Abstract
Objective
To provide an analysis of complications following eustachian tube balloon dilation as well as their treatments and outcomes.
Data Sources
PubMed, Ovid Embase, and MAUDE Database.
Review Methods
A systematic approach following PRISMA guidelines was used to identify publications pertaining to balloon dilation of the eustachian tube from PubMed and Ovid Embase databases was used. Once these publications were critically reviewed, the primary outcome extracted were reported complications. Additional complications were collected in the Manufacturer and User Facility Device Experience (MAUDE) database using the product class “eustachian tube dilation device” and searching through relevant manufacturers. Complications and outcomes were compared between these sources.
Results
Fifty five full‐length manuscripts involving 7155 patients were included and 98 complications reported for a 1.4% complication rate. The most frequently reported adverse events were subcutaneous emphysema of the head and neck (19%), epistaxis (12%), and acute otitis media (11%). The MAUDE search returned 18 distinct patient entries, of which 12 (67%) reported complications. The most reported complications in the MAUDE database included subcutaneous emphysema (8, 67%) and pneumomediastinum (3, 25%). The most serious complication was a carotid artery dissection reported in one patient in the MAUDE database.
Conclusion
Eustachian tube dilation is rarely associated with complications, which nevertheless may lead to morbidity and medical emergencies. Patients and providers should recognize potential risks associated with this intervention as well as methods to manage complications.
Keywords: complications, comprehensive otolaryngology, eustachian tube dilation, MAUDE database
A rapid review was performed reporting the significant adverse events reported in the modern literature and compared this with the domestic manufacturing database MAUDE.
1. INTRODUCTION
The eustachian (pharyngotympanic or auditory) tube is an anatomic structure in the head and neck that is essential for physiologic homeostasis of the middle ear including: pressure equalization, mucociliary transport, and protection from retrograde pathogens/secretions from the nasopharynx. 1 Eustachian tube dysfunction (ETD) is a common pathologic entity in adults and children, with a prevalence in the United States of approximately 4% to 6%. 2 , 3 ETD can lead to otitis media with effusion (OME), tympanic membrane retraction/perforation or more long term sequelae including middle ear atelectasis, chronic otitis media (COM), or cholesteatoma. 4
Balloon dilation of the eustachian tube (BDET) is a novel therapeutic approach for patients suffering from ETD, approved by the United States Food and Drug Administration (FDA) in 2016 for obstructive ETD. 5 The American Academy of Otolaryngology—Head and Neck Surgery released a consensus statement in 2018 supporting BDET as a therapeutic option for patients suffering from obstructive ETD. 4 Although rare, complications associated with BDET occur and their characterization is warranted. 6 , 7 , 8 Herein, we queried the Manufacturer and User Facility Device Experience (MAUDE) database utilized by the FDA to report medical device‐related complications. 9 Additionally, we performed a comprehensive literature review to identify complications reported in the published literature.
2. METHODS
This study did not require institutional review board approval since data were collected from a publicly accessible database and previously published studies.
2.1. Literature search
PubMed and Ovid Embase databases were searched with an open‐ended date until September 18, 2022. The use of limiters and filters was minimized, and manual review and selection was relied upon to avoid missing potentially relevant studies. Each database was searched utilizing the advanced search feature with respective database nomenclature. Further details on search queries for each database can be found in the Appendix (Supporting information). The web‐based systematic review application Covidence was used as the author platform for including and excluding publications and generating the PRISMA diagram. 10
Publication review was performed in an iterative manner according to PRISMA guidelines. 11 The review began with a title/abstract screen, followed by a successive full text review should the article pass the inclusion/exclusion criteria. All titles and abstracts identified from the search procedure were independently evaluated by at least two reviewers (PFC and AAH). Full‐length articles then followed a similar review process.
Basic patient demographics, study design, balloon dilator manufacturer, and reported SAEs to patients were then extracted for descriptive analysis. For each publication, the results and associated tables/figures were reviewed for complications and SAEs. This included both the number reported, but also types of complications (e.g., epistaxis, etc.).
2.2. Inclusion and exclusion criteria
For this review, the authors included randomized‐controlled trials, retrospective/prospective cohort studies, and case series/reports that reported either short‐ or long‐term outcomes and major complications of patients undergoing BDET and other concomitant procedures for ETD and were original research articles. Publications describing both pediatric and adult patients were included. Review articles, meta‐analyses, editorials, cadaveric/animal studies, conference papers, and abstracts without companion full text were excluded. Articles without English full‐text translations were also excluded.
