Abstract
Citrus assamensis, commonly known as Adajamir, is an underutilized fruit with distinctive sensory and nutritional properties. The limited amount of research on this particular citrus type was recognized as one of the research gaps for this study. The objective of this study was to evaluate and compare the impacts of sonication, pasteurization, and thermosonication techniques on the quality and sensory attributes of Adajamir juice. A randomized experimental design was used in the study, wherein the juice underwent three different treatments. The results indicate that there were no significant changes in pH or titratable acidity following all treatments. Yet, notable differences in juice color were observed. The use of sonication and thermosonication resulted in an increase in β-carotenoid levels. Additionally, total phenolic content and antioxidant activities were observed to increase. All three treatments led to a reduction in ascorbic acid levels relative to the control. However, the complete elimination of microbial growth was observed during the thermal treatment. Compared to other approaches, sonication has been shown to be notably more efficacious in enhancing both the flavor and aroma. Sonication has been observed to improve the perceived bitterness to a certain degree. These findings support the potential of sonication as an alternative preservation method for Adajamir juice, offering enhanced quality and sensory acceptance.
Keywords: Adajamir, Antioxidant, Bioactive compounds, Citrus, Fruit juice, Power BI
1. Introduction
Citrus assamensis, commonly known as Adajamir, is a distinctive and relatively untapped species of citrus fruit cultivated in the larger Sylhet regions of Bangladesh and in the Northeast part of India [1]. It is a most prominent and expensive variety of citrus fruit that is grown in the larger Sylhet region of Bangladesh [1]. Adajamir is recognized for its typical aromatic flavor and significant nutritional value, containing a variety of vitamins, minerals, dietary fibers, and phytochemicals. Consumption of this fruit, whether fresh or juiced, provides a plethora of physiological benefits, including increased immune function, anti-inflammatory effects, and antioxidative properties, among others [1,2]. Although Adajamir has several advantages, the juice industry has not fully explored its potential, resulting in it being an unexplored resource for the beverage market. As people become more health-conscious, they look for alternatives to carbonated and sugar-laden beverages. It is essential to use appropriate preservation methods during processing to produce high-quality juice that preserves its sensory, nutritional, and functional qualities.
The processing of juices is a crucial step in extending their shelf life. Juices are frequently thermally processed in order to inhibit enzyme activity and microbe development, lowering the number of the most thermo-resistant pathogens to 5-log levels [3]. Heat processing can result in the loss of volatile aroma compounds, color deterioration, vitamin loss, and off-flavors in fruit juices [4]. Hence, it is recommended to put emphasis on processing technologies for juices that exhibit minimal or negligible impact on their quality [5]. In response to consumer demand for processed, fresh-tasting fruit juices, a variety of non-thermal processing techniques have been developed in recent years. On a variety of juices, the efficacy of non-thermal techniques, such as high-pressure processing, pulse electric fields, ionizing radiations, and ultrasonication, in preventing the deterioration of fruit juice quality was evaluated [4,5].
Sonication, an emerging non-thermal technology, has gained popularity as a potential alternative to traditional preservation methods due to its ability to preserve the sensory and nutritional properties of fruit juices. Sonication offers several benefits, including decreased time and energy usage, extraction at a lower temperature, and maintenance of the product's quality. The utilization of this technique has been observed to yield several advantages in terms of both environmental and economic aspects [6]. Sonication uses high-frequency sound waves to create cavitation bubbles in a liquid media, which expand and collapse rapidly, causing shear stresses and microstreaming that can damage microbial cell membranes and structures [7]. This method has advantages such as shorter processing periods, lower energy use, and little impact on the quality attributes of the treated juices [8]. Ultrasonic treatment has less effect on functional compounds and sensory characteristics of foods [9], such as orange juice [10] and increases shelf life and improves organoleptic properties of some food products [11]. The use of sonication has been demonstrated to have the potential to enhance the microbial quality of fruit juices, while simultaneously preserving bioactive compounds such as polyphenols and anthocyanins that are beneficial to human health [12]. The process of combining ultrasound with mild heat, known as thermosonication, has also been found to be effective in terms of microorganism and enzyme inactivation [13] compared to thermal treatment alone [14]. The ultrasound induces cell membrane disruption, making bacteria more vulnerable to subsequent heat treatment, and the increase in temperature results in system deactivation [15]. Thermosonication is considered an effective approach for food processing due to its ability to decrease processing time [16], improve antioxidant properties and quality parameters, and deactivate microorganisms present in fruit and vegetable juices [17]. Pasteurization, on the other hand, uses a specified temperature and time to kill pathogenic microorganisms by denaturing proteins and enzymes critical for microbial growth [18].
Although pasteurization and sonication have been extensively studied on various fruit juices, nothing is known regarding their influence on the quality of Adajamir juice. Additionally, the synergistic effects of combining both methods on juice quality are unexplored. Therefore, certain knowledge gaps have been identified for the continuation of this research. First, there is a notable absence of research on the processing and preservation of Adajamir juice. Second, while extensive research has been conducted on the effects of sonication and pasteurization on a variety of fruit juices, there is no comparative analysis for this juice. Finally, the influence of sonication intensity, frequency, treatment time, pasteurization temperature, and duration on the flavor of Adajamir juice is understudied. Understanding these interactions is essential, as the sensory properties of juice determine consumer satisfaction and acceptance.
Addressing these research gaps will contribute to the optimization of Adajamir juice processing. The present study aims to evaluate and compare the effects of thermal, sonication and thermosonication treatment on physicochemical, bio active, and sensory properties of Adajamir juice as well as microorganism inactivation. The novelty of the work lies in its focus on a lesser-known and underutilized fruit species, which could offer new opportunities in the development of innovative and health-promoting beverages. The primary component of the study compares sonication and pasteurization of Adajamir juice in different conditions. A comprehensive investigation is needed to determine the ideal circumstances for preserving and improving the juice's sensory qualities, which boost customer satisfaction. Our investigation will also reveal the intricate dynamics between processing factors and sensory qualities. These insights may help manufacturers provide nutritious, safe Adajamir juice. It promotes sustainable exploitation of this underutilized fruit, increasing its recognition and use.
2. Materials and methods
2.1. Sample preparation
Fresh and matured Adajamir (Citrus assamensis) were collected from the Citrus Research Center of Bangladesh Agricultural Research Institute (BARI), Sylhet, Bangladesh in the month January 2020. The solvents and standards were procured from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Adajamir was properly washed with clean water. The peel was manually removed with a steel cutter. The white albedo and pulp were sliced and mixed in a juicer, and the juice was collected. The juice was then homogenized by filtering it through a sterile muslin cloth. The final juice was then stored in sterile glass bottles and refrigerated at 4 °C for further analysis.
