Abstract
Introduction
Carbon-tetrachloride (CCl4) is well-known to cause liver damage due to severe oxidative stress. Nerol, on the other hand, is a monoterpene that is antioxidant, antiviral, antibacterial, anti-inflammatory, and anxiolytic. This study set out to determine if nerol may be used as a prophylactic measure against the oxidative stress mediated hepatic injury caused by CCl4.
Materials and methods
For the aim of this experiment, 35 male Sprague-Dawley rats ranging in body weight (BW) from 140 to 180 g were split into five separate groups. With the exception of vehicle control group 1, all experimental rats were subjected to carbon tetrachloride exposure through intra-peritoneal injection at a 0.7 mL/kg body weight dose once a week for 4 weeks (28 days). The treatment groups 3 and 4 received oral administration of nerol at 50 and 100 mg/kg BW for 28 days. In the same time period, the standard control group received 100 mg/kg BW silymarin.
Results
Serum hepatic markers, lipid profiles, albumin, globulin, bilirubin, and total protein were all substantially improved in nerol-treated rats in a dose-dependent manner that had been exposed to CCl4 compared to the only CCl4-treated group. Carbon tetrachloride-exposed rats had lower glutathione, superoxide dismutase, and catalase levels and higher thio-barbituric acid reactive substances (TBARS) levels than normal rats. In contrast, administration of nerol shown a significant augmentation in the concentrations of these antioxidant compounds, while concurrently inducing a decline in the levels of TBARS in the hepatic tissue. In a similar vein, the histo-pathological examination yielded further evidence indicating that nerol offered protection to the hepatocyte against damage generated by CCl4.
Conclusion
According to the findings of our investigation, nerol has potential as a functional element to shield the liver from harm brought on by ROS that are caused by CCL4.
Keywords: Nerol, Carbon-tetrachloride, Hepatotoxicity, Oxidative-stress, Hepatoprotective
1. Introduction
The liver is an essential body part responsible for regulating metabolic processes, facilitating detoxification, and eliminating various foreign substances known as xenobiotics. Certain toxic substances may lead to the disruption of these processes and induce hepatic damage via the formation of free radicals [1]. Previous research investigations have shown that liver illnesses are responsible for an estimated annual global mortality rate of around 2.0 million individuals. Though a variety of standard hepato-protective medicines are accessible, however, some of them have possible negative effects. Since study numbers reveal that the prevalence of hepatic damage continues to rise, there is rising focus to phytochemicals to treat liver illnesses that are effective and safe [2].
Phytochemicals that possess therapeutic properties are regarded as valuable contributions to human due to their ability to effectively treat ailments while exhibiting minimal adverse effects. Phytochemicals refer to plant-derived substances that lack nutritional value but possess qualities that may guard against or prevent diseases. It is well acknowledged that many plant species synthesize these chemical compounds as a means of self-defense. However, current research findings indicate that these compounds has the potential to provide protective effects against illnesses in people as well. Phytochemicals may be classified into two main groups: primary metabolites, which include sugars, proteins, and chlorophylls, and secondary metabolites, which consist of alkaloids, phenolics, flavonoids, steroids, curcumins, and saponins. These classifications are based on the different functions these compounds have in plant metabolism [3].
Nerol has the chemical formula C10H18O and is also known as (2Z)-3,7-Dimethylocta-2,6-dien-1-ol. The presence of this particular monoterpene has been observed in several therapeutic plants, including Lippia spp and Melissa officinalis. Prior research has shown evidence for the existence of antibacterial, anxiolytic, antioxidant, anti-inflammatory, and antiviral effects in these species, which may be due to nerol [4].
Carbon tetrachloride is a well-recognized toxic compound used in various working models of hepatic injury, making it one of the most effective xenobiotic damage induction techniques and a frequently employed method for testing hepatoprotective or liver therapeutic drugs. Cytochrome P450 metabolizes carbon tetrachloride to generate trichloromethyl and trichloroperoxymethyl free radicals. These radicals then interact with other vital components, including proteins, fatty acids, nucleic acids, lipids, and amino acids [5]. Free radicals furthermore produce other reactive oxygen species (ROS) and lipid radicals, which inactivate natural antioxidant enzymes and cause oxidative stress-mediated hepatic damage. Hence, the elevation of antioxidant levels and the lowering of ROS are crucial factors in preserving the liver, thus increasing the significance of natural antioxidant phytochemicals for our well-being [6]. Prior studies have demonstrated that nerol has strong antioxidant properties, which are evident in its capacity to combat free radicals and the observed preventative benefits against cardiovascular diseases [7]. Oxidative damage also prompts inflammation along with the discharge of pro-inflammatory molecules and immune cells, exacerbating both inflammation and redox imbalance, potentially leading to fibrosis and liver dysfunction [8]. A prior study has shown that nerol has anti-nociceptive and anti-inflammatory properties, hence contributing to its efficacy in alleviating pathological manifestations in oxazolone-induced ulcerative colitis [9].
To the best of our current understanding, there is a lack of scientific investigation into the potential protective benefits of nerol against hepatotoxicity generated by carbon tetrachloride. The activation of oxidative stress and inflammatory pathways is closely linked to the hepatotoxicity caused by carbon tetrachloride. Conversely, nerol has notable antioxidant and anti-inflammatory capabilities. Therefore, the primary goal of this investigation was to evaluate the protective effects of nerol in a rat model with CCl4-induced hepatotoxicity, using silymarin as a reference drug for comparison.
2. Materials and methods
2.1. Chemicals
Phosphate-buffered saline, tri-chloro-acetic acid (TCA), thio-barbituric acid, hydrogen peroxide, acetic acid, ellman's reagent, pyrogallol and sodium-dodecyl-sulfate were obtained from Merck (Germany). Nerol (purity 98 %), Ethylenediaminetetraacetic acid (EDTA), carbon tetrachloride, and pyridine were all supplied by Sigma-Aldrich Co., USA.
2.2. Animals
A cohort of male Sprague-Dawley rats (140–180 g BW), was procured from Jahangirnagar University. The rats were then acclimated to standard experimental settings for a duration of one week. Subsequently, they were provided with a pellet-based food and unrestricted access to water. The experimental methodology received approval from the Ethical Committee of Jahangirnagar University [BBEC- JU/M 2018 (1)3]. The rats were housed in regular laboratory settings, with a temperature of 25 ± 2 °C, humidity of 55 %, and a 12-h light/dark cycle.
