Abstract
Objective
Auricular/periauricular cutaneous malignancies can be challenging to manage surgically due to the complex anatomy of the region. Otologists/neurotologists have unique skillsets that are well‐suited to surgically treat these patients. We aim to highlight the role of otologists and neurotologists in providing surgical care of patients with auricular and periauricular malignancies by describing the experience of a single fellowship‐trained neurotologist over a 10‐year period.
Methods
Retrospective chart review of 387 patients with auricular and periauricular malignancy treated by a single neurotologist between 2012 and 2022 was completed. Tumor histology and procedures performed for each patient were extracted. Additional data was collected for a subset of 84 patients with complex cases requiring selective neck dissection, parotidectomy, lateral temporal bone resection, regional advancement or rotational flap reconstruction, and/or free tissue transfer reconstruction.
Results
Within the series of 387 patients, squamous cell carcinoma was the most common histology (42.6%, n = 165), followed by basal cell carcinoma (40.8%, n = 158), and melanoma (9.8%, n = 38). Common surgical procedures included wide local excision (61.8%, n = 239), partial/sub‐total auriculectomy 18.3% (n = 71), or total auriculectomy 5.2% (n = 20). Within the 84‐patient subset, median age at diagnosis was 71.9 years. Dermatologists provided most patient referrals (50.0%, n = 42). Most common tumor locations included: auricular (58.3%, n = 49), pre‐auricular (21.4%, n = 18), and parotid (27.4%, n = 23). Revision surgery occurred in 22.6% of cases (n = 19), of which 26.3% (n = 5) for positive margins and 31.6% (n = 6) for recurrence. Mean follow‐up was 22.8 months. Disease‐specific 5‐year survival was 91%.
Conclusions
We demonstrate the feasibility of an otologist/neurotologist incorporating the surgical management of auricular and periauricular malignancies into their practice.
Level of Evidence
4.
Keywords: auricle, basal cell carcinoma, melanoma, otology, skin cancer, squamous cell carcinoma
Auricular/periauricular cutaneous malignancies can be challenging to manage surgically due to the complex anatomy of the region. Otologists/neurotologists have unique skillsets that are well‐suited to surgically treat these patients. We demonstrate the importance and feasibility of an otologist/neurotologist incorporating the surgical management of auricular and periauricular malignancies into their practice.
1. INTRODUCTION
The incidence of cutaneous malignancies including squamous cell carcinoma (SCCA), basal cell carcinoma (BCCA), and melanoma is rising in the United States. 1 , 2 , 3 Approximately 30% of cutaneous SCCA and BCCA cases and 20% of melanoma cases are diagnosed in the head and neck region. 4 , 5 , 6 As many cutaneous malignancies are associated with ultraviolet (UV) light (sun) exposure, the head and neck are especially vulnerable regions. 7 , 8 Data suggests that especially in melanoma, auricular and periauricular sites portend worse prognosis, likely due to the high UV light exposure and variable lymphatic drainage of the ear. 9 , 10
Due to increasing incidence and the aggressive nature of the many auricular and periauricular cutaneous malignancies, prompt diagnosis and treatment are imperative. 7 , 8 , 10 , 11 Because of the complex three‐dimensional anatomy of the external ear, cutaneous malignancies in this region may go unnoticed by the patient and may be difficult to examine for providers who do not have the proper equipment such as a microscope or endoscope available. Otologists and neurotologists are well‐suited to provide comprehensive surgical care for patients with auricular and periauricular cutaneous malignancies. The otologist or neurotologist has the experience and equipment necessary to examine and diagnose these conditions. With their prior training in head and neck surgery, they are ideally‐suited to treat patients requiring partial or total auriculectomy, ear canal sleeve resection, parotidectomy, cervical lymphadenectomy/neck dissection, local and regional reconstruction, or lateral temporal bone resection (LTBR). While collaboration with additional surgical specialists may be necessary in some cases, otologists may certainly be able to provide much of the required surgical care for patients with auricular or periauricular cutaneous malignancies. Though treatment of cancers involving the temporal bone and skull base are described in the literature, there are few reports of an otologist's experience treating tumors involving auricular and periauricular soft tissues as well as the temporal bone. 12 , 13 , 14 Gidley and colleagues have previously highlighted the importance of an otologic surgeon's involvement in treating a series of 157 patients with primary tumors of the periauricular skin, parotid gland, ear canal, external ear, and skull base. 12
Here, we describe a large series of 387 patients with auricular and periauricular cutaneous, temporal bone, and parotid gland malignancies treated by a single fellowship‐trained neurotologist over a span of approximately 10 years from August 2012 to November 2022. Detailed retrospective analysis for a subset of 84 patients with more advanced tumors, requiring neck dissection, parotidectomy, LTBR, regional flap reconstruction, and/or free flap reconstruction is performed. We aim to highlight the role of otologists and neurotologists in providing comprehensive surgical care of patients with auricular and periauricular malignancies by describing the experience of a single fellowship‐trained neurotologist over a 10‐year period. The rationale and approach for creating a practice treating auricular and periauricular cutaneous malignancies from the perspective of an otologist or neurotologist is also discussed.
2. MATERIALS AND METHODS
2.1. Data source and study population
Following approval by the University of Michigan Institutional Review Board (HUM00115814), a retrospective review was conducted of all patients diagnosed with an auricular and/or a periauricular malignancy treated at our institution between August 2012 and November 2022 by a single surgeon. The senior author's personal surgical records and the electronic medical record (Epic Hyperspace, Madison, Wisconsin) were reviewed and a total of 387 patients were identified for inclusion. Only patients treated surgically at our institution by a single fellowship‐trained otologist/neurotologist (senior author) were included. An in‐depth review of the electronic medical records was performed for a subset of patients that required more complex surgical procedures including neck dissection, parotidectomy, LTBR, regional flap reconstruction, and/or free flap reconstruction. Any patients treated prior to the institution's transition to the Epic Hyperspace electronic medical record were excluded (n = 10) as their records were unavailable for review. A total of 84 patients were included for the deeper analysis of the variables listed below (Figure 1).
FIGURE 1.
