Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare and potentially life-threatening complication of acute pulmonary embolism. It is characterised by persistent fibro-thrombotic pulmonary vascular obstructions and elevated pulmonary artery pressure leading to right heart failure. The diagnosis is based on two steps, as follows: 1) suspicion based on symptoms, echocardiography and ventilation/perfusion scan and 2) confirmation with right heart catheterisation, computed tomography pulmonary angiography and, in most cases, digital subtraction angiography. The management of CTEPH requires a multimodal approach, involving medical therapy, interventional procedures and surgical intervention. This clinical–radiological–pathological correlation paper illustrates the diagnostic and therapeutic management of two patients. The first had chronic thromboembolic pulmonary disease without pulmonary hypertension at rest but with significant physical limitation and was successfully treated with pulmonary endarterectomy. The second patient had CTEPH associated with splenectomy and was considered unsuitable for surgery because of exclusive subsegmental lesions combined with severe pulmonary hypertension. The patient benefited from multimodal treatment involving medical therapy followed by multiple sessions of balloon pulmonary angioplasty. Both patients had normalised functional capacity and pulmonary haemodynamics 3–6 months after the interventional treatment. These two examples show that chronic thromboembolic pulmonary diseases are curable if diagnosed promptly and referred to CTEPH centres for specialist treatment.
Tweetable abstract
Multidisciplinary treatment of chronic thromboembolic pulmonary diseases with or without pulmonary hypertension achieves normalisation of exercise capacity and pulmonary haemodynamics as illustrated in two exemplative cases. https://bit.ly/45ZXWNv
Introduction
The definition of chronic thromboembolic pulmonary hypertension (CTEPH) has recently been updated [1–3]. It is now defined as the association of symptoms with the presence of post-thrombotic deposits within the pulmonary arteries (PAs) after 3 months of therapeutic anticoagulation, with pre-capillary pulmonary hypertension (PH) defined by a mean PA pressure (mPAP) >20 mmHg and with a pulmonary vascular resistance (PVR) ≥2 Wood units (WU). Recently, the concept of chronic thromboembolic pulmonary disease (CTEPD) was introduced. It has the same definition as CTEPH but without the PH criterion.
Methods
The contents of this paper formed the basis of the fifth session in a recent European Respiratory Society webinar series on PH [4]. Cases representative of CTEPD with or without PH at rest and of both surgical and interventional treatments were chosen.
Key publications related to clinical, radiological and pathological features of CTEPH were identified.
Epidemiology
The incidence of CTEPH has been a matter of debate, with an important variation between epidemiological studies. This relatively high variation is due to the variability of populations studied, the screening strategies performed and the utilisation of right heart catheterisation (RHC) or transthoracic echocardiography (TTE) for diagnosis. A recent meta-analysis has shown that CTEPH develops in approximatively 3.2% of patients who survive an acute pulmonary embolism (PE) [5]. The presence of deposits within the PAs is more common than CTEPH after acute PE, with an incidence up to 29% [6]. However, the natural history of these residual deposits remains largely unclear, but they do not seem to evolve in the majority of cases [7].
The main symptoms in CTEPH are progressive exercise intolerance and/or exertional dyspnoea, which can be explained by a limitation in cardiac output (CO) and an increased dead space ventilation [8, 9]. Without treatment, patients will develop severe functional limitations, leading to a poor quality of life and reduced survival [10].
Pathology
The pathology of CTEPH is complex, multifactorial and not yet fully understood, encompassing both macroscopic and microscopic components. The clots present in the pulmonary vascular bed at the time of acute PE may not be completely dissolved by the endogenous fibrinolysis and may remain in the form of fibrotic tissue. The mechanisms precluding a complete resolution of clots include defects in fibrinolysis and increased coagulation [11–13]. Mechanisms such as inflammation and abnormal angiogenesis also play a role [14, 15]. Finally, several risk factors have shed light on the particular mechanisms leading to CTEPH, such as the presence of intravenous devices [16] or splenectomy [12], which result in abnormal platelet activation [17]. Obstruction of the pulmonary vascular bed results in increased pulmonary pressure and afterload for the right ventricle (RV), which may lead to right heart failure and death [8]. The increased pressure triggers vascular shear stress in nonoccluded vessels [18]. This shear stress may lead to endothelial dysfunction and vascular remodelling of the small blood vessels in vascular territories not affected by fibrotic clots. Interestingly, microvasculopathy is also found in parts of the lung where the blood flow from the PAs has been totally interrupted [19]. It has been suggested that this could be due to collateral flow from the systemic circulation, mainly through hypertrophic bronchial arteries. This compensates for the absence of PA flow but may also trigger microvascular remodelling, affecting both the pre-capillary compartment and small veins [20]. Remodelling of the small pulmonary vessels increases PVR, aggravates RV dysfunction and may explain why some patients present residual PH after surgery. Importantly, it is also the rationale for medical therapy in CTEPH. Clinically, the haemoptysis sometimes seen in CTEPH patients is likely associated with the hypertrophied bronchial artery collateral circulation [21].
Diagnosis and evaluation
The underdiagnosis and diagnostic delay of CTEPH are well known and cannot be ignored. The true incidence of new CTEPH is estimated to be two to three times higher than the observed incidence in registry data [22–24]. Progressive remodelling of the distal arteries and increase of PVR are both important determinants of outcome but are also part of the natural course of the disease. Therefore, early CTEPH diagnosis and referral to a CTEPH centre appear to be critical for optimal treatment. Hence a longer diagnostic delay seems to be associated with a less favourable haemodynamic profile and shorter survival in CTEPH patients, possibly reflecting poorer RV function and more severe concomitant secondary vascular disease [25].
The diagnosis of CTEPH should mainly be suspected in patients with persistent complaints of dyspnoea 3 months after an acute PE effectively treated with anticoagulation. It should also be considered during the diagnostic workup of patients without a history of acute PE, but with otherwise unexplained dyspnoea, as 25% of all CTEPH patients have no history of documented PE [26]. The first step when screening for CTEPH is performing TTE, which may indicate the presence of PH [27]. However, subjecting all survivors of acute PE to TTE would result in a low diagnostic yield, potential overdiagnosis and prove to be cost-ineffective [28]. A dedicated screening algorithm, as described in the InShapeII study, could potentially exclude CTEPH promptly and accurately after an acute PE, thereby avoiding TTE in 81% of patients [29]. Most physicians will refer patients for TTE based on their clinical symptoms. However, educating cardiologists to screen for PH during TTE and, if present in the absence of left-sided valvular pathology, to then refer the patient for the next step, a ventilation/perfusion (V′/Q′) scan to identify CTEPD, is challenging [24]. V′/Q′ lung scans are highly sensitive (96–97.4%) in terms of detecting perfusion abnormalities and have a negative predictive value of nearly 100%. Therefore, a normal V′/Q′ lung scan rules out the diagnosis of CTEPH [30]. It is currently considered the “gold standard” screening method for CTEPH. However, the nonspecificity of this modality necessitates additional diagnostic imaging in cases of mismatched perfusion defects to definitively confirm CTEPH. Therefore, computed tomography pulmonary angiography (CTPA) must be performed to provide substantial information regarding the localisation and extent of endovascular lesions [1]. CTPA can be used to assess operability as it can provide detailed structural information, including endovascular thrombi, vascular wall thickness, intraluminal fibrous bands or webs, stenosis, and bronchial artery collateral circulation [31]. It may also reveal indirect signs of CTEPH, such as a mosaic perfusion pattern in the pulmonary parenchyma. This pattern implies CTEPH and corresponds to a vascular redistribution of blood flow. Other generic signs of PH have also been described, such as dilatation and calcification of the main PAs, RV hypertrophy, right atrial dilatation, and pericardial effusion [31]. Additional benefits of CTPA are the assessment of possible underlying parenchymal lung and mediastinal disease and the detection of other pulmonary vessel disorders that may present with perfusion defects on V′/Q′ lung scanning, such as PA sarcoma, large vessel vasculitis or fibrosing mediastinitis [32]. If findings of pre-existing CTEPD are present on the CTPA performed to diagnose the acute PE, the screening steps should of course be skipped and the patient directly referred to a CTEPH centre [24]. However, difficulties with this imaging modality relate to the interpretive expertise required and the lower sensitivity for detecting CTEPH in subsegmental vessels [33, 34]. Therefore, it is frequently combined with conventional digital subtraction angiography (DSA). DSA has been considered the “gold standard” for imaging in the evaluation of CTEPH. It can confirm the presence of CTEPH to the level of subsegmental vessels, exclude other possible diagnoses and accurately localise or “map out” lesions in the determination of surgical accessibility or targets for balloon pulmonary angioplasty (BPA) [32, 35]. Characteristic pulmonary angiographic findings suggestive of CTEPH include webs or bands, intimal irregularities, pouch defects, abrupt vascular narrowing, decreased perfusion, and complete obstruction of PAs [36].
