Abstract
Background
Cutaneous angiosarcoma is an aggressive tumor commonly found in the head and neck region. There is no consensus regarding the definitive treatment for angiosarcoma.
Methods
This was a retrospective chart review that evaluated 64 patients from 1983 to 2019. Demographic and clinical variables were examined for impact on recurrence using the time to recurrence and the overall survival in Kaplan–Meier curves.
Results
Average age at diagnosis was 71 (32–95) years, with a 2.8 male: female ratio. Surgery was utilized in 62% of patients, with mean defect size of 11.4 ± 8.1 cm. Recurrence was found in 70% of patients, and mean time to recurrence was 15.3 ± 12.3 months. Decreased recurrence was associated with use of intraoperative frozen section analysis (p = .036) and negative margins (p = .086). Two‐year overall survival was 80%, and recurrence free survival was 30%.
Conclusions
Negative margins are associated with decreased recurrence, and intraoperative frozen section analysis may be considered to obtain preliminary surgical margins.
Level of Evidence: 4.
Keywords: angiosarcoma, cutaneous malignancy, scalp, skin cancer, soft tissue sarcoma
This manuscript serves to outline outcomes of patients with cutaneous angiosarcoma of the head and neck at our institution. We used Kaplan–Meier curves to analyze time to recurrence as cutaneous angiosarcoma is hallmarked by high rates of local recurrence, and this benchmark is not well studied. We found that negative margins were associated with decreased recurrence, and intraoperative frozen sections may be utilized to obtain negative surgical margins.

1. INTRODUCTION
Cutaneous angiosarcoma (CAS) is a rare, highly aggressive tumor arising from blood or lymphatic vessels. These soft tissue tumors are primarily found in the head and neck region and make up 1% of all soft tissue sarcomas. 1 CAS is primarily found in elderly males and commonly presents as a bruise‐like lesion with indistinct borders on the face or scalp. These lesions are typically present for months before a definitive diagnosis is made. 2 Immunohistochemistry aids in the diagnosis of angiosarcoma, via detection of erythroblast transformation specific related gene (ERG) and associated CD31 and CD24. 3 CAS is associated with lymphedema and radiation and toxin exposure; this malignancy portends a high local recurrence and poor survival rates. 4 , 5 , 6
Recent studies have identified several negative factors that can be used to prognosticate patients diagnosed with CAS; these include increasing age, large tumor size, and positive margin status. 7 , 8 Other studies have identified other risk factors such as male sex, cardiovascular disease, a history of smoking and distant metastases as poor prognostic indicators associated with decreased survival rates. 9 Additionally, it has been shown that younger patients are more likely to have resectable tumors and respond to multimodal treatment, thereby having a more favorable prognoses than their older counterparts. 10 , 11
Despite ongoing research, no consensus has been made as to the definitive treatment for angiosarcoma. Although multiple studies suggest that surgical intervention with wide margins is the most effective treatment, 12 , 13 others advocate for a multimodal approach that combines surgical resection in addition to chemotherapy and/or radiotherapy. 8 , 11 , 14 , 15 , 16 , 17 , 18 , 19 Newer literature has also emerged advocating for the use of immunotherapy. 12 Although these articles have identified radiation and chemotherapy as effective adjuvant treatments to CAS, the data is conflicting as other reports exist that found no statistically significant increase in survival rates. 7 , 20 Due to the rarity and poor prognosis associated with CAS, these intervention recommendations are based on studies with small patient populations and no clear consensus has been made delineating the best approach for treatment of CAS.
The current study aims to detail the UCLA experience of patients with cutaneous angiosarcoma of the head and neck, with a novel approach of examining locoregional recurrence in terms of time to recurrence.
2. METHODS
This retrospective review was approved by the University of California, Los Angeles (UCLA) Institutional Review Board. Records of patients diagnosed with angiosarcoma of the head and neck who were treated from January 1983 to December 2019 were identified by a computer‐assisted search performed by the UCLA Tumor Registry. Identified patients were stratified into cutaneous and non‐cutaneous angiosarcoma of the head and neck. A total of 64 patients were then identified as having cutaneous angiosarcoma of the head and neck region. Six patients held diagnoses of non‐cutaneous angiosarcoma of the head and neck and were excluded from analysis.
