Abstract
Background
Huntington's disease is an inherited progressive neurodegenerative disorder caused by an expansion of the polyglutamine tract leading to malformation and aggregation of the mutant huntingtin protein in the cell cytoplasm and nucleus of affected brain regions. The development of neuroprotective agents from plants has received considerable research attention.
Objective
Our study aims to investigate the neuroprotective effects of luteolin and the mechanisms that underline its potential mediated protection in the mutant htt neuroblastoma cells.
Methods
The mutant htt neuroblastoma cells were transfected with 160Q, and the control wild-type neuroblastoma cells were transfected with 20Q htt for 24 h and later treated with luteolin. Cell viability was determined by MTT and PI staining in both groups, while western blotting was used to evaluate caspase 3 protein expression. Aggregation formation was assessed via immunofluorescence microscopy. Also, western blotting was utilized to measure the protein expression of mutant htt aggregated and soluble protein, Nrf2 and HO-1. The impact of Nrf2 on luteolin-treated neuroblastoma cells was assessed using small interfering RNAs.
Results
Our study reports that luteolin can protect cultured cells from mutant huntingtin cytotoxicity, evidenced by increased viability and decreased apoptosis. Also, luteolin reduced the accumulation of soluble and insoluble mutant huntingtin aggregates in mutant htt neuroblastoma cells transfected with 160Q compared to the control wild-type. The mutant htt aggregate reduction mediated by luteolin appeared to be independent of the Nrf2 –HO-1 antioxidant pathway.
Conclusion
Luteolin presents a new potential therapeutic and protective agent for the treatment and decreasing the cytotoxicity in neurodegenerative diseases such as Huntington's disease.
Keywords: Aggregation; Huntington’s disease; Luteolin, misfolded protein; Neurodegenerative diseases
1. Introduction
Huntington's disease (HD) is an inherited progressive neurodegenerative disorder belonging to the polyglutamine disorders (McColgan and Tabrizi, 2018). Polyglutamine disorders share adult onset of symptoms, which include chorea, general motor impairment, psychiatric disorders, and cognitive decline (Arning and Nguyen, 2021). The disease usually begins in midlife, with progressive neurodegeneration leading to death within ten years of onset (Niemann and Jankovic, 2019). The prevalence of HD is estimated to be 2.71 per 100,000 worldwide. However, the figure is much higher in Western countries (Medina et al., 2022). A Canadian study estimated that the patient's mean healthcare cost was $23,211 [$38,599] per person-year, with hospitalizations accounting for 57.8% of costs. (Shaw et al., 2022). Furthermore, as predicted, cost estimates show that HD patients and their caregivers bear an enormous financial burden as the disease advances (Rodriguez-Santana et al., 2023). Alarmingly, it concluded that patients diagnosed with HD are at increased risk of suicide compared to individuals without the disease (Alothman et al., 2022).
HD is caused by an abnormal (CAG)N-trinucleotide repeat expansion in the mutated gene, located in the first exon of 67 exons of the huntingtin gene, which is translated into a polyglutamine tract that encodes the protein huntingtin (Donaldson et al., 2021). Expansion of the polyglutamine tract by more than 35 glutamines leads to misfolding and aggregation of the mutant huntingtin protein in the cell's cytoplasm and nucleus. Huntingtin aggregates mark the presence of misfolded mutant Huntingtin in susceptible cell populations and are a hallmark of HD (Lieberman et al., 2019). All mutant huntingtin constructs form aggregates in the cytoplasm, but only shorter N-terminal fragments produce nuclear aggregates (Vieweg et al., 2021). Increased aggregation correlates with increased susceptibility to apoptosis and disease severity (Marcelo et al., 2021). Treatments that suppress aggregation, including chaperone overexpression and administration of small molecule aggregation inhibitors, have decreased neurodegeneration (Radbakhsh et al., 2021).
Regarding disease pathogenesis, mutant Huntingtin was shown to induce transcriptional dysregulation, impairment of protein degradation due to proteasomal and autophagy dysfunction, mitochondrial damage and apoptosis (Tobore, 2019, Bono-Yagüe et al., 2020). It has been shown that htt aggregates can sequester and alter the kinetics of transported organelles and proteins such as synaptic vesicles and block transport of organelles or vesicles into axons; this blockage can impair axonal function and lead to cell degeneration (Stavoe and Holzbaur, 2019). However, the mechanisms by which mutant Huntingtin causes neuronal dysfunction and degeneration remains unclear.
Oxidative stress increases DNA, protein, and lipid oxidation, contributing to cell death and degeneration. Indeed, oxidative stress is a primary mechanism in HD pathogenesis. Also, it has been demonstrated that mutant htt protein-mediated factors alter the expression of antioxidant factors in the mitochondria, enhancing cellular oxidative stress and cellular dysfunction. Furthermore, the mutant impairs the activity of proteasome and autophagy systems, which can hinder the removal of unfolded protein and damaged organelle such as mitochondria, resulting in increased ROS. Also, protein overload of unfolded protein triggers endoplasmic reticulum stress and free radical generation. Nuclear mhtt aggregates sequester transcription factors that regulate antioxidant gene expression. Various mechanistic studies have confirmed the link between mutant htt and oxidative stress (Abramov et al., 2020, Bono-Yagüe et al., 2020).
While protein aggregation can result in oxidative stress, on the other hand, oxidative stress can, in turn, result in aggregation in neurodegenerative disease. For instance, oxidation of critical amino acids results in an alteration of protein shape, making it prone to aggregation. Examples of protein that are prone to ROS oxidation and, subsequently, aggregation includes γD-crystallin and GAPDH. Carbonylation, a particular type of oxidation of specific amino acid residues, can also lead to protein aggregation. Furthermore, oxidation can affect chaperonin, proteasomal and autophagy activity. Oxidation of a specific protein can prevent its interaction with its partner, the molecular chaperone heat-shock protein, involved in controlling proper protein folding. The ubiquitin–proteasome system (UPS) is vital for the removal of unfolded, misfolded, or oxidized protein by proteolytic degradation. Hence, oxidized proteasomes can favour the accumulation of aggregation-prone proteins (Levy et al., 2019). Also, autophagy is a highly selective process in which damaged/aggregated proteins or damaged organelles are marked by ubiquitin for elimination via the autophagy-lysosomal pathway. Increased production of ROS can lead to disruption of lysosomal membrane integrity by peroxidation of lysosomal membrane lipids (Nagakannan et al., 2020), hence impairing the degradation pathway, resulting in misfolded protein buildup. Therefore, antioxidants are being tested for their neuronal anti-aggregatory and anti-apoptotic effects.