2.2. Pasteurization and sonication treatment
The juice was divided into seven portions: 1) control sample (CP), 2) low heat pasteurization at 63 °C for 30 min (LP), 3) high heat pasteurization at 90 °C for 2 min (HP), 4) sonication for 10 min at 100 W (US10), 5) sonication for 20 min at 100 W (US20), 6) low heat pasteurization at 63 °C, 10 min and sonication for 10 min at 100 W (TS10), 7) low heat pasteurization at 63 °C for 20 min and sonication for 20 min at 100 W (TS20). Freshly squeezed juice was poured (30 ml) in a small beaker in an unstirred covered water bath at 63 °C for 30 min (LP) and 90 °C for 2 min (HP). The temperature of the juice at the center was continuously monitored by means of a thermometer. When the necessary temperature was reached, the time of the treatment process for the juice samples was recorded [19]. Following the thermal treatment, the juice samples undergo prompt cooling to attain ambient temperature, specifically 25 ± 1 °C, through immersion in an ice water bath. All treatments were carried out in triplicates. On the other hand, a 750-W high-intensity ultrasonic processor (VCX 750, Sonics & Materials, Inc., Newtown, CT, USA) with a 13 mm (1/2″) probe was used for juice sonication at a constant frequency of 20 kHz. In a 100 ml beaker, 50 ml of Adajamir juice was deposited for each treatment. The tip was immersed 15 mm below the juice surface. The applied power level was adjusted to 100 % of the maximum equipment power. The time was adjusted to 10 min and 20 min for the power level. After the treatment, the physical and chemical properties of the treated and untreated juice was measured.
2.3. Combination of sonication and thermal treatment (thermosonication)
A high-intensity ultrasonic processor with a 750-W power output and a 13 mm (1/2″) probe was used to sonicate the juice. The samples were processed at a consistent frequency of 20 kHz. In each treatment, 50 ml of Adajamir juice was dispensed into a laboratory-grade 100 ml beaker. The tip was plunged 15 mm below the surface of the juice. The applied power level was adjusted to 100 % of the maximum equipment power (100 W). The time was adjusted to 10 min and 20 min for the power level. After the sonication treatment both Adajamir juice samples (S-10, S-20; 30 ml) were poured in two small beakers for pasteurization in an unstirred covered water bath at 63 °C for 30 min. After the thermal treatment, the juice samples were immediately cooled to ambient temperature (25 ± 1 °C) by immersing in an ice water bath. All treatments were carried out in triplicates. After the treatment the physical and chemical properties of the treated and untreated juice was measured [20].
2.4. Physiochemical analysis (pH, titratable acidity, and total soluble solids)
pH was determined using a pH meter (Benchtop pH meter, model: HI22091, Hanna Instruments). Before placing the pH meter's electrode into the Adajamir juice, it was first calibrated with a standard buffer solution and cleansed with distilled water. The TSS content of the juice sample was assessed utilizing a digital refractometer (Atago PR-1, Tokyo, Japan) at a controlled temperature of 25 ± 1 °C. The results were presented in standard °Brix unit. Titratable acidity (TA) of juice sample was performed according to the method of Jiménez-Sánchez et al. [21]. The expression of TA was quantified in terms of grams of citric acid per 100 ml of juice. A 5 ml sample of juice was introduced into a 50 ml conical flask containing 20 ml of distilled water. The given solution was subjected to titration with a standardized solution of 0.1 M sodium hydroxide (NaOH) until a distinct pale pink endpoint was achieved, which should endure for a minimum of 15 s. According to Sadler and Murphy [22], the volume of NaOH used for titration was converted to grams of citric acid per 100 ml of juice. TA was calculated using following equation:
| (1) |
| (2) |
2.5. The measurement of color
The color of the juice specimens was determined utilizing a colorimeter (Konica Minolta CM-600 d, Osaka, Japan). The parameters of color, namely L* (lightness), a* (redness/greenness), and b* (yellowness/blueness), were evaluated. The calculation of color differences (ΔE) was performed by means of the following equation, in relation to the control [23].
| (3) |
2.6. Determination of bio-active compounds
2.6.1. Determination of -carotene
To determine the total carotenoid content, 1 g of the sample was mixed with 10 mL of distilled water and homogenized for a duration of 2 min. A 5 mL quantity of hexane was subjected to vigorous stirring for a duration of 1 min, followed by a 5-min period of rest to facilitate mass transfer. Subsequently, the mixture was once again subjected to vigorous stirring for 1 min. The supernatant was obtained and absorbance was measured in a UV–Vis spectrophotometer (Shimadzu, UV-1800, Japan) at 452 nm [24]. The experiments were conducted in triplicate and the outcomes were reported as mg/100 g, utilizing β-carotene calibration curves as a reference standard.
2.7. Determination of ascorbic acid
The method used for the determination of ascorbic acid was in accordance with Silva et al.'s procedure [25]. A 5 mL juice sample was blended with 3 % HPO3 and subsequently diluted to 50 mL with 3 % HPO3 and then filtered. 10 mL from the aliquot was transferred into a 100 mL conical flask. This was underwent titration using a standardized dye until reaching the endpoint, characterized by a light pink color that remained for 15 s. The estimation of Vitamin C was conducted using equation (4).
| (4) |
2.8. Sample preparation for total polyphenol content and antioxidant activity determination
For determining bioactive compound of extract, the sample were extracted with 80 % methanol with a ratio of 1:10 (sample: solvent) and incubated in a shaking incubator (HYSC SI-300 R, Korea) at 20 for 90 min. Following the incubation period, the crude extract underwent centrifugation at 3000 rpm (DM0424, DLAB, China), for a duration of 15 min. The sample was preserved at a temperature of −20 °C until the next tests were conducted.
2.8.1. Total polyphenol content (TPC)
The total phenolic content of juice samples was determined using the Folin-Ciocalteu method according to Aadil et al.'s protocol [26]. The reaction mixture included 0.5 mL of juice samples, 1 mL of a 10 % Folin-Ciocalteu reagent, and 2 mL of a 20 % sodium carbonate solution. The mixture was allowed to stay at 30 °C in the absence of light for a duration of 60 min. The measurement of absorbance was carried out using a spectrophotometer at 765 nm (Shimadzu, UV-1800, Japan). Using a standard solution of Gallic acid (2–170 mg/mL, r2 = 0.99), a calibration curve was generated and the results were quantified as mg of Gallic acid equivalent (GAE) per gram of sample.
2.9. Antioxidant activity
The evaluation of the antioxidant activity was conducted through analysis of the DPPH free radical scavenging activities and the ferric reducing antioxidant power (FRAP). DPPH and FRAP were measured following the method of Silva et al. [25] and Thaipong et al. [27]. The absorbance readings for DPPH and FRAP were obtained using a UV spectrophotometer (Shimadzu, UV-1800, Japan) at 517 nm and 700 nm, respectively. The percentage of the control was utilized to express the DPPH radical scavenging activity. The standard curve for FRAP assay was prepared using ascorbic acid, and the results were denoted in terms of mg AAE (Ascorbic Acid Equivalent)/100 g dry matter (DM).
