Abstract
BACKGROUND
There is limited evidence regarding the association between muscle strength and metabolic dysfunction-associated fatty liver disease (MAFLD).
AIM
To investigate the association between muscle strength and MAFLD in the general population in Korea.
METHODS
This nationwide representative cross-sectional study included 31649 individuals aged ≥ 19 years who participated in the Korea National Health and Nutrition Examination Survey between 2015 and 2018. Odds ratios (ORs) and 95% confidence intervals (95%CIs) for MAFLD according to sex-specific quartiles of muscle strength, defined by relative handgrip strength, were calculated using multivariable logistic regression analysis. Additionally, multivariable logistic regression analysis was used to assess the association between muscle strength and probable liver fibrosis in patients with MAFLD.
RESULTS
Of all the participants, 29.3% had MAFLD. The prevalence of MAFLD was significantly higher in the lower muscle strength quartile groups for all participants, sexes, and age groups (P < 0.001). A 1.92-fold (OR = 1.92, 95%CI: 1.70–2.16) and 3.12-fold (OR = 3.12, 95%CI: 2.64–3.69) higher risk of MAFLD was observed in the lowest quartile (Q1) group than in the other groups (Q2–Q4) and the highest quartile (Q4) group, respectively. The ORs of MAFLD were significantly increased in the lower muscle strength quartile groups in a dose-dependent manner (P for trend < 0.001). These associations persisted in both sexes. An inverse association between muscle strength and the risk of MAFLD was observed in all subgroups according to age, obesity, and diabetes mellitus. In patients with MAFLD, the odds of severe liver fibrosis were higher in Q1 (OR = 1.83, 95%CI: 1.25–2.69) than in other groups (Q2–Q4).
CONCLUSION
Among Korean adults, low muscle strength was associated with an increased risk of MAFLD and liver fibrosis in patients with MAFLD.
Keywords: Muscle strength, Handgrip strength, Metabolic dysfunction-associated fatty liver disease, Liver fibrosis, Korea National Health and Nutrition Examination Survey
Core Tip: Limited evidence exists regarding the association between muscle strength and metabolic dysfunction-associated fatty liver disease (MAFLD). This nationwide cross-sectional study analyzed 17349 individuals in the general community who participated in the Korea National Health and Nutrition Examination Survey and measured their grip strength between 2015 and 2018. Among the participants, 29.3% had MAFLD. The prevalence of MAFLD was significantly higher in the lower muscle strength quartiles. The odds ratios of MAFLD were significantly increased in the lower muscle strength quartile groups in a dose-response manner. Among Korean adults, low muscle strength was associated with an increased risk of MAFLD and liver fibrosis in patients with MAFLD.
INTRODUCTION
The prevalence of non-alcoholic fatty liver disease (NAFLD) has surged alongside the obesity pandemic, making it a significant global public health issue, particularly within the Asian population. Over the last three decades, the overall burden of NAFLD has grown exponentially[1-3]. NAFLD has significant clinical implications because it causes liver cirrhosis and liver cancer, which are major causes of liver-related mortality[1]. In response to the broader multi-system nature of NAFLD and the rising prevalence of metabolic disorders, a recent introduction has been made regarding a new definition: metabolic dysfunction-associated fatty liver disease (MAFLD)[2]. While reports suggest that the prevalence of MAFLD in Asian countries ranges from 10%–30%, it exhibits a discernible upward trajectory[2]. Notably, a 23-year follow-up United States study revealed that MAFLD was associated with increased all-cause mortality; furthermore, advanced fibrosis in MAFLD had a higher all-cause mortality risk than that in NAFLD[4]. Given the clinical significance and the imperative to alleviate the disease burden associated with MAFLD, a thorough analysis of risk factors is essential.
Previous studies have suggested an association between sarcopenia and NAFLD. Specifically, muscle mass or strength has shown an inverse relationship with NAFLD[5-7]. Furthermore, it is worth noting that reduced muscle strength has shown a significant association with liver fibrosis in individuals afflicted with NAFLD[8]. Muscle strength, a marker for cardiometabolic fitness, is inversely associated with morbidity[9,10], encompassing conditions such as metabolic syndrome and mortality, particularly within the context of NAFLD[11-14]. Thus, we hypothesized that sarcopenia, particularly muscle strength, is associated with the risk of MAFLD and advanced fibrosis in MAFLD.
Although the body of evidence is evolving, it is worth noting that only a few cross-sectional studies have shown that sarcopenia, measured using dual-energy X-ray absorptiometry and mid-upper arm circumference, was associated with liver fibrosis in patients with MAFLD[15,16]. Limited evidence exists on the association between muscle strength and the prevalence of MAFLD and liver fibrosis in MAFLD in the general population[17]. In light of the public health burden of MAFLD in Asia, we investigated the association between muscle strength and MAFLD using a Korean nationally representative database.
MATERIALS AND METHODS
Survey description and study participants
This study used data from the Korea National Health and Nutrition Examination Survey (KNHANES). The Ministry of Health and Welfare and the Korea Disease Control and Prevention Agency jointly conduct the KNHANES to calculate national health statistics, which is the fundamental database for healthcare policymaking in South Korea. This annual examination recruits an average of 10000 participants in South Korea and consists of 11 different variables, including blood tests and physical examinations, such as measuring body weight, height, handgrip strength, and blood pressure (BP). The KNHANES ensures the quality of the data entered into the database through data collection by well-trained staff and quality control of procedures by internal and external professionals. Details of the KNHANES database have been covered in depth in the past[18].
Because the KNHANES database includes pediatric and adolescent participants, only Korean citizens aged ≥ 19 years who participated in the KNHANES during 2015-2018 were initially included in the analysis. Among the 31649 individuals who participated in the KNHANES between 2015 and 2018, we excluded individuals aged < 19 years (n = 6315), heavy drinkers who consumed ≥ 210 g of alcohol per week for men and ≥ 140 g per week for women (n = 2712) based on self-reported questionnaires, those who tested positive serological markers for hepatitis B or C virus (n = 976), those diagnosed with liver cirrhosis or hepatocellular carcinoma (n = 84), and those with missing data (n = 4213). Ultimately, the data from 17349 individuals were included in the analysis. All participants provided written informed consent for data collection.
