Abstract
In Escherichia coli, certain mutations in the cpxA gene (encoding a sensor kinase of a two-component signal transduction system) randomize the location of FtsZ ring assembly and dramatically affect cell division. However, deletion of the cpxRA operon, encoding the sensor kinase and its cognate regulator CpxR, has no effect on division site biogenesis. It appears that certain mutant sensor kinases (CpxA*) either exhibit hyperactivity on CpxR or extend their signalling activity to one or more noncognate response regulators involved in cell division.
In dividing Escherichia coli, a complex of different Fts proteins directs septum formation in the middle of the cell. Early in the division cycle, the FtsZ protein assembles into a ring structure at the future division site (1, 4, 16, 24–26, 35, 40). The Min proteins are involved in directing assembly of FtsZ to the medial division sites (2, 11, 12, 41). MinC and MinD, together, block FtsZ assembly at potential polar division sites, while MinE stimulates its assembly at the medial position (5, 12). Thus, when FtsZ is excessive or MinC or MinD is insufficient, FtsZ may assemble at the polar positions, resulting in minicell formation (3, 12, 46).
The cpxA gene encodes the sensor kinase (27, 28) of a two-component system, with CpxR as the response regulator (15, 31, 44). Numerous phenotypic changes (7, 27, 28, 32, 33, 38), many of which are associated with membrane function, have been attributed to cpxA* mutants (8, 10, 34). Recently, a CpxA* protein was found defective in CpxR-P phosphatase activity (39), which would explain the elevated expression of CpxR-P target operons, such as degP, dsbA, ppiA, and cpxP (8–10, 34). Here we describe the randomized location of FtsZ ring assembly and septation in cpxA* mutants (Table 1), a cell division phenotype heretofore not reported.
TABLE 1.
Strains and plasmids used in this studya
| Strain | Relevant genotype | Reference |
|---|---|---|
| ECL525 | MC4100 Δ(argF-lac)U169 araD139 Δfrd-101 rpsL150 relA1 deoC1 flb-5301 ptsF25 | 22 |
| JP406 | ECL525 F′pOXgen | This study |
| ECL1212 | JP406 ΔcpxRA-2 | This study |
| ECL1215 | JP406 ara+ | 34 |
| JP408 | JP406 zii-510::Tn10 cpxA9* | This study |
| JP466 | JP406 argE::Tn10 | 34 |
| JP467 | JP406 argE::Tn10 cpxA2.1* | 34 |
All of the strains used in this study are isogenic derivatives of ECL525. F′pOXgen (14) was used to introduce conjugative ability to ECL525. The defined deletion ΔcpxRA-2 was constructed as described by Blum et al. (6). The 1.2-kb deletion between the XhoI and EcoRI sites in the cpxRA operon removed most of the coding sequence of the two genes. The deletion was confirmed by PCR. Mutant cpxA alleles were introduced into strain JP406 by phage P1 cotransduction of linked zii-510::Tn10 or argE::Tn10. Strain JP408 received the cpxA9* allele from strain AE2293, which was provided by P. M. Silverman (38). The cpxA9* mutant was originally selected for amikacin resistance (38) and shown to have a Leu38→Phe (TTT) substitution in the periplasmic domain of CpxA (42). This mutation was confirmed in strain JP408 by DNA sequencing. The cpxA2.1* mutant was isolated in a selection for increased expression of a lacZ transcriptional fusion to the yajC-secDF operon. This cpxA allele was sequenced and shown to have a Val20→Ala (GCG) substitution plus an insertion of Leu and Val (CTGGTG) between Ala20 and Leu21 in the first membrane-spanning segment. For other characteristics of this mutation, see reference 34. Strains with cpxA* alleles were routinely grown at 30°C, except where indicated, to minimize reversion or suppression.
Irregular septation and nucleoid inheritance in cpxA* populations.