2.10. Microbial analysis
The primary objective of methods used for food processing is to ensure that there is no risks to consumers. In order to ascertain the microbiological safety of the juice samples, an analysis was conducted on the total plate counts, yeast, and mold counts [28]. The total plate count was determined using plate count agar (Merck, Germany) and incubation at 37 °C for 48 h. Yeast and mold were also counted using the spread plate technique and potato dextrose agar (Merck, Germany). The media was adjusted to a pH of 3.5 during preparation using 100 g/L tartaric acid. It was then incubated at of 25 °C for 120 h. The resulting colonies were counted and expressed as log CFU/ml.
2.11. Sensory analysis
A cohort of 50 untrained individuals (24 males and 26 females) with a mean age of 26 years, who were acquainted with the fruit, were employed to carry out sensory evaluations. The primary inclusion criterion for panel testers was their familiarity with the taste, aroma, and color of the juice. In the interim periods of sample assessments, the panelists were instructed to perform oral cleansing using drinking water. The juice samples were evaluated for taste, color, aroma, and overall acceptability using a nine-point hedonic scale (Table S1), where 9 represented extreme liking and 1 represented extreme disliking [29].
3. Statistics analysis
All experiments were carried out in triplicates (n = 3). Results are expressed as means ± standard deviations. A one-way analysis of variance (ANOVA) was performed to compare the comparison of various treatments (control, LP, HP, US-10, US-20, TS-10, & TS-20). Significant differences between mean values were determined by comparison test at a significance level of p < 0.05. The statistical analysis was conducted using the SPPS 16.0 software (SPSS Inc., Chicago, IL). The data visualization tasks in this study were performed using Microsoft Power BI.
4. Results and discussion
4.1. Physicochemical analysis
pH, TSS, and TA.
The pH, TSS, and TA of Adajamir juice showed no significant changes after LP, HP, sonication treatment (S-10, S-20), and thermosonication (TS-10, TS-20) treatment (Table 1). Values for pH (2.43a to 2.47a), TSS (8.88a to 8.93a) and TA (1.13a % to 1.17a %) of treated juice samples were found to be within the standard desirable range for untreated Adajamir juice. The obtained results could be attributed to the use of ultrasonic intensity and heat treatments on the juice. It is worth noting, however, that the amount of energy applied to the samples had no effect on the high molecular weight structures associated with these specific physicochemical properties [30]. A similar result for pH, TSS, and TA was found in Valencia and Novel orange juice [31]. A finding reported that there is no significant variations in pH, TSS and TA after sonication in Kasturi lime juice, even after 30 min of treatment [9]. There is no significant change in pH, TSS and TA of apple juice even after the thermo-sonication [32]. A number of studies report comparable observations regarding the physicochemical analysis of various fruit juices [26,30,[33], [34], [35], [36], [37]].
Table 1.
Effect of pasteurization, sonication, and thermosonication on physicochemical properties of Adajamir juice.
| Treatment | Time (min) | pH | TSS (⁰Brix) | Total acidity (%) |
|---|---|---|---|---|
| Control | 0 | 2.47 ± 0.02a | 8.93 ± 0.36a | 1.17 ± 0.02a |
| LP | 30 | 2.45 ± 0.02a | 8.91 ± 0.66a | 1.14 ± 0.01a |
| HP | 2 | 2.43 ± 0.02a | 8.89 ± 0.53a | 1.13 ± 0.01a |
| US-10 | 10 | 2.46 ± 0.01a | 8.88 ± 0.39a | 1.15 ± 0.02a |
| US-20 | 20 | 2.45 ± 0.02a | 8.91 ± 0.77a | 1.14 ± 0.02a |
| TS-10 | (30 + 10) | 2.45 ± 0.01a | 8.92 ± 0.70a | 1.14 ± 0.01a |
| TS-20 | (30 + 20) | 2.43 ± 0.01a | 8.90 ± 0.66a | 1.15 ± 0.02a |
Data are expressed as means ± standard deviation (SD). Values with different letters in the same column are significantly different (p < 0.05) from each other, and same letters (a) indicate no significant differences.
4.1.1. Color
The pasteurization, sonication, and thermo-sonication treatment significantly affected all color parameters compared to control (Table 2). The application of LP and HP treatments resulted in a decrease in the color intensity of the juice sample. The US-10 and US-20 treatments led the juice to turn more yellow, while the implementation of TS-10 and TS-20 treatments elicited a notable elevation in the degree of browning. Color changes caused by heat are triggered by a change in the physical state of the carotenoid. Because suspended pulp particles reflected juice color, thermal pasteurization is likely to change suspended pulp particles, causing color variations in juices [38]. The process of sonication has been observed to expedite the isomerization of carotenoids and the oxidation reactions that are triggered by the presence of free radicals. These reactions are known to cause color degradation [39]. Significant change during sonication treatment in Kasturi lime (Citrus microcarpa) juice quality is also reported [9]. But the color difference of juice samples subjected to thermosonication was greater than those treated with sonication or thermal treatment. Juice samples got a' well visible' color changes as a result of thermosonication. This is in line with a study that was conducted on heated citrus juice, where significant color variations were observed, resulting in less light color (decrease in L* value) and less saturated juice [40]. A decrease in lightness and increase in redness and yellowness were observed after sonication [9]. Color degradation in thermo-sonication was observed by Dabir and Ananthanarayan [32], which also reported resulting in less light color and more redness with yellowness.
Table 2.
Effect of pasteurization, sonication, and thermosonication on color of Adajamir juice.
| Treatments | Time (mins) | L* | a* | b* |
|---|---|---|---|---|
| 28.17 ± 0.44a | 0.69 ± 0.01a | 11.58 ± 0.53a | ||
| LP | 30 | 26.28 ± 0.38b | 0.60 ± 0.04a | 12.87 ± 0.30b |
| HP | 2 | 26.41 ± 0.37b | 0.66 ± 0.03a | 12.25 ± 0.30b |
| US-10 | 10 | 27.85 ± 0.19c | 0.68 ± 0.02a | 12.37 ± 0.49b |
| US-20 | 20 | 27.15 ± 0.34c | 0.57 ± 0.06a | 12.79 ± 0.27b |
| TS-10 | 30 + 10 | 26.38 ± 0.31b | 1.05 ± 0.01b | 12.38 ± 0.19b |
| TS-20 | 30 + 20 | 26.20 ± 0.28b | 1.20 ± 0.03b | 12.23 ± 0.18b |
Data are expressed as means ± standard deviation (SD). Values with different letters (a-c) in the same column are significantly different (p < 0.05) from each other.