2.3. Risk of bias assessment
The selected articles were reviewed and evaluated for risk of bias. The bias was assessed using the appropriately indicated National Institute of Health quality assessment tool based on the study design of each included article. 12 The heuristics of good, fair, and poor were utilized to have common nomenclature between heterogeneous study designs. The “N/a” designation was given to included case reports.
2.4. MAUDE database
MAUDE database is a repository of suspected device‐associated deaths, serious injuries and malfunctions submitted by mandatory and voluntary reporters, with the intent of ongoing risk and performance assessment of medical devices. 11 Utilizing the “Product Class” database search function, the MAUDE database was searched for “Eustachian Tube Dilation Device.” With these preliminary results, the search was then expanded using the “Manufacturer” function for any possible entries not found under the “Eustachian Tube Dilation Device.” Each result was assessed for basic patient demographics, balloon dilator manufacturer, and reported SAEs.
3. RESULTS
3.1. Literature search
A total of 146 citations resulted from searching the PubMed and Ovid Embase databases. Twenty duplicates were removed, leaving a total of 126 unique citations. Sixty potential full texts were reviewed for inclusion after a title and abstract screen was performed. After completion of the full‐text reviews, 55 manuscripts were selected to proceed for data abstraction (Figure 1).
FIGURE 1.
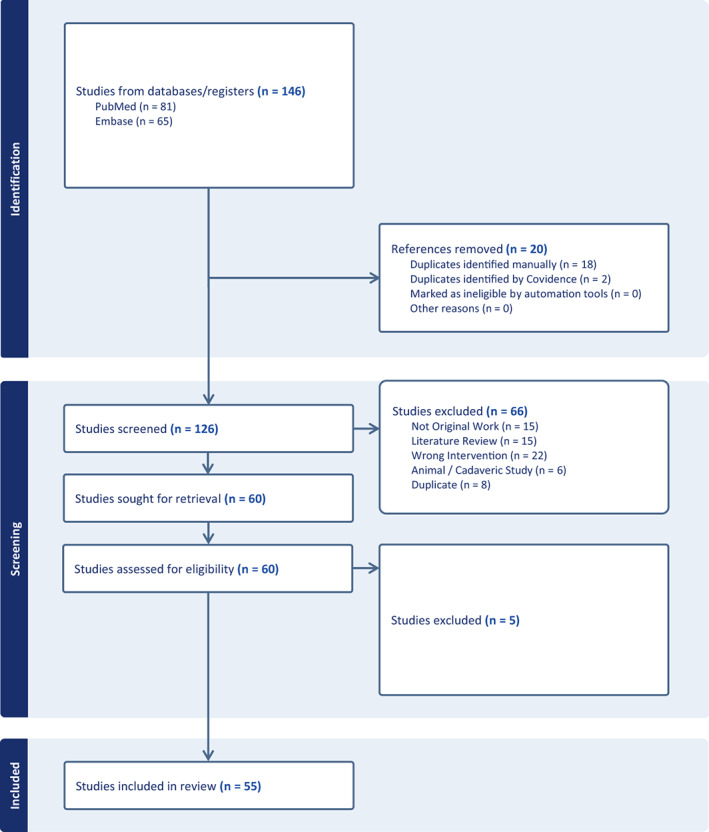
PRISMA diagram.
3.2. General study characteristics
Table 1 displays study characteristics for the 55 publications, including basic patient demographics, study design, balloon dilator manufacturer, and complications/SAEs reported. There was a median of 34 patients (Range: 1–2272) and 45 ears (Range: 1–3670) treated per publication. The average patient age was 39.1 years (SD ± 14.7) and the average follow‐up period was 11.9 months (SD ± 10.4 months).
TABLE 1.
