Abstract
Background
LBSL is a mitochondrial disorder caused by mutations in the mitochondrial aspartyl-tRNA synthetase gene DARS2, resulting in a distinctive pattern on brain magnetic resonance imaging (MRI) and spectroscopy. Clinical presentation varies from severe infantile to chronic, slowly progressive neuronal deterioration in adolescents or adults. Most individuals with LBSL are compound heterozygous for one splicing defect in an intron 2 mutational hotspot and a second defect that could be a missense, non-sense, or splice site mutation or deletion resulting in decreased expression of the full-length protein.
Aim
To present a new family with two affected members with LBSL and report a novel DARS2 mutation.
Results
An 8-year-old boy (Patient 1) was referred due to headaches and abnormal MRI, suggestive of LBSL. Genetic testing revealed a previously reported c.492 + 2 T > C mutation in the DARS2 gene. Sanger sequencing uncovered a novel variant c.228-17C > G in the intron 2 hotspot. Family studies found the same genetic changes in an asymptomatic 4-year-old younger brother (Patient 2), who was found on follow-up to have an abnormal MRI. mRNA extracted from patients' fibroblasts showed that the c.228-17C > G mutation caused skipping of exon 3 resulting in lower DARS2 mRNA level. Complete absence of DARS2 protein was also found in both patients.
Summary
We present a new family with two children affected with LBSL and describe a novel mutation in the DARS2 intron 2 hotspot. Despite findings of extensive white matter disease in the brain and spine, the proband in this family presented only with headaches, while the younger sibling, who also had extensive white matter changes, was asymptomatic. Our in-vitro results confirmed skipping of exon 3 in patients and family members carrying the intron 2 variant, which is consistent with previous reported mutations in intron 2 hotspots. DARS2 mRNA and protein levels were also reduced in both patients, further supporting the pathogenicity of the novel variant.
Keywords: Mitochondrial disorders, Aspartyl tRNA synthetase deficiency, Aminoacyl tRNA synthetase deficiency, Leukodystrophy, Splicing mutations
1. Introduction
Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL; MIM 611105) is a rare autosomal recessive disease caused by mutations in the DARS2 gene [1]. At least fifty DARS2 mutations have been identified worldwide [2], resulting in a broad clinical presentation. However, the most typical presentation is a mild, slowly progressive demyelinating disorder with characteristic magnetic resonance imaging (MRI) patterns involving the white matter, brainstem and spinal cord tracts. Elevated lactate levels are also present in the brain when studied by magnetic resonance spectroscopy (MRS) [3]. Most patients (88%) show clinical symptoms before 18 y, which include ataxia, spasticity, and dorsal column dysfunction, without loss of cognitive abilities [4]. Current treatments for LBSL focus on supportive management, including physical therapy and rehabilitation to improve motor function and prevent contractures and scoliosis. Anti-seizure medication, speech therapy and special education are sometimes needed. There is no cure for LBSL [5].
DARS2 encodes mitochondrial aspartyl-tRNA synthetase (mtAspRS), which is responsible for the transfer of aspartic acid to its mitochondrial cognate tRNA. The deficiency of mtAspRS impairs mitochondrial protein translation affecting mitochondrial energy production and other mitochondrial functions [6]. Most reported LBSL individuals harbor heterozygous DARS2 mutations, with one of them being a splice site mutation in a hotspot in intron 2 that results in the exclusion of exon 3, leading to a frameshift, truncated, non-functional protein. These intron 2 mutations are leaky, and result in the expression of both abnormal and normal mtAspRS, although recent studies have shown that some differentiated cells may only express transcripts without exon 3 [7]. Although these mutations in intron 2 will produce some functional DARS2 protein, when combined with a second, deleterious mutation, the amount of normal protein is not sufficient, leading to disease. Thus, the expression of functional mtAspRS is completely dependent on the allele with the intron 2 mutation [1].
In this study, we report a family with two affected siblings, diagnosed at 8 y and 4 y, in whom initial molecular testing identified only a previously reported splicing mutation in intron 5, c.492 + 2 T > C. Additional sequencing uncovered a novel mutation in intron 2, c.228-17C > G. Fibroblast studies confirmed that the c.228-17C > G mutation leads to skipping of exon 3 and, in combination with c.492 + 2 T > C results in abnormal DARS2 mRNA and protein levels.
