Abstract
Pulmonary hypertension (PH) is defined by the presence of a mean pulmonary arterial pressure >20 mmHg. Current guidelines describe five groups of PH with shared pathophysiological and clinical features. In this paper, the first of a series covering all five PH classification groups, the clinical, radiological and pathological features of pulmonary arterial hypertension (PAH) will be reviewed. PAH may develop in the presence of associated medical conditions or a family history, following exposure to certain medications or drugs, or may be idiopathic in nature. Although all forms of PAH share common histopathological features, the presence of certain pulmonary arterial abnormalities, such as plexiform lesions, and extent of co-existing pulmonary venous involvement differs between the different subgroups. Radiological investigations are key to diagnosing the correct form of PH and a systematic approach to interpretation, especially of computed tomography, is essential.
Tweetable abstract
A review of clinical, radiological and pathological features of pulmonary arterial hypertension (PAH). Emphasis on systematic evaluation of CTPA in making the correct diagnosis and expert discussion of features of PAH under the microscope. https://bit.ly/3PfuAp7
Introduction
Pulmonary hypertension (PH) describes a number of conditions characterised haemodynamically by a mean pulmonary arterial pressure (mPAP) >20 mmHg [1]. Pre-capillary PH is present when the left atrial (LA) pressure, usually assessed by the pulmonary arterial wedge pressure (PAWP), is ≤15 mmHg and the pulmonary vascular resistance (PVR) is >2 Wood units (WU). Post-capillary PH is diagnosed when the PAWP is >15 mmHg. Current classification defines five groups based on shared clinical and pathophysiological features with groups 1 (pulmonary arterial hypertension, PAH), 3 (PH associated with lung disease and/or hypoxia) and 4 (PH associated with pulmonary arterial obstructions) being pre-capillary and group 2 (PH associated with left heart disease) post-capillary. Group 5 consists of PH with unclear and/or multifactorial mechanisms. This paper, the first of a series exploring clinical features, pathological changes and radiological findings in PH, will focus on PAH. Throughout the series, illustrative cases will be presented and the importance of a systematic approach to radiological interpretation will be emphasised.
Methods
The contents of this paper formed the basis of the first in a recent European Respiratory Society webinar series [2]. Cases representative of different forms of PAH were chosen, with alterations of certain features of clinical histories. Pathological specimens of one case were available; specimens demonstrating typical features of other forms of PAH were chosen for the other cases. Key publications related to clinical, radiological and pathological features of PAH were identified.
Epidemiology and clinical classification
PAH (group 1 disease) is a rare condition with an estimated incidence of six cases per million per year and a prevalence of ≈50 million per year [3]. Idiopathic PAH (IPAH) historically presented in young and predominantly female patients, with the National Institute of Health registry from the 1980s reporting a mean age of 36 years [4]. The nature of patients receiving a diagnosis of IPAH has changed with more recent registry data reporting a mean age of >60 years, a more equal distribution of sexes and frequent comorbidities [5–9]. Approximately 5–10% of patients with IPAH have a very different phenotype with acute vasoreactivity to inhaled nitric oxide at right heart catheterisation and long-term response to high-dose calcium channel blockade [10]. Heritable PAH (HPAH) remains a disease predominantly of younger patients who are more likely to be female. A number of genetic mutations associated with HPAH have been identified, the commonest being the bone morphogenetic protein receptor type 2 (BMPR2), which is present in 70% of patients with a family history of PAH but is also present in up to 20% of patients with a clinical diagnosis of IPAH [11]. A number of illicit and prescription drugs have been associated with the development of PAH, including amphetamine-related appetite suppressants, methamphetamines and tyrosine kinase inhibitors such as dasatanib [12].
PAH can also develop in association with other medical conditions, most notably connective tissue disease (CTD) [13, 14]. 6.4–9.0% of patients with systemic sclerosis (SSc) will develop PAH and it is therefore recommended that patients undergo regular screening [14–16]. PAH is less commonly seen in other forms of CTD including mixed CTD and systemic lupus erythematosus [13]. PAH is relatively common in patients with congenital heart disease (CHD) and may take the form of Eisenmenger syndrome (patients with large initial systemic-to-pulmonary shunts who develop high PVR and subsequent shunt reversal), patients with predominant left-to-right shunts, patients with small “bystander lesions” and patients who develop PAH subsequent to defect closure [17]. Up to 0.5% of patients with HIV may develop PAH while around 5% of patients with portal hypertension develop PAH, where it is termed portopulmonary hypertension [18, 19]. Schistosomiasis-associated PAH is a leading cause of PAH in Africa, Asia and South America [20]. The three most common forms of PAH reported in European and US registries are IPAH, CTD-associated PAH (CTD-PAH) and CHD-associated PAH (CHD-PAH); illustrative cases of these forms of PAH are presented below. Survival varies between forms of PAH which may reflect differences in patient characteristics, such as age, as well as disease-specific differences in the ability of the right ventricle (RV) to adapt to increased RV afterload (RV-PA coupling) and concomitant myocardial disease [6, 14, 21].
Clinical presentation and diagnosis
The commonest symptom of PAH is progressive exertional dyspnoea [22]. RV dysfunction in more advanced disease leads to elevated right atrial (RA) pressure and the development of peripheral oedema and exertional pre-syncope and syncope [1]. These symptoms should raise suspicion of PAH, especially in patients with the associated medical conditions or drugs discussed above. Patients with significant RV hypertrophy (RVH) or compression of the left main stem coronary artery as a result of pulmonary arterial dilatation may also develop exertional chest pain [23]. If PAH is suspected, then initial investigations to detect PH should be performed, which include ECG and N-terminal pro B-type natriuretic peptide (NT-proBNP) analysis followed by chest X-ray, lung function and echocardiography [1]. Echocardiography provides estimates of pulmonary artery (PA) and RA pressure and an assessment of chamber size and function. These data can be integrated to assign an echocardiographic probability of PH into low, intermediate or high levels [24]. Patients with a high-probability echocardiogram (or an intermediate-probability echocardiogram in the presence of risk factors) should proceed to further investigations to confirm the presence and nature of PH. Radiological investigations are an integral part of a comprehensive diagnostic work-up, which also includes right heart catheterisation. It should be emphasised that echocardiography alone should not be used to diagnose PAH.