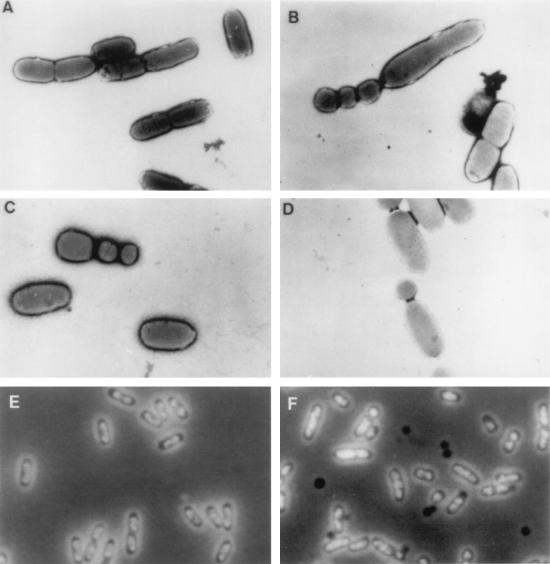
Since cpxA* mutations seem to impair membrane functions, we wondered whether cell morphology was also affected. Therefore, strains JP406 (cpxA+) and JP408 (cpxA9*) were grown exponentially (glucose medium, 37°C) and examined by electron microscopy. Unexpectedly, the mutant cells did not show uniform morphology: a minority exhibited irregular shapes and sizes which seemed to result from aberrant cell septation (Fig. 1A to D). When 250 dividing cells of each strain were surveyed, JP406 (cpxA+) almost invariably cleaved at the cell midpoint (97% of the population). By contrast, 38% of the JP408 (cpxA9*) cells cleaved at random positions along the cell axis and sometimes showed multiple septum formation. Phase-contrast microscopy of cells (Luria-Bertani [LB] medium, 37°C) stained for DNA by 4′-6-diamidino-2-phenylindole showed that, in contrast to wild-type JP406 (cpxA+), mutant strains JP408 (cpxA9*) and JP467 (cpxA2.1*) often formed subsized cell bodies devoid of DNA (nucleoids) during division (Fig. 1E and F).
FIG. 1.
(A to D) Electron micrographs. Cells were fixed on an electron microscopy grid with 1% phosphotungstic acid and observed at a magnification of ×5,000. Strains: JP406 (cpxA+), A; JP408 (cpxA9*), B to D. (E and F) Phase-contrast micrographs. Division products without DNA appear as dark bodies. Strains: JP406 (cpxA+), E; JP467 (cpxA2.1*), F.
Whereas the division anomaly of JP408 (cpxA9*) occurred at 42 and 37°C but not at 30°C (LB or minimal-glucose medium), this growth defect occurred at all three temperatures with JP467 (cpxA2.1*). The cpxA* mutation alone seems to account for the growth defect, since this phenotype was P1 transduced to wild-type strains. It is noteworthy that a cpx deletion mutant, ECL1212, divided normally.
Relationship between FtsZ ring formation and cell length in cpxA* mutants.
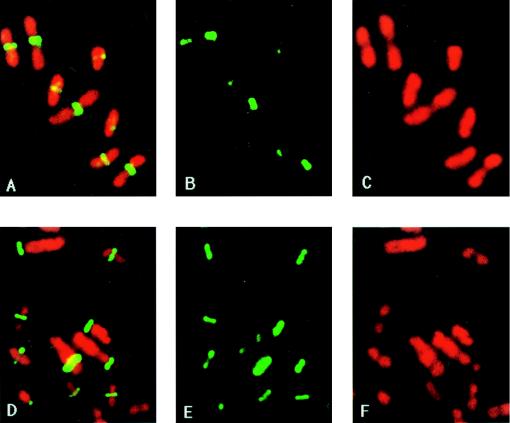
Because of the role of FtsZ in septation, the assembly of FtsZ and the positioning of the FtsZ ring during mutant cell division were compared with that in wild-type cells (Fig. 2). Consistent with the results of cell division analysis, the FtsZ ring (stained green by immunofluorescence) formed almost invariably at the cell midpoint in dividing wild-type cells, whereas the location of the FtsZ ring was random in almost half of the JP467 (cpxA2.1*) population. The patterns of nucleoid inheritance, as revealed by DNA staining (red), confirmed the results of phase-contrast microscopy.
FIG. 2.