2. Material and methods
2.1. Study approval and subjects
All human tissue-based experiments were approved by the Children's Hospital of Orange County (CHOC) Institutional Review Board (IRB), IRB # 130990. Informed consent was obtained for all subjects. There was a total of four siblings in the family. Sibling 1, Sibling 2, and Patient 1 are the product of a 28-week triplet pregnancy. Patient 2 is the younger brother of the triplets. There was no history of consanguinity; ancestry was mixed European and Ashkenazi Jewish. Both patients were diagnosed and followed by the Division of Metabolic Disorders at CHOC Children's Hospital.
2.2. Genetic testing and Sanger sequencing
Initial genetic testing was performed using a 300-gene leukodystrophy panel in a certificated clinical laboratory (GeneDX, Gaithersburg, USA). The exonic regions and flanking splice junctions (−13 to +6 within the intron) of the genome were captured using a proprietary system and sequenced by massively parallel (NextGen) sequencing on an Illumina system with 100 bp or greater paired end reads. Reads were aligned to human genome build GRCh37/UCSC hg19 and analyzed for sequence variants using a custom-developed analysis tool. Sequence and copy number alterations were reported according to the Human Genome Variation Society (HGVS) and International System for Human Cytogenetic Nomenclature (ISCN) guidelines, respectively.
Sanger sequencing was used to confirm the results from the initial genetic testing and to uncover the second mutation in intron 2. Primers for sequencing were designed by Primer3 Input [8]. Primers for amplifying exon 3 were 5′-TTGCATGAATGTAACTAATGAAGGT-3′ and 5′-AGCTTACCCCAGCAATAGCA-3′, and exon 5 were 5′-TCTGAACACATCAGCCACATA-3′ and 5′-TTTCAGGACAGTGTGCCAAGA-3′ (Integrated DNA Technologies, Coralville, USA). The amplified fragments were Sanger sequenced (Retrogen Inc., San Diego, USA) and the results were analyzed by Sequencher® (V5.4.6, Gene Codes Corporation, Ann Arbor, USA).
2.3. Cell culture
Primary skin fibroblasts were derived from punch biopsies. The tissues were grown in tissue cultured-treated flasks in minimum essential medium (MEM) α (Gibco, Cat. 32,571,036, Waltham, USA) supplemented with 100 μM non-essential amino acids (NEAA) (Gibco, Cat. 11,140,050), 100 μg/mL Primocin (InvivoGen, Cat. ant-pm-1, San Diego, USA) and 15% fetal bovine serum (FBS) (HyClone™ FetalClone™ III Serum, Cytiva, Cat. SH30109.03, Marlborough, USA). Once cell cultures were established, the fibroblasts were maintained in Dulbecco's modified eagle medium (Gibco, Cat.11966025) supplemented with 5 mM glucose (Sigma, Cat. G8644, St. Louis, USA), 100 μM NEAA (Gibco, Cat. 11,140,050), 1 mM sodium pyruvate (Gibco, Cat. 11,360,070), antibiotic/antimycotic (Gibco, Cat. 15,240,062) and 10% FBS at 37 °C (growth medium) in 5% CO2 at 37 °C. Stocks of cells were cryopreserved in liquid nitrogen in a solution with 20% (V/V) FBS and 10% (V/V) dimethyl sulfoxide (Sigma, Cat. D2650). Cultured cells from control and family members were at similar passage number and had similar growth rate at the time of the experiments.
2.4. RNA extraction and RT-PCR
RNA was extracted using PureLink™ RNA Mini Kit (Invitrogen, Cat. 12–183-018A, Waltham, USA) and samples were treated with PureLink™ DNase Set (Invitrogen, Cat. 12,185,010) to produce pure RNA. Two μg of RNA were reverse transcribed using the Superscript VILO cDNA synthesis kit (Invitrogen, Cat. 11,754,050). The exclusion of exon 3 was analyzed by PCR using the Q5® Hot Start High-Fidelity 2× Master Mix (New England BioLabs, Cat. M0494S, Ipswich, USA), and the forward and reverse primers 5′- GAGGAGAATTCCAGAATTCAGTAG-3′ and 5′- CTGTTGGCATTTTTGGATTCTC-3′, respectively. PCR products were visualized after 1 h electrophoresis at 140 V on 2% agarose gel. Sanger sequencing was performed in the PCR products to confirm the skipping of exon 3.
2.5. Quantitative PCR
RNA expression levels of DARS2 were measured using a CFX Connect Real-Time PCR Detection System (Bio-Rad Inc., Hercules, USA). Quantitative PCR was performed via the DARS2 Taqman gene expression assay, Hs01016220_m1 (Thermo Fisher, Waltham, USA), spanning the exon 8 and 9 boundary and using actin beta as housekeeping gene. Samples were run in triplicate. Gene expression was quantified via the ∆∆Ct method using Bio-Rad CFX Manager Software version 3.0 (Bio-Rad Inc).