Systematic radiological assessment
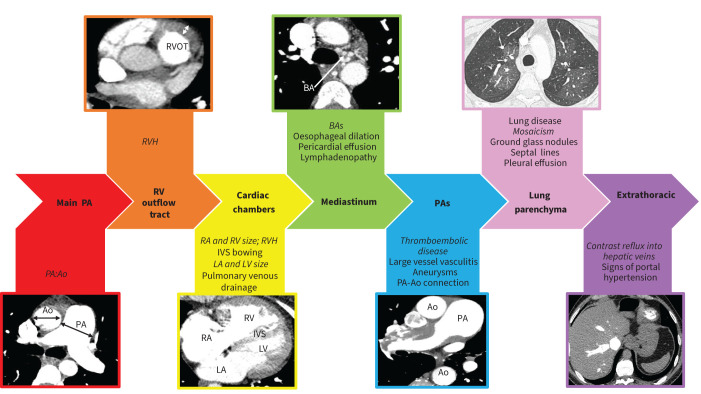
A systematic approach to the assessment of patients with suspected PH is vital [25]. Ventilation/perfusion (V′/Q′) scintigraphy (planar or single-photon emission computed tomography) has a high sensitivity for the presence of haemodynamically important chronic thromboembolic pulmonary disease (CTEPD) with a normal perfusion scan having a 98% negative predictive value [26, 27]. V′/Q′ scanning can also demonstrate and quantify the presence of a right-to-left shunt by assessing tracer uptake in the brain or kidneys. Computed tomography (CT) scanning is a key investigation and can provide a comprehensive cardiopulmonary assessment. Although ECG-gated multidetector CT may provide additional information and cardiac magnetic resonance imaging is the gold-standard tool for assessment of RV function, this review will focus on CT pulmonary angiography (CTPA) in view of its wide clinical use [28]. A routine systematic approach to interpreting standard CTPA axial images is suggested to ensure that maximal information is gained (figure 1).
FIGURE 1.
Systematic approach to computed tomography pulmonary angiogram interpretation. Ao: aorta; BA: bronchial artery; IVS: interventricular septum; LA: left atrium; LV: left ventricle; PA: pulmonary artery; RA: right atrium; RV: right ventricle; RVH: right ventricular hypertrophy; RVOT: right ventricular outflow tract. Main features demonstrated in each image are in italics.
1) Main PA
Absolute PA diameter and PA diameter in relation to the aorta (PA:Ao) provide clues to the presence of PH. Ninetieth centile thresholds for normality range from 28.9 to 31.3 mm in males and from 26.9 to 29.6 mm in females [29, 30]. The sensitivity and specificity of PA diameter or PA:Ao for predicting the presence of PH depends on the nature of the individual patient. The Fleischner Society guidelines suggest thresholds of PA diameter and PA:Ao of >30 mm and >0.9 in patients with conditions conferring a high risk (>10%) of PH (including left heart disease, COPD, interstitial lung disease, obstructive sleep apnoea, SSc, chronic kidney disease requiring dialysis, CHD and sickle cell disease), >32 mm and >1.0 in patients with conditions conferring an intermediate risk (1–10%) of PH (including non-SSc CTD, portal hypertension, HIV, previous pulmonary embolism, thalassaemia and schistosomiasis), and >34 mm and >1.1 in those with no known risk factors for PH [31]. Severe aneurysmal PA dilatation may suggest very longstanding PH or chronic left-to-right shunting.
2) RV outflow tract
Compensatory RVH suggests chronic elevation in RV afterload. It is assessed most easily at the RV outflow tract (RVOT), with RVOT thickness ≥6 mm suggesting PH [32]. Severe RVH should raise the possibility of Eisenmenger syndrome.
3) Cardiac chambers
Maximal RV transverse diameter can be compared to maximal left ventricular (LV) diameter (RV:LV) with an RV:LV >1 suggesting the presence of PH [32]. Atrial area can also be measured with an LA area >26.8 cm2 being suggestive of elevated LA pressure [33]. In normal individuals, the interventricular septum is mildly convex to the right [34]. With progressive PH, flattening or reversal of septal curvature occurs. Furthermore, an increase in the septal angle has been shown to have high specificity for the presence of PH [32]. To exclude anomalous pulmonary venous drainage as a cause of RV dilatation, the insertion of left and right superior and inferior pulmonary veins into the LA should be confirmed [35]. The interatrial and interventricular septum should be inspected for the presence of an atrial or ventricular septal defect. In some cases, a blush of contrast entering the LA from the RA (suggesting right-to-left shunting) or a blush of unopacified blood entering the RA from the LA (suggesting left-to-right shunting) may suggest the presence of an atrial septal defect.
4) Mediastinal features
Bronchial artery dilatation (>2 mm) suggests areas of chronic pulmonary arterial hypoxaemia which may be regional in chronic thromboembolic PH (CTEPH) or global in Eisenmenger syndrome. A dilated oesophagus may suggest underlying SSc as the cause for PAH. A pericardial effusion may suggest elevated RA pressures or an underlying inflammatory cause such as systemic lupus erythematosus.
5) Pulmonary arterial tree
Before assessing for signs of acute or CTEPD, the adequacy of the scan in terms of slice thickness (≤1 mm), contrast load (≥250 Hounsfield units in the main PA) and lack of significant breathing and motion artefact should be assessed [31]. Switching to bone windows is preferable to avoid missing signs of CTEPD. Direct features of CTEPD and CTEPH are discussed more fully later in this series but include mural and eccentric thrombus, webs, stenosis and abrupt tapering. Dual-energy CT may be performed in addition to a standard CTPA to produce iodine perfusion maps with high levels of agreement with V′/Q′ scintigraphy [36]. Other vascular abnormalities that may be associated with different forms of PH include calcification, aneurysms and wall thickening in vasculitis, pulmonary arteriovenous malformations in patients with hereditary haemorrhagic telangiectasia and peripheral systemic-to-pulmonary shunts in patients with CHD [37]. The PA should also be assessed for the presence of a persistent aortic connection (persistent ductus arteriosus (PDA)).