Demographic information and clinical variables were collected using the electronic medical record, including age, sex, ethnicity, site of primary tumor, presence of multifocal or satellite lesions, nodular versus diffuse presentation, and presenting symptoms and misdiagnoses. Additional variables collected included prior history of radiation, lymphedema, or environmental toxins, time from lesion occurrence to diagnosis, time from diagnosis to treatment, immunohistochemistry, pathological grade, tumor size, AJCC tumor stage, metastasis status, comorbidities, previous cancer diagnoses, treatment modalities, surgical margin status, defect size, reconstruction method, death, follow‐up time, recurrence, time to recurrence, type of surgeon, and use of intraoperative frozen section analysis (IFSA).
Variables were examined for impact on recurrence and overall survival. Age was categorized into greater than or less than 70 years old, based on the mean age. Categorization of nodular versus diffuse nature of the lesion was based on chart descriptors of the lesion, or clinical photos when available. Multifocal nature was categorized based on multiple lesions arising from separate distinct areas, whereas satellite lesions referred to surrounding lesions near the primary tumor site. Time from lesion occurrence to diagnosis was calculated as the time from identification of the lesion to time of pathologic diagnosis. Time from diagnosis to treatment spanned from the pathologic diagnosis date to the first chemotherapy visit, initiation of radiation, or surgery, whichever occurred first. Surgical defect size was recorded as the largest dimension of defect, and operative reports were utilized for details on reconstructive strategies.
Demographic information was compiled, and all information was analyzed via counts, mean, range, and variance. Of all included patients, 30 had very few to no missing clinical variables and were analyzed for statistical significance. Chi‐square analyses were conducted to assess above variables and association with scalp subsite, use of surgery as treatment for disease, and recurrence. Kaplan–Meier curves were created using the variable time to recurrence, in which the outcome of interest was locoregional CAS recurrence. Time to recurrence was calculated in months, from the conclusion of treatment, until the first clinical, radiographic, or pathologic sign of recurrence. Overall survival, using death as the outcome of interest, via Kaplan–Meier was also generated from the data. Significance was set at alpha of .05. All statistical analysis was performed using SPSS Version 26 (Armonk, New York).
3. RESULTS
3.1. Patient characteristics
The average age at diagnosis was 71 years (range, 32–95), with a 2.8 male to female ratio. Of all patients, 70% self‐identified as White. A previous diagnosis of skin cancer was noted in 35% (9/26) among those with adequate history documented. History of prior radiation was present in 8% (2/26) and history of lymphedema in 4% (1/26) of patients. No cases noted chemical exposure prior to malignancy development.
Of all patients, 38% (10/26) were staged as AJCC stage IV; additional tumor stage and grade among the cohort are presented in Table 1. Average tumor size was 3.9 ± 3.1 cm. In 64% (21/33) of cases, the lesions were nodular and multifocal in 57% (21/37). Satellite lesions were noted on presentation in 65% (24/37). Lymph node metastases were present in 41% (12/26) of patients. Pathology specimens displayed positivity for the following markers: CD31 in 97% (29/30), CD34 in 93% (13/14), and ERG in 100% (4/4). Immunohistochemistry was only conducted on samples after 2002.
TABLE 1.
Demographic and clinical characteristics of patients with cutaneous angiosarcoma.
| Category | Characteristic | N (%) |
|---|---|---|
| Sex | Male | 47 (74.6) |
| Female | 16 (25.3) | |
| Age | ≥70 years | 40 (63.4) |
| <70 years | 23 (36.5) | |
| Self‐reported race | White | 26 (70.3) |
| Latino | 2 (5.4) | |
| Asian | 5 (13.5) | |
| Black | 2 (5.4) | |
| Other | 2 (5.4) | |
| Location | Scalp | 34 (53.9) |
| Nose | 12 (19) | |
| Cheek | 7 (11.1) | |
| Neck | 4 (6.3) | |
| Temple | 4 (6.3) | |
| Forehead | 2 (3.2) | |
| Pathologic grade | Low‐grade | 11 (26.8) |
| Intermediate‐grade | 3 (7.3) | |
| High‐grade | 27 (65.89 | |
| AJCC tumor stage | Stage I | 9 (37.5) |
| Stage II | 1 (4.2) | |
| Stage III | 4 (16.7) | |
| Stage IV | 10 (41.7) | |
| Treatment | Surgery (S) | 5 (15.6) |
| Chemotherapy (C) | 6 (18.8) | |
| Radiotherapy (RT) | 0 (0) | |
| S + C | 3 (18.8) | |
| S + RT | 1 (3.1) | |
| RT + C | 6 (18.8) | |
| S + RT + C | 11 (34.3) | |
| Ablative surgeon type | Surgical oncology | 10 (55.6) |
| Head and neck surgery | 7 (38.9) | |
| Plastic surgery | 1 (3.1) |
Note: Characteristics are shown of the available data for the patients.
