Abstract
Objectives
This study aimed to investigate the association between salivary matrix metalloproteinase‐1 (MMP‐1) and clinicopathological parameters of oral cavity squamous cell carcinoma (OSCC) and compare the prognostic efficacy of salivary MMP‐1 and other established circulating markers for OSCC.
Methods
Saliva specimens from 479 OSCC subjects were examined using an enzyme‐linked immunosorbent assay. The area under the curve (AUC) values of salivary MMP‐1 and other markers were calculated, and survival analyses were conducted using Kaplan–Meier and multivariate regression methods.
Results
Salivary MMP‐1 showed good discrimination in predicting overall survival, with an AUC of 0.638, which was significantly higher than that of albumin (0.530, p = .021) and Charlson comorbidity index (0.568, p = .048) and comparable with neutrophil‐to‐lymphocyte ratio (0.620, p = .987), platelet‐to‐lymphocyte ratio (0.575, p = .125), and squamous cell carcinoma antigen (0.609, p = .605). Elevated levels of salivary MMP‐1 were significantly associated with higher pT classification, pN classification, overall pathological stage, positive extranodal extension, tumor differentiation, positive lymphovascular invasion, positive perineural invasion, and tumor depth (p all <.05). Multivariate analyses indicated that a higher level of salivary MMP‐1 (≥2060.0 pg/mL) was an independent predictive factor of poorer overall survival (adjusted hazard ratio: 1.421 [95% confidential interval: 1.014–1.989], p = .041).
Conclusion
The study found that the salivary MMP‐1 level was significantly associated with many adverse clinicopathological parameters of OSCC. In OSCC, it was found to have superior efficacy in predicting prognosis and was an independent prognostic factor of post‐treatment outcome.
Level of evidence
3.
Keywords: matrix metalloproteinase‐1, oral squamous cell carcinoma, saliva
Salivary MMP‐1 showed a significantly higher AUC than albumin and CCI and a higher or comparable AUC to the NLR, the PLR, and SCCA in predicting overall survival in OSCC patients. The study also indicates that patients with high levels of salivary MMP‐1 have poorer prognosis in terms of OS and DSS. Overall, these findings may facilitate development of a noninvasive and user‐friendly salivary detection tool for OSCC treatment.
1. INTRODUCTION
Head and neck cancers are the seventh most prevalent cancers in the world. 1 Over one million new cases were reported in 2016, accounting for approximately 50,000 deaths globally. 2 Squamous cell carcinoma is the most frequent histological type of malignancy and constitutes more than 90% of oral cavity cancer histopathological types. 3 , 4 For patients with oral cavity squamous cell carcinoma (OSCC), the reported 5‐year overall survival rate is approximately 50%–70%, and the chances of survival decrease when the tumor is detected at advanced stages. 5 Over 50% of OSCC patients have delayed diagnosis at initial presentation. 6 Early detection of OSCC would substantially improve the survival rate and lower treatment costs. 7 Although biopsy of suspected lesions remains the gold standard for determination of OSCC, it is time‐consuming, not well accessible and requires invasive procedures. 8 In contrast, saliva is a physiological fluid of the oral cavity, and the procedure for its sampling is easy and noninvasive. In 2016, we quantified levels of 49 potential protein candidates in saliva for OSCC detection using multiple reaction monitoring mass spectrometry, and matrix metalloproteinase‐1 (MMP‐1) was recognized as the most significant biomarker. 9 An enzyme‐linked immunosorbent assay (ELISA) was subsequently developed by our group to further analyze the level of salivary MMP‐1 in 1160 enrolled subjects; salivary MMP‐1 effectively detected OSCC and that it was significantly associated with OSCC progression. 8 However, the association between pathological features/survival outcomes and salivary MMP‐1 levels in OSCC patients has not been fully elucidated. Previous literature reports that levels of squamous cell carcinoma antigen (SCCA), systemic inflammation and malnutrition before treatment are associated with OSCC tumorigenesis, progression and distant metastasis. 10 , 11 , 12 , 13 , 14 As a result, several biomarkers, including the neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR) and albumin level, are used to predict OSCC prognosis. 10 , 11 , 12 , 13 , 14 This study aimed to achieve three objectives. First, we compared the area under the curve (AUC) of salivary MMP‐1 with other established clinic/blood markers for predicting posttreatment outcome. Second, we sought to validate the correlation between salivary MMP‐1 and various clinicopathological characteristics. Finally, we evaluated the role of salivary MMP‐1 in posttreatment prognosis for OSCC.
2. MATERIALS AND METHODS
2.1. Study subjects
For this retrospective study, a total of 792 saliva specimens were collected from 313 subjects with oral potentially malignant disorder (OPMD) and 479 subjects with OSCC tumors were collected from subjects with OSCC tumors between May 2012 and September 2021. The informed consent form was signed by all the enrolled patients before collection of saliva samples (pretreatment), and the present study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (Number: 10012‐L2).