2.3. Design of hepatoprotective experiment
The dosing regimen scheduling was conducted using the carbon tetrachloride model as given by Wong et al. (2011), with minor adjustments [10]. Hepatic damage was induced by administering four weekly intra-peritoneal injections of carbon tetrachloride dissolved in olive oil (1:1 ratio) at 0.7 mL/kg BW dosage. Thirty-five rats were separated into five groups and each group comprises seven rats. Group 1 was designated as the vehicle control group and administered normal saline. Group 2 was administered normal saline and subjected to treatment with the aforementioned dose of CCl4. Experimental animals in groups 3 and 4 were administered nerol at 50 and 100 mg/kg BW doses, correspondingly, along with a hepatotoxic dose of CCl4. Additionally, silymarin at a dosage of 100 mg/kg BW was administered to rats in group 6 in addition to CCl4 treatment. The rats were subjected to a continuous 28-day treatment, where they were administered precise oral dosages of nerol and silymarin. Based on a prior study, rats were exposed to nerol via two different dose levels [11]. The rats were subjected to euthanasia using a carbon-dioxide chamber, precisely 24 h after the last injection of CCl4. Blood samples were collected through cardiac puncture using sterile disposable syringes, while serum was obtained through centrifugation process (speed = 3000 rpm, duration = 15 min). After removing any trace of blood and other contaminants, the livers from each rat were refrigerated at −20 °C for additional examination. Successively, hepatic tissue samples were subjected to homogenization in a phosphate buffer saline solution with a concentration of 25 mM and a pH value of 7.4. This process resulted in the formation of a homogenate with an approximate weight-to-volume ratio of 10 %. The supernatant was obtained by centrifuging at 4000 rpm and 4 °C for 15 min. This supernatant was then utilized to evaluate the levels of hepatic enzymatic and non-enzymatic reduced antioxidants, as well as to determine the levels of TBARS. The determination of relative liver weight was carried out using the following equation: relative liver weight (%) = wet liver weight/body weight × 100.
2.4. Assessment of biochemical markers in the serum
In this study, various biochemical markers pertaining to liver function were assessed. These included the analysis of serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl-transferase (GGT), lactate dehydrogenase (LDH), total bilirubin (TB), total protein (TP), albumin (ALB), globulin (GLB), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). The quantification of these parameters followed established protocols using Human commercial kits and was performed using the Humalyzer 3500 instrument (Human, Wiesbaden, Germany) [2].
2.5. Assay for measuring lipid peroxidation
The evaluation of lipid peroxidation, enumerated by TBARS, was conducted in the experimental arrangement as shown below: at first 0.2 mL of liver tissue homogenate was combined with 0.2 mL of 8.1 % sodium dodecyl sulfate, 1.5 mL of 20 % acetic acid, and 1.5 mL of 8 % thio-barbituric acid. Following the addition of 4 mL of distilled water, the mixture was subjected to boiling in a water bath maintained at a temperature of 95 °C for a duration of 60 min. After reaching room temperature, a mixture of butanol and pyridine at a ratio of 15:1 (5 mL) was added. Following an intense vortex process lasting 2 min, the mixture was subjected to centrifugation at a speed of 3000 revolutions per minute for a duration of 10 min. Subsequently, the top organic layer was precisely collected. The measurement of lipid peroxidation was represented as the amount of TBARS in Nano-moles per milligram of protein. The absorbance was determined at a wavelength of 532 nm using a Shimadzu UV-VIS spectrophotometer (UV PC-1600, Japan), with a blank sample serving as a reference [12].
2.6. Assessment of reduced GSH levels
The approach used in this research, as per Islam et al. was adjusted with some modifications [13]. At first, 1 mL of liver homogenate was combined with an equivalent amount of 10 % TCA and left to incubate for 5 min. Subsequently, the mixture was subjected to centrifugation at a speed of 2000 revolutions per minute for a duration of 10 min at a temperature of 4 °C. The resultant liquid was mixed with 5 mL of 0.2 M phosphate-buffered saline with a pH of 8. Subsequently, a volume of 2 mL of a 0.6 mM solution of Ellman's reagent was added into the test tube. The solution was homogeneously blended, and the level of light absorption was quantified at a wavelength of 412 nm using a Shimadzu UV-VIS spectrophotometer (UV PC-1600, Japan) within a time frame of 15 min later. The measurement of reduced glutathione (GSH) was reported as Nano-moles per milligram of protein.
2.7. Evaluation of superoxide dismutase (SOD) levels
The quantification of superoxide dismutase (SOD) was assessed using the methodology outlined in the study conducted by Mondal et al. (2021) [14]. To demonstrate 2.86 mL of tris-buffer solution with a concentration of 50 mM and a pH of 8.5 was combined with 0.1 mL of EDTA solution. Afterwards, a 0.02 mL portion of liver homogenate sample was added to the reaction mixture, along with a varying amount of pyrogallol ranging from 0.02 mL to 0.08 mL. The mixture's absorbance was quantified at a wavelength of 420 nm relative to a blank using a Shimadzu UV PC-1600 spectrophotometer from Japan. The data obtained was quantified in units relative to the amount of protein, namely in milligrams.
2.8. Evaluation of catalase (CAT) levels
According to the research done by Mondal et al. (2021) [14], hydrogen peroxide was used as a substrate to measure the enzyme activity of CAT. The experiment started by adding 1 mL of hydrogen peroxide (with a concentration of 30 M) to a combination consisting of 0.1 mL of homogenized sample and 1.9 mL of phosphate-buffered saline (with a pH of 7.0) inside a 3 mL cuvette, in accordance with the specified procedure. A Shimadzu UV PC-1600 UV-VIS spectrophotometer, made in Japan, was used to measure the absorbance at a wavelength of 240 nm. The results of the CAT test were expressed in units per milligram of protein.
2.9. Histo-pathological assessment
The liver tissues were immersed in a 10 % neutral buffered formalin solution to facilitate their preparation for further histological examinations. Tissue sections of 6 μm in thickness were acquired from paraffin wax-embedded biopsies using a rotary microtome (HM 325, Thermo Scientific, U.K.) [15]. Afterwards, the tissue slices received staining with hematoxylin and eosin, and pictures were captured using an Olympus DP 72 microscope (Tokyo, Japan) at a magnification of 40× [16].
