Abstract
Singlet oxygen (1O2) has a very short half-life of 10−5 s; however, it is a strong oxidant that causes growth arrest and necrotic lesions on plants. Its signaling pathway remains largely unknown. The Arabidopsis flu (fluorescent) mutant accumulates a high level of 1O2 and shows drastic changes in nuclear gene expression. Only two plastid proteins, EX1 (executer 1) and EX2 (executer 2), have been identified in the singlet oxygen signaling. Here, we found that the transcription factor abscisic acid insensitive 4 (ABI4) binds the promoters of genes responsive to 1O2-signals. Inactivation of the ABI4 protein in the flu/abi4 double mutant was sufficient to compromise the changes of almost all 1O2-responsive-genes and rescued the lethal phenotype of flu grown under light/dark cycles, similar to the flu/ex1/ex2 triple mutant. In addition to cell death, we reported for the first time that 1O2 also induces cell wall thickening and stomatal development defect. Contrastingly, no apparent growth arrest was observed for the flu mutant under normal light/dim light cycles, but the cell wall thickening (doubled) and stomatal density reduction (by two-thirds) still occurred. These results offer a new idea for breeding stress tolerant plants.
Keywords: ABI4, cell wall, singlet oxygen, stomatal development, transcriptome reprogramming
In plants, reactive oxygen species (ROS) are produced continuously as byproducts of multiple metabolic pathways and are localized in multiple cellular compartments. Under normal conditions, ROS are scavenged by various antioxidative defense systems (1, 2). The equilibrium between the generation and the scavenging of ROS may be perturbed by many environmental stresses. To date, research on the physiological activities of ROS in plant cells has mainly been restricted to hydrogen peroxide and superoxide, which are released upon abiotic and biotic stress and work as signaling molecules to regulate various processes, such as stomatal behavior, programmed cell death, and pathogen defense (1, 2).
In photosynthetic organisms, excited chlorophylls or tetrapyrrole intermediates can stimulate the formation of singlet oxygen (1O2) upon illumination; a highly toxic molecule that in addition to its damaging nature acts as a crucial signaling molecule. 1O2 signaling has been shown to interact with the signal cascades of other ROS, lipid hydroperoxide-derived reactive electrophile species, and oxidized carotenoids, which may induce programmed cell death (3). 1O2 is responsible for more than 80% of nonenzymatic lipid peroxidation (4). The FLU (fluorescent) protein is a nuclear-encoded plastid protein that plays a key role in the negative-feedback control of chlorophyll biosynthesis (5). Inactivation of this protein in the flu mutant leads to the over-accumulation of free protochlorophyllide (Pchlide), which may work as a potent photosensitizer. Thus, the flu mutant generates 1O2 in plastids in a controlled but noninvasive manner. Immediately after the release of 1O2, mature flu plants stop growing, whereas young seedlings bleach and die, when grown under dark/light cycles (5). Inactivation of the plastid proteins executer1 (EX1) and executer2 (EX2) attenuate the extent of 1O2-induced upregulation of nuclear gene expression, thereby reversing the growth arrest and seedling lethality of the flu mutant under light/dark cycles (6, 7).
Under severe light stress, the signaling is initiated independently of EX1 by singlet oxygen that is thought to be generated at the acceptor side of active photosystem II (PSII) within the core of grana stacks. The second source of 1O2 formation was found in the grana margins, close to the place of chlorophyll biosynthesis, where EX1 is localized and the disassembly of damaged PSII and reassembly of active PSII take place (8). The initiation of 1O2 signaling in grana margins depends on EX1 and the ATP-dependent zinc metallo-protease FtsH2 (9). Alternatively, 1O2 could oxidize β-carotene to release β-cyclocitral, which has emerged as a 1O2-induced stress signal in plants (10).
Despite these research progresses, little is known about how and where the 1O2 signals are sensed and transmitted to the nucleus, and no nuclear signaling factor has been identified. The 1O2 signal is a type of plastid retrograde signal (2, 3, 6, 7), in which plastid genomes uncoupled 1 (GUN1) protein and nuclear Apetala 2 (AP2)–type transcription factor abscisic acid insensitive 4 (ABI4) may be involved (11). A previous study demonstrated that the gun1 mutation did not prevent the 1O2-mediated bleaching and cell death response of the flu mutant grown under light/dark cycles (12). Thus, the possible role of ABI4 in 1O2 signaling was investigated in this study. Previous studies suggested that ABI4 may function as a master switch, required for the regulation of nuclear genes in response to developmental cues (such as carbon source changes), environmental stress tolerance (responsive to abscisic acid, ethylene, and jasmonic acid), as well as chloroplast retrograde signaling (e.g., norflurazon or lincomycin-trigged signals) (11, 13). In this report, we found that the inactivation of ABI4 was sufficient to abrogate 1O2-mediated signaling, resulting in flu mutant survival. In addition to cell death, we reported for the first time that 1O2 also induces cell wall thickening and stomatal development defect.
Results
ABI4 mutation rescued the lethal phenotype of flu
Two abi4 mutants were used to perform genetic crossing with the flu mutant: abi4-104 mutant (CS3839; single nucleotide substitution at codon 69 leading to missense E to K) and abi4-2 mutant (SALK_080095; T-DNA insertion at codon 152) (13). In contrast to WT plants, all flu mutants (flu, flu/abi4-2, flu/abi4-104, and flu/ex1/ex2) accumulated five times higher levels of free Pchlide in the dark (Fig. 1, A and B). After transfer to the light, flu/abi4-2, flu/abi4-104, and flu/ex1/ex2 generated singlet oxygen in amounts similar to that of flu (Fig. 1C). Despite their high Pchlide levels, both flu/abi4-2 and flu/abi4-104 rescued the lethal phenotype of flu (Fig. 1D). When grown under light/dark cycles, the flu seedlings ceased growth, whereas the flu/abi4 and flu/ex1/ex2 seedlings continued to grow at similar levels to the WT, except that their growth was slightly reduced and flu/abi4 flowered a little earlier than the WT plants (Fig. 1, E and F). Under continuous light, all four lines grew equally well and finally reached the same flowering stage (Fig. S1).
Figure 1.
Inactivation of either EXECUTERs or ABI4 results in flu mutant survival under light/dark cycles.A, fluorescence of 7-day-old WT (Col-0), flu, flu/abi4 (flu/abi4-104 and flu/abi4-2), and flu/ex1/ex2 etiolated seedlings. B, Pchlide contents of 7-day-old etiolated seedlings. F.W., fresh weight. C, singlet oxygen in 7-day-old de-etiolated seedlings during 100 min of illumination (100 μmol photons m−2 s−1). D, forty-day-old seedlings grown under light/dark cycles. E, increasing fresh weight during 40 days of growth under light/dark cycles. F, flowering times of all four lines of plants grown under light/dark cycles. N.A., not available. Error bars show standard deviations (n = 3). Different lowercase letters indicate significant differences at the 0.05 (p < 0.05) level. ABI4, abscisic acid insensitive 4.
It is interesting to note that Pchlide/1O2 accumulation levels in the dark were almost the same in the flu mutant, flu/abi4 double-mutant, and flu/ex1/ex2 triple-mutant (Fig. 1, A–C); however, only the flu mutant showed a lethal phenotype after illumination (Fig. 1D). This demonstrated that the growth inhibition and seedling lethality do not result from the physicochemical damage caused by 1O2 after the dark-to-light shift but are rather caused by the activation of a genetically determined stress response program (6, 7).
Rose Bengal (RB) functions as a photosensitizer by transferring energy to O2, generating 1O2 (14, 15). To confirm the specificity of RB action in nuclei, we grew 5-day-old abi4-2, abi4-104, and WT (Col-0) seedlings under 16-h light (100 μmol·m−2·s−1)/8-h dark cycles without RB for 5 days and then transferred to 1/2 Murashige and Skoog (MS) medium with 1, 10, or 100 μM RB for additional 6 days. Again, abi4 mutants showed less photobleaching upon 100 μM RB treatment. However, all plants showed mild growth arrest at 10 μM RB treatment and severe growth arrest at 100 μM RB treatment may because of its toxicity at high concentrations (Fig. S2).