6) Lung parenchyma
The inspiratory or expiratory nature of the scan should be identified by assessing the level of tracheal expansion or collapse. Emphysema +/− interstitial lung disease may suggest the presence of group 3 disease. A “geographic” mosaic perfusion pattern with dilated pulmonary arteries in areas of high (brighter) attenuation and reduced pulmonary arterial filling, often in combination with peripheral scarring, in areas of low (darker) attenuation suggests CTEPD [38]. This should not be confused with the commoner “airway” mosaicism centred around the polyhedral secondary pulmonary lobule suggestive of air trapping. Subtle centrilobular ground glass changes may be present in PAH while patients with SSc can exhibit a pattern of reduced peripheral attenuation [39, 40]. Centrilobular ground glass changes may also suggest pulmonary veno-occlusive disease (PVOD), which should be suspected in the presence of a very low diffusing capacity of the lung for carbon monoxide (DLCO) and especially if accompanied by interlobular septal thickening and mediastinal lymphadenopathy [41]. PVOD is covered in a subsequent paper in this series. Centrilobular ground glass nodules may alternatively represent pulmonary disease such as subacute hypersensitivity pneumonitis or smoking-related respiratory bronchiolitis.
7) Extrathoracic features
Infradiaphragmatic features suggestive of possible portopulmonary PH include an irregular liver margin, splenomegaly or varices. Contrast reflux into the hepatic vein suggests increase tricuspid regurgitation with its extent correlating with RA pressure [42].
Pulmonary vascular histopathology in health and in PAH
The lung vasculature comprises two main vascular networks that are linked to their provenance, namely the pulmonary vasculature that carries deoxygenated blood from the right heart (so-called vasa publica) and the systemic lung vasculature, carrying oxygenated blood from the aorta (so-called vasa privata). Both networks are involved in the setting of PAH, but whilst systemic vessels play only a minor role in healthy conditions and are merely perceivable under the microscope, typical and characteristic lesions that appear to be the morphological correlate of pre-capillary pressure increase in PAH are found in the pulmonary vasculature. Within the pulmonary vasculature, all compartments, namely PAs, arterioles, capillaries, veins and venules, are involved in the remodelling process to different degrees, depending on the subgroup and hence the underlying cause (figure 2). The quality of remodelling depends on the vascular layers that are involved. Heath and Edwards [43] assessed these qualitative changes for CHD-PAH in a scoring system, which is still sporadically used in the literature and in pathology reports. It must be stated, however, that this scoring system of “gravity” cannot be applied to the whole of group 1 PAH, since sub-entities such as PVOD or CTD-PAH will never (or exceptionally) display grade 5 or 6 lesions and patients still die from the disease. This reflection reminds us that even subtle histologic differences may correspond to different aetiologies and therefore pathomechanisms.
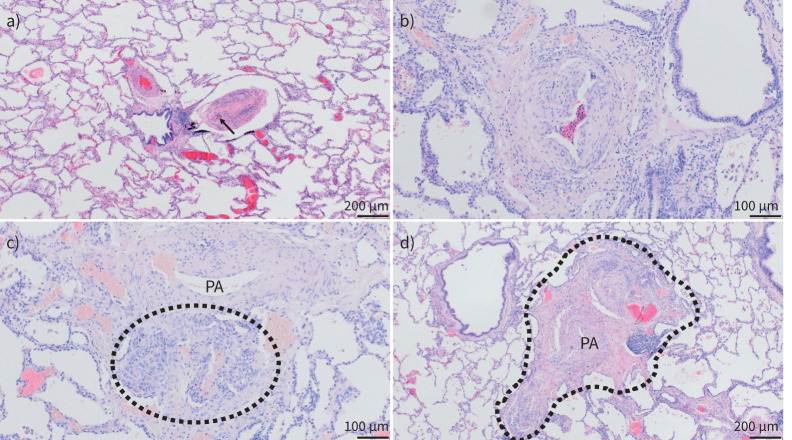
FIGURE 2.
Pulmonary vascular histopathology in idiopathic and hereditary pulmonary arterial hypertension (PAH). Lungs from patients suffering from idiopathic and hereditary PAH; haematoxylin and eosin staining. a) Well-preserved lung parenchyma with slender alveolar septa and extended alveolar spaces; note the central bronchiole with two pulmonary arterial (PA) branches: the left one with medial and intimal concentric thickening, the right one displaying near-occlusive concentric intimal fibrosis (arrow indicates remaining lumen) and moderate lymphocytic infiltrate. b) PA with eccentric intimal fibrosis and cushion-like intimal thickening, suggesting an organised thrombotic event or in situ thrombosis. c) Plexiform lesion (circled) arising from the adventitia of a PA (top) and representing a connection between systemic vasa vasorum and pulmonary vasculature. d) Singular millimetric fibro-vascular lesion in a patient with hereditary PAH (bone morphogenetic protein type II receptor mutation); this vascular and fibrotic conglomerate comprising systemic and pulmonary vessels measures about 1.5 mm (circled area). a) and d) magnification ×40; b) and c) magnification ×100.
PAs of the elastic type (above 500 µm in diameter) have a mix of smooth muscle cells and elastic fibres within their tunica media. Changes will affect rather the intimal layer in these large arteries. Moderate atherosclerosis can be found in patients with longlasting PAH that resembles aortic changes in the metabolic syndrome. Increased stiffness of the media and adventitia has been discussed research-wise that could contribute to increased pressures, but a morphological correlate for this kind of remodelling is difficult to identify [44]. In contrast, muscular-type arteries (beneath 500 µm in diameter) show typical remodelling of all layers. The muscle layer, the tunica media, displays hyperplasia in PAH (and many other forms of PH) with substantial thickening and is understood as a potentially reversible change that may appear, e.g., under hypoxic conditions. The intima will show thickening through concentric laminar or nonlaminar fibrosis, the former leading to so-called onion-skin lesions. Eccentric intimal fibrosis can also be found in all forms of PH, sometimes displaying a cushion-like appearance of the so-called neo-intima. These lesions tend to be interpreted as organised thrombotic lesions, leaving an irregular wall-thickening behind. The adventitia shows rather unspecific broadening with increased collagen deposition in PAH and other groups of PH. However, the arterial adventitia is also home to systemic vasa vasorum, the vascular pendant to bronchial arteries. It has been shown in the near past that interconnections between these systemic vessels and PAs can increase in size and complexity and will develop to become plexiform lesions or larger atypical plexiform lesions that have been termed singular millimetric fibro-vascular lesions and appear more frequently in heritable PAH linked to BMPR2 mutations [45, 46]. The classic plexiform lesion is a peculiar and easily recognisable lesion that is rather typical for PAH group 1, with the exception of PVOD and PAH-CTD. Lungs from patients suffering from PAH are notable in that the lung parenchyma, in most cases, is perfectly preserved, alveolar septa are slender and alveoli are extended. Chronic inflammatory infiltrates are present in many cases, but inflammation is rather moderate, of peri-vascular distribution and rather a low-level sustaining glow than a vasculitis-like fire. In this mellow, near-normal histologic landscape, unusual plexiform lesions and small remodelled PAs stand out; this is when PAH group 1 can be suspected by the observer.