The combination of CTPA and DSA is frequently used to obtain the benefits of both imaging techniques to optimally diagnose and assess the operability of CTEPH. In order to address the disadvantages associated with those imaging techniques, numerous new developments in CTEPH imaging have been described in the last decade. One of them, dual-energy computed tomography (DECT), increases the sensitivity and specificity of the diagnosis of CTEPH. DECT can also be utilised to assess pulmonary perfusion in patients with CTEPH by calculating perfused blood volume maps from iodine distribution in the lung parenchyma [37]. Merging anatomical and physiologic data with DECT angiography improves the detection of distal CTEPH and provides more accurate information about the overall pulmonary vascular reserve and parenchymal arterial perfusion [38].
RHC is mandatory to confirm the presence of PH, evaluate pulmonary haemodynamics and assess the severity of the disease and is frequently performed at the same time as DSA [2, 3]. The severity of the disease may also be assessed by evaluating 6-min walk distance (6MWD), RV function on TTE and biomarkers such as N-terminal pro-brain natriuretic peptide (NT-proBNP). In addition, other specific tests may be relevant, especially in CTEPD, such as exercise RHC, showing a sharp increase in the mPAP/CO relationship [39], or cardiopulmonary exercise testing (CPET), demonstrating an increase in ventilatory inefficiency and dead space ventilation [40].
Treatments
The general measures recommended for patients with CTEPH are anticoagulation treatments and common PH management strategies, such as the use of diuretics, oxygen therapy and avoidance of pregnancy. Direct oral anticoagulants and vitamin K antagonists are used, but vitamin K antagonists seem to be more effective and are associated with a lower risk of recurrence of acute PE [41, 42].
Pulmonary endarterectomy (PEA) is the recommended treatment for patients with CTEPH who are deemed suitable for surgery. During a PEA, the abnormal intima of the pulmonary vascular bed is surgically removed, thereby retrieving the obstructive lesions [24]. In patients deemed operable, it may improve survival [43], haemodynamics [36], RV function [44] and functional capacity [45]. In experienced centres, post-operative mortality is now less than 3% [46]. Operability is frequently considered up to the segmental level, although some experienced centres operate on patients with subsegmental disease with good outcomes [47]. PEA leads to normalisation or near normalisation of haemodynamics in the vast majority of cases. However, some patients present some degree of residual PH or may experience a recurrence of PH after PEA [48].
Determining surgical candidacy involves two separate processes, namely 1) an evaluation of technical operability and 2) an assessment of the potential risks and benefits of surgery that should be performed by a multidisciplinary CTEPH team, including at least one experienced surgeon [2, 3]. Technical operability ultimately depends on both the anatomic location of CTEPH and the experience of the surgeon. The patient must have sufficient surgically accessible thromboembolic material, with a proportional PVR indicative of the absence of extensive secondary microvasculopathy [2, 3]. The only absolute contraindication for PEA is severe lung disease (mainly severe emphysema or interstitial lung disease), as these patients will derive little benefit from PEA because the attempted improvement in perfusion may not improve symptoms if ventilation is severely compromised and the risk of respiratory failure after surgery is significant [49]. With the exception of patients with terminal or end-stage cancer, no other comorbid conditions are absolute contraindications to PEA. Age per se and obesity are not contraindications, with studies demonstrating excellent results in children, octogenarians and obese patients [50–53]. However, some coexisting conditions impose an increased risk on less favourable peri-operative and long-term outcomes [54]. These risk factors do not represent an absolute contraindication to PEA and should be weighed by the multidisciplinary CTEPH team against the potential symptomatic benefits of PEA.
Initially, symptomatic CTEPH represents the sole indication for PEA. However, even in the absence of resting PH, patients with CTEPD may have functional limitations due to increased dead space ventilation or an exaggerated PAP increase during exercise and may also benefit from PEA [55, 56]. Therefore, PEA can be offered to CTEPD patients without PH to improve symptoms.
For patients considered as inoperable or who present with persistent PH after PEA, medical therapy and interventional treatments are the remaining options. The only approved medication for CTEPH patients is riociguat, a soluble stimulator of guanylate cyclase [57]. It increases the nitric oxide intracellular content of pulmonary vascular smooth muscle cells, leading to vasodilatation and antiproliferative properties [58]. Riociguat may increase functional capacity, improve RV function and decrease PVR in CTEPH patients with inoperable disease or residual PH after PEA [59–61]. Intravenous treprostinil has also been shown to improve haemodynamic and functional capacity in inoperable patients and in those with persistent PH after PEA [62]. No other pulmonary arterial hypertension-specific therapies have been proven to be efficient in randomised clinical trials but some are used off label in experienced centres.
Finally, BPA may be proposed for patients with subsegmental disease [2, 3]. This interventional treatment uses a guiding catheter and a guidewire in the pulmonary bed, crossing the vascular lesions and allowing the deployment of a coronary dilatation balloon [63]. Low-pressure inflation of the balloon pushes fibrotic material against the vascular wall, thereby restoring blood flow. BPA has been shown to improve haemodynamics [64], RV function [65], functional capacity [66] and survival [67]. It can be complicated by haemoptysis and BPA-related lung injury [68]. The rate of these complications may dramatically decrease after a sufficient learning curve and the procedure is safe in the hands of experienced practitioners [64, 69]. Selection of patients for BPA is still a field of active research. Most centres consider patients with subsegmental disease as good candidates; however, segmental disease may also be treated with BPA. Different target lesions have been described [70] and the easiest ones to treat are focal stenoses such as webs. Diffuse irregularity of the lumen or subtotal occlusion can also be treated successfully. Total occlusions are quite challenging to treat and are associated with high levels of failure and complications. Some authors have proposed treating more proximal disease with BPA [71]. BPA could eventually be proposed for patients who have been formally contraindicated for PEA. However, the benefits of BPA in these patients should be studied further.
The various interventions available for CTEPH patients have led to the development of the concept of multimodal treatments, involving combinations of different therapeutic approaches. The complexity of the therapeutic approach and the high level of expertise required to adequately treat CTEPH patients underscore the importance of conducting a multidisciplinary evaluation involving different experts (PH physicians, radiologists, PEA surgeons and BPA specialists) [1].
Case presentation
The following two cases highlight the variety of clinical presentations of CTEPH and CTEPD and the complexity of treatments and their association. The first case is a patient with CTEPD and proximal disease treated with PEA, resulting in an improvement of haemodynamics during exercise and ventilatory efficiency. The second case is a patient with a distal form of CTEPH related to splenectomy and treated with combination therapy (riociguat and bosentan) before BPA. In the following section, we will present the step-by-step diagnostic workup, the evaluation of disease severity, the selection of the most appropriate treatment and the results obtained in these two patients.