Both 35S:ABI4-GFP/flu/abi4-104 and 35S:ABI4-GFP/flu/abi4-2 complemented lines showed growth arrest and photobleaching under light/dark cycles (Fig. S3), confirming the irreplaceable role of ABI4 in 1O2 signaling. Given that both WT ABI4 protein and the single-point mutant ABI4 protein are expressed in the 35S-ABI4-GFP/flu/abi4-104 plants, they did not show the lethal phenotype of flu, but showed moderate growth arrest and photobleaching under light/dark cycles (Fig. S3). It could also be due to a dimer formation between the mutant ABI4 and WT ABI4-GFP, making a nonfunctional complex, or competition between them. To rule out the possibility that other proteins may interact with ABI4 and function in 1O2 signaling, the single-point mutant abi4-104 with the full-length ABI4 ORF preserved was selected for the following experiments.
Singlet oxygen signals regulate gene expression depending on ABI4
The binding strength of ABI4 with 1O2-responsive gene promoters was then evaluated using the chromatin immunoprecipitation (ChIP)-PCR method. For generating the antibody used for the ChIP assay, five epitopes of the ABI4 protein were synthesized chemically. The polyclonal antibodies against these epitopes were generated by inoculation in a mouse. The immune specificity of each antibody was verified by Western blotting (Fig. S4, A and B), and the antibody against epitope#11 was selected for the following experiments.
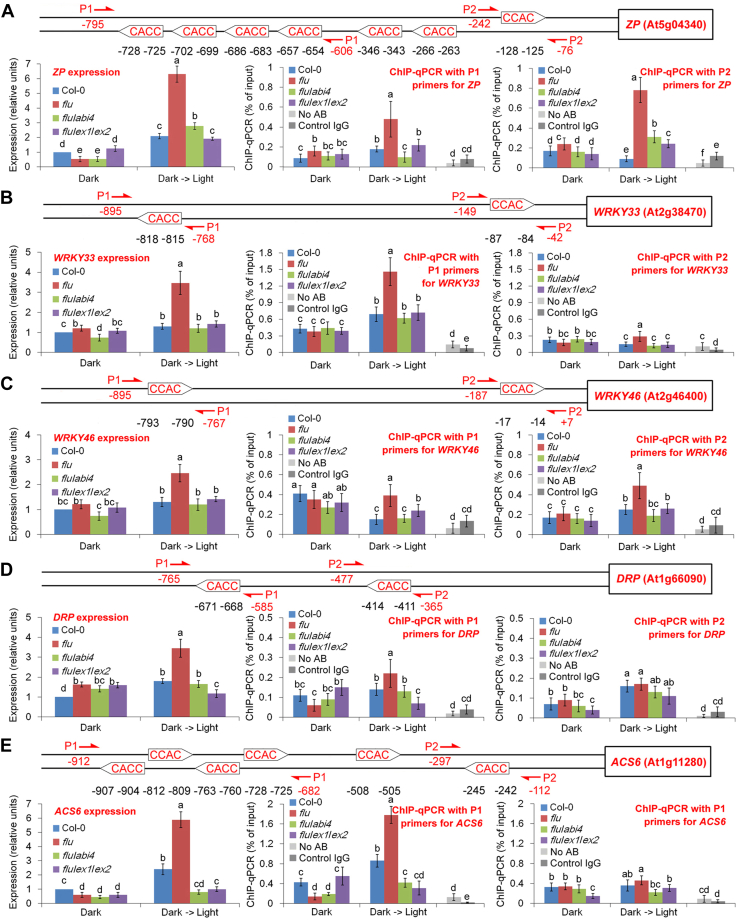
Previous studies indicated that the CCAC motif is a core element required for ABI4 binding (11, 16). This core binding element was found to be present at high frequencies in the promoters of five representative 1O2-inducible genes, including ZP (a putative C2H2 zinc finger transcription factor; At5g04340) (17), WRKY33 (a transcription factor; At2g38470), WRKY46 (a transcription factor; At2g46400), DRP (a disease resistance protein; At1g66090), and ACS6 [1-amino-cyclopropane-1 carboxylic acid (ACC) synthase 6; At1g11280] (7). As shown in Figure 2, A–E, the dark-to-light shift significantly induced ABI4 binding to these gene promoters as well as their expression in the flu mutant, whereas these levels were only slightly increased or downregulated in flu/abi4, flu/ex1/ex2, or WT seedlings, also implying the indispensable roles of EXECUTER1/2 and ABI4 in 1O2 signaling. WRKY46 primer pair 1 showed an unexpected pattern of high binding in the dark which was alleviated in the light (Fig. 2C). However, the fragment in WRKY46 promoter contains both light-induced and light-repressed elements. Detailed regulatory mechanism needs further investigations.
Figure 2.
Dark-to-light shift induces ABI4 binding to1O2-responsive gene promoters. WT (Col-0), flu, flu/abi4 (flu/abi4-104), and flu/ex1/ex2 plants were grown for 21 days under continuous light, transferred to the dark for 8 h (dark), and in some cases reexposed to light for 30 min (dark -> light). Five representative genes, ZP (A), WRKY33 (B), WRKY46 (C), DRP (D), and ACS6 (E) were studied. The positions of the CCAC motif and the corresponding ChIP-PCR primers (two pairs of primers were designed for each promoter: P1 and P2) are marked on the promoters. The binding strength of ABI4 with gene promoters was detected by ChIP-PCR. No AB shows the control signals in the “Dark -> Light” flu sample without the antibody (AB). Control IgG shows the control signals in the “dark -> light” flu sample with the mouse control IgG. Gene expression levels were detected by quantitative real-time PCR. The expression levels of the WT seedlings subjected to 8 h of dark were normalized to 100%. Error bars show standard deviations (n = 3). Different lowercase letters indicate significant differences at the 0.05 (p < 0.05) level. ABI4, abscisic acid insensitive 4; ACS6, 1-amino-cyclopropane-1 carboxylic acid (ACC) synthase 6; ChIP, chromatin immunoprecipitation; DRP, disease resistance protein; IgG, immunoglobulin IgG.
Nevertheless, the induction rates of ABI4 mRNA levels were much lower than the increasing rates of ABI4 binding (Fig. S5A), and no significant changes in ABI4 protein levels were observed after the dark-to-light shift in all four lines (Figs. S4C and S5B), indicating some other regulatory mechanism besides transcriptional or translational enhancement. Previous studies (11, 16) showed similar results whereby ABI4 expression was barely affected by plastid signals. ABI4 might transmit the signals (bind to the target promoters) through a posttranslational regulation, such as phosphorylation or activity modulation through protein-protein interaction (18).
Recently, Li et al. (15) interestingly found that, upon light irradiation or RB treatment, EX1 transiently accumulated in the nucleus. Thus EX1 might interact with ABI4 to regulate 1O2-responsive genes. However, we failed to address a direct binding of EX1 to ABI4 by Coimmunoprecipitation (Co-IP) experiments (Fig. S6). Whether ABI4 is a direct mediator of the signal or a downstream target of EX1 in the signaling network requires further studies.
The 1O2-dependent nuclear gene expression changes were clarified by RNA-seq [the principal component analysis is shown in Fig. S7; heatmaps of differentially expressed genes (DEGs) are shown in Fig. S8]. Genes with a 2-fold or greater transcript level than the control were considered to be significantly upregulated. After 30 min of reillumination, a total of 896 genes had been upregulated in flu or flu/abi4 or flu/ex1/ex2 relative to the WT (Fig. 3A). Among them, 200 transcripts were 1O2-induced genes in flu specifically, including 30 genes regulating cell wall organization (29 genes) or lignin catabolic process (1 gene), 22 phytohormone-regulated genes (six cytokinin-regulated genes; five abscisic-acid-regulated genes; four gibberellin-regulated genes; three brassinosteroid-regulated genes; one ethylene-regulated gene; one auxin-regulated gene; one salicylic-acid-regulated gene; one jasmonic-acid-regulated gene), 20 stress-responsive genes (11 genes responsive to water deprivation; five genes responsive to cold; two genes responsive to salt stress; two genes responsive to oxidative stress), 15 photosynthesis-related genes, 11 lipid metabolic process genes, two genes related to stomatal development, and two genes related to cell death (Data sheet S1). We also analyze the DEGs between flu and flu/abi4. Among them, top ten DEGS with the biggest differences included three cell wall organization genes, one ethylene-responsive gene, one stress-responsive gene and one cell death-related gene (Data sheet S1). Gene Ontology (GO) enrichment analysis also showed overrepresented GO terms related to cell wall organization, pectin biosynthesis, and salicylic acid binding (Fig. S9). A previous study also indicated that genes in jasmonic acid signaling pathway were induced by the application of RB in the light or dark-to-light transitions in the flu mutant independently of the hormone (14).
Figure 3.
