Abstract
The gut microbiota works in unison with the host, promoting its health. In particular, it has been shown to exert protective, metabolic and structural functions. Recent evidence has revealed the influence of the gut microbiota on other organs such as the central nervous system, cardiovascular and the endocrine-metabolic systems and the digestive system. The study of the gut microbiota is outlining new and broader frontiers every day and holds enormous innovation potential for the medical and pharmaceutical fields. Prevention and treatment of specific women’s diseases involves the need to deepen the function of the gut as a junction organ where certain positive bacteria can be very beneficial to health. The gut microbiota is unique and dynamic at the same time, subject to external factors that can change it, and is capable of modulating itself at different stages of a woman’s life, playing an important role that arises from the intertwining of biological mechanisms between the microbiota and the female genital system. The gut microbiota could play a key role in personalized medicine.
Keywords: Gut microbiota, Women, Metabolites, Health promotion, Immune system, Gut-brain axis
Core Tip: The function of the gut microbiota on health is of primary importance, as it educates and controls the immune system, allows to metabolize and absorb nutrients correctly and protects from pathogens invasion. This paper focuses on the importance of the microbiota for women’s physical and psychological well-being. The gut microbiota has a strategic role in crucial moments at every stage of a woman’s life: From childhood to adolescence, from fertile age to pregnancy-partum, up to menopause. In the future, the study of the gut microbiota could be useful in the treatment of autoimmune and metabolic diseases and even in the fight against tumors, allowing the latest generation of oncological treatments, including immunotherapy, to be more effective.
INTRODUCTION
Symbiotic microbes are present in several sites across the human body and contribute to the healthy physiology of our organism. The microbiota consists of all these microorganisms (bacteria, viruses, fungi and parasites) in a multicellular living organism that live in symbiosis with it without harming it. The assemblage of microbes and their respective genomes constitute our “microbiome” and contribute to shape and regulate many aspects of healthy bodily function. Gastrointestinal, skin, vaginal, and respiratory microbiomes are featured across those respective anatomical and functional sites[1].
In particular, the gastrointestinal tract is composed of different anatomical structures and is the theatre of complex biochemical processes, as well as parallel interactions with sensory, neurological, and endocrinological networks. Not surprisingly, the gut environment is characterized by a heterogeneous collection of distinct habitats along the rostral-caudal axis, which host the most abundant and diverse microbiota in the human body[2].
In recent years, a large body of studies on the human gut microbiota has increased impressively, deepening the awareness that the composition of the gut microbiota can greatly influence health status. Taking part in the digestive process, the gut microbiota plays a fundamental role in the synthesis of short-chain fatty acids, certain vitamins and essential amino acids, which contribute to the health of the body and the gut[3]. The gut microbiota has a strong influence on the immunoregulation, and on metabolic and cardiovascular health[4,5]. Furthermore, it can contribute to the correct functioning of the central nervous system and can even condition the response to drugs[3,6]. States of dysbiosis can adversely affect host health by promoting enrichment of pathogenic species, compromising the permeability of the intestinal barrier, and contributing to localized or generalized inflammatory states[7]. These conditions can lead to the onset of diseases such as cancer, inflammatory bowel disease, metabolic diseases, or even affect the health of other body districts e.g., through gynaecological and dermatological diseases.
The gut microbiota is directly involved not only in the genesis of gastroenterological diseases, such as chronic inflammatory bowel disease, but also in the neurological and psychiatric fields due to the role of the so-called gut-brain axis[8]. The microbiota-gut-brain axis is a bidirectional communication system that connects the central nervous system with the gut microbiota. This axis describes a bidirectional interaction between the inside of the enteric environment (the intestinal epithelium, microbiota, enteric nervous system) and the outside (the central nervous system), connecting centers of the cognitive and emotional spheres, endocrine, and immunological activity. Growing scientific evidence indicates that the gut microbiota can modulate the functions of the central nervous system and vice versa[9]. The marked synergy and continuous exchange of information along this axis is possible because of the vast neurochemical pool available to the enteric nervous system, which innervates the gastrointestinal tract, comparable only to that of the central nervous system. Cells in both systems use the same chemical mediators[3]. Under stressful conditions, the autonomic nervous system can alter intestinal motility and blood flow, and lead to an excessive secretion of hormones and neurotransmitters such as adrenaline and cortisol[10]: All this translates into an alteration in the composition and functional activity of the gut microbiota (Figure 1).