Immunolocalization of FtsZ in fixed samples of strains JP406 (cpxA+) and JP467 (cpxA2.1*). Cultures growing exponentially in LB medium at 37°C were harvested and processed for microscopy essentially as previously described (36). Photographs of the same visual field were then taken with two different filter sets, one for visualizing the green-fluorescent antibodies against the FtsZ protein (B and E) and one for the red-stained DNA (C and F). A composite picture of both FtsZ and DNA stained was obtained by sequentially exposing the same frame of film to light emitted by each fluorophore (A and D). A to C, strain JP406 (cpxA+); D to F, strain JP467 (cpxA2.1*). Separate panels of the FtsZ rings and the nucleoids are presented because their superimposition rendered some of the individual images obscure.
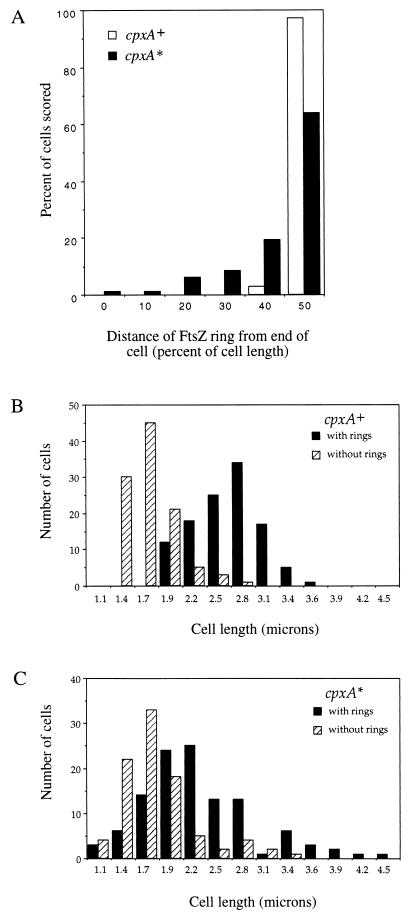
During steady-state growth of wild-type cells, length is an approximate indicator of age. Hence, most long cells have an FtsZ ring, whereas most short ones do not (1, 35), giving a bimodal distribution when the numbers of cells with an FtsZ ring and those without one are plotted against cell length. In the JP467 (cpxA2.1*) population, a significant fraction of dwarf cells is present, some of which nonetheless possess the FtsZ ring. Also, a large fraction of the cells is oversized, and yet some of these lack an FtsZ ring. Consequently, the presence or absence of an FtsZ ring, and thus cell age, can no longer be statistically predicted by cell length. The results of FtsZ analysis are summarized in Fig. 3.
FIG. 3.
Position analysis of the FtsZ ring in wild-type and mutant cells. (A) Percentage of cells containing an FtsZ ring (ordinate) versus the distance between the FtsZ ring and the nearest cell end (abscissa). Scoring for the presence of the ring and precise measurements of cell length and FtsZ ring position were carried out on photographic films on which the cell envelope is visible (from the samples shown in Fig. 2). The distance between the FtsZ ring and the nearest cell end was divided by the total cell length and multiplied by 100. Hence, the FtsZ ring in the midpoint of the dividing cell is given a value 50. Open bars, results from 220 cells of JP406 (cpxA+); solid bars, results from 207 cells of JP467 (cpxA2.1*). (B and C) Presence or absence of the FtsZ ring as a function of cell length. The number of cells with (solid bars) or without (hatched bars) the ring is plotted versus cell length. Strains: JP406 (cpxA+), B; JP467 (cpxA2.1*), C.
FtsZ levels in cpxA* mutants.
Unlike the minB mutants, which simultaneously produce minicells and filaments (12), cpxA* mutants only occasionally produce cells that are moderately longer than cpxA+ cells. The difference in length distribution between wild-type and mutant cells does not appear to be attributable to a difference in growth rate, since the doubling times of strains JP467 (cpxA2.1*) and JP408 (cpxA9*) are only slightly longer than that of the wild-type parent (LB medium, 37°C). The aberrant cell division phenotype of cpxA* mutants, therefore, more closely resembles that of FtsZ overproduction. However, when rates of FtsZ synthesis and stability were measured by pulse-chase immunoprecipitation, no significant difference was found between strains JP406 (cpxA+) and JP467 (cpxA2.1*) (data not shown).
Normal septation in wild-type cells with NlpE-activated Cpx signal transduction.