Pulmonary veins and pre-septal venules are frequently remodelled in PAH, even outside subgroup 1.6 (PAH with overt involvement of pulmonary veins and capillaries, formerly PVOD/pulmonary capillary haemangiomatosis (PCH)). Smooth muscle cell hyperplasia and intimal fibrosis are common phenomena, though generally to a moderate degree. It has been shown in hereditary PAH with BMPR2 mutations that venous remodelling is correlated with hypertrophy of the systemic vasculature in this condition [46]. Since bronchopulmonary shunting in different groups of PH is common (e.g. CTEPH), one forwarded hypothesis is that venous remodelling occurs due to increased draining of high-pressure systemic blood via bronchial arterioles and venules. The haemodynamic relevance of post-capillary remodelling in PAH group 1 (non-1.6), however, is unclear. It is noteworthy that CTD-PAH (group 1.4.1) frequently displays important similarities to group 1.6 and may be clinically more challenging than other subgroups in PAH group 1 [47, 48].
It should be emphasised that pathological study pre-mortem or pre-transplantation is very rarely performed to establish a diagnosis of PAH.
Case 1
Clinical assessment
A 52-year-old lifelong nonsmoker presented in 2014 with a 2-year history of progressive breathlessness. He had developed some ankle swelling but denied exertional pre-syncope. He was previously fit with no significant past medical history. He took minimal alcohol and had never used recreational drugs. He had no Raynaud's phenomenon or other features suggestive of CTD. There was no family history of unexplained breathlessness. On examination there was mild ankle oedema and his jugular venous pressure was elevated. His body mass index was within normal limits. He had no stigmata of CTD or liver disease. Autoantibody screen and HIV serology were negative. Spirometry revealed: forced expiratory volume in 1 s (FEV1) of 3.4 L (93%), forced vital capacity (FVC) of 4.9 L (107%) and FEV1/FVC of 69% while DLCO was 78% predicted. ECG demonstrated p-pulmonale, right axis deviation, RVH and a right heart strain pattern. Echocardiography suggested a high probability of PH with a tricuspid regurgitant velocity (TRV) of 3.8 m·s−1. Other features suggestive of PH included systolic septal flatting, a dilated and impaired RV, and RA dilatation. LA size was normal. Incremental shuttle walking distance (ISWD) was reduced at 280 m. He was assessed as being in World Health Organization Functional Class (WHO FC) III. NT-proBNP was elevated at 1350 ng·L−1.
Radiology
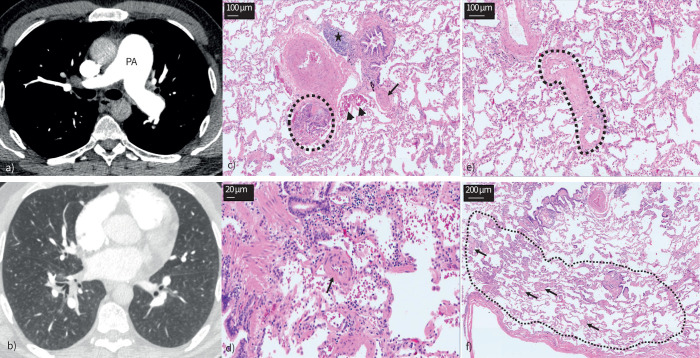
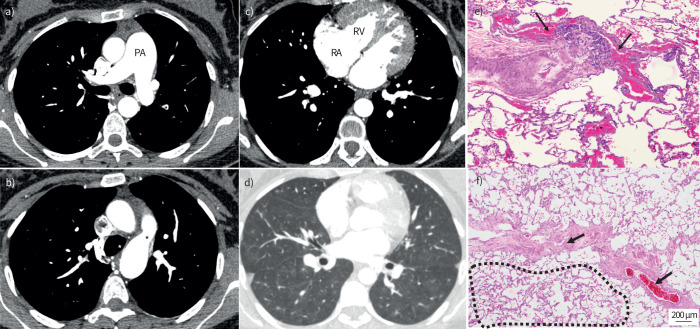
V′/Q′ scan was unremarkable. CTPA demonstrated a dilated main PA with RVOT hypertrophy (figure 3). The RV and RA were dilated while the LA was not enlarged. Pulmonary venous drainage was normal and there were no features indicative of a shunt. There were no signs of CTEPD while his lung parenchyma had a pattern of diffuse subtle centrilobular ground glass changes. There were no interlobular septal lines or mediastinal lymphadenopathy. Appearances of liver and spleen were unremarkable while there was significant contrast reflux into the hepatic vein.
FIGURE 3.
Case 1: idiopathic pulmonary arterial hypertension. a) Dilated main pulmonary artery (PA). b) Centrilobular ground glass changes in the absence of interlobular septal thickening or mediastinal lymphadenopathy. c–f) Lungs from the same patient. Haematoxylin and eosin staining; magnification: c) and e) ×100; d) ×200; and f) ×40. c) Classic plexiform lesion (circled), with adjacent dilation lesions (arrowheads), representing an open anastomosis between the pulmonary and the systemic (bronchial) vasculature; note the completely occluded small PA with concentric intimal fibrosis (arrow); also note the lymphocytic follicle between bronchiole and larger PA (asterisk). d) Small arteriole (about 50 µm in diameter, arrow) with important muscularisation and constriction of its lumen. e) Septal vein (circled) displaying prominent collagen-rich intimal fibrosis that appears focally constrictive. f) Peripheral lung with limiting visceral pleura (bottom); the arrows indicate multiple remodelled microvessels <70 µm in diameter, down to the pre-capillary level; in fact, the alveolar septa between the arterioles and venules appear focally thickened (circled area) this is probably due to the microvasculopathy; together with an increase in intra-alveolar macrophages that can be depicted as well, these histologic changes would be the morphological correlate for ground glass opacities in panel b.
