Abstract
Background
Exercise‐based swallowing training (EBST) and transcutaneous neuromuscular electrical stimulation (TNMES) are common modalities used to treat late dysphagia after radiotherapy for nasopharyngeal carcinoma (NPC). We aimed to investigate and compare the efficacies of EBST and TNMES as proactive treatments administered early after radiotherapy.
Methods
Patients with early post‐radiotherapy NPC (n = 120) underwent either TNMES or EBST. Flexible endoscopic evaluation of swallowing (FEES), quality of life (QOL), and swallowing function questionnaires were completed before the intervention as well as immediately, 6, and 12 months after the intervention. Outcome measures included the scores for the swallowing function score (SFS), penetration and aspiration scale (PAS), dynamic imaging grade of swallowing toxicity (DIGEST), functional oral intake scale (FOIS), swallowing performance status scale (SPSS), pharyngeal motor impairment (PMI), pharyngeal function impairment (PFI), and functional assessment after cancer therapy–nasopharyngeal (FACT‐NP) questionnaire.
Results
Three months after radiotherapy, 31 and 34 patients underwent TNMES and EBST, respectively, and completed swallowing assessments at all four assessment timepoints. All patients showed post‐radiotherapy impairments in the SFS, PAS, DIGEST, PMI, and PFI. Compared with the EBST group, the TNMES group showed significant improvements in the PFI and PMI scores, with small‐to‐medium effect sizes. Additionally, compared with the EBST group, the TNMES group demonstrated a trend toward slightly better improvements in the PAS, DIGEST, FOIS, and SPSS scores immediately and 6 months after the intervention. The SFS scores improved from baseline in both groups; however, the TNMES group showed an earlier improvement. Finally, the TNMES group showed better QOL according to the FACT‐NP than the EBST group.
Conclusion
Proactive TMNES and EBST are safe and feasible modalities for improving swallowing in patients with NPC when administered early after radiotherapy. Although TNMES showed better results than EBST, these results should be interpreted with caution given the study limitations.
Level of evidence
1B.
Keywords: exercise‐based swallowing training, nasopharyngeal carcinoma, neuromuscular electrical stimulation, proactive swallowing training, quality of life, radiotherapy
This study investigated the outcomes of proactive swallowing training by electric neuromuscular stimulation and swallowing exercise in early post irradiated patients with nasopharyngeal carcinoma and results shows neuromuscular stimulation and swallowing exercise are feasible to improve swallowing in 12 months follow up assessment. Electrical neuromuscular stimulation is better than swallowing exercise in some aspect of swallowing function.

1. INTRODUCTION
Nasopharyngeal carcinoma (NPC) is endemic in south‐eastern Asia, which is the region with the highest incidence worldwide. 1 Over the past decade, advances in radiotherapy and chemotherapy have improved disease control and survival rates. 2 , 3 Since 60%–90% of patients with NPC have cervical lymph node metastases at initial presentation, 4 , 5 radiotherapy fields usually cover the nasopharynx and bilateral neck from the skull‐base to the clavicles in order to achieve improved long‐term local and regional disease control. 6 , 7 Radiotherapy may cause cranial neuropathies in the pharynx and larynx that affect sensory and motor function 8 , 9 ; fibrosis of soft tissues and muscles that are crucial for swallowing 10 ; soft tissue swelling resulting from chronic lymphedema 11 ; and osteoradionecrosis of the skull base. 12 , 13 These adverse effects may lead to dysphagia and aspiration over time. We previously reported that 22% and 50% of patients experienced aspiration at 5 and 7 years after external beam radiotherapy, respectively. 14 , 15
The introduction of intensity‐modulated radiotherapy (IMRT) for treating NPC, which relatively spares swallowing‐related organs, has reduced the incidence of post‐radiotherapy dysphagia. 16 , 17 However, it only delays the onset of some symptoms rather than prevent all post‐radiotherapy complications. 18 Accordingly, a sizable proportion of long‐term NPC survivors who are treated with radiotherapy or chemoradiotherapy may still experience different severities of dysphagia, which affect their quality of life (QOL). 19 , 20
Transcutaneous neuromuscular electrical stimulation (TNMES) is used to treat dysphagia of different etiologies. 21 It involves application of a low‐voltage current to the neck skin via two bipolar surface electrodes, which triggers the contraction of target muscles to increase their strength and prevent muscle fibrosis. 22 , 23 This technique is administered as an adjunct to traditional exercise‐based swallowing training (EBST) to enhance its efficacy in improving swallowing function. Bhatt et al. 24 and Ryu et al. 25 reported that there were advantages of administering both TNMES and EBST in patients with dysphagia after radiotherapy for head and neck cancer. However, Langmore et al. 26 reported no advantages of EBST alone or in combination with TNMES in these patients.
There remains limited evidence regarding the efficacy of TNMES alone or in combination with the operating procedure such as esophageal dilatation for dysphagia in patients treated with radiotherapy for NPC or other head and neck cancers. A small case series on the use of TNMES with esophageal balloon dilatation in patients with dysphagia after radiotherapy for NPC demonstrated post‐intervention improvements in the pharyngeal transit time, laryngeal excursion, and laryngeal inlet closure. 27 Further, Lin et al. reported that TNMES alone was better than EBST in treating dysphagia in patients with NPC following radiotherapy. 28 These preliminary data suggest that TNMES can be used without EBST to treat dysphagia after radiotherapy, which may enhance adherence by eliminating the need for round‐the‐clock home‐based swallowing exercises. 29 , 30
Prolonged disuse of swallowing muscles can cause irreversible muscular atrophy, which can result in chronic dysphagia with poor response to swallowing rehabiltation. 31 Moreover, proactive treatment with EBST before or during radiotherapy for head and neck cancer has yielded encouraging outcomes, with a reduction in the post‐radiotherapy severity of dysphagia and tube feeding dependence. 32 , 33 , 34 , 35 , 36 However, there is limited evidence regarding the potential benefits of proactive swallowing training, as well as the efficacy of EBST or TNMES alone, in patients treated with radiotherapy or chemoradiotherapy for NPC. Accordingly, we aimed to investigate and compare the efficacies of EBST and TNMES as proactive treatments administered early after radiotherapy. We hypothesized that early proactive swallowing training with TNMES or EBST alone would yield different outcomes in terms of swallowing function and QOL in patients with NPC treated using radiotherapy.
2. MATERIALS AND METHODS
2.1. Patient recruitment, randomization of intervention training, and swallowing evaluation
This prospective, randomized, controlled study was conducted at the Prince of Wales Hospital (PWH) and Queen Elizabeth Hospital, which are tertiary referral centers for otolaryngology and head and neck surgery in Hong Kong. This study was approved by the Research Ethics Committee of the New Territories East Hospital Cluster (CRE‐2010.223‐T) and the Kowloon West Cluster (KC/KE‐10‐0229/ER‐1), which oversee all research activities in the participating institutions. The clinical trial certificate number is NCT01237704.