The final diagnosis of OPMD, including leukoplakia, erythroplakia, erythorleukoplakia, oral lichen planus, oral lichenoid lesions, oral submucous fibrosis and verrucous hyperplasia, was performed by head and neck surgeons according to pathological diagnosis or clinical findings. Final diagnosis of OSCC was achieved via surgical biopsy of the oral lesion. Subjects with other concomitant primary or recurrent malignancies, subjects who presented with distant metastasis at initial diagnosis, or subjects with a previous history of other cancers were excluded from the present study. The tumor, node, metastasis (TNM) classifications of all OSCC cases were assigned in accordance with the seventh edition of the American Joint Committee on Cancer (AJCC) Cancer staging manual. 15
2.2. Clinical specimens
Salivary samples were collected and processed as described in our previous article. 9 Briefly, saliva samples of the enrolled subjects were collected after food and water fasting for a minimum of 60 min. The donors were also asked to avoid using oral hygiene substances or cigarette smoking before collection. The enrolled subjects were instructed to mouth rinse with clean water, and the saliva specimen (approximately 7.5 mL) was collected using the passive drool method into sterile 50‐mL‐volume centrifuge tubes. The saliva samples were centrifuged at 3000 × g at 4°C for 15 min. The supernatant was aspirated, and a cocktail of protease inhibitors (Roche) was added. The processed saliva was aliquoted and stored at −80°C. Laboratory tests for blood samples from the OSCC patients were performed before curative surgery, and variables including the NLR and PLR, albumin and SCCA were also measured in the present study. The preoperative value of the NLR in peripheral blood was obtained by dividing the absolute neutrophil count by the absolute lymphocyte count. The preoperative PLR value of peripheral blood was obtained by dividing the absolute platelet count by the absolute lymphocyte count.
2.3. Treatment protocol
Before surgery, a detailed medical history with physical examination, blood test results, status of Charlson comorbidity index (CCI), abdominal ultrasonography, computed tomography or magnetic resonance imaging of the head and neck, fluorodeoxyglucose‐positron emission tomography or bone scan, and chest radiography were performed for each OSCC patient. Patients with OSCC received surgery as their primary treatment, and OSCC tumors were radically resected en bloc with adequate margins. 15 Pathological evaluations were performed intraoperatively on frozen sections to confirm all negative margins. The oral cavity defect was closed primarily or reconstructed using a local flap or free‐tissue transfer immediately after OSCC tumor excision. Cervical lymphadenectomy, including selective or modified radical neck dissection, was performed simultaneously. 16 , 17 The final pathological staging was determined by the AJCC staging manual, 8th edition. 15 Postoperative radiotherapy or chemotherapy was usually recommended in accordance with National Comprehensive Cancer Network guidelines after our multidisciplinary team discussion. 18 In general, adjuvant radiation therapy was suggested when a close margin (less than 5 mm), advanced T classification (T4), perineural invasion or bone invasion was identified. Adjuvant chemoradiation was suggested when a positive surgical margin, extranodal extension (ENE) or pathological metastasis in multiple lymph nodes or multiple adverse pathological features was identified. The total dose of adjuvant radiotherapy was delivered in 2 Gy per fraction 5 days per week, and the total target dose was 60–72 Gy. Patients received cisplatin‐based chemotherapy along with radiotherapy when adjuvant chemoradiation was recommended. All patients had follow‐up visits every 2 months for the first year, every 3 months for the second and third years, and every 6 months thereafter after discharge.
2.4. Measurement of MMP‐1 in saliva
The collected saliva specimens were analyzed with a Human MMP‐1 ELISA kit (S&T BioMed Co., Ltd., Hsinchu, Taiwan) for determination of MMP‐1 levels.
Each assay plate included a standard curve, 3 control samples and 38 plasma samples. The standard curve was generated using a twofold dilution series of an 8000 pg/mL MMP‐1 protein standard down to 125 pg/mL. Optical density levels of specimens were retested at 10‐ and 50‐fold dilutions if they were close to or higher than the standard point; 0 pg/mL served as a blank control. ELISAs were conducted following the general protocol. (1) Blank, standards, controls or samples (no dilution) were added to capture antibody‐precoated 96‐well strip microplates and incubated at room temperature for 60 min on a shaker. (2) A detection antibody was added to the wells after washing them four times, and the plates were incubated for 40 min at room temperature on a shaker. (3) After washing again, a 3,3′,5,5′‐tetramethylbenzidine solution was added to the wells, and the plates were incubated for 15 min at room temperature on a shaker. (4) After adding stop solution to each well, the plates were incubated on a shaker for 2 min. (5) The optical density value at 450 nm of each well was determined within 30 min using a microplate reader with 540 or 570 nm as the reference wavelength. (6) The MMP‐1 level of each specimen was calculated according to a standard curve.
2.5. Statistical analysis
Statistical data are presented as the mean with the standard deviation (SD) or the number with the percentage. The association between clinicopathological factors and the salivary concentration of MMP‐1 was calculated using the chi‐squared test. Intergroup differences in salivary MMP‐1 levels were assessed using the Wilcoxon test. Receiver operator characteristic (ROC) curves were created by plotting data pairs for sensitivity and (1 − specificity). Areas under the curve (AUC) were analyzed using the pROC package. 19 Comparison of AUCs was performed using the DeLong test. The Youden index was utilized to determine the optimal cutoff values for ROC curves.
All patients were followed up regularly at our outpatient clinic until October 2021 or death. Survival status was plotted using the Kaplan–Meier method, and differences were evaluated using the log‐rank test. Multivariate regression analyses were applied to define specific outcomes for overall survival (OS) and disease‐specific survival (DSS). All analyses were conducted using Statistical Analysis System software (version 9.4; SAS Institute). All p values were two‐sided, and statistical significance was indicated by a p value less than .05.
3. RESULTS
3.1. Patient characteristics
The mean ages of the 479 OSCC and 313 OPMD patients were 55.0 ± 11.4 and 51.4 ± 11.3 years, respectively. Among the included 479 OSCC patients, 82.0% were younger than 65 years old, and 91.6% were male. The predominant primary site of the OSCC was the buccal mucosa (n = 191, 39.9%), followed by the tongue (n = 185, 38.6%). Other important clinicopathological characteristics regarding OSCC treatment in our study group are provided in Table 1.