Summary of included publications from PubMed and Ovid Embase literature search with adverse events reported.
| Authors | No. of patients | No. of ears | Patient age, in y (mean) | Study design | Risk of bias | Balloon manufacturer | Average follow‐up (in months) | No. of complications (%) | Complications (n) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abdel‐Aziz et al. (2014) | 284 | 510 | Unknown | Retrospective case series | Good | Spiggle and theiss | 2.00 | 3 (1%) | Hypoglossal Paresis (1), Subcutaneous emphysema (2) |
|
2 |
Ashry et al. (2017) | 48 | 67 | 50 | Retrospective cohort study | Good | Acclarent | 15.60 | 1 (2%) | Patulous ET (1) |
| 3 | Bowles et al. (2017) | 39 | 55 | 45.5 | Prospective cohort study | Good | Acclarent | 6.00 | 0 | NRAE |
|
4 |
Catalano, Peter et al. (2012) | 70 | 100 | 45 | Retrospective cohort study | Good | Acclarent | 7.58 | 1 (1%) | Subcutaneous Emphysema (1) |
|
5 |
Chen, Nina et al. (2022) | 35 | 49 | 38.4 | Retrospective cohort study | Poor | Unknown | 36.00 | 0 | NRAE |
|
6 |
Chen et al. (2020) | 49 | 25 | 10.2 | Retrospective cohort study | Good | Spiggle and theiss | 18.00 | 3 (6%) | Epistaxis (3) |
|
7 |
Cheng et al. (2021) | 62 | 96 | 47.3 | Retrospective cohort study | Good | Spiggle and theiss | 10.00 | 0 | NRAE |
| 8 | Choi et al. (2021) | 31 | 38 | 44.7 | Randomized controlled trial | Good | Mega medical | 1.50 | 0 | NRAE |
| 9 | Cutler et al. (2019) | 47 | 83 | 52.5 | Randomized controlled trial | Good | Entellus | 29.40 | 0 | NRAE |
|
10 |
Dai et al. (2016) | 8 | 12 | 53.1 | Retrospective case series | Fair | Spiggle and theiss | 6.40 | 0 | NRAE |
| 11 | Dalchow et al. (2016) | 217 | 342 | 45.58 | Prospective cohort study | Good | Spiggle and theiss | 7.50 | 0 | NRAE |
| 12 | Dean (2019) | 33 | 43 | Unknown | Retrospective cohort study | Fair | Acclarent | 1.50 | 0 | NRAE |
| 13 | Demir and Batman (2020) | 62 | 55 | 7 | Retrospective cohort study | Good | Spiggle and theiss | 12.00 | 4 (6%) | Hemotympanum (2), OME (2) |
| 14 | Giunta et al. (2019) | 20 | 21 | Unknown | Retrospective cohort study | Good | Spiggle and theiss | 34.80 | 4 (20%) | AOM (1), Tinnitus (1), SNHL (1), Subcutaneous Emphysema (1) |
| 15 | Gürtler et al. (2015) | 21 | 21 | 37.5 | Retrospective cohort study | Good | Spiggle and theiss | 26.00 | 1 (5%) | Epistaxis (1) |
| 16 | Howard et al. (2021) | 43 | 81 | 12.4 | Retrospective cohort study | Good | Acclarent | 0.96 | 2 (5%) | Epistaxis (1), Vertigo (1) |
| 17 | Jenckel et al. (2015) | 33 | 56 | 11 | Retrospective cohort study | Fair | Spiggle and theiss | 6.00 | 0 | NRAE |
| 18 | Jurkiewicz et al. (2013) | 4 | 7 | 45.75 | Prospective case series | Fair | Spiggle and theiss | 1.50 | 0 | NRAE |
| 19 | Kapadia and Tarabichi (2018) | 40 | 40 | 30 | Retrospective case series | Good | Foley catheter | 16.50 | 4 (10%) | Tinnitus (1), SNHL (1), Vertigo (2) |
| 20 | Kim et al. (2018) | 10 | 10 | 52.2 | Prospective case series | Good | Acclarent | 3.00 | 1 (10%) | Mucosal Laceration (1) |
| 21 | Kim et al. (2022) | 1 | 2 | 22 | Retrospective case report | N/a | Unknown | 0.75 | 1 (100%) | Perilymph Fistula (1) |
| 22 | Lee et al. (2020) | 1 | 2 | 55 | Retrospective case report | N/a | Acclarent | 12 | 1 (100%) | Nasopharyngeal Mucocele (1) |
| 23 | Leichtle et al. (2017) | 52 | 97 | 7 | Retrospective cohort study | Good | Spiggle and theiss | 5.18 | 4 (8%) |