The calculated estimated sample size was 84 participants based on the following considerations: alpha = 5%, power = 80%, effect size = 0.1, number of groups = 2, correlation between repeated measures = 0.7, and number of measurements = 4 for the four timepoints of the swallowing assessment. Considering a dropout rate of 25%, 110 participants were required. Additionally, assuming that 20% of the participants would not meet the selection criteria, we invited 140 patients from two hospitals to participate in the study. The inclusion criteria were as follows: (i) age ≥ 18 years; (ii) diagnosed with NPC; (iii) completed primary radiotherapy or chemoradiotherapy within ≤3 months; and (iv) being of Chinese ethnicity. The exclusion criteria were as follows: (i) a history of a head and neck cancer other than NPC treated with radiotherapy, chemotherapy, or surgery; (ii) a synchronous neoplasm along with NPC; (iii) residual or recurrent NPC after radiotherapy; (iv) other causes of dysphagia; (v) preexisting dysphagia before radiotherapy; (vi) contraindications to electrical stimulation; (vii) poor cognitive function impeding completion of the assessment; viii) presence of cerebral vascular disease or other neurological disorders including cranial neuropathies; and (ix) inability to attend all swallowing training sessions or post‐treatment follow‐up assessments. All the patients provided informed consent.
All patients underwent flexible endoscopic evaluation of swallowing (FEES); subsequently, they were randomized using computer‐generated numbers to receive either EBST or TNMES (Figure 1). EBST and TNMES were administered to 57 and 50 patients, respectively. The FEES was then performed immediately, 6, and 12 months after completion of the swallowing training. Along with the FEES, the patients completed the Functional Assessment after Cancer Therapy‐Nasopharyngeal 37 (FACT‐NP) questionnaire, which assess various health‐related QOL aspects, including swallowing function, in patients with NPC.
FIGURE 1.

Consort flow diagram. EBST, exercise‐based swallowing training; TNMES, transcutaneous neuromuscular electrical stimulation.
2.2. Flexible endoscopic evaluation of swallowing
FEES was performed by otolaryngologists and speech‐language pathologists in PWH who had >10 years of experience in assessing and managing patients with dysphagia. During the FEES examination, each patient sequentially received foods with different consistencies in 5‐mL boluses, including thin liquid (International Dysphagia Diet Standardization Initiative [IDDSI] 38 level 0), thick liquid (IDDSI level 2), puree (IDDSI level 4), gastric rice (IDDSI level 5), and biscuit (IDDSI level 7). Three swallowing attempts were allowed for each food bolus. During each swallowing attempt, the degree of lateral pharyngeal wall contraction, nasal regurgitation, premature spillage, food residue, laryngeal excursion, and epiglottic retroflexion were graded on a four‐point scale (0, normal; 1, mild; 2, moderate; 3, severe) during the pharyngeal swallowing phase. Additionally, penetration and aspiration were rated using the Rosenbek's penetration and aspiration scale (PAS) (Table S1). 39 The FEES procedure was video recorded and used for rating by other experienced investigators blinded to the patients' oncological condition and assigned training intervention.
2.3. Proactive swallowing training early after radiotherapy for NPC
Swallowing training sessions were performed by speech‐language pathologists who were certified to use TNMES (VitalStim® Therapy) and had extensive experience in administering EBST to patients with dysphagia. All patients underwent twelve 40‐min training sessions in a clinic over a 4‐week period, which were monitored by the speech‐language pathologists to ensure good adherence. No home‐based swallowing training was allowed during the study period. To ensure valid outcome comparisons, a similar frequency and duration was applied for the TNMES or EBST since there were no published data to inform equivalent training intensities for both modalities. Moreover, since TNMES is contraindicated in patients with untreated, residual, or recurrent head and neck cancer, proactive swallowing training was only offered to patients who were confirmed to be in remission through a negative nasopharyngeal biopsy at 3 months after radiotherapy or chemoradiotherapy.
Patients in the TNMES group underwent VitalStim® electrical stimulation therapy (VitalStim® Therapy; Chattanooga Group, TN, USA). Two electrodes were applied to the bilateral anterior neck, covering the suprahyoid and infrahyoid regions (Figure 2). Specifically, the electrodes were placed based on an adapted type “3A” configuration following the manufacturer‐provided training manual. This configuration covered muscles involved in tongue base retraction, pharyngeal contraction, laryngeal excursion, and opening of the upper esophageal sphincter. 40 During each session, the electrical current to each surface electrode was gradually increased until muscular contraction occurred in the anterior neck with tolerable discomfort. The current amplitudes at the start and end of each session were recorded. During the current‐induced muscular contractions of the pharynx, oral floor, and anterior neck, the patients were also instructed to practice dry swallowing or sip water in order to practice power‐assisted swallowing.
FIGURE 2.
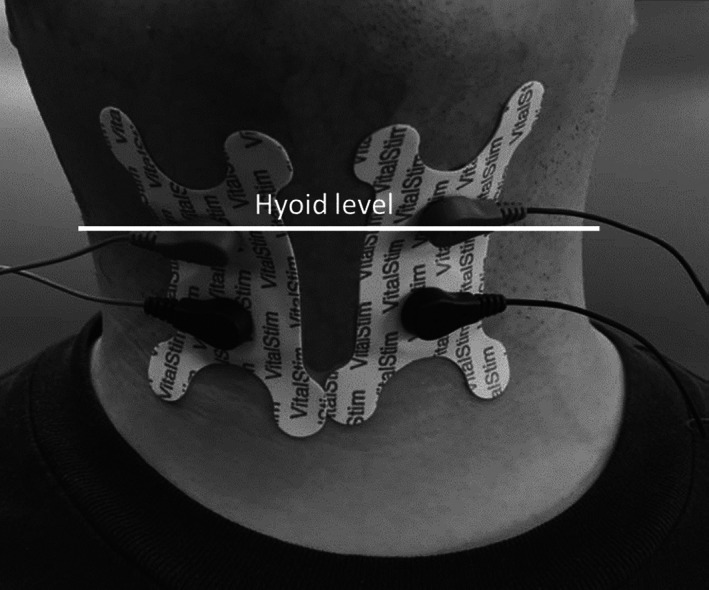
Transcutaneous neuromuscular electric stimulation (VitalStim®) with two electrode placements on the anterior neck.
Patients in the EBST group performed the Shaker Exercise (lying supine with three sustained 1‐min head lifts and 1‐min rest intervals, followed by 30 quick lifts), the Masako maneuver (five sets of 10 Masako swallows), effortful swallows (one set of 10 effortful swallows), and the Mendelsohn maneuver (one set of 10 swallows) as instructed by a speech‐language pathologist. The objectives of each training exercise are summarized in Appendix A. 41 Surface electromyography (MyoTrac Infiniti 2 Channel System, Thought Technology Ltd., USA) was used to provide feedback waveforms corresponding to muscle movement and strength during effortful swallowing and Mendelsohn maneuvers, which were used by a speech‐language pathologist to confirm optimal training outcomes.
2.4. Outcomes evaluation after swallowing training
The primary study endpoint was to investigate between‐group differences in swallowing outcomes, including the swallowing function score (SFS), pharyngeal motor impairment (PMI), pharyngeal function impairment (PFI), PAS, dynamic imaging grade of swallowing toxicity (DIGEST), 42 and QOL as measured using the FACT‐NP. The FACT‐NP is a validated self‐administered or interview questionnaire that contains 43 items scored on a 5‐point Likert‐type scale and has been validated in Chinese. 37 It is specifically used for QOL evaluation in patients with NPC treated with radiotherapy or chemoradiotherapy. Its items assess physical well‐being (PWB), functional well‐being (FWB), social well‐being (SWB), and emotional well‐being (EWB). Further, it comprises a 6‐item nasopharyngeal subscale (NPS) that evaluates swallowing function.