4.1.2. β-carotenoid content
Fig. 1(a) displays the β-carotene levels of both the control and treated samples. Following the ultrasound treatment, a significant rise in β-carotenoid was observed in the juice samples, with values of 71.51 μg/100 ml (US-10) and 76.2 μg/100 ml (US-20), respectively, as compared to the control sample (60.20 μg/100 ml). The US-20 (100w for 20 min) resulted in the highest increase in carotenoid content (26.59 %). The rise in carotenoid levels found in sonicated juice can be attributed to mechanical breakage of cell walls, which facilitates the liberation of these compounds. As a result of cavitation processes, this may increase the concentration of unbound carotenoids in the juice [17]. A study showed that sonication treatment has the potential to increase the concentration of β-carotenoid in Cape gooseberry juice [30]. Thermosonication treatment resulted in a significant increase of carotenoids in the juice samples. Upon subjecting the LP to sonication for 10 min, a carotenoid content of 62.10 μg/100 ml was observed. Similarly, sonication for a duration of 20 min with the same LP sample resulted in 65.75 μg/100 ml carotenoid content. The application of thermosonication treatment resulted in the disruption of the cellular membrane, facilitating the release of compounds. Additionally, the process of cavitation led to the deactivation of the enzyme lipoxygenase, thereby increasing the concentration of carotenoids in fruit juice [8].
Fig. 1.
Comparison of effects of thermal, sonication, and thermosonication on (a) β-carotenoid content, (b) ascorbic acid content of Adajamir juice.
4.1.3. Ascorbic acid
Fruits and vegetables are considered highly vulnerable to the degradation of ascorbic acid. The present study showed a reduction in the concentration of ascorbic acid in comparison to the control group that underwent sonication treatment, as depicted in Fig. 1(b). The US-10 exhibited a concentration of 36.77 mg/100 ml of ascorbic acid, indicating a decrease of approximately 3.80 % compared to the control sample. However, this difference was not deemed statistically significant. The US-20 exhibited a concentration of 35.29 mg/100 ml, indicating a reduction of about 7.67 % in comparison to the control sample. Tiwari et al. also observed a significant decrease of ascorbic acid content in strawberry juice after sonication treatment [41]. Ascorbic acid loss findings are in line with the observation of sonicated orange juice. It was observed that the ascorbic acid content decreased subsequent to a 15-min sonication treatment at 40 kHz [37]. Thermosonication also results in a reduction of ascorbic acid compared to the control samples. Both TS-10 and TS-20 exhibited a decrease in ascorbic acid content of 11.90 % and 18.32 %, respectively, compared to the control sample, with values of 33.67 mg/100 ml and 31.22 mg/100 ml.
4.2. Bioactive compounds
4.2.1. TPC & TFC content
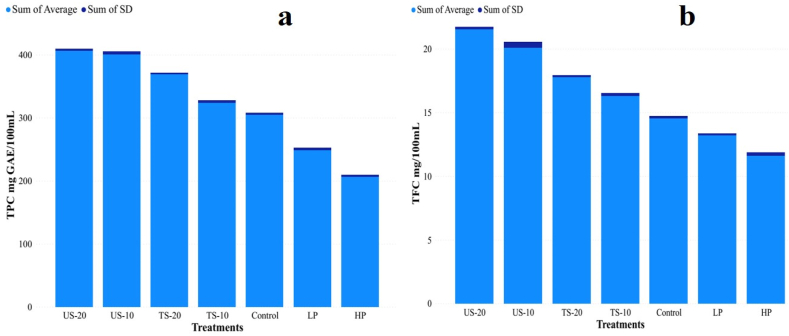
After pasteurization treatment (LP and HP), significant decreases were observed from Fig. 2(a) in the TPC (248.51 ± 3.39 mg GAE/100 ml and 206.08 ± 3.16 mg GAE/100 ml) when compared to control (304.79 ± 3.07 mg GAE/100 ml). Thermal pasteurization of fruit juices induces intricate chemical and physical changes that impact the phenolic structures. This process leads to the release of phenolic compounds from their bound forms, degradation of polyphenols, and alteration of phenolic composition through breakdown and transformation [42]. Similar kinds of the phenomenon were previously observed in mango juice [36,37]. Fig. 2(b) displays the consistent trend of a significant reduction in overall flavonoid content subsequent to the pasteurization process, in comparison to the control. The TFC values of the samples subjected to LP and HP were 13.21 ± 0.13 mg GAE/100 g and 11.60 ± 0.25 mg GAE/100 g, respectively. These values were compared to the control sample, which had a TFC value of 14.53 ± 0.18 mg GAE/100 g. The reduction in polyphenolic compounds observed during pasteurization can be linked to the thermal degradation, leaching loss, and diffusion of these compounds [42]. Similar outcomes was also observed by Turkmen et al. [43].
Fig. 2.
Comparison of effects of thermal, sonication and thermosonication treatment on (a) total phenolic content (TPC), (b) total flavonoid content (TFC) of Adajamir juice.
According to the data presented in Fig. 2(a), following exposure to both US-10 and US-20, the TPC (400.35 ± 4.65 mg GAE/100 ml and 406.60 ± 2.64 mg GAE/100 ml) exhibited a statistically significant increase compared to the control group (304.79 ± 3.07 mg GAE/100 ml). The sonication treatment resulted in an increase in TPC, with a rise of 29.65 % observed in the US-10 and 31.67 % in the US-20, as compared to the control. Similar to phenols, flavonoids also exhibit an increase subsequent to sonication. Specifically, the TFC was observed to be 20.08 ± 0.45 mg GAE/100 ml and 21.56 ± 0.17 mg GAE/100 ml, which represents a statistically significant increase when compared to the control (14.53 ± 0.18 mg GAE/100 ml). A comparable increase in phenolic compounds was also found in carrot juice [36], lime juice [9], and pear juice [44] under ultrasound treatment. This increase in polyphenol content is a result of increased cell wall disruption, which releases phenolic binding facilities to the cell wall traces of pectin, cellulose, hemicellulose, and lignin [21]. The production of hydroxyl radicals that contribute to the hydroxylation of the aromatic ring of phenolic compounds in the ortho-, meta-, and para-positions can increase phenolic compounds [35]. Thermosonication treatment resulted in a significant increase of both TPC and TFC in the juice samples. The TS-10 and TS-20 samples showed TPC values of 324.09 ± 3.34 mg GAE/100 ml and 368.94 ± 2.29 mg GAE/100 ml, respectively, in comparison to control (304.79 ± 3.07 mg GAE/100 ml). The thermosonication treatments also showed 16.28 ± 0.23 mg GAE/100 ml and 17.79 ± 0.15 mg GAE/100 ml of TFC.
4.2.2. Radical scavenging activity (DPPH)
The majority of DPPH free radical scavenging and antioxidant capacity is attributed to phenolic chemicals and vitamin C, particularly in citrus fruits. These chemicals have the ability to scavenge free radicals, lowering the risk of oxidative stress-related disorders in the human body [26]. After the pasteurization treatment, DPPH radical scavenging activity was observed to decrease significantly. Based on Fig. 3, the values of DPPH radical scavenging activity at LP and HP were 64.39 % and 58.20 %, respectively. Maximum reduction of 11.22 % was observed in HP samples (90 °C) and the control (69.42 %). Scalzo et al. also showed decreases in DPPH radical scavenging activity after thermal treatment in blood orange juice [45].