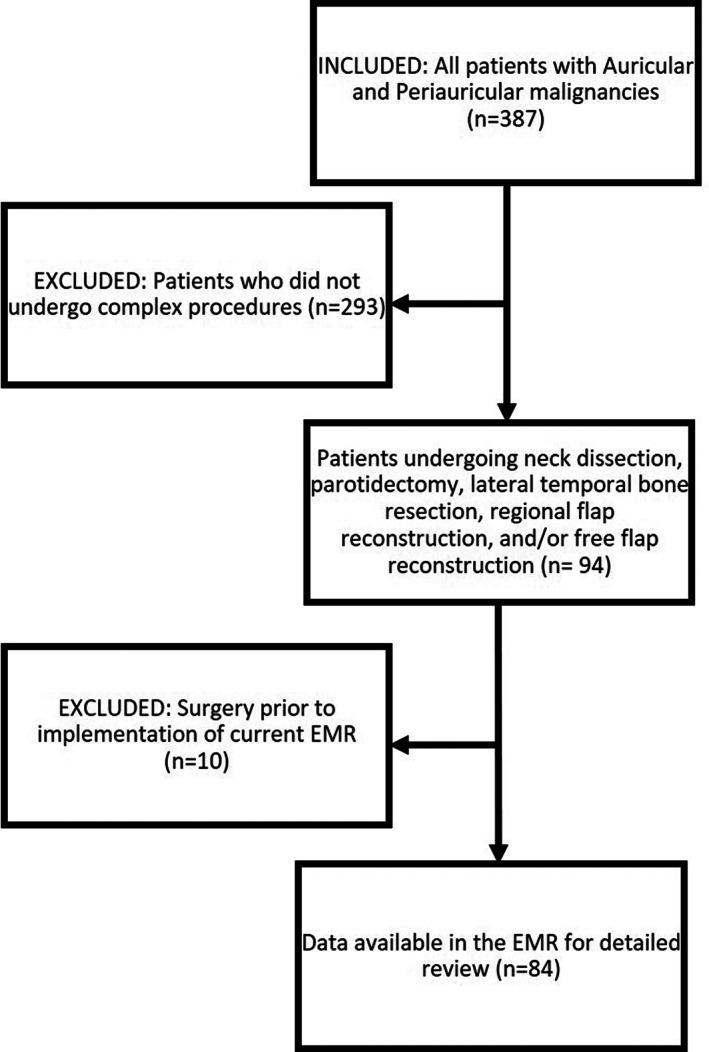
Flow chart outlining patient inclusion and exclusion criteria.
2.2. Extracted data and analysis
The data collected for the entire 387 patient cohort included procedure type and tumor pathology. Comprehensive data collection of the subset of 84 more complex cases included patient demographics (age, sex, race, ethnicity); co‐morbidities (alcohol use, tobacco use, body mass index, hypertension, diabetes, heart disease, lung disease, antiplatelet drug use, anticoagulant use); pre‐operative workup (referral source, imaging, tumor boar discussion); tumor characteristics (anatomic location, size, final tumor pathology, perineural invasion, lymphovascular invasion, margin status, lymph node status, stage);complications (wound issues, hematoma, cranial nerve injury); adjuvant treatment (radiation, chemotherapy, immunotherapy); and follow‐up (length of follow‐up, recurrence, revision surgery). Tumor staging was performed using the Modified Pittsburgh Staging System for primary temporal bone cancers and the 8th edition of the American Joint Commission on Cancer Guidelines for cutaneous and parotid gland primary tumors. For the purposes of staging, the true primary site of advanced locally invasive tumors assigned using the final pathology report.
Descriptive statistical analysis was performed for each of the variables listed here. For continuous variables, mean values and standard deviations were calculated. For categorical variables, frequency counts were reported. Patients who had missing data are reported as “unknown” in the results tables below. Survival curves were generated using the Kaplan–Meier method.
2.3. Surgical indications and techniques
For the purpose of this study, wide local excision (WLE) is defined as a split‐thickness resection typically involving either the anterior auricular skin, posterior auricular skin or periauricular skin. The underlying auricular cartilage was commonly excised with the overlying primary lesion as one en bloc specimen, however, the skin on the opposite side of the auricle was left intact for reconstruction (i.e., posterior skin is left for an anterior tumor and vice versa). Typically, a 0.5 cm margin was taken surrounding the gross tumor edge for biopsy‐proven BCCA and a 1.0 cm margin for SCCA and melanoma. Partial or total auriculectomy is defined as a full‐thickness excision of anterior skin, posterior skin, and the intervening auricular cartilage.
External auditory canal (EAC) skin sleeve resection was performed for less aggressive tumors (BCCA) involving the skin of the EAC without erosion of the underlying bony canal or to obtain a margin for other auricular tumors approaching the external acoustic meatus. Additionally, in our series, it was performed for patients who were medically unstable for a prolonged surgical procedure such as a LTBR. To perform the sleeve resection, the EAC skin lateral to tumor was incised, leaving a 5–10 mm margin. Medially, the EAC skin was typically incised at the level of the tympanic annulus. Additional incisions may be made around the tumor as needed, ensuring there was an appropriate margin. The skin was elevated off the underlying bone en bloc. The specimen was carefully oriented and frozen section analysis was performed intraoperatively. This differs from the LTBR in which the entire bony EAC as well as the tympanic ring are resected. 15 , 16 LTBR was performed on patients who had primary temporal bone cancers or tumors of other adjacent sites that involved the bony EAC.
Parotidectomy was performed for patients with parotid primary tumors, temporal bone primary tumors, tumors, cutaneous SCCAs, and in other tumors that showed evidence of positive parotid lymph nodes or local invasion into the gland. Sentinel lymph node biopsy (SLNB) was performed for patients with melanoma or T2 cutaneous SCCA who did not have a clinically positive lymph node. Selective neck dissection (SND) (typically levels 2–5) is performed for patients who have parotid primaries, advanced (T3 or greater) cutaneous or temporal bone cancer, clinically positive cervical lymph nodes, or a history of a positive SLNB.
3. RESULTS
A total of 387 patients with auricular and/or periauricular malignancy treated by a single otologist/neurotologist were identified. The tumor pathology for these patients is listed in Table 1. The majority of treated malignancies were cutaneous primary cancers. SCCA was the most common histology (42.6%, n = 165; Figure 2) followed by BCCA (40.8%, n = 158; Figure 3), and melanoma (9.8%, n = 38; Figure 4). Primary parotid malignancies were less commonly treated as the referral pattern for these lesions were often directly referred to a traditional head and neck oncology surgical provider. The distribution of pathology for parotid gland tumors is listed in Table 1.
TABLE 1.
Surgical procedures and tumor histology for full cohort.
| (n = 387) Count (%) | ||
|---|---|---|
| Ablative procedures | Wide local excision | 239 (61.8%) |
| Partial auriculectomy | 71 (18.3%) | |
| Parotidectomy | 60 (15.5%) | |
| Sentinel lymph node biopsy | 37 (9.6%) | |
| Neck dissection | 27 (7.0%) | |
| Lateral temporal bone resection | 27 (7.0%) | |
| Sleeve resection | 25 (6.5%) | |
| Total auriculectomy | 20 (5.2%) | |
| Facial nerve decompression | 19 (4.9%) | |
| Infratemporal fossa approach | 1 (0.3%) | |
| Reconstruction/closure | Split thickness skin graft | 153 (39.5%) |
| Full thickness skin graft | 67 (17.3%) | |
| Local/regional flap | 49 (12.7%) | |
| Primary closure | 44 (11.4%) | |
| Free flap | 11 (2.8%) | |
| Facial reanimation | 9 (2.3%) | |
| EAC overclosure | 7 (1.8%) | |
| Tumor type | SCCA | 165 (42.6%) |
| BCCA | 158 (40.8%) | |
| Melanoma | 38 (9.8%) | |
| Adenoid cystic | 8 (2.1%) | |
| Adenocarcinoma | 3 (0.8%) | |
| Acinic cell | 3 (0.8%) | |
| Salivary ductal carcinoma | 2 (0.5%) | |
| Sarcoma | 2 (0.5%) | |
| Mucoepidermoid | 2 (0.5%) | |
| Merkel cell | 2 (0.5%) | |
| Basosquamous | 2 (0.5%) | |
| Unknown | 2 (0.5%) | |
Abbreviations: BCCA, basal cell carcinoma; EAC, external auditory canal; SCCA, squamous cell carcinoma.