Nine of the 35 patients with available presenting symptoms were initially misdiagnosed with another disorder before angiosarcoma was confirmed, including rosacea, nevi, allergic reaction, trauma, and epidermal inclusion cyst. Average time from lesion appearance to pathologic diagnosis was 4.6 months (median 3, SD 4.8), spanning anywhere from 1 to 21 months. Eighty percent (19/23) of patients began treatment in 1 month or less of pathologic diagnosis, with the remainder beginning treatment between 1 and 2 months after diagnosis.
Scalp subsite accounted for the majority of cases (54%, 34/64), whereas 19% were located on the nose, 11% on the cheek, 6% each on the neck and temporal region, and 3% on the forehead. Scalp subsite was found to be associated with age less than 70 years old (X 2 = 6.451, p = .011) when compared to non‐scalp head and neck subsites, but was not found to be associated with diffuse nature (p = .071), multifocality (p = .089), distant metastasis (p = .134), stage (p = .239), positive margins (p = .074), radiotherapy (p = .696), surgery (p = .484), recurrence (p = .643), grade (p = 1.00), or mortality (p = .511).
3.2. Treatment and reconstruction
The most common treatment regimen included triple modality treatment of surgery with chemotherapy and radiotherapy, which occurred in 32% (10/31) of patients. Ablative surgery was most often performed by surgical oncology, followed by head and neck surgery (Table 1). Surgery was pursued in 62% (20/32) of patients and was more often utilized for treatment of nodular cutaneous lesions when compared to diffuse lesions, (X 2 = 5.511, p = .024). There were no significant findings when examining multifocality (p = .249), satellite lesion presence (p = .149), age (p = .671), pathologic grade (p = .919), or stage (p = .715) with patients who received surgical therapy.
IFSA was performed in 62% (8/13) of surgical resections. Positive permanent section margins were present in 54% (14/26) cases. One case occurred where initial negative IFSA resulted in positive margins on permanent pathology. Defect size after surgical removal ranged from the largest dimension of 2 to 30 cm, with an average of 11.4 ± 8.1 cm. Surgical closure was most often pursued using a split thickness skin graft alone, followed by local advancement flaps, then free flaps (Table 2).
TABLE 2.
Closure type and reconstruction strategies after ablative surgery for angiosarcoma in the head and neck.
| Closure type | Location | Defect size (cm) | Flap type |
|---|---|---|---|
| Primary closure | Neck | NR | – |
| Secondary intention | Scalp, forehead | 6 | – |
| STSG alone | Parietal scalp | 5 | – |
| Parietal scalp | 12 | – | |
| Nose | NR | – | |
| Frontal scalp | 4.8 | – | |
| Frontal scalp | 6 | – | |
| Parietal scalp | 10 | – | |
| FTSG alone | Parietal scalp | 5 | – |
| Local flap | Nasal dorsum | 2 | Glabellar and frontal advancement flaps |
| Cheek | 13 | Advancement flaps | |
| Frontal scalp | 5 | Circumferential advancement/rotational flaps | |
| Scalp | 9 | Scalp flap based on superficial temporal and occipital arteries + STSG | |
| Regional flap | Occipital scalp | 13 | Lower island trapezius flap |
| Free flap | Scalp | 25 | Latissimus dorsi and serratus anterior free flap + STSG |
| Temporal | 30 | Radial forearm free flap | |
| Temporal | 12 | Radial forearm free flap |
Abbreviations: −, not applicable; FTSF, full thickness skin graft; NR, data was not recorded in the medical record; STSG, split thickness skin graft.