Luteolin (3′,4′,5,7-tetrahydroxyflavone) (Fig. 1) is a flavonoid known as a free radical scavenger, found mainly in the glycosylated form in many plant species, including fruits, vegetables, and medicinal herbs. It is among the most important antioxidant, anti-inflammatory and anticancer substances (Ashaari et al., 2018). The Luteolin anticancer effects and associated mechanisms are well-established in several cancer types, including lung, breast, glioblastoma, prostate, colon, and pancreatic cancers (Seelinger et al., 2008, Imran et al., 2019). A plethora of cellular mediators/mechanisms/processes were shown to facilitate its anticarcinogenic effects, including cell cycle arrest in the G2/M, S, or G0/1 phase, inhibition of MMP2 and MMP9 secretion, which impedes migration, activation of caspases and consequently apoptosis, inhibition of EGFR, bFGF and VEGF hindering angiogenesis. Luteolin was also shown to reverse epithelial-mesenchymal transition, evident by the increased expression of epithelial markers such as E-cadherin and decreasing mesenchymal cell markers such as N-cadherin, snail, and vimentin (Imran et al., 2019). Importantly, it was found to increase the levels of antioxidant enzymes and compounds such antioxidant enzymes, superoxide dismutase (SOD), glutathione peroxidase and catalase (CAT) in colon cancer cells (Kang et al. 2017).
Fig. 1.

Chemical structure of luteolin.
The neuroprotective effects of luteolin have been well illustrated in several Parkinson's and Alzheimer's Disease models. Luteolin's neuroprotective antioxidant effect was illustrated in Wistar rats exposed to cobalt chloride. Luteolin and gallic acid inhibited hydrogen peroxide and nitric oxide and restored the antioxidants glutathione S-transferase (GST) and SOD in the cobalt chloride exposed rats (Akinrinde and Adebiyi, 2019). Also, luteolin attenuated hydrogen peroxide-induced neurotoxicity in vitro (Duarte et al. 2023). Notably, Luteolin has been reported to induce nuclear factor E2-related factor 2 (Nrf2), the master regulator of antioxidative.
Induced Nrf2 will be released from Keap1, escaping from proteasomal degradation, and translocating to the nucleus. In the nucleus, Nfr2 binds to the antioxidant response element (ARE) and starts the transcription of an array of antioxidant enzymes such as heme oxygenase-1 (HO-1), GST, SOD, CAT, glutathione reductase, NAD(P)H and many more. These enzymes act by reducing the cell oxidative stress and free radicals’ formation (Francisqueti-Ferron et al. 2019). Indeed luteolin increased heme oxygenase-1 (HO-1) mRNA expression and protein levels (Li et al., 2019) and enhance the binding of Nrf2 to ARE (Siddique, 2021) that functions as an enhancer sequence in the HO-1 promoter. It is noteworthy to indicate that luteolin penetrated the blood–brain barrier in vivo and attenuated scopolamine-induced amnesia in rats (Bakoyiannis et al., 2019).
1.1. Objective/purpose of the study
This study aimed to assess the neuroprotective effects of flavonoid against apoptosis and oxidative stress in a neuro-2a (N2a) cells that express the mutant huntingtin. Furthermore, to identify the cellular mechanism that mediates the neuroprotective effect.
1.2. Significance
Our study can provide supporting evidence for utilising the natural plant product luteolin as a neuroprotective agent.
2. Material and methods
2.1. Cell culture and treatment
Murine neuroblastoma Neuro2A (ATCC) cells were plated in a 6-well culture plate and maintained in Dulbecco's modified Eagles medium (DMEM; Invitrogen) supplemented with 2% heat-inactivated fetal bovine serum and with penicillin/streptomycin (Invitrogen). The cells were maintained at 37 °C in a humidified incubator with 5% CO2. After seeding, cells were transfected with amino-terminal huntingtin fragments containing 20 or 160 CAG repeats using Lipofectamine 2000 (Invitrogen) (according to the manufacturer's instructions). Expression constructs, Cherry-20Q Huntingtin and Cherry-160Q Huntingtin, were used. Six hours later, the transfection medium was replaced with complete DMEM. The luteolin (Sigma, St. Louis, MO) (1.25–10 ng/ml in DMSO) or vehicle (DMSO) was added to the transfected cells and then kept in the incubator for 48 h.
2.2. RNA interference and transfection
Silencing of Nrf2 expression in Neuro2a cells was achieved by the small interfering RNA (siRNA) technique. The siRNA (GCAGGAGAGGTAAGAATAA) targeting specific Nrf2 sequences was designed and synthesized by RiBoBio (Guangzhou RiBoBio Co. Ltd., China). A siRNA vector was used as a control. SiRNA transfection was performed using Lipofectamine 2000 according to the manufacturer's instructions.
2.3. Measurement of cytotoxicity
Cell viability was measured by the MTT (dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Briefly, the cells were plated in 96-well plates and transfected with the plasmids encoding an N-terminal fragment of htt containing 20Q (htt20Q) or 160Q (htt160Q), followed by luteolin or vehicle treatment for 48 h. At the end of the treatment period, a sterile solution of MTT (0.5 mg/mL) in DMEM was added to each well, and incubated at 37 °C for 4 h. The MTT medium was discarded, and 200 µl of sterile DMSO was added to lyse the cells for 10 min at 37 °C. MTT absorbance (OD) was measured at 565 nm using a spectrophotometer. The results were obtained by comparing the mean absorbance of treated cells to the mean absorbance of untreated cells and expressed as a percentage (%) of the absorbance of control cells.
For analysis of cell death rate, cultured wild-type 20Q and mutant 160Q-N2a cells treated with luteolin or DMSO were stained with PI (propidium iodide five µg/ml, 10 min) and then observed under an inverted fluorescence microscope to count the number of non-viable (red stained) and viable (unstained) cells in 10 different fields and averaged from 6 independent experiments.
2.4. Fluorescent microscopy
The cultured cells were fixed with 4% paraformaldehyde for 10 min at room temperature and then treated with 0.5% saponin (Fluka). They were then stained with 1:3,000 Hoechst nuclear labelling solution. The plates were then used directly for fluorescence microscopy to examine the expression of mhtt-160Q cherry and htt-20Q cherry.
2.5. Western immunoblot
For the cytoplasm and nuclear protein extraction, a buffer consisting of NP40 buffer (50 mM Tris pH 7.4, 50 mM NaCl, 0.1% Triton X-100, 1% NP40, protease inhibitor, Cocktail Pierce 78430) and 1 ml of PMSF, Sigma P-7626 was added to the cultured N2a cells based on the protocol previously described [Lin et al., 2016]. Culture lysates were sonicated, and the protein concentrations were determined using BCA assay. An equal amount of protein (80 ng/40 µl/lane) was separated on 12% SDS-PAGE gels. Proteins transferred to nitrocellulose membrane (GE Healthcare Life Sciences, Marlborough, MA, USA) were blocked in 5% non-fat dry milk in PBS for 30 min and then incubated in primary antibodies htt anti-mouse 1:1000, Nrf2 anti-rabbit 1:500, HO-1 anti-rabbit 1:500, and Caspase3 rabbit 1:500, in 3% BSA/PBS overnight at 4℃. Mouse tubulin 1:10000 was used as a loading control. Secondary HRP-conjugated antibodies (Jackson Immuno-Research) in 5% milk were added to the membranes, incubated for two hours, and later visualized using Super Signal ECL (Pierce). The results were analyzed using a Bio-Rad Imaging Densitometer.