FIGURE 2.
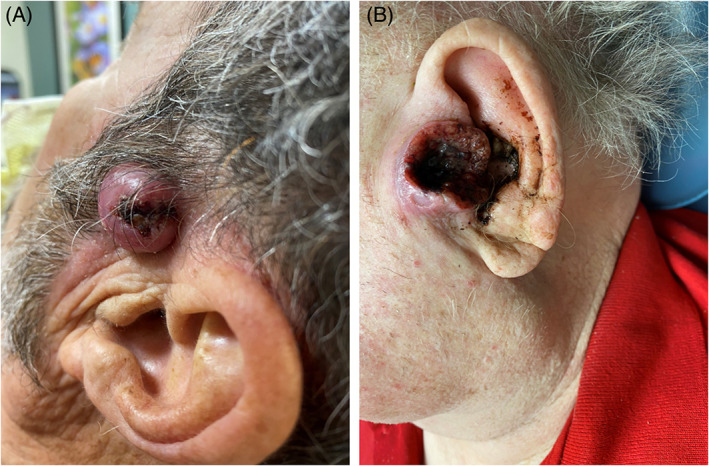
Squamous cell carcinoma (SCCA) involving the pre‐auricular skin (A), tragus (B).
FIGURE 3.
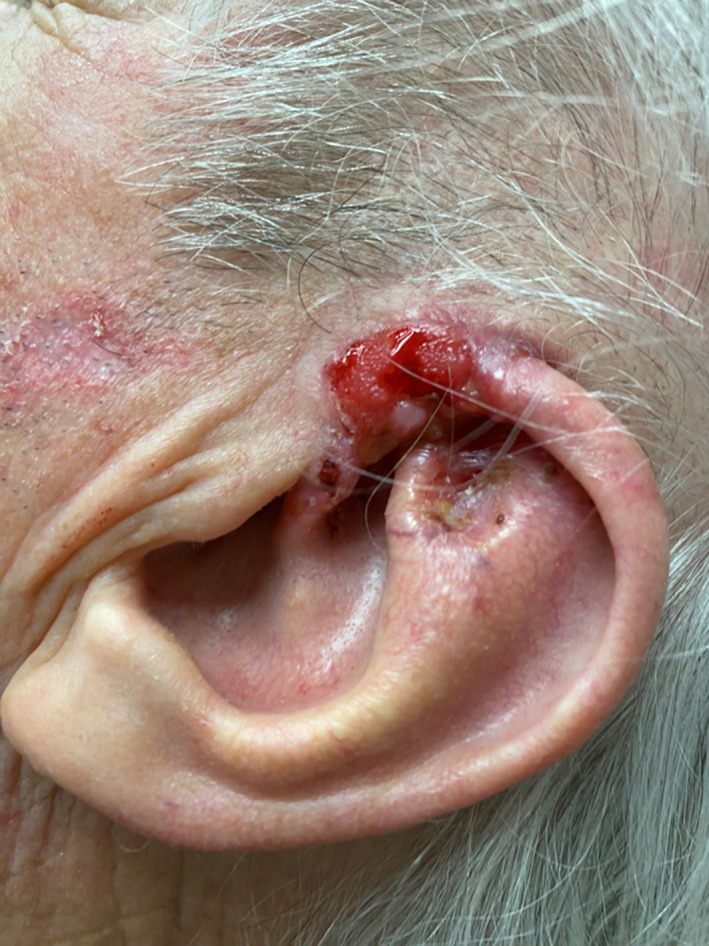
Basal cell carcinoma (BCCA) involving the helical root.
FIGURE 4.
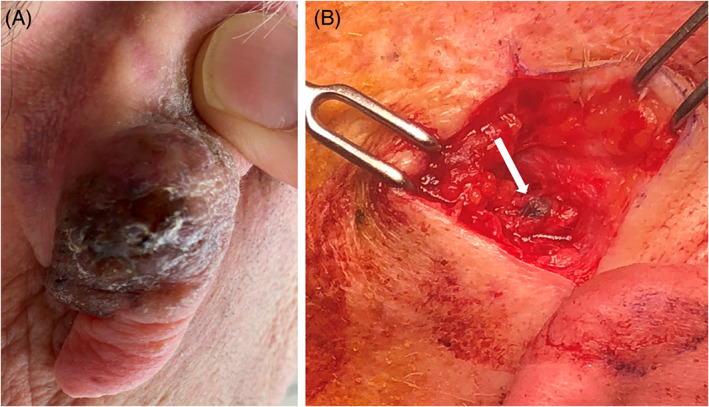
Patient with a melanoma involving the skin of the posterior auricle (A) who also underwent sentinel lymph node biopsy (B). Methylene blue uptake into sentinel node indicated by white arrow.
The surgical procedures performed for these patients are listed in Table 1. Patients with cutaneous malignancy of the auricle underwent either WLE (61.8%, n = 239), partial/sub‐total auriculectomy 18.3% (n = 71), or total auriculectomy 5.2% (n = 20). Other surgical procedures performed included superficial parotidectomy (15.5%, n = 60), SLNB (9.6%, n = 37; for melanoma and SCCA lesion greater than 2 cm on the auricle proper or if in the pre‐auricular region), LTBR (7.0%, n = 27), SND (7.0%, n = 27; typically neck levels 2–5), EAC skin sleeve resection (6.5%, n = 25), mastoidectomy (4.1%, n = 16), and infratemporal fossa approach and dissection (0.3%, n = 1).
Reconstructive methods included primary closure for small defects that were amenable (11.4%, n = 44). More complex reconstruction methods included full‐thickness (17.3%, n = 67), or split‐thickness (39.5%, n = 153) skin grafts (Figure 5), local or regional advancement or rotational flaps (12.7%, n = 49; Figure 6), free tissue transfer (2.8%, n = 11), and EAC overclosure (1.8%, n = 7). Facial nerve decompression was performed in 4.9% (n = 19) of cases and facial nerve reanimation was performed in 2.3% (n = 9) of cases.
FIGURE 5.