3.3. Outcomes
Mean follow‐up time was 13.8 ± 11.9 months. Only 10 patients had sufficient follow‐up time to determine 5‐year overall survival (OS) and recurrence free survival (RFS). Five‐year OS was 60%, and 5‐year RFS was 30%. Two‐year OS was 80%, and 2‐year RFS was 30%. Angiosarcoma locoregional recurrence was found overall in 70% (19/27). Among those who had IFSA utilized, 50% (4/8) experienced locoregional recurrence overall. Dichotomous locoregional recurrence as calculated via chi‐square analysis was increased with multifocal presentation (X 2 = 5.753, p = .056), higher AJCC stage (X 2 = 12.42, p = .006), use of radiotherapy treatment (X 2 = 4.63, p = .031), and distant metastasis (Fisher's exact = 0.079, p = .040). Dichotomous recurrence as calculated by chi‐square analysis was not associated with satellite lesions (p = .102), surgery (p = .484), grade (p = .477), age (p = .389) or margin (p = .551). Average time to recurrence was 15.3 ± 12.3 months.
Kaplan–Meier analysis was used to investigate time to recurrence; the use of IFSA (X 2 = 4.398, p = .036) and negative margins (X 2 = 2.952, p = .086) was associated with decreased recurrence (Figures 1 and 2). No other factors were significant in decreasing recurrence in this Kaplan–Meier analysis. Distant metastasis occurred in 10 patients (37%) and was the cause of death in four patients. Kaplan–Meier survival analysis found an association between the presence of distant metastases and decreased survival (X 2 = 5.208, p = .022).
FIGURE 1.
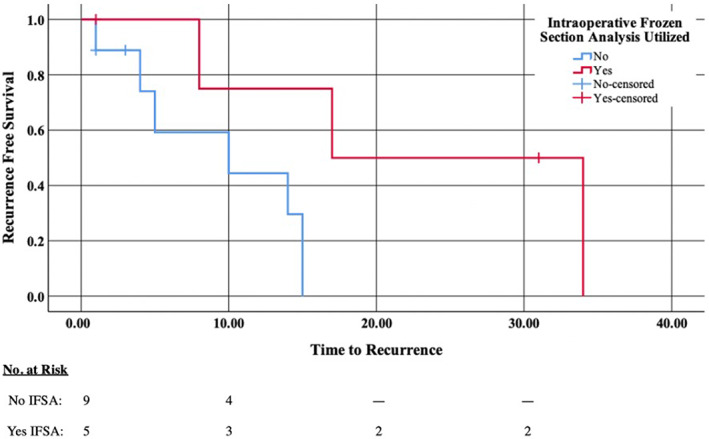
Kaplan–Meier of time to recurrence (in months) and whether intraoperative frozen section analysis (IFSA) was utilized in the operating room during resection. Decreased recurrence was associated with use of IFSA (X 2 = 4.398, p = .036). No for no IFSA performed, Yes for yes IFSA performed, No‐censored for no IFSA performed though patient was lost to follow‐up at this timepoint, and Yes‐censored for yes IFSA performed though patient was lost to follow‐up at this timepoint.
FIGURE 2.
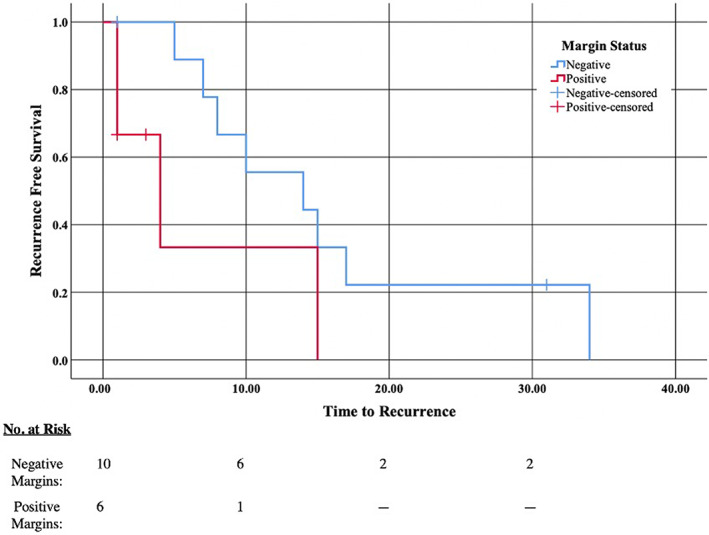
Kaplan–Meier analysis of time to recurrence (in months) and negative pathologic margins. There was a decreased recurrence associated with negative margins (X 2 = 2.952, p = .086). Negative for negative final pathologic margins, Positive for positive final pathologic margins, Negative‐censored for negative final pathologic margins though patient was lost to follow‐up at this timepoint, and Positive‐censored for positive final pathologic margins though patient was lost to follow‐up at this timepoint.