The PMI, PFI, and PAS were rated by an experienced speech‐language pathologist and a senior otolaryngologist using the video recordings of the FEES examination. The scores of the swallowing performance status scale (SPSS) 43 (Table S2) and functional oral and intake scale (FOIS) 44 (Table S3) were determined based on the PAS findings and pharyngeal performance, as reflected by the PMI and PFI.
The SFS was calculated as the sum of the scores for all six NPS items (Table 1) to allow independent analysis of the intervention efficacy measured using the aforementioned outcome measures. Regarding the FACT‐NP, the subdomain and total scores were positively correlated with the QOL.
TABLE 1.
Dysphagia symptom enquiry derived from nasopharyngeal subscale (NPS) items in the Functional Assessment after Cancer Therapy‐Nasopharyngeal (FACT‐NP).
| Questions | Scoring scale | |||||
|---|---|---|---|---|---|---|
| 1. | I am able to eat the foods that I like | 0 | 1 | 2 | 3 | 4 |
| 2. | My mouth is dry | 0 | 1 | 2 | 3 | 4 |
| 3. | I am able to eat as much food as I want | 0 | 1 | 2 | 3 | 4 |
| 4. | I can swallow naturally and easily | 0 | 1 | 2 | 3 | 4 |
| 5. | I can eat solid foods | 0 | 1 | 2 | 3 | 4 |
| 6. | I can enjoy the taste of food | 0 | 1 | 2 | 3 | 4 |
Note: Scoring scale: 0, not a bit; 1, a little bit; 2, somewhat; 3, quite a bit; 4, very much.
PMI was assessed as the sum of the scores of the (i) swallowing response, (ii) epiglottic retroflexion, (iii) laryngeal excursion, and (iv) pharyngeal contraction in the FEES examination during fluid intake. Nasal regurgitation impairment (NRI) and premature spillage impairment (PSI) were assessed as the sum of the scores of the aforementioned items during intake of (i) thin fluid, (ii) thick fluid, (iii) puree, (iv) gastric rice, and (v) biscuits. Pharyngeal residue impairment (PRI) was assessed as the sum of the scores for the (i) pharyngeal location (vallecula, pyriform fossa, or diffuse) and (ii) severity of food stasis during food intake with the same order of consistency. PFI was assessed as the sum of the PMI, NRI, PSI, and PRI scores. It reflected the overall performance of the pharyngeal swallowing phase for all food consistencies and was used to evaluate the efficacy of each intervention. Table 2 summarizes the scoring systems for the PMI, NRI, PSI, PRI, and PFI.
TABLE 2.
Scoring of pharyngeal function by flexible endoscopic evaluation of swallowing (FEES).
| Scoring methods and ranges | |
|---|---|
| Pharyngeal motor impairment (PMI) | Summation of score for SR, LE, ER, PC for thin or thick liquid |
| Scoring range | |
| Swallowing response (SR) | 0 (normal) to 7 (the worst) |
| Laryngeal elevation (LE) | 0 (normal) to 7 (the worst) |
| Epiglottic retroflexion (ER) | 0 (normal) to 7 (the worst) |
| Pharyngeal contraction (PC) | 0 (normal) to 7 (the worst) |
| Nasal regurgitation impairment (NRI) | Summation of score for NRI in thin liquid, thick liquid, puree, gastric and biscuit |
| Scoring range | 0 (absent), 1 (present) |
| Premature spillage impairment (PSI) | Summation of score for PSI in thin liquid, thick liquid, puree, gastric and biscuit |
| Scoring range | 0 (absent), 1 (vallecula), 2 (pyriform fossa), 3 (arytenoid) |
| Pharyngeal residue impairment (PRI) | Summation of scores for RA and RD with thin liquid, thick liquid, puree, gastric and biscuit |
| Scoring range | |
| Pharyngeal residue amount (RA) | 0 (nil), 1 (<10%), 2 (10–50%), 3 (>50%) |
| Food residue distribution (RD) | 0 (none), 1 (vallecula), 2 (vallecula, pyriform fossa), 3 (diffuse) |
| Pharyngeal function impairment (PFI) | Summation of PMI, NRI, PSI and PRI |
Regarding outcome analysis, the efficacy of each intervention was assessed based on the changes in the SFS; total and sub‐scores of FACT‐NP, PMI, and PFI; and the sub‐scores of PAS, DIGEST, SPSS, and FOIS at each assessment time points.
2.5. Statistical analysis
All statistical analyses were performed using SPSS software (version 23.0; IBM, Armonk, NY, USA). The Chi‐square test and Mann–Whitney U test were used for between‐group comparisons of demographic data. NPC stage, radiation dose, and time interval from radiotherapy to swallowing intervention.
The intraclass correlation coefficient (ICC) was used to measure the intra‐ and inter‐rater reliabilities of two raters for all measured FEES parameters at the four assessment timepoints. The kappa coefficient was used to measure the inter‐rater reliability for the PMI, PFI, and their different scoring components. Additionally, in case of between‐rater inconsistency in the ratings of any parameter, discussions were held until a consensus was reached regarding the value to be included in the data analysis.
The Mann–Whitney U test was for between‐group comparisons of PMI, including nasal regurgitation, premature spillage, laryngeal excursion, and pharyngeal contraction. Two‐way repeated‐measures analysis of variance (ANOVA) was used to analyze the PAS, PMI, NRI, PSI, PRI, PFI, DIGEST, SPSS, and FOIS scores in order to determine whether there was an interaction effect between the training modalities and the assessment time points. Moreover, two‐way repeated‐measures ANOVA was used to evaluate the effect of the training modalities on the subscale and total FACT‐NP scores at different assessment time point. The Wilcoxon signed‐rank test was used for within‐group pairwise comparisons of the scores. Cohen's D was used to measure the effect size of the post‐intervention changes in the total and subscale FACT‐NP scores at each assessment time‐point. Partial eta squared (η p 2) was used to assess the effect size of the post‐intervention changes in the PMI, PFI, DIGEST, PAS, FOIS, SPSS, and SFS scores. Pearson's correlation coefficient was used to assess the correlation between the total FACT‐NP scores and SFS.
3. RESULTS
3.1. Demographic characteristics
There were 34 and 31 patients in the EBST and TNMES groups, respectively. The reasons for dropping out after swallowing training included tumor recurrence (n = 1), refusal of follow‐up assessments due to poor health (n = 8), declining follow‐up due to personal affairs (n = 7), and loss contact (n = 12). There was no adverse side effect reported during training by EBST or TNMES. Moreover, there were no cases of training termination due to discomfort; accordingly, we achieved 100% adherence in both groups. There were no significant between‐group differences in the demographic characteristics (Table 3).
TABLE 3.