2.6. Western blot
Fibroblasts were lysed in radioimmunoprecipitation assay buffer (Sigma, D2650-100ML) containing 1× HALT protease/phosphatase inhibitor cocktail (Thermo Fisher, Cat. PI78442). The antibodies used were anti-DARS2 (Abcam, Cat. ab154606, Cambridge, USA; dilution: 1:2000), anti-actin (Abcam, Cat. ab184220, dilution: 1:80,000), and horseradish peroxidase-conjugated anti-rabbit or -mouse IgG (H & L,) secondary antibody (Abcam, Cat. ab6721 & ab6728, dilution: 1:20,000). The signals were developed by SuperSignal Chemi-luminescent substrate (ThermoFisher, Cat. PI34577), and imaged on CL-Xposure film (ThermoFisher, Cat. PI34090,). The images were analyzed using ImageJ [9].
2.7. Statistical analysis
Statistical analyses were performed by student's t-test or one-way ANOVA as indicated in each figure legend. The accepted level of significant for all tests was P < 0.05. Statistics and graphs were performed by using Graphpad Prism 9® (GraphPad, San Diego, USA).
3. Results
3.1. Clinical reports of two LBSL patients
Patient 1, male, was the 28-week product of a triplet pregnancy who remained in the neonatal intensive care unit (NICU) for three months and required patent ductus arteriosus ligation. Mild hydrocephalus, detected at two weeks, did not require shunting. After discharge from the NICU he had no significant illnesses. At 4.9 y a brain MRI, obtained due to headaches, showed mild prominence of the lateral ventricles with mild T2-hyperintensities in the deep periventricular and subcortical white matter (Fig. 1a & 1b), which were considered secondary to the patient's perinatal complications. There were also abnormal T2-hyperintense signals in the dorsal aspect of the proximal cervical spinal cord (Fig. 1c & 1d). At 5.4 y the patient had an episode of transient loss of consciousness following a head trauma. A computed tomography (CT) scan was not concerning for acute lesions, and the patient fully recovered. The patient's headaches remained mild and intermittent until age 8.4 y when headaches became more intense. A new MRI showed mild progression of the abnormal T2-hyperintensities within the supratentorial white matter with sparing of the subcortical U fibers with unchanged subtle signal hyperintensity in the posterior limb of the internal capsule (Fig. 2a & 2b). There were no changes in the previously seen signal abnormalities in the dorsal columns of the proximal cervical spinal cord, however, new signal abnormalities were identified in middle cerebellar peduncles and bilateral pyramids (Fig. 2c, d, & e).
Fig. 1.
Brain and spinal cord magnetic resonance imaging of Patient 1 at 4.9 y. Axial T2-weighted image shows focal and confluent signal abnormalities in the deep periventricular and subcortical white matter of both cerebral hemispheres sparing u-fibers, subtle signal hyperintensity in the posterior limb of the internal capsule (a & b). There is also signal abnormalities in the dorsal aspect of the medulla oblongata (c) and dorsal columns of the proximal cervical spinal cord (d).
Fig. 2.
Brain and spinal cord magnetic resonance imaging of Patient 1 at 8.4 y. Axial T2-weighted image shows persistent focal and confluent signal abnormalities in the deep periventricular and subcortical white matter of both cerebral hemispheres sparing u-fibers, subtle signal hyperintensity in the posterior limb of the internal capsule (a & b). Previously seen signal abnormality in the dorsal aspect of the medulla oblongata and dorsal columns of the proximal cervical spinal cord demonstrated (d & e). New signal abnormalities identified in middle cerebellar peduncles (c) and bilateral pyramids (d).
Due to the abnormal MRI, the patient was referred for a metabolic evaluation. The patient was intellectually normal, attended regular school with good performance, and had no significant findings in the physical exam. An MRI of the spine was obtained at 9 y and showed extensive signal abnormality involving the lateral cortical spinal tracts, as well as the dorsal columns throughout the cervical and thoracic spine (Fig. 3).
Fig. 3.
Spinal cord magnetic resonance imaging (MRI) of Patient 1 at 9 y. MRI of the spine axial T2-weighted images show signal abnormality involving the lateral cortical spinal tracts (b) as well as the dorsal columns (c). This extends throughout the cervical and thoracic spine (a). Axial cuts (b & c) correspond to the yellow lines drawn in the sagittal view (a).