Assessment of muscle strength
Muscle strength was assessed using relative handgrip strength, which has been utilized in prior studies as an indicator of muscle strength[8,19]. To measure handgrip strength, the participants were instructed to squeeze a handgrip dynamometer (Digital grip strength dynamometer, T.K.K 5401, Takei Scientific Instruments Co., Ltd., Tokyo, Japan) for at least 3 S using their dominant arm with the elbow extended and the participant in a standing position. Handgrip strength was measured thrice with a 1-min interval for rest between each measurement. Muscle strength was defined as the mean handgrip strength (kg) divided by the body mass index (BMI, kg/m2). As the present study aimed to understand the association between muscle strength and MAFLD, the study participants were divided into sex-specific quartile groups of muscle strength, with Q1 and Q4 being the lowest and highest quartiles, respectively. The cutoff values for the quartiles were 1.30, 1.53, and 1.77 in men, and 0.76, 0.94, and 1.11 in women, respectively.
Definition of MAFLD and liver fibrosis
NAFLD was defined using a validated fatty liver prediction model called the hepatic steatosis index (HSI)[20]. HSI was defined as 8 × alanine aminotransferase (ALT)/aspartate aminotransferase (AST) + body mass index (BMI, + 2, if diabetic; +2, if female). HSI > 36 was defined as NAFLD[20]. Previous studies have reported that HSI could predict NAFLD with high sensitivity and specificity in the Korean population[20,21].
MAFLD was defined as NAFLD (HSI > 36) with the presence of at least one of the following metabolic risk factors[2]: (1) Overweight or obesity (BMI ≥ 23 kg/m2) based on Asian standards[22]; (2) Type 2 diabetes [physician diagnosis, fasting serum glucose ≥ 126 mg/dL, or glycated hemoglobin (HbA1c) ≥ 6.5%]; and (3) Normal BMI (< 23 kg/m2) with two or more of the following metabolic risk factors[23]: (1) Waist circumference (WC) ≥ 90 cm and ≥ 80 cm for men and women; (2) BP ≥130/85 mmHg or being administered anti-hypertensive medication(s); (3) Triglyceride level ≥ 150 mg/dL or being administered lipid-lowering medication(s); (4) High-density lipoprotein cholesterol (HDL-C) < 40 mg/dL for men and < 50 mg/dL for women, or being administered lipid-lowering medication(s); (5) Diagnosis of prediabetes state, defined as fasting serum glucose of 100-125 mg/dL or HbA1c of 5.7%–6.4%; and (6) Serum high-sensitivity C-reactive protein (hs-CRP) level > 2 mg/L.
To evaluate advanced liver fibrosis in patients with MAFLD, we used the following prediction equation: Fibrosis-4 (FIB-4) score = age (years) × AST (IU/L)/[platelet (109/L)] × [ALT (IU/L)]1/2. The risk of advanced fibrosis in MAFLD was classified as either 1.3 ≤ FIB-4 score < 2.67 (intermediate risk) or FIB-4 ≥ 2.67 (high risk)[24].
Measurements and covariates
Participants’ sociodemographic information and data on health behaviors were assessed using a self-report questionnaire. Smoking status was classified based on whether the participant was a current smoker. Based on the modified version of the International Physical Activity Questionnaire[25,26], regular physical activity was defined as: (1) Moderate-intensity physical activity for ≥ 150 min/wk; (2) High-intensity physical activity for ≥ 75 min/week; or (3) A combination of moderate- and high-intensity physical activity per week, where 1 min of high-intensity physical activity is equivalent to 2 min of moderate-intensity physical activity, with the collective minutes satisfying either one of the above criteria. Household income was segmented into quartiles, and educational attainment was assessed based on whether participants had completed more than 12 years of education (or high school graduate).
The physical examination was conducted by certified staff. Height, body weight, and WC were measured, and BMI was defined as the weight in kilograms divided by the square of height in meters. Using a standard sphygmomanometer, three BP measurements were conducted at 5-min intervals, and the mean values of the second and third BP measurements were recorded. Blood samples were drawn after fasting for ≥ 8 h, and the serum concentrations of AST, ALT, total cholesterol, HDL-C, low-density lipoprotein cholesterol (LDL-C), triglycerides, fasting glucose, HbA1c, hs-CRP, hepatitis B surface antigen, hepatitis C virus antibody, and platelet count were assessed.
Hypertension was defined as either a medical diagnosis by a physician or a systolic/diastolic BP reading of ≥ 140/90 mmHg[27]. Diabetes mellitus (DM) was defined as having a physician's diagnosis, a fasting glucose level of ≥ 126 mg/dL, or an HbA1c level of ≥ 6.5%[28]. Dyslipidemia was characterized by either a physician's diagnosis or a total cholesterol level of ≥ 240 mg/dL[29]. Obesity was defined as BMI ≥ 25 kg/m2[30].
Statistical analyses
Continuous variables were summarized as mean ± SE and categorical variables as percentages and were compared using analysis of variance and the Rao–Scott chi-square test, respectively. We performed multivariable logistic regression analysis to evaluate the association between muscle strength and the risk of MAFLD and calculated the odds ratios (ORs) and 95% confidence intervals (95%CIs). Model 1 was not adjusted, and Model 2 was adjusted for age, sex, income, education, smoking status, and physical activity. In Model 3, adjustments were made for ALT, obesity, hypertension, DM, dyslipidemia, and hs-CRP levels, in addition to the confounders in Model 2. The adjusted variables were selected from the statistically significant variables in Table 1, the clinical factors that were expected to be associated with muscle strength and MAFLD, and based on the results of the preliminary logistic regression analysis between baseline variables and MAFLD (Supplementary Table 1) and a literature search. The association between muscle strength and the risk of MAFLD was also evaluated in subgroups stratified by sex, age, obesity, and DM. Multivariable logistic regression analysis was used to assess the association between muscle strength and probable liver fibrosis in patients with MAFLD. Statistical analyses, including pairwise comparison, were performed using IBM SPSS Statistics ver. 22.0 (IBM Corp., Armonk, NY, United States). Complex sample procedures were performed based on the survey design. Statistical significance was set at P < 0.05. The statistical methods used in this study were reviewed by Dr. Youn Huh from Uijeongbu Eulji Medical Center.
Table 1.