Fig. 3.
Comparison of effects of thermal, sonication and thermosonication treatment on radical scavenging activity (DPPH) in Adajamir juice.
In contrast, significant increases in DPPH scavenging activity were observed in sonicated juice samples compared to the control. The US-20 exhibited the highest DPPH radical scavenging activity, at 82.62 %, compared to the control. And 77.40 % radical scavenging activity was observed at US-10 DPPH. Cavitation, which is occurred by sonication can increase the extraction of antioxidant compounds. Sonicated samples showed significant increase in the percent DPPH inhibition compared to control in some studies [9]. This research also indicated that sonication can increase the extractability of antioxidant compounds. Slightly increase of the percentage was observed after thermosonication in Adajamir juice sample compared to control samples. After thermosonication treatments, the values were 73.54 % (TS-10) & 74.73 % (TS-20). Same kind of increases in carrot juice and star fruit juice were observed after thermosonication treatment [46,47].
4.2.3. Microbial inactivation analysis
The use of sonication treatments has been recognized as a promising technique that has the potential to satisfy the Food and Drug Administration's (FDA's) standards for attaining a 5-log reduction in the microorganisms that are linked with fruit juices [48]. The assessment of the microbiological quality of Adajamir juice samples was conducted using two indicators, namely the aerobic plate count (APC) and yeast and mold count (YMC) (Table 3). The application of sonication treatment resulted in a significant reductions in the aerobic plate count and yeast-mold count in the juice samples. A higher reduction rate was observed in the aerobic plate count and yeast-mold count of the US-20 sample in comparison to the US-10 sample. It has been reported that the power ultrasound treatment is effective in minimizing the presence of foodborne pathogens in orange, guava and tomato juices [[49], [50], [51]]. The thermosonication treatment resulted in a decrease in the total plate count of the samples. The thermosonication reduced total plate count to below the detection limits. The application of ultrasound has been found to increase the susceptibility of microorganisms to heat, owing to the phenomenon of acoustic cavitation and changes in the cellular membrane of the microbes [52]. The thermosonication treatment in carrot juice containing pulp extract showed no detectable microbial activity [47]. In a previous study conducted, it was observed that ultrasound treatment together with heat has been more efficient in inactivating microorganism compared to ultrasound treatment alone [53]. Under the Spanish Regulation [54], the overall limit of microorganisms in minimally processed foods is 7 log CFU/mL (at the expiry date). Hence, it can be inferred that the untreated control sample and US-10 fail to meet commercial acceptability standards at the end of the experimental storage period of 10 days. The sonication treated sample for 20 min (U-20), TS-10 and TS-20, exhibit a safe consumption profile even after the aforementioned storage period (Table 3). The results of this study support the utilization of sonication as a viable substitute for thermal processing in extending the microbiological shelf life of Adajamir juice.
Table 3.
Comparison of effects of pasteurization, sonication and thermosonication treatment on aerobic plate count, yeast, and mold count of Adajamir juice.
| Microorganisms | Storage (days) | Control | Treatments |
|||||
|---|---|---|---|---|---|---|---|---|
| LP | HP | US-10 | US-20 | TS-10 | TS-20 | |||
| Aerobic Plate Count (APC) (CFU/mL) |
0 3 7 10 |
2.83 ± 0.15a 3.76 ± 0.06a 5.34 ± 0.10a 7.13 ± 0.07a |
ND ND ND 2.28 ± 0.19de |
ND ND ND 2.1 ± 0.09e |
2.11 ± 0.02b 2.53 ± 0.06b 4.63 ± 0.18a 7.09 ± 0.08a |
1.58 ± 0.02c 1.59 ± 0.05c 3.24 ± 0.10b 5.26 ± 0.10b |
ND ND 2.76 ± 0.12bc 3.79 ± 0.30c |
ND ND 1.53 ± 0.19c 3.57 ± 0.13d |
| Yeast and Molds Count (YMC) (CFU/mL) |
0 3 7 10 |
2.81 ± 0.08a 3.59 ± 0.08a 6.39 ± 0.95a 8.53 ± 0.29a |
ND ND ND ND |
ND ND ND ND |
2.09 ± 0.1b 2.40 ± 1.5b 5.1 ± 0.04a 7.05 ± 0.09b |
1.55 ± 0.13c 2.85 ± 0.11c 4.28 ± 0.10b 5.04 ± 0.10c |
ND ND 1.86 ± 0.04c 3.15 ± 0.06d |
ND ND 1.15 ± 0.14d 2.62 ± 0.24d |
Data are expressed as means ± SD, mean values in a row with different superscript letters are significantly different at P < 0.05.
5. Sensory evaluation
The sensory attributes, namely aroma, taste, color, and overall acceptability, of all the treatments were evaluated and the outcomes were illustrated in Fig. 4. The results of the general evaluations carried out by the panelists indicate that the US20 sample obtained the most favorable outcomes, as demonstrated by its ratings for taste (6.82), aroma (7.86), color (8.71), and overall acceptability (7.80). Conversely, LP exhibits the least favorable ratings in terms of taste (5.74), aroma (7.55), color (8.54), and overall acceptability (7.28), rendering it the most undesirable sample in these regards. TS20 exhibits the least favorable scoring in terms of aroma (7.46). When two attributes demonstrate a strong positive correlation, it implies that any enhancements made to one variable are likely to result in improvements in the other variable as well. Empirical evidence suggests a significant correlation between taste and aroma, as indicated by the superior ratings in both categories being attributed to US20, whereas LP and TS20 received the least favorable scores. The highest-scoring sample (US20) demonstrates the highest ratings for flavor, aroma, and color as well. The sample with the lowest score (LP) displays the lowest scores in taste and color, and the second-lowest score in Aroma. Usually Adajamir juice has a bitter taste. In the current study, according to the panelist, the sonication treatment somewhat improve the taste and aroma of the sample. Naringin and limonin are flavonoids and limonoids, respectively, which are recognized as the representative compounds responsible for the bitter taste in citrus fruits. However, quercetin has been identified as a potential contributor to the bitterness observed in lemon and other corresponding fruits [55]. Sonication has been found to improve the bitter taste in a number of studies [56,57]. It can also be observed that thermosonication significantly affects the sensory ratings in comparison with pasteurized juices as reported earlier by Nayak et al. [34]. The sensory scores of thermosonicated samples in this study found to be higher than the pasteurized samples. Purewal and Sandhu identified the hot water treatment (HWT) as a notable physical technique for mitigating the issue of bitterness in citrus fruits and juices [55].
Fig. 4.