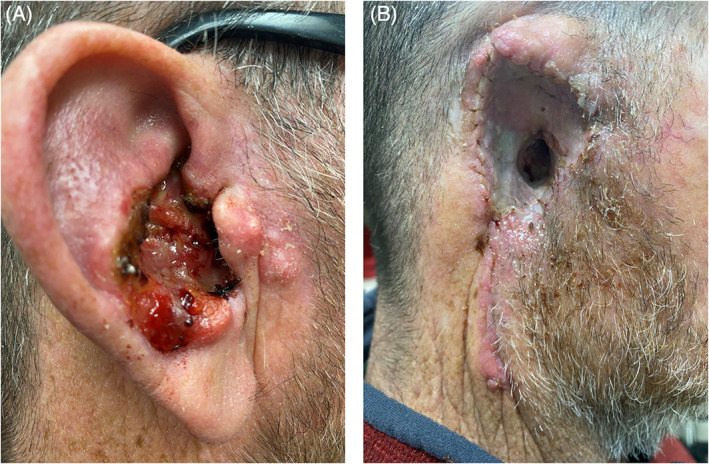
Patient on chronic immunosuppression due to renal transplant who presented with squamous cell carcinoma (SCCA) involving the concha bowl and extending to the external auditory canal (EAC) (A), following auriculectomy and split thickness skin graft reconstruction (B).
FIGURE 6.
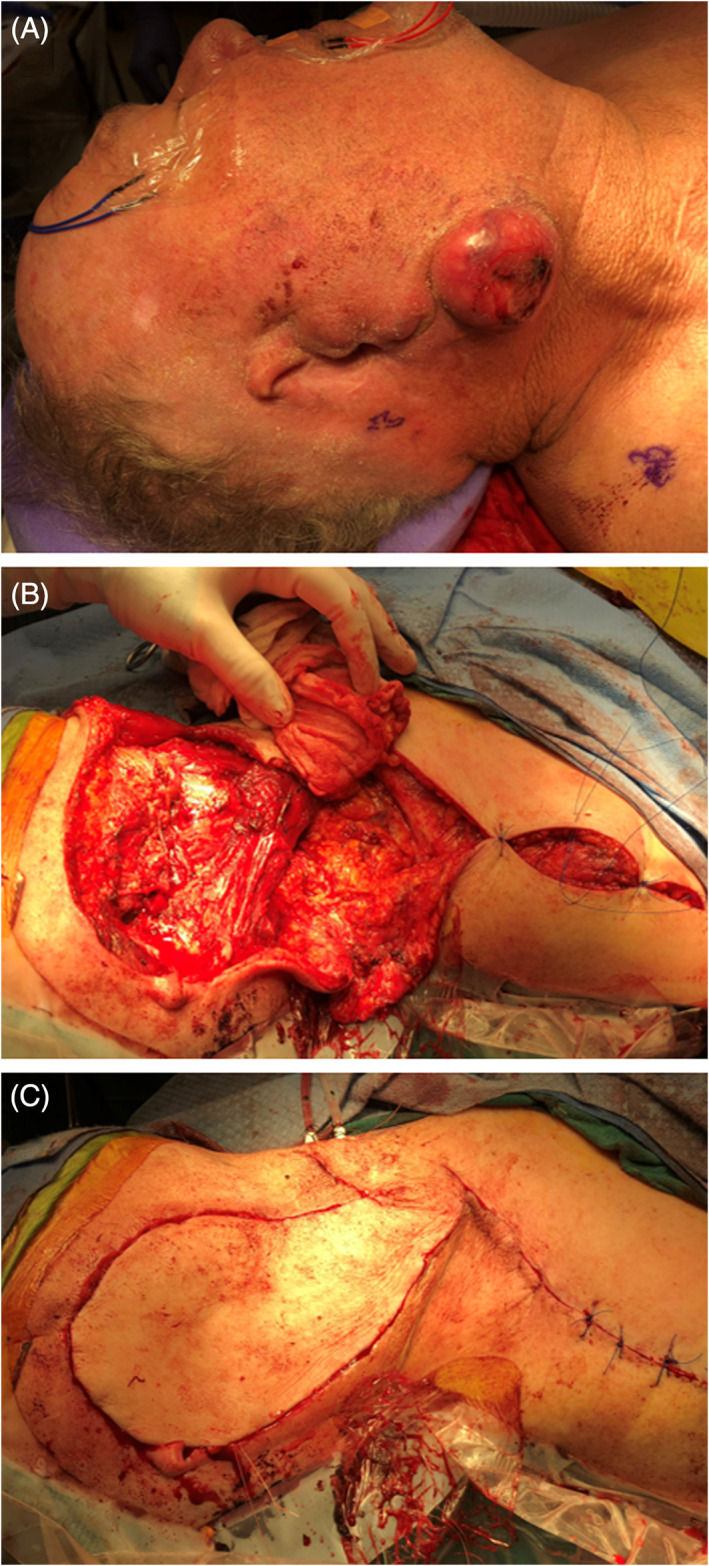
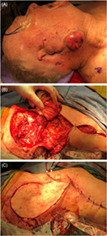
Operative photographs showing wide local excision, parotidectomy, selective neck dissection, and supraclavicular artery island pedicled flap reconstruction, for a patient with a prior sub‐total auriculectomy for a recurrent aggressive auricular squamous cell carcinoma (SCCA). Pre‐operative (A), following tumor resection and raising of supraclavicular artery island pedicled flap (B), and following completion of reconstruction (C).
Of the 387 patients meeting inclusion criteria, a subset of 84 patients who required complex procedures including SND (typically neck levels 2a, 2b, 3, 4, and 5), parotidectomy, LTBR, regional advancement or rotational flap reconstruction, and/or free tissue transfer reconstruction was identified (Figure 6). Six of the 84 patients had prior surgical resection and the remainder received primary treatment. Of these six patients, four had locoregional recurrence requiring an operation. One patient had a prior positive SLNB which required a subsequent neck dissection. One patient had negative frozen section margin results reversed to positive on final pathology analysis, requiring additional surgery. Further in‐depth analysis for this subset of patients was performed and is described below.
3.1. Patient demographics and co‐morbidities
Within the 84‐patient subset, median age at diagnosis was 71.9 years (±12.6; Table 2). Most patients were male (79.8%, n = 67) and Caucasian (94.0%, n = 79). Underlying risk factors included smoking (16.7% current, n = 14; 38.1% former, n = 32; 45.2% never, n = 38), alcohol use (47.0%, n = 39), anticoagulation (21.4%, n = 18), and antiplatelet drug use (33.3%, n = 28). Additional comorbidities include diabetes (23.8%, n = 20), hypertension (54.8%, n = 46), heart disease (32.1%, n = 27), and COPD/Asthma (13.1%, n = 11).
TABLE 2.