Right heart catheterisation
Mean RA pressure (mRAP) of 12 mmHg, mPAP of 48 mmHg, PAWP of 13 mmHg, cardiac output (CO) of 3.5 L·min−1, cardiac index (CI) of 1.7 L·min−1·m−2, PVR of 10 WU, superior vena cava saturations of 60% and PA saturations of 64%. There was no significant response to inhaled nitric oxide.
Clinical diagnosis
IPAH.
Clinical course
Upfront intravenous therapy was discussed but the patient chose initial dual combination therapy with a phospodiesterase-5 inhibitor and an endothelin-1 receptor antagonist. He was also commenced on diuretics. Genetic analysis was performed which did not demonstrate any variants associated with PAH. Due to a suboptimal response, intravenous epoprostenol was added after 9 months of therapy. This was well tolerated and his ISWD improved to 350 m and NT-proBNP fell to 550 ng·L−1; however, he remained in WHO FC III. He subsequently deteriorated despite an increasing dose of intravenous epoprostenol and underwent successful double lung transplantation.
Histopathological review
Intimal fibrosis of small PAs, muscularisation of pulmonary arterioles and classic plexiform lesions were present. A detailed description of the histopathological abnormalities is presented in detail in figure 3.
Case 2
Clinical assessment
A 65-year-old with a 15 pack-year smoking history presented in 2019 with an 18-month history of progressive breathlessness. She had developed ankle swelling and described some dizziness on exertion. Her past medical history was remarkable for a cardiac bypass graft in 2018 and for a previous iron deficiency anaemia which was ascribed to gastric antral vascular ectasia. She took no alcohol and had never used recreational drugs. She had developed Raynaud's phenomenon 1 year previously. There was no family history of unexplained breathlessness. On examination there was ankle swelling and the jugular venous pressure was elevated. She had evidence of telangiectasia on her fingers and some dilated nailfold capillaries but no skin thickening. Her chest was clear to auscultation. Autoantibody screen demonstrated an anti-centromere antibody pattern. Spirometry revealed an FEV1 of 1.94 L (85%), FVC of 2.5 L (82%) and FEV1/FVC of 78% while DLCO was reduced at 45% pred. ECG demonstrated right axis deviation and a right heart strain pattern. Echocardiography suggested a high probability of PH with a TRV of 4.4 m·s−1 together with systolic septal flatting and a dilated and impaired RV. ISWD was reduced at 40 m. She was assessed as being in WHO FC IV while NT-proBNP was elevated at 2524 ng·L−1.
Radiology
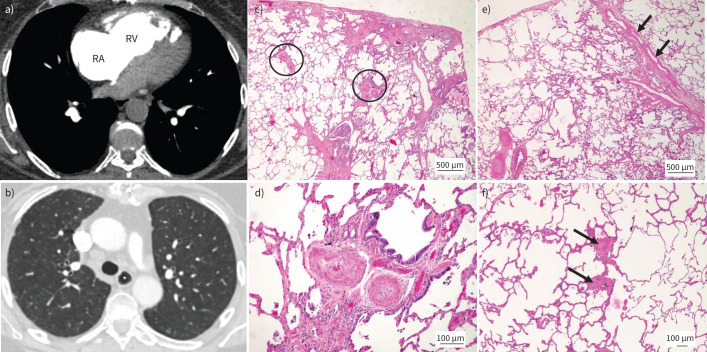
V′/Q′ scan was unremarkable. CTPA demonstrated a mildly dilated main PA with mild RVOT hypertrophy (figure 4). The RV and RA were moderately dilated while the LA was not enlarged. Pulmonary venous drainage was normal. There were no signs of CTEPD, while lung parenchyma was unremarkable apart from some minor basal subpleural fibrotic changes. The oesophagus was prominent. Appearances of liver and spleen were unremarkable while there was significant reflux of contrast into the hepatic vein.
FIGURE 4.
Pulmonary arterial hypertension (PAH) in association with systemic sclerosis. a) Dilated right atrium (RA) and right ventricle (RV). b) Prominent oesophagus (*). c–f) Lungs from a different patient suffering from systemic sclerosis-associated PAH. Haematoxylin and eosin staining; magnification: c) and e) ×20; d) ×100; and f) ×40. c) Peripheral lung parenchyma with mild subpleural fibrosis (top). Note heavily remodelled muscular-type pulmonary arteries (PAs) (circled) in preserved areas, independent of fibrosis. d) Magnification of right circled PA. Medial muscular thickening and important near-occlusive concentric nonlaminar intimal fibrosis is present. e) Remodelled PAs (bottom left) and septal veins with substantial smooth muscle cell hyperplasia (arrows). f) Muscularisation of small pulmonary arterioles (arrows) and thickening of alveolar septa to the left of the arterioles; these changes are vaguely reminiscent of PAH group 1.6 (pulmonary veno-occlusive disease/pulmonary capillary haemangiomatosis).
Right heart catheterisation
mRAP of 13 mmHg, mPAP of 42 mmHg, PAWP of 7 mmHg, CO of 2.03 L·min−1, CI of 1.25 L·min−1·m−2, PVR of 19 WU, SVC saturations of 34% and PA saturations of 41%.
Clinical diagnosis
PAH in association with undiagnosed CTD (SSc sine scleroderma). Underlying CTD should always be considered in patients presenting with PH with close attention to clinical features in the history and on examination and the performing of an autoimmune screen.
Clinical course
She was assessed as being at high risk of 1-year mortality according to the European Society of Cardiology/European Respiratory Society approach and agreed to commence combination therapy including intravenous epoprostenol [1]. She also started diuretic therapy. Her clinical condition improved and at 4 months had improved to an intermediate–low risk state [49].