2.10. Statistical analysis
The findings are shown as the average ± standard error of the average (SEM) based on a sample size of 7. The data analysis in this research used SPSS (Statistical Packages for Social Science, version 20.0, IBM Corporation, New York, United States of America) and Microsoft Excel 2013 (Redmond, Washington, U.S.A.). The data was subjected to one-way analysis of variance (ANOVA), and statistical analysis was performed using Dunnett's and Tukey's multiple comparisons to assess the datasets. Statistical significance was evaluated at the significance levels of 0.1 % and 5 %, indicated by p < 0.001 and p < 0.05, respectively.
3. Results
3.1. Impacts of nerol on body weight and relative organ weigh
Carbon tetrachloride, either alone or in combination with nerol and silymarin, has distinct effects on both body weight increase and relative liver weight, as seen in Fig. 1. In comparison to the rats in group 1, the rats in group 2 that were treated with carbon tetrachloride saw a notable decrease in body weight growth and a considerable increase in comparative hepatic mass. Compared to the rats in group 2, the rats in groups 3 and 4 that were administered with nerol exhibited a statistically insignificant rise in changes in body weight growth and a statistically significant drop in comparative hepatic mass in a way that depended on the dosage. The hepatic masses of rats in Group 5, who received silymarin treatment, showed a substantial decrease compared to Group 2.
Fig. 1.
Carbon tetrachloride, nerol, and silymarin affect body and liver weights The data shown are the mean ± SEM from a sample size of n = 7. Denoted significance levels: “x" and “y" (p < 0.05 and p < 0.01, respectively) to Group 2, and “a" and “b" (p < 0.05 and p < 0.01, respectively) to Group 1.
3.2. Impacts on biochemical parameters
The consequences of CCl4, nerol, and silymarin on serum hepatic diagnostic biomarkers are shown in Table 1. Group 2 rats exhibited elevated levels of serum hepatic enzymes, including ALT, AST, ALP, GGT, and LDH, as a result of the CCl4 therapy, as compared to group 1. In contrast, administration of nerol (in groups 3 and 4) substantially reduced the levels of these serum markers in a way that depended on the dosage. This effect was also seen in group 5, which received the standard silymarin therapy.
Table 1.
Impacts of CCl4, nerol and silymarin on serum hepatic bio-markers.
| Parameter | Groups |
||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
| ALT (U/L) | 79.13 ± 5.07 | 148.38 ± 6.47 b | 109.29 ± 4.45a,x | 92.38 ± 6.56 y | 86.57 ± 6.48 y |
| AST (U/L) | 118.17 ± 4.03 | 198.07 ± 6.52 b | 142.75 ± 4.31a,x | 126.74 ± 5.37 y | 122.25 ± 5.53 y |
| ALP (U/L) | 191.45 ± 7.42 | 320.25 ± 6.64 b | 261.66 ± 8.46 b,y | 234.82 ± 6.74a,y | 193.52 ± 5.46 y |
| GGT (U/L) | 8.29 ± 0.16 | 11.17 ± 0.10 b | 8.67 ± 0.09 b,x | 8.02 ± 0.08a,y | 8.76 ± 0.09 y |
| LDH (U/L) | 66.19 ± 3.35 | 103.39 ± 4.74 b | 78.89 ± 3.73x | 72.48 ± 2.66 y | 65.69 ± 3.63 y |
| The data shown are the mean ± SEM from a sample size of n = 7. Denoted significance levels: “x" and “y" (p < 0.05 and p < 0.01, respectively) to Group 2, and “a" and “b" (p < 0.05 and p < 0.01, respectively) to Group 1. | |||||
TB, TP, ALB, and GLB are crucial indicators used in the assessment of liver function, alongside blood hepatic enzymes. The administration of CCl4 resulted in a noteworthy upsurge in blood TB and GLB levels than rats in group 1. However, the levels of these markers were progressively reduced with the administration of nerol. In contrast, group 2 that treated with CCl4 had considerably lower levels of TP and ALB. Following treatment with nerol and silymarin, the biochemical markers in group 1 were seen to be restored to values that were close to those of the control group, as shown in Table 2.
Table 2.
Impacts of CCl4, nerol and silymarin on TB, TP, ALB, and GLB.
| Parameter | Groups |
||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
| TB (mg/dL) | 0.175 ± 0.007 | 0.238 ± 0.009 b | 0.193 ± 0.008x | 0.167 ± 0.007 y | 0.174 ± 0.006 y |
| TP (g/dL) | 8.23 ± 0.37 | 5.13 ± 0.16 b | 6.61 ± 0.14 b | 7.56 ± 0.30 y | 7.97 ± 0.20 y |
| ALB (U/L) | 4.92 ± 0.06 | 3.61 ± 0.11 b | 4.01 ± 0.13 b | 4.45 ± 0.08a,x, | 4.79 ± 0.11 y |
| GLB (g/dL) | 5.84 ± 0.14 | 7.87 ± 0.12 b | 6.81 ± 0.07 b | 6.33 ± 0.08a,x | 6.11 ± 0.07 y |
| The data shown are the mean ± SEM from a sample size of n = 7. Denoted significance levels: “x" and “y" (p < 0.05 and p < 0.01, respectively) to Group 2, and “a" and “b" (p < 0.05 and p < 0.01, respectively) to Group 1. | |||||
Group 2 rats treated with CCl4 showed a significant increase (p < 0.001) in blood levels of TC, TG, and LDL, while HDL levels decreased compared to group 1 rats. Conversely, standard silymarin (group 5) significantly reversed (p < 0.01) the changes, restoring them to normal levels. Table 3 shows that nerol treatment (groups 3 and 4) significantly ameliorated CCl4-induced alterations in rats in a dose-dependent manner.
Table 3.