2.6. Reverse transcription-polymerase chain reaction
Total RNA from the mutant and wild-type htt cells treated with luteolin or vehicle was prepared using TRIZOL reagent (Invitrogen) according to the manufacturer's instructions. RNA concentration and purity were determined using UV spectroscopy by measuring the absorbance at 260 and 280 nm, respectively.
RNA was reversed transcribed into cDNA using QuantiTect Reverse Transcription Kit (Qiagen). Then, 1 µl of cDNA template was amplified by PCR in 50 µl total reaction volume containing 25 µl master mix and four pmol of the Cherry primers (5CTGCCCTCTCGCCTGGGACATCCTGT3 (forward) 5GGCCTCCCAGCCCATGGTCTTCTTC3 (reverse)). PCR amplification was performed under the following: 94 °C for 3 min; 30 cycles of 94 °C for 30 s, 55 °C for 40 s, and 72 °C for 50 s, followed by 72 °C for 5 min. After amplification, the products were separated in a 2% agarose gel containing 0.03% ethidium bromide and visualized using the FastGene FAS-DIGI PRO Gel imaging system.
2.7. Statistical analysis
All images were analyzed using Image-pro Plus 6.0 image analysis software. Data were presented as the mean ± SD from six independent experiments. Statistical analysis was performed using SPSS Statistics 17.0 software using one-tailed ANOVA and Student's t-test. Differences between the means were considered significant if p < 0.05.
3. Results
3.1. Luteolin treatment protected N2a cells expressing mutant huntingtin from cytotoxicity
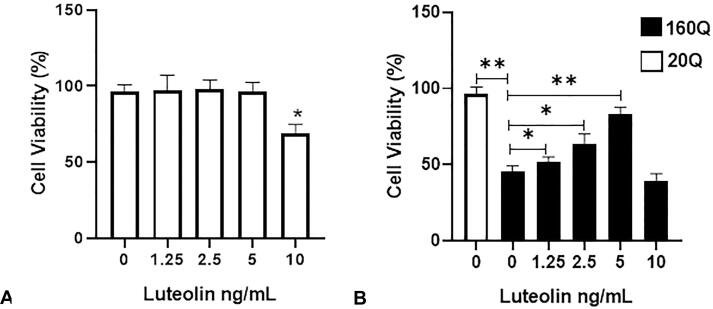
The cells expressing the normal (20Q htt) or the mutant (160Q htt) were treated with luteolin concentrations of 1.25, 2.5, 5, and 10 ng/ml, respectively. There were insignificant differences in the mean percentage of MTT transformation between the luteolin-treated (1.25––5 ng/ml) normal cell-type (20 Q) cultures and untreated cells. However, the mutant (160Q htt) htt-expressing cells displayed an approximately 45% reduction in MTT conversion to formazan blue compared to the normal (20Q htt) expressing cells, indicating a reduction in cell survival (Fig. 2). On the other hand, the luteolin-treated htt mutant cells (160Q) had a significantly higher mean percentage of MTT conversion, 52% (p < 0.05), 66% (p < 0.05), and 85% (p < 0.01), for luteolin concentrations of 1.25, 2.5, and 5 ng/mL, respectively, compared to the untreated mutant htt cells which is indicative of higher cellular survival. The use of treatments with a luteolin concentration of 10 ng/ml showed cytotoxicity for both the mutant htt (160Q) and wild-type htt (20Q) cells (Fig. 2). Thus, the 5 ng/ml luteolin concentration, which showed the highest protective activity against mutant htt cytotoxicity, was used to evaluate further their effectiveness in reducing htt aggregates.
Fig. 2.
Luteolin treatment protected the neuroblastoma cells against mutant htt cytotoxicity. The neuroblastoma cells were transfected either with the wild type htt 20 Q or the mutant htt 160Q, then treated with vehicle (0) or different concentrations of luteolin for 48 hrs. MTT assay was used to assess cell viability. Luteolin concentrations, 1.25, 2.5, and 5 ng/ml, show no viability reduction of the Wild type 20Q treated cells (A). Vehicle-treated 160 Q cells show a significant reduction in cell viability compared to the vehicle-treated Wild type 20 Q cells (B). The mutant 160 Q cells treated with 1.25, 2.5, and 5 ng/ml Luteolin concentrations show a significant increase in cell viability compared to the vehicle-treated mutant cells. Data represented as mean ± SD *, p < 0.05; **, p < 0.01 vs. control. n = 6 in triplicates.
To confirm the protective activities of luteolin against mutant htt cytotoxicity, we utilized propidium iodide (PI) staining. The number of dye-positive cells stained red was significantly higher in the mutant htt cells than the wild-type cells (60%, p < 0.01, Fig. 3). Luteolin treatment significantly reduced PI-positive staining in mutant cells (20%), indicating more cell viability (p < 0.05, Fig. 3). Caspase-3 is an essential mediator of apoptosis. Hence, caspase 3 expression was assessed via western blotting. Western blot analysis showed that the htt 160Q mutant cells had an increased level of the pro-apoptotic active caspase3 compared to the wild-type htt 20Q cells (p < 0.01, Fig. 4). Luteolin treatment significantly inhibited the active caspase3 expression in the mutant and wild-type htt cells, with a more pronounced effect in the mutant cells which demonstrates luteolin's anti-apoptotic activities (Fig. 4).
Fig. 3.
Luteolin increased the viability of mutant neuroblastoma cells. PI stain shows the cell viability of the wild type 20 Q and mutant 160Q htt cells after being treated with vehicle or luteolin (5 ng/ml) for 48 hrs. Arrowheads indicate htt aggregation (Green), while the remaining arrows show PI-positive cells (Red) (A). B) Shows quantifications of the PI-positive cells in the wild type and mutant htt cells as a percentage of average density 150X104/ ml. Mutant htt 160 Q cells show higher PI-positive cells than wild type 20Q cells. Luteolin treated 160 Q cells show a significant reduction in the PI-positive cells compared to vehicle cells. Scale bar: 50 µm. Data represented as mean ± SD *, p < 0.05; **, p < 0.01 vs. control. n = 6 in triplicates.
Fig. 4.
Caspase 3 expression was reduced in luteolin treated cells. A) Western blot detection of the cleaved caspase3 in the wild type 20 Q and mutant 160 Q cells treated with vehicle or luteolin (5 ng/ml) for 48 hrs. B) Western blot quantification shows significant inhibitions of the cleaved caspase 3 in the wild type and mutant htt cells treated with luteolin compared with the vehicle cells. Data represented as mean ± SD **, p < 0.01 vs control. n = 6 in triplicates.
3.2. Luteolin treatment reduced the aggregated and soluble mutant htt in N2a cells expressing mutant huntingtin
The mutated htt forms were shown to aggregate in the neurons at different parts of the brain of HD mice (Alpaugh et al., 2022). These aggregations have been linked to neuronal dysfunction and premature death. It has been proposed to form a spectrum of oligomeric species and different huntingtin oligomers in vitro that exhibit varying degrees of cytotoxicity (Subramaniam, 2019, Feng et al., 2018).