Oversynthesis of the outer membrane protein NlpE activates the Cpx pathway in wild-type cells (8, 10, 34). To determine whether this activation affects septation during cell division, strain ECL1215 was transformed with plasmid pND18, expressing nlpE from an l-arabinose-inducible promoter (10, 34). Cells were grown in LB medium (37°C), induced with l-arabinose, stained for DNA, and examined by phase-contrast microscopy. No cell division defects similar to those seen in cpxA* mutants were observed.
No epistatic effect of a degP null mutation on cpxA* phenotypes.
Induction of degP in cpxA* mutants is implicated in suppressing toxic phenotypes associated with accumulation of the LamB-LacZ-PhoA hybrid protein (8). To see whether some of the pleiotropic defects are attributable to elevated levels of the DegP protease, causing nonspecific damage to cell envelope proteins, strain JP467 (cpxA2.1*) was compared with a cpxA2.1* degP::Tn5 double mutant. In particular, the growth phenotypes on succinate and serine as the sole carbon source, resistance to low levels of amikacin, and aberrant cell division were analyzed. Abolition of degP function had no effect on the cpxA* phenotypes tested.
How CpxA* may affect cell division.
Since CpxA* probably enhances CpxR-P levels (39), we attempted to identify CpxR-P target operons associated with cell division. By using the proposed CpxR-P-binding consensus 5′-GTAAAN5–7GTAAA-3′ (34), we found no obvious candidates among the known cell division genes, including ftsQAZ (17, 43), ftsYEX (18), minCDE (12), and zipA (19). However, the fic gene, implicated in cell division (23), is preceded by ppiA, a CpxR-P-controlled gene. Whether fic expression is altered in cpxA* mutants remains to be determined.
It is also possible that CpxA* cross-phosphorylates one or more noncognate two-component regulators that have a role in cell division. In Bacillus subtilis, a two-component signal transduction system directly regulates the localization of the division site during sporulation (21, 25). Two-component signal transduction systems also play essential roles in cell cycle control in Caulobacter crescentus (13, 20, 29, 30, 37, 45, 47, 48). Further characterization of the cpxA* cell division phenotype may therefore cast new light on control of cell division in E. coli.
Acknowledgments
We thank P. M. Silverman for strain AE2293 (cpxA9*) and for communicating the base change in the mutation and J. Lutkenhaus for polyclonal antibodies against FtsZ. We thank P. Levin, W. Margolin, and J. Beckwith for helpful discussions.
This work was supported by Public Health Service grants GM-40993 and GM-30693 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Addinall S G, Bi E, Lutkenhaus J. FtsZ ring formation in fts mutants. J Bacteriol. 1996;178:3877–3884. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akerlund T, Bernander R, Nordstrom K. Cell division in Escherichia coli minB mutants. Mol Microbiol. 1992;6:2073–2083. doi: 10.1111/j.1365-2958.1992.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 3.Bi E, Lutkenhaus J. FtsZ regulates frequency of cell division in Escherichia coli. J Bacteriol. 1990;172:2765–2768. doi: 10.1128/jb.172.5.2765-2768.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi E, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1990;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 5.Bi E, Lutkenhaus J. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol. 1993;175:1118–1125. doi: 10.1128/jb.175.4.1118-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum P, Holzschu D, Kwan H-S, Riggs D, Artz S. Gene replacement and retrieval with recombinant M13mp bacteriophages. J Bacteriol. 1989;171:538–546. doi: 10.1128/jb.171.1.538-546.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosma C L, Danese P N, Carlson J H, Silhavy T J, Snyder W B. Mutational activation of the Cpx signal transduction pathway of Escherichia coli suppresses the toxicity conferred by certain envelope-associated stresses. Mol Microbiol. 1995;18:491–505. doi: 10.1111/j.1365-2958.1995.mmi_18030491.x. [DOI] [PubMed] [Google Scholar]
- 8.Danese P N, Silhavy T J. The ςE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 1997;11:1183–1193. doi: 10.1101/gad.11.9.1183. [DOI] [PubMed] [Google Scholar]
- 9.Danese P N, Silhavy T J. CpxP, a stress-combative member of the Cpx regulon. J Bacteriol. 1998;180:831–839. doi: 10.1128/jb.180.4.831-839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danese P N, Snyder W B, Cosma C L, Davis L J B, Silhavy T J. The Cpx two component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 1995;9:387–398. doi: 10.1101/gad.9.4.387. [DOI] [PubMed] [Google Scholar]
- 11.de Boer P. Chromosome segregation and cytokinesis in bacteria. Curr Opin Cell Biol. 1993;5:232–237. doi: 10.1016/0955-0674(93)90108-3. [DOI] [PubMed] [Google Scholar]
- 12.de Boer P, Crossley R E, Rothfield L I. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E. coli. Cell. 1989;56:641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- 13.Domian I J, Quon K C, Shapiro L. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell. 1997;90:415–424. doi: 10.1016/s0092-8674(00)80502-4. [DOI] [PubMed] [Google Scholar]
- 14.Dong, J. M. Unpublished data.