1O2-induced genes in flu mutant specifically. WT (Col-0), flu, flu/abi4 (flu/abi4-104), flu/ex1/ex2 and 35S:ABI4-GFP/flu/abi4-2 plants were grown for 21 days under continuous light, transferred to the dark for 8 h, and then reexposed to light for 30 min. A, The relationships of three selected groups of genes that were upregulated by at least 2-fold in flu versus the WT, flu/abi4 versus the WT, and flu/ex1/ex2 versus the WT were analyzed using a Venn diagram. B–G, the expression levels of six representative genes were detected by quantitative real-time PCR: two cell-death genes XCP1 (B) and AED3 (C), two cell wall organization genes F8H (D) and LRX2 (E), and two stomatal development genes TMM (F) and BCA1 (G). The expression levels of the WT seedlings were normalized to 100%. Error bars show standard deviations (n = 3). Different lowercase letters indicate significant differences at the 0.05 (p < 0.05) level. AED3, apoplastic EDS1 (enhanced disease susceptibility 1)-dependent 3; BCA1, beta carbonic anhydrase 1; F8H, fragile fiber 8 homolog; LRX2, leucine-rich repeat/extensin 2; TMM, too many mouths; XCP1, xylem cysteine peptidase 1.
Interestingly, 95% (521/548) of upregulated genes in flu/ex1/ex2 relative to the WT were also upregulated in flu/abi4 relative to the WT (Fig. 3A), suggesting that ABI4 functions downstream of EXECUTER1/2. The expression levels of six representative genes were detected by quantitative real-time PCR, including two cell-death genes XCP1 (xylem cysteine peptidase 1; At4g35350) (19) and AED3 [apoplastic EDS1 (enhanced disease susceptibility 1)-dependent 3; At1g09750] (20), two cell wall organization genes F8H (fragile fiber 8 HOMOLOG; At5g22940) (21) and LRX2 (leucine-rich repeat/extensin 2; At1g62440) (22), and two stomatal development genes TMM (too many mouths; At1g80080) (23) and beta carbonic anhydrase 1(BCA1); At3g01500) (24). These genes were all induced more than three times in flu mutant or 35S:ABI4-GFP/flu/abi4-2 complemented plants after the dark-to-light shift but were nonsignificantly or less induced in the WT, flu/abi4 or flu/ex1/ex2 seedlings (Fig. 3, B–G), further confirming the key roles of EXECUTER1/2 and ABI4 in 1O2 signaling.
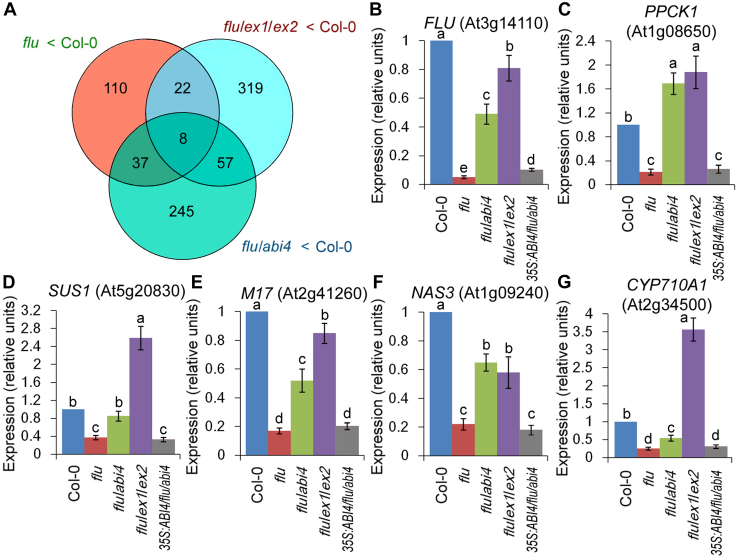
1O2-repressed genes were also analyzed. After 30 min of reillumination, a total of 798 genes had been downregulated in flu or flu/abi4 or flu/ex1/ex2 relative to the WT (Fig. 4A). Among them, 110 transcripts were 1O2-repressed genes in flu specifically, including seven genes encoding lipid metabolic or lipid-binding proteins, seven genes responsive to water deprivation, six genes regulating pollen development or pollen tube growth (including two nicotianamine synthase genes), five genes controlling carbohydrate metabolism or transport, four genes related to seed dormancy or development and four cytochrome p450 genes (Data sheet S2). We also analyze the DEGs between flu and flu/abi4. Among them, top ten DEGS with the biggest differences included three lipid (sterol) metabolic genes, two phenylpropanoid biosynthetic (water deprivation-responsive) gene and one chlorophyll biosynthetic gene (Data sheet S2). GO enrichment analysis also showed overrepresented GO terms related to sterol biosynthetic process, water deprivation, and nicotianamine biosynthesis (Fig. S10).
Figure 4.
1O2-repressed genes in flu mutant specifically. WT (Col-0), flu, flu/abi4 (flu/abi4-104), flu/ex1/ex2 and 35S:ABI4-GFP/flu/abi4-2 plants were grown for 21 days under continuous light, transferred to the dark for 8 h, and then reexposed to light for 30 min. A, the relationships of three selected groups of genes that were downregulated by at least 2-fold in flu versus the WT, flu/abi4 versus the WT, and flu/ex1/ex2 versus the WT were analyzed using a Venn diagram. B–G, the expression levels of six representative genes were detected by quantitative real-time PCR: FLU (B), PPCK1 (C), SUS1 (D), and M17 (E), NAS3 (F) and CYP710A1 (G). The expression levels of the WT seedlings were normalized to 100%. Error bars show standard deviations (n = 3). Different lowercase letters indicate significant differences at the 0.05 (p < 0.05) level. ABI4, abscisic acid insensitive 4; CYP, cytochrome P450; NAS3, nicotianamine synthase 3; PPCK1, phosphoenolpyruvate carboxylase kinase; SUS1, SUS1, sucrose synthase 1.
Interestingly, FLU gene was 20-fold repressed by the 1O2 signal (Fig. 4B). The expression levels of other five representative genes were detected by quantitative real-time PCR, including two carbohydrate metabolism genes PPCK1 (encoding a phosphoenolpyruvate carboxylase kinase; At1g08650) (25) and SUS1 (encoding a water-deprivation-responsive sucrose synthase; At5g20830) (26), one seed development genes M17 (encoding a late embryosis abundant protein; At2g41260) (27), one pollen development gene NAS3 (encoding a nicotianamine synthase; At1g09240) (28) and a sterol metabolic cytochrome (CYP) P450 gene CYP710A1 (At2g34500) (29). These genes were all reduced more than 2.5 times in flu mutant or 35S:ABI4-GFP/flu/abi4-2 complemented plants after the dark-to-light shift but were nonsignificantly or less reduced in the WT, flu/abi4 or flu/ex1/ex2 seedlings (Fig. 4, C–G), further confirming the key roles of EXECUTER1/2 and ABI4 in 1O2 signaling.
Singlet oxygen signals induce stomatal development defect and cell wall thickening under light/dark cycles
Consistent with the RNA-seq data, under the condition of light/dark cycles, abrupt ROS accumulation, lipid peroxidation, and cell death were observed in the flu mutant, but not in the flu/abi4 or flu/ex1/ex2 mutants (Fig. S11). Although all flu mutants accumulated the same high level of Pchlide in the dark (Fig. 1, A and B), the Pchlide was converted into chlorophylls in flu/abi4 and flu/ex1/ex2 under light, and thus their Pchlide declined to low levels similar to that of the WT seedlings (Fig. S12, B and C). By contrast, although a certain level of chlorophylls (about one-fourth of the WT; Fig. S12F) was synthesized in the flu mutant grown under 7-days light/dark cycles and then transferred to reillumination for 1 h, the plants became bleached (the steady-state chlorophyll level declined to 1/25 of the WT; Fig. S12E), and their Pchlide could not be converted into chlorophylls and therefore accumulated (Fig. S12C). Abrupt ROS accumulation (Fig. S11) and dramatically declined FLU gene expression (Fig. 4B) in flu mutant after dark-to-light shifts may be two of the reasons.
Besides oxidative damage, interestingly, no stomata have been observed on the flu cotyledons grown under light/dark cycles (Fig. 5, B–D). Furthermore, apparent cell wall thickening (by quantifying propidium iodide fluorescence signals; Fig. 6, A and B) and increases in pectin (uronic acid) and cellulose contents of cell walls (Fig. 6, C and D) were also observed in the flu mutant grown under light/dark cycles, but not in the other lines. As an integrated effect of stomatal development defect and cell wall thickening, following the dark-to-light shifts, flu stopped growing, whereas flu/abi4 and flu/ex1/ex2 grew normally.