Patient 2, the younger brother of Patient 1, was ascertained at 4 y of age after molecular testing was performed. Patient 2 had no history of head trauma. Physical exam and intellect were normal; however, a brain MRI obtained at 5.4 y showed abnormalities in the supraventricular and periventricular white matter as well as hyperintense signals in the posterior limbs of the internal capsule, the pyramids at the level of the medulla, and the dorsal columns of the spinal cord (Fig. 4 a-e). Additionally, the MRI uncovered a cyst in the right ventricle, which has not been described in LBSL, and was interpreted as an incidental finding [10].
Fig. 4.
Brain magnetic resonance imaging of Patient 2 at 5.4 y. Axial T2-weighted image shows focal and confluent signal abnormalities in the deep periventricular and subcortical white matter of both cerebral hemispheres sparing u-fibers, and subtle signal hyperintensity in the posterior limb of the internal capsule (a & b). Signal abnormalities seen in the middle cerebellar peduncles (c), dorsal aspect of the medulla oblongata (d), and dorsal columns of the proximal cervical spinal cord (e). Dilated posterior horn of the right lateral ventricle is shown with cerebrospinal fluid signal on all sequences, consistent with a cyst (b).
3.2. Clinical genetic testing and Sanger sequencing revealed a novel DARS2 mutation in LBSL patients
Due to the abnormal MRI in Patient 1, a 300-gene leukodystrophy panel test was performed in a certified clinical laboratory, which uncovered a previously reported intron 5 mutation (c.492 + 2 T > C) in DARS2 inherited from mother, with a GnomAD frequency of 0.000335 (47/140240), which was classified as pathogenic according to American College of Medical Genetics (ACMG) criteria (PS_A, PS1, PP4) [1,11]. However, no second mutation in DARS2 or any other gene was identified.
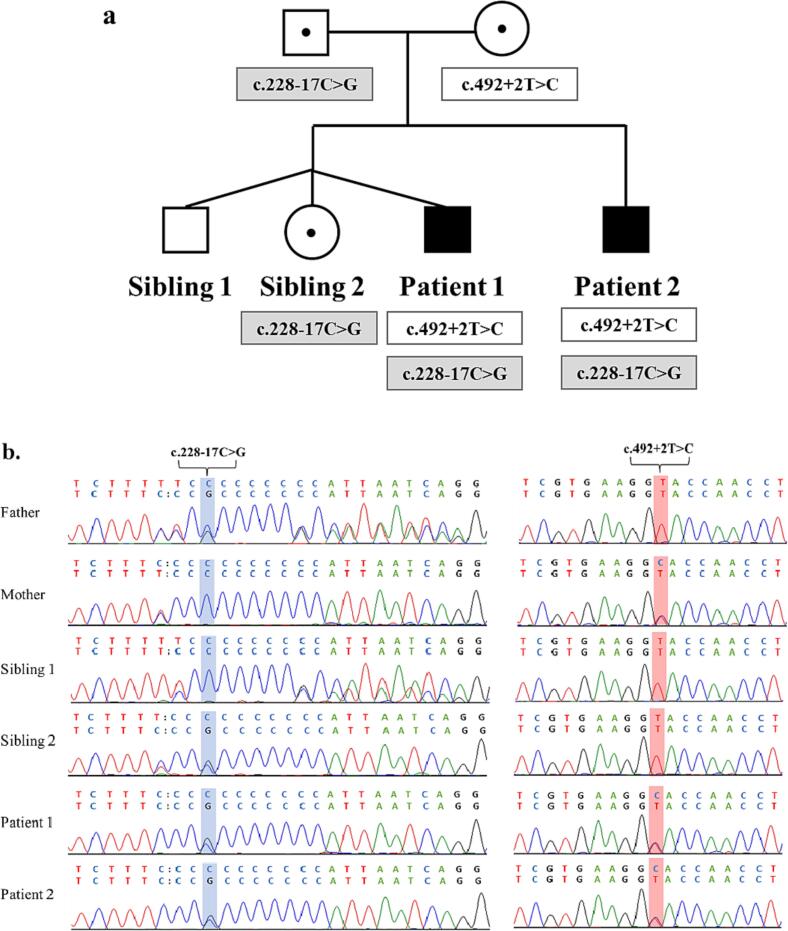
Since most LBSL affected individuals are compound heterozygous for a splice defect in the mutational intron 2 hotspot [1,4], Sanger sequencing was performed in that region in the proband and family members uncovering a novel c.228-17C > G variant (Fig. 5b). In addition, the common polymorphism, c.228-20 T > C, was present in cis with both the c.228–17C > G and the c.492 + 2 T > C variants. Because of this, both Patient 1 and Patient 2 were homozygous for the polymorphism, while mother, father, and sibling 2 were heterozygous. Of note, the father and Sibling 1 were also heterozygous for c.228-20dupT, which was in trans to the c.228-20 T > C and c.228-17C > G.