We then assessed whether the observed protection of mutant htt cells after luteolin treatment resulted from the reduction of mutant huntingtin aggregations. Cells expressing N-terminal wild-type (htt 20Q) and mutant htt (mhtt 160Q) were treated with the luteolin or vehicle (control). The control cells expressing the mutant htt 160Q showed a marked time-dependent accumulation of the soluble and aggregates of the mutant htt protein (Fig. 5). These aggregates were found to be diffused into the cell cytoplasm, observed through a fluorescence microscope. These diffused aggregates were markedly reduced upon luteolin treatment (Fig. 5). Furthermore, there was a decrease in aggregated htt expression in luteolin-treated htt 160Q (Fig. 5). The mutant htt protein aggregates were more abundant 48 h post transfection. Accordingly, 48 h was used as a time point to examine the protective efficacy of luteolin treatment against mutant htt aggregates. The observed accumulation of the mutant htt aggregates was drastically prevented by luteolin treatment, significantly reducing both soluble and aggregated mutant htt protein in a concentration- and time-dependent manner (p < 0.01 vs. non-treated group, Fig. 6).
Fig. 5.
Luteolin reduced aggregates in mutant htt cells. A) Immunofluorescence detection of the mutant htt 160 Q cells shows that luteolin (5 ng/ml) treatment reduces mutant htt aggregates (white head arrows) after 48 h of transfection. B) Western blotting detection of the mutant Htt aggregates and soluble protein in the cells treated with vehicle or luteolin for 24 and 48 h. Scale bar: 50 µm.
Fig. 6.
Luteolin reduced soluble and aggregated htt in a dose-dependent manner. A) Western blot detection of the htt protein in neuroblastoma cells transfected with mutant 160 Q and treated with vehicle or luteolin (2.5 and 5 ng/ml) for 48 hrs. B) and C) show a significant decrease in the soluble and aggregated mutant htt protein in cells treated with different concentrations of luteolin. Data represented as mean ± SD **, p < 0.01 vs control. n = 6 in triplicates.
Evaluation of the selectivity effect of luteolin treatment showed that luteolin treatment (5 ng/ml) affected the mutant form. Indeed, the decrease in mutant htt 160 Q protein expression (aggregate and soluble forms) was evident compared to non-luteolin treated cells (Fig. 7). To ensure that the observed reduction in mutant htt aggregates was due to the effects of luteolin treatment on mutant htt protein degradation rather than htt gene expression, the htt mRNA expression level was assessed using RT-PCR. The luteolin-treated cells showed no significant difference in the htt mRNA expression level compared to the vehicle-treated cells mutant htt 160 Q transfected cells. Also, the htt mRNA expression was similar in the normal htt 20 Q and mutant htt 160 cells. This suggests that luteolin reduction of the mutated htt protein is not due to changes in RNA expression (Fig. 7).
Fig. 7.
Luteolin reduced htt protein but not transcript expression. A) Western blot detection of the normal htt 20 Q and mutant htt 160 Q's soluble and aggregated forms treated with luteolin 5 ng/ml or vehicle. B) Detection of htt mRNA expression in normal 20 Q and mutant htt 160 Q cells treated with luteolin 5 ng/ml or vehicle using RT-PCR. C) shows the insignificant difference in the mRNA expression level of the normal 20 Q and mutant htt 160 Q cells treated with luteolin. Data represented as mean ± SD. n = 6 in triplicates.
3.3. Luteolin treatment induced the Nrf2 ARE pathway
Oxidative stress has been described as a significant mechanism in the pathogenesis of HD (Zheng et al., 2018). The htt mutant cells treated with H2O2 increased the accumulation of htt aggregates and cell death susceptibility (Iuchi et al., 2021). Hence, using antioxidants or enhancing and activating the antioxidant pathways will be a promising method to reduce mutant htt aggregates and inhibit their toxicity. Luteolin has been reported to activate the antioxidant pathway Nrf2- HO-1 pathway (Li et al., 2019, Siddique, 2021). To study whether treatment with luteolin can induce the antioxidant enzymes and protect the mutant htt cells from oxidative stress and cytotoxicity, Nrf2-ARE antioxidant pathway was assessed in the cells expressing the mutant htt 160Q or the wild-type htt 20Q treated with the luteolin or the vehicle. Western blotting analysis shows that luteolin treatment significantly increased protein levels of Nrf2, the transcription factor responsible for activating many antioxidant enzymes, compared to vehicle-treated cells (p < 0.01, Fig. 8). Similarly, the Nrf2 downstream antioxidant target enzyme Ho-1 also showed a significant increase in protein expression levels in both mutant and wild-type luteolin-treated cells (p < 0.01 vs. non treated cells, Fig. 8). Note that the increased expression was more apparent in the mutant group compared to the wild-type (Fig. 8).
Fig. 8.
Antioxidants expression was elevated in luteolin treated cells. Western blot detection of the Nrf2 (A) and HO-1, (B) in the wild type 20 Q and mutant 160 Q cells treated with vehicle or luteolin (5 ng/ml) for 48 hrs (C) shows a significant increase in protein expression of Nrf2 in the wild type and mutant htt cells treated with luteolin compared to the vehicle cells. (D) shows a significant upregulation in Nrf2 downstream target HO-1 in the wild type and mutant htt cells treated with luteolin compared to the vehicle cells. Data represented as mean ± SD **, p < 0.01 vs control. n = 6 in triplicates.
This activation of the Nrf2-ARE signaling pathway in luteolin treated could be how it mediates its protective effects. We showed a significant accumulation of the soluble and aggregated mutant htt after inhibiting the Nrf2-ARE signaling pathway using Nrf2 siRNA in the mutant htt 160Q. While the Nrf2 siRNA abrogates Nrf2 ARE function, luteolin treatment still reduced mutated and soluble htt aggregates (Fig. 9). Thus, luteolin reduction of soluble and aggregated mutant htt is independent of antioxidant Nrf2 ARE activation.
Fig. 9.
Silencing Nrf2 did not affect Luteolin mediated reduction of mutant Htt protein. (A) Western blot detection of mutant Htt protein in neuroblastoma cells transfected with 160Q, followed by silencing of Nrf2 or vector as control, then treated with vehicle or 5 ng/ml luteolin for 48 hrs. Quantification of htt protein shows a significant increase in mutant htt aggregates, (B) and soluble mutant htt, (C) in Nrf2 silenced cells compared to the vector control cells. Luteolin treatment significantly reduced both mutant htt soluble and aggregated proteins in the silenced Nrf2 and the vector control cells compared to the vehicle-treated cells. Data represented as mean ± SD **, p < 0.01 vs. control. n = 6 in triplicates.