Figure 5.
1O2-signal induces stomatal development defect under light/dark cycles. Cotyledons from 11-day-old WT (Col-0), flu, flu/abi4 (flu/abi4-104) and flu/ex1/ex2 seedlings grown under light/dark cycles or continuous light were collected (A). The cotyledons were fixed and observed with a light microscope. Stomata are false colored in blue for easier identification (B). And the cotyledons were also fixed and observed with a scanning microscope. Red arrows indicate the stomata (B). Stomatal index (C) and stomatal density (D) were counted. Error bars show standard deviations (n = 5). Different lowercase letters indicate significant differences at 0.05 (p < 0.05) levels.
Figure 6.
1O2-signal induces cell wall thickening under light/dark cycles. Cotyledons from 11-day-old WT (Col-0), flu, flu/abi4 (flu/abi4-104) and flu/ex1/ex2 seedlings grown under continuous light (A) or light/dark cycles (B) were collected. The paraffin cross sections of cotyledons were made by staining with safranine and solid green (A and B). Cell walls of the roots were stained with propidium iodide (PI). And the PI fluorescence signals were quantified and shown in the right panel (A and B). Uronic acid content (C) and cellulose content (D) of cell walls were detected. F.W., fresh weight. Error bars show standard deviations (n = 5). Different lowercase letters indicate significant differences at 0.05 (p < 0.05) levels.
Moderate singlet oxygen signals enhance plant tolerance to environmental stresses
When culturing, we noticed that, if the complete darkness was replaced by a dim light, the flu mutant could grow up. Although about 50% higher Pchlide (Figs. S13A) and 120% higher 1O2 (Fig. S13B) accumulated, no apparent growth arrest was observed for the flu mutant under 16-h normal light (100 μmol·m−2·s−1)/8-h dim light (10 μmol·m−2·s−1) cycles (Fig. 7A and Fig. S13, C–E). Nevertheless, stomatal density reduction (by two-thirds; Fig. 7, B, D, and E) and cell wall thickening (doubled; Fig. 7, C, F, and G) and still occurred under normal light/dim light cycles.
Figure 7.
1O2-signal induces cell wall thickening with much less stomata in the flu mutant under normal light/dim light cycles. Seedlings of 11-day-old WT (Col-0), flu, flu/abi4 (flu/abi4-104) and flu/ex1/ex2 seedlings grown under 16-h normal light (100 μmol·m−2·s−1)/8-h dim light (10 μmol·m−2·s−1) cycles (A) were collected. The cotyledons were fixed and observed with a light microscope. Stomata (B) are false colored in blue for easier identification. Cell walls of the roots were stained with propidium iodide (PI). And the PI fluorescence signals were quantified and shown in the right panel (C). Stomatal index (D) and stomatal density (E) were counted. Uronic acid content (F) and cellulose content (G) of cell walls were detected. F.W., fresh weight. Error bars show standard deviations (n = 5). Different lowercase letters indicate significant differences at 0.05 (p < 0.05) levels.
The stress tolerance of 1O2-signaling mutants has been investigated further. The plants were grown under 16-h normal light (100 μmol·m−2·s−1)/8-h dim light (10 μmol·m−2·s−1) cycles for 21 days, and then subjected to environmental stresses. After 3-days osmotic stress with 16% PEG 6000 solution, 14-days drought treatment (withholding water), 3-h high-light stress (1500 μmol of photon m−2 s−1) or 3-days saline treatment (100 mM NaCl), malondialdehyde contents and electrolyte leakage (EL) increased dramatically in WT seedlings and flu/abi4 and flu/ex1/ex2 mutants (Fig. 8). On the contrary, the flu mutant showed a greater resistance to all the four types of stresses (Fig. 8). All the data suggested that moderate 1O2-signals (induced by normal light/dim light cycles) play a positive role in plant’s adaptation to environmental stresses.
Figure 8.
The flu mutant shows greater tolerance to environmental stresses under normal light/dim light cycles. Seedlings of 21-day-old WT (Col-0), flu, flu/abi4 (flu/abi4-104) and flu/ex1/ex2 seedlings grown under 16-h normal light (100 μmol·m−2·s−1)/8-h dim light (10 μmol·m−2·s−1) cycles were subjected to environmental stresses (A). After 3-days osmotic stress with 16% PEG 6000 solution, 14-days drought treatment (withholding water), 3-h high-light stress (1500 μmol of photon m−2 s−1) or 3-days saline treatment (100 mM NaCl), malondialdehyde (MDA) contents (B) and electrolyte leakage (C) were measured. F.W., fresh weight. Error bars show standard deviations (n = 5). Different lowercase letters indicate significant differences at 0.05 (p < 0.05) levels.
Discussion
A recent study indicated that EX1 protein undergoes tryptophan (Trp) 643 oxidation by singlet oxygen (30), leading to the generation of oxidized Trp variants and priming EX1 degradation via a membrane-bound FtsH protease (9). While EX2 also encounters 1O2-dependent Trp530 oxidation and FtsH-dependent turnover, and functions as a negative regulator of the EX1 (30). Future studies should clarify how the 1O2 signals are transducted. All 1O2-signaling factors identified previously (FLU, EX1, and EX2) were plastid proteins, but how the 1O2-signals are transferred from the plastids into the nucleus is still unknown. Some plastid proteins may be secreted and thus a part of the signaling network, such as chloroplast preprotein and amino acid transporters (31, 32).
Alternatively, upon induction by 1O2, EX1 may transiently accumulate and translocate from plastids to the nucleus. Then nuclear EX1 may act as a transcriptional coactivator and interact with the transcription factors WRKY18 and WRKY40 to promote the expression of 1O2-responsive genes (15). In this study, we found that the transcription factor ABI4 may also be involved in the 1O2-signaling transcriptional network, although EX1 may not bind to ABI4 directly. It has been reported that WRKY18, WRKY40, and WRKY60 interacted with the W-box in the promoters of ABI4 and ABI5 genes (33). Therefore, EX1 might modulate the 1O2 signaling pathway by interacting with multiple transcription factors (e.g., WRKY18 and WRKY40) and then operate ABI4 gene indirectly, which requires further studies.
1O2-induced genes include 20 stress-responsive genes, 30 cell wall biosynthesis or organization genes, and two genes related to stomatal development (Data sheet S1). Exponentially upregulation of two negative stomatal development regulators TMM and BCA1 by 1O2 signals (Fig. 3, F and G) might be associated with the stomatal development defect in the flu cotyledons. However, the stomatal development phenotype of flu mutant could not be only attributed to higher expression of TMM and BCA1. Cell wall organization also regulates stomatal development. For example, pectin methylesterification in guard cell walls plays a key role in stomatal dynamics and stomatal response to external stimuli (34). The Arabidopsis sensitive-to-freezing 8 (sfr8) mutant exhibits reduced cell wall fucose levels and larger stomatal pore complexes, and its guard cell lacks a fully developed cuticular ledge. Fucosylation-dependent dimerization of the cell wall pectic domain rhamnogalacturonan-II may be essential for normal cuticular ledge development (35). Detailed cell wall component/structure changes and stomatal development regulations trigged by 1O2 signals need further explorations.
A large number of 1O2-repressed genes have also been identified in the flu mutant specifically (Data sheet S2). Given that genes related to pollen and seed development were significantly suppressed in the flu mutant, the regulation to plant reproductive growth by singlet oxygen signaling would be an interesting future research direction.
The phenotype of stomatal density reduction and cell wall thickening induced by singlet oxygen has important implications for agricultural industry. Declines in stomatal density would enhance plant's tolerance to drought (36, 37, 38, 39, 40), salinity/osmotic stress (38, 39, 40, 41, 42), and heat stress (40, 43, 44) by reducing water transpiration. There are interests in developing crop varieties that require less water yet still maintain the grain yield. This requirement may be fulfilled by altering stomatal development that the crop varieties with fewer stomata may be more conservative in their water use and therefore more tolerant to drought, salinity, or heat stress (45). Plant cell walls are the first physical barrier against pathogenic invasion. Under biotic stresses, plants thicken their cell walls to strengthen them and restrain pathogenic infections (46, 47, 48). And cell wall thickening would enhance plant's tolerance to drought (48, 49), salinity/osmotic stress (50, 51), metal stress (52, 53), and especially waterlogging stress (49, 54, 55) by improving mechanical strength and facilitating water transport. Tailoring the content and structure of plant cell walls could modulate growth, abiotic/biotic stress resistance, biomass yield, and other important agronomic traits (55). Illuminated by this study, all these goals of cell wall thickening and stomatal reduction may be simply achieved by knocking out the FLU gene, accompanying with appropriate light cycles (periodic alternation of light intensity). Future experiments with FLU-null plants are warranted.