4. DISCUSSION
Despite ongoing research, the standard of care for CAS of the head and neck region remains debated, due to data limited to small retrospective studies and case reports. Definitive diagnosis can be challenging and may lead to misdiagnosis or delay in treatment. This study demonstrated a delay in diagnosis of 4.6 months from initial presentation to diagnosis, similar to Pawlik et al.'s study demonstrating a delay of 5.1 months. 21 A diagnosis of CAS carries with it a high local recurrence rate, high propensity for distant metastasis, and poor survival rates. These devastating prognostic factors underscore the need for an effective standard of care treatment that will increase survival and decrease local recurrence in this patient population.
Although there is debate surrounding surgical excision with wide margins alone or in conjunction with other treatment modalities, it has been shown that a surgical excision with negative margins is an important prognostic factor for CAS. 7 , 11 The use of IFSA is a method utilized in multiple surgical specialties that aims to decrease the number of positive margins associated with resection procedures. This method has been shown to increase the number of negative margins in various cancer removal surgeries, including breast lumpectomies and laparoscopic prostatectomies. 22 , 23 It is also the premise for Mohs micrographic surgery. 24 One study has explored the use of this technique to ensure negative margins during the initial surgical resection of a cutaneous angiosarcoma, identifying this approach to have a positive predictive value of 100%, but a negative predictive value of 33.3%. 21 These conclusions were made based on a study population of 28 patients.
IFSA can help identify positive margins and assist with a thorough resection at the time of initial surgery. A study by Kohler et al. in 2008 reported that surgical resection with IFSA resulted in a complete resection in all patients. 25 However, the use of IFSA in cutaneous head and neck angiosarcoma is heavily debated as other studies report unreliability in determining the adequacy of the surgical resection due to the high false‐negative rate. 26 , 27 , 28 Another study by Pawlick et al. reported IFSA sensitivity to be 64.7% with a positive predictive value of 100%, however the negative predictive value was 33.3% as nine patients in the study had negative IFSA margins and six (67%) went on to later have positive margins on permanent sections. Our experience is similarly conflicted, as we found time to recurrence and subsequent recurrence rates to be decreased with the utilization of IFSA and negative margins, whereas also identifying one case where initial negative IFSA resulted in positive margins on permanent pathology. 21 Due to this contrast, larger studies are needed to determine the effectiveness of IFSA in CAS of the head and neck.
Much of the literature surrounding CAS investigates survival outcomes using Kaplan–Meier curves to analyze time to death; this study rather analyzed time to recurrence, which may serve as an important prognostic factor that has not been well studied in the CAS literature. As this malignancy is one hallmarked by high rates of local recurrence, 11 this factor is one that warrants further investigation. In the current study, recurrence was found in 70% of patients, with decreased time to recurrence associated with use of IFSA and negative margins. A study by Pawlik et al. from 2003 analyzed survival and time to recurrence, and noted the burden and distribution of disease at presentation decreased time to recurrence. 21 No other studies, to our knowledge, analyzed time to recurrence in this way.
The current study's 5‐year survival rates are higher than other published studies due to skewed data from lack of 5 year follow‐up by patients and resultant small sample size; however, the 2‐year recurrence free survival was comparable to other recurrence rates at 30%. 11
Due to our small sample size, with 4 patients (6.25%) expiring due to complications of metastatic disease, significant associations with survival were generally difficult to detect. Due to this small subset of death among participants and resulting low power, a larger sample size is needed to detect significant correlations with the survival outcome.
Although absolute guidelines for surgical resection do not exist, it is generally accepted that resection should be completed with wide margins. This can be problematic in certain patient groups, including the elderly or in patients with diffuse cutaneous disease. In these patients, a multimodal approach is necessary with adjuvant radiation and chemotherapy. Outcomes such as recurrence and mortality in this study did not differ based on whether surgical resection was undertaken. Oashi et al. investigated complete surgical resection with curative intent and found complete removal of the tumor load to be an optimal treatment option; they noted total resection to enhance the efficacy of radiotherapy and chemotherapy, even in patients with primary lesions larger than 5 cm and regardless of the histological surgical margin status. 28 Risk factors for recurrence and optimal treatment strategy based on size warrant further investigation.