Characteristics of study participants according to the quartiles of muscle strength
|
|
Q1
|
Q2
|
Q3
|
Q4
|
P for trend
|
| N (unweighted) | 4305 | 4347 | 4397 | 4300 | |
| Age (yr) | 55.9 ± 0.4 | 49.2 ± 0.4 | 44.6 ± 0.3 | 39.9 ± 0.2 | < 0.001 |
| Sex (men) | 45.2 (1.0) | 45.9 (0.9) | 46.2 (0.9) | 48.0 (0.9) | 0.172 |
| Current smoker | 13.6 (0.7) | 16.6 (0.7) | 17.7 (0.7) | 20.3 (0.8) | < 0.001 |
| Physical activity | 38.5 (1.0) | 46.4 (1.0) | 51.1 (0.9) | 53.1 (0.9) | < 0.001 |
| Income (lowest quartile) | 28.8 (1.0) | 16.5 (0.7) | 10.7 (0.6) | 8.0 (0.5) | < 0.001 |
| Education (≤ 12 yr) | 45.4 (1.2) | 28.1 (0.9) | 16.9 (0.7) | 8.5 (0.5) | < 0.001 |
| BMI (kg/m2) | 26.0 ± 0.1 | 24.7 ± 0.1 | 23.5 ± 0.1 | 21.8 ± 0.1 | < 0.001 |
| Waist circumference (cm) | 88.0 ± 0.2 | 84.0 ± 0.2 | 80.5 ± 0.2 | 76.3 ± 0.2 | < 0.001 |
| Handgrip strength (kg) | 21.8 ± 0.2 | 27.8 ± 0.2 | 31.2 ± 0.2 | 35.6 ± 0.2 | < 0.001 |
| Muscle strength (handgrip strength/body mass index) | 0.8 ± 0.01 | 1.1 ± 0.01 | 1.3 ± 0.01 | 1.6 ± 0.01 | < 0.001 |
| Systolic BP (mmHg) | 122.7 ± 0.4 | 118.2 ± 0.3 | 115.2 ± 0.3 | 112.1 ± 0.3 | < 0.001 |
| Diastolic BP (mmHg) | 75.3 ± 0.2 | 75.8 ± 0.2 | 75.2 ± 0.2 | 74.2 ± 0.2 | < 0.001 |
| AST (IU/L) | 24.5 ± 0.2 | 23.1 ± 0.2 | 21.5 ± 0.2 | 20.2 ± 0.1 | < 0.001 |
| ALT (IU/L) | 26.0 ± 0.5 | 24.2 ± 0.4 | 21.0 ± 0.3 | 18.4 ± 0.2 | < 0.001 |
| Total cholesterol (mg/dL) | 191.4 ± 0.7 | 194.3 ± 0.7 | 194.1 ± 0.6 | 188.8 ± 0.6 | < 0.001 |
| HDL-C (mg/dL) | 47.4 ± 0.2 | 49.9 ± 0.2 | 51.6 ± 0.2 | 54.0 ± 0.2 | < 0.001 |
| LDL-C (mg/dL) | 115.0 ± 0.7 | 116.9 ± 0.6 | 116.5 ± 0.6 | 112.3 ± 0.5 | < 0.001 |
| Triglycerides (mg/dL) | 145.6 ± 2.0 | 137.5 ± 2.0 | 130.1 ± 1.9 | 112.5 ± 1.6 | < 0.001 |
| Fasting glucose (mg/dL) | 106.1 ± 0.5 | 100.8 ± 0.4 | 97.6 ± 0.4 | 93.8 ± 0.3 | < 0.001 |
| HbA1c (%) | 5.9 ± 0.02 | 5.7 ± 0.01 | 5.6 ± 0.01 | 5.4 ± 0.01 | < 0.001 |
| hs-CRP (mg/L) | 1.7 ± 0.04 | 1.2 ± 0.04 | 1.1 ± 0.03 | 0.8 ± 0.03 | < 0.001 |
| Obesity | 56.7 (0.9) | 44.0 (1.0) | 28.7 (0.8) | 11.6 (0.6) | < 0.001 |
| Hypertension | 45.4 (1.0) | 30.5 (0.8) | 21.5 (0.7) | 12.4 (0.6) | < 0.001 |
| Diabetes mellitus | 22.5 (0.7) | 13.1 (0.6) | 7.8 (0.4) | 3.9 (0.3) | < 0.001 |
| Dyslipidemia | 31.8 (0.9) | 28.1 (0.9) | 21.2 (0.7) | 14.1 (0.6) | < 0.001 |
Data are presented as mean ± SE or percentage (SE). Q: Quartile; BMI: Body mass index; BP: Blood pressure; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; HbA1c: Glycated hemoglobin; hs-CRP: High-sensitivity C-reactive protein.
RESULTS
Characteristics of study participants
Among a total of 31649 potentially eligible individuals, 17349 individuals were included and analyzed in the study. The characteristics of the participants according to the muscle strength quartiles are presented in Table 1. Individuals with weaker muscle strength were older and had lower socioeconomic status. Those with weaker muscle strength were less likely to be current smokers and engaged in regular physical activities (P for trend < 0.001). The mean values of cardiometabolic parameters, such as BMI, WC, BPs, AST, ALT, total cholesterol, LDL-C, triglycerides, fasting glucose, HbA1c, and hs-CRP, tended to be higher in the lower muscle strength quartile groups (P for trend < 0.001). The proportion of those with obesity, hypertension, DM, and dyslipidemia also increased as muscle strength decreased (P for trend < 0.001).
Prevalence of MAFLD according to muscle strength
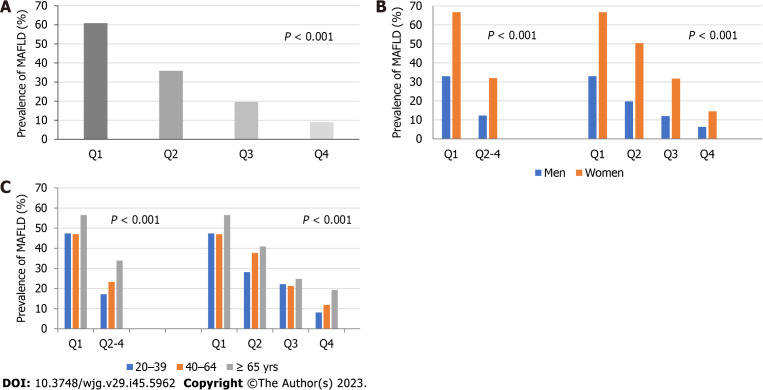
Among all participants, including within various sex and age groups, the prevalence of MAFLD was significantly higher in the lower muscle strength quartile groups (P < 0.001 in Figure 1). Additionally, regardless of sex and age group, the prevalence was higher in the lowest quartile (Q1) group of muscle strength than in the remaining quartile (Q2–Q4) groups (P < 0.001 in Figure 1B and C).
Figure 1.
Prevalence of metabolic dysfunction-associated fatty liver disease by muscle strength. A: The prevalence of metabolic dysfunction-associated fatty liver disease (MAFLD) in the total participants; B: the prevalence in men and women; C: the prevalence in different age groups. The prevalence of MAFLD was higher in the lower muscle strength quartile groups in total participants and age and sex groups. The prevalence was higher in the lowest muscle strength quartile (Q1) group than in the remaining groups (Q2–Q4) in both sexes and all age groups. A dose-response relationship between lower muscle strength quartile and MAFLD was also observed. Q: Quartile; MAFLD: Metabolic dysfunction-associated fatty liver disease.