TABLE 1.
Baseline clinicopathological characteristics of patients with oral cavity squamous cell carcinoma (n = 479).
| Variable | Characteristics |
|---|---|
| Age (years) | |
| <65 | 393 (82.0%) |
| ≥65 | 86 (18.0%) |
| Gender | |
| Male | 439 (91.6%) |
| Female | 40 (8.4%) |
| Personal habits | |
| Alcohol consumption | 319 (66.6%) |
| Betel nut chewing | 394 (82.3%) |
| Cigarettes smoking | 403 (84.1%) |
| Site of the primary tumor | |
| Buccal mucosa | 191 (39.9%) |
| Tongue | 185 (38.6%) |
| Others | 103 (21.5%) |
| Overall stage | |
| I | 98 (20.5%) |
| II | 111 (23.2%) |
| III | 64 (13.3%) |
| IV | 206 (43.0%) |
| pT classification | |
| T1 | 121 (25.1%) |
| T2 | 155 (32.4%) |
| T3 | 54 (11.3%) |
| T4 | 149 (31.2%) |
| pN classification | |
| N0 | 309 (64.5%) |
| N1 | 57 (11.9%) |
| N2 | 85 (17.7%) |
| N3 | 28 (5.9%) |
| PNI | 156 (32.6%) |
| ENE | 102 (21.3%) |
| LVI | 49 (10.2%) |
| DOI ≥5 mm | 362 (75.6%) |
| Surgical margin | |
| ≥5 mm | 314 (65.6%) |
| <5 mm | 165 (34.4%) |
| Adjuvant therapy | |
| Absent | 205 (42.8%) |
| Radiotherapy | 66 (13.8%) |
| Chemoradiotherapy | 208 (43.4%) |
| Neutrophil (×103 μL−1) a | 6.8 ± 23.1 |
| Platelet (×103 μL−1) a | 266.8 ± 106.3 |
| Lymphocyte (×103 μL−1) a | 2.6 ± 12.3 |
| NLR a | 4.1 ± 14.1 |
| PLR a | 151.6 ± 111.5 |
| Albumin (g/dL) a | 4.2 ± 0.5 |
| SCCA (ng/mL) a | 1.6 ± 2.0 |
| CCI a | 1.3 ± 1.2 |
| Salivary MMP‐1 (pg/mL) a | 6818.7 ± 21114.5 |
Abbreviations: PNI, perineural invasion; ENE, extranodal extension; LVI, lymphovascular invasion; DOI, depth of invasion; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; SCCA, squamous cell carcinoma antigen; CCI, Charlson comorbidity index.
Mean ± SD.
3.2. Salivary MMP‐1 levels in OPMD and OSCC patients
As shown in Figure 1A, there was a significant difference between the salivary MMP‐1 levels between the OSCC and OPMD groups (p < .0001). The median salivary MMP‐1 levels for the OSCC and OPMD groups were 1055.0 (interquartile range, 344.2–3432.0) and 83.2 (interquartile range, 46.5–178.0) pg/mL, respectively. The area under the curve in the current study was 0.8869 (p < .0001) (Figure 1B). The sensitivity of the cut‐off value (223.8 pg/mL) determined by the maximized Youden's index to differentiate OSCC and OPMD in the current cohort was 82.7%. The specificity was 82.1%, and the accuracy was 82.5%.
FIGURE 1.
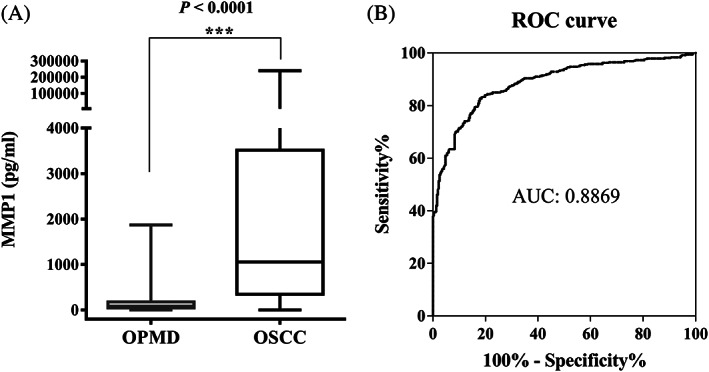
Matrix metalloproteinase‐1 (MMP‐1) levels in saliva from 792 clinical specimens. (A) Salivary levels of MMP‐1 in oral potentially malignant disorders (OPMD) and oral squamous cell carcinoma (OSCC) patients were determined using an enzyme‐linked immunosorbent assay kit. The OSCC patients had significantly higher saliva MMP‐1 levels than the OPMD patients. (B) Receiver operating characteristic (ROC) curve showing the diagnostic efficacy of salivary MMP‐1 for discriminating OSCC and OPMD patients. The area under the ROC curve (AUC) was 0.8869, p < .0001.