Review of typical histopathology of SSc-associated PAH (SSc-PAH)
SSc-PAH can display similar pulmonary vascular lesions to those found in IPAH (figure 4). Of note, plexiform lesions are less frequently present, as has been shown by different groups [47, 50]. Concentric intimal fibrosis is typically found in small muscular-type PAs. However, it has been demonstrated that SSc-PAH patients may show a vasculopathy resembling PAH group 1.6 (PVOD/PCH).
Case 3
Clinical assessment
A 23-year-old lifelong nonsmoker was referred in 2007. She had moved to the UK at the age of 10 from the Indian subcontinent. She gave a history of longstanding mild breathlessness which had worsened over the preceding 12 months. Her past medical history was unremarkable. On examination there was no leg swelling and the jugular venous pressure was not elevated. She had a systolic murmur over the left lower sternal edge. Oxygen saturation measured via her fingers was 93% and when measured via her toes, which were clubbed, was 85%. Spirometry revealed: FEV1 of 1.77 L (63%), FVC of 2.3 L (71%) and FEV1/FVC of 77% while DLCO was 65% predicted. ECG demonstrated right axis deviation, RVH and a right heart strain pattern. Echocardiography suggested a high probability of PH with a TRV of 4.5 m·s−1 and a hypertrophied and mildly dilated RV. No intracardiac shunt was demonstrated. Haemoglobin was 135 g·dL−1 with mean corpuscular volume of 79, ferritin of 25 ng·mL−1 (normal >30) and transferrin saturations of 10% (normal >15%). She was in WHO FC III.
Radiology
CTPA demonstrated a dilated main PA and significant RVH (figure 5). A large PDA was visualised. There was no evidence of thromboembolic disease. Centrilobular ground glass changes were present.
FIGURE 5.
Eisenmenger syndrome secondary to patent ductus arteriosus. a) Dilated main pulmonary artery (PA). b) Large patent ductus arteriosus (*). c) Right atrial (RA) and right ventricular (RV) dilatation with severe RV hypertrophy. d) Centrilobular ground glass change. e–f) Lungs from two other patients with congenital heart disease-associated pulmonary arterial hypertension. Haematoxylin and eosin staining; magnification ×40 and ×20, respectively. e) PAs of a patient with Eisenmenger syndrome show classic plexiform lesions of muscular-type arteries; note the blood-filled dilatation lesions (arrows) in direct vicinity to the plexiform core. f) Pulmonary septal vein with important smooth muscle cell hyperplasia in another patient with Eisenmenger syndrome due to an open ductus arteriosus; in this case, venous remodelling (arrows) and even pulmonary capillary haemangiomatosis-like capillary proliferation (thicker alveolar septa beneath the septal vein, circled area) were more prominent than arterial changes, demonstrating that different patients with Eisenmenger syndrome may be associated with different phenotypes of pulmonary vascular disease.
Clinical diagnosis
Eisenmenger Syndrome secondary to a PDA. The differential oxygen saturations between upper and lower limbs reflects the location of the PDA, and hence right to left shunt, being distal to the left subclavian artery. A PDA may not be visualised at transthoracic echocardiography and so the CTPA should always be specifically reviewed for its presence or absence.
Clinical course
She was commenced initially on a phospodiesterase-5 inhibitor and iron replacement (in view of the iron deficiency and lack of expected compensatory secondary erythrocytosis) with subsequent improvement to WHO FC II. Other aspects of management in Eisenmenger syndrome include close attention to dental hygiene (to reduce the risk of endocarditis or cerebral abscesses) [51]. In this patient, as in all patients with child-bearing potential, clear advice regarding the risks of pregnancy and the importance of adequate contraception was also given [52].
Review of typical histopathology of Eisenmenger syndrome
Lungs from patients with longstanding Eisenmenger syndrome are in fact indistinguishable from IPAH. Plexiform lesions are the typical hallmark, but medial hyperplasia and intimal fibrosis of muscular-type PAs are also always present (figure 5). In fact, the Heath and Edwards [43] classification of vascular lesions in PH that is frequently used for PAH group 1 in general was originally created for the study of 67 cases with PH due to congenital cardiac septal defects. Although exceptional, capillary and post-capillary remodelling may occur even in CHD-PAH.
Points for clinical practice
• PAH may be idiopathic, have a genetic association or may present in the context of risk factors including CTD, CHD, portal hypertension, HIV and a number of prescription or illicit drugs.
• A systematic approach to radiological investigation, especially to CTPA, is integral to making the correct diagnosis.
• The clinical presentation of PAH reflects increased RV afterload resulting from pathological changes in the resistance of PAs and arterioles. Changes in pulmonary veins and pre-septal venules may also be present in patients without other clinical features of PVOD.
Conclusion
PAH may develop in the presence of associated medical conditions or a family history, following exposure to certain medications or drugs, or may be idiopathic in nature. Although all forms of PAH share common histopathological features, the presence of certain pulmonary arterial abnormalities, such as plexiform lesions, and extent of co-existing pulmonary venous involvement differs between the different subgroups. Radiological investigations are key to diagnosing the correct form of PH and a systematic approach to interpretation of CTPA is essential. Subsequent manuscripts in this series will focus on other forms of PH.
Acknowledgements
This manuscript includes work carried out at the National Institute for Health and Care Research (NIHR) Sheffield Biomedical Research Centre (BRC). This work used biological samples provided by Manchester University NHS Foundation Trust and the University of Sheffield from the Sheffield Teaching Hospitals Observational Study of Patients with Pulmonary Hypertension, Cardiovascular and Lung Disease.
Provenance: Commissioned article, peer reviewed.
Number 1 in the Series “Clinical–radiological–pathological correlation in pulmonary hypertension” Edited by Robin Condliffe, Anton Vonk Noordegraaf, Olivier Sitbon, Peter Dorfmüller and Deepa Gopalan
This article has an editorial commentary: https://doi.org/10.1183/16000617.0237-2023
Conflicts of interest: R. Condliffe, C. Durrington, A. Hameed, R.A. Lewis, R. Venkateswaran, D. Gopalan and P. Dorfmüller report no conflicts of interest related to this work.