Patient 1 is a 62-year-old man working as a farmer and welder. He is a nonsmoker, consumes 5–6 units alcohol per day and has no relevant family history. 5 months before referral to our CTEPH centre, deep vein thrombosis was diagnosed in one of his legs. 1 month later, he was admitted to the hospital with an acute PE with a systolic PA pressure (sPAP) of 55 mmHg on TTE, with D-dimers of 1500 µg·L−1 and was treated with dabigatran. He received antibiotics (amoxicillin and clavulanic acid) because of a subsequent episode of pleurodynia (differential diagnosis PE infarction). A few weeks later, dyspnoea and inflammation decreased but D-dimers increased above 4000 µg·L−1. A lung function test showed restrictive pulmonary disease with a total lung capacity (TLC) of 63% and a diffusing capacity of lung for carbon monoxide (DLCO) of 38%. Dabigatran treatment was temporarily switched to tinzaparin. 1 month later, he complained of persistent dyspnoea, but inflammatory markers and D-dimers had clearly decreased. Another month later, he still complained of dyspnoea at exercise New York Heart Association (NYHA) class III with important oxygen desaturation during a 6MWD test, with a persistent decreased DLCO (57%) and an sPAP of 55 mmHg on TTE. Subsequently, he was referred to our CTEPH centre. Central venous pressure (CVP) was increased, with bimalleolar oedema and an NT-proBNP of 528 mg·dL−1. ECG showed no abnormalities. On TTE, the right atrium had an increased surface of 20.1 cm2, the RV/left ventricular (LV) area ratio was 0.90, tricuspid insufficiency was grade 1 with an sPAP of 37 mmHg+CVP, the Tei index was 0.33 and the tricuspid annular plane systolic excursion (TAPSE) was 28 mm. There was no LV dysfunction, valvular disease or pericardial fluid. Pulmonary function tests showed relatively preserved lung volumes (forced vital capacity of 85%, forced expiratory volume in 1 s of 72%, TLC of 87%) with a moderate decrease of DLCO of 57%. Arterial blood gas showed a trend to respiratory alkalosis with normal oxygen saturation (pH 7.45, arterial carbon dioxide tension (PaCO2) of 35 mmHg, arterial oxygen tension (PaO2) of 79 mmHg) without supplemental oxygen.
Patient 2 is a 57-year-old woman with a history of splenectomy due to spherocytosis as a child. At the age of 55, she presented a segmental acute PE treated with rivaroxaban over 3 months, followed by aspirin. 2 years later, at time of presentation, she experienced progressive shortness of breath and chest pain, requiring hospitalisation for further workup. Clinically, the patient described severe dyspnoea on exertion (NYHA functional class III) with signs of increased CVP and bimalleolar oedema. Blood tests were normal except for an increased NT-proBNP (1370 mg·dL−1). Arterial blood gas at ambient air showed a manifest hypoxaemia (PaO2 58 mmHg) with compensatory respiratory alkalosis (pH 7.47, PaCO2 31 mmHg). ECG did not show significant abnormalities. TTE showed preserved LV function without significant valvular pathology. Right chambers were slightly dilated with a right atrial area of 16 cm2 and an RV/LV area ratio at 0.90. There was a discrete tricuspid regurgitation with sPAP estimated at 76 mmHg. RV function was relatively preserved with a TAPSE of 21 mm. There was no pericardial effusion. Lung function tests showed normal lung volumes with slightly decreased DLCO (61%). 6MWD was 495 m with significant desaturation. CPET demonstrated a decreased exercise capacity with a peak oxygen uptake (V′O2) of 15.7 mL·min−1·kg−1 and an abnormal ventilation response with an increased minute ventilation (V′E)/carbon dioxide production (V′CO2) slope and an abnormal evolution of dead space during exercise. The exercise test was limited by cardiac function.
V′/Q′ scintigraphy
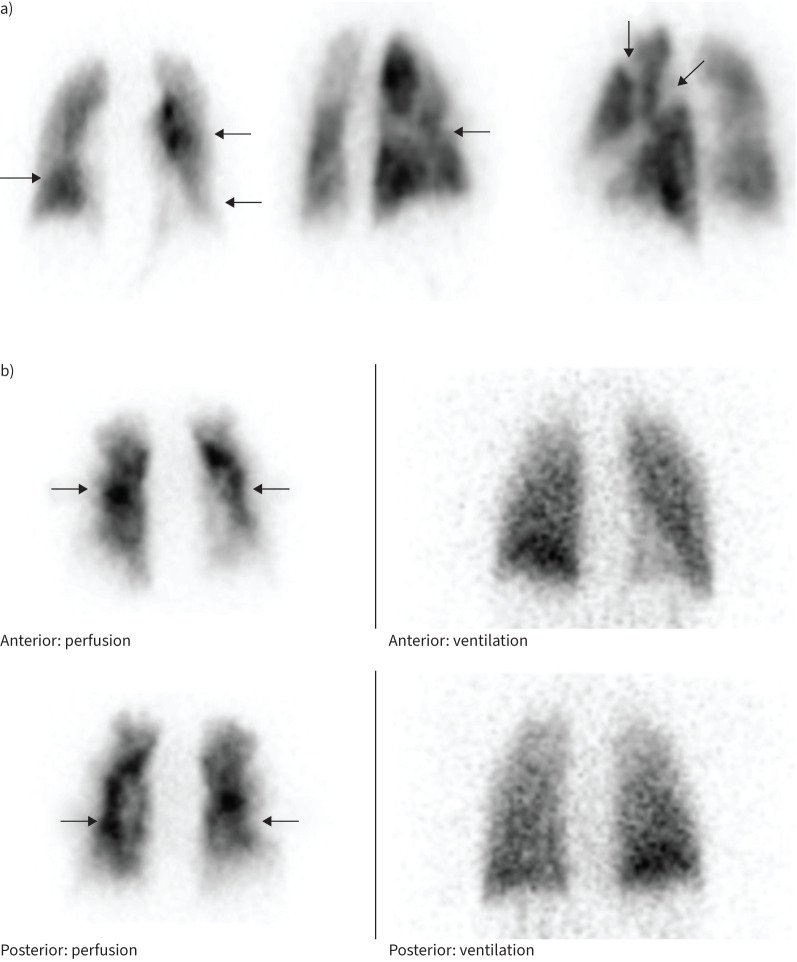
The V′/Q′ lung scanning of patient 1 showed decreased perfusion in the posterior and lateral parts of the left upper lobe, in the lateral part of the left lower lobe and small perfusion defects in the right middle lobe (figure 1a). Ventilation was homogenous.
FIGURE 1.
a) Bilateral segmental and subsegmental perfusion defects on frontal and oblique views. Ventilation (not shown) was homogenous. b) Ventilation (right)/perfusion (left) scintigraphy shows bilateral predominantly subsegmental perfusion defects (arrows) with relatively normal ventilation.
The V′/Q′ lung scanning of patient 2 showed normal ventilation with a subsegmental perfusion defect and a marked subpleural decreased perfusion. This pattern has been associated with more distal disease and inoperability (figure 1b) [72].