3.3. Comparison of AUC values of salivary MMP‐1 and other clinical parameters for overall survival
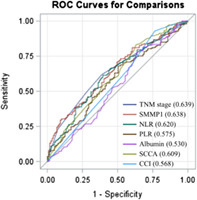
ROC curves of salivary MMP‐1 and other clinical markers for OS are depicted in Figure 2. The AUC value of salivary MMP‐1 for OS was 0.638 (p = .016), revealing adequate discrimination ability. Clinical biomarkers, including the NLR, PLR, serum albumin, SCCA and CCI, had AUC values of 0.620 (p = .003), 0.575 (p = .120), 0.530 (p = .111), 0.609 (p = .006) and 0.568 (p = .002), respectively. We further compared AUCs between salivary MMP‐1 and the markers mentioned above and found the AUC of salivary MMP‐1 to be significantly higher than that of albumin and CCI (p = .021 and p = .048, respectively) and comparable with neutrophil‐to‐lymphocyte ratio (p = .987), platelet‐to‐lymphocyte ratio (p = .125) and squamous cell carcinoma antigen (p = .605) (Table 2). The Youden index was utilized to determine the optimal cutoff value for salivary MMP‐1 levels, and the analysis revealed that 2060.0 pg/mL was the value that maximized Youden's index.
FIGURE 2.
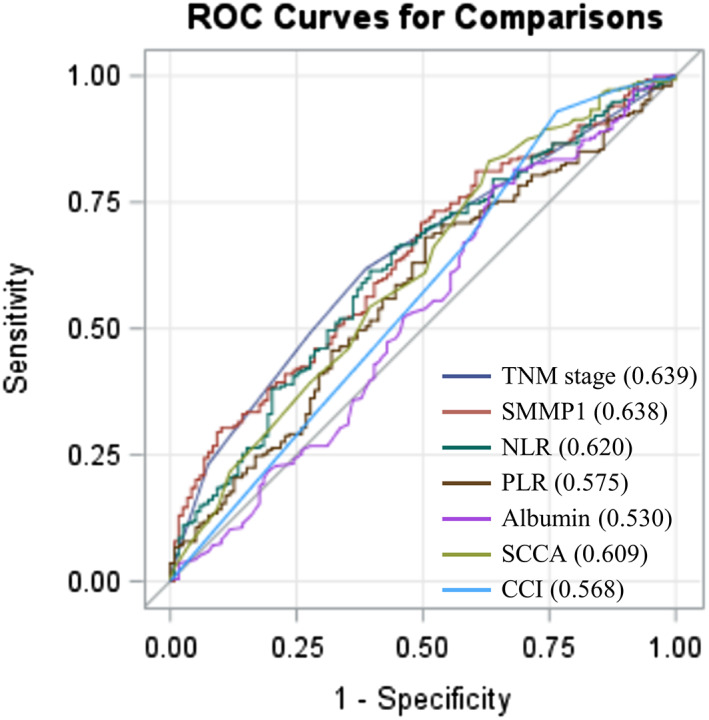
Receiver operating characteristic (ROC) curve analysis of salivary MMP‐1 and several clinical markers in patients with OSCC who underwent surgery. To compare the prognostic discrimination between MMP‐1 and several prognosticators, that is, TNM stage, the neutrophil‐to‐lymphocyte ratio (NLR), the platelet‐to‐lymphocyte ratio (PLR), albumin, squamous cell carcinoma antigen (SCCA), and Charlson comorbidity index (CCI), we performed ROC curve analysis of these markers and compared the corresponding areas under the curve (AUCs) indicated within parentheses.
TABLE 2.
Comparison of the AUC values of salivary MMP‐1 and several clinical markers.
| AUC | 95% CI | p‐value | p‐value | |
|---|---|---|---|---|
| Salivary MMP‐1 | 0.638 | 0.580–0.697 | .041 a | ‐ |
| TNM stage | 0.639 | 0.585–0.693 | <.001 a | .187 |
| NLR | 0.620 | 0.559–0.681 | .018 a | .987 |
| PLR | 0.575 | 0.513–0.637 | .143 | .125 |
| Albumin | 0.530 | 0.464–0.595 | .110 | .021 a |
| SCCA | 0.609 | 0.547–0.672 | .006 a | .605 |
| CCI | 0.568 | 0.505–0.631 | .002 a | .048 a |
Note: The AUC values between the salivary MMP‐1 and other markers were compared using the Z test.
Abbreviations: AUC, area under the curve; CCI, Charlson Comorbidity Index; CI, confidence interval; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; SCCA, squamous cell carcinoma antigen.
Statistically significant.
3.4. Association of salivary MMP‐1 levels with clinicopathological parameters
The OSCC patients were stratified according to salivary MMP‐1 concentration (2060.0 pg/mL) to demonstrate its association with various clinical and pathological parameters. Elevated levels of MMP‐1 in saliva were associated with higher pT classification (p < .001), higher pN classification (p < .001), higher overall pathological stage (p < .001), positive extranodal extension (ENE) (p < .001), poor tumor differentiation (p = .027), positive lymphovascular invasion (p = .010), positive perineural invasion (p < .001) and depth of invasion (DOI) (p < .001). The related details are described in Table 3.
TABLE 3.
Baseline clinicopathological characteristics according to the salivary MMP‐1.