Baseline demographic characteristics of the patients in the exercise‐based swallowing training (EBST) and transcutaneous neuromuscular electrical stimulation (TNMES) groups before prophylactic swallowing training.
| EBST | TNMES | p‐value* | |
|---|---|---|---|
| Number of patients | 34 | 31 | |
| Age (years) | 49.75 ± 11.32 | 53.77 ± 12.25 | .185 |
| Sex | |||
| Male | 25 | 18 | .188 |
| Female | 9 | 13 | |
| Height (cm) | 164.24 ± 10.15 | 161.48 ± 8.66 | .258 |
| Weight (kg) | 57.73 ± 10.81 | 54.47 ± 13.40 | .291 |
| Cancer stage | |||
| Stage I | 3 | 4 | .642 |
| Stage II | 6 | 8 | |
| Stage III | 16 | 10 | |
| Stage IV | 9 | 9 | |
| Intensity‐modulated radiotherapy (IMRT) | 34 | 31 | ‐ |
| Chemotherapy | 27 | 19 | .109 |
| Radiation dose (Gy) | 70 ± 3.81 | 71.48 ± 4.38 | .149 |
| Post‐radiotherapy interval (days) | 86.09 ± 24.98 | 95.55 ± 34.17 | .205 |
| Tube feeding | 0 | 0 | ‐ |
| Swallowing function score (SFS) | 9.79 ± 4.22 | 8.52 ± 3.75 | .087 |
| Penetration and aspiration scale (PAS) | 6.71 ± 2.66 | 6.55 ± 2.13 | .944 |
| Dynamic Imaging Grade of Swallowing Toxicity (DIGEST) | 1.38 ± 0.60 | 1.39 ± 0.56 | .861 |
| Swallowing performance status score (SPSS) | 1.76 ± 0.99 | 1.81 ± 1.11 | .977 |
| Functional oral and intake score (FOIS) | 6.35 ± 0.73 | 6.32 ± 0.79 | .943 |
| Pharyngeal function impairment (PFI) | 27.44 ± 7.48 | 27.06 ± 6.95 | .777 |
| Total FACT‐NP | 112.2 ± 18.59 | 109.26 ± 20.05 | .533 |
| Nasopharyngeal subscale (NPS) | 37.06 ± 7.60 | 35.71 ± 7.88 | .361 |
Note: FACT‐NP, Functional Assessment After NPC Therapy.
p‐value is significant at p < .05.
3.2. Post‐intervention swallowing outcomes
Regarding intra‐rater reliability for all measured FEES parameters, the ICC was 0.87–1, indicating good to excellent intra‐rater reliability. Moreover, regarding the inter‐rater reliability, the ICCs for the PAS and PMI ranged from 0.68 to 0.98 and 0.81 to 0.97, respectively. The Kappa coefficients for the different PFI components, including the NRI, PSI, and PRI, ranged from 0.6 to 1. Table 4 shows the PMI, NRI, PSI, PRI, PFI, PAS, and DIGEST scores at the four assessment timepoints.
TABLE 4.
Scores for the penetration and aspiration score (PAS), dynamic imaging grade of swallowing toxicity (DIGEST), and pharyngeal function impairment (PRI), as well as the sub‐scores of pharyngeal motor impairment (PMI), nasal regurgitation impairment (NRI), premature spillage impairment (PSI), and pharyngeal residue impairment (PRI), at four timepoints.
| EBST | TNMES | EBST | TNMES | EBST | TNMES | EBST | TNMES | |
|---|---|---|---|---|---|---|---|---|
| Pre‐train | Pre‐train | Immediate | Immediate | 6‐month | 6‐month | 12‐month | 12‐month | |
| Pharyngeal motor impairment (PMI) | 10.03 ± 2.69 | 10.23 ± 2.17 | 10.76 ± 2.78 | 9.13 ± 1.8 | 9.82 ± 2.66 | 9.16 ± 2.35 | 9.03 ± 2.14 | 9.71 ± 2.57 |
| Swallowing response | 1.91 ± 0.67 | 2.23 ± 0.62 | 2.03 ± 0.52 | 1.9 ± 0.47 | 2.03 ± 0.76 | 1.94 ± 0.68 | 1.82 ± 0.67 | 2.03 ± 0.55 |
| Laryngeal elevation | 2.44 ± 0.82 | 2.29 ± 0.78 | 2.59 ± 0.78 | 2.29 ± 0.74 | 2.29 ± 0.91 | 2.13 ± 0.85 | 2.21 ± 0.81 | 2.23 ± 0.99 |
| Epiglottic retroflexion | 3.21 ± 1.25 | 3.19 ± 0.95 | 3.59 ± 1.42 | 2.81 ± 1.25 | 3.09 ± 1.08 | 2.81 ± 0.87 | 2.97 ± 1.14 | 2.97 ± 1.22 |
| Pharyngeal contraction | 2.47 ± 1.05 | 2.52 ± 0.77 | 2.56 ± 1.02 | 2.13 ± 0.67 | 2.41 ± 0.86 | 2.29 ± 0.82 | 2.03 ± 0.67 | 2.48 ± 0.81 |
| Nasal regurgitation impairment (NRI) | 0.35 ± 0.92 | 0.23 ± 0.76 | 0.24 ± 0.5 | 0.16 ± 0.45 | 0.21 ± 0.64 | 0.16 ± 0.52 | 0.24 ± 0.86 | 0.1 ± 0.4 |
| Premature spillage impairment (PSI) | 2.03 ± 1.73 | 1.87 ± 1.84 | 2.53 ± 1.58 | 2.1 ± 1.87 | 1.97 ± 1.68 | 1.97 ± 1.52 | 2.41 ± 2.62 | 2.81 ± 2.56 |
| Pharyngeal residue impairment (PRI) | 15.03 ± 4.76 | 14.74 ± 5.14 | 15.44 ± 5.22 | 14.48 ± 5.08 | 14.91 ± 5.33 | 13.65 ± 6.03 | 14.59 ± 4.72 | 14.81 ± 4.79 |
| Pharyngeal residue amount | 5.71 ± 1.93 | 5.06 ± 1.79 | 5.12 ± 1.47 | 4.97 ± 1.83 | 5.06 ± 1.69 | 4.52 ± 1.71 | 5.44 ± 1.67 | 5.03 ± 1.38 |
| Food residue distribution | 9.32 ± 3.48 | 9.6 ± 3.95 | 10.32 ± 4.1 | 9.7 ± 3.56 | 9.85 ± 4.05 | 9.13 ± 4.57 | 9.15 ± 3.51 | 9.83 ± 3.92 |
| Pharyngeal function impairment (PFI) | 27.44 ± 7.48 | 27.06 ± 6.95 | 28.97 ± 7.62 | 25.87 ± 6.83 | 26.91 ± 7.46 | 24.94 ± 8.5 | 26.26 ± 7.51 | 27.42 ± 7.85 |
| Penetration and aspiration (PAS) | 6.71 ± 2.66 | 6.55 ± 2.13 | 6.41 ± 2.3 | 6.1 ± 1.99 | 6.94 ± 3.27 | 5.97 ± 1.43 | 6.85 ± 3.53 | 6.61 ± 2.54 |
| Dynamic imaging grade of swallowing toxicity (DIGEST) | 1.38 ± 0.6 | 1.39 ± 0.56 | 1.26 ± 0.51 | 1.23 ± 0.5 | 1.44 ± 0.71 | 1.16 ± 0.37 | 1.62 ± 0.89 | 1.32 ± 0.54 |
Note: *p < .05.