Sensory analysis values chart of Adajamir juice; CS = control sample, LP = low heat pasteurization at 63 °C for 30 min, HP = high heat pasteurization at 90 °C for 2 min, US10 = sonication for 10 min at 100 W, US = sonication for 20 min at 100 W, TS10 = low heat pasteurization at 63 °C, 10 min and sonication for 10 min at 100 W, TS20 = low heat pasteurization at 63 °C, 20 min and sonication for 20 min at 100 W.
Fig. 5 shows a different viewpoint for the measure of overall acceptability. It indicates the average overall acceptability of the juice sample based on its individual sensory characteristics. In terms of taste, Fig. 5 indicates that the US-20 (taste score: 6.82) was the most acceptable, followed by the US-10 (taste score: 6.74). LP (taste score: 5.74) appears to have the lowest acceptability based on taste. Likewise, the remaining two parameters, namely color and aroma, are observable through the corresponding figure.
Fig. 5.
The measure of overall acceptability based on individual sensory attributes.
6. Conclusion and future perspective
In the present study, the effect of thermal (pasteurization), nonthermal (sonication), and combined (thermosonication) approaches on the quality of Adajamir juice was evaluated. When compared to pasteurization and combined pasteurization-sonication techniques, the use of sonication solely resulted in a good percentage of retention in the majority of quality parameters in Adajamir juice. The pH and TA values did not exhibit any significant differences (p < 0.01) during the processes of sonication and pasteurization. However, significant changes in color were observed. The application of sonication resulted in significant increases in the total phenolic and β-carotenoid content in the juice samples. It was observed that the implementation of pasteurization resulted in significant reductions in the total phenols and β-carotene levels. Thermosonication was observed to result in significant enhancements in the total phenols and β-carotenoid in the juice samples. Sonication and thermosonication led to a significant increase in the antioxidant activities. It was observed that pasteurization had a detrimental effect on the antioxidant activities of the juice. All treatments reduced the amount of ascorbic acid. In general, sonication has the potential to serve as a viable alternative to thermal treatment due to its ability to enhance the antioxidant activity and minimize nutrient loss in juice samples. Still, the complete eradication of microbial load was not observed through the process of sonication. Based on our findings, we propose some recommendations for future research. It would be useful to study how the intensity and duration of sonication affect juice quality and taste. Sonication can improve the juice's flavor and aroma, but blindfolded taste tests or comprehensive surveys are needed to determine if consumers would prefer sonicated Adajamir juice in everyday situations. An area deserving attention is the improvement of microbial stability post-sonication or thermosonication. Researchers might consider amalgamating these methods with alternative non-thermal preservation strategies. In light of our study's promising results, it might be worthwhile to study the effects of these techniques on other lesser-known fruit varieties to see if a universal approach can be adopted or if individualized strategies are necessary. Finally, a compelling aspect to explore would be examining the influence of these methods on the ease of digestion and nutrient absorption from Adajamir juice.
Funding
No funding was received for this work. The authors incurred expenses for sample collecting, experimentation, writing, reviewing, and editing the manuscript.
Ethical approval
The study was approved by the department of Food Engineering and Tea Technology, Shahjalal University of Science and Technology, Sylhet, Bangladesh. The experiments pertaining to sensory evaluation were carried out in adherence to established ethical guidelines, wherein each panelist was required to provide informed written consent prior to participating in the study. It is important to note that there are no strict ethical approval requirements for sensory tests in Bangladesh.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Hasan Ahmad: Conceptualization, Formal analysis, Methodology, Writing – original draft. Tariqul Islam: Formal analysis, Writing – original draft, Writing – review & editing. Zohurul Islam: Data curation, Resources, Writing – original draft, Writing – review & editing. Fahad Jubayer: Conceptualization, Formal analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. Rahmatuzzaman Rana: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Nothing to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23074.
Contributor Information
Fahad Jubayer, Email: fahadbau21@hotmail.com, jubayer.fet@sau.ac.bd.
Rahmatuzzaman Rana, Email: rzaman-fet@sust.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Nudar J.T., Roy M., Ahmed S. Combined osmotic pretreatment and hot air drying: Evaluation of drying kinetics and quality parameters of adajamir (Citrus assamensis) Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e19545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Snafi A.E. Nutritional value and pharmacological importance of citrus species grown in Iraq. IOSR J. Pharm. 2016;6(8):76–108. doi: 10.9790/3013-0680176108. [DOI] [Google Scholar]
- 3.Cervantes-Elizarrarás A., Piloni-Martini J., Ramírez-Moreno E., Alanís-García E., Güemes-Vera N., Gómez-Aldapa C.A., Zafra-Rojas Q.Y., del Socorro Cruz-Cansino N. Enzymatic inactivation and antioxidant properties of blackberry juice after thermo ultrasound: optimization using response surface methodology. Ultrason. Sonochem. 2017;34:371–379. doi: 10.1016/j.ultsonch.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Ashokkumar M., Sunartio D., Kentish S., Mawson R., Simons L., Vilkhu K., Versteeg C.K. Modification of food ingredients by ultrasound to improve functionality: a preliminary study on a model system. Innovat. Food Sci. Emerg. Technol. 2008;9(2):155–160. doi: 10.1016/j.ifset.2007.05.005. [DOI] [Google Scholar]
- 5.Basumatary B., Nayak P.K., Chandrasekar C.M., Nath A., Nayak M., Kesavan R.K. Impact of thermo sonication and pasteurization on the physicochemical, microbiological and anti-oxidant properties of pomelo (Citrus maxima) juice. Int. J. Fruit Sci. 2020;20:S2056–S2073. doi: 10.1080/15538362.2020.1848751. [DOI] [Google Scholar]