| Variable | Salivary MMP‐1 (pg/mL) | p‐value | |
|---|---|---|---|
| <2060 (n = 305) | ≥2060 (n = 174) | ||
| Age (years) | |||
| <65 | 257 (84.3%) | 136 (78.2%) | .094 |
| ≥65 | 48 (15.7%) | 38 (21.8%) | |
| Gender | |||
| Male | 278 (91.2%) | 161 (92.5%) | .599 |
| Female | 27 (8.8%) | 13 (7.5%) | |
| Alcohol consumption | |||
| No | 104 (34.1%) | 56 (32.2%) | .669 |
| Yes | 201 (65.9%) | 118 (67.8%) | |
| Betal nut chewing | |||
| No | 60 (19.7%) | 25 (14.4%) | .143 |
| Yes | 245 (80.3%) | 149 (85.6%) | |
| Cigarettes smoking | |||
| (−) | 49 (16.1%) | 27 (15.5%) | .874 |
| (+) | 256 (83.9%) | 147 (84.5%) | |
| pT classification | |||
| T1–T2 | 222 (72.8%) | 54 (31.0%) | <.001 a |
| T3–T4 | 83 (27.2%) | 120 (69.0%) | |
| pN classification | |||
| N0 | 224 (73.4%) | 85 (48.9%) | <.001 a |
| N1–N3 | 81 (26.6%) | 89 (51.1%) | |
| Overall stage | |||
| I–II | 172 (56.4%) | 37 (21.3%) | <.001 a |
| III–IV | 133 (43.6%) | 137 (78.7%) | |
| ENE | |||
| Absent | 258 (84.6%) | 119 (68.4%) | <.001 a |
| Present | 47 (15.4%) | 55 (31.6%) | |
| Cell differentiation | |||
| W‐D/M‐D | 282 (92.5%) | 150 (86.2%) | .027 a |
| P‐D | 23 (7.5%) | 24 (13.8%) | |
| LVI | |||
| Absent | 282 (92.5%) | 148 (85.1%) | .010 a |
| Present | 23 (7.5%) | 26 (14.9%) | |
| PNI | |||
| Absent | 230 (75.4%) | 92 (53.2%) | <.001 a |
| Present | 75 (24.6%) | 81 (46.8%) | |
| DOI | |||
| <5 mm | 101 (33.1%) | 16 (9.2%) | <.001 a |
| ≥5 mm | 204 (66.9%) | 158 (90.8%) | |
Abbreviations: DOI, depth of invasion; ENE, extranodal extension; LVS, lymphovascular invasion; M‐D, moderately differentiated; P‐D: poorly differentiated; PNI, perineural invasion; W‐D, well‐differentiated.
Statistically significant.
3.5. Association of salivary MMP‐1 levels with survival
The Kaplan–Meier survival curve analysis revealed that the subgroup of patients with salivary MMP‐1 levels ≥2060.0 pg/mL had significantly worse survival probability for both OS and DSS compared to those with levels <2060.0 pg/mL, with respective five‐year survival rates of 56.0% versus 74.5% (p < .001) and 64.0% versus 79.9% (p < .001). (Figure 3A,B). Overall, the presence of high salivary MMP‐1 levels (≥2060.0 pg/mL) was a significant predictor of poorer OS and DSS by univariate analysis with the Cox proportional hazard model (p < .001 and p < .001, respectively) (Table 4 and Table S1). To confirm whether salivary MMP‐1 expression was an independent predictor of survival in OSCC patients, we conducted multivariate regression analysis using age, sex, overall pathological stage, ENE, surgical margin, DOI, cell differentiation, perineural invasion, lymphovascular invasion, CCI, adjuvant therapy status and salivary MMP‐1 level. The statistical results indicated that older age (≥65 years), presence of ENE, and higher levels of salivary MMP‐1 (≥2060.0 pg/mL) were independent predictive factors of OS (p = .007, p < .001, p = .041, respectively) (Table 4). In multivariate analysis, only older age (≥65 years) and the presence of ENE were independent prognostic factors for DSS (p = .002 and p < .001, respectively), and salivary MMP‐1 showed marginal significance for predicting DSS (p = .067, 95% CI: 0.974–2.094; Table S1).
FIGURE 3.
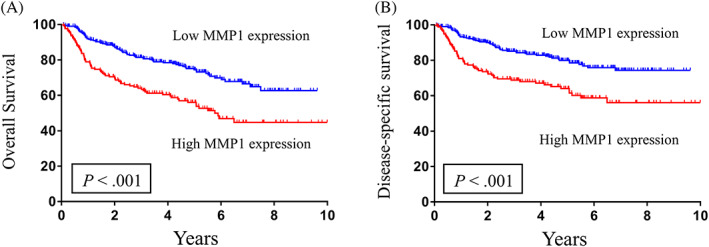
Kaplan–Meier survival curves demonstrate that the 5‐year (A) overall survival and (B) disease‐specific survival rates for patient subgroups stratified by MMP‐1 levels using 2060.0 pg/mL as the cutoff value were 56.0% versus 74.5% and 64.0% versus 79.9%, respectively (p < .001 and <.001). p values were calculated with the log‐rank test.
TABLE 4.