Impacts of CCl4, nerol and silymarin on serum lipid profiles.
| Parameter | Groups |
||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
| TC (mg/dL) | 174.18 ± 4.35 | 209.85 ± 4.72 b | 199.58 ± 4.46a | 185.62 ± 3.83x | 178.68 ± 5.69 y |
| TG (mg/dL) | 290.18 ± 13.11 | 495.35 ± 20.97 b | 358.70 ± 11.96a,y | 312.98 ± 8.09 y | 303.59 ± 10.96 y |
| LDL-C (mg/dL) | 43.48 ± 4.01 | 98.98 ± 7.12 b | 59.34 ± 4.77 y | 54.55 ± 2.85 y | 50.17 ± 5.20 y |
| HDL-C (mg/dL) | 36.13 ± 2.45 | 20.75 ± 2.06 b | 30.00 ± 1.47 | 34.25 ± 1.65x | 38.75 ± 2.06 y |
| The data shown are the mean ± SEM from a sample size of n = 7. Denoted significance levels: “x" and “y" (p < 0.05 and p < 0.01, respectively) to Group 2, and “a" and “b" (p < 0.05 and p < 0.01, respectively) to Group 1. | |||||
3.3. Impacts on TBARS and GSH, SOD and CAT levels
Table 4 shows TBARS levels in rats' liver tissue after lipid peroxidation induction through CCl4. Rats intoxicated with carbon tetrachloride (group 2) exhibited considerably higher TBARS levels (p < 0.01) than the control group. In contrast, rats treated with silymarin showed significantly amended (p < 0.01) in their TBARS measurements. Similarly, co-administration of nerol with CCl4 decreased TBARS levels, showing that nerol protected hepatocytes against CCl4-induced lipid peroxidation.
Table 4.
Impacts of CCl4, and silymarin on TBARS and GSH, SOD and CAT levels.
| Parameter | Groups |
||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
| TBARS (nmol/mg of protein) | 8.15 ± 0.80 | 15.13 ± 0.90 b | 10.95 ± 0.31a,y | 8.98 ± 0.17 y | 8.78 ± 0.13 y |
| GSH (nmol/mg of protein) | 3.12 ± 0.48 | 8.23 ± 0.83 b | 6.45 ± 0.43x | 5.01 ± 0.15 y | 4.28 ± 0.15 y |
| SOD (U/mg of protein) | 16.56 ± 0.20 | 10.87 ± 0.37 b | 13.67 ± 0.25a,y | 15.75 ± 0.21 y | 15.83 ± 0.28 y |
| CAT (U/mg of protein) | 38.57 ± 2.35 | 21.53 ± 2.71 b | 28.98 ± 2.09a,x | 34.67 ± 1.79 y | 35.62 ± 1.72 y |
| The data shown are the mean ± SEM from a sample size of n = 7. Denoted significance levels: “x" and “y" (p < 0.05 and p < 0.01, respectively) to Group 2, and “a" and “b" (p < 0.05 and p < 0.01, respectively) to Group 1. | |||||
GSH, SOD, and catalase were used to assess hepatic antioxidant activity. Experimental animals with CCl4 had lower endogenous antioxidant levels than group 1. However, silymarin dramatically improved these levels in group 5. Table 4 shows that groups 3 and 4's levels rose significantly after receiving nerol, depending on the dose.
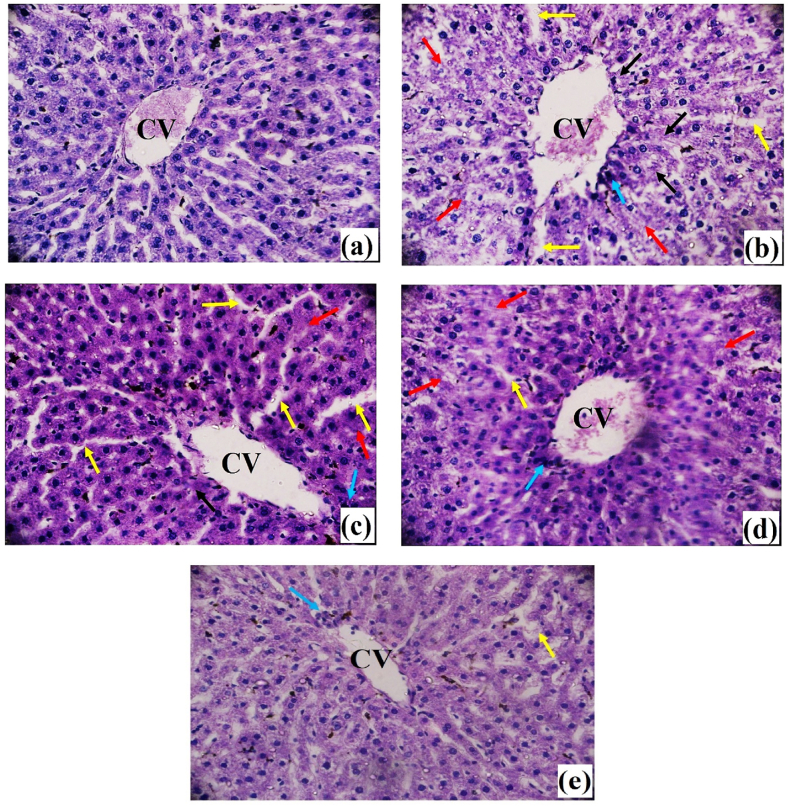
3.4. Impacts on histo-pathological findings
The typical cellular structure of sinusoids, sinusoidal gaps, and central vein may be seen in the control (group 1) hepatic tissues (Fig. 2 (a)). The presence of carbon tetrachloride poisoning resulted in hepatocyte degeneration, characterized by central and lobular necrosis, inflammatory cell infiltrations, and vascular edematous congestion (Fig. 2 (b)). Treatment with Nerol at a dosage of 100 mg/kg body weight caused a moderate level of inflammation and tissue death (Fig. 2 (d)). On the other hand, therapy with Nerol at a dosage of 50 mg/kg resulted in a significant level of inflammation, congested blood vessels, and infiltration of inflammatory cells (Fig. 2(c)). In the control group, the therapeutic medications silymarin had a considerable reduction in these pathological anomalies, as shown in Fig. 2 (e).
Fig. 2.
Effects of Carbon tetrachloride, Nerol and Silymarin on the histological examination of liver tissue against CCl4-induced histological changes in investigated animals. (a): Group 1-Normal Control, (b): Group 2-CCl4 Control, (c): Group 3-Treatment Group treated by Nerol at 50 mg/kg, (d): Group 4-Treatment Group treated by Nerol at 100 mg/kg, (e): Group 5-Standard Group treated by silymarin at 100 mg/kg [Magnification: 40×; scale bar: 20 μm]. (The red and black arrows denote necrosis in the peripheral area of the central vein and the lobule, respectively, while the blue arrow denotes congestion in the central vein along with extensive inflammatory infiltrates, and the yellow arrow denotes edema in various locations).