- 15.Dong J M, Iuchi S, Kwan H S, Lu Z, Lin E C C. The deduced amino acid sequence of the cloned cpxR gene suggests the protein is the cognate regulator for the membrane sensor, CpxA, in a two-component signal transduction system of Escherichia coli. Gene. 1993;136:227–230. doi: 10.1016/0378-1119(93)90469-j. [DOI] [PubMed] [Google Scholar]
- 16.Erikson H P. FtsZ, a tubulin homologue in prokaryotic cell division. Trends Cell Biol. 1997;7:362–367. doi: 10.1016/S0962-8924(97)01108-2. [DOI] [PubMed] [Google Scholar]
- 17.García-Lara J, Shang L H, Rothfield L I. An extracellular factor regulates expression of sdiA, a transcriptional activator of cell division genes in Escherichia coli. J Bacteriol. 1996;178:2742–2748. doi: 10.1128/jb.178.10.2742-2748.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbs T W, Gill D R, Salmond G P. Localised mutagenesis of the ftsYEX operon: conditionally lethal missense substitutions in the FtsE cell division protein of Escherichia coli are similar to those found in the cystic fibrosis transmembrane conductance regulator protein (CFTR) of human patients. Mol Gen Genet. 1992;234:121–128. doi: 10.1007/BF00272353. [DOI] [PubMed] [Google Scholar]
- 19.Hale C A, de Boer P A J. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell. 1997;88:175–185. doi: 10.1016/s0092-8674(00)81838-3. [DOI] [PubMed] [Google Scholar]
- 20.Hecht G B, Lane T, Ohta N, Sommer J M, Newton A. An essential single domain response regulator required for normal cell division and differentiation in Caulobacter crescentus. EMBO J. 1995;14:3915–3924. doi: 10.1002/j.1460-2075.1995.tb00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoch J A. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol. 1993;47:441–465. doi: 10.1146/annurev.mi.47.100193.002301. [DOI] [PubMed] [Google Scholar]
- 22.Iuchi S, Lin E C C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci USA. 1988;85:1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komano T, Utsumi R, Kawamukai M. Functional analysis of the fic gene involved in regulation of cell division. Res Microbiol. 1991;142:269–277. doi: 10.1016/0923-2508(91)90040-h. [DOI] [PubMed] [Google Scholar]
- 24.Levin P, Losick R. Transcription factor SpoOA switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- 25.Lutkenhaus J. FtsZ ring in bacterial cytokinesis. Mol Microbiol. 1993;9:403–409. doi: 10.1111/j.1365-2958.1993.tb01701.x. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Ehrhardt D W, Margolin W. Colocalization of cell division proteins FtsZ and FtsA to cytoskeletal structures in living Escherichia coli cells by using green fluorescent protein. Proc Natl Acad Sci USA. 1996;93:12998–13003. doi: 10.1073/pnas.93.23.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEwen J, Silverman P. Chromosomal mutations of Escherichia coli that alter expression of the conjugative plasmid functions. Proc Natl Acad Sci USA. 1980;77:513–517. doi: 10.1073/pnas.77.1.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McEwen J, Silverman P. Mutations in genes cpxA and cpxB alter the protein composition of Escherichia coli inner and outer membranes. J Bacteriol. 1982;151:1553–1559. doi: 10.1128/jb.151.3.1553-1559.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohta N, Lane T, Ninfa E G, Sommer J M, Newton A. A histidine protein kinase homologue required for regulation of bacterial cell division and differentiation. Proc Natl Acad Sci USA. 1992;89:10297–10301. doi: 10.1073/pnas.89.21.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohta N, Newton A. Signal transduction in the cell cycle regulation of Caulobacter differentiation. Trends Microbiol. 1996;4:326–332. doi: 10.1016/0966-842x(96)10050-0. [DOI] [PubMed] [Google Scholar]