Patient demographics and co‐morbidities.
| (n = 84) Count (%) | ||
|---|---|---|
| Age (years) | Mean ± SD | 71.9 ± 12.6 |
| Sex | Male | 67 (79.8%) |
| Female | 17 (20.2%) | |
| Race | White | 79 (94.0%) |
| Black | 2 (2.4%) | |
| Other | 3 (3.6%) | |
| Ethnicity | Hispanic | 1 (1.2%) |
| Non‐Hispanic | 81 (96.4%) | |
| Other | 2 (2.4%) | |
| Tobacco use | Current | 14 (16.7%) |
| Former | 32 (38.1%) | |
| Never | 38 (45.2%) | |
| Alcohol use | Yes | 39 (47.0%) |
| No | 44 (53.0%) | |
| BMI | Mean ± SD | 28.3 ± 5.7 |
| Hypertension | Yes | 46 (54.8%) |
| No | 38 (45.2%) | |
| Diabetes | Yes | 20 (23.8%) |
| No | 64 (76.2%) | |
| Heart disease | Yes | 27 (32.1%) |
| No | 57 (67.9%) | |
| COPD/asthma | Yes | 11 (13.1%) |
| No | 73 (86.9%) | |
| Anticoagulant use | Yes | 18 (21.4%) |
| No | 66 (78.6%) | |
| Antiplatelet use | Yes | 28 (33.3%) |
| No | 56 (66.7%) | |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; SD, standard deviation.
3.2. Referral sources and pre‐operative imaging
On analysis of the 84 patients, dermatologists provided the majority of patient referrals (50.0%, n = 42), followed by other otolaryngologists (33.3%, n = 28), and primary care providers (7.1%, n = 6) (Table 3). Most patients obtained preoperative imaging; 90.5% (n = 76) underwent computed tomography (CT) of the temporal bone and/or neck with contrast, 20.2% (n = 17) underwent magnetic resonance imaging (MRI), and 13.1% (n = 11) had positron emission tomography (PET) scans. Imaging was indicated in most cases if the patient had long‐standing disease, clinically positive periauricular or regional (neck) lymph nodes, concerning locations (intertragal notch, EAC, pre‐auricular skin overlying the parotid gland, pre‐auricular lymph node drainage basin, or retro‐auricular crease), and for concerns for bone or nerve involvement. Tumor board discussion occurred in more than half of cases 53.6% (n = 45). Tumor board discussion occurred when there was concern for metastatic disease, questions about imaging findings, rare histology, or highly invasive tumors requiring large resection and possible free tissue transfer reconstruction.
TABLE 3.
Pre‐operative workup.
| (n = 84) Count (%) | ||
|---|---|---|
| Referral source | Primary care | 6 (7.1%) |
| Dermatology | 42 (50.0%) | |
| Otolaryngology | 28 (33.3%) | |
| Other | 8 (9.5%) | |
| Tumor board discussion | Yes | 45 (53.6%) |
| No | 39 (46.4%) | |
| Pre‐operative imaging | None | 8 (9.5%) |
| CT | 76 (90.5%) | |
| MRI | 17 (20.2%) | |
| PET | 11 (13.1%) | |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
3.3. Tumor characteristics
Tumor location was distributed among the following sites: auricular (58.3%, n = 49), pre‐auricular (21.4%, n = 18), post‐auricular (2.4%, n = 2), infra‐auricular (6.0%, n = 5), parotid (27.4%, n = 23), and definitive temporal bone (4.8%, n = 4; Figure 7). Among auricular lesions, specific subsites on the external ear included: conchal bowl (7.1%, n = 6), helix (8.3%, n = 7), antihelix (1.2%, n = 1), intertragal notch (1.2%, n = 1), scaphoid fossa (1.2%, n = 1), EAC (10.7%, n = 9), and lobule skin (1.2%, n = 1). The true subsite was unspecified or unclear in (27.4%, n = 23) of tumors due to their locally invasive nature.
FIGURE 7.
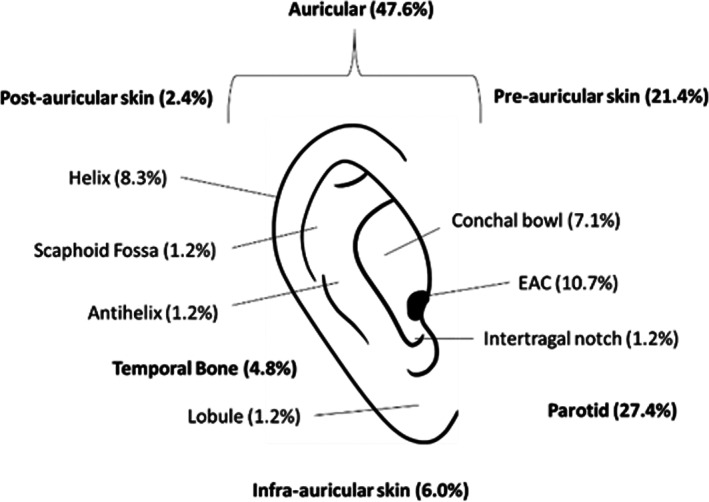
Distribution of malignancies among subsites of the external ear.
3.4. Surgical procedures
All 84 patients in this cohort were selected because they had received more complex procedures including either parotidectomy, LTBR, SND, regional advancement (i.e., cervicofacial) or rotational flap reconstruction, free tissue transfer, or some combination of those procedures. Details of surgical procedures performed are listed in Table 4. Many patients required multiple concurrent procedures as a part of their tumor resection and reconstruction.
TABLE 4.
Total number of surgical procedures.
| (n = 84) Count (%) | ||
|---|---|---|
| Ablative procedures | Parotidectomy | 56 (66.7%) |
| Wide local excision | 28 (33.3%) | |
| Neck dissection | 26 (31.0%) | |
| Partial auriculectomy | 23 (27.4%) | |
| Lateral temporal bone resection | 20 (23.8%) | |
| Total auriculectomy | 13 (15.5%) | |
| Sentinel lymph node biopsy | 13 (15.5%) | |
| Facial nerve decompression | 13 (15.5%) | |
| Sleeve resection | 10 (11.9%) | |
| Infratemporal fossa approach | 1 (1.2%) | |
| Reconstruction/closure | Local/regional flap | 45 (53.6%) |
| Full thickness skin graft | 21 (25.0%) | |
| Split thickness skin graft | 15 (17.9%) | |
| EAC overclosure | 12 (14.3%) | |
| Free flap | 11 (13.1%) | |
| Facial reanimation | 9 (10.7%) | |
Abbreviation: EAC, external auditory canal.
3.5. Histology and stage
As in the full 387‐patient cohort, cutaneous malignancies were most prevalent in the 84‐patient subset that was analyzed in detail. SCCA was the most common histology (61.9%, n = 52), followed by BCCA (27.4, n = 23), and melanoma (4.8%, n = 4; Table 5). Other tumor types were rare (i.e., Merkel cell carcinoma [MCC]; adenoid cystic carcinoma; Table 5). The mean size of the tumors was 3.7 cm (±2.4 cm). In the final pathology reports of the 84‐patient cohort, 28 specimens (33.3%) exhibited perineural invasion, 12 specimens (14.3%) had lymphovascular invasion, 12 specimens (14.3%) had positive lymph nodes, and 14 specimens (16.7%) had positive margins.
TABLE 5.