Univariate and multivariate analysis of poor prognostic factors for overall survival in 479 patients with oral cavity squamous cell carcinoma.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p‐value | HR (95% CI) | p‐value | |
| Age (years) | ||||
| <65 | Reference | Reference | ||
| ≥65 | 1.963 (1.359–2.835) | <.001 a | 1.952 (1.197–3.184) | .007 a |
| Gender | ||||
| Female | Reference | Reference | ||
| Male | 0.924 (0.524–1.632) | .785 | 0.986 (0.554–1.756) | .962 |
| Overall stage | ||||
| I–II | Reference | Reference | ||
| III–IV | 2.882 (2.009–4.134) | <.001 a | 1.488 (0.863–2.567) | .152 |
| ENE | ||||
| Absent | Reference | Reference | ||
| Present | 3.456 (2.495–4.789) | <.001 a | 2.195 (1.480–3.255) | <.001 a |
| Surgical margin | ||||
| <5 mm | Reference | Reference | ||
| ≥5 mm | 1.598 (1.160–2.200) | .004 a | 1.206 (0.861–1.690) | .275 |
| DOI | ||||
| <5 mm | Reference | Reference | ||
| ≥5 mm | 2.687 (1.679–4.300) | <.001 a | 1.542 (0.873–2.721) | .135 |
| Cell differentiation | ||||
| W‐D/M‐D | Reference | Reference | ||
| P‐D | 1.944 (1.248–3.028) | .003 a | 1.301 (0.809–2.094) | .277 |
| PNI | ||||
| Absent | Reference | Reference | ||
| Present | 2.292 (1.669–3.149) | <.001 a | 1.131 (0.766–1.671) | .536 |
| LVI | ||||
| Absent | Reference | Reference | ||
| Present | 2.775 (1.813–4.247) | <.001 a | 1.460 (0.916–2.327) | .111 |
| CCI | ||||
| 0, 1 | Reference | Reference | ||
| >1 | 1.436 (1.043–1.976) | .026 a | 1.152 (0.748–1.775) | .519 |
| Adjuvant therapy | ||||
| No | Reference | Reference | ||
| Yes | 2.625 (1.836–3.754) | <.001 a | 0.951 (0.547–1.653) | .859 |
| Salivary MMP‐1 | ||||
| <2060 pg/mL | Reference | Reference | ||
| ≥2060 pg/mL | 2.098 (1.531–2.875) | <.001 a | 1.421 (1.014–1.989) | .041 a |
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; ENE, extranodal extension; DOI, depth of invasion; HR, hazard ratio; LVI, lymphovascular invasion; M‐D, moderately differentiated; OS, overall survival; P‐D, poorly differentiated; PNI, perineural invasion; W‐D, well‐differentiated.
Statistically significant.
4. DISCUSSION
MMP‐1 is a collagenase that primarily breaks down collagen types I, II, and III. 20 MMP‐1 is involved in remodeling of the extracellular matrix via hydrolysis of several matrix proteins. The MMP family, which includes MMP‐1, plays a crucial role in the normal remodeling process the human body, such as wound healing, embryonic development and tissue inflammation. 21 The function of these factors is tightly regulated, from the level of gene expression to endogenous inhibition and zymogen conversion. 22 Expression of MMP‐1, at either the protein or mRNA level, is reported in various neoplasms, and upregulation of MMP‐1 has been associated with poor treatment outcomes in cancers, including nasopharyngeal carcinoma, 23 salivary gland cancers, 24 esophageal cancers, 25 and OSCC. 26 Several studies have also suggested that MMP‐1 is involved in several steps of cancer progression, including angiogenesis, tumor cell invasion and metastasis. 27 , 28
Over the past decade, our group has focused on the use of salivary biomarkers to detect OSCC and identification of their potential roles related to clinical parameters. In 2016, we identified elevated levels of MMP‐9, MMP‐3, and MMP‐1 in the saliva of OSCC patients, and further analysis supported that MMP‐1 was the most crucial biomarker for detecting OSCC. 9 We then further investigated the correlation between salivary MMP‐1 levels and OSCC progression, and our findings show that salivary MMP‐1 levels exhibit a significant association with the TNM stage of OSCC. 8 However, no further research was performed to elucidate the association of salivary MMP‐1 levels and other clinicopathological features of OSCC patients. The current study demonstrates a significant association between salivary MMP‐1 levels and various pathological features of OSCC patients, including pT classification, pN classification, overall pathological stage, ENE, tumor cell differentiation, lymphovascular invasion, perineural invasion, and tumor DOI. Univariate and Kaplan–Meier survival analyses indicated higher salivary MMP‐1 levels to be associated with lower OS and DFS. Moreover, multivariate analysis demonstrated that higher salivary MMP‐1 levels are an independent prognostic indicator of OS. The results from our analysis are consistent with upregulation of MMP‐1 in OSCC tissue and the extracellular matrix remodeling function of MMP‐1 in tumor progression and spread. To our knowledge, this is the first study to establish the prognostic value of salivary MMP‐1 with regard to treatment outcomes of OSCC patients.
Many previous studies have found that the status of comorbidity, nutrition and systemic inflammation are all linked to oncologic outcomes. 10 , 11 , 12 , 13 , 14 OSCC patients with a higher comorbidity burden, as measured by CCI, have worse survival outcomes, especially OS. 29 Consistent with previous research, analysis by our study group showed that CCI in OSCC patients has adequate discrimination ability for OS. In addition, nutritional status is related to OSCC prognosis, and malnourished patients become immunocompromised, with a particular decrease in cell‐mediated immunity. 30 Previous research has shown that serum albumin is an indicator of a patient's overall nutritional status, and low serum albumin is significantly related to decreased OSCC survival. 13 Compared with serum albumin and CCI status, the AUC of salivary MMP‐1 for OS prediction is significantly higher than these two known predictors, indicating that salivary MMP‐1 has sufficient discrimination ability for determining the OS of OSCC patients. Although TNM stage was not identified as an independent prognostic indicator in the current model's multivariate analysis, its impact on posttreatment outcomes could become more evident due to its highest AUC value on ROC curves.