4. Discussion
Although the exact pathogenesis of HD remains unclear, it has been suggested that mutated huntingtin is responsible for mediating the pathogenesis process (Ghosh and Tabrizi, 2018). The accumulation of mutant htt neuronal aggregates is the main feature of the disease (Chung et al., 2018). Abnormal huntingtin (htt) protein interactions (Zhu et al., 2019, Wanker et al., 2019), oxidative stress and transcription factor deregulations are implicated in several poly-Q neurodegenerative diseases, including HD (Gkekas et al., 2021). The increase in reactive oxygen species production and oxidative stress cause damage to cellular macromolecules such as DNA, lipids, and proteins, ultimately resulting in necrosis and apoptotic cell death and damage of proteasome and autophagy pathways, resulting in increased aggregation and toxicity of mutant htt (Abramov et al., 2020). Therefore, inhibiting oxidative stress could represent an attractive therapeutic target to delay neurodegeneration in HD, although the origin of oxidative stress in the disease has been complicated (Sharma et al., 2022). This study examined the potential neuroprotective effects of the well-known antioxidant luteolin and the mechanisms underlying the protection in the treated htt mutant cells. Our findings show that treatment with luteolin significantly protected the mutant htt cells and prevented cell death and degeneration in this HD invitro model. Also, luteolin significantly reduced soluble and aggregated mutant htt cells aggregates. Furth more, luteolin induced the expression of the antioxidant pathway Nrf2-HO-1.
Similar to our findings, amyloid plaque aggregate deposition was less in the Alzheimer's disease (AD) fruit fly model that received 20 μM of luteolin than the unexposed ones. Furthermore, the apoptotic markers caspase 3 and caspase 9 activity was reduced in the luteolin recipient flies relative to the unexposed ones (Ali et al. 2019). Likewise, in an AD’s mouse model, where amyloid-beta oligomers were injected intracerebroventricularly into mice's brains, Luteolin-treated mice's amyloid-beta expression was reduced compared to the non-treated group. Also, the expression of the pro-apoptotic proteins Bax, Bcl-2, and Caspase-3 was reduced, while the anti-apoptotic marker Cox-2 was increased in luteolin-treated mice compared to the brains of the amyloid-beta oligomers -injected group (Ahmad et al. 2021). These pro- and anti-apoptotic markers expression changes were also observed in another Triple transgenic (3 × Tg-AD) AD’s mouse model (He et al., 2023), in addition to the reduction of TUNEL-positive neurons. Luteolin neuroprotective effect was also observed in the in vitro neurodegenerative model (SH-SY5Y), where monomers/aggregates and caspase-3 activity were decreased in Luteolin-7-O-Glucoside treated cells compared to the non-treated cells (Rehfeldt et al. 2022). In a Parkinson's disease transgenic Drosophila model that expresses wild-type human synuclein, luteolin decreased the expression of the pro-apoptotic caspases in a dose-dependent manner and preserved the dopamine content and dopamine neurons in the brain of PD flies in comparison to the unexposed ones (Siddique et al. 2018). luteolin-mediated reduction of brain cell death was also observed in HD transgenic fly lines that express the first coding exon of human htt encoding 96 glutamines. Additionally, molecular docking analysis revealed strong binding stabilization through hydrophobic and Vander Waals interaction between luteolin and htt protein (Siddique, 2021). Please refer to Table 1, Table 2 for further elaboration pertaining to luteolin anti-apoptotic and anti-aggregation effects.
Table 1.
Luteolin and its anti-apoptotic property in neurodegenerative disease models.
| Relevant studies ↓ | Molecule | Study samples | Neurodegenerative disease | Effect |
|---|---|---|---|---|
|
Current Study Abuelnor Mohammed et al., 2023 (unpublished) |
Luteolin | Neuroblastoma Cells |
Huntington Disease |
Cleaved Caspase 3 expression was decreased in luteolin treated mutant Htt cells compared to the control. |
| He et al., 2023 | Luteolin | Triple transgenic AD (3 × Tg-AD) mice | Alzheimer’s disease | Luteolin supplement significantly ameliorated memory and cognitive impairment of Alzheimer’s diseased mice. Luteolin treatment effectively increased the bcl-2 expression levels and decreased Bax, CytC, cleaved-caspase9 and cleaved-caspase3 expression levels. Also, luteolin treatment reduced the ratio of TUNEL-positive neurons. |
| Rehfeldt et al., 2022 | Luteolin-7-O-Glucoside | Neurotoxin 6-OHDA-Induced Damage in vitro neurodegenerative model (SH-SY5Y) | Neuroblastoma cells |
Caspase-3 activity was decreased in Luteolin-7-O-Glucoside-treated cells. vs. the non-treated cells. Lut7 decreased the occurrence of nuclear condensation and fragmentation, characteristic features of apoptosis induced by 6-OHDA. |
| Ahmad et al., 2021 | Luteolin | amyloid-beta oligomers were injected intracerebroventricularly into mice's brains | Alzheimer's mouse model | Pro-apoptotic proteins Bax, Bcl-2, and Caspase-3 were reduced, while the anti-apoptotic marker Cox-2 was increased in luteolin treated mice compared to the untreated ones. |
| Siddique et al., 2021 | Luteolin | Transgenic fly lines that expresses first coding exon of human htt encoding 96 glutamines | Huntington’s disease | Brains of flies fed a diet supplemented with luteolin had a decreased expression of the apoptotic markers caspase 3 and 9 compared to the control. |
| Ali et al., 2019 | Luteolin | fruit fly model expressing human Aβ42 | Alzheimer's disease | Caspase 3 and caspase 9 activity was less in the luteolin recipient flies relative to the unexposed ones. |
| Siddique et al., 2018 | Luteolin | Transgenic fly (Drosophila) lines that express wild-type human synuclein |
Parkinson’s disease | The expression of caspase 3 and 9 in Parkinson’s diseased flies exposed to luteolin was reduced in a dose-dependent manner in the brain of Parkinson’s diseased flies vs. unexposed ones. |
Table 2.
Luteolin anti-aggregation property and protein deposition reduction in neurodegenerative disease models.
| Relevant studies ↓ | Molecule | Study samples | Neurodegenerative disease | Effect |
|---|---|---|---|---|
|
Current Study Abuelnor Mohammed et al., 2023 (unpublished) |
Luteolin | Neuroblastoma Cells |
Huntington Disease |
Reduction of soluble and aggregated htt protein. |
| Rehfeldt et al., 2022 | Luteolin-7-O-Glucoside | Neurotoxin 6-OHDA-Induced Damage in vitro neurodegenerative model (SH-SY5Y) | Neuroblastoma cells |
Monomers/aggregates were decreased in Luteolin-7-O-Glucoside-treated cells. vs. the non-treated cells |
| Ahmad et al., 2021 | Luteolin | Amyloid-beta oligomers injected intracerebroventricularly into mice's brains | Alzheimer's mouse model | Amyloid-beta expression was reduced compared to the non-treated group. |
| Ali et al., 2019 | Luteolin | Fruit fly model expressing human Aβ42 |
Alzheimer's disease | Deposition of amyloid plaque was less in the Alzheimer's disease fruit fly model. |
| Siddique et al., 2018 | Luteolin | Transgenic fly (Drosophila) lines that express wild-type human synuclein |
Parkinson’s disease | The presence of dopaminergic neurons in the Parkinson’s diseased flies exposed to various doses of luteolin. Also, the exposure to luteolin showed a dose-dependent increase in the dopamine content in the brains of Parkinson’s diseased flies. |
Our finding of luteolin-mediated antioxidant activity, evident by the induction of the antioxidant pathway, agrees with findings in several neurodegenerative disease models. For instance, in an AD transgenic model, luteolin induced the activity of the antioxidants SOD and glutathione (GSH). Furthermore, luteolin increased the expression of uncoupling protein 2 (UCP2), a key player that prevents ROS production and hence protects neurons from oxidative stress (He et al., 2023, Table 3). Similarly, luteolin decreased the protein carbonyl content, lipid peroxidation and malondialdehyde (MDA), a biomarker of oxidative damage and O2 − levels in Parkinson’s disease models compared to non-luteolin recipients (Siddique et al., 2018, Reudhabibadh et al., 2021) (Table 3). Luteolin mediated increase the antioxidant enzymes SOD and CAT was also observed in an HD model (Siddique, 2021) (Table 3).