Tumor histology and pathology.
| (n = 84) Count (%) | ||
|---|---|---|
| Tumor type | SCCA | 52 (61.9%) |
| BCCA | 23 (27.4%) | |
| Melanoma | 4 (4.8%) | |
| Adenoid cystic carcinoma | 2 (2.4%) | |
| Mucoepidermoid | 1 (1.2%) | |
| Acinic cell carcinoma | 1 (1.2%) | |
| Salivary ductal carcinoma | 1 (1.2%) | |
| Perineural invasion | Yes | 28 (33.3%) |
| No | 45 (53.6%) | |
| Unknown | 11 (13.1%) | |
| Positive lymph nodes | Yes | 12 (14.3%) |
| No | 62 (73.8%) | |
| Unknown | 10 (11.9%) | |
| Lymphovascular invasion | Yes | 12 (14.3%) |
| No | 58 (69.0%) | |
| Unknown | 14 (16.7%) | |
| Positive margin | Yes | 14 (16.7%) |
| No | 68 (81.0%) | |
| Unknown | 2 (2.4%) | |
Abbreviations: BCCA, basal cell carcinoma; SCCA, squamous cell carcinoma.
Tumor stage distribution is presented in Table 6. Tumors with unknown or unclear primary sites were assigned a primary site classification based on the final pathology report so that the appropriate staging paradigm may be applied. A majority of patients (64%) who presented with advanced T‐stage disease of T3 or greater. Lymph nodes were not assessed in 33% of patients. Of the patients whose lymph nodes were assessed, 75% were pN0.
TABLE 6.
Tumor staging using modified Pittsburgh staging system for temporal bone primary sites and AJCC 8th edition for cutaneous and parotid primary sites.
| Cutaneous (n = 65) | Parotid (n = 10) | Temporal bone (n = 9) | ||
|---|---|---|---|---|
| T stage | Tx | 17 (26.2%) | 0 | 0 |
| Tis | 0 | 0 | 1 (11.1%) | |
| T1 | 5 (7.7%) | 1 (10.0%) | 4 (44.4%) | |
| T2 | 6 (9.2%) | 4 (40.0%) | 3 (33.3%) | |
| T3 | 35 (53.8%) | 3 (30.0%) | 1 (11.1%) | |
| T4a | 2 (3.1%) | 2 (20.0%) | 0 | |
| T4b | 0 | 0 | n/a | |
| pN stage | pNx | 19 (29.2%) | 4 (40.0%) | 5 (55.6%) |
| pN0 | 34 (52.3%) | 4 (40.0%) | 4 (44.4%) | |
| pN1 | 4 (6.2%) | 0 | 0 | |
| pN2a | 2 (3.1%) | 0 | 0 | |
| pN2b | 1 (1.5%) | 1 (10.0%) | 0 | |
| pN2c | 0 | 0 | 0 | |
| pN3a | 0 | 0 | 0 | |
| pN3b | 5 (7.7%) | 1 (10.0%) | n/a | |
3.6. Complications, adjuvant treatment, follow‐up, survival
Wound complications occurred in 21.4% (n = 18) of cases and included wound dehiscence in eight cases, of which two required operative revision. Other less common wound complications included failure of skin graft take, partial flap necrosis, and exposure of mastoid bone. Hematomas occurred in 6% (n = 5) of cases, and transient facial nerve weakness occurred in 8.3% (n = 7; Table 7). There was no inadvertent permanent facial nerve weakness. Adjuvant radiation was required in 48.8% of patients (n = 41), immunotherapy in 8.3% (n = 7), and chemotherapy in 6% (n = 5). The mean follow‐up time was 22.8 months. Recurrent disease occurred in 17.9% of patients (n = 15). Of these, 40% (n = 6) received repeat surgical resection, 40% (n = 6 received adjuvant therapy, and 20% (n = 3) pursued hospice care. Revision surgery occurred in 22.6% of cases (n = 19). Of these revision surgeries, 26.3% (n = 5) were for positive margins (reversed on final pathology from an intra‐operatively cleared frozen margin at the time of primary tumor resection), 31.6% (n = 6) for recurrence, 10.5% (n = 2) for wound dehiscence, and 31.6% (n = 6) for cosmetic concerns. Disease‐specific 5‐year survival was 91% (Figure 8).
TABLE 7.
Complications, adjuvant treatment, and follow‐up.
| (n = 84) Count (%) | ||
|---|---|---|
| Admission length (days) | Mean ± SD | 2.6 ± 3.5 |
| Wound complication | Yes | 18 (21.4%) |
| No | 66 (78.6%) | |
| Hematoma | Yes | 5 (6.0%) |
| No | 79 (94.0%) | |
| Cranial nerve injury | Transient weakness | 7 (8.3%) |
| Permanent weakness | 0 (0.0%) | |
| None | 76 (90.5%) | |
| Unknown | 1 (1.2%) | |
| Other complications | Urinary retention | 4 (4.8%) |
| DVT | 1 (1.2%) | |
| Clostridium difficile colitis | 1 (1.2%) | |
| Delayed emergence | 3 (3.6%) | |
| Renal injury | 2 (2.4%) | |
| ICU admission requirement | 3 (3.6%) | |
| EAC stenosis | 1 (1.2%) | |
| Adjuvant treatment | None | 42 (50.0%) |
| Chemotherapy | 5 (6.0%) | |
| Immunotherapy | 7 (8.3%) | |
| Radiation therapy | 41 (48.8%) | |
| Follow‐up duration (months) | Mean ± SD | 22.8 ± 25.7 |
| Recurrence | Yes | 15 (17.9%) |
| No | 69 (82.1%) | |
| Revision surgery | Yes | 19 (22.6%) |
| No | 64 (76.2%) | |
| Unknown | 1 (1.2%) | |
Abbreviations: DVT, deep venous thrombosis; EAC, external auditory canal; SD, standard deviation.
FIGURE 8.
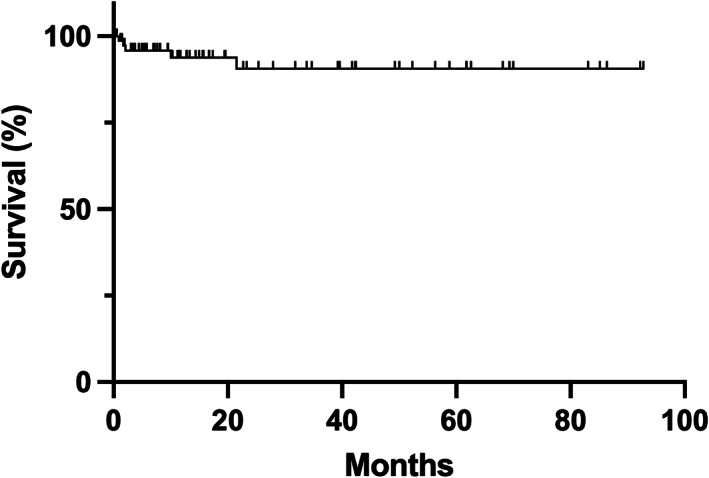
Kaplan–Meier curve showing disease‐specific survival. Tick marks indicate censored cases that were lost to follow‐up.