Abbreviations: EBST, exercise‐based swallowing training; TNMES, transcutaneous neuromuscular electrical stimulation.
Compared with the baseline values, there was a significant improvement in the PFI scores immediately and 6 months after TNMES; however, it returned near to the baseline level at 12 months. Contrastingly, compared with baseline values, there was a deterioration in the PFI scores immediately after EBST, which gradually improved at 6 months and plateaued at 12 months (Figure 3A). Compared with the EBST group, the TNMES group showed significant improvement in the PFI score at different assessment timepoints, with a small effect size (η p 2: 0.05). This indicated that TNMES allowed better improvement in pharyngeal swallowing than EBST, with a similar trend being observed for PMI. Compared with EBST, TNMES allowed better swallowing improvement as shown by improved PMI score at each assessment timepoint, with a medium effect size (η p 2: 0.065; Figure 3B). Other parameters such as NRI, PSI, and PRI did not show significant between‐group differences at each assessment point. Moreover, the TNMES group tended to show better performance than the EBST group in terms of the DIGEST, PAS, FOIS, and SPSS scores immediately and 6 months after swallowing training. However, the scores in both groups returned to near baseline at 12 months. There was a small effect size for the interaction between the EBST and TNMES (η p 2: 0.009–0.020; Figure 3C–F).
FIGURE 3.

Swallowing outcomes after proactive swallowing training through exercise‐based swallowing training (EBST) and transcutaneous neuromuscular electrical stimulation (TNMES).
Figure 4 shows the SFS at each assessment timepoint. In both groups, there was a post‐intervention improvement in the SFS scores; however, only the TNMES group showed improvement at all three post‐intervention timepoints. Contrastingly, the EBST group only showed a significant SFS improvement at 6 months, which then plateaued at 12 months. There was a significant interaction between the EBST and TNMES, with a small effect size (η p 2: 0.04). Table 5 shows the scores for each SFS item at each assessment timepoint.
FIGURE 4.

Swallowing function score (SFS) before and after proactive swallowing training by exercise‐based swallowing training (EBST) and transcutaneous neuromuscular electrical stimulation (TNMES) at the four assessment timepoints.
TABLE 5.
Swallowing function score (SFS) at before intervention as well as immediately, 6, and 12 months after intervention by exercise‐based swallowing training (EBST) and transcutaneous neuromuscular electrical stimulation (TNMES).
| Pre‐training SFS | Post‐immediate SFS | Post 6 months SFS | Post 12 months SFS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| EBST | TNMES | EBST | TNMES | EBST | TNMES | EBST | TNMES | ||
| 1 | I am able to eat the foods that I like | 1.56 ± 0.99 | 1.19 ± 1.08 | 1.74 ± 1.02 | 1.42 ± 1.12 | 1.74 ± 0.96 | 1.87 ± 1.12 | 1.82 ± 1.03 | 1.97 ± 0.95 |
| 2 | My mouth is dry | 0.79 ± 0.84 | 0.58 ± 0.72 | 1.18 ± 0.94 | 1.06 ± 1.12 | 1.41 ± 0.86 | 1.03 ± 1.08 | 1.44 ± 0.93 | 1.45 ± 1.09 |
| 3 | I am able to eat as much food as I want | 1.97 ± 1.06 | 1.81 ± 0.91 | 2.03 ± 1.03 | 2.39 ± 0.95 | 2.26 ± 1.08 | 2.39 ± 1.12 | 2.21 ± 0.95 | 2.71 ± 0.78 |
| 4 | I can swallow naturally and easily | 1.88 ± 1.07 | 1.65 ± 1.11 | 2.12 ± 0.95 | 2.23 ± 1.02 | 2.03 ± 1.11 | 2.16 ± 1.07 | 1.97 ± 0.97 | 2.29 ± 0.90 |
| 5 | I can eat solid foods | 1.94 ± 0.95 | 2.00 ± 1.29 | 2.21 ± 0.84 | 2.29 ± 1.07 | 2.09 ± 1.03 | 2.32 ± 1.08 | 2.21 ± 0.98 | 2.52 ± 0.93 |
| 6 | I can enjoy the taste of food | 1.56 ± 0.99 | 1.29 ± 1.04 | 1.85 ± 1.10 | 1.55 ± 1.18 | 2.12 ± 1.25 | 1.97 ± 1.28 | 2.15 ± 1.10 | 2.03 ± 1.11 |
| Total score | 9.79 ± 4.22 | 8.52 ± 3.75 | 11.09 ± 4.03 | 10.94 ± 4.07 | 11.62 ± 4.31 | 11.74 ± 4.38 | 11.79 ± 4.31 | 13.00 ± 3.36 | |
3.3. QOL according to the FACT‐NP and swallowing function scores
Table 6 shows the mean total and subscale FACT‐NP scores in both groups at each assessment timepoint, with confidence intervals and effect sizes measured by Cohen's D. Figure 5 shows a graphical presentation of the mean total and subscale FACT‐NP scores in both groups at each assessment timepoint, as well the effect size of their interaction. The total FACT‐NP scores showed improvement in both groups at all post‐intervention timepoints. Further, TNMES allowed significant improvement in the total FACT‐NP score at 6 and 12 months, with a greater effect size than the EBST. Regarding the PWB subscale, both groups showed deterioration in the PWB subscale scores immediately after intervention, which significantly improved at 6 and 12 months, with a small‐to‐medium effect size. The TNMES group showed a significant improvement in the FWB sub‐score at all post‐intervention timepoints. Contrastingly, the EBST group showed improvement in these subscale scores immediately after training, which subsequently dropped at 6 and 12 months after training. Compared with the EBST group, the TNMES group showed greater effect sizes at 6 and 12 months. There were significant between‐group differences in terms of the effect size between pretreatment versus 6 and 12 months. Contrastingly, there were no significant post‐intervention improvements in the EWB and SWB scores. Regarding the NPS, both groups showed significant post‐intervention improvement at all timepoints, with the TNMES group showing a greater effect size than the EBST group. Pearson's correlation coefficient analysis revealed a strong positive correlation between the SFS and FACT‐NP scores (r: 0.56–0.76, ‘moderate strong’ to ‘strong’).
TABLE 6.