Secondary to its rarity, data is limited on reconstructive strategies following CAS resection. In general, current strategies are similar to those of other cutaneous malignancies, with scalp reconstructive options mirroring similar reconstructive options as presented above and including biosynthetic matrixes such as Integra when necessary. 29
Although this study presents additional information on CAS that is valuable to the larger picture of CAS, it is not without limitations. As this study is retrospective in nature, it is inherently subject to bias and not all patients have complete data sets that were able to be analyzed. Additionally, this study spans a large time frame of 36 years, which may introduce selection bias due to heterogeneous treatment among patients as progression and advancement in treatment modalities changed over time. Finally, although this work adds patients to the already small number of patients in the literature, the small sample size of 63 patients is small and may limit the study's generalizability. Future studies are needed that investigate prospective case series to better analyze best treatment and outcomes for cutaneous angiosarcoma.
Cutaneous angiosarcoma in the head and neck region is an aggressive cutaneous tumor that is locally invasive and has a high predilection for distant metastases. The most effective treatment method for this tumor is debated and warrants further investigation. Using a robust patient population, this study demonstrates the importance of obtaining negative margins on the preliminary excision of an angiosarcoma. Given the success of this technique in various other cancer resection procedures, we propose that IFSA should be used intraoperatively to ensure negative margins are obtained. Further studies should focus on this proposed recommendation in conjunction with other treatments of CAS including chemotherapy, radiation therapy and immunotherapy.
5. CONCLUSION
Cutaneous angiosarcoma of the head and neck can present as aggressive, diffuse tumors locally, with a propensity for distant metastases. Negative margins are associated with decreased recurrence, and IFSA may be considered to obtain preliminary surgical margins.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Evans LK, Sutton S, Echanique K, et al. Cutaneous head and neck angiosarcoma: The 30‐year UCLA experience. Laryngoscope Investigative Otolaryngology. 2023;8(6):1557‐1563. doi: 10.1002/lio2.1173
Results of this project were previously presented by the author group at the American Academy of Otolaryngology Annual Meeting on October 5, 2021.
REFERENCES
- 1. Wanebo HJ, Koness RJ, MacFarlane JK, et al. Head and neck sarcoma: report of the Head and Neck Sarcoma Registry. Society of Head and Neck Surgeons Committee on research. Head Neck. 1992;14(1):1‐7. doi: 10.1002/hed.2880140102 [DOI] [PubMed] [Google Scholar]
- 2. Lydiatt WM, Shaha AR, Shah JP. Angiosarcoma of the head and neck. Am J Surg. 1994;168(5):451‐454. doi: 10.1016/s0002-9610(05)80097-2 [DOI] [PubMed] [Google Scholar]
- 3. Sullivan HC, Edgar MA, Cohen C, Kovach CK, HooKim K, Reid MD. The utility of ERG, CD31 and CD34 in the cytological diagnosis of angiosarcoma: an analysis of 25 cases. J Clin Pathol. 2015;68(1):44‐50. doi: 10.1136/jclinpath-2014-202629 [DOI] [PubMed] [Google Scholar]
- 4. Conic RRZ, Damiani G, Frigerio A, et al. Incidence and outcomes of cutaneous angiosarcoma: a SEER population‐based study. J Am Acad Dermatol. 2020;83(3):809‐816. doi: 10.1016/j.jaad.2019.07.024 [DOI] [PubMed] [Google Scholar]
- 5. Choi JH, Ahn KC, Chang H, Minn KW, Jin US, Kim BJ. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:e321896. doi: 10.1155/2015/321896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moon IJ, Kim YJ, Won CH, et al. Clinicopathological and survival analyses of primary cutaneous angiosarcoma in an Asian population: prognostic value of the clinical features of skin lesions. Int J Dermatol. 2020;59(5):582‐589. doi: 10.1111/ijd.14828 [DOI] [PubMed] [Google Scholar]
- 7. Shin JY, Roh SG, Lee NH, Yang KM. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta‐analysis. Head Neck. 2017;39(2):380‐386. doi: 10.1002/hed.24554 [DOI] [PubMed] [Google Scholar]
- 8. Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27(4):A3‐A8. doi: 10.1016/j.suronc.2018.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee BL, Chen CF, Chen PCH, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 Suppl 2):S41‐S46. doi: 10.1097/SAP.0000000000001004 [DOI] [PubMed] [Google Scholar]