Figure 1.
Gut-brain axis interaction.
Due to dysbiosis, injurious molecules released from the gut and mediators of the immune response (especially cytokines and interleukins) released during the subsequent chronic inflammatory process can damage and overcome the blood-brain barrier. As a result, brain areas that are critical for the control of emotions and behaviour, such as the limbic and frontal lobes, can be damaged[11]. The study conducted by Carloni et al[12] highlighted that alteration of the blood-brain barrier, induced by chronic intestinal inflammation, can be a cause of severe depressive and anxiety disorders.
The emerging role of the gut microbiota in women
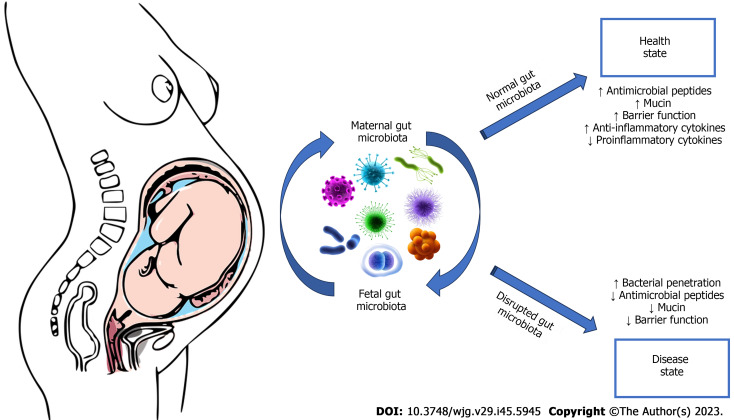
There is a complex controlled exchange of bacteria and other microorganisms from mother to child, even before birth. This dialogue prepares the newborn’s immune system to face the outside world. Each individual acquires at birth his or her own personal gut microbiota, which will be an integral part of his or her defence mechanisms: An extremely heterogeneous set of bacteria, protozoa, fungi, and viruses that form the barrier between the external environment and the internal part of the organism (Figure 2). Its strength lies in diversity: A microbiota with high diversity can maintain certain functions and is usually a guarantee of a healthy immune system, while a low diversity microbiota can more easily undergo deficiencies and can cause impaired immune defences[13]. At birth, the digestive tract of newborns is completely sterile and is colonized immediately, starting from birth, by the microorganisms with which it comes into contact[13]. The gut microbiota of newborns delivered by caesarean section and/or those artificially breastfed appears profoundly different and takes longer to stabilize. In the first 4-36 mo of life, as a result of contact with parents, the external environment and food, the gut microbiota develops and changes rapidly.
Figure 2.
Interplay between maternal and fetal microbiota.
In the first years of life, a real genetic “imprinting” of the gut microbiota takes place, which then determines the state of health in the rest of existence: There it is written whether or not there is predisposition toward certain diseases[14]. This is a crucial time in an individual’s life, it occurs within the age of 4-6 years, and for this reason all factors that can alter it should be avoided[15].
At this age, any intervention on the bacterial flora takes on a very important meaning as it will leave an indelible mark on what will be the adult gut microbiota. From this individual basic nucleus, various changes continue to be observed at different stages of life or if particular pathological conditions are established.
Differences in gut microbiota between men and women
The factors that can influence the composition and balance of the gut microbiota in adulthood are the most diverse: Environmental conditions, stress levels, genetic predisposition, hormonal structure (such as pregnancy, menopause, premenstrual period), pharmacological therapies, eating habits, styles of life[16]. There are diversities between male and female gut microbiota[17] related to hormonal[18], autoimmune[19], ethnic[20] or purely physiological differences such as body mass index body mass index[21] and age[22]. Since microbes of the same species can produce different metabolites depending on the gender of the host and interact differently with sex hormones producing different effects[23] for better characterization it is important to take into account gender differences. These differences appear after puberty, suggesting that sexual hormones have a key role in influencing composition of gut microbiota[24]. The gut microbiota progressively develops along with the maturation of the immune and nervous system throughout the lifespan in men and women with resultant different microbial communities as well as immune and neuro-inflammatory pathways in adult males and females[24].