Functional Assessment after Cancer Therapy‐Nasopharyngeal (FACT‐NP).
| Mean score | Mean difference | Std. error | p‐value | 95% confidence interval for difference | ||||
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | Cohen's D | ||||||
| Exercise‐based swallowing training | ||||||||
| FACT‐NP | Pretreatment | 112.2 | ||||||
| Posttreatment | 114.6 | −2.448 | 2.206 | 0.276 | −6.947 | 2.051 | 0.196 | |
| 6 months | 117.3 | −5.083 | 3.016 | 0.102 | −11.235 | 1.069 | 0.298 | |
| 12 months | 118.1 | −5.953* | 2.439 | 0.021 | −10.928 | −0.978 | 0.431 | |
| Transcutaneous neuromuscular electrical stimulation | ||||||||
| FACT‐NP | Pretreatment | 109.2 | ||||||
| Posttreatment | 113.8 | −4.567 | 3.099 | 0.151 | −10.906 | 1.771 | 0.269 | |
| 6 months | 119.5 | −10.272* | 3.460 | 0.006 | −17.348 | −3.195 | 0.542 | |
| 12 months | 122.4 | −13.206* | 3.314 | 0.000 | −19.983 | −6.429 | 0.728 | |
| Exercise‐based swallowing training | ||||||||
| Physical well‐being (PWB) | Pretreatment | 20.9 | ||||||
| Posttreatment | 19.1 | 1.755* | 0.584 | 0.005 | 0.568 | 2.942 | 0.531 | |
| 6 months | 22.6 | −1.706* | 0.746 | 0.029 | −3.224 | −0.187 | 0.404 | |
| 12 months | 23.1 | −2.235* | 0.654 | 0.002 | −3.566 | −0.904 | 0.604 | |
| Transcutaneous neuromuscular electrical stimulation | ||||||||
| Physical well‐being (PWB) | Pretreatment | 21.3 | ||||||
| Posttreatment | 20.1 | 1.258* | 0.568 | 0.034 | 0.098 | 2.418 | 0.404 | |
| 6 months | 23.1 | −1.774* | 0.654 | 0.011 | −3.109 | −0.439 | 0.495 | |
| 12 months | 23.6 | −2.323* | 0.834 | 0.009 | −4.026 | −0.619 | 0.509 | |
| Exercise‐based swallowing training | ||||||||
| Functional well‐being (FWB) | Pretreatment | 15.9 | ||||||
| Posttreatment | 17.0 | −1.147* | 0.560 | 0.049 | −2.287 | −0.007 | 0.362 | |
| 6 months | 16.3 | −0.471 | 0.996 | 0.640 | −2.496 | 1.555 | 0.084 | |
| 12 months | 16.3 | −0.412 | 1.014 | 0.687 | −2.475 | 1.652 | 0.072 | |
| Transcutaneous neuromuscular electrical stimulation | ||||||||
| Functional well‐being (FWB) | Pretreatment | 15.0 | ||||||
| Posttreatment | 16.3 | −1.290 | 1.044 | 0.226 | −3.423 | 0.843 | 0.226 | |
| 6 months | 18.1 | −3.065* | 1.132 | 0.011 | −5.377 | −0.753 | 0.494 | |
| 12 months | 18.5 | −3.419* | 1.119 | 0.005 | −5.706 | −1.133 | 0.558 | |
| Exercise‐based swallowing training | ||||||||
| Emotional well‐being (EWB) | Pretreatment | 17.0 | ||||||
| Posttreatment | 18.2 | −1.176* | 0.551 | 0.040 | −2.298 | −0.055 | 0.377 | |
| 6 months | 18.1 | −1.147 | 0.581 | 0.057 | −2.328 | 0.034 | 0.349 | |
| 12 months | 17.9 | −0.912 | 0.519 | 0.088 | −1.969 | 0.145 | 0.311 | |
| Transcutaneous neuromuscular electrical stimulation | ||||||||
| Emotional well‐being (EWB) | Pretreatment | 17.7 | ||||||
| Posttreatment | 18.3 | −0.581 | 0.562 | 0.310 | −1.728 | 0.567 | 0.189 | |
| 6 months | 17.5 | 0.198 | 0.534 | 0.713 | −0.893 | 1.289 | 0.068 | |
| 12 months | 18.6 | −0.839 | 0.581 | 0.159 | −2.026 | 0.348 | 0.264 | |
| Exercise‐based swallowing training | ||||||||
| Social well‐being (SWB) | Pretreatment | 20.3 | ||||||
| Posttreatment | 19.9 | 0.382 | 0.528 | 0.474 | −0.692 | 1.456 | 0.128 | |
| 6 months | 19.7 | 0.657 | 0.871 | 0.456 | −1.115 | 2.429 | 0.133 | |
| 12 months | 19.4 | 0.985 | 0.826 | 0.242 | −0.696 | 2.666 | 0.211 | |
| Transcutaneous neuromuscular electrical stimulation | ||||||||
| Social well‐being (SWB) | Pretreatment | 19.5 | ||||||
| Posttreatment | 20.1 | −0.554 | 1.009 | 0.587 | −2.615 | 1.507 | 0.100 | |
| 6 months | 20.4 | −0.914 | 1.208 | 0.455 | −3.380 | 1.552 | 0.138 | |
| 12 months | 20.8 | −1.328 | 0.894 | 0.148 | −3.154 | 0.498 | 0.271 | |
| Exercise‐based swallowing training | ||||||||
| Nasopharyngeal subscale (NPS) | Pretreatment | 37.1 | ||||||
| Posttreatment | 39.5 | −2.412* | 1.080 | 0.032 | −4.609 | −0.215 | 0.395 | |
| 6 months | 39.6 | −2.529* | 1.213 | 0.045 | −4.997 | −0.062 | 0.369 | |
| 12 months | 40.5 | −3.441* | 1.099 | 0.004 | −5.678 | −1.204 | 0.553 | |
| Transcutaneous neuromuscular electrical stimulation | ||||||||
| Nasopharyngeal subscale (NPS) | Pretreatment | 35.7 | ||||||
| Posttreatment | 39.2 | −3.447* | 1.269 | 0.011 | −6.039 | −0.855 | 0.496 | |
| 6 months | 40.6 | −4.864* | 1.361 | 0.001 | −7.643 | −2.086 | 0.652 | |
| 12 months | 41.1 | −5.350* | 1.258 | 0.000 | −7.919 | −2.781 | 0.776 | |
Note: Effect size interpretation by Cohen's D: 0.2–0.5: small effect; 0.5–0.8: medium effect; 0.8–1: large effect.
FIGURE 5.

Functional Assessment after Cancer Therapy‐Nasopharyngeal (FACT‐NP), total score and subscale scores. EBST, exercise‐based swallowing training; TNMES, transcutaneous neuromuscular electrical stimulation.
4. DISCUSSION
In our study, patients with NPC showed post‐radiotherapy impairments in pharyngeal motor function and swallowing efficiency. Compared with the EBST group, the TNMES group showed significant improvements in the SFS, PMI, and PFI scores. Moreover, the TNMES group showed earlier significant improvement and better QOL according to the FACT‐NP than the EBST group. These findings suggest that proactive TMNES and EBST are safe and feasible modalities for improving swallowing in patients with NPC when administered early after radiotherapy when symptoms are still mild. To our knowledge, this is the first prospective randomized controlled trial to investigate the efficacy of early TNMES for proactive swallowing training in patients with NPC treated with radiotherapy or chemoradiotherapy, using EBST as a control.