- 6.Mazumder M.A.R., Rana J., Jubayer M.F., Ranganathan T.V., Ansari M.J. Ultrasound and Microwave for Food Processing. Academic Press; 2023. Sonication microwave synergistic extraction of bioactive compounds from plant source; pp. 239–267. [DOI] [Google Scholar]
- 7.Guimarães J.T., Scudino H., Ramos G.L., Oliveira G.A., Margalho L.P., Costa L.E.…Cruz A.G. Current applications of high-intensity ultrasound with microbial inactivation or stimulation purposes in dairy products. Curr. Opin. Food Sci. 2021;42:140–147. doi: 10.1016/j.cofs.2021.06.004. [DOI] [Google Scholar]
- 8.Aadil R.M., Zeng X.A., Zhang Z.H., Wang M.S., Han Z., Jing H., Jabbar S. Thermosonication: a potential technique that influences the quality of grapefruit juice. Int. J. Food Sci. Technol. 2015;50(5):1275–1282. doi: 10.1111/ijfs.12766. [DOI] [Google Scholar]
- 9.Bhat R., Kamaruddin N.S.B.C., Min-Tze L., Karim A.A. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason. Sonochem. 2011;18(6):1295–1300. doi: 10.1016/j.ultsonch.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Schuina G.L., Moraes V.P., Silva P.I., Carvalho R.V. Effect of thermosonication on pectin methylesterase activity and on quality characteristics of orange juice. Rev. Cienc. Agron. 2021;52(4) doi: 10.5935/1806-6690.20210055. [DOI] [Google Scholar]
- 11.Bhargava N., Mor R.S., Kumar K., Sharanagat V.S. Advances in application of ultrasound in food processing: a review. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farhadi Chitgar M., Aalami M., Maghsoudlou Y., Milani E. Comparative study on the effect of heat treatment and sonication on the quality of barberry (Berberis vulgaris) juice. J. Food Process. Preserv. 2017;41(3) doi: 10.1111/jfpp.12956. [DOI] [Google Scholar]
- 13.Parreiras P.M., Nogueira J.A.V., da Cunha L.R., Passos M.C., Gomes N.R., Breguez G.S., Falco T.S., Bearzoti E., Menezes C.C. Effect of thermosonication on microorganisms, the antioxidant activity and the retinol level of human milk. Food Control. 2020;113 doi: 10.1016/j.foodcont.2020.107172. [DOI] [Google Scholar]
- 14.Tahi A.A., Sousa S., Madani K., Silva C.L., Miller F.A. Ultrasound and heat treatment effects on Staphylococcus aureus cell viability in orange juice. Ultrason. Sonochem. 2021;78 doi: 10.1016/j.ultsonch.2021.105743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perera C.O., Alzahrani M.A.J. Ultrasound as a pre-treatment for extraction of bioactive compounds and food safety: a review. Lebensm. Wiss. Technol. 2021;142 doi: 10.1016/j.lwt.2021.111114. [DOI] [Google Scholar]
- 16.Rani M., Sood M., Bandral J.D., Bhat A., Gupta I. Thermosonication technology and its application in food industry. Int. J. Chem. Stud. 2020;8(3):922–928. doi: 10.22271/chemi.2020.v8.i3l.9317. [DOI] [Google Scholar]
- 17.Abid M., Jabbar S., Hu B., Hashim M.M., Wu T., Lei S., Khan M.A., Zeng X. Thermosonication as a potential quality enhancement technique of apple juice. Ultrason. Sonochem. 2014;21(3):984–990. doi: 10.1016/j.ultsonch.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Bermudez-Aguirre D., Niemira B.A. Pasteurization of foods with ultrasound: the present and the future. Appl. Sci. 2022;12(20) doi: 10.3390/app122010416. [DOI] [Google Scholar]
- 19.Putnik P., Kresoja Ž., Bosiljkov T., Jambrak A.R., Barba F.J., Lorenzo J.M., Roohinejad S., Granato D., Žuntar I., Kovačević D.B. Comparing the effects of thermal and non-thermal technologies on pomegranate juice quality: a review. Food Chem. 2019;279:150–161. doi: 10.1016/j.foodchem.2018.11.131. [DOI] [PubMed] [Google Scholar]
- 20.Demirdöven A., Baysal T. The use of ultrasound and combined technologies in food preservation. Food Rev. Int. 2008;25(1):1–11. doi: 10.1080/87559120802306157. [DOI] [Google Scholar]
- 21.Jiménez-Sánchez C., Lozano-Sánchez J., Segura-Carretero A., Fernández-Gutiérrez A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 1: techniques and applications. Crit. Rev. Food Sci. Nutr. 2017;57(3):501–523. doi: 10.1080/10408398.2013.867828. [DOI] [PubMed] [Google Scholar]
- 22.Sadler G.D., Murphy P.A. pH and titratable acidity. Food Analysis. 2010;4:219–238. [Google Scholar]
- 23.Pathare P.B., Opara U.L., Al-Said F.A.J. Colour measurement and analysis in fresh and processed foods: a review. Food Bioprocess Technol. 2013;6:36–60. doi: 10.1007/s11947-012-0867-9. [DOI] [Google Scholar]
- 24.Rodriguez-Amaya D.B. vol. 71. ILSI press; Washington: 2001. (A Guide to Carotenoid Analysis in Foods). [Google Scholar]
- 25.Silva E.M., Souza J.N.S., Rogez H., Rees J.F., Larondelle Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2007;101(3):1012–1018. doi: 10.1016/j.foodchem.2006.02.055. [DOI] [Google Scholar]
- 26.Aadil R.M., Zeng X.A., Han Z., Sun D.W. Effects of ultrasound treatments on quality of grapefruit juice. Food Chem. 2013;141(3):3201–3206. doi: 10.1016/j.foodchem.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Byrne D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006;19(6–7):669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 28.Nayak P.K., Rayaguru K., Radha Krishnan K. Quality comparison of elephant apple juices after high‐pressure processing and thermal treatment. J. Sci. Food Agric. 2017;97(5):1404–1411. doi: 10.1002/jsfa.7878. [DOI] [PubMed] [Google Scholar]
- 29.Basu S., Shivhare U.S., Singh T.V., Beniwal V.S. Rheological, textural and spectral characteristics of sorbitol substituted mango jam. J. Food Eng. 2011;105(3):503–512. doi: 10.1016/j.jfoodeng.2011.03.014. [DOI] [Google Scholar]
- 30.Ordóñez-Santos L.E., Martínez-Girón J., Arias-Jaramillo M.E. Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice. Food Chem. 2017;233:96–100. doi: 10.1016/j.foodchem.2017.04.114. [DOI] [PubMed] [Google Scholar]
- 31.Bull M.K., Zerdin K., Howe E., Goicoechea D., Paramanandhan P., Stockman R., Sellahewa J., Szabo E.A., Johnson R.L., Stewart C.M. The effect of high pressure processing on the microbial, physical and chemical properties of Valencia and Navel orange juice. Innovat. Food Sci. Emerg. Technol. 2004;5(2):135–149. doi: 10.1016/j.ifset.2003.11.005. [DOI] [Google Scholar]
- 32.Dabir M.P., Ananthanarayan L. Effect of thermosonication on peroxidase, pectin methylesterase activities and on bioactive compounds in custard apple juice. J. Food Meas. Char. 2017;11:1623–1629. doi: 10.1007/s11694-017-9542-1. [DOI] [Google Scholar]