The embryo differentiates in a male direction due to the Y chromosome and the genes contained therein, which condition circulating testosterone levels. In mice, the microbiota also seems to follow the same paradigm. There is a basic female-type microbiota, which progressively differentiates into a male-type microbiota in parallel with the increase in circulating testosterone[25,26]. Boys and girls during childhood show significant differences in Actinomycetota, Bacillota, and Bacteroidota phyla amount with higher Bacteroidota/Bacillota ratio in boys compared to girls. In adult population men and women maintain different amount of gut microbiota phyla (Bacillota, Verrucomicrobia, Bacteroidota, Fusobacteria and Actinomycetota)[27].
Gut microbiota during different stages of a woman’s life
Puberty: In women, estrogens are important for differentiating the microbiota with health-promoting characteristics. The vaginal microbiota, whose population of microorganisms changes radically after puberty, is essential for the defence of the vagina throughout the fertile age, with a particular value for the pregnancy protection. After menopause, the impoverishment of the microbiota by sexual hormones deficiency leads to a depletion of many of its local, loco-regional and systemic beneficial health functions[24-26].
The estrobolome is capable of producing and metabolizing estrogen, which plays a key physiological role in maintaining women’s health therefore modulating estrogen levels can have effects on weight, libido, and even mood, important dimensions for well-being of every person[28]. As regards estrogens in particular, intestinal microorganisms can modify their levels mainly through the activity of certain enzymes with which some bacteria are equipped, thus transforming estrogens into their active form capable of triggering physiological effects. An altered gut microbiota may play a leading role in the onset and development of reproductive system disorders with hormonal imbalances, such as polycystic ovary syndrome, endometriosis, and infertility[29]. Similarly, it has been suggested that microbiota imbalances with impairment of the gut’s physiological “barrier” function and consequent translocation of microbes and/or microbial components have an active role in the development of vaginal and urinary tract infections[30,31].
Pregnancy: Pregnancy represents the period most clearly exemplifying the existing relationships between microbiota and hormone levels, where the modification of gut bacteria produces relevant and evident alterations ranging from a “low-grade” inflammatory condition typical of the pregnant state, to a reduced glucose tolerance, increased adiposity, among other transmissible as has been demonstrated in some experimental observations conducted on laboratory mice transplanted with the microbiota of women in the third trimester of pregnancy[30,31]. During pregnancy, the gut microbiota primarily defends the health of the mother, for example, by helping her not to develop gestational diabetes, and at the same time, it also preserves that of the child who will be exposed to the first “transfer” of microorganisms at the time of birth[32]. The vertical transmission of the microbiota from mother to fetus, which appears to begin during intrauterine life, contributes to the development of the child’s gastrointestinal microbiota[33]. Going toward the third trimester, the relative abundance of some bacteria that are then transferred to the newborn in the peripartum (in the case of vaginal birth) increases: In fact, the child’s gut microbiota consists of 22% bacteria derived from the mother’s gut microbiota, particularly Bifidobacteria, Bacteroides, and Escherichia coli[31]. A more recent study showed important differences in the gut microbiota related to the sex of the individual and allowed the identification of microorganisms that remain in the female gut until fertile age and are then transmitted from mother to child during birth[34]. These strains are part of the genus Bifidobacterium, a group of microorganisms that has been much studied because they are associated with a positive impact on host health. Thus, knowing the composition of the microbiota during pregnancy can be a most useful tool both for preserving a woman’s health status and for “working” to ensure that a healthy stock of microorganisms is inherited at the time of birth.
Menopause: In menopausal women it has been observed a depletion of Bacillota and a progressive reorganization of the gut microbiome (post-menopausal women tend to be more similar to age-matched men as to pre-menopausal women regarding e.g., Bacillota to Bacteroidota ratio). This could suggest that testosterone may play a major role in shaping the gut microbiota, but more studies focusing on different ages with data comparing both male and female subjects are warranted in order to enlighten the distinct role of sexual hormones as direct or indirect choirmasters of human gut microbiota[27].
Gut microbiota and diseases in women
The fact that the gut microbiota differs between males and females can cause some sex-specific changes in immunity and it has been demonstrated that differences in male and female microbiota can drive chronic diseases, ranging from gastrointestinal inflammatory and metabolic conditions to neurological, cardiovascular, and respiratory illnesses[35]. Gut microbiota has a bidirectional relationship with inflammation and depending on its composition, it can inhibit or stimulate inflammatory pathways. Altered gut microbiota can contribute to subacute systemic inflammation reinforcing the disease state[36].