Most previous studies on proactive swallowing training using EBST only reported follow‐up assessment up to 6 months after radiotherapy, with only a few studies reporting outcomes at ≥12 post‐training months. 33 , 45 , 46 Additionally, prophylactic swallowing training has been shown to improve swallowing function, oral intake, malnutrition, and aspiration/aspiration pneumonia. 47 , 48 A unique aspect of the present study is the provision of swallowing training at around 3 months after radiotherapy upon confirmed remission status of NPC, with follow‐up assessments up to 12 post‐intervention months. Notably, most acute toxicities of chemoradiotherapy, including mucositis, dermatitis, and salivary gland injury, resolve within 12 weeks. 49
Impaired tongue movement, pharyngeal contraction, epiglottic retroflexion, and laryngeal excursion have been reported following radiotherapy for treating symptomatic NPC. 14 , 15 , 50 , 51 , 52 These oropharyngeal swallowing impairments can be mitigated using EBST exercises, including Shaker's exercise, Masako maneuver, Mendelsohn maneuver, and effortful swallowing. In our study, we applied vertical placement of the bipolar electrodes in a paramedian position on both sides of the neck to stimulate the suprahyoid and infrahyoid musculature, the hypoglossal nerves, and the deeper pharyngeal constrictor muscles. 53 This could have contributed to the significant improvement in PMI immediately and 6 months after swallowing training in the TNMES group. However, the eventual return to baseline at 12 months suggested that this improvement was transient.
We used FEES for objective swallowing assessment since it can detect penetration and aspiration during swallowing, as well describe the anatomical integrity of the pharynx and larynx and the handling of secretions such as saliva and sputum. 54 In FEES, the PAS, DIGEST, PMI, and PFI represent the general condition of the pharyngeal phase of swallowing with various food consistencies. We observed post‐radiotherapy mild deterioration in the PAS and DIGEST scores in both groups, which non‐significantly improved following proactive swallowing training, with the TNMES group tending to demonstrate a more persistent improvement over time. A similar trend was observed for the PMI and PFI. Although the observed trend of improvement in the DIGEST, PAS, FOIS, and SPSS scores in both groups may be partially attributed to the natural recovery process after radiation therapy, the training may have yielded an additional positive impact on these scores.
We observed significant impairment in patient‐rated subjective swallowing functions in both groups early after radiotherapy or chemoradiotherapy, which was indicated by deterioration in the SFS. The TNMES group showed significant improvement in the SFS at all post‐intervention assessment timepoints; contrastingly, the post‐intervention improvement in the EBST group was only significant at 6 and 12 months. Notably, our patients generally rated their subjective swallowing function as poor, which differed from the mild impairment observed in the PAS, DIGEST, PMI, and PFI on FEES. This is inconsistent with our previous report that patients with NPC often underestimated their swallowing functions compared with the objective findings of videofluoroscopic swallowing assessment. 55
Our preliminary results showed that the TNMES group tended to perform better than the EBST group with respect to the SFS, PMI, and PFI scores. Regarding QOL, both groups showed post‐intervention improvements in the total FACT‐NP score. The improvement in QOL was significant in the TNMES group at 6 and 12 months; contrastingly, it was only significant at 12 months in the EBST group. Notably, there was a strong correlation between the SFS and total FACT‐NP scores. The graphs for the SFS, NPS, and FACT‐NP scores showed similar changes over the four assessment timepoints, with the TNMES slightly outperforming EBST at 6 and 12 months. Specifically, the TNMES group showed steeper curves than the EBST group, indicating a more protracted gain in swallowing function and QOL. Our findings suggested that both EBST and TNMES had a significant positive influence on patients' swallowing function and QOL scores.
Patient adherence to the swallowing rehabilitation program is critical to achieving the expected outcomes. However, most studies have reported a suboptimal adherence rate ranging from 13% to 64%. 47 To ensure 100% training adherence in our study, all training sessions were conducted under close supervision by qualified speech and language pathologists in hospital‐based clinics. This was a strength of our study since it allowed patients to receive consistent and high‐quality care throughout the training process.
Most studies on swallowing rehabilitation for dysphagia in patients with head and neck cancer applied adjunct TNMES to traditional EBST. 24 , 25 , 26 A potential limitation of our study is the lack of a treatment arm involving administration of both TNMES and EBST in order to observe their synergistic effect as well as the lack of a non‐treatment arm without swallowing training as a control group. We previously observed a high proportion of patients with progressive deterioration in neck stiffness, jaw opening, tongue movement, pharyngeal contraction, food residues, penetration, and aspiration at 6 and 12 months after radiotherapy for NPC. 50 Accordingly, we anticipated ethical issues in enrolling a control group for the non‐treatment arm; instead, we used baseline swallowing parameters as the control values.
To the best of our knowledge, there have been no studies comparing the training intensities of TNMES and EBST. Therefore, our findings could serve as a good reference for future studies exploring a new standard of care for TNMES and EBST in patients with head and neck cancer. TNMES requires less time and frequency, which may yield better adherence. However, our findings do not conclusively determine that TNMES is superior to EBST since standard EBST requires three daily sessions which was not applied in our study. Instead, our findings only suggest that TNMES may be better than EBST in certain aspects under the same training duration. Future large‐scale studies are warranted to administer both EBST and TNMES, with home‐based EBST being administered before or during radiotherapy or chemoradiotherapy and TNMES being administered after radiotherapy completion. Additionally, they should enroll a control group in order to compare changes in swallowing function and QOL over time in these patients. Given the aforementioned limitations, it is important to interpret our findings with caution.
5. CONCLUSION
Our findings suggested that early proactive swallowing training with TNMES and EBST is safe and feasible for patients with NPC after radiotherapy or chemoradiotherapy. Nonetheless, our result suggests that TNMES is a promising modality for improving swallowing symptoms, pharyngeal motor function, and QOL in patients with NPC after radiotherapy. Moreover, our findings can inform personalized protocols for TNMES, EBST, or both modalities for swallowing rehabilitation following elucidation of the efficacy of each modality using a standardized adherence rate, frequency, and duration of training.
FUNDING INFORMATION
This study was supported by research funds from the Research Grants Council, Hong Kong Special Administrative Region, China (RGC reference number: 475210).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests or financial relationships to disclose.
Supporting information
TABLE S1: Rosenbek's penetration and aspiration scale (PAS).
TABLE S2: Swallowing Performance and Severity Scale (SPSS).
TABLE S3: Functional Oral Intake Scale (FOIS).
ACKNOWLEDGEMENTS
The authors would like to dedicate this paper to the memory of Dr. Leung Sing Fai of the Department of Clinical Oncology, The Chinese University of Hong Kong, who contributed to the methodology, recruitment of patients, and data collection, as well as enthusiastically, humorously, and generally shared his experience with this research colleagues. Additionally, the authors would like to thank Tristel Asia Limited for sponsoring the Tristel Trio Wipes System, which was used to clean the flexible endoscopes used in this study.