Association between muscle strength and the risk of MAFLD
Among all participants, the Q1 group had higher odds of MAFLD than the other groups (Q2–Q4) (OR = 1.92, 95%CI: 1.70–2.16) (Model 3, Table 2). Compared with the Q4 group, the ORs of MAFLD significantly increased in the Q3 (OR = 1.41, 95%CI: 1.22–1.65), Q2 (OR = 2.19, 95%CI: 1.85–2.58), and Q1 (OR = 3.12, 95%CI: 2.64–3.69) groups. The ORs were higher in the lower muscle strength quartile groups in a dose-dependent manner (P for trend < 0.001). Among both men and women, higher odds of MAFLD were observed in the Q1 group than in the Q2–Q4 (OR = 2.05, 95%CI: 1.70–2.47 in men and OR = 1.76, 95%CI: 1.47–2.10 in women) and Q4 groups (OR = 2.88, 95%CI: 2.20–3.78 in men and OR = 3.09, 95%CI: 2.43–3.92 in women). The ORs of MAFLD tended to be higher in the lower muscle strength quartile groups for both sexes (P for trend < 0.001). Furthermore, the receiver operating characteristic analysis to assess the relationship between muscle strength and prevalence of MAFLD revealed that the area under the curve for the entire participant group, as well as for men and women separately, were 0.764, 0.701, and 0.740, respectively (all P < 0.001, Supplementary Figure 1).
Table 2.
Odds ratios (95% confidence intervals) of metabolic dysfunction-associated fatty liver disease according to the categories of muscle strength
|
Muscle strength
|
Model 1
|
Model 2
|
Model 3
|
| Total | |||
| Q1 | 3.63 (3.34–3.95) | 3.24 (2.94–3.58) | 1.92 (1.70–2.16) |
| Q2–Q4 | 1 (reference) | 1 (reference) | 1 (reference) |
| P value | |||
| Q1 | 9.12 (7.98–10.41) | 8.61 (7.41–10.01) | 3.12 (2.64–3.69) |
| Q2 | 4.92 (4.29–5.64) | 4.75 (4.10–5.50) | 2.19 (1.85–2.58) |
| Q3 | 2.48 (2.17–2.84) | 2.42 (2.10–2.78) | 1.41 (1.22–1.65) |
| Q4 | 1 (reference) | 1 (reference) | 1 (reference) |
| P for trend | < 0.001 | < 0.001 | < 0.001 |
| Men | |||
| Q1 | 3.51 (3.02–4.09) | 3.31 (2.80–3.90) | 2.05 (1.70–2.47) |
| Q2–Q4 | 1 (reference) | 1 (reference) | 1 (reference) |
| P value | < 0.001 | < 0.001 | |
| Q1 | 7.30 (5.67–9.39) | 7.20 (5.52–9.38) | 2.88 (2.20–3.78) |
| Q2 | 3.68 (2.83–4.78) | 3.68 (2.82–4.80) | 1.75 (1.32–2.31) |
| Q3 | 2.02 (1.54–2.66) | 2.03 (1.55–2.68) | 1.23 (0.93–1.63) |
| Q4 | 1 (reference) | 1 (reference) | 1 (reference) |
| P for trend | < 0.001 | < 0.001 | < 0.001 |
| Women | |||
| Q1 | 4.20 (3.74–4.71) | 3.17 (2.79–3.60) | 1.76 (1.47–2.10) |
| Q2–Q4 | 1 (reference) | 1 (reference) | 1 (reference) |
| P value | < 0.001 | < 0.001 | < 0.001 |
| Q1 | 11.76 (9.87–14.02) | 9.14 (7.56–11.05) | 3.09 (2.43–3.92) |
| Q2 | 6.09 (5.15–7.20) | 5.30 (4.47–6.30) | 2.52 (2.05–3.10) |
| Q3 | 2.76 (2.34–3.26) | 2.59 (2.20–3.06) | 1.52 (1.25–1.85) |
| Q4 | 1 (reference) | 1 (reference) | 1 (reference) |
| P for trend | < 0.001 | < 0.001 | < 0.001 |
Odds ratios (95% confidence intervals) were calculated using multivariable logistic regression analysis; Model 1 = not adjusted; Model 2 = adjusted for age, sex, income, education, smoking status, and physical activity; Model 3 = Model 2 + alanine aminotransferase, obesity, hypertension, diabetes mellitus, dyslipidemia, and high-sensitivity C-reactive protein. Q: Quartile.
Subgroup analyses on the association between muscle strength and MAFLD
As indicated in Table 3, a noteworthy inverse relationship between muscle strength and the risk of MAFLD remained consistent across all subgroups. Importantly, no significant interactions were observed based on sex, age, obesity, or DM status in the association between muscle strength and MAFLD.
Table 3.
Subgroup analysis for the association between muscle strength and metabolic dysfunction-associated fatty liver disease
|
|
Muscle strength
|
OR (95%CI)
|
P for interaction
|
| Sex | 0.126 | ||
| Men | Q2–Q4 | 1 (reference) | |
| Q1 | 2.05 (1.70–2.47) | ||
| Women | Q2–Q4 | 1 (reference) | |
| Q1 | 1.76 (1.47–2.10) | ||
| Age | 0.057 | ||
| < 65 yr | Q2–Q4 | 1 (reference) | |
| Q1 | 1.97 (1.70–2.29) | ||
| ≥ 65 yr | Q2–Q4 | 1 (reference) | |
| Q1 | 1.74 (1.44–2.09) | ||
| Obesity | 0.264 | ||
| No | Q2–Q4 | 1 (reference) | |
| Q1 | 1.63 (1.36–1.96) | ||
| Yes | Q2–Q4 | 1 (reference) | |
| Q1 | 2.09 (1.75–2.48) | ||
| Diabetes mellitus | 0.622 | ||
| No | Q2–Q4 | 1 (reference) | |
| Q1 | 1.97 (1.72–2.26) | ||
| Yes | Q2–Q4 | 1 (reference) | |
| Q1 | 1.71 (1.33–2.22) |
Odds ratios (95% confidence intervals) were calculated using multivariable logistic regression analysis after adjusting for age, sex, income, education, smoking status, physical activity, alanine aminotransferase, obesity, hypertension, diabetes mellitus, dyslipidemia, and high-sensitivity C-reactive protein; Stratified variables (sex, age, obesity, and diabetes mellitus) were omitted from the adjusted variables during the respective subgroup analyses. OR: Odds ratio; 95%CI: 95% confidence interval; Q: Quartile.