Epistaxis (3), Hemotympanum (1) |
| 24 | Liang et al. (2016) | 90 | 90 | 36 | Randomized controlled trial | Good | Spiggle and theiss | 6 | 0 | NRAE |
| 25 | Long et al. (2021) | 1 | 2 | 51 | Retrospective case report | N/a | Entellus | Unknown | 2 (100%) | Epistaxis (1), Pneumomediastinum (1) |
| 26 | Luukkainen et al. (2018) | 34 | 52 | 41 | Retrospective cohort study | Fair | Acclarent | 37.20 | 0 | NRAE |
| 27 | Luukkainen et al. (2019) | 18 | 27 | 39.5 | Prospective case series | Good | Spiggle and theiss | Unknown | 0 | NRAE |
| 28 | McCoul and Anand (2012) | 22 | 35 | 55.1 | Prospective cohort study | Good | Acclarent | 10.00 | 1 (5%) | Hemotympanum (1) |
| 29 | McMurran et al. (2020) | 25 | 36 | 44 | Prospective cohort study | Good | Spiggle and theiss | 28 | 2 (8%) |
Subcutaneous Emphysema (1), TM Perforation (1) |
| 30 | Meyer et al. (2018) | 60 | 91 | 49.4 | Randomized controlled trial | Good | Entellus | 10.92 | 0 | NRAE |
| 31 | Ockermann et al. (2010) | 8 | 13 | 44.1 | Quasi‐experimental study | Good | Spiggle and theiss | 2.00 | 0 | NRAE |
| 32 | Park et al. (2022) | 1 | 1 | 63 | Prospective case report | N/a | Mega medical | 12.00 | 1 (100%) | Patulous ET (1) |
| 33 | Poe et al. (2011) | 11 | 11 | 51.8 | Quasi‐experimental study | Good | Acclarent | 9.12 | 5 (45%) | Mucosal Laceration (5) |
| 34 | Poe et al. (2018) | 296 | 444 | 55.6 | Randomized controlled trial | Good | Acclarent | 1.50 | 0 | NRAE |
| 35 | Ramakrishnan and Kadambi (2020) | 10 | 15 | 32 | Retrospective cohort study | Good | Eustacare | 2.00 | 0 | NRAE |
| 36 | Satmis and van der Torn (2018) | 42 | 66 | 43 | Retrospective cohort study | Good | Spiggle and theiss | 3.00 | 5 (12%) | Epistaxis (1), Otalgia (2), Patulous ET (1), TM Perforation (1) |
| 37 | Schmitt et al. (2018) | 38 | 45 | 49.9 | Retrospective cohort study | Good | Spiggle and theiss | 14.27 | 2 (5%) | Rhinitis (1), Tongue dysesthesia (1) |
| 38 | Schroder et al. (2015) | 622 | 1076 | Unknown | Retrospective cohort study | Good | Spiggle and theiss | Unknown | 3 (0.5%) | Subcutaneous Emphysema (3) |
| 39 | Shah et al. (2018) | 1 | 1 | 28 | Retrospective case report | N/a | Entellus | Unknown | 2 (100%) |
Subcutaneous emphysema (1), Pneumomediastinum (1) |
| 40 | Si et al. (2018) | 200 | 200 | 41.02 | Retrospective cohort study | Good | Spiggle and theiss | 11.09 | 1 (0.5%) | Patulous ET (1) |
| 41 | Si et al. (2019) | 120 | 120 | 43 | Randomized controlled trial | Good | 24.00 | 2 (1.7%) | Patulous ET (2) | |
| 42 | Silvola et al. (2014) | 41 | 41 | 48 | Prospective cohort study | Good | Acclarent | 30.00 | 0 | NRAE |
| 43 | Singh et al. (2017) | 11 | 13 | 42.5 | Prospective cohort study | Fair | Spiggle and theiss | Unknown | 0 | NRAE |
|
44 |
Skevas et al. (2018) | 2272 | 3670 | Unknown | Retrospective cohort study | Good | Spiggle and theiss | Unknown | >18 (0.8%) | AOM (1), Epistaxis (?), a Patulous ET (1), Pneumomediastinum (3), Subcutaneous emphysema (10), Tinnitus (3) |
| 45 | Standring et al. (2021) | 169 | 309 | 52.4 | Prospective cohort study | Good | Stryker | 6.00 | 3 (2%) | AOM (1), OME (1), Otalgia (1) |
| 46 | Sun et al. (2020) | 58 | 74 | 50.1 | Retrospective cohort study | Good | Unknown | 24.00 | 0 | NRAE |
| 47 | Swain et al. (2020) | 21 | 25 | 44.9 | Retrospective cohort study | Fair | Spiggle and theiss | 2.00 | 3 (14%) | AOM (1), Mucosal Laceration (2) |