References
- 1.Humbert M, Kovacs G, Hoeper MM, et al. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 2.European Respiratory Society . Pulmonary hypertension: clinical–radiological–pathological case series. Date last accessed: 8 July 2023. Date last updated: 31 March 2023. www.ersnet.org/events/pulmonary-hypertension-clinical-radiological-pathological-case-series
- 3.Leber L, Beaudet A, Muller A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review. Pulm Circ 2021; 11: 2045894020977300. doi: 10.1177/2045894020977300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 1987; 107: 216–223. doi: 10.7326/0003-4819-107-2-216 [DOI] [PubMed] [Google Scholar]
- 5.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. doi: 10.1161/CIRCULATIONAHA.109.898122 [DOI] [PubMed] [Google Scholar]
- 6.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 2012; 39: 945–955. doi: 10.1183/09031936.00078411 [DOI] [PubMed] [Google Scholar]
- 7.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186: 790–796. doi: 10.1164/rccm.201203-0383OC [DOI] [PubMed] [Google Scholar]
- 8.Chang KY, Duval S, Badesch DB, et al. Mortality in pulmonary arterial hypertension in the modern era: early insights from the pulmonary hypertension association registry. J Am Heart Assoc 2022; 11: e024969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoeper MM, Dwivedi K, Pausch C, et al. Phenotyping of idiopathic pulmonary arterial hypertension: a registry analysis. Lancet Respir Med 2022; 10: 937–948. doi: 10.1016/S2213-2600(22)00097-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sitbon O, Humbert M, Jais X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005; 111: 3105–3111. doi: 10.1161/CIRCULATIONAHA.104.488486 [DOI] [PubMed] [Google Scholar]
- 11.Morrell NW, Aldred MA, Chung WK, et al. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801899. doi: 10.1183/13993003.01899-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez RL, 3rd, Pienkos SM, de Jesus Perez V, et al. Pulmonary arterial hypertension secondary to drugs and toxins. Clin Chest Med 2021; 42: 19–38. [DOI] [PubMed] [Google Scholar]
- 13.Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 2009; 179: 151–157. doi: 10.1164/rccm.200806-953OC [DOI] [PubMed] [Google Scholar]
- 14.Haque A, Kiely DG, Kovacs G, et al. Pulmonary hypertension phenotypes in patients with systemic sclerosis. Eur Respir Rev 2021; 30: 210053. doi: 10.1183/16000617.0053-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avouac J, Airo P, Meune C, et al. Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasians and metaanalysis of 5 studies. J Rheumatol 2010; 37: 2290–2298. doi: 10.3899/jrheum.100245 [DOI] [PubMed] [Google Scholar]
- 16.Rubio-Rivas M, Homs NA, Cuartero D, et al. The prevalence and incidence rate of pulmonary arterial hypertension in systemic sclerosis: systematic review and meta-analysis. Autoimmun Rev 2021; 20: 102713. doi: 10.1016/j.autrev.2020.102713 [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner H, De Backer J. The ESC clinical practice guidelines for the management of adult congenital heart disease 2020. Eur Heart J 2020; 41: 4153–4154. doi: 10.1093/eurheartj/ehaa701 [DOI] [PubMed] [Google Scholar]
- 18.Sitbon O, Lascoux-Combe C, Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 2008; 177: 108–113. doi: 10.1164/rccm.200704-541OC [DOI] [PubMed] [Google Scholar]
- 19.Krowka MJ, Miller DP, Barst RJ, et al. Portopulmonary hypertension: a report from the US-based REVEAL registry. Chest 2012; 141: 906–915. doi: 10.1378/chest.11-0160 [DOI] [PubMed] [Google Scholar]
- 20.Fernandes CJC, Piloto B, Castro M, et al. Survival of patients with schistosomiasis-associated pulmonary arterial hypertension in the modern management era. Eur Respir J 2018; 51: 1800307. doi: 10.1183/13993003.00307-2018 [DOI] [PubMed] [Google Scholar]
- 21.Ramjug S, Hussain N, Hurdman J, et al. Idiopathic and systemic sclerosis-associated pulmonary arterial hypertension: a comparison of demographic, hemodynamic, and MRI characteristics and outcomes. Chest 2017; 152: 92–102. doi: 10.1016/j.chest.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 22.Kiely DG, Elliot CA, Sabroe I, et al. Pulmonary hypertension: diagnosis and management. BMJ 2013; 346: f2028. doi: 10.1136/bmj.f2028 [DOI] [PubMed] [Google Scholar]
- 23.Galie N, Saia F, Palazzini M, et al. Left main coronary artery compression in patients with pulmonary arterial hypertension and angina. J Am Coll Cardiol 2017; 69: 2808–2817. doi: 10.1016/j.jacc.2017.03.597 [DOI] [PubMed] [Google Scholar]
- 24.Hadinnapola C, Bleda M, Haimel M, et al. Phenotypic characterization of EIF2AK4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation 2017; 136: 2022–2033. doi: 10.1161/CIRCULATIONAHA.117.028351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiely DG, Levin D, Hassoun P, et al. EXPRESS: statement on imaging and pulmonary hypertension from the Pulmonary Vascular Research Institute (PVRI). Pulm Circ 2019; 9: 2045894019841990. doi: 10.1177/2045894019841990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tunariu N, Gibbs SJ, Win Z, et al. Ventilation–perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med 2007; 48: 680–684. doi: 10.2967/jnumed.106.039438 [DOI] [PubMed] [Google Scholar]
- 27.He J, Fang W, Lv B, et al. Diagnosis of chronic thromboembolic pulmonary hypertension: comparison of ventilation/perfusion scanning and multidetector computed tomography pulmonary angiography with pulmonary angiography. Nucl Med Commun 2012; 33: 459–463. doi: 10.1097/MNM.0b013e32835085d9 [DOI] [PubMed] [Google Scholar]
- 28.Hahn RT, Lerakis S, Delgado V, et al. Multimodality imaging of right heart function: JACC scientific statement. J Am Coll Cardiol 2023; 81: 1954–1973. doi: 10.1016/j.jacc.2023.03.392 [DOI] [PubMed] [Google Scholar]