Association between muscle strength and probable liver fibrosis assessed by FIB-4 in MAFLD
After adjusting for all confounding factors, muscle strength was not significantly associated with the intermediate risk for advanced liver fibrosis (defined as 1.3 ≤ FIB-4 < 2.67) in patients with MAFLD (Table 4). However, the lowest muscle strength group (Q1) had a higher odds of a high risk of advanced fibrosis (defined by FIB-4 ≥ 2.67) than the Q2–Q4 group (OR = 1.83, 95%CI: 1.25–2.69).
Table 4.
Association between muscle strength and probable liver fibrosis assessed using fibrosis-4 among patients with metabolic dysfunction-associated fatty liver disease
| Muscle strength |
OR (95%CI)
|
|
|
|
Model 1
|
Model 2
|
Model 3
|
|
| 1.3 ≤ FIB-4 < 2.67 | |||
| Q1 | 2.67 (2.35–3.02) | 0.96 (0.82–1.12) | 1.03 (0.87–1.21) |
| Q2–Q4 | 1 (reference) | 1 (reference) | 1 (reference) |
| P value | < 0.001 | 0.588 | 0.773 |
| Q1 | 4.09 (3.50–4.79) | 0.89 (0.72–1.09) | 1.01 (0.81–1.26) |
| Q2 | 2.31 (2.00–2.66) | 0.85 (0.71–1.01) | 0.94 (0.78–1.13) |
| Q3 | 1.67 (1.43–1.94) | 0.97 (0.81–1.16) | 1.04 (0.86–1.25) |
| Q4 | 1 (reference) | 1 (reference) | 1 (reference) |
| P for trend | < 0.001 | 0.117 | 0.809 |
| FIB-4 ≥ 2.67 | |||
| Q1 | 4.71 (3.38–6.56) | 1.72 (1.19–2.50) | 1.83 (1.25–2.69) |
| Q2–Q4 | 1 (reference) | 1 (reference) | 1 (reference) |
| P value | < 0.001 | 0.004 | 0.002 |
| Q1 | 8.13 (4.74–13.95) | 1.68 (0.89–3.17) | 1.85 (0.92–3.74) |
| Q2 | 2.25 (1.22–4.15) | 0.78 (0.41–1.47) | 0.82 (0.41–1.66) |
| Q3 | 2.20 (1.18–4.13) | 1.26 (0.65–2.46) | 1.31 (0.65–2.65) |
| Q4 | 1 (reference) | 1 (reference) | 1 (reference) |
| P for trend | < 0.001 | 0.113 | 0.073 |
Odds ratios (95% confidence intervals) were calculated using multivariable logistic regression analysis; Model 1 = not adjusted; Model 2 = adjusted for age, sex, income, education, smoking status, and physical activity; Model 3 = Model 2 + alanine aminotransferase, obesity, hypertension, diabetes mellitus, dyslipidemia, and high-sensitivity C-reactive protein. OR: Odds ratio; 95%CI: 95% confidence interval; FIB-4: Fibrosis-4; Q: Quartile.
DISCUSSION
In this large-scale nationwide study, we found that the prevalence of MAFLD was greater among individuals with lower muscle strength, which was associated with a higher risk of MAFLD after adjusting for potential confounding variables. The lowest muscle strength quartile group had 3.12-fold, 2.88-fold, and 3.09-fold higher odds of MAFLD than the highest quartile group in all participants, men, and women, respectively. These associations persisted in the subgroups stratified by age, obesity, and DM. Furthermore, in patients with MAFLD, the lowest muscle strength quartile group had 1.83-fold increased odds of high risk of advanced liver fibrosis compared to the other groups.
To the best of our knowledge, no prior studies have examined the association between muscle strength and the risk of MAFLD. However, few studies have examined the association between muscle strength and NAFLD[31]. One cross-sectional study, utilizing data from the KNHANES database, found an association between low muscle strength and NAFLD[8]. In our study, we have effectively demonstrated a significant association between muscle strength and MAFLD. Importantly, our findings suggest that low muscle strength may be a modifiable risk factor for MAFLD. This study also suggests an association between low muscle strength and a high probability of advanced liver fibrosis in patients with MAFLD. Our findings are in line with previous studies reporting an association between low muscle strength and advanced liver fibrosis[8,32]. Although the association did not persist when each muscle strength group was analyzed, the current study demonstrated that low muscle strength might be associated with advanced liver fibrosis in patients with MAFLD.
The mechanisms underlying the association between low muscle strength and MAFLD have not yet been fully elucidated. However, potential pathways may include insulin resistance due to hepatic steatosis[33] and physical inactivity and disuse, leading to decreased muscle function[34]. Metabolically inactive muscles undergo disuse atrophy, in which sarcomeres are catabolized and capillaries decrease in number[35]. This risk is especially pronounced in individuals with metabolic syndrome and a sedentary lifestyle. Weight gain leads to adipocyte dysfunction and increased local inflammation, eventually resulting in insulin resistance. Disturbances in fat storage in adipocytes increase the release of fatty acids. Excess fatty acids produce strain in the hepatic mitochondria and subsequently result in the production of reactive oxygen species and mitochondrial damage. Excess fatty acids also increase stress on the endoplasmic reticulum, contributing to mitochondrial dysfunction and cellular death[33]. In particular, reduced muscle strength may be linked to mitochondrial dysfunction in both skeletal muscles and the liver, as suggested by previous studies[36-41]. Data accumulated thus far have indicated that mitochondrial dysfunction may play a role in the development of insulin resistance, and MAFLD and mitochondrial dysfunction may additionally contribute to low muscle strength[42-46]. In relation to hepatic fibrosis, stellate cells are thought to be involved in inflammatory and fibrotic changes in fatty liver disease in response to damage-associated molecular patterns from dying hepatocytes, free cholesterol, toll-like receptors, and oxidative stress[33,47].
Sarcopenia is a muscle disease of both young and old and increases the risks of falls, fractures, and mortality[34]. Studies have revealed that muscle strength, rather than muscle mass, is a predictor of mortality[48-50]; thus, the guideline from the European Working Group on Sarcopenia in Older People highlighted low muscle strength as the main characteristic of sarcopenia[34]. Low muscle strength alone is adequate for making a clinical diagnosis of probable sarcopenia. Subsequently, diagnostic assessments for potential underlying causes and appropriate interventions can be initiated[34,51.] Additionally, the guideline recommended handgrip strength as a proxy for whole-body strength[34]. Overall, these findings suggest that muscle strength is a crucial parameter in detecting sarcopenia[52]. Accordingly, we used muscle strength, defined by handgrip strength, as the primary independent variable in this study.