Fig. 5.
Pedigree of two patients affected with leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation (LBSL) and Sanger sequencing result of mutations. Family tree of the affected siblings. Open square, unaffected male. Solid square affected male. Black dot in the circles and squares, female carriers and male carrier. c.228-17C > G, newly discovered mutation in intron 2. c.492 + 2 T > C, previous reported mutation in intron 5 (a). Electropherogram of the Sanger sequencing of intron 2 and intron 5, show segregation of the mutations in the family (b).
Subsequently, the clinical laboratory was contacted and, after sequencing the intron 2 hot spot region, confirmed the intron 2 variant, which was found to have ExAC frequency of 0.000008 (1/120908) and was classified as likely pathogenic according to ACMG criteria (PM2, PM3, PP4, PP) [11]. The laboratory also confirmed the presence of the c.228-20 T > C polymorphism as well as the c.228-20dupT variant, which was classified as likely benign as it has been seen frequently (817 times) as homozygous in population cohorts [12].
Fig. 5a depicts the segregation of the mutations within the family with the identification of the second affected sibling (Patient 2).
3.3. Splicing abnormality and low gene expression in LBSL patients
Analysis of a PCR amplified fragment from exon 2 to exon 4 showed that both patients had an extra band at 229 bp besides the normal expected band at 296 bp, consistent with the skipping of the exon. Moreover, the father and Sibling 2, who also carry the intron 2 mutation, showed a second smaller fragment (Fig. 6a & 6b).
Fig. 6.
Effects of the DARS2 mutations on mRNA splicing in a family affected with leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. RNA and protein were extracted from fibroblasts and RT-PCR was performed to obtain cDNA. Scheme for exon 3 skipping (a). The exclusion of exon 3 was visualized by PCR with primers amplifying a fragment from exon 2 to exon 4 (inverted image). The expected sizes of 296 bp (for the full transcript with exon 3) and 229 bp (without exon 3) are shown (b). Electropherograms from Sanger sequencing confirmed lack of exon 3 in the two patients and the family members carrying the c.228-17G > C mutation (c).
To further confirm exon 3 skipping in family members carrying the c.228-17C > G variant, cDNA obtained from fibroblasts of all family members was sequenced (Fig. 6c). The electropherograms showed multiple peaks within the exon 3 region in the two patients and the carriers of the intron 2 mutation (father and Sibling 2), indicative of the different mRNA transcripts due to the leaking effect of the intron 2 mutation.
As expected, both patients showed a moderate decrease in DARS2 expression (F(6, 14) = 129.8; P < 0.0001) and reduced DARS2 protein content, while all carriers had a mildly reduced DARS2 gene expression. (Fig. 7a & 7b).
Fig. 7.
mRNA expression and protein content of the DARS2 mutations in a family affected with leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. DARS2 mRNA levels were assessed using the TaqMan qPCR assay and normalized by actin beta (a). DARS2 protein levels were measured using Western Blot. Bars represent mean ± standard error of mean. Parents were compared to a normal Adult Control, and Sibling 1 was used as a control for both patients and Sibling 2. ***P < 0.001; ****P < 0.0001; one-way ANOVA, followed by the post hoc Tukey test (b).
4. Discussion
LBSL is characterized in most affected individuals by slowly progressive ataxia and spasticity with dorsal column dysfunction starting before 18 y. More than one hundred individuals with mutations spread throughout the DARS2 gene have been reported. Clinical presentation ranges from antenatal onset in patients harboring two severe mutations [13] to a milder disease in patients with one severe and one “leaky” mutation in intron 2 [4], while no affected individuals have been described harboring two “leaky” mutations.
Due to the unique combination of one severe and one “leaky” mutations, it is challenging to establish a clear genotype and phenotype correlation in patients with mild disease. However, a subgroup of patients with a combination of the c.228-21_20delTTinsC together with c.455 G > T (n = 9), or with c.492 + 2 T > C (n = 11) consistently showed a mildly progressive neurological deterioration [4].
Like this subgroup, Patient 1 and Patient 2 have an intron 2 mutation in the hot spot, in combination with the c.492 + 2 T > C and present with a slowly progressive clinical course [4]. As previously reported by van Berge et al. [4], the common polymorphism c.228-20 T > C was also found segregating with the novel c.228–17C > G intron 2 variant.