Potential role of the gut microbiota and its dysbiosis has been described in many diseases affecting women, such as polycystic ovarian syndrome, female cancers (breast, cervical, and ovarian cancer) and postmenopausal period illnesses such as menopausal obesity, Alzheimer’s disease, and bone diseases[37]. The International Cancer Microbiome Consortium has suggested a key role of the gut microbiota in disease development, tumour protection, response to therapies, and control of side effects of cancer treatments[38]. Wang et al[39] found that the feces composition of women with benign pathology differed from that of women suffering from ovarian carcinoma where Akkermansia were low. The researchers showed that transferring gut microbes from ovarian carcinoma patients to mice with the same tumour increased their growth. Subsequent addition of Akkermansia bacteria to the microbiota transplant slowed tumour growth, probably due to increased T-cell activation. Akkermansia supplementation increased levels of short-chain fatty acids, which are associated with an improved ability of specific T lymphocytes to kill tumour cells.
The profile of the gut microbiota and its metabolites can serve as biomarkers for breast cancer. Some potentially pathogenic bacteria such as Clostridium, Citrobacter, and Escherichia may negatively influence the development and progression of breast cancer cells[40,41]. However, some bacterial species have shown a protective effect against this disease. Faecalibacterium prausnitzii and Roseburia intestinalis are producers of butyrate, this molecule has powerful anti-inflammatory and intestinal permeability-reducing activities, demonstrating a protective effect against the development of tumour cells[42,43].
The gut appears to be directly connected to the breast through an “axis”, which may underlie a correlation between gut and breast microbiota dysfunction: The gut microbiota-mammary axis[44]. Altered composition of the various bacterial populations, no longer harmoniously represented within the microbiota, could affect not only local intestinal inflammation, but also systemic inflammation through “hyperactivation” of the immune system. In fact, several studies have shown the presence of dysbiosis in the breast microbiota of women with cancer, but not of healthy women[45]. Costantini et al[46] pointed out that the microbiota may predispose to neoplastic transformation. The clinical importance of this observation suggests that “working” on the microbiota to promote its rebalancing could result in a subsequent reduction in the risk of tumour transformation (Table 1).
Table 1.
Summary of gut microbiota in women
|
|
Summary of gut microbiota
|
| Healthy female[27] | Decreased: Bacteroides abundance with ↑diversity than in men |
| Increased: Lactobacillus, Bifidobacterium and Parabacteroides than in men | |
| Menstruation[29] | Decreased: Bacteroidota, Butyricicoccus, Extibacter, Megasphaera, Parabacteroides |
| Pregnancy[31-33] | Increased: Actionbacteria, Proteobacteria, Akkermansia, Bifidobacterium, Bacillota |
| Decreased: Short chain fatty acids producers | |
| Polycistic ovarian syndrome[36] | Increased: Phocaeicola vulgatus, Bacillota, Streptococcus, Escherichia/Shigella |
| Decreased: Tenericutes, Akkermansia, Oscillospiraceae | |
| Menopause[22] | Increased: Bacillota, Roseburia, Lachnospira, Bacteroidota |
| Decreased: Bilophila, Prevotella, Parabacteroides | |
| Breast cancer[40,41] | Increased: Eubacteriales, Bacillus, Enterobacteriaceae, Staphylococcus |
| Cervical cancer[37] | Increased: Proteobacteria, Prevotella, Porphyromonas, Dialister |
| Decreased: Bacteroides, Alistipes, Lachnospiraceae | |
| Ovarian cancer[39] | Increased: Prevotella, Coriobacteriaceae, Bifidobacterium |
The gut microbiota also actively and importantly influences the effectiveness of the response to immunotherapy. Several studies have observed that patients in whom immunotherapy is effective have a gut microbiome very rich in different species, whereas in patients who are resistant to this treatment the microbiota repertoire is more limited. Thus, the gut microbiota represents a first important modulation tool in regulating the anti-tumour immune response[47]. For example, Bacteroidota are biomarkers of response in melanoma patients: Their presence is associated with a possible reduction in response rates. In melanoma patients, the presence of three types of bacteria (Bifidobacterium pseudocatenulatum, Roseburia spp. and Akkermansia muciniphila) appears to be associated with better response to immunotherapy, but the link between microbiota and response to therapy involves different species in different patient groups[48]. On the other hand, Faecalibacterium, Bifidobacterium and Oscillospiraceae can improve the response to immune checkpoint inhibitors[49]. In addition, the gut microbiota may also play a role in the response to chemotherapy and in reducing the impact of treatment side effects, such as oral mucositis and inflammation[50,51].
