Abstract
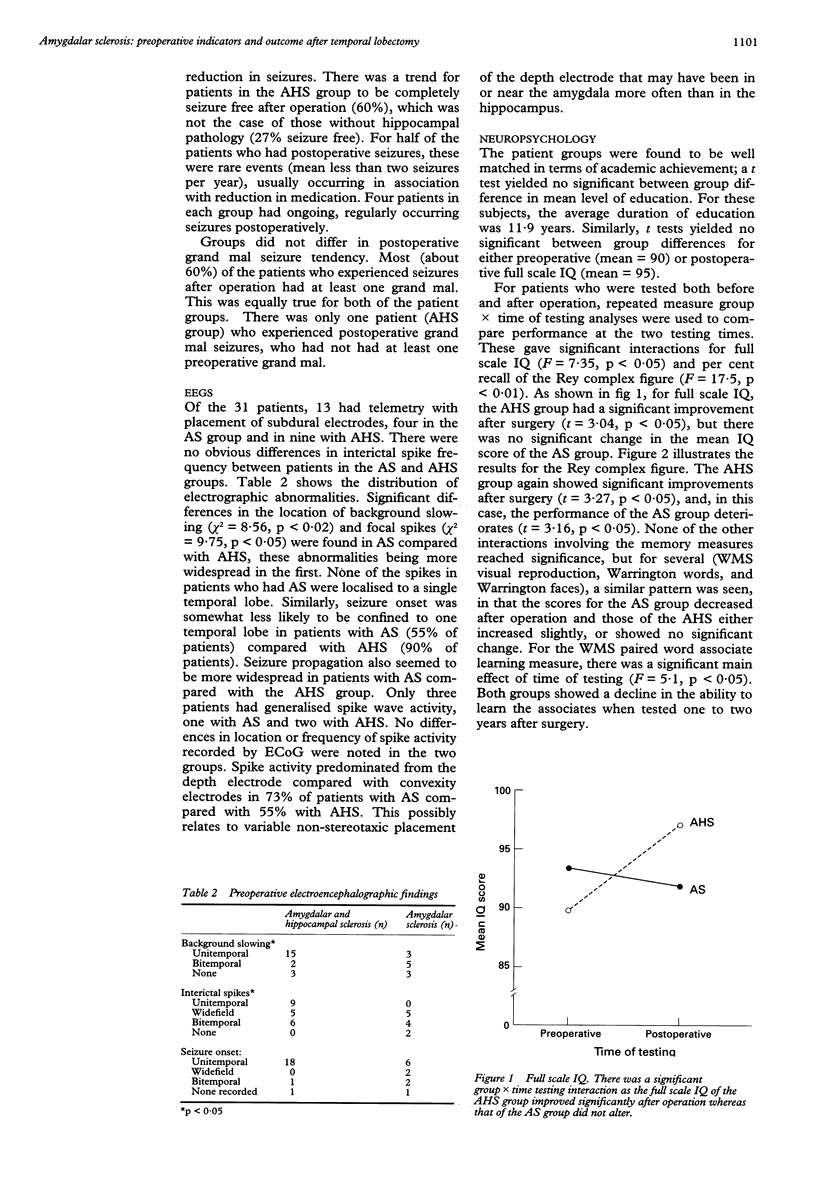
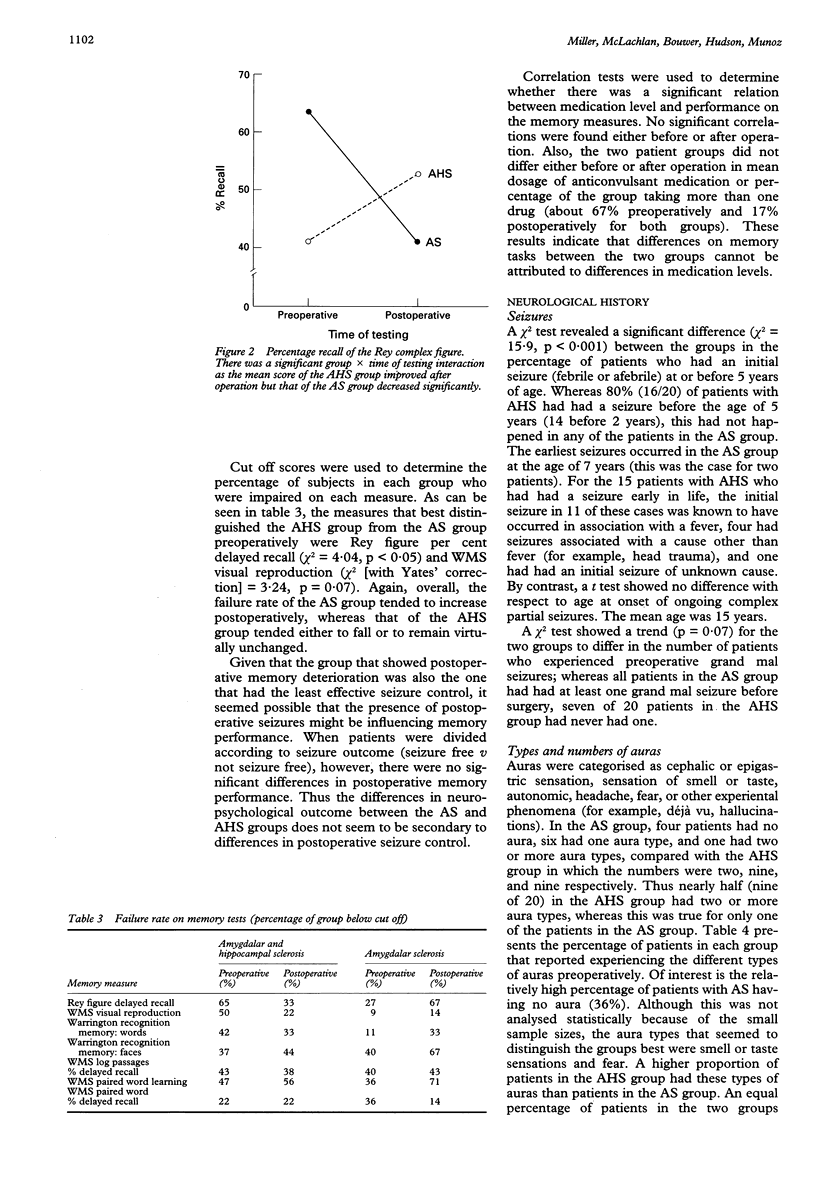
Isolated amygdalar sclerosis (AS) in the presence of an intact hippocampus has been described in a subset of patients who have undergone a temporal lobectomy for the relief of seizures. Clinical observation suggested that these patients might be distinguishable, before and after operation, from those with typical mesial temporal sclerosis, which implies combined amygdalar and hippocampal sclerosis (AHS). From a three year series, all 11 patients classified as having AS were included in this study. These patients were compared with a group of 20 randomly chosen patients with AHS. The groups were found to be well matched in duration of ongoing seizures, full scale IQ, and duration of follow up (mean 19 months). Compared with patients with AHS, patients in the AS group were less likely to have had a seizure in early childhood, a variety of auras, EEG abnormalities localised to one temporal lobe, or an abnormal MRI before operation. They also performed better on preoperative memory tests. At follow up, patients in the AS group were less likely to be seizure free and more likely to have a deterioration in memory after undergoing anterior temporal lobectomy, including part of the hippocampus. The results show that there are preoperative indicators of mesial temporal pathology that are also of prognostic importance given the differences in outcome between the two pathological groups.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkovic S. F., Andermann F., Olivier A., Ethier R., Melanson D., Robitaille Y., Kuzniecky R., Peters T., Feindel W. Hippocampal sclerosis in temporal lobe epilepsy demonstrated by magnetic resonance imaging. Ann Neurol. 1991 Feb;29(2):175–182. doi: 10.1002/ana.410290210. [DOI] [PubMed] [Google Scholar]
- Bronen R. A., Cheung G., Charles J. T., Kim J. H., Spencer D. D., Spencer S. S., Sze G., McCarthy G. Imaging findings in hippocampal sclerosis: correlation with pathology. AJNR Am J Neuroradiol. 1991 Sep-Oct;12(5):933–940. [PMC free article] [PubMed] [Google Scholar]
- Duncan J. S., Sagar H. J. Seizure characteristics, pathology, and outcome after temporal lobectomy. Neurology. 1987 Mar;37(3):405–409. doi: 10.1212/wnl.37.3.405. [DOI] [PubMed] [Google Scholar]