Experimental procedures
Plant materials and growth conditions
Arabidopsis mutants were in the Col-0 background. The flu mutant and flu,ex1,ex2 triple mutant were provided by Prof. Chanhong Kim (Chinese Academy of Sciences, China). Seeds of 35S:EX1-GFP transgenic plants were a gift from Prof. Rongcheng Lin (Institute of Botany, Chinese Academy of Sciences). Seeds of abi4-102 mutant (CS3837; W to STOP at codon 80), abi4-104 mutant (CS3839; single nucleotide substitution at codon 69 leading to missense E to K) and abi4-2 mutant (SALK_080095; T-DNA insertion at condon 152) were obtained from the Arabidopsis Biological Resource Center at Ohio State University (Columbus, OH, USA). flu,abi4-2 double mutant and flu,abi4-104 double mutant were generated by performing genetic crossing respectively, and the progenies were analyzed by PCR. The 35S:ABI4-GFP construct (56) was crossed into either flu,abi4-2 or flu,abi4-104 double mutant background. After vernalization at 4 °C for 3 days, the seeds were sterilized in 75% (v/v) alcohol and 0.1% (v/v) HgCl2, and then grown on 1/2 MS medium with 1% sucrose or on soils under continuous light (100 μmol·m−2·s−1), 16-h light (100 μmol·m−2·s−1)/8-h dark cycles or 16-h normal light (100 μmol·m−2·s−1)/8-h dim light (10 μmol·m−2·s−1) cycles at 21 °C ± 1 deg. C.
Stress treatments
WT plants (Col-0), flu mutant, flu,abi4-104 double mutant, and flu,ex1,ex2 triple mutant were grown under 16-h normal light (100 μmol·m−2·s−1)/8-h dim light (10 μmol·m−2·s−1) cycles for 21 days, and then subjected to four types of environmental stresses. Osmotic stress was imposed by submerging roots into half strength Hoagland solution containing 16% (w/v) PEG-6000 with an osmotic potential of −0.5 MPa for 3 days. Drought treatment was performed by withholding water for 14 days. For high-light stress, the seedlings were exposed to 1500 μmol of photon m−2 s−1 for 3 h. For salt stress, plants were treated with 100 mM NaCl for 3 days.
Analysis of flowering time
For seedlings grown in 1/2 MS media, we scored the number of rosette leaves at the stage of bolting when stems were about 3 mm (57). We also scored days to bolting. 20 rosettes for each sample were counted. The experiments were repeated three times.
Measurement and visualization of protochlorophyllide
Protochlorophyllide (Pchlide) were extracted from 7-days etiolated seedlings (0.2 g seedlings to 0.5 ml alkaline acetone) as described by Lay et al. (58). Etiolated cotyledons of 5-day-old seedlings were exposed to blue light and the emitted Pchlide fluorescence was recorded using the Leica M165C/FC fluorescent microscope (Leica Microsystems) (9).
Pchlide and chlorophylls were also extracted with 80% acetone from the seedlings grown under continuous light, 16 h/8 h light/dark cycles or 16-h normal light/8-h dim light cycles for 7 days (at the time-points of 0-h and 1-h illumination after the seventh cycle). Pchlide was quantified by using the standard chemical, which was purchased from Frontier Scientific (Logan Comp). Chlorophyll (Chl) content was measured according to the method of Lichtenthaler and Wellburn (59).
ROS visualization and cell-death staining
Superoxide and H2O2 levels were visually detected with nitro blue tetrazolium and 3,3-diaminobenzidine, respectively, as described previously (60). Seven-day-old seedlings were excised at the base with a razor blade and supplied through the cut ends with nitro blue tetrazolium (0.5 mg ml−1) or 3,3-diaminobenzidine (2 mg ml−1) solutions for 8 h. Leaves were then decolorized in boiling ethanol (95%) for 20 min. At least three leaves were used for each treatment.
The singlet oxygen level was determined with the fluorescence probe Singlet Oxygen Sensor Green Reagent (Molecular Probes Inc). Singlet oxygen generation ratios were determined for 7-day-old etiolated seedlings after transfer to normal light (100 μmol photons m−2 s−1) (61), or 7-day-old seedlings grown under 16-h normal light/8-h dim light cycles and then transferred to normal light for 1 h.
The photobleaching (cell death) was visually detected by trypan-blue staining (1.25 mg mL−1) (62).
Measurements of malonaldehyde and electrolyte leakage
The extraction of malondialdehyde in 7-day-old seedlings was performed using thiobarbituric acid solution according to the method of Chen et al. (63) After centrifugation, the absorbance of the supernatant was monitored at 532 nm and corrected for nonspecific turbidity by subtracting the absorbance at 600 nm. EL was determined by using a conductivity meter (DDSJ-308A, Shanghai Precision Instruments Co Ltd) according to the method of Chen et al. (63) The relative EL was obtained according to the ratio of the initial conductivity to the absolute conductivity.
Pectin and cellulose content determination
Extraction of cell wall components from 11-days-old seedlings grown under continuous light, 16 h/8 h light/dark cycles or 16-h normal light/8-h dim light cycles was carried out according to Xiao et al. (64) and Du et al. (65). Pectin was extracted from the cell wall extract by boiling 2 mg of the extract with 1 ml ultrapure water three times, 1 h each. Each boiling was followed by centrifugation for 3 min at 4500g and collection of the supernatant into one tube. Uronic acid content was measured as described by Xiao et al. (64) and Du et al. (65), using galacturonic acid as reference. Crystalline cellulose content was measured following a procedure described by Xiao et al. (64) and Du et al. (65).
Microscopy analysis of root and cotyledon cell walls
To visualize root cell walls, roots of 11-day-old seedlings grown under continuous light, 16 h/8 h light/dark cycles or 16-h normal light/8-h dim light cycles were stained with 10 μg/ml propidium iodide for 20 min at room temperature (66). Signals of the stained roots were then excited by light with wave length of 595 nm and imaged under an Olympus FluoView FV1000 confocal microscope.
To visualize cotyledon cell walls, the paraffin cross sections of cotyledons were made by staining with safranine and solid green. The slices were scanned and imaged under the stereo microscope (Leica Microsystems M165C/FC).
Stomatal count
Seedlings of 11-day-old seedlings grown under continuous light or under continuous light, 16 h/8 h light/dark cycles or 16-h normal light/8-h dim light cycles were collected in 70% ethanol, cleared overnight at room temperature, and then stored in Hoyer’s solution and observed with a light microscope (Leica Microsystems M165C). Stomatal density is the number of stomata per unit of area. To count the stomatal density, five square areas of 0.5 mm2 adaxial epidermises were examined for each cotyledon, and the amounts were averaged to yield a predicted stomatal density. Cotyledons from at least eight different plants were selected for all genotypes. The stomatal index was calculated using the following formula: stomatal index = (number of stomata)/(total epidermal cells) × 100% (67).
A scanning microscope (GeminiSEM300, Carl Zeiss) was also applied to determine the surface characteristics of adaxial epidermises and stomata of cotyledons. The samples were prefixed overnight at 4 °C in 3% glutaraldehyde and 0.1 M sodium cacodylate buffer (pH 6.9).
RNA-seq
WT (Col-0), flu, flu,abi4, and flu,ex1,ex2 mutant plants were grown for 21 days under continuous light, shifted to the dark for 8 h, and reexposed to light for 30 min. Equal quantities of RNA from three independent biological replicates of each plant were pooled for complementary DNA (cDNA) library construction. Oligo-(dT) magnetic beads were used to isolate poly-(A) mRNA from total RNA, and the mRNA was fragmented in a fragmentation buffer. Using these short fragments (about 200 bp in length) as templates, random hexamer-primers were used in first-strand cDNA synthesis. Second-strand cDNA was synthesized using the appropriate buffer, dNTPs, RNaseH, and DNA polymerase I. Short double-stranded cDNA fragments were purified with a Qiaquick PCR extraction kit (Qiagen), resolved with an buffer EB (Qiagen) for end-reparation and for adding poly (A), and then ligated to sequencing adapters. After purification via agarose gel electrophoresis, suitable fragments were enriched by PCR amplification. Finally, the libraries were sequenced on an Illumina HiSeq4000 platform by the Majorbio Comp, following the manufacturer’s protocols. The raw data have been deposited in the NCBI Sequence Read Archive (accession number: SRP133889).