CONCLUSION
Although there is a growing interest on influence of the gut microbiota on other organs, our knowledge on the role of gut microbiota in diseases is currently still limited. Some researchers have focused on the variation of the gut microbiome via diet and through supplementation with pre/pro/postbiotics in various female health issues. Interdisciplinary studies on the microbiota are achieving progress toward a better understanding of the molecular basis responsible for microorganism-host interaction and possible positive or negative implications on women’s health. The modulation of the gut microbiome as a both preventative and therapeutic strategy needs to be accomplished. Theoretically, enriching the gut microbiota with “good” bacteria at the expense of “bad” bacteria, good health is promoted. However, there cannot be an ideal microbiota that is the same for everyone: Genes and individual characteristics play a determining role. Increasingly advanced techniques for analyzing the gut microbiota and its functions may also contribute to the development of precision medicine.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 3, 2023
First decision: October 23, 2023
Article in press: November 21, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Angelidi AM, United States; Mandal P, India S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
Contributor Information
Giuseppe Marano, Department of Neurosciences, Università Cattolica del Sacro Cuore, Rome 00168, Italy; Unit of Psychiatry, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy.
Gianandrea Traversi, Unit of Medical Genetics, Department of Laboratory Medicine, Fatebenefratelli Isola Tiberina-Gemelli Isola, Rome 00186, Italy.
Eleonora Gaetani, Department of Medical and Surgical Sciences, Università Cattolica del Sacro Cuore, Rome 00168, Italy; Internal Medicine and Gastroenterology Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy.
Antonio Gasbarrini, Medicina Interna e Gastroenterologia, CEMAD Digestive Disease Center, Università Cattolica del Sacro Cuore, Fondazione Policlinico Universitario A Gemelli IRCCS, Rome 00168, Italy.
Marianna Mazza, Department of Neurosciences, Università Cattolica del Sacro Cuore, Rome 00168, Italy; Unit of Psychiatry, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy. marianna.mazza@policlinicogemelli.it.
References
- 1.Kennedy MS, Chang EB. The microbiome: Composition and locations. Prog Mol Biol Transl Sci. 2020;176:1–42. doi: 10.1016/bs.pmbts.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marano G, Mazza M, Lisci FM, Ciliberto M, Traversi G, Kotzalidis GD, De Berardis D, Laterza L, Sani G, Gasbarrini A, Gaetani E. The Microbiota-Gut-Brain Axis: Psychoneuroimmunological Insights. Nutrients. 2023;15 doi: 10.3390/nu15061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg S, Sharma N, Bharmjeet, Das A. Unraveling the intricate relationship: Influence of microbiome on the host immune system in carcinogenesis. Cancer Rep (Hoboken) 2023:e1892. doi: 10.1002/cnr2.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamjane N, Mechita MB, Nourouti NG, Barakat A. Gut microbiota dysbiosis -associated obesity and its involvement in cardiovascular diseases and type 2 diabetes. A systematic review. Microvasc Res. 2023;151:104601. doi: 10.1016/j.mvr.2023.104601. [DOI] [PubMed] [Google Scholar]
- 6.Khan I. Drugs and gut microbiome interactions-an emerging field of tailored medicine. BMC Pharmacol Toxicol. 2023;24:43. doi: 10.1186/s40360-023-00684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helwig U, Lammers KM, Rizzello F, Brigidi P, Rohleder V, Caramelli E, Gionchetti P, Schrezenmeir J, Foelsch UR, Schreiber S, Campieri M. Lactobacilli, bifidobacteria and E. coli nissle induce pro- and anti-inflammatory cytokines in peripheral blood mononuclear cells. World J Gastroenterol. 2006;12:5978–5986. doi: 10.3748/wjg.v12.i37.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Chen Y, He H, Peng M, Zeng M, Sun H. The role of the indoles in microbiota-gut-brain axis and potential therapeutic targets: A focus on human neurological and neuropsychiatric diseases. Neuropharmacology. 2023;239:109690. doi: 10.1016/j.neuropharm.2023.109690. [DOI] [PubMed] [Google Scholar]