The two LBSL affected individuals presented here share the distinctive MRI pattern that has been reported in most LBSL patients [14]. Of note, initial MRI abnormalities in Patient 1 at 4.9 y and Patient 2 at 5.4 y were very similar, with characteristic brain leukodystrophy and signal abnormalities in the cerebellar peduncles, dorsal aspect of the medulla oblongata, and dorsal columns of the proximal cervical spinal cord. A follow-up MRI in Patient 1 showed mild progression of the disease in the brain and spine. This second MRI was obtained 3 years after Patient 1 had a concussion. It is known that head trauma can contribute to progression of the disease in patients with leukodystrophies [15,16], however it is not clear if the worsening MRI changes in Patient 1 are associated to head trauma or simply represent age-related disease progression. Potentially follow-up MRI in the sibling, who has not experienced head trauma, could help answer this question.
At the time of diagnosis, 8 y, Patient 1 presented only with a long history of headaches, which have not been described in a comprehensive review of 67 LBSL patients [2]. To our knowledge, there is only one report of a patient presenting with headaches and vision loss at age 9 y, who also had congenital cataracts. This patient had a splice site mutation in intron 2 (c.228-21_228-20delTTinsC), and a missense alteration in exon 5 (c.455G > T p.C152F), which significantly decreased dimerization of the wild-type enzyme [17], different from Patient 1. Of note, other patients with the above genotype have had a benign phenotype with no reported cataracts or headaches [4].
Patient 1 is currently 14 y and headaches have become less frequent and Patient 2, has not complained of headaches and remains asymptomatic at 9 y. Additionally several asymptomatic patients with LBSL and extensive lesions demonstrated in brain and spine have been reported [4,18]. Therefore, it is unclear whether headaches in Patient 1 are indeed a clinical manifestation of LBSL.
Genetic testing confirmed the presence of a c.492 + 2 T > C mutation in Patient 1 and Patient 2. This previously reported intron 5 defect results in exon skipping without a frameshift and is the most common pathogenic variant reported in LBSL [5].
The reporting clinical laboratory did not identify the c.228-17C > G since the reporting boundaries were at −13 and + 6 base pairs within the intron, while the novel mutation described here was deeper into intron 2. Lack of complete coverage of intronic regions is a known limitation of any exome based genetic testing. Therefore, additional sequencing for the known intron 2 hot spot region should be offered by laboratories when a potential pathogenic variant is identified somewhere else in the gene.
This novel mutation is in a stretch of C residues just upstream of exon 3 and is predicted to affect the splicing of this exon, leading to a frameshift and the transcript of a truncated, non-functional protein [1,4]. PCR results shown here proved that this intron 2 mutation caused skipping of exon 3 and are consistent with the electropherograms showing a clear overlap of exon 3 and exon 4, in patients and carriers. As expected, the combined effect of both mutations resulted in low DARS2 mRNA levels and absent protein content in Patients 1 & 2, which could be secondary to decay. This can also explain why Western Blot assay did not identify the mutated DARS2 protein with a smaller size in carriers [1].
Currently, there is no available treatment for LBSL. Palliative care is mostly focused on physical therapy and rehabilitation to maintain or improve motor function and prevent complications. While availability of genetic testing allows earlier diagnosis of the disease, further research is needed for a deeper understanding of pathophysiology and to develop potential treatments.
In summary, we present a family with two affected children with LBSL with ambiguous clinical presentation despite extensive MRI abnormalities and describe a new mutation in the intron 2 hotspot, proving its pathogenicity.
Declaration of Competing Interest
None.
Acknowledgements
We thank all members of the family for their participation in this study. This work was supported by grants from the Ralph and Sue Stern Family (16984011), the Cure LBSL Foundation (16984017). Latini A is a CNPq fellow (PQ #312854/2019-6).
Data availability
Data will be made available on request.