CTPA
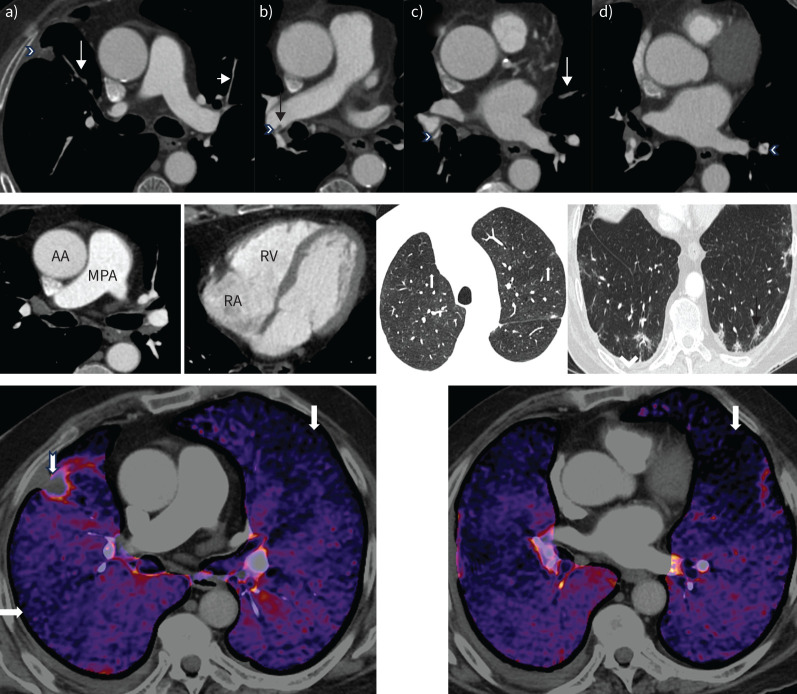
CTPA of patient 1 showed segmental occlusion in both upper lobes and lingula, a linear filling defect in the right interlobar artery that was in keeping with an intravascular web, segmental stenoses in the right lower lobe, and segmental webs in the left lower lobe. In addition, there was mosaic attenuation evidenced by heterogenous lung parenchymal attenuation. A focal subpleural anterobasal consolidation in the right upper lobe adjacent to the fissure with spiculated margins was compatible with pulmonary infarction. Ill-defined ground glass opacities with spiculated margins in the left upper lobe as well as posterobasally in subpleural locations in both lower lobes were likely to be pulmonary infarction/parenchymal scarring (figure 2).
FIGURE 2.
Computed tomography (CT) pulmonary angiography of patient 1. Upper panel: axial views. a) Occluded anterior segment of right upper lobe (long arrow) with a small peripheral infarct (arrowhead). The anterior segment of the left upper lobe is very attenuated (short arrow). b) Intravascular shelf in the right interlobar artery (arrow) and stenosis at the origin of the right lower lobe vessel (arrowhead). c) Segmental web in the right lower lobe (arrowhead) and occluded segmental lingula (arrow). d) Segmental web in the left lower lobe (arrowhead). Middle panel: mediastinal windows showing a normal calibre main pulmonary artery (MPA) and an MPA/ascending aorta (AA) ratio <1.0. The right atrium (RA) and right ventricle (RV) are not dilated. There is no right ventricular hypertrophy. Lung windows demonstrate mosaic attenuation (left, white arrows point to poorly perfused regions), peripheral infarct (right, black arrow) and a small right pleural effusion (arrowhead). Lower panel: dual-energy CT pulmonary angiography demonstrates multiple segmental perfusion defects in both lungs (arrows). There is a small peripheral pulmonary infarct in the right upper lobe (notched arrow).
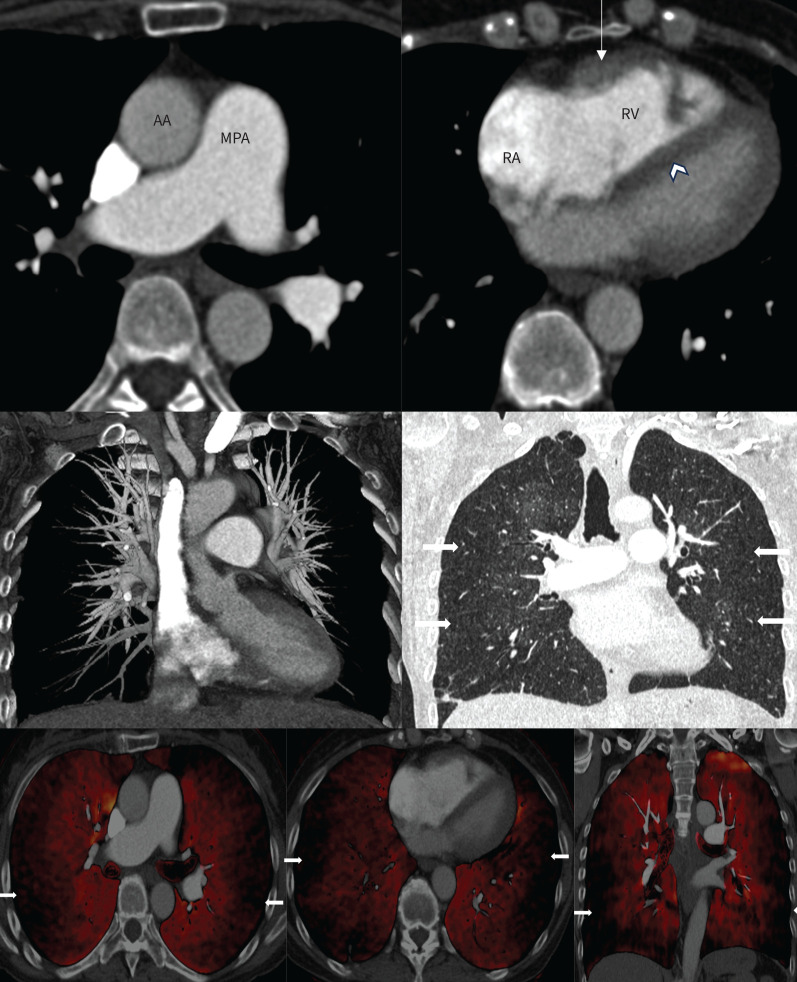
CTPA of patient 2 showed no involvement of proximal PAs until the segmental level. Adequate multiplanar reconstructions of the pulmonary angiogram with maximum-intensity projection showed diffuse narrowing of the calibres of the PA at subsegmental levels, irregular lumen and attenuated peripheral vasculature. All these findings could have been missed if they had not been carefully assessed by an experienced radiologist. Indirect signs of CTEPH were also present, such as a mosaic attenuation pattern of the lung parenchyma with areas of normal perfusion (higher density) alternating with areas of decreased perfusion (lower density). There were generic features of PH, such as enlargement of the main PA with dilatation of right atrium and RV, RV hypertrophy, flattening of the interventricular septum, and reflux of contrast medium into the hepatic veins, suggesting severe RV dysfunction (figure 3).
FIGURE 3.
Computed tomography (CT) pulmonary angiography of patient 2. Upper panel: mediastinal windows showing mild dilatation of the main pulmonary artery (MPA) and an MPA/ascending aorta (AA) ratio >1.0. The right atrium (RA) and right ventricle (RV) are dilated. There is moderate right ventricular hypertrophy (arrow) and flattening of the interventricular septum (arrowhead). Middle panel: coronal maximum intensity projection (left) demonstrating relatively intact proximal vessels but absent distal segmental and subsegmental vasculature. The corresponding lung window (right) shows mosaic attenuation with preserved central and poor peripheral perfusion (arrows). Lower panel: dual-energy CT pulmonary angiography axial and coronal views elegantly depicting the subpleural diffuse perfusion defect.
Furthermore, DECT images of patients 1 and 2 are presented in figures 2 and 3, respectively.