4. DISCUSSION
This study characterized a large series of patients with auricular and periauricular malignancy who underwent surgical treatment with a fellowship‐trained neurotologist (senior author) over a span of over 10 years. Malignancies treated by the senior author included several primary locations arising from cutaneous, parotid, and temporal bone sites. Most of the cases examined in this report were primary cutaneous malignancies. Surgical treatment for many of these patients included a WLE of the lesion with cleared intra‐operative frozen margins and skin graft reconstruction, parotidectomy, neck dissection, local reconstruction, and regional reconstruction. An in‐depth review of a subset of 84 patients requiring more complex surgeries was performed. This study suggests that otologists and neurotologists have a unique and poised role in caring for patients with auricular and periauricular cutaneous malignancy as they can provide considerable expertise in surgery within this anatomically complex area and associated structures.
4.1. Rationale for otology/neurotology involvement
Auricular and periauricular cutaneous malignancies are common. Long‐term UV‐light exposure to the anatomically exposed auricles is a primary risk factor for these lesions. 1 While BCCA is the most common UV‐light exposure‐related cutaneous malignancy on or immediately around the external ear, more aggressive lesions such as SCCA, melanoma, and MCC can all form on the external ear and surrounding adjacent skin. 1 , 2 , 3 Cumulative UV‐light exposure over time leads to increased incidence and prevalence of auricular and periauricular cutaneous malignancies as patients age. 17 Important prevention measures for limiting UV exposure include avoiding exposed skin in the sun, use of a wide brimmed‐hat, sunscreen use, and avoidance of tanning beds. 18 Patients should be counseled to perform regular self‐exams and should undergo skin examination regularly with a primary care provider or dermatologist.
The importance of establishing the otologist as a single provider to surgically manage auricular and periauricular cutaneous malignancies also lies in the aggressive nature of many of the tumors (i.e., SCCA). Establishing the otologist as the primary surgical specialist to treat periauricular malignancy may reduce the lag time required for referral to multiple different specialists and subspecialists, during which tumors can rapidly change size and invade deeper structures or metastasize to lymph nodes. 19 In building this unique “otologic oncology” practice, the senior author has been able to reliably receive a new patient referral, evaluate the patient within 24–48 h and operate on these patients in a timely fashion. This reason, coupled with an intricate understanding of the anatomy in and around the ear, are key elements in the observed high rates of surgical successes we have had and in the resultant advocacy for otologists to build this type of practice.
4.2. Beginning an otologic oncology practice
To establish an auricular and periauricular cutaneous oncology practice, there must first be an identified clinical need. One must examine current monthly case volume, the providers currently managing these cases, and the potential for volume growth. If the volume outpaces the ability of the existing providers to manage it, if considerable delays in evaluating and surgically treating these lesions, or other colleagues are not interested in managing these lesions, then the otologist has an opportunity to establish a practice like the one described here.
Building the practice requires close communication to other providers involved in the care of patients with head and neck cutaneous malignancies. The otologist must meet with dermatologists, Mohs surgeons, primary care providers and otolaryngologists in the community to alert these individuals of the practice patterns so that they may refer patients requiring surgical care. Presenting more complex cases at a multi‐disciplinary head and neck tumor board is an ideal means to communicate with colleagues and coordinate surgical care on a case‐by‐case basis. In our series, tumor board discussion was performed for patients with complex medical history or disease with advanced stage, pathology, or imaging requiring discussion to establish a treatment plan and coordinate care. Formal tumor board discussion was not performed for cases with early‐stage disease or well‐recognized treatment protocols, at the senior author's discretion (Table 3). Regardless of whether their case was presented in tumor board, patients had the opportunity to discuss treatment with any provider who may need to be involved in their care.
It is crucial to establish a working relationship with existing head and neck oncology and facial plastics and reconstructive surgery providers if simultaneous free tissue transfer reconstruction or facial nerve reanimation may be required for any patient. Many patients after surgery will have tumors that show aggressive features on final pathology (i.e., lymphovascular involvement, perineural invasion, regional metastasis) and will require post‐operative adjunctive radiation therapy, chemotherapy, immunotherapy, or a combination of treatments. As such, a working relationship with radiation oncology and medical oncology colleagues is also critical. A partnership with nuclear medicine will be necessary for patients requiring lymphoscintigraphy for SLNB. Finally, many patients with auricular and periauricular cutaneous malignancies have significant medical co‐morbidities. A pathway to obtain timely and efficient pre‐operative medical/surgical clearances from cardiology, pulmonology, transplant services and anesthesia may be required.
The senior author, a fellowship‐trained neurotologist, started treating auricular and periauricular cutaneous malignancies in practice 11 years ago, based on the frequency and severity of these lesions and the resultant need for timely surgical management. In announcing to the head and neck oncology division as well as the Mohs and dermatology colleagues, the senior author established the practice by seamlessly building reliable and timely referral patterns. Head and neck surgery colleagues were assured that they would be called upon if needed to assist in especially challenging cases or if a free‐tissue transfer was required for reconstruction. Over the last 11 years, the senior author's practice has now become the primary referral pathway within their home institution for any auricular and periauricular cutaneous malignancies that dermatology/Mohs surgery providers (both within the institution and in the outside immediate referral areas) feel is beyond their scope of practice due to tumor extension to the ear canal or parotid, for example.
4.3. Follow‐up protocols
Follow‐up protocols for patients with periauricular malignancies are variable and depend upon several factors including pathology, staging, and extent of surgical resection. In the senior author's practice, patients are followed every 2–6 weeks for the first 3 months to ensure proper wound healing after surgery, discuss surgical pathology results, and help coordinate adjuvant treatment, as necessary. Once wounds have stabilized, frequency of follow‐up examination is decreased to once every 3–12 months. Patients are recommended to follow‐up in clinic on a minimum yearly basis for at least for 5 years after surgery.
A multi‐disciplinary approach to surveillance for cutaneous malignancies including primary care providers, dermatology, and/or medical oncology is strongly advised. Establishment of a multi‐disciplinary team prior to treatment helps facilitate a smooth transition to post‐treatment surveillance. Patients with a diagnosis of BCCA or SCCA are recommended to undergo skin cancer screening examinations at least yearly for life. 20 , 21 There is no clear consensus on surveillance recommendations for melanoma and published protocols are highly variable. 22 , 23 According to the American Academy of Dermatology (AAD), early‐stage melanoma patients should undergo follow‐up visits with emphasis on examination of the nodal basin every 6–12 months for the first 5 years and at least annually thereafter. 24 Routine surveillance imaging for early‐stage melanoma patients is not recommended and should be performed only if indicated by symptoms or examination findings. Advanced‐stage melanoma patients are recommended to have follow‐up visit every 3–6 months for 2 years, then every 6–12 months for the subsequent 3 years and at least yearly thereafter. Surveillance imaging may be performed during the first 3 years and may include chest radiography, CT, brain MRI, and/or PET scans. Preference on modality or frequency of imaging is not specified by the AAD. 24
4.4. Limitations
There are several limitations and caveats to the study that warrant discussion. There are inherent limitations of a retrospective study, including incorrect, incomplete, or missing data. We have attempted to clearly identify incomplete or missing data. This retrospective case series only highlights the experience of a single surgeon at a single institution and results may not be generalizable. Differences in institutional resources, patient need, and referral patterns may present significant challenges to otologists and neurotologists practicing in other locations. There may be bias in which patients are referred to the senior author or how surgical candidates are selected. We attempted to limit further selection bias by including all patients who underwent surgical excision of auricular or periauricular malignancy in our study, a vast majority of which were cutaneous primaries. Detailed analysis was only performed on patients who were deemed as having undergone more advanced procedures, as defined previously. Therefore, the data are not representative of all patients with auricular or periauricular malignancy. There is significant heterogeneity in the types of cancers (primary site, pathology, stage) that are included in this case series, making it difficult to draw definitive conclusions regarding treatment outcomes, including survival. Disease‐specific survival was comparable to other studies of early‐stage temporal bone cancers. 14 Our reported survival is greater than that reported in previous studies examining advanced temporal bone cancers, likely because our cohort included a subset of auricular and periauricular tumors that did not involve the temporal bone. 12 , 13 Despite the above limitations, this study illustrates the extent of an otologist's possible involvement in treating auricular and periauricular malignancies, even in more advanced cases.