Peripheral blood tests can also help to assess a patient's status of systemic inflammation and the level of biomarkers released by tumor cells. Indices developed from peripheral blood samples, including the NLR and PLR or SCCA levels, have all been reported as independent factors related to OSCC prognosis. 10 , 11 , 12 , 14 , 31 The AUC values of salivary MMP‐1 in our study were higher than or comparable to those of established blood markers, such as the NLR and PLR and SCCA. These findings highlight the potential usefulness of salivary MMP‐1 as a clinical biomarker for both detecting OSCC and predicting OS in OSCC patients. This adds value to the current use of saliva testing in the management of OSCC. Compared with other sampling techniques, such as direct tissue biopsy or peripheral blood tests, collection of saliva specimens is stress‐free and noninvasive. In addition, the ease of storage and transport and patient cooperation as well as the significantly reduced risk of procedure‐related infection are also known advantages of salivary testing. 32 Our study group's investigations into salivary tests suggest that salivary MMP‐1 could serve as a potential biomarker for detecting OSCC, and it may also be associated with the progression of OSCC. 8 , 9 Furthermore, salivary MMP‐1 may be used as an independent prognosticator for OS. These findings are beneficial to both patients and clinicians in this field, and routine examinations of salivary fluid may be considered in OSCC management.
As this study was conducted at a single institution, the generalizability of the findings to other cohorts may be limited. The results of multivariate analysis indicated that salivary MMP‐1 is an independent prognostic indicator for OS in OSCC patients, with marginal significance in predicting DSS. The limited sample size may be another potential reason for these results, and a larger study population may be necessary to detect a statistically significant difference regarding the prognostic role of salivary MMP‐1 regarding DSS. Despite these limitations, the study strongly demonstrates, for the first time, the prognostic value of salivary MMP‐1 levels for OSCC patients.
5. CONCLUSIONS
In summary, this study demonstrates a strong association between salivary MMP‐1 levels and various adverse and clinicopathological characteristics. Additionally, salivary MMP‐1 showed a significantly higher AUC than albumin and CCI and a higher or comparable AUC to the NLR, the PLR, and SCCA in predicting overall survival in OSCC patients. The study also indicates that patients with high levels of salivary MMP‐1 have poorer prognosis in terms of OS and DSS. Moreover, high salivary MMP‐1 was identified as an independent prognostic indicator of poor OS, emphasizing its potential value as a biomarker for OSCC patients. Overall, these findings may facilitate development of a noninvasive and user‐friendly salivary detection tool for OSCC treatment. Future longitudinal studies are needed to explore further utilities of salivary MMP‐1 in OSCC treatment.
AUTHOR CONTRIBUTIONS
Yi‐Chan Lee, Li‐Yu Lee, Yenlin Huang, and Huang‐Kai Kao: Write the manuscript and substantial contributions to the acquisition of data; giving final approval of the version to be published. Ya‐Ting Chang, Shao‐Yu Hung, and Chuieng‐Yi Lu: Analysis and interpretation of data; giving final approval of the version to be published. Yu‐Sun Chang, Jau‐Song Yu, and Kai‐Ping Chang: Substantial contributions to conception of the manuscript; revising the manuscript critically for important intellectual content; giving final approval of the version to be published.
FUNDING INFORMATION
This study was supported by a grant (MOST 111‐2314‐B‐182A‐078‐MY3) from the Ministry of Science and Technology and by grants (CMRPG3J1253 and CMRPG3M0101) from Chang Gung Memorial Hospital, Taiwan. The authors thank all the members of the Cancer Center at Chang Gung Memorial Hospital for their invaluable help.
CONFLICT OF INTEREST STATEMENT
None declared.
Supporting information
TABLE S1. Univariate and multivariate analysis of poor prognostic factors for disease‐specific survival in 479 patients with oral cavity squamous cell carcinoma.
ACKNOWLEDGMENTS
The authors thank all members of the Cancer Center, Chang Gung Memorial Hospital for their invaluable help. This study was supported by a grant (MOST 111‐2314‐B‐182A‐078‐MY3) from the Ministry of Science and Technology, Taiwan and by grants (CMRPG3J1253 and CMRPG3M0101) from Chang Gung Memorial Hospital, Taiwan.
Lee Y‐C, Lee L‐Y, Huang Y, et al. Comparison between a novel salivary marker and several clinical prognosticators in oral cavity cancer. Laryngoscope Investigative Otolaryngology. 2023;8(6):1547‐1556. doi: 10.1002/lio2.1166
Yi‐Chan Lee, Li‐Yu Lee, and Yenlin Huang contribute equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Mehanna H, Paleri V, West C, Nutting C. Head and neck cancer—part 1: epidemiology, presentation, and prevention. BMJ. 2010;341:c4684. [DOI] [PubMed] [Google Scholar]
- 2. Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Featherston T, Brasch HD, Siljee SD, et al. Cancer stem cells in head and neck cutaneous squamous cell carcinoma express cathepsins. Plast Reconstr Surg Glob Open. 2020;8(8):e3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin. 2015;24:491‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ong T, Murphy C, Smith A, Kanatas A, Mitchell D. Survival after surgery for oral cancer: a 30‐year experience. Br J Oral Maxillofac Surg. 2017;55:911‐916. [DOI] [PubMed] [Google Scholar]