DSA and RHC
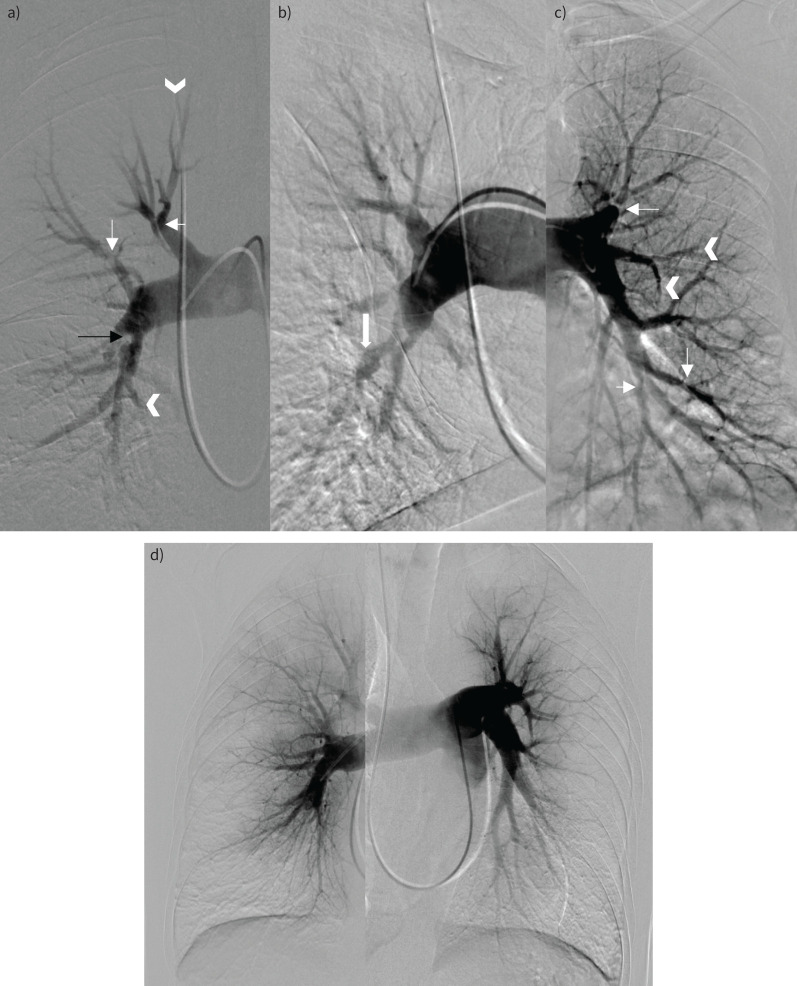
The pulmonary angiography of patient 1 (figure 4a) shows multiple stenoses in the proximal right lower lobe segmental arteries (mostly posterobasal), on the artery towards the posterior segment of the right upper lobe and on the bifurcation of the right upper lobe artery splitting in the segmental branches of the apical and anterior segment. Wall-adherent material can be seen in the right upper lobe artery and the posterior segmental artery of this right upper lobe. A stenosis is seen proximal in the left upper lobe artery, together with some variation in the calibre of multiple proximal segmental left lower lobe arteries and truncation of some lingular branches and of some basal branches in the left lower lobe.
FIGURE 4.
Digital subtraction angiography. a) Patient 1. Pulmonary vascular signs of chronic thromboembolic disease. There are bilateral segmental webs and stenoses (thin white arrows in panels a and c), post-stenotic dilatation (block arrow in b), intimal irregularity (black arrow in a), multifocal occlusions, and abrupt vessel truncation (arrowheads in a and c). d) Patient 2. Diffuse narrowing of the subsegmental vessels with abrupt calibre change. The distal vessels are spindly with a corresponding peripheral perfusional defect. There was no significant disease in the proximal vessels.
The pulmonary angiography of patient 2 (figure 4b) shows diffuse narrowing of the subsegmental vessels with all segments involved, steep calibre changes and spindly aspects of distal vessels. A diffuse peripheral defect of perfusion is apparent in the subpleural region. There is no major involvement of proximal vessels (main PAs, lobar arteries) and all the segmental branches are present without occlusion at this level.
RHC of patient 1 showed a right atrial pressure (RAP) of 3 mmHg, mPAP of 18 mmHg, a PA wedge pressure (PAWP) of 5 mmHg, a cardiac index (CI) of 2.22 L·min−1·m−2, PVR of 3 WU and a mixed venous oxygen saturation (SvO2) of 73.2%. These results correspond to an absence of PH with discretely elevated PVR.
RHC of patient 2 showed RAP of 7 mmHg, mPAP of 50 mmHg, PAWP of 7 mmHg, a decreased CI of 2.14 L·min−1·m−2, PVR of 12 WU and an SvO2 of 69.7%. Altogether, these results correspond to severe pre-capillary PH with moderate RV dysfunction.
Cardiac function and haemodynamics during exercise
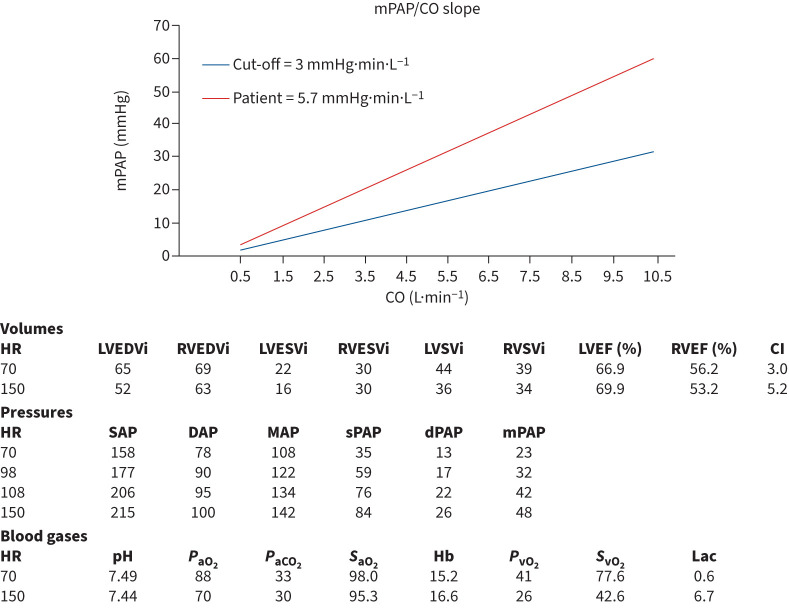
To better explain the mechanism of dyspnoea in CTEPD and therefore assess the potential benefit of a PEA in these particular patients, we performed CPET and cardiac magnetic resonance imaging (MRI) during exercise in order to disclose an impaired cardiac reserve and significant pulmonary vascular disease [63]. Patient 1 was such a CTEPD patient without PH at rest. Figure 5 shows the results of the stress MRI. Exercise induced an increase of LV ejection fraction of 3% but a decrease of RV ejection fraction of 3% indicating the absence of an RV contractile reserve. The ratio of LV/RV volume decreased from 0.94 to 0.83, indicating RV overload. In addition, mPAP increased abnormally compared to CO with a slope of the mPAP/CO curve of 5.7 mmHg·min·L−1, clearly indicating exercise-induced PH.
FIGURE 5.
Magnetic resonance imaging at rest and at maximal exercise. CI: cardiac index; CO: cardiac output; DAP: diastolic arterial pressure; dPAP: diastolic pulmonary arterial pressure; Hb: haemoglobin; HR: heart rate; lac: lactate; LVEDVi: left ventricle end diastolic volume index; LVEF: left ventricle ejection fraction; LVESVi: left ventricle end systolic volume index; LVSVi: left ventricle stroke volume index; MAP: mean arterial pressure; mPAP: mean pulmonary arterial pressure; PaCO2: arterial carbon dioxide tension; PaO2: arterial oxygen tension; PvO2: mixed venous oxygen partial tension; RVEDVi: right ventricle end diastolic volume index; RVEF: right ventricle ejection fraction; RVESVi: right ventricle end systolic volume index; RVSVi: right ventricle stroke volume index; SAP: systolic arterial pressure; SaO2: arterial oxygen saturation; sPAP: systolic pulmonary arterial pressure; SvO2: mixed venous oxygen saturation.