Raw reads from the sequencing machine were generated by base calling and saved in FASTQ format. Clean reads were generated by removing reads with adapters, reads where the number of unknown bases was more than 10%, and low-quality reads (the percentage of the low-quality bases with which value ≤5 was more than 50% in one read). Three biological replicates of each sample, showing correlation coefficient value of ≥0.95 were considered for subsequent gene expression analysis.
Twelve RNA libraries from the four plants were analyzed by using the RNA-Seq approach and comparative DEG profiling analysis (68). Approximately 43.64∼52.61 million raw RNA-Seq reads were produced from each individual sample. After filtering the dirty reads, 43.25∼52.10 million clean sequences were acquired and 95.2∼96.4% sequences were mapped to the reference genome of Arabidopsis thaliana. On the basis of these mapped reads, gene expression levels were calculated by using the reads per kb per million reads (RPKM) method (68).
Real-time quantitative PCR
WT (Col-0), flu, flu,abi4 and flu,ex1,ex2 mutants and 35S:ABI4-GFP/flu/abi4-2 complemented plants were grown for 21 days under continuous light, shifted to the dark for 8 h, and reexposed to light for 30 min. ABI4, XCP1, AED3, F8H, LRX2, TMM, BCA1, FLU, PPCK1, SUS1, M17, NAS3, and CYP710A1 expression levels in above samples were detected by quantitative real-time PCR analysis. The cDNA was amplified by using SYBR Premix Ex Taq (TaKaRa). The Ct (threshold cycle), defined as the PCR cycle at which a statistically significant increase of reporter fluorescence was first detected, was used as a measure for the starting copy numbers of the target gene (57). Three technical replicates were performed for each experiment. ACTIN1 gene (At2g37620) was used as internal controls. The expression levels of the WT seedlings in darkness are normalized to 100%. All primers are shown in Table S1.
Antibody generation and Western blotting
Five epitopes of ABI4 protein were synthesized chemically. The polyclonal antibodies against these epitopes were generated by inoculation of mice and purified by Abmart Comp. The immune specificity of each antibody was verified by Western blotting. According to the Western blots (Fig. S4, A and B), the antibody against epitope#11 was selected for the following experiments.
Arabidopsis nuclear extracts were prepared by following the ChIP method (69). Briefly, nuclei were purified from total protein extracts with Percoll extraction buffer (95% V/V Percoll, 0.25 M sucrose, 10 mM Tris–HCl, pH 8.0 10 mM MgCl2, 5 mM β-mercaptoethanol, 5 mM NaF, 1× protease inhibitor cocktail) and washed twice with nuclei resuspension buffer (10% glycerol, 50 mM Tris–HCl, pH 8.0 5 mM MgCl2, 10 mM β-mercaptoethanol, 5 mM NaF, 1× protease inhibitor cocktail) by 10 min of centrifugation at 12,000g. Then nuclei were lysised in nuclei lysis buffer [50 mM Tris–HCl, pH 8.0 10 mM EDTA, 1% SDS, 5 mM NaF, 1× protease inhibitor cocktail].
SDS-PAGE and Western blotting analysis of the extracts was processed according to the method as described previously (16). For Western blots, 50 μg nuclear proteins were loaded for each sample.
ChIP-quantitative PCR assay
The chromatin samples for ChIP experiments were obtained following the methods by Saleh et al. (69). The plants seedlings were first cross-linked by formaldehyde, and the purified cross-linked nuclei were then sonicated to shear the chromatin into suitably sized fragments. The antibody that specifically recognizes the recombinant ABI4 was used to immuno-precipitate DNA/protein complexes from the chromatin preparation. The DNA in the precipitated complexes was recovered and analyzed by qPCR methods. The chosen primer combinations (Table S1) could amplify fragments of 90 to 200 bp within the promoters of ZP, WRKY33, WRKY46, DRP and ACC6 genes. To ensure the reliability of ChIP data, the input sample and no antibody (NoAB) control sample were analyzed with each primer set. Rabbit control IgG was purchased from Abcam Comp. The results were quantified with a calibration line made with DNA isolated from cross-linked and sonicated chromatin (69).
Co-immunoprecipitation (Co-IP) assay
35S:EX1-GFP transgenic plants were grown for 5 days in the dark before transfer to 1/2 MS medium containing 100 μM RB and incubation in white light (100 μmol·m−2·s−1) for 0 or 30 min. For the possible interaction between ABI4 and EX1, nuclear proteins were extracted from the seedlings in Co-IP buffer (100 mM NaCl, 10 mM Tris–HCl, 10 mM MgCl2, 1 mM EDTA, 5% [v/v] glycerol, 1% [v/v] Triton X-100, 0.4% [v/v] NP-40 and 1 × complete protease inhibitor cocktail, pH 7.5). Then the proteins were immuno-precipitated with 20 μl anti-GFP–agarose beads or anti-ABI4–agarose beads by gentle shaking for 1 h at 4 °C in the dark (15). The pellets were washed with wash buffer four times before subjecting the immuno-precipitates to SDS-PAGE separation followed by immuno-blotting with mouse anti-GFP antibody (Abcam Comp.) or the anti-ABI4 antibody.
Statistics analysis
All experiments were repeated three to five times, and average values were presented and the standard deviations (n ≥ 3) were shown. Student’s t test was used for comparison between different treatments. A difference was considered to be statistically significant when p <0.05. Statistically significant difference among plant strains were determined by one way ANOVA (Duncan’s multiple range test; p < 0.05).
Data availability
Experimental raw data are available to be shared upon request to the corresponding authors.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank Prof. Rongcheng Lin (Chinese Academy of Sciences) for the seeds of 35S:EX1-GFP transgenic plants and Prof. Chanhong Kim (Chinese Academy of Sciences) for the seeds of flu mutant and flu,ex1,ex2 triple mutant and critical comments. And we thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author contributions
Z.-W. Z., Y.-F. F., X.-Y. Y., M. Y., X.-J. Z., X.-F. L., M.-Y. Z., L.-B. X., K. S., S. R., C. R., F. W., L.-Y. F., J.-B. D., C.-Q. W., X.-S. G., Y. E. C., Y.-Y. Z., Y. L., Q. T., T. L., X.-Y. T., J. Z., G.-D. C., and S. Y. data analysis; Z.-W. Z., Y.-F. F., X.-Y. Y., M. Y., X.-J. Z., X.-F. L., M.-Y. Z., L.-B. X., K. S., S. R., C. R., F. W., L.-Y. F., J.-B. D., C.-Q. W., X.-S. G., Y.-E. C., Y.-Y. Z., Y. L., Q. T., T. L., X.-Y. T., J. Z., G.-D. C., and S. Y. writing–review and editing; Z.-W. Z., Y.-F. F., X.-Y. Y., M. Y., X.-J. Z., X.-F. L., M.-Y. Z., L.-B. X., and K. S. investigation; Z.-W. Z., Y.-F. F., X.-Y. Y., M. Y., and X.-J. Z. methodology; Z. W. Z. and S. Y. conceptualization; G.-D. C. and S. Y. funding acquisition; S. Y. writing–original draft.
Funding and additional information
This work was supported by the National Natural Science Foundation of China (31971944) to G.-D. C., Sichuan Province Science Foundation for Distinguished Young Scholars (2022JDJQ0006) to G.-D. C. and the National Natural Science Foundation of China (31770322) to S. Y.
Reviewed by members of the JBC Editorial Board. Edited by Joseph Jez
Contributor Information
Guang-Deng Chen, Email: gdchen@sicau.edu.cn.
Shu Yuan, Email: roundtree318@hotmail.com.