4. Discussion
The liver parenchyma is often harmed and hepatocellular injury occurs as a consequence of the generation of free radicals during the metabolism and removal of xenobiotic substances. Several substances that are toxic to the liver, including paracetamol, di-methyl nitrosamine, Dgalactosamine/lipopolysaccharide, carbon tetrachloride, as well as alcoholism and viral infections, have been associated with this kind of damage [17]. Due to the limited availability of pharmaceutical options for liver problems, it is crucial to choose suitable hepatoprotective medications derived from natural sources. Hence, it is important to discover a secure and effective alternative for managing liver treatment [18]. Propolis, chamomile, silymarin, naringin, and several other phytochemicals obtained from fruits, plants, yeasts, and algae have undergone testing in models of liver damage [19]. In earlier research, nerol has shown significant hepatoprotective effects against paracetamol-induced liver damage in rats [15].
We choose the CCl4-induced hepatic injury model due to its ability to produce hepatic changes that closely resemble cirrhosis/hepatitis, including the invasion of mononuclear cells and the steatotic foamy degeneration of hepatocytes [20]. CCl4 is a highly toxic substance that may easily penetrate cell membranes and rapidly distribute throughout tissues due to its solubility in lipids. Previous research [21] has shown that the carbon-chlorine link (C–Cl bond) of carbon tetrachloride may be broken, resulting in the formation of the trichloromethyl free radical. This free radical is believed to have a role in the development of liver damage. The cytochrome P450 oxygenase enzyme transforms these unbound radicals into tri-chloro-methyl-peroxy radicals, resulting in oxidative stress. This causes oxidative degradation of lipids by attaching to polyunsaturated cytoplasmic membrane fatty acids, which damages cell components and finally causes liver failure [22].
The present study used changes in BW gain and relative liver weights of experimental animals as strong indicators of CCl4-induced toxicity. Rats exposed to CCl4 for 4 weeks lost a lot of body weight, which may have been caused by CCl4's direct cytotoxic effects. Our findings are supported by previous research showing that mice whose weight was substantially lowered with CCl4 [23]. CCl4-induced liver damage can also lead to several metabolic changes, including decreased food intake, increased lipid peroxidation, and decreased protein synthesis [24]. These changes may lead to weight loss in rats. Our analysis revealed that the administration of CCl4 led to a significant augmentation in liver mass. This conclusion aligns with a prior research that documented similar results [25]. There are many pathways by which CCl4 might elevate relative liver weight. After 2–4 weeks of CCl4 therapy, fibrosis may emerge, which forms scar tissue in the liver, as discovered by Scholten et al. (2015) [26]. It has also been demonstrated that CCl4 presence stimulates the release of inflammatory mediators. The presence of inflammatory cytokines has been shown to induce hepatocyte swelling and contribute to the development of edema, as evidenced by our histological findings. Nevertheless, the administration of nerol to animals treated with CCl4 resulted in the improvement of both body and relative liver weights. This beneficial effect may be related to the antioxidant capabilities of nerol, which effectively mitigate the oxidative-stress initiated by CCl4.
Excessive ROS impair the functionality of many endogenous antioxidant enzymes, such as SOD, catalase, and glutathione peroxidase. Therefore, these enzymes may experience a decline in their capacity to safeguard cellular integrity [27]. As a result, ALT, AST, and ALP become more able to pass through the membranes of hepatocellular cells, and this increases the likelihood that they will enter the bloodstream [28]. It is widely known that serum hepatic enzyme activity are indicators of the degree of hepatotoxicity. Estimating liver cell structural integrity through measuring blood levels of these enzymes [29]. Carbon tetrachloride was shown to raise blood levels of AST, ALT, and ALP because it injured hepatocytes by disrupting mitochondrial structure and increasing membrane fluidity. Previous investigations have found that following carbon tetrachloride therapy, these enzymes activity are dramatically increased [30]. Experimental animals given nerol exhibited a strong protective effect against carbon tetrachloride-induced hepatotoxicity, as demonstrated by lower blood ALT, ALP, and AST levels, which agrees with findings from Islam et al. (2021). The oxido-reductase enzyme LDH converts pyruvate and lactate in the liver and other physiological tissues. Serum LDH, similar to other hepatic enzymes, exhibits an elevation in cases of hepatic failure, making it a valuable biomarker for assessing the extent of hepatotoxicity [31]. GGT, a microsomal enzyme, is one of the strongest biomarkers of hepatic disease [32]. In our investigation, administration of carbon tetrachloride resulted in decreased levels of gamma-glutaryl-transferase and lactate-dehydrogenase, which were restored by nerol administration in a dose-dependent approach.
In most cases, hemoglobin is broken down into bilirubin and excreted in the bile. After serious liver damage, less bilirubin is secreted, producing in hyper-bilirubinemia that suggests hepatic destruction (necrosis) [33]. The impaired capacity of the damaged hepatic tissue to attach, conjugate, and eliminate bilirubin may explain the observed elevation in total blood bilirubin levels after CCl4 treatment. In contrast to the CCl4-treated group, nerol treatment inhibited serum bilirubin levels from rising. These results suggest an enhancement in liver secretion after treatment with the nerol treatment. The liver, on the other hand, is recognized to take an active part in serum protein production. Consequently, a decrease in TP occurs due to defective protein synthesis [34]. Total protein content was observed to be lower in CCl4-treated rats, suggesting the severity of hepatic-toxicity. Nevertheless, the groups treated with nerol demonstrated an improvement in the functional condition of hepatic cells, as seen by the increased amounts of serum protein. ALB and GLB levels in the blood may be used as diagnostic markers for diseases of the liver. Serum albumin levels were shown to be lower after CCl4 injection, but globulin levels were often found to be higher [35].