Patient selection and treatment
As there were no significant operative risk factors identified in patient 1 and given the imaging findings and the findings of significant exercise impairment on exercise RHC, CPET and stress MRI, we decided to propose surgery. Patient 1 underwent an uncomplicated PEA according to the operative principles described by Madani et al. [49]. Circulatory arrest times were 20 and 13 min for the right side and 20 min and 20 min for the left side. Figure 6 shows the resection specimens, exhibiting University of California San Diego classification level 2 (lobar) for the right side and level 3 (segmental) for the left side. The patient was extubated 5 h post-surgery but had however a prolonged stay of 4 days on the intensive care unit because of an atrial fibrillation episode with haemodynamic compromise, which was successfully treated with amiodarone. After another 8 days on the ward, the patient went home. 6 months later, RHC revealed an mPAP of 16 mmHg, a CI of 2.35 L·min−1·m−2 and a PVR of 1.35 WU. The patient was functioning at NYHA class I.
FIGURE 6.
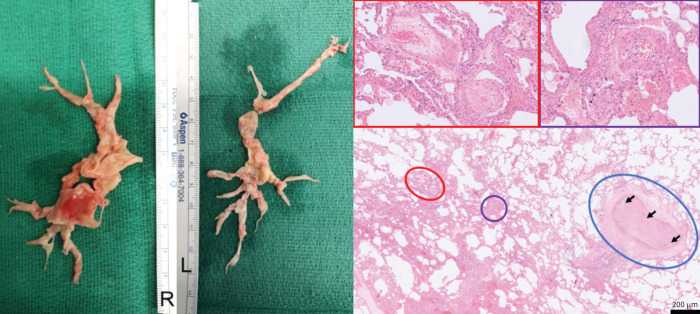
Left panel: resected material during pulmonary endarterectomy in patient 1. Right panel: lungs from another patient with chronic thromboembolic pulmonary hypertension with peripheral, inoperable disease who underwent a lung transplantation (haematoxylin and eosin staining; magnification: ×40, insets ×200). A pulmonary artery of a cross-sectional diameter of about 400 µm (circled in blue) is obliterated by organised thromboembolic material. Note the recanalisation of vessels within the obstruction (arrows). In the vicinity of this obstructed artery, microvascular remodelling can be found in arterioles (circled in purple), as well as in post-capillary venules (circled in red), both of around 70 µm in diameter, and depicted in the magnified purple and red inset boxes above. Note the concentric character of the microvascular remodelling, which indicates reactive changes, rather than thrombotic lesion, with smooth muscle cell hyperplasia and intimal fibrosis (venules).
Patient 2 presented with a distal disease, without evidence of the involvement of proximal arteries on CTPA and pulmonary angiography. The medical history of splenectomy was another argument suggesting a distal disease. The patient also had high levels of PVR and mPAP (>40 mmHg), suggesting a risk of complications during BPA. Therefore, the decision was made to add riociguat treatment. After a treatment period of 3 months, there was haemodynamic improvement, but this was considered insufficient and it was decided that medical treatment should be optimised with the endothelin receptor antagonist bosentan before the first BPA. Patient 2 underwent a total of six BPA sessions, with successful dilatations of subsegmental arteries in the vast majority of the segments. The final RHC evaluation showed a final mPAP of 21 mmHg, a decreased PVR at 2 WU and a CI of 3.14 L·min−1·m−2. The patient's symptoms dramatically decreased (NYHA I). Exercise capacity improved, with a 6MWD of 579 m and oxygen uptake of 19 mL·min−1·kg−1. V′E/V′CO2 also improved to 52. The angiogram showed persistence of target lesions and perfusion defects, although a clear improvement was noted. After discussion with the patient and regarding the excellent haemodynamic evolution, it was decided not to propose further BPA.
Points for clinical practice
Awareness of potential CTEPH after acute PE.
Diagnosis in two steps, as follows: 1) suspicion (symptoms, echocardiography and V′/Q′ scan), followed by 2) confirmation (RHC, CTPA and DSA).
Treatment of choice is PEA when lesions are reachable.
Alternatively, BPA and/or PH medication can be used, alone or in combination, or adjuvant to PEA in a so-called multimodality treatment strategy.
Conclusions
This clinical–radiological–pathological correlation paper illustrates the diagnostic and therapeutic management of two patients with CTEPD without PH or CTEPH. It describes the diagnostic approach as well as the therapeutic decision process. Evaluation of operability results in two different decisions, based on comorbidities, imaging and pulmonary haemodynamics. If selected appropriately, both PEA and BPA can be successful in terms of restoring normal exercise capacity. These two examples confirm that CTEPH is a curable disease when patients are referred to CTEPH centres for specialist treatment.
Footnotes
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Condliffe R, Durrington C, Hameed A, et al. Clinical–radiological–pathological correlation in pulmonary arterial hypertension. Eur Respir Rev 2023; 32: 230138. No. 2: Lichtblau M, Mayer L, Gopalan D, et al. Clinical–radiological–pathological correlation in pulmonary hypertension with unclear and/or multifactorial mechanisms. Eur Respir Rev 2023; 32: 230119.
Number 3 in the Series “Clinical–radiological–pathological correlation in pulmonary hypertension” Edited by Robin Condliffe, Anton Vonk Noordegraaf, Olivier Sitbon, Peter Dorfmüller and Deepa Gopalan
This article has an editorial commentary: https://doi.org/10.1183/16000617.0237-2023
Conflict of interest: T. Verbelen reports lecture fees from Medtronic, and was a Board member for European Society for Artificial Organs (ESAO). L. Godinas reports consulting and lecture fees from Janssen and support for attending meetings and/or travel from MSD. P. Dorfmüller reports support for attending meetings and/or travel from Stanford University, EULAR Society, and Amsterdam University (AMC). D. Gopalan reports no conflicts of interest. R. Condliffe reports consulting fees from Janssen and MSD, lecture fees and support for attending meetings and/or travel from Janssen, and participation on a data safety monitoring board or advisory board with Boston Scientific. M. Delcroix reports a research grant from Janssen, consulting fees from Actelion/Janssen, Bayer/MSD, Acceleron, Ferrer, AOP, United therapeutics, Altavant and INARI, and lecture fees from Actelion/Janssen and Bayer/MSD.