MAFLD increases the risk of liver fibrosis, hepatocellular carcinoma, and cardiovascular diseases; hence, controlling metabolic disorders is essential in the management of MAFLD[2]. Treatment objectives encompass the reversal of hepatic steatosis, steatohepatitis, and hepatic fibrosis, as well as the reduction of cardiovascular risk associated with MAFLD. The mainstay of treatment is the modification of metabolic risk factors and lifestyle[2]. Weight loss can reduce liver steatosis and reverse steatohepatitis or fibrosis[53]. Generally, low-carbohydrate, low-fat, and Mediterranean-type dietary plans with moderate-intensity exercise for 30 min/d for ≥ 5 d/wk or ≥ 150 min/wk or vigorous-intensity exercise for ≥ 20 min/d for ≥ 3 d/wk are recommended[2,54]. The guidelines endorse the inclusion of both aerobic and resistance exercises[2]. In light of the observed association between low muscle strength and a higher prevalence of MAFLD in this study, it is imperative to underscore the importance of resistance training during patient education.
While this study has provided valuable insights, it is important to acknowledge that it also had several limitations. First, the findings of this study are not generalizable to other ethnic groups since only Koreans were included in the analysis. Second, although liver biopsy is the gold standard for diagnosis, the present study used HSI to diagnose NAFLD and MAFLD. However, the diagnostic reliability of the HSI has been validated in previous studies[20,21]. Third, causal relationships could not be fully determined owing to the cross-sectional design of the study. Future prospective longitudinal studies are needed to confirm the role of muscle strength in MAFLD. Fourth, the lack of data on medications for chronic diseases and those potentially affecting liver steatosis is a limitation. Thus, we defined hypertension, DM, and dyslipidemia using a combination of prior physician diagnoses and laboratory blood tests. Fifth, handgrip strength was used to diagnose probable sarcopenia in the present study. The prevalence of probable sarcopenia can vary depending on the diagnostic methods, especially in liver steatosis[55]. Nonetheless, handgrip strength is easy to incorporate into clinical settings and is also a well-studied parameter of sarcopenia in many studies. Despite these shortcomings, the use of KNHANES enabled us to study important exposures and muscle strength in a large representative Korean population and make adjustments for a variety of potential confounders. Furthermore, we were able to successfully assess the new definition of MAFLD and study its prevalence and association with muscle strength, which extends beyond previous studies where only NAFLD was considered.
CONCLUSION
In this nationwide study of the Korean adult population, low muscle strength was associated with a dose-dependent higher risk of MAFLD in all participants and subgroups. Low muscle strength is associated with a high probability of liver fibrosis in patients with MAFLD. The identification and management of low muscle strength may play a crucial role in preventing MAFLD and liver fibrosis. Nonetheless, additional research is necessary to validate this association.
ARTICLE HIGHLIGHTS
Research background
More evidence is needed regarding the association between muscle strength and metabolic dysfunction-associated fatty liver disease (MAFLD) and only a few cross-sectional studies have shown that sarcopenia was associated with liver fibrosis in patients with MAFLD. In response to the increasing public health burden of MAFLD in Asia, we investigated the association between muscle strength and MAFLD using a Korean nationally representative database.
Research motivation
A recent introduction has been made regarding a new definition: MAFLD. Importantly, MAFLD is associated with increased all-cause mortality and advanced fibrosis in MAFLD had a higher all-cause mortality risk than that in non-alcoholic fatty liver disease. However, the link between muscle strength and MAFLD is not well studied.
Research objectives
We aimed to investigate the association between muscle strength and MAFLD in the general population in Korea. Additionally, we sought to study the risk of liver fibrosis in patients with MAFLD according to muscle strength.
Research methods
This study used data from the Korea National Health and Nutrition Examination Survey. Muscle strength was assessed using relative handgrip strength and the participants were categorized into muscle strength quartiles. We performed multivariable logistic regression analysis to evaluate the association between muscle strength and the risk of MAFLD and calculated the odds ratios and 95% confidence intervals.
Research results
Twenty-nine point three per cent of the participants had MAFLD. The lowest quartile was significantly associated with higher prevalence of MAFLD for all participants, sexes, and age groups. In patients with MAFLD, the odds of severe liver fibrosis were higher in Q1 than in other groups (Q2–Q4). However, causality should be investigated in future studies.
Research conclusions
The nationwide study of the Korean adult population revealed that low muscle strength was associated with a dose-dependent higher risk of MAFLD in all participants and subgroups. Additionally, low muscle strength is associated with a high probability of liver fibrosis in patients with MAFLD. The identification and management of low muscle strength may play a crucial role in preventing MAFLD and liver fibrosis.
Research perspectives
Prospective cohort or randomized controlled trials are needed to confirm the relationship between muscle strength and MAFLD. Future studies should focus on whether physical activity can prevent or reverse MAFLD and liver fibrosis in patients with MAFLD.
Footnotes
Institutional review board statement: The institutional review board of the Korea University Guro Hospital in Seoul, Republic of Korea approved the study protocol (No. 2022GR0322).
Informed consent statement: Signed informed consent was obtained from all participants.
Conflict-of-interest statement: The authors disclose no conflicts.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 1, 2023
First decision: September 30, 2023
Article in press: November 17, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hu JH, China; Xing H, China; Yang ZG, China S-Editor: Lin C L-Editor: A P-Editor: Zhao S
Contributor Information
Gyu Bae Lee, Department of Family Medicine, Korea University College of Medicine, Seoul 02841, South Korea.
Youn Huh, Department of Family Medicine, Uijeongbu Eulji Medical Center, EULJI University, Daejeon 11759, South Korea.
Sang Hyun Lee, School of Electrical Engineering, Korea University, Seoul 02841, South Korea.
Byoungduck Han, Department of Family Medicine, Korea University College of Medicine, Seoul 02841, South Korea.
Yang-Hyun Kim, Department of Family Medicine, Korea University College of Medicine, Seoul 02841, South Korea.
Do-Hoon Kim, Department of Family Medicine, Korea University College of Medicine, Seoul 02841, South Korea.
Seon Mee Kim, Department of Family Medicine, Korea University College of Medicine, Seoul 02841, South Korea.
Youn Seon Choi, Department of Family Medicine, Korea University College of Medicine, Seoul 02841, South Korea.
Kyung Hwan Cho, Department of Family Medicine, Korea University College of Medicine, Seoul 02841, South Korea.
Ga Eun Nam, Department of Family Medicine, Korea University College of Medicine, Seoul 02841, South Korea. silver79@korea.ac.kr.
Data sharing statement
No additional data are available.
