Abstract
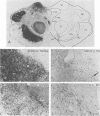
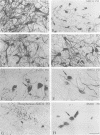
The distribution of neurofilament immunoreactivity in the substantia nigra was examined by immunohistochemistry in five patients dying with Parkinson's disease and six control patients dying without neurological disease. In controls, pigmented neurons in the substantia nigra were intensively labelled by SMI32, a monoclonal antibody to non-phosphorylated neurofilament protein. In the substantia nigra from patients who had Parkinson's disease, there was a pronounced reduction of SMI32 labelling intensity in surviving pigmented neurons. By contrast, tyrosine hydroxylase immunoreactivity in surviving pigmented neurons was normal. SMI32 labelling was normal in regions of the brainstem not affected by the neuropathological process of Parkinson's disease. Findings with either antibodies to phosphorylated neurofilament, or enzymatic dephosphorylation followed by SMI32 labelling, indicated that loss of SMI32 immunostaining in Parkinson's disease was not due to masking of the neurofilament epitopes by phosphorylation. Our results indicate that neurofilament proteins are particularly likely to be disrupted or destroyed by the neuropathological process of Parkinson's disease. Nevertheless, the normal appearance of tyrosine hydroxylase indicates that protein synthesising systems may be intact in surviving neurons. Loss of neurofilament immunoreactivity may prove a sensitive neuropathological marker for characterisation of degenerating neurons in Parkinson's disease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancher C., Lassmann H., Budka H., Jellinger K., Grundke-Iqbal I., Iqbal K., Wiche G., Seitelberger F., Wisniewski H. M. An antigenic profile of Lewy bodies: immunocytochemical indication for protein phosphorylation and ubiquitination. J Neuropathol Exp Neurol. 1989 Jan;48(1):81–93. doi: 10.1097/00005072-198901000-00007. [DOI] [PubMed] [Google Scholar]
- Campbell M. J., Morrison J. H. Monoclonal antibody to neurofilament protein (SMI-32) labels a subpopulation of pyramidal neurons in the human and monkey neocortex. J Comp Neurol. 1989 Apr 8;282(2):191–205. doi: 10.1002/cne.902820204. [DOI] [PubMed] [Google Scholar]
- Carden M. J., Schlaepfer W. W., Lee V. M. The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state. J Biol Chem. 1985 Aug 15;260(17):9805–9817. [PubMed] [Google Scholar]
- Fearnley J. M., Lees A. J. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991 Oct;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Forno L. S., Sternberger L. A., Sternberger N. H., Strefling A. M., Swanson K., Eng L. F. Reaction of Lewy bodies with antibodies to phosphorylated and non-phosphorylated neurofilaments. Neurosci Lett. 1986 Mar 14;64(3):253–258. doi: 10.1016/0304-3940(86)90337-x. [DOI] [PubMed] [Google Scholar]
- Gai W. P., Geffen L. B., Denoroy L., Blessing W. W. Loss of C1 and C3 epinephrine-synthesizing neurons in the medulla oblongata in Parkinson's disease. Ann Neurol. 1993 Apr;33(4):357–367. doi: 10.1002/ana.410330405. [DOI] [PubMed] [Google Scholar]
- Gai W. P., Halliday G. M., Blumbergs P. C., Geffen L. B., Blessing W. W. Substance P-containing neurons in the mesopontine tegmentum are severely affected in Parkinson's disease. Brain. 1991 Oct;114(Pt 5):2253–2267. doi: 10.1093/brain/114.5.2253. [DOI] [PubMed] [Google Scholar]
- Galloway P. G., Mulvihill P., Perry G. Filaments of Lewy bodies contain insoluble cytoskeletal elements. Am J Pathol. 1992 Apr;140(4):809–822. [PMC free article] [PubMed] [Google Scholar]
- German D. C., Manaye K., Smith W. K., Woodward D. J., Saper C. B. Midbrain dopaminergic cell loss in Parkinson's disease: computer visualization. Ann Neurol. 1989 Oct;26(4):507–514. doi: 10.1002/ana.410260403. [DOI] [PubMed] [Google Scholar]
- Gibb W. R., Lees A. J. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1991 May;54(5):388–396. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb W. R., Mountjoy C. Q., Mann D. M., Lees A. J. The substantia nigra and ventral tegmental area in Alzheimer's disease and Down's syndrome. J Neurol Neurosurg Psychiatry. 1989 Feb;52(2):193–200. doi: 10.1136/jnnp.52.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J. E., Yen S. H., Chiu F. C., Peress N. S. Lewy bodies of Parkinson's disease contain neurofilament antigens. Science. 1983 Sep 9;221(4615):1082–1084. doi: 10.1126/science.6308771. [DOI] [PubMed] [Google Scholar]
- Halliday G. M., Li Y. W., Joh T. H., Cotton R. G., Howe P. R., Geffen L. B., Blessing W. W. Distribution of monoamine-synthesizing neurons in the human medulla oblongata. J Comp Neurol. 1988 Jul 15;273(3):301–317. doi: 10.1002/cne.902730303. [DOI] [PubMed] [Google Scholar]