References
- 1.Delcroix M, Torbicki A, Gopalan D, et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J 2021; 57: 2002828. doi: 10.1183/13993003.02828-2020 [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2022; 43: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 4.European Respiratory Society . Pulmonary hypertension: clinical-radiological-pathological case series. Date last accessed: 23 November 2023. www.ersnet.org/events/pulmonary-hypertension-clinical-radiological-pathological-case-series/#Programme
- 5.Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J 2017; 49: 1601792. doi: 10.1183/13993003.01792-2016 [DOI] [PubMed] [Google Scholar]
- 6.Sanchez O, Helley D, Couchon S, et al. Perfusion defects after pulmonary embolism: risk factors and clinical significance. J Thromb Haemost 2010; 8: 1248–1255. doi: 10.1111/j.1538-7836.2010.03844.x [DOI] [PubMed] [Google Scholar]
- 7.Reddy SA, Swietlik EM, Robertson L, et al. Natural history of chronic thromboembolic pulmonary disease with no or mild pulmonary hypertension. J Heart Lung Transplant 2023; 42: 1275–1285. doi: 10.1016/j.healun.2023.04.016 [DOI] [PubMed] [Google Scholar]
- 8.Marchetta S, Verbelen T, Claessen G, et al. A comprehensive assessment of right ventricular function in chronic thromboembolic pulmonary hypertension. J Clin Med 2022; 12: 47. doi: 10.3390/jcm12010047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Plas MN, Reesink HJ, Roos CM, et al. Pulmonary endarterectomy improves dyspnea by the relief of dead space ventilation. Ann Thorac Surg 2010; 89: 347–352. doi: 10.1016/j.athoracsur.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 10.Riedel M, Stanek V, Widimsky J, et al. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982; 81: 151–158. doi: 10.1378/chest.81.2.151 [DOI] [PubMed] [Google Scholar]
- 11.Bonderman D, Turecek PL, Jakowitsch J, et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thromb Haemost 2003; 90: 372–376. doi: 10.1160/TH03-02-0067 [DOI] [PubMed] [Google Scholar]
- 12.Bonderman D, Wilkens H, Wakounig S, et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur Respir J 2009; 33: 325–331. doi: 10.1183/09031936.00087608 [DOI] [PubMed] [Google Scholar]
- 13.Li JF, Lin Y, Yang YH, et al. Fibrinogen Aα Thr312Ala polymorphism specifically contributes to chronic thromboembolic pulmonary hypertension by increasing fibrin resistance. PLoS One 2013; 8: e69635. doi: 10.1371/journal.pone.0069635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quarck R, Wynants M, Verbeken E, et al. Contribution of inflammation and impaired angiogenesis to the pathobiology of chronic thromboembolic pulmonary hypertension. Eur Respir J 2015; 46: 431–443. doi: 10.1183/09031936.00009914 [DOI] [PubMed] [Google Scholar]
- 15.Quarck R, Willems L, Tielemans B, et al. Impairment of angiogenesis-driven clot resolution is a key event in the progression to chronic thromboembolic pulmonary hypertension: validation in a novel rabbit model. Arterioscler Thromb Vasc Biol 2023; 43: 1308–1321. doi: 10.1161/ATVBAHA.122.317262 [DOI] [PubMed] [Google Scholar]
- 16.Jevnikar M, Montani D, Savale L, et al. Chronic thromboembolic pulmonary hypertension and totally implantable central venous access systems. Eur Respir J 2021; 57: 2002208. doi: 10.1183/13993003.02208-2020 [DOI] [PubMed] [Google Scholar]
- 17.Frey MK, Alias S, Winter MP, et al. Splenectomy is modifying the vascular remodeling of thrombosis. J Am Heart Assoc 2014; 3: e000772. doi: 10.1161/JAHA.113.000772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonneau G, Dorfmüller P, Guignabert C, et al. Chronic thromboembolic pulmonary hypertension: the magic of pathophysiology. Ann Cardiothorac Surg 2022; 11: 106–119. doi: 10.21037/acs-2021-pte-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boulate D, Perros F, Dorfmuller P, et al. Pulmonary microvascular lesions regress in reperfused chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2015; 34: 457–467. doi: 10.1016/j.healun.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 20.Dorfmüller P, Günther S, Ghigna MR, et al. Microvascular disease in chronic thromboembolic pulmonary hypertension: a role for pulmonary veins and systemic vasculature. Eur Respir J 2014; 44: 1275–1288. doi: 10.1183/09031936.00169113 [DOI] [PubMed] [Google Scholar]
- 21.Clements W, Venn G, McGiffin D, et al. Chronic thromboembolic pulmonary hypertension (CTEPH) and massive hemoptysis: the rationale for bronchial artery embolization. Respir Med 2022; 195: 106784. doi: 10.1016/j.rmed.2022.106784 [DOI] [PubMed] [Google Scholar]
- 22.Kramm T, Wilkens H, Fuge J, et al. Incidence and characteristics of chronic thromboembolic pulmonary hypertension in Germany. Clin Res Cardiol 2018; 107: 548–553. doi: 10.1007/s00392-018-1215-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delcroix M, Kerr K, Fedullo P. Chronic thromboembolic pulmonary hypertension. Epidemiology and risk factors. Ann Am Thorac Soc 2016; 13: Suppl. 3, S201–S206. doi: 10.1513/AnnalsATS.201509-621AS [DOI] [PubMed] [Google Scholar]
- 24.Verbelen T, Godinas L, Maleux G, et al. Chronic thromboembolic pulmonary hypertension: diagnosis, operability assessment and patient selection for pulmonary endarterectomy. Ann Cardiothorac Surg 2022; 11: 82–97. doi: 10.21037/acs-2021-pte-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klok FA, Barco S, Konstantinides SV, et al. Determinants of diagnostic delay in chronic thromboembolic pulmonary hypertension: results from the European CTEPH registry. Eur Respir J 2018; 52: 1801687. doi: 10.1183/13993003.01687-2018 [DOI] [PubMed] [Google Scholar]
- 26.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011; 124: 1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008 [DOI] [PubMed] [Google Scholar]
- 27.Klok FA, Ageno W, Ay C, et al. Optimal follow-up after acute pulmonary embolism: a position paper of the European Society of Cardiology working group on pulmonary circulation and right ventricular function, in collaboration with the European Society of Cardiology working group on atherosclerosis and vascular biology, endorsed by the European Respiratory Society. Eur Heart J 2022; 43: 183–189. doi: 10.1093/eurheartj/ehab816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klok FA, van Kralingen KW, van Dijk AP, et al. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica 2010; 95: 970–975. doi: 10.3324/haematol.2009.018960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boon G, Ende-Verhaar YM, Bavalia R, et al. Non-invasive early exclusion of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: the InShape II study. Thorax 2021; 76: 1002–1009. doi: 10.1136/thoraxjnl-2020-216324 [DOI] [PubMed] [Google Scholar]
- 30.Tunariu N, Gibbs SJR, Win Z, et al. Ventilation–perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J Nucl Med 2007; 48: 680–684. doi: 10.2967/jnumed.106.039438 [DOI] [PubMed] [Google Scholar]
- 31.Kiely DG, Levin D, Hassoun P, et al. EXPRESS: statement on imaging and pulmonary hypertension from the Pulmonary Vascular Research Institute (PVRI). Pulm Circ 2019; 9: 2045894019841990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmud E, Madani MM, Kim NH, et al. Chronic thromboembolic pulmonary hypertension: evolving therapeutic approaches for operable and inoperable disease. J Am Coll Cardiol 2018; 71: 2468–2486. doi: 10.1016/j.jacc.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 33.Rogberg AN, Gopalan D, Westerlund E, et al. Do radiologists detect chronic thromboembolic disease on computed tomography? Acta Radiol Stockh Swed 1987 2019; 60: 1576–1583. [DOI] [PubMed] [Google Scholar]
- 34.Sugiura T, Tanabe N, Matsuura Y, et al. Role of 320-slice CT imaging in the diagnostic workup of patients with chronic thromboembolic pulmonary hypertension. Chest 2013; 143: 1070–1077. doi: 10.1378/chest.12-0407 [DOI] [PubMed] [Google Scholar]
- 35.Auger WR, Fedullo PF, Moser KM, et al. Chronic major-vessel thromboembolic pulmonary artery obstruction: appearance at angiography. Radiology 1992; 182: 393–398. doi: 10.1148/radiology.182.2.1732955 [DOI] [PubMed] [Google Scholar]
- 36.Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg 2003; 76: 1457–1462. doi: 10.1016/S0003-4975(03)00828-2 [DOI] [PubMed] [Google Scholar]
- 37.Hoey ETD, Mirsadraee S, Pepke-Zaba J, et al. Dual-energy CT angiography for assessment of regional pulmonary perfusion in patients with chronic thromboembolic pulmonary hypertension: initial experience. Am J Roentgenol 2011; 196: 524–532. doi: 10.2214/AJR.10.4842 [DOI] [PubMed] [Google Scholar]