Supporting information
References
- 1.Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 2.Kim C. ROS-driven oxidative modification: its impact on chloroplasts-nucleus communication. Front. Plant Sci. 2020;10:1729. doi: 10.3389/fpls.2019.01729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer B.B., Hideg É., Krieger-Liszkay A. Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxid. Redox Signal. 2013;18:2145–2162. doi: 10.1089/ars.2012.5124. [DOI] [PubMed] [Google Scholar]
- 4.Triantaphylidès C., Krischke M., Hoeberichts F.A., Ksas B., Gresser G., Havaux M., et al. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 2008;148:960–968. doi: 10.1104/pp.108.125690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meskauskiene R., Nater M., Goslings D., Kessler F., op den Camp R., Apel K. FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12826–12831. doi: 10.1073/pnas.221252798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner D., Przybyla D., Op den Camp R., Kim C., Landgraf F., Lee K.P., et al. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science. 2004;306:1183–1185. doi: 10.1126/science.1103178. [DOI] [PubMed] [Google Scholar]
- 7.Lee K.P., Kim C., Landgraf F., Apel K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10270–10275. doi: 10.1073/pnas.0702061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L., Kim C., Xu X., Piskurewicz U., Dogra V., Singh S., et al. Singlet oxygen- and EXECUTER1-mediated signaling is initiated in grana margins and depends on the protease FtsH2. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E3792–E3800. doi: 10.1073/pnas.1603562113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dogra V., Li M., Singh S., Li M., Kim C. Oxidative post-translational modification of EXECUTER1 is required for singlet oxygen sensing in plastids. Nat. Commun. 2019;10:2834. doi: 10.1038/s41467-019-10760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramel F., Birtic S., Ginies C., Soubigou-Taconnat L., Triantaphylidès C., Havaux M. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5535–5540. doi: 10.1073/pnas.1115982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koussevitzky S., Nott A., Mockler T.C., Hong F., Sachetto-Martins G., Surpin M., et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;316:715–719. [PubMed] [Google Scholar]
- 12.Kim C., Apel K. 1O2-mediated and EXECUTER-dependent retrograde plastid-to-nucleus signaling in norflurazon-treated seedlings of Arabidopsis thaliana. Mol. Plant. 2013;6:1580–1591. doi: 10.1093/mp/sst020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kacprzak S.M., Mochizuki N., Naranjo B., Xu D., Leister D., Kleine T., et al. Plastid-to-nucleus retrograde signalling during chloroplast biogenesis does not require ABI4. Plant Physiol. 2019;179:18–23. doi: 10.1104/pp.18.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh E., Cohen D., Brandis A., Fluhr R. Attenuation of cytosolic translation by RNA oxidation is involved in singlet oxygen-mediated transcriptomic responses. Plant Cell Environ. 2021;44:3597–3615. doi: 10.1111/pce.14162. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Liu H., Ma T., Li J., Yuan J., Xu Y.C., et al. Arabidopsis EXECUTER1 interacts with WRKY transcription factors to mediate plastid-to-nucleus singlet oxygen signaling. Plant Cell. 2023;35:827–851. doi: 10.1093/plcell/koac330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z.W., Feng L.Y., Cheng J., Tang H., Xu F., Zhu F., et al. The roles of two transcription factors, ABI4 and CBFA, in ABA and plastid signalling and stress responses. Plant Mol. Biol. 2013;83:445–458. doi: 10.1007/s11103-013-0102-8. [DOI] [PubMed] [Google Scholar]
- 17.op den Camp R.G., Przybyla D., Ochsenbein C., Laloi C., Kim C., Danon A., et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sibéril Y., Doireau P., Gantet P. Plant bZIP G-box binding factors. Modular structure and activation mechanisms. Eur. J. Biochem. 2001;268:5655–5666. doi: 10.1046/j.0014-2956.2001.02552.x. [DOI] [PubMed] [Google Scholar]
- 19.Funk V., Kositsup B., Zhao C., Beers E.P. The Arabidopsis xylem peptidase XCP1 is a tracheary element vacuolar protein that may be a papain ortholog. Plant Physiol. 2002;128:84–94. [PMC free article] [PubMed] [Google Scholar]
- 20.Breitenbach H.H., Wenig M., Wittek F., Jordá L., Maldonado-Alconada A.M., Sarioglu H., et al. Contrasting roles of the apoplastic aspartyl protease apoplastic, enhanced disease susceptibility1-dependent1 and legume lectin-like protein1 in Arabidopsis systemic acquired resistance. Plant Physiol. 2014;165:791–809. doi: 10.1104/pp.114.239665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong R., Peña M.J., Zhou G.K., Nairn C.J., Wood-Jones A., Richardson E.A., et al. Arabidopsis fragile fiber8, which encodes a putative glucuronyltransferase, is essential for normal secondary wall synthesis. Plant Cell. 2005;17:3390–3408. doi: 10.1105/tpc.105.035501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumberger N., Steiner M., Ryser U., Keller B., Ringli C. Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J. 2003;35:71–81. doi: 10.1046/j.1365-313x.2003.01784.x. [DOI] [PubMed] [Google Scholar]
- 23.Yang M., Sack F.D. The too many mouths and four lips mutations affect stomatal production in Arabidopsis. Plant Cell. 1995;7:2227–2239. doi: 10.1105/tpc.7.12.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engineer C.B., Ghassemian M., Anderson J.C., Peck S.C., Hu H., Schroeder J.I. Carbonic anhydrases, EPF2 and a novel protease mediate CO2 control of stomatal development. Nature. 2014;513:246–250. doi: 10.1038/nature13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartwell J., Gill A., Nimmo G.A., Wilkins M.B., Jenkins G.I., Nimmo H.G. Phosphoenolpyruvate carboxylase kinase is a novel protein kinase regulated at the level of expression. Plant J. 1999;20:333–342. doi: 10.1046/j.1365-313x.1999.t01-1-00609.x. [DOI] [PubMed] [Google Scholar]
- 26.Déjardin A., Sokolov L.N., Kleczkowski L.A. Sugar/osmoticum levels modulate differential abscisic acid-independent expression of two stress-responsive sucrose synthase genes in Arabidopsis. Biochem. J. 1999;344:503–509. [PMC free article] [PubMed] [Google Scholar]
- 27.Cadman C.S., Toorop P.E., Hilhorst H.W., Finch-Savage W.E. Gene expression profiles of Arabidopsis Cvi seeds during dormancy cycling indicate a common underlying dormancy control mechanism. Plant J. 2006;46:805–822. doi: 10.1111/j.1365-313X.2006.02738.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Zhang W.Z., Song L.F., Zou J.J., Su Z., Wu W.H. Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiol. 2008;148:1201–1211. doi: 10.1104/pp.108.126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morikawa T., Mizutani M., Ohta D. Cytochrome P450 subfamily CYP710A genes encode sterol C-22 desaturase in plants. Biochem. Soc. Trans. 2006;34:1202–1205. doi: 10.1042/BST0341202. [DOI] [PubMed] [Google Scholar]
- 30.Dogra V., Singh R.M., Li M., Li M., Singh S., Kim C. EXECUTER2 modulates the EXECUTER1 signalosome through its singlet oxygen-dependent oxidation. Mol. Plant. 2022;15:438–453. doi: 10.1016/j.molp.2021.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Rossig C., Reinbothe C., Gray J., Valdes O., von Wettstein D., Reinbothe S. New functions of the chloroplast Preprotein and Amino acid Transporter (PRAT) family members in protein import. Plant Signal. Behav. 2014;9 doi: 10.4161/psb.27693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reinbothe S., Rossig C., Gray J., Rustgi S., von Wettstein D., Reinbothe C., et al. tRNA-dependent import of a transit sequence-less aminoacyl-tRNA synthetase (LeuRS2) into the mitochondria of Arabidopsis. Int. J. Mol. Sci. 2021;22:3808. doi: 10.3390/ijms22083808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z.Q., Yan L., Wu Z., Mei C., Lu K., Yu Y.T., et al. Cooperation of three WRKY-domain transcription factors WRKY18, WRKY40, and WRKY60 in repressing two ABA-responsive genes ABI4 and ABI5 in Arabidopsis. J. Exp. Bot. 2012;63:6371–6392. doi: 10.1093/jxb/ers293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X., Guo H., Xiao C., Yan Z., Ning N., Chen G., et al. Pectin methylesterase inhibitor18 functions in stomatal dynamics and stomatal dimension. Plant Physiol. 2023;192:1603–1620. doi: 10.1093/plphys/kiad145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panter P.E., Seifert J., Dale M., Pridgeon A.J., Hulme R., Ramsay N., et al. Cell wall fucosylation in Arabidopsis influences control of leaf water loss and alters stomatal development and mechanical properties. J. Exp. Bot. 2023;74:2680–2691. doi: 10.1093/jxb/erad039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masle J., Gilmore S.R., Farquhar G.D. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature. 2005;436:866–870. doi: 10.1038/nature03835. [DOI] [PubMed] [Google Scholar]