Lipids are metabolized by the liver. A considerable increase in TG, TC, and LDL quantities, and a substantial reduction in HDL levels were all seen after CCl4 delivery. Protein synthesis may be reduced, and phospholipid metabolism may be disrupted, resulting in aberrant lipoprotein levels [5]. Synthesis of protein may be reduced, and phospholipid metabolism may be disrupted, resulting in aberrant lipoprotein levels. Higher blood cholesterol may result from elevated lipid esterification, suppressed lipid acid-oxidation, and delayed lipid clearance [36]. CCl4 increases acetate transport into hepatocytes, thereby promoting cholesterol production. It promotes fatty acid and triglyceride synthesis from acetate and improves lipid esterification. Suppressing lysosomal lipase function and VLDL release may lead to hepatic triglyceride accumulation. Studies indicate that lipids from peripheral fat tissue move to the organs of the liver and kidney during CCl4 intoxication, causing accumulation [5]. Administration of nerol at dosages of fifty and one hundred mg/kg resulted in a decrease in TC, TG, and LDL levels, whilst simultaneously boosting HDL concentrations. Nerol is thought to be able to decrease the oxidation of LDL and cell membranes by inhibiting the harmful effects of ROS.
Peroxidation of polyunsaturated-fatty-acids (PUFAs) in liver cell membranes may be caused by the interaction of free radicals generated by carbon tetrachloride. As a result of this process, the generation of TBARS, a main reactive aldehyde formed by the peroxidation of PUFAs, rises [33]. The level of TBARS in the CCl4-treated rat liver was substantially greater than in the healthy control group, indicating oxidative stress mediated by CCl4. In the CCl4-treated rat liver homogenates, nerol administration reduced TBARS generation. To clarify, nerol decreased the process of lipid peroxidation in rats that were also treated with CCl4, so mitigating the occurrence of oxidative stress. This led us to believe that the phytochemicals' high antioxidant and free radical scavenging capabilities were responsible for this impact.
GSH, which is present in high quantities in biological tissues, is thought to be the most important detoxifying and antioxidant molecule generated by cells. To reduce the harmful effects of foreign substances, it gets conjugated with them. As a result, determining its level in the liver can offer insight into the amount of cell damage induced by a particular substance [37]. By establishing covalent connections with the free radicals generated during CCl4 metabolism, GSH unquestionably protects cells from the harm brought on by CCl4 toxicity. These free radicals cause a chain of events to start that result in the oxidation of lipids in cellular membranes. When there are not enough antioxidants present, this process eventually results in cell membrane rupture, affecting their fluidity and permeability [23]. Treatment of rats with CCl4 had significantly lower levels of liver GSH than control rats, a finding that was corrected by the administration of nerol. These findings are consistent with past research [15,38].
Enzymes that fight free radicals are vulnerable to severe cell damage. A significant amount of liver damage caused by CCl4 is shown by the drop in SOD, and CAT levels. Lipid peroxidation brought on by CCl4-derived free radicals causes damage to the liver after CCl4 injection. To prevent liver damage caused by CCl4, it is essential to have antioxidant activity and decrease the production of free radicals. The body's excellent defense systems can inhibit and eliminate free radicals [36]. The management of natural antioxidants, such as catalase, glutathione peroxidase, and superoxide dismutase, is executed with great proficiency in this activity. These enzymes together provide a mutual support system to counteract ROS. Mitochondrial failure disrupts cellular activity and leads to liver damage and necrosis. This dysfunction also alters the balance between the formation of ROS by enzymes and the antioxidant defense system in liver damage produced by CCl4. Typically, the antioxidant system, including enzymatic and non-enzymatic elements, neutralizes ROS. SOD is a distinctive antioxidant that transforms surplus superoxide into hydrogen peroxide, which is subsequently counteracted by catalase [39]. In our investigation, we saw a drop in enzyme levels in the CCl4 treatment group. However, we noticed significant increases in SOD and CAT activity in animals treated with nerol. Prior research has shown that nerol enhances the levels of SOD and CAT in mice exposed to substances that promote oxidative stress, such as acetaminophen [15]. The assessment of histopathology plays a vital role in determining the extent of aberrant tissue structure in liver cells [40]. The histopathological data showed a correlation with the biochemical improvements seen after nerol treatment. The liver samples exposed alone to CCl4 exhibited hepatocyte degeneration and necrosis, infiltration of inflammatory cells, and vascular blockage accompanied by regions of edema. The findings are consistent with the characteristics often seen in rat hepatocytes intoxicated with CCl4 [41]. In contrast, the histological section of the rats treated with nerol and silymarin exhibited a significant degree of cellular protection, as seen by the reduction in necrosis, inflammatory cell infiltrations, and vascular edematous congestion. Hence, the ability of nerol to mitigate hepatic damage caused by CCl4 can be deduced from the fact that it rectified abnormalities and enhanced the structural organization of hepatocytes in the treated group. This was evidenced by the elevated levels of endogenous antioxidant enzymes and reduced lipid peroxidation levels.
5. Conclusion
To summarize, the liver is an essential organ in the body, playing a crucial role in several metabolic processes and the elimination of toxins. Nevertheless, the liver is vulnerable to harm inflicted by free radicals that arise from metabolic processes and exposure to hepatotoxins, including carbon tetrachloride and paracetamol. The presence of these harmful substances may cause oxidative stress, the breakdown of lipids, and damage to liver cells, eventually leading to impaired liver function. The results showed that administering nerol resulted in positive changes in body weight, organ weights, and a decrease in TBRAS. This was achieved by increasing the activities of hepatic anti-oxidative enzymes such as CAT, GSH, and SOD, leading to a significant decrease in serum ALT, AST, triglyceride, and cholesterol levels. The protective effects of nerol against CCl4-induced liver damage are likely attributed to its antioxidant properties, since it may effectively neutralize free radicals and reduce oxidative stress.
Funding
During the academic year 2020–2021, the University Grants Commission of Bangladesh and Bangabandhu Sheikh Mujibur Rahman Science and Technology University provided funding for this study (Reference No.:3631108, Bank Advice no:1516).
Ethical approval
The research project was approved by Jahangirnagar University's Biosafety, Biosecurity, and Ethical Committee (Approval Number: BBEC, JU/M 2018 (1)3) in Savar, Dhaka, Bangladesh.
Data availability statement
The data are not publicly available because these are under processing for further research.
CRediT authorship contribution statement
Milon Mondal: Writing - original draft, Methodology, Investigation, Data curation, Conceptualization. Jibanananda Bala: Methodology, Investigation, Data curation. Kakoli Rani Mondal: Formal analysis. Sadia Afrin: Software, Investigation, Formal analysis. Protyaee Saha: Software, Investigation, Formal analysis. Moumita Saha: Methodology, Investigation, Formal analysis. Sarmin Jamaddar: Resources, Methodology, Investigation. Uttam Kumar Roy: Resources, Methodology, Investigation. Chandan Sarkar: Writing - review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Lab space and equipment were generously donated by the Department of Pharmacy at Jahangirnagar University for the purposes of this investigation.