Table 3.
Luteolin and its antioxidant property in neurodegenerative disease models.
| Relevant studies ↓ | Molecule | Study samples | Neurodegenerative disease | Effect |
|---|---|---|---|---|
|
Current Study Abuelnor Mohammed et al., 2023 (unpublished) |
Luteolin | Neuroblastoma Cells |
Huntington Disease |
Activation of Nrf2-OH1 |
| He et al., 2023 | Luteolin | Triple transgenic AD (3 × Tg-AD) mice | Alzheimer’s disease | Luteolin treatment increased both the antioxidants SOD activity and GSH levels while decreasing the level of MDA. Also, UCP2, a key part of the mechanism preventing ROS production and plays a vital role in protecting neurons from oxidative stress, levels were increased in luteolin treated Alzheimer’s diseased mice compared to wild type mice. |
| Reudhabibadh et al., 2021 | Luteolin | Neurotoxicity induction by 1-methyl-4-phenylpyridinium iodide (MPP + ), a neurotoxin in neuroblastoma SH-SY5Y cells | Parkinson’s Disease | Treatment with luteolin significantly attenuated MPP + -induced O2 − elevation compared to the MPP + treated group. Also, luteolin reduced MPP + induction of MDA, a biomarker of oxidative damage and O2 − levels, compared to the control group. |
| Siddique, 2021 | Luteolin | Transgenic fly lines that expresses first coding exon of human htt | Huntington’s disease | The activity of GSH, SOD and CAT in luteolin exposed Huntington’s diseased flies was increased compared to controls. |
| Siddique et al., 2018 | Luteolin | Transgenic fly (Drosophila) lines that express wild-type human synuclein |
Parkinson’s disease | PD flies exposed to luteolin showed an increase in the GSH activity in a dose dependent manner while a decrease in protein carbonyl content and lipid peroxidation compared to unexposed flies. |
Interestingly, Ali and Siddique (2019) indicated that in terms of the bioavailability of luteolin, it remains as glucuronides and sulphate-conjugates in the plasma after being metabolized. Also, toxicity was not observed at a dose of 100 mg/day in clinical trials (Ali and Siddique, 2019).
Several studies have highlighted the protective effects of Nrf2 activation in reducing oxidative stress in both in vitro and in vivo models of neurodegenerative diseases (Brandes and Gray, 2020). Luteolin has been reported to upregulate the expression of HO-1 and Nrf2 (Xiao et al., 2019, Al-Megrin et al., 2020). Similarly, we found that Nrf2 and HO-1 expression was elevated in luteolin-treated cells, an effect that was enhanced in the mutant group. It is important to note that oxidative stress can result in protein aggregation due to oxidation of critical amino acids, resulting in protein structural changes, modulation of chaperone, proteasome and autophagy activity and many more mechanisms (Levy et al., 2019). Hence, we wanted to investigate if inhibiting the luteolin-mediated activation of the antioxidant Nrf2 would exacerbate htt cell aggregates. However, despite blocking the Nrf2 ARE antioxidant pathway, luteolin was still able to reduce the mutant htt aggregate. Our findings suggest that the luteolin antiaggregatory effect is independent of its antioxidant properties.
With the mentioned results and relatively low toxicity, luteolin represents a new potential therapeutic and protective agent for treating and preventing neurodegenerative diseases such as HD, which are caused by proteins prone to intracytoplasmic aggregates.
5. Conclusion
Luteolin is a small-molecule flavonoid natural product found in many plants that can cross the blood–brain barrier. Our study concluded that luteolin effectively reduced the mutant huntingtin cytotoxicity, protected the neuroblastoma cells, and reduced soluble and aggregated mutant huntingtin proteins. The luteolin-mediated reduction of the aggregates was independent of the Nrf2 ARE antioxidant pathway. The findings will be validated in Huntington's transgenic animal models. Furthermore, the cellular pathways involved in luteolin-mediated protection will be further investigated.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Azza Ramadan, Email: azza.ramadan@aau.ac.ae.
Abuelnor Mohammed, Email: Abuelnor88@yahoo.com.
Asim Ahmed Elnour, Email: asim.ahmed@aau.ac.ae.
Adel Sadeq, Email: adel.sadeq@aau.ac.ae.
Nadia Al Mazrouei, Email: nalmazrouei@sharjah.ac.ae.
Khalid Awad Al-Kubaisi, Email: kalkubaissi@sharjah.ac.ae.
Semira Abdi Beshir, Email: dr.semira@dpc.edu.
Vineetha Menon, Email: dr.vineetha@gmu.ac.ae.
Abdulla AlAmoodi, Email: aalamoodi@seha.ae.
Kishore Ganana Sam, Email: dr.kishore@dpc.edu.
Sami Fatehi Abdalla, Email: sbillal@um.edu.sa.