- 6. Seoane‐Romero J‐M, Vázquez‐Mahía I, Seoane J, Varela‐Centelles P, Tomás I, López‐Cedrún J‐L. Factors related to late stage diagnosis of oral squamous cell carcinoma. Med Oral Patol Oral Cirug Bucal. 2012;17:e35‐e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saman Warnakulasuriya O. Global Oral Cancer Forum (Group 2). University of Buenos Aires; 2016. [Google Scholar]
- 8. Chang Y‐T, Chu LJ, Liu Y‐C, et al. Verification of saliva matrix metalloproteinase‐1 as a strong diagnostic marker of oral cavity cancer. Cancer. 2020;12:2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu J‐S, Chen Y‐T, Chiang W‐F, et al. Saliva protein biomarkers to detect oral squamous cell carcinoma in a high‐risk population in Taiwan. Proc Natl Acad Sci. 2016;113:11549‐11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen M‐F, Tsai M‐S, Chen W‐C, Chen P‐T. Predictive value of the pretreatment neutrophil‐to‐lymphocyte ratio in head and neck squamous cell carcinoma. J Clin Med. 2018;7:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kao H‐K, Löfstrand J, Loh CY‐Y, et al. Nomogram based on albumin and neutrophil‐to‐lymphocyte ratio for predicting the prognosis of patients with oral cavity squamous cell carcinoma. Sci Rep. 2018;8:13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Zheng L, Quan L, Du L. Prognostic role of platelet‐to‐lymphocyte ratio in oral cancer: a meta‐analysis. J Oral Pathol Med. 2021;50:274‐279. [DOI] [PubMed] [Google Scholar]
- 13. Bao X, Liu F, Lin J, et al. Nutritional assessment and prognosis of oral cancer patients: a large‐scale prospective study. BMC Cancer. 2020;20:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen I‐H, Liao C‐T, Wang H‐M, Huang J‐J, Kang C‐J, Huang S‐F. Using SCC antigen and CRP levels as prognostic biomarkers in recurrent oral cavity squamous cell carcinoma. PLoS One. 2014;9:e103265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population‐based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93‐99. [DOI] [PubMed] [Google Scholar]
- 16. Chen T‐Y, Hsin L‐J, Lin W‐N, Tsai M‐S, Tsai Y‐T, Lee Y‐C. LigaSure small jaw versus conventional neck dissection: a systematic review and meta‐analysis. J Otolaryngol Head Neck Surg. 2021;50:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee Y‐C, Hsin L‐J, Yang S‐W, Tsai M‐S, Tsai Y‐T, Ho C‐F. Endoscope‐assisted versus conventional neck dissection in patients with oral cancer: a systematic review and meta‐analysis. J Otolaryngol Head Neck Surg. 2022;51:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pfister DG, Spencer S, Adelstein D, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2020;18:873‐898. [DOI] [PubMed] [Google Scholar]
- 19. Robin X, Turck N, Hainard A, et al. pROC: an open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sunami E, Tsuno N, Osada T, et al. MMP‐1 Is a Prognostic Marker for Hematogenous Metastasis of Colorectal Cancer. Oxford University Press; 2000:108‐114. [DOI] [PubMed] [Google Scholar]
- 21. Lemaître V, D'Armiento J. Matrix metalloproteinases in development and disease. Birth Defects Res C Embryo Today. 2006;78:1‐10. [DOI] [PubMed] [Google Scholar]
- 22. Gaffney J, Solomonov I, Zehorai E, Sagi I. Multilevel regulation of matrix metalloproteinases in tissue homeostasis indicates their molecular specificity in vivo. Matrix Biol. 2015;44:191‐199. [DOI] [PubMed] [Google Scholar]
- 23. Yang R, Xu Y, Li P, et al. Combined upregulation of matrix metalloproteinase‐1 and proteinase‐activated receptor‐1 predicts unfavorable prognosis in human nasopharyngeal carcinoma. Onco Targets Ther. 2013;6:1139‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luukkaa H, Klemi P, Hirsimäki P, et al. Matrix metalloproteinase (MMP)‐1, ‐9 and ‐13 as prognostic factors in salivary gland cancer. Acta Otolaryngol. 2008;128:482‐490. [DOI] [PubMed] [Google Scholar]
- 25. Tao Y‐S, Ma X‐Y, Chai D‐M, et al. Overexpression of MMP‐1 and VEGF‐C is associated with a less favorable prognosis in esophageal squamous cell carcinoma. Onkologie. 2012;35:651‐656. [DOI] [PubMed] [Google Scholar]
- 26. Fan H‐X, Chen Y, Ni B‐X, et al. Expression of MMP‐1/PAR‐1 and patterns of invasion in oral squamous cell carcinoma as potential prognostic markers. Onco Targets Ther. 2015;8:1619‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pulukuri SMK, Rao JS. Matrix metalloproteinase‐1 promotes prostate tumor growth and metastasis. Int J Oncol. 2008;32:757‐765. [PMC free article] [PubMed] [Google Scholar]
- 28. Eck S, Hoopes P, Petrella B, Coon C, Brinckerhoff C. Matrix metalloproteinase‐1 promotes breast cancer angiogenesis and osteolysis in a novel in vivo model. Breast Cancer Res Treat. 2009;116:79‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peter F, Wittekindt C, Finkensieper M, Kiehntopf M, Guntinas‐Lichius O. Prognostic impact of pretherapeutic laboratory values in head and neck cancer patients. J Cancer Res Clin Oncol. 2013;139:171‐178. [DOI] [PubMed] [Google Scholar]
- 30. Alshadwi A, Nadershah M, Carlson ER, Young LS, Burke PA, Daley BJ. Nutritional considerations for head and neck cancer patients: a review of the literature. J Oral Maxillofac Surg. 2013;71:1853‐1860. [DOI] [PubMed] [Google Scholar]
- 31. Lin WH, Chen IH, Wei FC, et al. Clinical significance of preoperative squamous cell carcinoma antigen in oral‐cavity squamous cell carcinoma. Laryngoscope. 2011;121:971‐977. [DOI] [PubMed] [Google Scholar]
- 32. Li Q, Ouyang X, Chen J, Zhang P, Feng Y. A review on salivary proteomics for oral cancer screening. Curr Issues Mol Biol. 2020;37:47‐56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Univariate and multivariate analysis of poor prognostic factors for disease‐specific survival in 479 patients with oral cavity squamous cell carcinoma.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