- Hill W. D., Arai M., Cohen J. A., Trojanowski J. Q. Neurofilament mRNA is reduced in Parkinson's disease substantia nigra pars compacta neurons. J Comp Neurol. 1993 Mar 15;329(3):328–336. doi: 10.1002/cne.903290304. [DOI] [PubMed] [Google Scholar]
- Hill W. D., Arai M., Cohen J. A., Trojanowski J. Q. Neurofilament mRNA is reduced in Parkinson's disease substantia nigra pars compacta neurons. J Comp Neurol. 1993 Mar 15;329(3):328–336. doi: 10.1002/cne.903290304. [DOI] [PubMed] [Google Scholar]
- Hof P. R., Cox K., Morrison J. H. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer's disease: I. Superior frontal and inferior temporal cortex. J Comp Neurol. 1990 Nov 1;301(1):44–54. doi: 10.1002/cne.903010105. [DOI] [PubMed] [Google Scholar]
- Javoy-Agid F., Hirsch E. C., Dumas S., Duyckaerts C., Mallet J., Agid Y. Decreased tyrosine hydroxylase messenger RNA in the surviving dopamine neurons of the substantia nigra in Parkinson's disease: an in situ hybridization study. Neuroscience. 1990;38(1):245–253. doi: 10.1016/0306-4522(90)90389-l. [DOI] [PubMed] [Google Scholar]
- Jellinger K. A. Pathology of Parkinson's disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol. 1991 Jun;14(3):153–197. doi: 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- Jellinger K. New developments in the pathology of Parkinson's disease. Adv Neurol. 1990;53:1–16. [PubMed] [Google Scholar]
- Jenner P., Schapira A. H., Marsden C. D. New insights into the cause of Parkinson's disease. Neurology. 1992 Dec;42(12):2241–2250. doi: 10.1212/wnl.42.12.2241. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. The distribution of phosphorylation sites among identified proteolytic fragments of mammalian neurofilaments. J Biol Chem. 1983 Mar 25;258(6):4019–4025. [PubMed] [Google Scholar]
- Kosik K. S., Rogers J., Kowall N. W. Senile plaques are located between apical dendritic clusters. J Neuropathol Exp Neurol. 1987 Jan;46(1):1–11. doi: 10.1097/00005072-198701000-00001. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W., Trojanowski J. Q. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987 Nov;7(11):3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Trojanowski J. Q. Novel monoclonal antibodies provide evidence for the in situ existence of a nonphosphorylated form of the largest neurofilament subunit. J Neurosci. 1986 Mar;6(3):850–858. doi: 10.1523/JNEUROSCI.06-03-00850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Carden M. J., Hollosi M., Dietzschold B., Lazzarini R. A. Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1998–2002. doi: 10.1073/pnas.85.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. H., Lewis D. A., Campbell M. J., Huntley G. W., Benson D. L., Bouras C. A monoclonal antibody to non-phosphorylated neurofilament protein marks the vulnerable cortical neurons in Alzheimer's disease. Brain Res. 1987 Jul 28;416(2):331–336. doi: 10.1016/0006-8993(87)90914-0. [DOI] [PubMed] [Google Scholar]
- Nixon R. A., Sihag R. K. Neurofilament phosphorylation: a new look at regulation and function. Trends Neurosci. 1991 Nov;14(11):501–506. doi: 10.1016/0166-2236(91)90062-y. [DOI] [PubMed] [Google Scholar]
- Pant H. C. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem J. 1988 Dec 1;256(2):665–668. doi: 10.1042/bj2560665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollanen M. S., Bergeron C., Weyer L. Deposition of detergent-resistant neurofilaments into Lewy body fibrils. Brain Res. 1993 Feb 12;603(1):121–124. doi: 10.1016/0006-8993(93)91307-e. [DOI] [PubMed] [Google Scholar]
- Schmidt M. L., Murray J., Lee V. M., Hill W. D., Wertkin A., Trojanowski J. Q. Epitope map of neurofilament protein domains in cortical and peripheral nervous system Lewy bodies. Am J Pathol. 1991 Jul;139(1):53–65. [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schmidt M. L., Shin R. W., Bramblett G. T., Rao D., Lee V. M. Altered tau and neurofilament proteins in neuro-degenerative diseases: diagnostic implications for Alzheimer's disease and Lewy body dementias. Brain Pathol. 1993 Jan;3(1):45–54. doi: 10.1111/j.1750-3639.1993.tb00725.x. [DOI] [PubMed] [Google Scholar]
- Vickers J. C., Costa M. The neurofilament triplet is present in distinct subpopulations of neurons in the central nervous system of the guinea-pig. Neuroscience. 1992 Jul;49(1):73–100. doi: 10.1016/0306-4522(92)90077-f. [DOI] [PubMed] [Google Scholar]
- Vickers J. C., Costa M., Vitadello M., Dahl D., Marotta C. A. Neurofilament protein-triplet immunoreactivity in distinct subpopulations of peptide-containing neurons in the guinea-pig coeliac ganglion. Neuroscience. 1990;39(3):743–759. doi: 10.1016/0306-4522(90)90258-6. [DOI] [PubMed] [Google Scholar]
- Vickers J. C., Delacourte A., Morrison J. H. Progressive transformation of the cytoskeleton associated with normal aging and Alzheimer's disease. Brain Res. 1992 Oct 30;594(2):273–278. doi: 10.1016/0006-8993(92)91134-z. [DOI] [PubMed] [Google Scholar]