References
- 1.Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, Jia J, Tian Q, Aggarwal R, Muljono DH, Omata M, Ooka Y, Han KH, Lee HW, Jafri W, Butt AS, Chong CH, Lim SG, Pwu RF, Chen DS. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2020;5:167–228. doi: 10.1016/S2468-1253(19)30342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Huh Y, Cho YJ, Nam GE. Recent Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease. J Obes Metab Syndr. 2022;31:17–27. doi: 10.7570/jomes22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim D, Konyn P, Sandhu KK, Dennis BB, Cheung AC, Ahmed A. Metabolic dysfunction-associated fatty liver disease is associated with increased all-cause mortality in the United States. J Hepatol. 2021;75:1284–1291. doi: 10.1016/j.jhep.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi T, Takahashi H, Gerber LH. Clinics in Liver Disease: Update on Nonalcoholic Steatohepatitis: Sarcopenia and Nonalcoholic Fatty Liver Disease. Clin Liver Dis. 2023;27:275–286. doi: 10.1016/j.cld.2023.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Rigor J, Vasconcelos R, Lopes R, Moreira T, Barata P, Martins-Mendes D. Associations between muscle mass, strength, and performance and non-alcoholic fatty liver disease. Minerva Gastroenterol (Torino) 2023;69:374–381. doi: 10.23736/S2724-5985.22.03097-2. [DOI] [PubMed] [Google Scholar]
- 7.Lee SB, Kwon YJ, Jung DH, Kim JK. Association of Muscle Strength with Non-Alcoholic Fatty Liver Disease in Korean Adults. Int J Environ Res Public Health. 2022;19 doi: 10.3390/ijerph19031675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang S, Moon MK, Kim W, Koo BK. Association between muscle strength and advanced fibrosis in non-alcoholic fatty liver disease: a Korean nationwide survey. J Cachexia Sarcopenia Muscle. 2020;11:1232–1241. doi: 10.1002/jcsm.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silventoinen K, Magnusson PK, Tynelius P, Batty GD, Rasmussen F. Association of body size and muscle strength with incidence of coronary heart disease and cerebrovascular diseases: a population-based cohort study of one million Swedish men. Int J Epidemiol. 2009;38:110–118. doi: 10.1093/ije/dyn231. [DOI] [PubMed] [Google Scholar]
- 10.Cho J, Johnson BD, Watt KD, Kim CH. Greater Muscular Strength Is Associated with a Lower Risk of Pulmonary Dysfunction in Individuals with Non-Alcoholic Fatty Liver Disease. J Clin Med. 2022;11 doi: 10.3390/jcm11144151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–B365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 12.García-Hermoso A, Cavero-Redondo I, Ramírez-Vélez R, Ruiz JR, Ortega FB, Lee DC, Martínez-Vizcaíno V. Muscular Strength as a Predictor of All-Cause Mortality in an Apparently Healthy Population: A Systematic Review and Meta-Analysis of Data From Approximately 2 Million Men and Women. Arch Phys Med Rehabil. 2018;99:2100–2113.e5. doi: 10.1016/j.apmr.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37:1849–1855. doi: 10.1249/01.mss.0000175865.17614.74. [DOI] [PubMed] [Google Scholar]
- 14.Charatcharoenwitthaya P, Karaketklang K, Aekplakorn W. Muscle strength, but not body mass index, is associated with mortality in patients with non-alcoholic fatty liver disease. J Cachexia Sarcopenia Muscle. 2022;13:2393–2404. doi: 10.1002/jcsm.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun HS, Kim MN, Lee JS, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, Kim SU. Risk stratification using sarcopenia status among subjects with metabolic dysfunction-associated fatty liver disease. J Cachexia Sarcopenia Muscle. 2021;12:1168–1178. doi: 10.1002/jcsm.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Li X, Jin R, Yang J, Huang R, Wei L, Liu F, Rao H. Mid-upper arm circumference is associated with liver steatosis and fibrosis in patients with metabolic-associated fatty liver disease: A population based observational study. Hepatol Commun. 2022;6:2262–2272. doi: 10.1002/hep4.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos CML, Brito MD, de Castro PASV, de Vries TP, Viana NL, Coelho MPP, Malheiro OB, Bering T, Gonzalez MC, Teixeira R, Cambraia RD, Rocha GA, Silva LD. Metabolic-associated fatty liver disease is associated with low muscle mass and strength in patients with chronic hepatitis B. World J Hepatol. 2022;14:1652–1666. doi: 10.4254/wjh.v14.i8.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kweon S, Kim Y, Jang MJ, Kim K, Choi S, Chun C, Khang YH, Oh K. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES) Int J Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo JI, Choi H, Ha YC. Mean Hand Grip Strength and Cut-off Value for Sarcopenia in Korean Adults Using KNHANES VI. J Korean Med Sci. 2017;32:868–872. doi: 10.3346/jkms.2017.32.5.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, Kim YJ, Yoon JH, Cho SH, Sung MW, Lee HS. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Sviklāne L, Olmane E, Dzērve Z, Kupčs K, Pīrāgs V, Sokolovska J. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J Gastroenterol Hepatol. 2018;33:270–276. doi: 10.1111/jgh.13814. [DOI] [PubMed] [Google Scholar]
- 22.Pacific WHOROftW. The Asia-Pacific perspective: redefining obesity and its treatment. 2020 Oct 24; Sydney: Health Communications Australia, 2000: 55. [Google Scholar]
- 23.Kim BY, Kang SM, Kang JH, Kang SY, Kim KK, Kim KB, Kim B, Kim SJ, Kim YH, Kim JH, Kim EM, Nam GE, Park JY, Son JW, Shin YA, Shin HJ, Oh TJ, Lee H, Jeon EJ, Chung S, Hong YH, Kim CH Committee of Clinical Practice Guidelines, Korean Society for the Study of Obesity (KSSO) 2020 Korean Society for the Study of Obesity Guidelines for the Management of Obesity in Korea. J Obes Metab Syndr. 2021;30:81–92. doi: 10.7570/jomes21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chun MY. Validity and reliability of korean version of international physical activity questionnaire short form in the elderly. Korean J Fam Med. 2012;33:144–151. doi: 10.4082/kjfm.2012.33.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput JP, Chastin S, Chou R, Dempsey PC, DiPietro L, Ekelund U, Firth J, Friedenreich CM, Garcia L, Gichu M, Jago R, Katzmarzyk PT, Lambert E, Leitzmann M, Milton K, Ortega FB, Ranasinghe C, Stamatakis E, Tiedemann A, Troiano RP, van der Ploeg HP, Wari V, Willumsen JF. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54:1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertoia ML, Waring ME, Gupta PS, Roberts MB, Eaton CB. Implications of new hypertension guidelines in the United States. Hypertension. 2012;60:639–644. doi: 10.1161/HYPERTENSIONAHA.112.193714. [DOI] [PubMed] [Google Scholar]