| 48 | Tisch et al. (2017) | 94 | 90 | 9.6 | Retrospective cohort study | Good | Spiggle and theiss | 11.73 | 5 (5%) | AOM (3), Epistaxis (2) |
| 49 | Todt et al. (2021) | 1547 | 2614 | Unknown | Retrospective case series | Good | Spiggle and theiss | Unknown | 7 (0.5%) | SNHL (7) |
| 50 | Toivonen et al. (2021) | 26 | 46 | 12.5 | Retrospective cohort study | Good | Acclarent | 27.60 | 2 (8%) | Patulous ET (2) |
| 51 | Utz et al. (2020) | 15 | 26 | 31.3 | Retrospective cohort study | Good | Acclarent | 9.50 | 0 | NRAE |
| 52 | Utz and Wise (2019) | 1 | 1 | 20 | Retrospective case report | N/a | Acclarent | 12.00 | 0 | NRAE |
| 53 | Wanscher and Svane‐Knudsen (2014) | 34 | 50 | 45 | Prospective cohort study | Fair | Spiggle and theiss | 2.00 | 4 (12%) | AOM (4) |
| 54 | Williams et al. (2016) | 18 | 25 | 40.6 | Retrospective cohort study | Good | Spiggle and theiss | 7.10 | 0 | NRAE |
| 55 | Wong and Prepageran (2021). | 12 | 14 | 39.5 | Prospective cohort study | Good | Entellus | 6.00 | 0 | NRAE |
Abbreviations: AOM, acute otitis media; ET, eustachian tube; OME, otitis media with effusion; SNHL, sensorineural hearing loss; TM, tympanic membrane.
Publication by Skevas et al. (2018) did not specify the number of epistaxis episodes after BDET.
3.3. Complications and SAEs reporting
The median number of complications per study was 1 (Range: 0–19), approximately a 1.4% complication rate across all included publications. The most reported adverse event was subcutaneous emphysema of the head and neck (n = 19) and there were several case reports of singular complications/SAEs including nonspecific rhinitis, tympanic membrane perforation, perilymph fistula, nasopharyngeal mucocele, hypoglossal paresis, and tongue dysesthesia. There were no reported mortality events among all included papers.
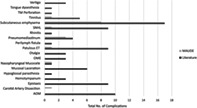
The MAUDE database was cross‐referenced with the literature search complications and SAEs. Through the MAUDE database search described above, there were a total of 18 entries. Of these, 12 entries were direct patient complications. The most reported complication in the MAUDE database was subcutaneous emphysema of the head and neck (n = 8, 67%). These adverse events are included in Table 2 and their relative rates are compared with the Literature Search results in Figure 2. There were no mortality events related to BDET in the MAUDE database.
TABLE 2.
Complications and adverse events as reported on the MAUDE database.
| Manufacturer | Event | Result |
|---|---|---|
| ACCLARENT, INC. | Hearing loss, tinnitus | Persistent symptoms at 6 months |
| ACCLARENT, INC. | Subcutaneous emphysema | Recovered |
| ENTELLUS MEDICAL, INC. | Subcutaneous emphysema | Recovered |
| ENTELLUS MEDICAL, INC. | Subcutaneous emphysema | Recovered |
| ENTELLUS MEDICAL, INC. | Patulous ET | Recovered |
| ENTELLUS MEDICAL, INC. | Carotid Artery Dissection | Recovered |
| ACCLARENT, INC. | Subcutaneous emphysema, pneumomediastinum | Recovered |
| ACCLARENT, INC. | Subcutaneous emphysema, pneumomediastinum | Recovered |
| ENTELLUS MEDICAL | Subcutaneous emphysema | Recovered |
| ENTELLUS MEDICAL, INC. | Subcutaneous emphysema, neumomediastinum | Recovered |
| ACCLARENT, INC. | Nasopharyngeal Mucocele | Recovered |
| ENTELLUS MEDICAL | Subcutaneous emphysema | Recovered |
FIGURE 2.