- 38.Dong C, Zhou M, Liu D, et al. Diagnostic accuracy of computed tomography for chronic thromboembolic pulmonary hypertension: a systematic review and meta-analysis. PLoS One 2015; 10: e0126985. doi: 10.1371/journal.pone.0126985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claeys M, Claessen G, La Gerche A, et al. Impaired cardiac reserve and abnormal vascular load limit exercise capacity in chronic thromboembolic disease. JACC Cardiovasc Imaging 2019; 12: 1444–1456. doi: 10.1016/j.jcmg.2018.07.021 [DOI] [PubMed] [Google Scholar]
- 40.Godinas L, Sattler C, Lau EM, et al. Dead-space ventilation is linked to exercise capacity and survival in distal chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2017; 36: 1234–1242. doi: 10.1016/j.healun.2017.05.024 [DOI] [PubMed] [Google Scholar]
- 41.Humbert M, Simonneau G, Pittrow D, et al. Oral anticoagulants (NOAC and VKA) in chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2022; 41: 716–721. doi: 10.1016/j.healun.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 42.Jeong I, Alotaibi M, Fernandes TM, et al. Direct oral anticoagulants in patients with chronic thromboembolic pulmonary hypertension and the presence of recent thrombus during pulmonary endarterectomy. Pulm Circ 2022; 12: e12110. doi: 10.1002/pul2.12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delcroix M, Lang I, Pepke-Zaba J, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation 2016; 133: 859–871. doi: 10.1161/CIRCULATIONAHA.115.016522 [DOI] [PubMed] [Google Scholar]
- 44.D'Armini AM, Zanotti G, Ghio S, et al. Reverse right ventricular remodeling after pulmonary endarterectomy. J Thorac Cardiovasc Surg 2007; 133: 162–168. doi: 10.1016/j.jtcvs.2006.08.059 [DOI] [PubMed] [Google Scholar]
- 45.Ghio S, Morsolini M, Corsico A, et al. Pulmonary arterial compliance and exercise capacity after pulmonary endarterectomy. Eur Respir J 2014; 43: 1403–1409. doi: 10.1183/09031936.00195313 [DOI] [PubMed] [Google Scholar]
- 46.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011; 141: 702–710. doi: 10.1016/j.jtcvs.2010.11.024 [DOI] [PubMed] [Google Scholar]
- 47.Fernandes TM, Kim NH, Kerr KM, et al. Distal vessel pulmonary thromboendarterectomy: results from a single institution. J Heart Lung Transplant 2023; 42: 1112–1119. doi: 10.1016/j.healun.2023.02.1500 [DOI] [PubMed] [Google Scholar]
- 48.Godinas L, Verbelen T, Delcroix M. Residual pulmonary hypertension after pulmonary thromboendarterectomy: incidence, pathogenesis and therapeutic options. Ann Cardiothorac Surg 2022; 11: 163–165. doi: 10.21037/acs-2021-pte-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madani M, Mayer E, Fadel E, et al. Pulmonary endarterectomy. patient selection, technical challenges, and outcomes. Ann Am Thorac Soc 2016; 13: Suppl. 3, S240–S247. doi: 10.1513/AnnalsATS.201601-014AS [DOI] [PubMed] [Google Scholar]
- 50.Madani MM, Wittine LM, Auger WR, et al. Chronic thromboembolic pulmonary hypertension in pediatric patients. J Thorac Cardiovasc Surg 2011; 141: 624–630. doi: 10.1016/j.jtcvs.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 51.Verbelen T, Cools B, Fejzic Z, et al. Pulmonary endarterectomy in a 12-year-old boy with multiple comorbidities. Pulm Circ 2019; 9: 2045894019886249. doi: 10.1177/2045894019886249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grazioli V, Ghio S, Pin M, et al. Pulmonary endarterectomy in the octogenarian population: safety and outcomes. J Cardiovasc Med 2021; 22: 567–571. doi: 10.2459/JCM.0000000000001138 [DOI] [PubMed] [Google Scholar]
- 53.Fernandes TM, Auger WR, Fedullo PF, et al. Baseline body mass index does not significantly affect outcomes after pulmonary thromboendarterectomy. Ann Thorac Surg 2014; 98: 1776–1781. doi: 10.1016/j.athoracsur.2014.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim NH, Delcroix M, Jais X, et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1801915. doi: 10.1183/13993003.01915-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taboada D, Pepke-Zaba J, Jenkins DP, et al. Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Respir J 2014; 44: 1635–1645. doi: 10.1183/09031936.00050114 [DOI] [PubMed] [Google Scholar]
- 56.van Kan C, van der Plas MN, Reesink HJ, et al. Hemodynamic and ventilatory responses during exercise in chronic thromboembolic disease. J Thorac Cardiovasc Surg 2016; 152: 763–771. doi: 10.1016/j.jtcvs.2016.05.058 [DOI] [PubMed] [Google Scholar]
- 57.Ghofrani HA, D'Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013; 369: 319–329. doi: 10.1056/NEJMoa1209657 [DOI] [PubMed] [Google Scholar]
- 58.Hoeper MM. Pharmacological therapy for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2015; 24: 272–282. doi: 10.1183/16000617.00001015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim NH, D'Armini AM, Grimminger F, et al. Haemodynamic effects of riociguat in inoperable/recurrent chronic thromboembolic pulmonary hypertension. Heart Br Card Soc 2017; 103: 599–606. doi: 10.1136/heartjnl-2016-309621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murata M, Kawakami T, Kataoka M, et al. Clinical significance of guanylate cyclase stimulator, riociguat, on right ventricular functional improvement in patients with pulmonary hypertension. Cardiology 2021; 146: 130–136. doi: 10.1159/000510860 [DOI] [PubMed] [Google Scholar]
- 61.Benza RL, Ghofrani HA, Grünig E, et al. Effect of riociguat on right ventricular function in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2021; 40: 1172–1180. doi: 10.1016/j.healun.2021.06.020 [DOI] [PubMed] [Google Scholar]
- 62.Sadushi-Kolici R, Jansa P, Kopec G, et al. Subcutaneous treprostinil for the treatment of severe non-operable chronic thromboembolic pulmonary hypertension (CTREPH): a double-blind, phase 3, randomised controlled trial. Lancet Respir Med 2019; 7: 239–248. doi: 10.1016/S2213-2600(18)30367-9 [DOI] [PubMed] [Google Scholar]
- 63.Lang IM, Andreassen AK, Andersen A, et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: a clinical consensus statement of the ESC working group on pulmonary circulation and right ventricular function. Eur Heart J 2023; 44: 2659–2671. doi: 10.1093/eurheartj/ehad413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brenot P, Jaïs X, Taniguchi Y, et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J 2019; 53: 1802095. doi: 10.1183/13993003.02095-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukui S, Ogo T, Morita Y, et al. Right ventricular reverse remodelling after balloon pulmonary angioplasty. Eur Respir J 2014; 43: 1394–1402. doi: 10.1183/09031936.00012914 [DOI] [PubMed] [Google Scholar]
- 66.Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–755. doi: 10.1161/CIRCINTERVENTIONS.112.971077 [DOI] [PubMed] [Google Scholar]
- 67.Taniguchi Y, Jaïs X, Jevnikar M, et al. Predictors of survival in patients with not-operated chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant 2019; 38: 833–842. doi: 10.1016/j.healun.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 68.Lang I, Meyer BC, Ogo T, et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160119. doi: 10.1183/16000617.0119-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Godinas L, Bonne L, Budts W, et al. Balloon pulmonary angioplasty for the treatment of nonoperable chronic thromboembolic pulmonary hypertension: single-center experience with low initial complication rate. J Vasc Interv Radiol 2019; 30: 1265–1272. doi: 10.1016/j.jvir.2019.03.023 [DOI] [PubMed] [Google Scholar]
- 70.Kawakami T, Ogawa A, Miyaji K, et al. Novel angiographic classification of each vascular lesion in chronic thromboembolic pulmonary hypertension based on selective angiogram and results of balloon pulmonary angioplasty. Circ Cardiovasc Interv 2016; 9: e003318. doi: 10.1161/CIRCINTERVENTIONS.115.003318 [DOI] [PubMed] [Google Scholar]
- 71.Darocha S, Araszkiewicz A, Kurzyna M, et al. Balloon pulmonary angioplasty in technically operable and technically inoperable chronic thromboembolic pulmonary hypertension. J Clin Med 2021; 10: 1038. doi: 10.3390/jcm10051038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanabe N, Sugiura T, Jujo T, et al. Subpleural perfusion as a predictor for a poor surgical outcome in chronic thromboembolic pulmonary hypertension. Chest 2012; 141: 929–934. doi: 10.1378/chest.11-0769 [DOI] [PubMed] [Google Scholar]