- 9.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. doi: 10.3389/fphys.2011.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Generoso JS, Giridharan VV, Lee J, Macedo D, Barichello T. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz J Psychiatry. 2021;43:293–305. doi: 10.1590/1516-4446-2020-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carloni S, Bertocchi A, Mancinelli S, Bellini M, Erreni M, Borreca A, Braga D, Giugliano S, Mozzarelli AM, Manganaro D, Fernandez Perez D, Colombo F, Di Sabatino A, Pasini D, Penna G, Matteoli M, Lodato S, Rescigno M. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science. 2021;374:439–448. doi: 10.1126/science.abc6108. [DOI] [PubMed] [Google Scholar]
- 13.Pantazi AC, Balasa AL, Mihai CM, Chisnoiu T, Lupu VV, Kassim MAK, Mihai L, Frecus CE, Chirila SI, Lupu A, Andrusca A, Ionescu C, Cuzic V, Cambrea SC. Development of Gut Microbiota in the First 1000 Days after Birth and Potential Interventions. Nutrients. 2023;15 doi: 10.3390/nu15163647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel E, Arzoomand A, Olin A, Wang J, Mikes J, Tan Z, Chen Y, Ehrlich AM, Bernhardsson AK, Mugabo CH, Ambrosiani Y, Gustafsson A, Chew S, Brown HK, Prambs J, Bohlin K, Mitchell RD, Underwood MA, Smilowitz JT, German JB, Frese SA, Brodin P. Bifidobacteria-mediated immune system imprinting early in life. Cell. 2021;184:3884–3898.e11. doi: 10.1016/j.cell.2021.05.030. [DOI] [PubMed] [Google Scholar]
- 15.Xiao L, Zhao F. Microbial transmission, colonisation and succession: from pregnancy to infancy. Gut. 2023;72:772–786. doi: 10.1136/gutjnl-2022-328970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L, Yan Y, Webb RJ, Li Y, Mehrabani S, Xin B, Sun X, Wang Y, Mazidi M. Psychological Stress and Gut Microbiota Composition: A Systematic Review of Human Studies. Neuropsychobiology. 2023;82:247–262. doi: 10.1159/000533131. [DOI] [PubMed] [Google Scholar]
- 17.Kim YS, Unno T, Kim BY, Park MS. Sex Differences in Gut Microbiota. World J Mens Health. 2020;38:48–60. doi: 10.5534/wjmh.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukui H, Xu X, Miwa H. Role of Gut Microbiota-Gut Hormone Axis in the Pathophysiology of Functional Gastrointestinal Disorders. J Neurogastroenterol Motil. 2018;24:367–386. doi: 10.5056/jnm18071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft HJ, Doré J, Blaut M. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72:1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, Zhang M, Xue J, Huang J, Zhuang R, Zhou X, Zhang H, Fu Q, Hao Y. Body Mass Index Differences in the Gut Microbiota Are Gender Specific. Front Microbiol. 2018;9:1250. doi: 10.3389/fmicb.2018.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salles N. Basic mechanisms of the aging gastrointestinal tract. Dig Dis. 2007;25:112–117. doi: 10.1159/000099474. [DOI] [PubMed] [Google Scholar]
- 23.Jahng J, Kim YS. Why Should We Contemplate on Gender Difference in Functional Gastrointestinal Disorders? J Neurogastroenterol Motil. 2017;23:1–2. doi: 10.5056/jnm16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon K, Kim N. Roles of Sex Hormones and Gender in the Gut Microbiota. J Neurogastroenterol Motil. 2021;27:314–325. doi: 10.5056/jnm20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Hokanson KC, Hernández C, Deitzler GE, Gaston JE, David MM. Sex shapes gut-microbiota-brain communication and disease. Trends Microbiol. 2023 doi: 10.1016/j.tim.2023.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Valeri F, Endres K. How biological sex of the host shapes its gut microbiota. Front Neuroendocrinol. 2021;61:100912. doi: 10.1016/j.yfrne.2021.100912. [DOI] [PubMed] [Google Scholar]