- 33.Oladunjoye A.O., Adeboyejo F.O., Okekunbi T.A., Aderibigbe O.R. Effect of thermosonication on quality attributes of hog plum (Spondias mombin L.) juice. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nayak P.K., Basumatary B., Chandrasekar C.M., Seth D., Kesavan R.K. Impact of thermosonication and pasteurization on total phenolic contents, total flavonoid contents, antioxidant activity, and vitamin C levels of elephant apple (Dillenia indica) juice. J. Food Process. Eng. 2020;43(8) doi: 10.1111/jfpe.13447. [DOI] [Google Scholar]
- 35.Tomadoni B., Cassani L., Viacava G., Moreira M.D.R., Ponce A. Effect of ultrasound and storage time on quality attributes of strawberry juice. J. Food Process. Eng. 2017;40(5) doi: 10.1111/jfpe.12533. [DOI] [Google Scholar]
- 36.Jabbar S., Abid M., Wu T., Muhammad Hashim M., Hu B., Lei S., Zhu X., Zeng X. Study on combined effects of blanching and sonication on different quality parameters of carrot juice. Int. J. Food Sci. Nutr. 2014;65(1):28–33. doi: 10.3109/09637486.2013.836735. [DOI] [PubMed] [Google Scholar]
- 37.Santhirasegaram V., Razali Z., Somasundram C. Effects of thermal treatment and sonication on quality attributes of Chokanan mango (Mangifera indica L.) juice. Ultrason. Sonochem. 2013;20(5):1276–1282. doi: 10.1016/j.ultsonch.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Lee H.S., Coates G.A. Thermal pasteurization effects on color of red grapefruit juices. J. Food Sci. 1999;64(4):663–666. doi: 10.1111/j.1365-2621.1999.tb15106.x. [DOI] [Google Scholar]
- 39.Liu Y., Wu J., Chong C., Miao S. Ultrasound assisted osmotic dehydration as pretreatment for hot-air drying of carrot. Food Sci. Technol. Res. 2014;20(1):31–41. doi: 10.3136/fstr.20.31. [DOI] [Google Scholar]
- 40.Mohd-Hanif H., Shamsudin R., Adzahan N.M. UVC dosage effects on the physico-chemical properties of lime (Citrus aurantifolia) juice. Food Sci. Biotechnol. 2016;25:63–67. doi: 10.1007/s10068-016-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiwari B.K., O'Donnell C.P., Muthukumarappan K., Cullen P.J. Ascorbic acid degradation kinetics of sonicated orange juice during storage and comparison with thermally pasteurized juice. LWT--Food Sci. Technol. 2009;42(3):700–704. doi: 10.1016/j.lwt.2008.10.009. [DOI] [Google Scholar]
- 42.Chen Y., Yu L.J., Rupasinghe H.V. Effect of thermal and non‐thermal pasteurisation on the microbial inactivation and phenolic degradation in fruit juice: a mini‐review. J. Sci. Food Agric. 2013;93(5):981–986. doi: 10.1002/jsfa.5989. [DOI] [PubMed] [Google Scholar]
- 43.Turkmen N., Sari F., Velioglu Y.S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005;93(4):713–718. doi: 10.1016/j.foodchem.2004.12.038. [DOI] [Google Scholar]
- 44.Saeeduddin M., Abid M., Jabbar S., Hu B., Hashim M.M., Khan M.A., Xie M., Wu T., Zeng X. Physicochemical parameters, bioactive compounds and microbial quality of sonicated pear juice. Int. J. Food Sci. Technol. 2016;51(7):1552–1559. doi: 10.1111/ijfs.13124. [DOI] [Google Scholar]
- 45.Scalzo R.L., Iannoccari T., Summa C., Morelli R., Rapisarda P. Effect of thermal treatments on antioxidant and antiradical activity of blood orange juice. Food Chem. 2004;85(1):41–47. doi: 10.1016/j.foodchem.2003.05.005. [DOI] [Google Scholar]
- 46.Nayak P.K., Chandrasekar C.M., Kesavan R.K. Effect of thermosonication on the quality attributes of star fruit juice. J. Food Process. Eng. 2018;41(7) doi: 10.1111/jfpe.12857. [DOI] [Google Scholar]
- 47.Adiamo O.Q., Ghafoor K., Al‐Juhaimi F., Mohamed Ahmed I.A., Babiker E.E. Effects of thermosonication and orange by‐products extracts on quality attributes of carrot (Daucus carota) juice during storage. Int. J. Food Sci. Technol. 2017;52(9):2115–2125. doi: 10.1111/ijfs.13490. [DOI] [Google Scholar]
- 48.Margean A., Lupu M.I., Alexa E., Padureanu V., Canja C.M., Cocan I., Negrea M., Calefariu G., Poiana M.A. An overview of effects induced by pasteurization and high-power ultrasound treatment on the quality of red grape juice. Molecules. 2020;25(7):1669. doi: 10.3390/molecules25071669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adekunte A., Tiwari B.K., Scannell A., Cullen P.J., O'donnell C. Modelling of yeast inactivation in sonicated tomato juice. Int. J. Food Microbiol. 2010;137(1):116–120. doi: 10.1016/j.ijfoodmicro.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Cheng L.H., Soh C.Y., Liew S.C., Teh F.F. Effects of sonication and carbonation on guava juice quality. Food Chem. 2007;104(4):1396–1401. doi: 10.1016/j.foodchem.2007.02.001. [DOI] [Google Scholar]
- 51.Valero M., Recrosio N., Saura D., Muñoz N., Martí N., Lizama V. Effects of ultrasonic treatments in orange juice processing. J. Food Eng. 2007;80(2):509–516. doi: 10.1016/j.jfoodeng.2006.06.009. [DOI] [Google Scholar]
- 52.Bermúdez-Aguirre D., Barbosa-Cánovas G.V. Inactivation of Saccharomyces cerevisiae in pineapple, grape and cranberry juices under pulsed and continuous thermo-sonication treatments. J. Food Eng. 2012;108(3):383–392. doi: 10.1016/j.jfoodeng.2011.06.038. [DOI] [Google Scholar]
- 53.Lee H., Zhou B., Liang W., Feng H., Martin S.E. Inactivation of Escherichia coli cells with sonication, manosonication, thermosonication, and manothermosonication: microbial responses and kinetics modeling. J. Food Eng. 2009;93(3):354–364. doi: 10.1016/j.jfoodeng.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 54.Boletìn Oficial del Estado (BOE) Ministerio de la Presidencia; Madrid: 2001. Real Decreto 3484/2000, of 29 December, Establishing Norms of Hygiene for the Production, Distribution and Commercialization of Prepared Food. BOE No. 11 of 12 January 2001; p. 1440. [Google Scholar]
- 55.Purewal S.S., Sandhu K.S. Debittering of citrus juice by different processing methods: a novel approach for food industry and agro-industrial sector. Sci. Hortic. 2021;276 doi: 10.1016/j.scienta.2020.109750. [DOI] [Google Scholar]
- 56.Gao X., Feng T., Liu E., Shan P., Zhang Z., Liao L., Ma H. Ougan juice debittering using ultrasound-aided enzymatic hydrolysis: impacts on aroma and taste. Food Chem. 2021;345 doi: 10.1016/j.foodchem.2020.128767. [DOI] [PubMed] [Google Scholar]
- 57.Gupta A.K., Sahu P.P., Mishra P. Ultrasound aided debittering of bitter variety of citrus fruit juice: effect on chemical, volatile profile and antioxidative potential. Ultrason. Sonochem. 2021;81 doi: 10.1016/j.ultsonch.2021.105839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.