- FALCONER M. A., CAVANAGH J. B. Clinico-pathological considerations of temporal lobe epilepsy due to small focal lesions. A study of cases submitted to operation. Brain. 1959 Dec;82:483–504. doi: 10.1093/brain/82.4.483. [DOI] [PubMed] [Google Scholar]
- FALCONER M. A., SERAFETINIDES E. A., CORSELLIS J. A. ETIOLOGY AND PATHOGENESIS OF TEMPORAL LOBE EPILEPSY. Arch Neurol. 1964 Mar;10:233–248. doi: 10.1001/archneur.1964.00460150003001. [DOI] [PubMed] [Google Scholar]
- Falconer M. A., Taylor D. C. Surgical treatment of drug-resistant epilepsy due to mesial temporal sclerosis. Etiology and significance. Arch Neurol. 1968 Oct;19(4):353–361. doi: 10.1001/archneur.1968.00480040019001. [DOI] [PubMed] [Google Scholar]
- Feindel W., Rasmussen T. Temporal lobectomy with amygdalectomy and minimal hippocampal resection: review of 100 cases. Can J Neurol Sci. 1991 Nov;18(4 Suppl):603–605. doi: 10.1017/s0317167100032807. [DOI] [PubMed] [Google Scholar]
- Gates J. R., Cruz-Rodriguez R. Mesial temporal sclerosis: pathogenesis, diagnosis, and management. Epilepsia. 1990;31 (Suppl 3):S55–S66. doi: 10.1111/j.1528-1157.1990.tb05860.x. [DOI] [PubMed] [Google Scholar]
- Gloor P., Olivier A., Quesney L. F., Andermann F., Horowitz S. The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Ann Neurol. 1982 Aug;12(2):129–144. doi: 10.1002/ana.410120203. [DOI] [PubMed] [Google Scholar]
- Hudson L. P., Munoz D. G., Miller L., McLachlan R. S., Girvin J. P., Blume W. T. Amygdaloid sclerosis in temporal lobe epilepsy. Ann Neurol. 1993 Jun;33(6):622–631. doi: 10.1002/ana.410330611. [DOI] [PubMed] [Google Scholar]
- Jackson G. D., Berkovic S. F., Tress B. M., Kalnins R. M., Fabinyi G. C., Bladin P. F. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990 Dec;40(12):1869–1875. doi: 10.1212/wnl.40.12.1869. [DOI] [PubMed] [Google Scholar]
- Kuzniecky R., de la Sayette V., Ethier R., Melanson D., Andermann F., Berkovic S., Robitaille Y., Olivier A., Peters T., Feindel W. Magnetic resonance imaging in temporal lobe epilepsy: pathological correlations. Ann Neurol. 1987 Sep;22(3):341–347. doi: 10.1002/ana.410220310. [DOI] [PubMed] [Google Scholar]