References
- 1.Scheper G.C., Van Der Klok T., Van Andel R.J., Van Berkel C.G.M., Sissler M., Smet J., Muravina T.I., Serkov S.V., Uziel G., Bugiani M., Schiffmann R., Krägeloh-Mann I., Smeitink J.A.M., Florentz C., Van Coster R., Pronk J.C., Van Der Knaap M.S. Mitochondrial aspartyl-tRNA synthetase deficiency causes leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Nat. Genet. 2007;39:534–539. doi: 10.1038/ng2013. [DOI] [PubMed] [Google Scholar]
- 2.Muthiah A., Housley G.D., Klugmann M., Fröhlich D. The Leukodystrophies HBSL and LBSL—correlates and distinctions. Front. Cell. Neurosci. 2021;14 doi: 10.3389/fncel.2020.626610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Der Knaap M.S., Van Der Voorn P., Barkhof F., Van Coster R., Krägeloh-Mann I., Feigenbaum A., Blaser S., Vles J.S.H., Rieckmann P., Pouwels P.J.W. A new leukoencephalopathy with brainstem and spinal cord involvement and high lactate. Ann. Neurol. 2003;53:252–258. doi: 10.1002/ana.10456. [DOI] [PubMed] [Google Scholar]
- 4.Van Berge L., Hamilton E.M., Linnankivi T., Uziel G., Steenweg M.E., Isohanni P., Wolf N.I., Krägeloh-Mann I., Brautaset N.J., Andrews P.I., De Jong B.A., Al Ghamdi M., Van Wieringen W.N., Tannous B.A., Hulleman E., Würdinger T., Van Berkel C.G.M., Polder E., Abbink T.E.M., Struys E.A., Scheper G.C., Van Der Knaap M.S., Alehan F., Appleton R.E., Boltshauser E., Brockmann K., Calado E., Carius A., de Coo I.F.M., van Coster R., Alehan F., Appleton R.E., Boltshauser E., Brockmann K., Calado E., Carius A., de Coo I.F.M., El-Zind S., Alehan F., Appleton R.E., Boltshauser E., Brockmann K., Calado E., Carius A., de Coo I.F.M., El-Zind S., Erturk O., Fadeeva L., Feigenbaum A., Gokben S., Gulati S., Hnevsova P., Joost K., Kohler W., Kolk A., Kristoferitsch W., Lemos Silveira E., Lin J., Lutz S., Mendonca C., Nuttin C., Opladen T., Savoiardo M., Schiffmann R., Seitz A., Serkov S., Sharma S., Stockler S., Temple I.K., Uluc K., Vojta S., Wilms G., Wong B., Yapici Z. Leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation: clinical and genetic characterization and target for therapy. Brain. 2014;137:1019–1029. doi: 10.1093/brain/awu026. [DOI] [PubMed] [Google Scholar]
- 5.Engelen M., Abbink T.E., Salomons G.S., van der Knaap M.S. University of Washington; Seattle: 2010 May 25. GeneReviews® [Internet] Leukoencephalopathy with Brain Stem and Spinal Cord Involvement and Lactate Elevation.https://www.ncbi.nlm.nih.gov/books/NBK43417/ [Updated 2021 Feb 18]. n.d. (accessed May 28, 2023) [Google Scholar]
- 6.Lin T.K., Chang Y.Y., Lin H.Y., Liou C.W., Wang P.W., Chuang J.H., der Chen S., Chuang Y.C., Huang S.T., Hsu T.Y., Peng C.H., Lan M.Y. Mitochondrial dysfunctions in leukoencephalopathy with brainstem and spinal cord involvement and lactate elevation (LBSL) PLoS One. 2019;14 doi: 10.1371/journal.pone.0224173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guang S., O’Brien B.M., Fine A.S., Ying M., Fatemi A., Nemeth C.L. Mutations in DARS2 result in global dysregulation of mRNA metabolism and splicing. Sci. Rep. 2023;13:13042. doi: 10.1038/s41598-023-40107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B.C., Remm M., Rozen S.G. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012;40 doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed A.K., Cohen A.R. Intracranial arachnoid cysts, child’s nervous. System. 2023;39:2771–2778. doi: 10.1007/s00381-023-06066-0. [DOI] [PubMed] [Google Scholar]