References
- Abramov A.Y., Potapova E.V., Dremin V.V., Dunaev A.V. Interaction of Oxidative Stress and Misfolded Proteins in the Mechanism of Neurodegeneration. Life (Basel, Switzerland) 2020;10(7):101. doi: 10.3390/life10070101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S., Jo M.H., Ikram M., Khan A., Kim M.O. Deciphering the Potential Neuroprotective Effects of Luteolin against Aβ1-42-Induced Alzheimer's Disease. Int. J. Mol. Sci. 2021;22(17):9583. doi: 10.3390/ijms22179583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinrinde A.S., Adebiyi O.E. Neuroprotection by luteolin and gallic acid against cobalt chloride-induced behavioural, morphological and neurochemical alterations in Wistar rats. Neurotoxicology. 2019;74:252–263. doi: 10.1016/j.neuro.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Ali F., Siddique Y.H. Bioavailability and Pharmaco-therapeutic Potential of Luteolin in Overcoming Alzheimer's Disease. CNS Neurol. Disord. Drug Targets. 2019;18(5):352–365. doi: 10.2174/1871527318666190319141835. [DOI] [PubMed] [Google Scholar]
- Ali F., Rahul, Jyoti S., Naz F., Ashafaq M.o., Shahid M., Siddique Y.H. Therapeutic potential of luteolin in transgenic Drosophila model of Alzheimer's disease. Neurosci. Lett. 2019;692:90–99. doi: 10.1016/j.neulet.2018.10.053. [DOI] [PubMed] [Google Scholar]
- Al-Megrin W.A., Alomar S., Alkhuriji A.F., Metwally D.M., Mohamed S.K., Kassab R.B., Abdel Moneim A.E., El-Khadragy M.F. Luteolin protects against testicular injury induced by lead acetate by activating the Nrf2/HO-1 pathway. IUBMB Life. 2020;72(8):1787–1798. doi: 10.1002/iub.2311. [DOI] [PubMed] [Google Scholar]
- Alothman D., Marshall C.R., Tyrrell E., Lewis S., Card T., Fogarty A. Risk of mortality from suicide in patients with Huntington's disease is increased compared to the general population in England. J. Neurol. 2022;269(8):4436–4439. doi: 10.1007/s00415-022-11085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpaugh M., Denis H.L., Cicchetti F. Prion-like properties of the mutant huntingtin protein in living organisms: the evidence and the relevance. Mol. Psychiatry. 2022;27(1):269–280. doi: 10.1038/s41380-021-01350-4. [DOI] [PubMed] [Google Scholar]
- Arning L., Nguyen H. Huntington disease update: new insights into the role of repeat instability in disease pathogenesis. Med. Gen. 2021;33(4):293–300. doi: 10.1515/medgen-2021-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashaari Z., Hadjzadeh M.A., Hassanzadeh G., Alizamir T., Yousefi B., Keshavarzi Z., Mokhtari T. The Flavone Luteolin Improves Central Nervous System Disorders by Different Mechanisms: A Review. J. Mol. Neurosci. : MN. 2018;65(4):491–506. doi: 10.1007/s12031-018-1094-2. [DOI] [PubMed] [Google Scholar]
- Bakoyiannis I., Daskalopoulou A., Pergialiotis V., Perrea D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. = Biomedecine & pharmacotherapie. 2019;109:1488–1497. doi: 10.1016/j.biopha.2018.10.086. [DOI] [PubMed] [Google Scholar]
- Bono-Yagüe J., Gómez-Escribano A.P., Millán J.M., Vázquez-Manrique R.P. Reactive Species in Huntington Disease: Are They Really the Radicals You Want to Catch? Antioxidants (Basel, Switzerland) 2020;9(7):577. doi: 10.3390/antiox9070577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes M.S., Gray N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro. 2020;12 doi: 10.1177/1759091419899782. 1759091419899782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C.G., Lee H., Lee S.B. Mechanisms of protein toxicity in neurodegenerative diseases. Cell. Mol. Life Sci. 2018;75:3159–3180. doi: 10.1007/s00018-018-2854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J., Powell S., Rickards N., Holmans P., Jones L., Jones L., Pearson C.E., Wheeler V. What is the Pathogenic CAG Expansion Length in Huntington's Disease? J. Huntington's Dis. 2021;10(1):175–202. doi: 10.3233/JHD-200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, G. M., de Araújo, F. E. A., da Rocha, J. M. C., Idalina Neta, F., do Rego, A. C. M., Araújo Filho, I., Pinheiro, F. I., de Azevedo, E. P., Cobucci, R. N., & Guzen, F. P. (2023). Neuroprotective Potential of Seed Extracts: Review of In Vitro and In Vivo Studies. Nutrients, 15(11), 2502. Doi: 10.3390/nu15112502. [DOI] [PMC free article] [PubMed]
- Feng X., Luo S., Lu B. Conformation Polymorphism of Polyglutamine Proteins. Trends Biochem. Sci. 2018;43(6):424–435. doi: 10.1016/j.tibs.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Francisqueti-Ferron F.V., Ferron A.J.T., Garcia J.L., Silva C.C.V.A., Costa M.R., Gregolin C.S., Moreto F., Ferreira A.L.A., Minatel I.O., Correa C.R. Basic Concepts on the Role of Nuclear Factor Erythroid-Derived 2-Like 2 (Nrf2) in Age-Related Diseases. Int. J. Mol. Sci. 2019;20(13):3208. doi: 10.3390/ijms20133208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, R., Tabrizi, S.J. (2018). Clinical Features of Huntington’s Disease. In: Nóbrega, C., Pereira de Almeida, L. (eds) Polyglutamine Disorders. Advances in Experimental Medicine and Biology, vol 1049. Springer, Cham. Doi: 10.1007/978-3-319-71779-1_1. [DOI] [PubMed]
- Gkekas I., Gioran A., Boziki M.K., Grigoriadis N., Chondrogianni N., Petrakis S. Oxidative Stress and Neurodegeneration: Interconnected Processes in PolyQ Diseases. Antioxidants (Basel, Switzerland) 2021;10(9):1450. doi: 10.3390/antiox10091450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Li X., Wang Z., Cao Y., Han S., Li N., Cai J., Cheng S., Liu Q. Protective effects of luteolin against amyloid beta-induced oxidative stress and mitochondrial impairments through peroxisome proliferator-activated receptor γ-dependent mechanism in Alzheimer's disease. Redox Biol. 2023;66 doi: 10.1016/j.redox.2023.102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M., Rauf A., Abu-Izneid T., Nadeem M., Shariati M.A., Khan I.A., Imran A., Orhan I.E., Rizwan M., Atif M., Gondal T.A., Mubarak M.S. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. = Biomedecine & pharmacotherapie. 2019;112 doi: 10.1016/j.biopha.2019.108612. [DOI] [PubMed] [Google Scholar]
- Iuchi K., Takai T., Hisatomi H. Cell Death via Lipid Peroxidation and Protein Aggregation Diseases. Biology. 2021;10(5):399. doi: 10.3390/biology10050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K.A., Piao M.J., Ryu Y.S., Hyun Y.J., Park J.E., Shilnikova K., Zhen A.X., Kang H.K., Koh Y.S., Jeong Y.J., Hyun J.W. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017;51(4):1169–1178. doi: 10.3892/ijo.2017.4091. [DOI] [PubMed] [Google Scholar]