- 31.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 32.Plate C. Mutant of Escherichia coli defective in response to colicin K and in active transport. Proc Natl Acad Sci USA. 1976;125:467–474. doi: 10.1128/jb.125.2.467-474.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plate C, Seely S A, Laffler T G. Evidence for a protonmotive force related regulatory system in Escherichia coli and its effects on lactose transport. Biochemistry. 1986;25:6127–6132. doi: 10.1021/bi00368a044. [DOI] [PubMed] [Google Scholar]
- 34.Pogliano J, Lynch A S, Belin D, Lin E C C, Beckwith J. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 1997;11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- 35.Pogliano J, Pogliano K, Weiss D, Losick R, Beckwith J. Inactivation of FtsI inhibits constriction of the FtsZ cytokinetic ring and delays the assembly of FtsZ rings at potential division sites. Proc Natl Acad Sci USA. 1997;94:559–564. doi: 10.1073/pnas.94.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pogliano K, Harry E, Losick R. Visualization of the subcellular location of sporulation proteins in Bacillus subtilis using immunofluorescence microscopy. Mol Microbiol. 1995;18:459–470. doi: 10.1111/j.1365-2958.1995.mmi_18030459.x. [DOI] [PubMed] [Google Scholar]
- 37.Quon K C, Marczynski G T, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84:83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 38.Rainwater S, Silverman P. The Cpx proteins of Escherichia coli K-12: evidence that cpxA, ecfB, ssd, and eup mutations all identify the same gene. J Bacteriol. 1990;172:2456–2461. doi: 10.1128/jb.172.5.2456-2461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raivio T L, Silhavy T J. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol. 1997;179:7724–7733. doi: 10.1128/jb.179.24.7724-7733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothfield L I, Justice S S. Bacterial cell division: the cycle of the ring. Cell. 1997;88:581–584. doi: 10.1016/s0092-8674(00)81899-1. [DOI] [PubMed] [Google Scholar]
- 41.Rothfield L I, Zhao C. How do bacteria decide where to divide? Cell. 1996;84:183–186. doi: 10.1016/s0092-8674(00)80971-x. [DOI] [PubMed] [Google Scholar]
- 42.Silverman, P. M. Personal communication.
- 43.Sitnikov D M, Schineller J B, Baldwin T O. Control of cell division in Escherichia coli: regulation of transcription of ftsQA involves both rpoS and SdiA-mediated autoinduction. Proc Natl Acad Sci USA. 1996;93:336–341. doi: 10.1073/pnas.93.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stock J B, Stock A M, Mottonen J M. Signal transduction in bacteria. Nature. 1990;344:395–400. doi: 10.1038/344395a0. [DOI] [PubMed] [Google Scholar]
- 45.Wang S P, Sharma P L, Schoenlein P V, Ely B. A histidine protein kinase is involved in polar organelle development in Caulobacter crescentus. Proc Natl Acad Sci USA. 1993;90:630–634. doi: 10.1073/pnas.90.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward J E, Lutkenhaus J. Overproduction of FtsZ induces minicell formation in E. coli. Cell. 1985;42:941–949. doi: 10.1016/0092-8674(85)90290-9. [DOI] [PubMed] [Google Scholar]
- 47.Wingrove J A, Gober J W. The molecular basis of asymmetric cell division in Caulobacter crescentus. Dev Biol. 1995;6:325–333. [Google Scholar]
- 48.Wingrove J A, Gober J W. The asymmetric localization of a sensor histidine kinase regulates temporal and spatial transcription. Science. 1996;274:597–601. doi: 10.1126/science.274.5287.597. [DOI] [PubMed] [Google Scholar]