Contributor Information
Milon Mondal, Email: milonmondal18@gmail.com.
Chandan Sarkar, Email: csarkar1053@gmail.com.
References
- 1.Delgado-Montemayor C., Cordero-Pérez P., Salazar-Aranda R., Waksman-Minsky N. Models of hepatoprotective activity assessment. Med. Univ. 2015;17:222–228. doi: 10.1016/j.rmu.2015.10.002. [DOI] [Google Scholar]
- 2.Mondal M., Hossain MdM., Hasan MdR., Tarun MdTI., Islam MdAF., Choudhuri M.S.K., et al. Hepatoprotective and antioxidant capacity of Mallotus repandus ethyl acetate stem extract against d-galactosamine-induced hepatotoxicity in rats. ACS Omega. 2020;5:6523–6531. doi: 10.1021/acsomega.9b04189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koche D., Shirsat R., Imran S., Bhadange D.G. Phytochemical screening of eight traditionally used ethnomedicinal plants from Akola District (MS) India. Int. J. Pharma Bio Sci. 2010;1 [Google Scholar]
- 4.Marques T.H.C., M.L.B.G.C.B. Marques, Lima D. dos S., Siqueira H.D.S., Branco M., do S.B.G.C., Souza AA de, et al. 2013. Evaluation of the Neuropharmacological Properties of Nerol in Mice 2013. [DOI] [Google Scholar]
- 5.Mahmoodzadeh Y., Mazani M., Rezagholizadeh L. Hepatoprotective effect of methanolic Tanacetum parthenium extract on CCl4-induced liver damage in rats. Toxicol Rep. 2017;4:455–462. doi: 10.1016/j.toxrep.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng H.-Y., Chao J., Chiu C.-S., Hsieh I.-C., Huang H.-C., Wu L.-Y., et al. Hepatoprotective and antioxidant effects of Wu-Zi-Yuan-Chung-Wan against CCl4-induced oxidative damage in rats. Eur. J. Inflamm. 2021;19 doi: 10.1177/20587392211014058. [DOI] [Google Scholar]
- 7.Cheng J., Zou Q., Xue Y. Nerol protects against hypoxia/reoxygenation-induced apoptotic injury by activating PI3K/AKT signaling in cardiomyocytes. STEMedicine. 2021;2:e87–e97. doi: 10.37175/stemedicine.v2i6.87. [DOI] [Google Scholar]
- 8.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C., Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid. Med. Cell. Longev. 2016;2016:1–9. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González-Ramírez A.E., González-Trujano M.E., Orozco-Suárez S.A., Alvarado-Vásquez N., López-Muñoz F.J. Nerol alleviates pathologic markers in the oxazolone-induced colitis model. Eur. J. Pharmacol. 2016;776:81–89. doi: 10.1016/j.ejphar.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 10.Wong L.L.Y., Fan S.T., Man K., Sit W.H., Jiang P.P., Jor I.W.Y., Lee C.Y.K., Ling W.L., Tam K.T., Wan J.M.F. Identification of liver proteins and their roles associated with carbon tetrachloride-induced hepatotoxicity. Hum. Exp. Toxicol. 2011;30(9):1369–1381. doi: 10.1177/0960327110391388. [DOI] [PubMed] [Google Scholar]
- 11.Ghashghaei S., Ghobeh M., Yaghmaei P. The effect of nerol on behavioral, biochemical and histological parameters in male Wistar Alzheimer's rats. Biomacromolecules J. 2019;5:12–22. [Google Scholar]
- 12.Mondal M., Hossen MdS., Rahman M.A., Saha S., Sarkar C., Bhoumik N.C., et al. Antioxidant mediated protective effect of Bridelia tomentosa leaf extract against carbofuran induced oxidative hepatic toxicity. Toxicol Rep. 2021;8:1369–1380. doi: 10.1016/j.toxrep.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mondal M., Kundu S.K., Islam M.T., Reiner Ž., Martorell M., Sharifi-Rad J. Protective effect of Bridelia tomentosa due to its phenolic acids and flavonoids against oxidative stress-mediated hepatic toxicity induced by carbofuran. South Afr. J. Bot. 2021;141:447–456. doi: 10.1016/j.sajb.2021.06.006. [DOI] [Google Scholar]
- 14.Islam M.T., Quispe C., Islam MdA., Ali E.S., Saha S., Asha U.H., et al. Effects of nerol on paracetamol-induced liver damage in Wistar albino rats. Biomed. Pharmacother. 2021;140 doi: 10.1016/j.biopha.2021.111732. [DOI] [PubMed] [Google Scholar]
- 15.Mondal M., Saha S., Sarkar C., Hossen M.S., Hossain M.S., Khalipha A.B.R., et al. Role of citrus medica L. Fruits extract in combatting the hematological and hepatic toxic effects of carbofuran. Chem. Res. Toxicol. 2021;34:1890–1902. doi: 10.1021/acs.chemrestox.1c00166. [DOI] [PubMed] [Google Scholar]
- 16.Naour F.L., Bralet M.-P., Debois D., Sandt C., Guettier C., Dumas P., et al. Chemical imaging on liver steatosis using synchrotron infrared and ToF-SIMS microspectroscopies. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahed T.B., Mondal M., Rahman M.A., Hossen MdS., Bhoumik N.C., Saha S., et al. Protective role of syzygium cymosum leaf extract against carbofuran-induced hematological and hepatic toxicities. Chem. Res. Toxicol. 2019;32:1619–1629. doi: 10.1021/acs.chemrestox.9b00164. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke H., Gores G.J., Cederbaum A.I., Hinson J.A., Pessayre D., Lemasters J.J. Mechanisms of hepatotoxicity. Toxicol. Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 19.Al-Harbi N.O., Imam F., Nadeem A., Al-Harbi M.M., Iqbal M., Ahmad S.F. Carbon tetrachloride-induced hepatotoxicity in rat is reversed by treatment with riboflavin. Int. Immunopharm. 2014;21:383–388. doi: 10.1016/j.intimp.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Madrigal-Santillán E., Madrigal-Bujaidar E., Álvarez-González I., Sumaya-Martínez M.T., Gutiérrez-Salinas J., Bautista M., et al. Review of natural products with hepatoprotective effects. World J. Gastroenterol. WJG. 2014;20:14787–14804. doi: 10.3748/wjg.v20.i40.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wahid A., Hamed A.N., Eltahir H.M., Abouzied M.M. Hepatoprotective activity of ethanolic extract of Salix subserrata against CCl4-induced chronic hepatotoxicity in rats. BMC Compl. Alternative Med. 2016;16:263. doi: 10.1186/s12906-016-1238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheeseman K.H., Albano E.F., Tomasi A., Slater T.F. Biochemical studies on the metabolic activation of halogenated alkanes. Environ. Health Perspect. 1985;64:85–101. doi: 10.1289/ehp.856485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abouzied M.M., Eltahir H.M., Taye A., Abdelrahman M.S. Experimental evidence for the therapeutic potential of tempol in the treatment of acute liver injury. Mol. Cell. Biochem. 2016;411:107–115. doi: 10.1007/s11010-015-2572-2. [DOI] [PubMed] [Google Scholar]