- 28.Marano G, Traversi G, Mazza M. Web-mediated Counseling Relationship in Support of the New Sexuality and Affectivity During the COVID-19 Epidemic: A Continuum Between Desire and Fear. Arch Sex Behav. 2021;50:753–755. doi: 10.1007/s10508-020-01908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bednarska-Czerwińska A, Morawiec E, Zmarzły N, Szapski M, Jendrysek J, Pecyna A, Zapletał-Pudełko K, Małysiak W, Sirek T, Ossowski P, Łach A, Boroń D, Bogdał P, Bernet A, Grabarek BO. Dynamics of Microbiome Changes in the Endometrium and Uterine Cervix during Embryo Implantation: A Comparative Analysis. Med Sci Monit. 2023;29:e941289. doi: 10.12659/MSM.941289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piancone E, Fosso B, Marzano M, De Robertis M, Notario E, Oranger A, Manzari C, Bruno S, Visci G, Defazio G, D'Erchia AM, Filomena E, Maio D, Minelli M, Vergallo I, Pesole G. Natural and after colon washing fecal samples: the two sides of the coin for investigating the human gut microbiome. Sci Rep. 2022;12:17909. doi: 10.1038/s41598-022-20888-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inversetti A, Zambella E, Guarano A, Dell'Avanzo M, Di Simone N. Endometrial Microbiota and Immune Tolerance in Pregnancy. Int J Mol Sci. 2023;24 doi: 10.3390/ijms24032995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, Armanini F, Truong DT, Manara S, Zolfo M, Beghini F, Bertorelli R, De Sanctis V, Bariletti I, Canto R, Clementi R, Cologna M, Crifò T, Cusumano G, Gottardi S, Innamorati C, Masè C, Postai D, Savoi D, Duranti S, Lugli GA, Mancabelli L, Turroni F, Ferrario C, Milani C, Mangifesta M, Anzalone R, Viappiani A, Yassour M, Vlamakis H, Xavier R, Collado CM, Koren O, Tateo S, Soffiati M, Pedrotti A, Ventura M, Huttenhower C, Bork P, Segata N. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe. 2018;24:133–145.e5. doi: 10.1016/j.chom.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tirone C, Paladini A, De Maio F, Tersigni C, D'Ippolito S, Di Simone N, Monzo FR, Santarelli G, Bianco DM, Tana M, Lio A, Menzella N, Posteraro B, Sanguinetti M, Lanzone A, Scambia G, Vento G. The Relationship Between Maternal and Neonatal Microbiota in Spontaneous Preterm Birth: A Pilot Study. Front Pediatr. 2022;10:909962. doi: 10.3389/fped.2022.909962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarracchini C, Alessandri G, Fontana F, Rizzo SM, Lugli GA, Bianchi MG, Mancabelli L, Longhi G, Argentini C, Vergna LM, Anzalone R, Viappiani A, Turroni F, Taurino G, Chiu M, Arboleya S, Gueimonde M, Bussolati O, van Sinderen D, Milani C, Ventura M. Genetic strategies for sex-biased persistence of gut microbes across human life. Nat Commun. 2023;14:4220. doi: 10.1038/s41467-023-39931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox LM, Abou-El-Hassan H, Maghzi AH, Vincentini J, Weiner HL. The sex-specific interaction of the microbiome in neurodegenerative diseases. Brain Res. 2019;1724:146385. doi: 10.1016/j.brainres.2019.146385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The Gut Microbiota and Inflammation: An Overview. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17207618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddiqui R, Makhlouf Z, Alharbi AM, Alfahemi H, Khan NA. The Gut Microbiome and Female Health. Biology (Basel) 2022;11 doi: 10.3390/biology11111683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott AJ, Alexander JL, Merrifield CA, Cunningham D, Jobin C, Brown R, Alverdy J, O'Keefe SJ, Gaskins HR, Teare J, Yu J, Hughes DJ, Verstraelen H, Burton J, O'Toole PW, Rosenberg DW, Marchesi JR, Kinross JM. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut. 2019;68:1624–1632. doi: 10.1136/gutjnl-2019-318556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Qin X, Hu D, Huang J, Guo E, Xiao R, Li W, Sun C, Chen G. Akkermansia supplementation reverses the tumor-promoting effect of the fecal microbiota transplantation in ovarian cancer. Cell Rep. 2022;41:111890. doi: 10.1016/j.celrep.2022.111890. [DOI] [PubMed] [Google Scholar]