- 37.Feng X., Xiong J., Zhang W., Guan H., Zheng D., Xiong H., et al. ZmLBD5, a class-II LBD gene, negatively regulates drought tolerance by impairing abscisic acid synthesis. Plant J. 2022;112:1364–1376. doi: 10.1111/tpj.16015. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y., Karthikeyan A., Yin J., Jin T., Ren R., Fang F., et al. The E3 ligase GmPUB21 negatively regulates drought and salinity stress response in soybean. Int. J. Mol. Sci. 2022;23:6893. doi: 10.3390/ijms23136893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra S., Sahu G., Shaw B.P. Insight into the cellular and physiological regulatory modulations of Class-I TCP9 to enhance drought and salinity stress tolerance in cowpea. Physiol. Plant. 2022;174 doi: 10.1111/ppl.13542. [DOI] [PubMed] [Google Scholar]
- 40.Djemal R., Khoudi H. The barley SHN1-type transcription factor HvSHN1 imparts heat, drought and salt tolerances in transgenic tobacco. Plant Physiol. Biochem. 2021;164:44–53. doi: 10.1016/j.plaphy.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 41.Chen Q., Guo L., Yuan Y., Hu S., Guo F., Zhao H., et al. Ectopic overexpression of histone H3K4 methyltransferase CsSDG36 from tea plant decreases hyperosmotic stress tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2021;22:5064. doi: 10.3390/ijms22105064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao R., Ding J., Li C., Zhu X., Guo W., Zhu M. Evaluating and screening of agro-physiological indices for salinity stress tolerance in wheat at the seedling stage. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.646175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen H., Zhong X., Zhao F., Wang Y., Yan B., Li Q., et al. Overexpression of receptor-like kinase ERECTA improves thermotolerance in rice and tomato. Nat. Biotechnol. 2015;33:996–1003. doi: 10.1038/nbt.3321. [DOI] [PubMed] [Google Scholar]
- 44.Pérez-Bueno M.L., Illescas-Miranda J., Martín-Forero A.F., de Marcos A., Barón M., Fenoll C., et al. An extremely low stomatal density mutant overcomes cooling limitations at supra-optimal temperature by adjusting stomatal size and leaf thickness. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.919299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buckley C.R., Caine R.S., Gray J.E. Pores for thought: can genetic manipulation of stomatal density protect future rice yields? Front. Plant Sci. 2020;10:1783. doi: 10.3389/fpls.2019.01783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie W., Ke Y., Cao J., Wang S., Yuan M. Knock out of transcription factor WRKY53 thickens sclerenchyma cell walls, confers bacterial blight resistance. Plant Physiol. 2021;187:1746–1761. doi: 10.1093/plphys/kiab400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozieł E., Otulak-Kozieł K., Bujarski J.J. Plant cell wall as a key player during resistant and susceptible plant-virus interactions. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.656809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khasin M., Bernhardson L.F., O'Neill P.M., Palmer N.A., Scully E.D., Sattler S.E., et al. Pathogen and drought stress affect cell wall and phytohormone signaling to shape host responses in a sorghum COMT bmr12 mutant. BMC Plant Biol. 2021;21:391. doi: 10.1186/s12870-021-03149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren Z., Zhang D., Cao L., Zhang W., Zheng H., Liu Z., et al. Functions and regulatory framework of ZmNST3 in maize under lodging and drought stress. Plant Cell Environ. 2020;43:2272–2286. doi: 10.1111/pce.13829. [DOI] [PubMed] [Google Scholar]
- 50.Tang Y., Wang M., Cao L., Dang Z., Ruan N., Wang Y., et al. OsUGE3-mediated cell wall polysaccharides accumulation improves biomass production, mechanical strength, and salt tolerance. Plant Cell Environ. 2022;45:2492–2507. doi: 10.1111/pce.14359. [DOI] [PubMed] [Google Scholar]
- 51.Gigli-Bisceglia N., van Zelm E., Huo W., Lamers J., Testerink C. Arabidopsis root responses to salinity depend on pectin modification and cell wall sensing. Development. 2022;149 doi: 10.1242/dev.200363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tao Q., Jupa R., Liu Y., Luo J., Li J., Kováč J., et al. Abscisic acid-mediated modifications of radial apoplastic transport pathway play a key role in cadmium uptake in hyperaccumulator Sedum alfredii. Plant Cell Environ. 2019;42:1425–1440. doi: 10.1111/pce.13506. [DOI] [PubMed] [Google Scholar]
- 53.Tao Q., Li M., Xu Q., Kováč J., Yuan S., Li B., et al. Radial transport difference mediated by root endodermal barriers contributes to differential cadmium accumulation between japonica and indica subspecies of rice (Oryza sativa L.) J. Hazard. Mater. 2022;425 doi: 10.1016/j.jhazmat.2021.128008. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen T.N., Son S., Jordan M.C., Levin D.B., Ayele B.T. Lignin biosynthesis in wheat (Triticum aestivum L.): its response to waterlogging and association with hormonal levels. BMC Plant Biol. 2016;16:28. doi: 10.1186/s12870-016-0717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voiniciuc C. It's time to go glyco in cell wall bioengineering. Curr. Opin. Plant Biol. 2023;71 doi: 10.1016/j.pbi.2022.102313. [DOI] [PubMed] [Google Scholar]
- 56.Luo X., Dai Y., Zheng C., Yang Y., Chen W., Wang Q., et al. The ABI4-RbohD/VTC2 regulatory module promotes reactive oxygen species (ROS) accumulation to decrease seed germination under salinity stress. New Phytol. 2021;229:950–962. doi: 10.1111/nph.16921. [DOI] [PubMed] [Google Scholar]
- 57.Yuan S., Zhang Z.W., Zheng C., Zhao Z.Y., Wang Y., Feng L.Y., et al. Arabidopsis cryptochrome 1 functions in nitrogen regulation of flowering. Proc. Natl. Acad. Sci. U. S. A. 2016;113:7661–7666. doi: 10.1073/pnas.1602004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lay P.L., Eullaffroy P., Juneau P., Popovic R. Evidence of chlorophyll synthesis pathway alteration in desiccated barley leaves. Plant Cell Physiol. 2000;41:565–570. doi: 10.1093/pcp/41.5.565. [DOI] [PubMed] [Google Scholar]
- 59.Lichtenthaler H.K., Wellburn A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983;11:591–593. [Google Scholar]
- 60.Yang Y.N., Qi M., Mei C.S. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J. 2004;40:909–919. doi: 10.1111/j.1365-313X.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- 61.Flors C., Fryer M.J., Waring J., Reeder B., Bechtold U., Mullineaux P.M., et al. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, singlet oxygen sensor green. J. Exp. Bot. 2006;57:1725–1734. doi: 10.1093/jxb/erj181. [DOI] [PubMed] [Google Scholar]
- 62.Koch E., Slusarenko A. Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell. 1990;2:437–445. doi: 10.1105/tpc.2.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y.E., Yuan S., Lezhneva L., Meurer J., Schwenkert S., Mamedov F., et al. The low molecular mass photosystem II protein PsbTn is important for light acclimation. Plant Physiol. 2019;179:1739–1753. doi: 10.1104/pp.18.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao C., Zhang T., Zheng Y., Cosgrove D.J., Anderson C.T. Xyloglucan deficiency disrupts microtubule stability and cellulose biosynthesis in Arabidopsis, altering cell growth and morphogenesis. Plant Physiol. 2016;170:234–249. doi: 10.1104/pp.15.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Du J., Kirui A., Huang S., Wang L., Barnes W.J., Kiemle S.N., et al. Mutations in the pectin methyltransferase QUASIMODO2 influence cellulose biosynthesis and wall integrity in Arabidopsis. Plant Cell. 2020;32:3576–3597. doi: 10.1105/tpc.20.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du J., Yin H., Zhang S., Wei Z., Zhao B., Zhang J., et al. Somatic embryogenesis receptor kinases control root development mainly via brassinosteroid-independent actions in Arabidopsis thaliana. J. Integr. Plant Biol. 2012;54:388–399. doi: 10.1111/j.1744-7909.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 67.Han X., Hu Y., Zhang G., Jiang Y., Chen X., Yu D. Jasmonate negatively regulates stomatal development in Arabidopsis cotyledons. Plant Physiol. 2018;176:2871–2885. doi: 10.1104/pp.17.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mortazavi A., Williams B.A., Mccue K., Schaeffer L., Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 69.Saleh A., Alvarez-Venegas R., Avramova Z. An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat. Protoc. 2008;3:1018–1025. doi: 10.1038/nprot.2008.66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experimental raw data are available to be shared upon request to the corresponding authors.