkekengi and P. Angulata[J] Chem. Nat. Compd. 2022;58(1):187–189. doi: 10.1007/s10600-022-03631-y. [DOI] [Google Scholar]
- 117.Gong S., Shan L.D., Zhang Y.Y., et al. Experimental study on the analgesic effect of Physalis alkekengi L.[J] Suzhou Univ. J. Med. Sci. 2002;22(4):380–382. doi: 10.3969/j.issn.1673-0399.2002.04.008. [DOI] [Google Scholar]
- 118.Zhao N., Feng X.C., Pan G.X., et al. Research progress on pharmacological actions of physalin A [J] Drug Evaluation Research. 2020;43(2):340–344. doi: 10.7501/j.issn.1674-6376.2020.02.030. [DOI] [Google Scholar]
- 119.Li Y.G., Han S.Y., Zhao H., et al. Evaluation of immunologic enhancement mediated by a polysaccharide isolated from the fruit of Physalis alkekengi L. var. francheti (Mast.) Makino. [J] Journal of Medicinal Plant Research. 2011;6(3):465–475. doi: 10.1002/cmdc.201000524. [DOI] [Google Scholar]
- 120.Shang D.J., Zhang L.L., Han S.Y., et al. Adjuvant effect of a novel water-soluble polysaccharide isolated from the stem of Physalis alkekengi L. var. francheti (Mast.) Makino [J] J. Med. Plants Res. 2011;5(16):3814–3818. doi: 10.1021/jm200686d. [DOI] [Google Scholar]
- 121.Han S.Y. Northeast Normal University; 2015. Effect of the Polysaccharide Isolated from the Fruit of Physalis Alkekengi L. On Immune Function of Murine Bone Marrow Dendritic cells[D] [Google Scholar]
- 122.Yang H.M., Han S.Y., Zhao D.Y., et al. Adjuvant effect of polysaccharide from fruits of Physalis alkekengi L. in DNA vaccine against systemic candidiasis [J] Carbohydrate Polymers. 2014;109:77–84. doi: 10.1016/j.carbpol.2014.03.054. [DOI] [PubMed] [Google Scholar]
- 123.Huang Y.F., Yu L.H., Cui L.H., et al. Experimental study on the effects of Jingdenglong ethanol extract on proliferation and apoptosis of colon cancer cells through the expression of IncRNA AK093987 gene[J] Hebei Medical Journal. 2022;44(9):1316–1320. doi: 10.3969/j.issn.1002-7386.2022.09.007. [DOI] [Google Scholar]
- 124.Laczkó-zöld E., Forgó P., Zupkó I., et al. Isolation and quantitative analysis of physalin D in the fruit and calyx of Physalis alkekengi L.[J] Acta Biol. Hung. 2017;68(3):300–309. doi: 10.1556/018.68.2017.3.7. [DOI] [PubMed] [Google Scholar]
- 125.Fu Y.F., Zhu F.F., Ma Z.J., et al. Physalis alkekengi var. franchetii extracts exert antitumor effects on non-small cell lung cancer and multiple myeloma by inhibiting STAT3 signaling. [J]. Onco Targets Ther. 2021;14:301–314. doi: 10.2147/OTT.S282334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.He H., Zang L.H., Feng Y.S., et al. Physalin A induces apoptotic cell death and protective autophagy in HT1080 human fibrosarcoma cells.[J] Journal of Natural Products. 2013;76(5):880–888. doi: 10.1021/np400017k. [DOI] [PubMed] [Google Scholar]
- 127.Zhu F.F., Dai C.Y., Fu Y.F., et al. Physalin A exerts anti-tumor activity in non-small cell lung cancer cell lines by suppressing JAK/STAT3 signaling. [J]. Oncotarget. 2016;7(8):9462–9476. doi: 10.18632/oncotarget.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kang N., Jian J.F., Cao S.J., et al. Physalin A induces G2/M phase cell cycle arrest in human non-small cell lung cancer cells: involvement of the p38 MAPK/ROS pathway. [J]. Mol Cell Biochem. 2016;415:145–155. doi: 10.1007/s11010-016-2686-1. [DOI] [PubMed] [Google Scholar]