- 40.Yang P, Wang Z, Peng Q, Lian W, Chen D. Comparison of the Gut Microbiota in Patients with Benign and Malignant Breast Tumors: A Pilot Study. Evol Bioinform Online. 2021;17:11769343211057573. doi: 10.1177/11769343211057573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sohail S, Burns MB. Integrating current analyses of the breast cancer microbiome. PLoS One. 2023;18:e0291320. doi: 10.1371/journal.pone.0291320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bobin-Dubigeon C, Bard JM, Luu TH, Le Vacon F, Nazih H. Basolateral Secretion from Caco-2 Cells Pretreated with Fecal Waters from Breast Cancer Patients Affects MCF7 Cell Viability. Nutrients. 2020;13 doi: 10.3390/nu13010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shrode RL, Knobbe JE, Cady N, Yadav M, Hoang J, Cherwin C, Curry M, Garje R, Vikas P, Sugg S, Phadke S, Filardo E, Mangalam AK. Breast cancer patients from the Midwest region of the United States have reduced levels of short-chain fatty acid-producing gut bacteria. Sci Rep. 2023;13:526. doi: 10.1038/s41598-023-27436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S, Zhang W, Ren H, Xue R, Wang Z, Lv Q. Mendelian randomization analysis revealed a gut microbiota-mammary axis in breast cancer. Front Microbiol. 2023;14:1193725. doi: 10.3389/fmicb.2023.1193725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filippone A, Rossi C, Rossi MM, Di Micco A, Maggiore C, Forcina L, Natale M, Costantini L, Merendino N, Di Leone A, Franceschini G, Masetti R, Magno S. Endocrine Disruptors in Food, Estrobolome and Breast Cancer. J Clin Med. 2023;12 doi: 10.3390/jcm12093158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costantini L, Magno S, Albanese D, Donati C, Molinari R, Filippone A, Masetti R, Merendino N. Characterization of human breast tissue microbiota from core needle biopsies through the analysis of multi hypervariable 16S-rRNA gene regions. Sci Rep. 2018;8:16893. doi: 10.1038/s41598-018-35329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasanisi P, Gariboldi M, Verderio P, Signoroni S, Mancini A, Rivoltini L, Milione M, Masci E, Ciniselli CM, Bruno E, Macciotta A, Belfiore A, Ricci MT, Gargano G, Morelli D, Apolone G, Vitellaro M. A Pilot Low-Inflammatory Dietary Intervention to Reduce Inflammation and Improve Quality of Life in Patients With Familial Adenomatous Polyposis: Protocol Description and Preliminary Results. Integr Cancer Ther. 2019;18:1534735419846400. doi: 10.1177/1534735419846400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee KA, Thomas AM, Bolte LA, Björk JR, de Ruijter LK, Armanini F, Asnicar F, Blanco-Miguez A Board R. Calbet-Llopart N, Derosa L, Dhomen N, Brooks K, Harland M, Harries M, Leeming ER, Lorigan P, Manghi P, Marais R, Newton-Bishop J, Nezi L, Pinto F, Potrony M, Puig S, Serra-Bellver P, Shaw HM, Tamburini S, Valpione S, Vijay A, Waldron L, Zitvogel L, Zolfo M, de Vries EGE, Nathan P, Fehrmann RSN, Bataille V, Hospers GAP, Spector TD, Weersma RK, Segata N. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat Med. 2022;28:535–544. doi: 10.1038/s41591-022-01695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halsey TM, Thomas AS, Hayase T, Ma W, Abu-Sbeih H, Sun B, Parra ER, Jiang ZD, DuPont HL, Sanchez C, El-Himri R, Brown A, Flores I, McDaniel L, Ortega Turrubiates M, Hensel M, Pham D, Watowich SS, Hayase E, Chang CC, Jenq RR, Wang Y. Microbiome alteration via fecal microbiota transplantation is effective for refractory immune checkpoint inhibitor-induced colitis. Sci Transl Med. 2023;15:eabq4006. doi: 10.1126/scitranslmed.abq4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruno JS, Al-Qadami GH, Laheij AMGA, Bossi P, Fregnani ER, Wardill HR. From Pathogenesis to Intervention: The Importance of the Microbiome in Oral Mucositis. Int J Mol Sci. 2023;24 doi: 10.3390/ijms24098274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mondal P, Meeran SM. The emerging role of the gut microbiome in cancer cell plasticity and therapeutic resistance. Cancer Metastasis Rev. 2023;2023 doi: 10.1007/s10555-023-10138-7. [DOI] [PubMed] [Google Scholar]