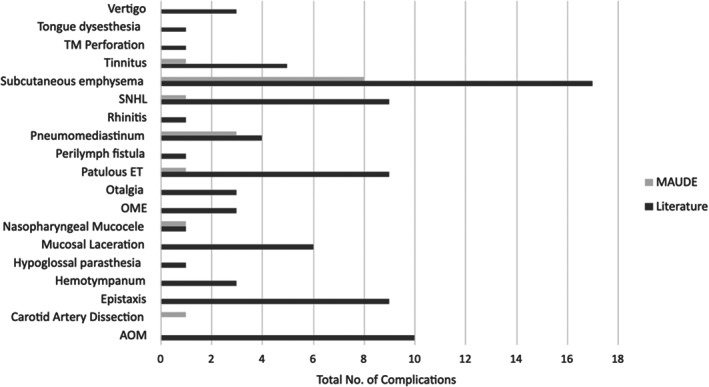
Absolute number of complications, comparing the Literature Search results to the MAUDE database. AOM, acute otitis media; ET, eustachian tube; OME, otitis media with effusion; TM, tympanic membrane.
4. DISCUSSION
Since the initial publications of clinical feasibility of BDET for ETD by Ockermann et al. in 2010 and Poe et al. in 2011, complication rates have been reported around 2%. 13 , 14 BDET was officially endorsed by AAO‐HNS in 2019 for ETD given its efficacy and generally benign, self‐limited complications. 4 , 15 Our present study reflects this low complication rate and, indeed, a large majority of the complications reported were minor. Even still, SAEs such as subcutaneous emphysema of the head and neck, pneumomediastinum, and trauma to the carotid artery carries a nonzero risk and is important during preoperative consultation with patients. Therefore, by cross‐referencing the MAUDE database with regards to complications, this work adds to the current literature on BDET by further characterizing the extent and occurrence of possible complications.
The MAUDE database is a domestic repository for the United States. In this present study, the ET balloon dilating system manufactured by the German company, Spiggle and Theiss, was the most used system reported in the literature (16/41 studies). Given that some of the included studies were performed in an international setting and the product was approved internationally before it was approved in the United States, there is not as much robust data in our MAUDE database. Germany does have a similar SAE reporting system available under the Federal Institute for Drugs and Medical Devices (BfArM), but the results were unavailable in an English translation and was deemed outside the scope of the current project. The proprietary nature and respective geographies of these medical device manufacturers could thusly be a contributing factor to the rates seen in the MAUDE database and represents a limitation of the present work.
First, there were much fewer complications and SAEs registered in the MAUDE database compared with the literature search. The authors hypothesize several plausible reasons for this. Second, the AEs reported to the MAUDE database were of greater severity on average when compared to the results from the literature review. Two‐thirds of the reported complications in the MAUDE database were subcutaneous emphysema (several with tracking pneumomediastinum as well) versus approximately 20% in the literature search. Subcutaneous emphysema is generally a benign, self‐limited pathology, but does portend a life‐threatening risk should the gaseous expansion cause compression of vascular or aerodigestive structures in the head and neck. 16 In the setting of recent BDET, the underlying pathophysiology for subcutaneous emphysema is thought to be from mucosal microtrauma allowing baro‐dissection into the adjacent soft tissue during episodes of increased upper airway pressure (e.g., Valsalva, sneezing, etc.). Further, the MAUDE database did have the only report of a cerebrovascular accident attributed to a carotid artery dissection 1 week after a patient had bilateral BDET. Hence, in accordance with the mission behind the MAUDE database, it does make plausible sense that, while fewer in number, the reporting of SAEs be relatively higher in severity than that of the literature search.
There are other limitations that warrant discussion. While reviewer bias was partially mitigated by multiple reviewers and a high (0.88) Cohen's Kappa was achieved, this cannot be fully eliminated from the literature review process. A broad criteria of study design was selected to include the most possible publications, but at the cost of standardized methods including the presence of adjunct procedures (e.g., Tympanostomy tube placement, sinus surgery, etc.) which could be possible confounders. Finally, while publication bias was limited by using multiple databases, English was the primary language referenced, representing a potential language bias. We recognize that the MAUDE database is limited. Although reports of device‐related complications are mandatory for industry, they are voluntary for providers/patients thus possibly under‐reporting complications.