- 29.Truong QA, Massaro JM, Rogers IS, et al. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging 2012; 5: 147–154. doi: 10.1161/CIRCIMAGING.111.968610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SH, Kim YJ, Lee HJ, et al. Comparison of CT-determined pulmonary artery diameter, aortic diameter, and their ratio in healthy and diverse clinical conditions. PLoS One 2015; 10: e0126646. doi: 10.1371/journal.pone.0126646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remy-Jardin M, Ryerson CJ, Schiebler ML, et al. Imaging of pulmonary hypertension in adults: a position paper from the Fleischner Society. Eur Respir J 2021; 57: 2004455. doi: 10.1183/13993003.04455-2020 [DOI] [PubMed] [Google Scholar]
- 32.Swift AJ, Dwivedi K, Johns C, et al. Diagnostic accuracy of CT pulmonary angiography in suspected pulmonary hypertension. Eur Radiol 2020; 30: 4918–4929. doi: 10.1007/s00330-020-06846-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Currie BJ, Johns C, Chin M, et al. CT derived left atrial size identifies left heart disease in suspected pulmonary hypertension: derivation and validation of predictive thresholds. Int J Cardiol 2018; 260: 172–177. doi: 10.1016/j.ijcard.2018.02.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruzzi JF, Remy-Jardin M, Delhaye D, et al. When, why, and how to examine the heart during thoracic CT: part 1, basic principles. AJR Am J Roentgenol 2006; 186: 324–332. doi: 10.2214/AJR.05.0717 [DOI] [PubMed] [Google Scholar]
- 35.Lewis RA, Billings CG, Bolger A, et al. Partial anomalous pulmonary venous drainage in patients presenting with suspected pulmonary hypertension: a series of 90 patients from the ASPIRE registry. Respirology 2020; 25: 1066–1072. doi: 10.1111/resp.13815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masy M, Giordano J, Petyt G, et al. Dual-energy CT (DECT) lung perfusion in pulmonary hypertension: concordance rate with V/Q scintigraphy in diagnosing chronic thromboembolic pulmonary hypertension (CTEPH). Eur Radiol 2018; 28: 5100–5110. doi: 10.1007/s00330-018-5467-2 [DOI] [PubMed] [Google Scholar]
- 37.Castaner E, Gallardo X, Rimola J, et al. Congenital and acquired pulmonary artery anomalies in the adult: radiologic overview. Radiographics 2006; 26: 349–371. doi: 10.1148/rg.262055092 [DOI] [PubMed] [Google Scholar]
- 38.Gopalan D, Delcroix M, Held M. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160108. doi: 10.1183/16000617.0108-201628298387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajaram S, Swift AJ, Condliffe R, et al. CT features of pulmonary arterial hypertension and its major subtypes: a systematic CT evaluation of 292 patients from the ASPIRE registry. Thorax 2015; 70: 382–387. doi: 10.1136/thoraxjnl-2014-206088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemnes AR, Leopold JA, Radeva MK, et al. Clinical characteristics and transplant-free survival across the spectrum of pulmonary vascular disease. J Am Coll Cardiol 2022; 80: 697–718. doi: 10.1016/j.jacc.2022.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montani D, Girerd B, Jais X, et al. Clinical phenotypes and outcomes of heritable and sporadic pulmonary veno-occlusive disease: a population-based study. Lancet Respir Med 2017; 5: 125–134. doi: 10.1016/S2213-2600(16)30438-6 [DOI] [PubMed] [Google Scholar]
- 42.Groves AM, Win T, Charman SC, et al. Semi-quantitative assessment of tricuspid regurgitation on contrast-enhanced multidetector CT. Clin Radiol 2004; 59: 715–719. doi: 10.1016/j.crad.2004.02.007 [DOI] [PubMed] [Google Scholar]
- 43.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 1958; 18: 533–547. doi: 10.1161/01.CIR.18.4.533 [DOI] [PubMed] [Google Scholar]
- 44.Schafer M, Kheyfets VO, Schroeder JD, et al. Main pulmonary arterial wall shear stress correlates with invasive hemodynamics and stiffness in pulmonary hypertension. Pulm Circ 2016; 6: 37–45. doi: 10.1086/685024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galambos C, Sims-Lucas S, Abman SH, et al. Intrapulmonary bronchopulmonary anastomoses and plexiform lesions in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2016; 193: 574–576. doi: 10.1164/rccm.201507-1508LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghigna MR, Guignabert C, Montani D, et al. BMPR2 mutation status influences bronchial vascular changes in pulmonary arterial hypertension. Eur Respir J 2016; 48: 1668–1681. doi: 10.1183/13993003.00464-2016 [DOI] [PubMed] [Google Scholar]
- 47.Dorfmuller P, Humbert M, Perros F, et al. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol 2007; 38: 893–902. doi: 10.1016/j.humpath.2006.11.022 [DOI] [PubMed] [Google Scholar]
- 48.Launay D, Sitbon O, Hachulla E, et al. Survival in systemic sclerosis-associated pulmonary arterial hypertension in the modern management era. Ann Rheum Dis 2013; 72: 1940–1946. doi: 10.1136/annrheumdis-2012-202489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoeper MM, Pausch C, Olsson KM, et al. COMPERA 2.0: a refined four-stratum risk assessment model for pulmonary arterial hypertension. Eur Respir J 2022; 60: 2102311. doi: 10.1183/13993003.02311-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cool CD, Kennedy D, Voelkel NF, et al. Pathogenesis and evolution of plexiform lesions in pulmonary hypertension associated with scleroderma and human immunodeficiency virus infection. Hum Pathol 1997; 28: 434–442. doi: 10.1016/S0046-8177(97)90032-0 [DOI] [PubMed] [Google Scholar]
- 51.Condliffe R, Clift P, Dimopoulos K, et al. Management dilemmas in pulmonary arterial hypertension associated with congenital heart disease. Pulm Circ 2018; 8: 2045894018792501. doi: 10.1177/2045894018792501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiely DG, Condliffe R, Wilson VJ, et al. Pregnancy and pulmonary hypertension: a practical approach to management. Obstet Med 2013; 6: 144–154. doi: 10.1177/1753495X13495193 [DOI] [PMC free article] [PubMed] [Google Scholar]