- 10. Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85(1019):e1127‐e1133. doi: 10.1259/bjr/31655219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141(4):335‐340. doi: 10.1001/jamaoto.2014.3584 [DOI] [PubMed] [Google Scholar]
- 12. Fujisawa Y, Yoshino K, Fujimura T, et al. Cutaneous angiosarcoma: the possibility of new treatment options especially for patients with large primary tumor. Front Oncol. 2018;8:46. doi: 10.3389/fonc.2018.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim SY, Pyon JK, Mun GH, Bang SI, Oh KS. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64(2):180‐182. doi: 10.1097/SAP.0b013e3181a2c5e6 [DOI] [PubMed] [Google Scholar]
- 14. Eitan T, Damico NJ, Pidikiti R, et al. Reirradiation for recurrent scalp angiosarcoma: dosimetric advantage of PBT over VMAT and EBT. Int J Part Ther. 2020;6(3):13‐18. doi: 10.14338/IJPT-19-00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hata M. Radiation therapy for angiosarcoma of the scalp: total scalp irradiation and local irradiation. Anticancer Res. 2018;38(3):1247‐1253. doi: 10.21873/anticanres.12346 [DOI] [PubMed] [Google Scholar]
- 16. Ihara H, Kaji T, Katsui K, et al. Single institutional experience of radiation therapy for angiosarcoma of the scalp without cervical lymph node metastases: impact of concurrent chemoradiation with maintenance chemotherapy using taxanes on patient prognosis. Mol Clin Oncol. 2019;11(5):498‐504. doi: 10.3892/mco.2019.1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ko S. Evolving radiation therapy techniques for scalp angiosarcoma. Surg Oncol. 2018;27(4):A1‐A2. doi: 10.1016/j.suronc.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 18. Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83(1):55‐62. doi: 10.1097/SAP.0000000000001865 [DOI] [PubMed] [Google Scholar]
- 19. Guadagnolo BA, Zagars GK, Araujo D, Ravi V, Shellenberger TD, Sturgis EM. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33(5):661‐667. doi: 10.1002/hed.21513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jasper KD, Holloway CL, DeVries KJ, Truong PT. Local relapse and survival outcomes in patients with scalp sarcoma: a retrospective study of 95 patients treated in a provincial cancer care institution over 25 years. Cureus. 2019;11(7):e5236. doi: 10.7759/cureus.5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98(8):1716‐1726. doi: 10.1002/cncr.11667 [DOI] [PubMed] [Google Scholar]
- 22. Bulbul MG, Zenga J, Tarabichi O, et al. Margin practices in oral cavity cancer resections: survey of American Head and Neck Society members. Laryngoscope. 2021;131(4):782‐787. doi: 10.1002/lary.28976 [DOI] [PubMed] [Google Scholar]
- 23. Choi SY, Chi BH, Kim TH, et al. Does intraoperative frozen section really predict significant positive surgical margins after robot‐assisted laparoscopic prostatectomy? A retrospective study. Asian J Androl. 2021;23(1):74‐79. doi: 10.4103/aja.aja_16_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mulvaney PM, Piris A, Besaw RJ, Schmults CD. Diagnostic biopsy via in‐office frozen sections for clinical nonmelanoma skin cancer. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2021;47(2):194‐199. doi: 10.1097/DSS.0000000000002473 [DOI] [PubMed] [Google Scholar]
- 25. Köhler HF, Neves RI, Brechtbühl ER, Mattos Granja NV, Ikeda MK, Kowalski LP. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Neck Surg. 2008;139(4):519‐524. doi: 10.1016/j.otohns.2008.07.022 [DOI] [PubMed] [Google Scholar]
- 26. Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84(1):55‐59. doi: 10.1097/00006534-198907000-00011 [DOI] [PubMed] [Google Scholar]
- 27. Kang MG, Park JL, Kim MG, Minn KW, Koh KS, Chang H. Surgical treatment of cutaneous angiosarcoma of scalp: usefulness of preoperative mapping biopsies. Korean J Head Neck Oncol. 2007;23(1):37‐40. [Google Scholar]
- 28. Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2018;44(6):823‐829. doi: 10.1016/j.ejso.2018.02.246 [DOI] [PubMed] [Google Scholar]
- 29. Johnson MB, Wong AK. Integra‐based reconstruction of large scalp wounds: a case report and systematic review of the literature. Plast Reconstr Surg Glob Open. 2016;4(10):e1074. doi: 10.1097/GOX.0000000000001074 [DOI] [PMC free article] [PubMed] [Google Scholar]