5. CONCLUSION
BDET is a relatively benign procedure for ETD but may result complications with a varying array of morbidity. While future peer‐reviewed studies will provide the strongest evidence on this topic, the MAUDE database highlights potential serious complications to following BDET. It is important that both patients and otolaryngologists be aware of these risks associated with the procedure and appropriate steps be taken to reduce potential significant sequelae.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to report.
Supporting information
DATA S1: Supporting information.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Chisolm PF, Hakimi AA, Maxwell JH, Russo ME. Complications of eustachian tube balloon dilation: Manufacturer and User Facility Device Experience (MAUDE) database analysis and literature review. Laryngoscope Investigative Otolaryngology. 2023;8(6):1507‐1515. doi: 10.1002/lio2.1185
Meeting Information: Poster Presentation, COSM 2023, May 3–7, 2023, Boston, MA, USA.
REFERENCES
- 1. Hamrang‐Yousefi SNJ, Andaloro C. Eustachian Tube Dysfunction. StatPearls Publishing; 2022. StatPearls [Internet]. [Google Scholar]
- 2. Patel MA, Mener DJ, Garcia‐Esquinas E, Navas‐Acien A, Agrawal Y, Lin SY. Tobacco smoke exposure and eustachian tube disorders in US children and adolescents. PLoS One. 2016;11(10):e0163926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shan A, Ward BK, Goman AM, et al. Prevalence of eustachian tube dysfunction in adults in the United States. JAMA Otolaryngol Head Neck Surg. 2019;145(10):974‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tucci DL, McCoul ED, Rosenfeld RM, et al. Clinical consensus statement: balloon dilation of the eustachian tube. Otolaryngol Head Neck Surg. 2019;161(1):6‐17. [DOI] [PubMed] [Google Scholar]
- 5. Poe D, Anand V, Dean M, et al. Balloon dilation of the eustachian tube for dilatory dysfunction: a randomized controlled trial. Laryngoscope. 2018;128(5):1200‐1206. [DOI] [PubMed] [Google Scholar]
- 6. Huisman JML, Verdam FJ, Stegeman I, De Ru JA. Treatment of eustachian tube dysfunction with balloon dilation: a systematic review. Laryngoscope. 2018;128(1):237‐247. [DOI] [PubMed] [Google Scholar]
- 7. Meyer TA, O'Malley EM, Schlosser RJ, et al. A randomized controlled trial of balloon dilation as a treatment for persistent eustachian tube dysfunction with 1‐year follow‐up. Otol Neurotol. 2018;39(7):894‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aboueisha MA, Attia AS, McCoul ED, Carter J. Efficacy and safety of balloon dilation of eustachian tube in children: systematic review and meta‐analysis. Int J Pediatr Otorhinolaryngol. 2022;154:111048. [DOI] [PubMed] [Google Scholar]
- 9. U.S. Food and Drug Administration MAUDE‐Manufacturer and User Facility Device Experience.
- 10. Covidence Systematic Review Software . Veritas Health Information, Melbourne, Australia. 2021. www.covidence.org
- 11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta‐analysis, and cliniical practice guideline: a systematic review. J Evid Based Med. 2015;8:2‐10. [DOI] [PubMed] [Google Scholar]
- 13. Ockermann T, Reineke U, Upile T, Ebmeyer J, Sudhoff HH. Balloon dilatation eustachian tuboplasty: a clinical study. Laryngoscope. 2010;120(7):1411‐1416. [DOI] [PubMed] [Google Scholar]
- 14. Poe DS, Silvola J, Pyykkö I. Balloon dilation of the cartilaginous eustachian tube. Otolaryngol Head Neck Surg. 2011;144(4):563‐569. [DOI] [PubMed] [Google Scholar]
- 15. McCoul ED, Weinreich HM, Mulder H, Man LX, Schulz K, Shin JJ. Health care utilization and prescribing patterns for adult eustachian tube dysfunction. Otolaryngol Head Neck Surg. 2019;160(6):1071‐1080. [DOI] [PubMed] [Google Scholar]
- 16. Kukuruza KAA. Subcutaneous Emphysema. StatPearls; 2022. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DATA S1: Supporting information.