5. CONCLUSIONS
Auricular and periauricular cutaneous malignancies can be challenging to treat due to the complex anatomy of the region and aggressiveness of the tumors. Otologists/neurotologists have the training and experience required to provide timely care for patients with these malignancies. Surgical management of auricular and periauricular malignancies can be incorporated into an otology/neurotology practice and otologists should consider treating this patient population in their practice.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Patel TR, Prince ADP, Benjamin WJ, Basura GJ. Role of the otologist/neurotologist in managing auricular and periauricular cutaneous malignancies: A 10‐year otologic oncology experience. Laryngoscope Investigative Otolaryngology. 2023;8(6):1637‐1647. doi: 10.1002/lio2.1171
REFERENCES
- 1. Asgari MM, Moffet HH, Ray GT, Quesenberry CP. Trends in basal cell carcinoma incidence and identification of high‐risk subgroups, 1998‐2012. JAMA Dermatol. 2015;151:976‐981. [DOI] [PubMed] [Google Scholar]
- 2. Guzman AK, Schmults CD, Ruiz ES. Squamous cell carcinoma: an update in staging, management, and postoperative surveillance strategies. Dermatol Clin. 2023;41:1‐11. [DOI] [PubMed] [Google Scholar]
- 3. Paulson KG, Gupta D, Kim TS, et al. Age‐specific incidence of melanoma in the United States. JAMA Dermatol. 2020;156:57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population‐based study in Queensland. Aus JAMA Dermatol. 2017;153:175‐182. [DOI] [PubMed] [Google Scholar]
- 5. Pruthi DK, Guilfoyle R, Nugent Z, Wiseman MC, Demers AA. Incidence and anatomic presentation of cutaneous malignant melanoma in Central Canada during a 50‐year period: 1956 to 2005. J Am Acad Dermatol. 2009;61:44‐50. [DOI] [PubMed] [Google Scholar]
- 6. Bray HN, Simpson MC, Zahirsha ZS, et al. Head and neck melanoma incidence trends in the pediatric, adolescent, and young adult population of the United States and Canada, 1995‐2014. JAMA Otolaryngol Head Neck Surg. 2019;145:1064‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kovatch KJ, Smith JD, Birkeland AC, et al. Institutional experience of treatment and outcomes for cutaneous periauricular squamous cell carcinoma. OTO Open. 2019;3:2473974X19875077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters M, Smith JD, Kovatch KJ, McLean S, Durham AB, Basura G. Treatment and outcomes for cutaneous periauricular basal cell carcinoma: a 16‐year institutional experience. OTO Open. 2020;4:2473974X20964735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Helsing P, Robsahm TE, Vos L, Rizvi SM, Akslen LA, Veierod MB. Cutaneous head and neck melanoma (CHNM): a population‐based study of the prognostic impact of tumor location. J Am Acad Dermatol. 2016;75:975‐982.e972. [DOI] [PubMed] [Google Scholar]
- 10. Mondin V, Rinaldo A, Shaha A, et al. Malignant melanoma of the auricle. Acta Otolaryngol. 2005;125:1140‐1144. [DOI] [PubMed] [Google Scholar]
- 11. Pacifico MD, Pearl RA, Grover R. The UK government two‐week rule and its impact on melanoma prognosis: an evidence‐based study. Ann R Coll Surg Engl. 2007;89:609‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gidley PW, Thompson CR, Roberts DB, DeMonte F, Hanna EY. The oncology of otology. Laryngoscope. 2012;122:393‐400. [DOI] [PubMed] [Google Scholar]
- 13. Muelleman T, Chowdhury NI, Killeen D, et al. Effect of piecemeal vs En bloc approaches to the lateral temporal bone on survival outcomes. Otolaryngol Head Neck Surg. 2018;158:716‐720. [DOI] [PubMed] [Google Scholar]
- 14. McCracken M, Pai K, Cabrera CI, et al. Temporal bone resection for squamous cell carcinoma of the lateral skull base: systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2023;168:154‐164. [DOI] [PubMed] [Google Scholar]
- 15. Lovin BD, Gidley PW. Squamous cell carcinoma of the temporal bone: a current review. Laryngosc Invest Otolaryngol. 2019;4:684‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang T, Li W, Dai C, Chi F, Wang S, Wang Z. Evidence‐based surgical management of T1 or T2 temporal bone malignancies. Laryngoscope. 2013;123:244‐248. [DOI] [PubMed] [Google Scholar]
- 17. Allanson BM, Low TH, Clark JR, Gupta R. Squamous cell carcinoma of the external auditory canal and temporal bone: an update. Head Neck Pathol. 2018;12:407‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ragi JM, Patel D, Masud A, Rao BK. Nonmelanoma skin cancer of the ear: frequency, patients' knowledge, and photoprotection practices. Dermatol Surg. 2010;36:1232‐1239. [DOI] [PubMed] [Google Scholar]
- 19. Rygalski CJ, Zhao S, Eskander A, et al. Time to surgery and survival in head and neck cancer. Ann Surg Oncol. 2021;28:877‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Work G, Invited R, Kim JYS, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Work G, Invited R, Kim JYS, et al. Guidelines of care for the management of basal cell carcinoma. J Am Acad Dermatol. 2018;78:540‐559. [DOI] [PubMed] [Google Scholar]
- 22. Trotter SC, Sroa N, Winkelmann RR, Olencki T, Bechtel M. A global review of melanoma follow‐up guidelines. J Clin Aesthet Dermatol. 2013;6:18‐26. [PMC free article] [PubMed] [Google Scholar]
- 23. Cromwell KD, Ross MI, Xing Y, et al. Variability in melanoma post‐treatment surveillance practices by country and physician specialty: a systematic review. Melanoma Res. 2012;22:376‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80:208‐250. [DOI] [PubMed] [Google Scholar]