- Leonard G. Temporal lobe surgery for epilepsy: neuropsychological variables related to surgical outcome. Can J Neurol Sci. 1991 Nov;18(4 Suppl):593–597. doi: 10.1017/s0317167100032777. [DOI] [PubMed] [Google Scholar]
- Margerison J. H., Corsellis J. A. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966 Sep;89(3):499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- Matsuda K., Yagi K., Mihara T., Tottori T., Watanabe Y., Seino M. MRI lesion and epileptogenic focus in temporal lobe epilepsy. Jpn J Psychiatry Neurol. 1989 Sep;43(3):393–400. doi: 10.1111/j.1440-1819.1989.tb02933.x. [DOI] [PubMed] [Google Scholar]
- McMillan T. M., Powell G. E., Janota I., Polkey C. E. Relationships between neuropathology and cognitive functioning in temporal lobectomy patients. J Neurol Neurosurg Psychiatry. 1987 Feb;50(2):167–176. doi: 10.1136/jnnp.50.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. A., Muñoz D. G., Finmore M. Hippocampal sclerosis and human memory. Arch Neurol. 1993 Apr;50(4):391–394. doi: 10.1001/archneur.1993.00540040051014. [DOI] [PubMed] [Google Scholar]
- Sagar H. J., Oxbury J. M. Hippocampal neuron loss in temporal lobe epilepsy: correlation with early childhood convulsions. Ann Neurol. 1987 Sep;22(3):334–340. doi: 10.1002/ana.410220309. [DOI] [PubMed] [Google Scholar]
- Squire L. R., Zola-Morgan S. Memory: brain systems and behavior. Trends Neurosci. 1988 Apr;11(4):170–175. doi: 10.1016/0166-2236(88)90144-0. [DOI] [PubMed] [Google Scholar]
- VAN BUREN J. M. The abdominal aura. A study of abdominal sensations occurring in epilepsy and produced by depth stimulation. Electroencephalogr Clin Neurophysiol. 1963 Feb;15:1–19. doi: 10.1016/0013-4694(63)90035-x. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R., Amaral D. G. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986 Oct;6(10):2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R., Amaral D. G. Lesions of the amygdala that spare adjacent cortical regions do not impair memory or exacerbate the impairment following lesions of the hippocampal formation. J Neurosci. 1989 Jun;9(6):1922–1936. doi: 10.1523/JNEUROSCI.09-06-01922.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