- 24.Sahreen S., Khan M.R., Khan R.A. Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. BMC Compl. Alternative Med. 2011;11:48. doi: 10.1186/1472-6882-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Althnaian T., Albokhadaim I., El-Bahr S.M. Biochemical and histopathological study in rats intoxicated with carbontetrachloride and treated with camel milk. SpringerPlus. 2013;2:57. doi: 10.1186/2193-1801-2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang S., Lu B., Han X., Xu L., Qi Y., Yin L., et al. Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2013;55:60–69. doi: 10.1016/j.fct.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerman H.J., Kodera Y., West M. Rate of increase in plasma levels of cytoplasmic and mitochondrial enzymes in experimental carbon tetrachloride hepatotoxicity. J. Lab. Clin. Med. 1965;66:315–323. doi: 10.5555/uri:pii:0022214365900132. [DOI] [PubMed] [Google Scholar]
- 28.Mondal M., Sarkar C., Saha S., Hossain M.N., Norouzi R., Mubarak M.S., et al. Hepatoprotective activity of andrographolide possibly through antioxidative defense mechanism in Sprague-Dawley rats. Toxicol Rep. 2022;9:1013–1022. doi: 10.1016/j.toxrep.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trivedi N.P., Rawal U.M., Patel B.P. Hepatoprotective effect of andrographolide against hexachlorocyclohexane-induced oxidative injury. Integr. Cancer Ther. 2007;6:271–280. doi: 10.1177/1534735407305985. [DOI] [PubMed] [Google Scholar]
- 30.Chang C.-Y., Chen Y.-L., Yang S.-C., Huang G.-C., Tsi D., Huang C.-C., et al. Effect of schisandrin B and sesamin mixture on CCl4-induced hepatic oxidative stress in rats. Phytother Res. 2009;23:251–256. doi: 10.1002/ptr.2602. [DOI] [PubMed] [Google Scholar]
- 31.Haque M.A., Haque M.U., Islam M.A.U. Evaluation of antioxidant and hepatoprotective effects of dendrophthoe pentranda leaves on CCl4 -induced hepatotoxic rat. Bangladesh Pharm. J. 2018;21:71–79. doi: 10.3329/bpj.v21i2.37916. [DOI] [Google Scholar]
- 32.Ogeturk M., Kus I., Kavakli A., Zararsiz I., Ilhan N., Sarsilmaz M. Effects of melatonin on carbon tetrachloride-induced changes in rat serum. J. Physiol. Biochem. 2004;60:205. doi: 10.1007/BF03167030. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez M.L., West K.L. Mechanisms by which dietary fatty acids modulate plasma lipids. J. Nutr. 2005;135:2075–2078. doi: 10.1093/jn/135.9.2075. [DOI] [PubMed] [Google Scholar]
- 34.Singh M., Gupta S., Pandey R., Aggarwal H., Aggarwal S. Oxidative stress and chronic alcohol liver disease: the current perspectives. IAJPR. 2014;4:1428–1446. [Google Scholar]
- 35.Parveen R., Baboota S., Ali J., Ahuja A., Vasudev S.S., Ahmad S. Effects of silymarin nanoemulsion against carbon tetrachloride-induced hepatic damage. Arch Pharm. Res. (Seoul) 2011;34:767–774. doi: 10.1007/s12272-011-0510-8. [DOI] [PubMed] [Google Scholar]
- 36.Mondal M., Hossain MdM., Rahman M.A., Saha S., Uddin N., Hasan MdR., et al. Hepatoprotective and antioxidant activities of Justicia gendarussa leaf extract in carbofuran-induced hepatic damage in rats. Chem. Res. Toxicol. 2019;32:2499–2508. doi: 10.1021/acs.chemrestox.9b00345. [DOI] [PubMed] [Google Scholar]
- 37.Vuda M., D'Souza R., Upadhya S., Kumar V., Rao N., Kumar V., et al. Hepatoprotective and antioxidant activity of aqueous extract of Hybanthus enneaspermus against CCl4-induced liver injury in rats. Exp. Toxicol. Pathol. 2012;64:855–859. doi: 10.1016/j.etp.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Zhang G., Wang X., Chung T.Y., Ye W., Hodge L., Zhang L., et al. Carbon tetrachloride (CCl4) accelerated development of non-alcoholic fatty liver disease (NAFLD)/steatohepatitis (NASH) in MS-NASH mice fed western diet supplemented with fructose (WDF) BMC Gastroenterol. 2020;20:1–13. doi: 10.1186/s12876-020-01467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okamoto T., Masuda Y., Kawasaki T., Okabe S. Zaltoprofen prevents carbon tetrachloride-induced reduction of body weight in rats. Int. J. Mol. Med. 2001;7:101–105. doi: 10.3892/ijmm.7.1.101. [DOI] [PubMed] [Google Scholar]
- 40.Yoshioka H., Nonogaki T., Fukaya S., Ichimaru Y., Nagatsu A., Yoshikawa M., et al. Sasa veitchii extract protects against carbon tetrachloride-induced hepatic fibrosis in mice. Environ. Health Prev. Med. 2018;23:1–10. doi: 10.1186/s12199-018-0739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scholten D., Trebicka J., Liedtke C., Weiskirchen R. The carbon tetrachloride model in mice. Lab. Anim. 2015;49:4–11. doi: 10.1177/0023677215571192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available because these are under processing for further research.