- 129.Shin J.M., Lee K.M., Lee H.J., et al. Physalin A regulates the Nrf2 pathway through ERK and p38 for induction of detoxifying enzymes.[J] BMC Complement Altern Med. 2019;19:101. doi: 10.1186/s12906-019-2511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.He H., Feng Y.S., Zang L.H., et al. Nitric oxide induces apoptosis and autophagy; autophagy down-regulates NO synthesis in physalin A-treated A375-S2 human melanoma cells. [J]. Food Chem Toxicol. 2014;71:128–135. doi: 10.1016/j.fct.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 131.Esmailpoor A., Ghasemian A., Dehnavi E., et al. Physalis alkekengi hydroalcoholic extract enhances the apoptosis in mouse model of breast cancer cells[J] Gene Reports. 2019;15 doi: 10.1016/j.genrep.2019.100366. [DOI] [Google Scholar]
- 132.Ghazy E., Taghi H.S. The autophagy-inducing mechanisms of Vitexin, cinobufacini, and physalis alkekengi hydroalcoholic extract against breast cancer in vitro and in vivo[J] J. Gastrointest. Cancer. 2022;53:592–596. doi: 10.1007/s12029-021-00668-0. [DOI] [PubMed] [Google Scholar]
- 133.Ko Y.C., Choi H.S., Liu R., et al. Physalin A, 13,14-seco-16, 24-cyclo-steroid, inhibits stemness of breast cancer cells by regulation of hedgehog signaling pathway and yes-associated protein 1 (YAP1)[J] Int. J. Mol. Sci. 2021;22(16):8718. doi: 10.3390/ijms22168718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang A.Q., Wang S.P., Zhou F.Y., et al. Physalin B induces cell cycle arrest and triggers apoptosis in breast cancer cells through modulating p53-dependent apoptotic pathway. [J]. Biomed Pharmacother. 2018;101:334–341. doi: 10.1016/j.biopha.2018.02.094. [DOI] [PubMed] [Google Scholar]
- 135.Cao C., Zhu L., Chen Y., et al. Physalin B induces G2/M cell cycle arrest and apoptosis in A549 human non-small-cell lung cancer cells by altering mitochondrial function. [J]. Anticancer Drugs. 2019;30(2):128–137. doi: 10.1097/CAD.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 136.Ji J.L. Southwest University; 2021. Study on the Anti-tumor Activity of Cynaroside in Gastric cancer[D] [Google Scholar]
- 137.Hong J.M., Kwon O.K., Shin I.S., et al. Anti-inflammatory activities of Physalis alkekengi var. franchetii extract through the inhibition of MMP-9 and AP-1 activation. [J]. Immunobiology. 2015;220(1):1–9. doi: 10.1016/j.imbio.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 138.Zhu F.F., Chen Z. Research progress on pharmacological action and clinical application of Physalis alkekengi L.var. franchetii[J] Journal of Gansu University of Chinese Medicine. 2015;32(2):66–69. [Google Scholar]
- 139.Liu H.H., Hao X.F. Observation of curative effect of Physalis alkekengi L.var. franchetii on allergic asthma in mice[J] Chin. Tradit. Pat. Med. 2009;31(2):285–287. doi: 10.3969/j.issn.1001-1528.2009.02.037. [DOI] [Google Scholar]
- 140.Wu X.Y. Shandong University; 2020. Investigation on Therapeutic Effect of Two Natural Flavonoids against Pulmonary Disorders and Their mechanism[D] [Google Scholar]
- 141.Dong H., Xu H.Y., Ding Y.H., et al. Study on lipid lowering pharmacodynamics of Brocade lantern [J] Asia-Pacific Traditional Medicine. 2023;19(1):27–33. doi: 10.11954/ytctyy.202301007. [DOI] [Google Scholar]
- 142.Liu X.D., Pan Z.W., Zhuang X.G., et al. The diastolic effect and mechanism of water extract of Physalis alkekengi L.var. franchetii on rat thoracic aorta[J] Chin. Tradit. Herb. Drugs. 2008;39(11):1709–1712. doi: 10.3321/j.issn:0253-2670.2008.11.036. [DOI] [Google Scholar]
- 143.Shangizi-ashtiyani C., Alizadeh M., najafi H., et al. Physalis alkekengi and Alhagi maurorum ameliorate the side effect of cisplatin-induced nephrotoxicity[Z] 2016;23:235–242. doi: 10.1038/cgt.2016.24. [DOI] [PubMed] [Google Scholar]
- 144.Vessal M., Mehrani H., Hossein Omrani G. Effects of an aqueous extract of Physalis Alkekengi fruit on estrus cycle, reproduction and uterine creatine kinase bb-isozyme in rats[J] J. Ethnopharmacol. 1991;34(1):69–78. doi: 10.1016/0378-8741(91)90190-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.