- 28.Kim MK, Ko SH, Kim BY, Kang ES, Noh J, Kim SK, Park SO, Hur KY, Chon S, Moon MK, Kim NH, Kim SY, Rhee SY, Lee KW, Kim JH, Rhee EJ, Chun S, Yu SH, Kim DJ, Kwon HS, Park KS Committee of Clinical Practice Guidelines, Korean Diabetes Association. 2019 Clinical Practice Guidelines for Type 2 Diabetes Mellitus in Korea. Diabetes Metab J. 2019;43:398–406. doi: 10.4093/dmj.2019.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin ES, Shim JS, Kim SE, Bae JH, Kang S, Won JC, Shin MJ, Jin HY, Moon J, Lee H, Kim HC, Jeong IK Committee of Public Relation of the Korean Society of Lipid and Atherosclerosis. Dyslipidemia Fact Sheet in South Korea, 2022. J Lipid Atheroscler. 2023;12:237–251. doi: 10.12997/jla.2023.12.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nam GE, Kim YH, Han K, Jung JH, Rhee EJ, Lee WY Obesity Fact Sheet of the Korean Society for the Study of Obesity. Obesity Fact Sheet in Korea, 2020: Prevalence of Obesity by Obesity Class from 2009 to 2018. J Obes Metab Syndr. 2021;30:141–148. doi: 10.7570/jomes21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bulur A, Sivritepe R. The Association between Non-Alcoholic Fatty Liver Disease and Dynapenia in Men Diagnosed with Type 2 Diabetes Mellitus. Healthcare (Basel) 2023;11 doi: 10.3390/healthcare11020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SH, Kim DJ, Plank LD. Association of grip strength with non-alcoholic fatty liver disease: investigation of the roles of insulin resistance and inflammation as mediators. Eur J Clin Nutr. 2020;74:1401–1409. doi: 10.1038/s41430-020-0591-x. [DOI] [PubMed] [Google Scholar]
- 33.Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, Bugianesi E, Sirlin CB, Neuschwander-Tetri BA, Rinella ME. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080. doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 34.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. doi: 10.1093/ageing/afz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, Kirkland JL, Sandri M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol Rev. 2019;99:427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andreux PA, van Diemen MPJ, Heezen MR, Auwerx J, Rinsch C, Groeneveld GJ, Singh A. Mitochondrial function is impaired in the skeletal muscle of pre-frail elderly. Sci Rep. 2018;8:8548. doi: 10.1038/s41598-018-26944-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prasun P, Ginevic I, Oishi K. Mitochondrial dysfunction in nonalcoholic fatty liver disease and alcohol related liver disease. Transl Gastroenterol Hepatol. 2021;6:4. doi: 10.21037/tgh-20-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirabara SM, Curi R, Maechler P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J Cell Physiol. 2010;222:187–194. doi: 10.1002/jcp.21936. [DOI] [PubMed] [Google Scholar]
- 39.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 40.Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 41.Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA. The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biol Sci Med Sci. 2010;65:119–128. doi: 10.1093/gerona/glp179. [DOI] [PubMed] [Google Scholar]
- 42.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 43.Ibdah JA, Perlegas P, Zhao Y, Angdisen J, Borgerink H, Shadoan MK, Wagner JD, Matern D, Rinaldo P, Cline JM. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology. 2005;128:1381–1390. doi: 10.1053/j.gastro.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Zhou M, Xu A, Tam PK, Lam KS, Chan L, Hoo RL, Liu J, Chow KH, Wang Y. Mitochondrial dysfunction contributes to the increased vulnerabilities of adiponectin knockout mice to liver injury. Hepatology. 2008;48:1087–1096. doi: 10.1002/hep.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587:1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rector RS, Morris EM, Ridenhour S, Meers GM, Hsu FF, Turk J, Ibdah JA. Selective hepatic insulin resistance in a murine model heterozygous for a mitochondrial trifunctional protein defect. Hepatology. 2013;57:2213–2223. doi: 10.1002/hep.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picca A, Fanelli F, Calvani R, Mulè G, Pesce V, Sisto A, Pantanelli C, Bernabei R, Landi F, Marzetti E. Gut Dysbiosis and Muscle Aging: Searching for Novel Targets against Sarcopenia. Mediators Inflamm. 2018;2018:7026198. doi: 10.1155/2018/7026198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 49.Li R, Xia J, Zhang XI, Gathirua-Mwangi WG, Guo J, Li Y, McKenzie S, Song Y. Associations of Muscle Mass and Strength with All-Cause Mortality among US Older Adults. Med Sci Sports Exerc. 2018;50:458–467. doi: 10.1249/MSS.0000000000001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim D, Dennis BB, Wijarnpreecha K, Cholankeril G, Ahmed A. Muscle strength in non-alcoholic fatty liver disease and all-cause and cause-specific mortality. Liver Int. 2023;43:513–516. doi: 10.1111/liv.15498. [DOI] [PubMed] [Google Scholar]
- 51.Mager DR, MacDonald K, Duke RL, Avedzi HM, Deehan EC, Yap J, Siminoski K, Haqq AM. Comparison of Body Composition, Muscle Strength and Cardiometabolic Profile in Children with Prader-Willi Syndrome and Non-Alcoholic Fatty Liver Disease: A Pilot Study. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232315115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mikami K, Endo T, Sawada N, Igarashi G, Kimura M, Hasegawa T, Iino C, Sawada K, Ando M, Sugimura Y, Mikami T, Nakaji S, Matsuzaka M, Sakuraba H, Fukuda S. Association of serum creatinine-to-cystatin C ratio with skeletal muscle mass and strength in nonalcoholic fatty liver disease in the Iwaki Health Promotion Project. J Clin Biochem Nutr. 2022;70:273–282. doi: 10.3164/jcbn.21-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367–78.e5; quiz e14. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Park Y, Sinn DH, Kim K, Gwak GY. Associations of physical activity domains and muscle strength exercise with non-alcoholic fatty liver disease: a nation-wide cohort study. Sci Rep. 2023;13:4724. doi: 10.1038/s41598-023-31686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Almeida NS, Rocha R, de Souza CA, da Cruz ACS, Ribeiro BDR, Vieira LV, Daltro C, Silva R, Sarno M, Cotrim HP. Prevalence of sarcopenia using different methods in patients with non-alcoholic fatty liver disease. World J Hepatol. 2022;14:1643–1651. doi: 10.4254/wjh.v14.i8.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.