- Levy E., El Banna N., Baïlle D., Heneman-Masurel A., Truchet S., Rezaei H., Huang M.E., Béringue V., Martin D., Vernis L. Causative Links between Protein Aggregation and Oxidative Stress: A Review. Int. J. Mol. Sci. 2019;20(16):3896. doi: 10.3390/ijms20163896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Luo W., Qian Y., Zhu W., Qian J., Li J., Jin Y., Xu X., Liang G. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomed.: Int. J. Phytother. Phytopharmacol. 2019;59 doi: 10.1016/j.phymed.2018.11.034. [DOI] [PubMed] [Google Scholar]
- Lieberman A.P., Shakkottai V.G., Albin R.L. Polyglutamine Repeats in Neurodegenerative Diseases. Annu. Rev. Pathol. 2019;14:1–27. doi: 10.1146/annurev-pathmechdis-012418-012857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelo A., Koppenol R., de Almeida L.P., Matos C.A., Nóbrega C. Stress granules, RNA-binding proteins and polyglutamine diseases: too much aggregation? Cell Death Dis. 2021;12(6):592. doi: 10.1038/s41419-021-03873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColgan P., Tabrizi S.J. Huntington's disease: a clinical review. Eur. J. Neurol. 2018;25(1):24–34. doi: 10.1111/ene.13413. [DOI] [PubMed] [Google Scholar]
- Medina A., Mahjoub Y., Shaver L., Pringsheim T. Prevalence and Incidence of Huntington's Disease: An Updated Systematic Review and Meta-Analysis. Movement Disorders: Off. J. Movement Disorder Society. 2022;37(12):2327–2335. doi: 10.1002/mds.29228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakannan P., Tabeshmehr P., Eftekharpour E. Oxidative damage of lysosomes in regulated cell death systems: Pathophysiology and pharmacologic interventions. Free Radic. Biol. Med. 2020;157:94–127. doi: 10.1016/j.freeradbiomed.2020.04.001. [DOI] [PubMed] [Google Scholar]
- Niemann N., Jankovic J. Juvenile parkinsonism: Differential diagnosis, genetics, and treatment. Parkinsonism Relat. Disord. 2019;67:74–89. doi: 10.1016/j.parkreldis.2019.06.025. [DOI] [PubMed] [Google Scholar]
- Radbakhsh S., Barreto G.E., Bland A.R., Sahebkar A. Curcumin: A small molecule with big functionality against amyloid aggregation in neurodegenerative diseases and type 2 diabetes. BioFactors (Oxford, England) 2021;47(4):570–586. doi: 10.1002/biof.1735. [DOI] [PubMed] [Google Scholar]
- Rehfeldt S.C.H., Silva J., Alves C., Pinteus S., Pedrosa R., Laufer S., Goettert M.I. Neuroprotective Effect of Luteolin-7-O-Glucoside against 6-OHDA-Induced Damage in Undifferentiated and RA-Differentiated SH-SY5Y Cells. Int. J. Mol. Sci. 2022;23(6):2914. doi: 10.3390/ijms23062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reudhabibadh R., Binlateh T., Chonpathompikunlert P., Nonpanya N., Prommeenate P., Chanvorachote P., Hutamekalin P. Suppressing Cdk5 Activity by Luteolin Inhibits MPP+-Induced Apoptotic of Neuroblastoma through Erk/Drp1 and Fak/Akt/GSK3β Pathways. Molecules (Basel, Switzerland) 2021;26(5):1307. doi: 10.3390/molecules26051307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Santana I., Mestre T., Squitieri F., Willock R., Arnesen A., Clarke A., D'Alessio B., Fisher A., Fuller R., Hamilton J.L., Hubberstey H., Stanley C., Vetter L., Winkelmann M., Doherty M., Wu Y., Finnegan A., Frank S. Economic burden of Huntington disease in Europe and the USA: Results from the Huntington's Disease Burden of Illness study. Eur. J. Neurol. 2023;30(4):1109–1117. doi: 10.1111/ene.15645. [DOI] [PubMed] [Google Scholar]
- Seelinger G., Merfort I., Wölfle U., Schempp C.M. Anti-carcinogenic effects of the flavonoid luteolin. Molecules (Basel, Switzerland) 2008;13(10):2628–2651. doi: 10.3390/molecules13102628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Advani D., Das A., Malhotra N., Khosla A., Arora V., Jha A., Yadav M., Ambasta R.K., Kumar P. Pharmacological intervention in oxidative stress as a therapeutic target in neurological disorders. J. Pharm. Pharmacol. 2022;74(4):461–484. doi: 10.1093/jpp/rgab064. [DOI] [PubMed] [Google Scholar]
- Shaw E., Mayer M., Ekwaru P., McMullen S., Graves E., Wu J.W., Budd N., Maturi B., Cowling T., Mestre T.A. Epidemiology and economic burden of Huntington's disease: a Canadian provincial public health system perspective. J. Med. Econ. 2022;25(1):212–219. doi: 10.1080/13696998.2022.2033493. [DOI] [PubMed] [Google Scholar]
- Siddique Y.H. Role of luteolin in overcoming Parkinson's disease. BioFactors (Oxford, England) 2021;47(2):198–206. doi: 10.1002/biof.1706. [DOI] [PubMed] [Google Scholar]
- Siddique Y.H., Jyoti S., Naz F. Protective effect of luteolin on the transgenic Drosophila model of Parkinson’s disease. Braz. J. Pharm. Sci... 2018;54(3) doi: 10.1590/s2175-97902018000317760. [DOI] [Google Scholar]
- Stavoe A.K.H., Holzbaur E.L.F. Autophagy in Neurons. Annu. Rev. Cell Dev. Biol. 2019;35:477–500. doi: 10.1146/annurev-cellbio-100818-125242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S. Selective Neuronal Death in Neurodegenerative Diseases: The Ongoing Mystery. Yale J. Biol. Med. 2019;92(4):695–705. [PMC free article] [PubMed] [Google Scholar]
- Tobore T.O. Towards a comprehensive understanding of the contributions of mitochondrial dysfunction and oxidative stress in the pathogenesis and pathophysiology of Huntington's disease. J. Neurosci. Res. 2019;97(11):1455–1468. doi: 10.1002/jnr.24492. [DOI] [PubMed] [Google Scholar]
- Vieweg S., Mahul-Mellier A.L., Ruggeri F.S., Riguet N., DeGuire S.M., Chiki A., Cendrowska U., Dietler G., Lashuel H.A. The Nt17 Domain and its Helical Conformation Regulate the Aggregation, Cellular Properties and Neurotoxicity of Mutant Huntingtin Exon 1. J. Mol. Biol. 2021;433(21) doi: 10.1016/j.jmb.2021.167222. [DOI] [PubMed] [Google Scholar]
- Wanker E.E., Ast A., Schindler F., Trepte P., Schnoegl S. The pathobiology of perturbed mutant huntingtin protein-protein interactions in Huntington's disease. J. Neurochem. 2019;151(4):507–519. doi: 10.1111/jnc.14853. [DOI] [PubMed] [Google Scholar]
- Xiao C., Xia M.L., Wang J., Zhou X.R., Lou Y.Y., Tang L.H., Zhang F.J., Yang J.T., Qian L.B. Luteolin Attenuates Cardiac Ischemia/Reperfusion Injury in Diabetic Rats by Modulating Nrf2 Antioxidative Function. Oxid. Med. Cell. Longev. 2019;2019:2719252. doi: 10.1155/2019/2719252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Winderickx J., Franssens V., Liu B. A Mitochondria-Associated Oxidative Stress Perspective on Huntington's Disease. Front. Mol. Neurosci. 2018;11:329. doi: 10.3389/fnmol.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Li C., Tao X., Brazill J.M., Park J., Diaz-Perez Z., Zhai R.G. Nmnat restores neuronal integrity by neutralizing mutant Huntingtin aggregate-induced progressive toxicity. PNAS. 2019;116(38):19165–19175. doi: 10.1073/pnas.1904563116. [DOI] [PMC free article] [PubMed] [Google Scholar]