- 11.Li M.M., Datto M., Duncavage E.J., Kulkarni S., Lindeman N.I., Roy S., Tsimberidou A.M., Vnencak-Jones C.L., Wolff D.J., Younes A., Nikiforova M.N. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Tukiainen T., Birnbaum D.P., Kosmicki J.A., Duncan L.E., Estrada K., Zhao F., Zou J., Pierce-Hoffman E., Berghout J., Cooper D.N., Deflaux N., DePristo M., Do R., Flannick J., Fromer M., Gauthier L., Goldstein J., Gupta N., Howrigan D., Kiezun A., Kurki M.I., Moonshine A.L., Natarajan P., Orozco L., Peloso G.M., Poplin R., Rivas M.A., Ruano-Rubio V., Rose S.A., Ruderfer D.M., Shakir K., Stenson P.D., Stevens C., Thomas B.P., Tiao G., Tusie-Luna M.T., Weisburd B., Won H.H., Yu D., Altshuler D.M., Ardissino D., Boehnke M., Danesh J., Donnelly S., Elosua R., Florez J.C., Gabriel S.B., Getz G., Glatt S.J., Hultman C.M., Kathiresan S., Laakso M., McCarroll S., McCarthy M.I., McGovern D., McPherson R., Neale B.M., Palotie A., Purcell S.M., Saleheen D., Scharf J.M., Sklar P., Sullivan P.F., Tuomilehto J., Tsuang M.T., Watkins H.C., Wilson J.G., Daly M.J., MacArthur D.G., Abboud H.E., Abecasis G., Aguilar-Salinas C.A., Arellano-Campos O., Atzmon G., Aukrust I., Barr C.L., Bell G.I., Bergen S., Bjørkhaug L., Blangero J., Bowden D.W., Budman C.L., Burtt N.P., Centeno-Cruz F., Chambers J.C., Chambert K., Clarke R., Collins R., Coppola G., Córdova E.J., Cortes M.L., Cox N.J., Duggirala R., Farrall M., Fernandez-Lopez J.C., Fontanillas P., Frayling T.M., Freimer N.B., Fuchsberger C., García-Ortiz H., Goel A., Gómez-Vázquez M.J., González-Villalpando M.E., González-Villalpando C., Grados M.A., Groop L., Haiman C.A., Hanis C.L., Hattersley A.T., Henderson B.E., Hopewell J.C., Huerta-Chagoya A., Islas-Andrade S., Jacobs S.B., Jalilzadeh S., Jenkinson C.P., Moran J., Jiménez-Morale S., Kähler A., King R.A., Kirov G., Kooner J.S., Kyriakou T., Lee J.Y., Lehman D.M., Lyon G., MacMahon W., Magnusson P.K., Mahajan A., Marrugat J., Martínez-Hernández A., Mathews C.A., McVean G., Meigs J.B., Meitinger T., Mendoza-Caamal E., Mercader J.M., Mohlke K.L., Moreno-Macías H., Morris A.P., Najmi L.A., Njølstad P.R., O’Donovan M.C., Ordóñez-Sánchez M.L., Owen M.J., Park T., Pauls D.L., Posthuma D., Revilla-Monsalve C., Riba L., Ripke S., Rodríguez-Guillén R., Rodríguez-Torres M., Sandor P., Seielstad M., Sladek R., Soberón X., Spector T.D., Tai S.E., Teslovich T.M., Walford G., Wilkens L.R., Williams A.L. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stellingwerff M.D., Figuccia S., Bellacchio E., Alvarez K., Castiglioni C., Topaloglu P., Stutterd C.A., Erasmus C.E., Sanchez-Valle A., Lebon S., Hughes S., Schmitt-Mechelke T., Vasco G., Chow G., Rahikkala E., Dallabona C., Okuma C., Aiello C., Goffrini P., Abbink T.E.M., Bertini E.S., Van der Knaap M.S. LBSL: case series and DARS2 variant analysis in early severe forms with unexpected presentations. Neurol. Genet. 2021;7 doi: 10.1212/NXG.0000000000000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steenweg M.E., Van Berge L., Van Berkel C.G.M., De Coo I.F.M., Temple I.K., Brockmann K., Mendonça C.I.P., Vojta S., Kolk A., Peck D., Carr L., Uziel G., Feigenbaum A., Blaser S., Scheper G.C., Van Der Knaap M.S. Early-onset LBSL: how severe does it get? Neuropediatrics. 2012;43:332–338. doi: 10.1055/s-0032-1329395. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton E.M.C., van der Lei H.D.W., Vermeulen G., Gerver J.A.M., Lourenço C.M., Naidu S., Mierzewska H., Gemke R.J.B.J., de Vet H.C.W., Uitdehaag B.M.J., Lissenberg-Witte B.I., V.W.M. Research Group, van der Knaap M.S. Natural history of vanishing white matter. Ann. Neurol. 2018;84:274–288. doi: 10.1002/ana.25287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raymond G.V., Seidman R., Monteith T.S., Kolodny E., Sathe S., Mahmood A., Powers J.M. Head trauma can initiate the onset of adreno-leukodystrophy. J. Neurol. Sci. 2010;290:70–74. doi: 10.1016/j.jns.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 17.van Berge L., Kevenaar J., Polder E., Gaudry A., Florentz C., Sissler M., van der Knaap M.S., Scheper G.C. Pathogenic mutations causing LBSL affect mitochondrial aspartyl-tRNA synthetase in diverse ways. Biochem. J. 2013;450:345–350. doi: 10.1042/BJ20121564. [DOI] [PubMed] [Google Scholar]
- 18.Labauge P., Dorboz I., Eymard-Pierre E., Dereeper O., Boespflug-Tanguy O. Clinically asymptomatic adult patient with extensive LBSL MRI pattern and DARS2 mutations. J. Neurol. 2011;258:335–337. doi: 10.1007/s00415-010-5755-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.