APPENDIX A. Swallowing exercises for proactive swallowing training after radiotherapy for nasopharyngeal carcinoma
A.1.
| Swallowing exercise | Effect of swallowing training |
|---|---|
| Shaker exercise | Improves the movement of the epiglottis and strengthens the opening of the esophagus. Also promotes upward movement of the larynx. |
| Masako maneuver | Helps strengthen tongue muscles needed for swallowing. |
| Mendelsohn maneuver | Promotes movement of the epiglottis. Improves the function of the larynx and strengthens the opening of the esophagus. |
| Effortful swallows | Improves movement of the tongue base and pharynx. |
Ku PKM, Vlantis AC, Wong RWM, et al. Quality of life and swallowing outcomes after early proactive swallowing rehabilitation by either transcutaneous neuromuscular electrical stimulation or exercise‐based swallowing training in patients with nasopharyngeal carcinoma after radiotherapy. Laryngoscope Investigative Otolaryngology. 2023;8(6):1532‐1546. doi: 10.1002/lio2.1162
Professor Michael C. F. Tong, Professor Kathy Y. S. Lee and Professor Andrew van Hasselt are co‐senior authors of this paper.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Zhang B, Mo Z, Du W, Wang Y, Liu L, Wei Y. Intensity‐modulated radiation therapy versus 2D‐RT or 3D‐CRT for the treatment of nasopharyngeal carcinoma: a systematic review and meta‐analysis. Oral Oncol. 2015;51:1041‐1046. [DOI] [PubMed] [Google Scholar]
- 3. Co J, Mejia MB, Dizon JM. Evidence on effectiveness of intensity‐modulated radiotherapy versus 2‐dimensional radiotherapy in the treatment of nasopharyngeal carcinoma: meta‐analysis and a systematic review of the literature. Head Neck. 2016;38:E2130‐E2142. [DOI] [PubMed] [Google Scholar]
- 4. Wang CC. Carcinoma of the nasopharynx. In: Wang CC, ed. Radiation Therapy for Head and Neck Neoplasms. 3rd ed. Wiley‐Liss; 1997:257‐280. [Google Scholar]
- 5. Perez CA, Brady LW. Principles and Practice of Radiation Oncology. Lippincott‐Raven Publishers; 1998:897‐939. [Google Scholar]
- 6. Sham JS, Choy D, Wei WI. Nasopharyngeal carcinoma: orderly neck node spread. Int J Radiat Oncol Biol Phys. 1990;19:929‐933. [DOI] [PubMed] [Google Scholar]
- 7. Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and pattern of failure. Int J Radiat Oncol Biol Phys. 1992;23:261‐270. [DOI] [PubMed] [Google Scholar]
- 8. Chow JCH, Cheung KM, Au KH, et al. Radiation‐induced hypoglossal nerve palsy after definitive radiotherapy for nasopharyngeal carcinoma: clinical predictors and dose‐toxicity relationship. Radiother Oncol. 2019;138:93‐98. [DOI] [PubMed] [Google Scholar]
- 9. Lin YS, Jen YM, Lin JC. Radiation‐related cranial nerve palsy in patients with nasopharyngeal carcinoma. Cancer. 2002;95:404‐409. [DOI] [PubMed] [Google Scholar]
- 10. Yeh SA, Tang Y, Lui CC, Huang YJ, Huang EY. Treatment outcomes and late complications of 849 patients with nasopharyngeal carcinoma treated with radiotherapy alone. Int J Radiat Oncol Biol Phys. 2005;62:672‐679. [DOI] [PubMed] [Google Scholar]
- 11. Brook I. Late side effects of radiation treatment for head and neck cancer. Radiat Oncol J. 2020;38:84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang XM, Zheng YQ, Mai HQ, et al. Diagnosis and treatment on osteoradionecrosis of skull base after radiotherapy for nasopharyngeal carcinoma. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2004;39:558‐561. [PubMed] [Google Scholar]
- 13. Ryu G, So YK, Seo MY, et al. Using the nasoseptal flap for reconstruction after endoscopic debridement of radionecrosis in nasopharyngeal carcinoma. Am J Rhinol Allergy. 2018;32:61‐65. [DOI] [PubMed] [Google Scholar]
- 14. Hughes PJ, Scott PM, Kew J, et al. Dysphagia in treated nasopharyngeal cancer. Head Neck. 2000;22:393‐397. [DOI] [PubMed] [Google Scholar]
- 15. Marshall JN, Ku PK, Kew J, et al. Assessment and management of dysphagia in patients with nasopharyngeal carcinoma. Asian J Surg. 1998;21:282‐287. [Google Scholar]
- 16. Wang L, Miao J, Huang H, et al. Long‐term survivals, toxicities and the role of chemotherapy in early‐stage nasopharyngeal carcinoma patients treated with intensity‐modulated radiation therapy: a retrospective study with 15‐year follow‐up. Cancer Res Treat. 2022;54:118‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kam MK, Teo PM, Chau RM, et al. Treatment of nasopharyngeal carcinoma with intensity‐modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60:1440‐1450. [DOI] [PubMed] [Google Scholar]
- 18. Patterson M, Brain R, Chin R, et al. Functional swallowing outcomes in nasopharyngeal cancer treated with IMRT at 6 to 42 months post‐radiotherapy. Dysphagia. 2014;29:663‐670. [DOI] [PubMed] [Google Scholar]
- 19. Fang FM, Chiu HC, Kuo WR, et al. Health‐related quality of life for nasopharyngeal carcinoma patients with cancer‐free survival after treatment. Int J Radiat Oncol Biol Phys. 2002;53:959‐968. [DOI] [PubMed] [Google Scholar]
- 20. Liao KC, Chuang HC, Chien CY, et al. Quality of life as a mediator between cancer stage and long‐term mortality in nasopharyngeal cancer patients treated with intensity‐modulated radiotherapy. Cancers (Basel). 2021;13:5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun Y, Chen X, Qiao J, et al. Effects of transcutaneous neuromuscular electrical stimulation on swallowing disorders: a systematic review and meta‐analysis. Am J Phys Med Rehabil. 2020;99:701‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frost J, Robinson HF, Hibberd J. A comparison of neuromuscular electrical stimulation and traditional therapy, versus traditional therapy in patients with longstanding dysphagia. Curr Opin Otolaryngol Head Neck Surg. 2018;26:167‐173. [DOI] [PubMed] [Google Scholar]
- 23. Peng G, Masood K, Gantz O, Sinha U. Neuromuscular electrical stimulation improves radiation‐induced fibrosis through Tgf‐Β1/MyoD homeostasis in head and neck cancer. J Surg Oncol. 2016;114:27‐31. [DOI] [PubMed] [Google Scholar]
- 24. Bhatt AD, Goodwin N, Cash E, et al. Impact of transcutaneous neuromuscular electrical stimulation on dysphagia in patients with head and neck cancer treated with definitive chemoradiation. Head Neck. 2015;37:1051‐1056. [DOI] [PubMed] [Google Scholar]
- 25. Ryu JS, Kang JY, Park JY, et al. The effect of electrical stimulation therapy on dysphagia following treatment for head and neck cancer. Oral Oncol. 2009;45:665‐668. [DOI] [PubMed] [Google Scholar]
- 26. Langmore SE, McCulloch TM, Krisciunas GP, et al. Efficacy of electrical stimulation and exercise for dysphagia in patients with head and neck cancer: a randomized clinical trial. Head Neck. 2016;38:E1221‐E1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Long YB, Wu XP. A randomized controlled trail of combination therapy of neuromuscular electrical stimulation and balloon dilatation in the treatment of radiation‐induced dysphagia in nasopharyngeal carcinoma patients. Disabil Rehabil. 2013;35:450‐454. [DOI] [PubMed] [Google Scholar]
- 28. Lin PH, Hsiao TY, Chang YC, et al. Effects of functional electrical stimulation on dysphagia caused by radiation therapy in patients with nasopharyngeal carcinoma. Support Care Cancer. 2011;19:91‐99. [DOI] [PubMed] [Google Scholar]
- 29. Krekeler BN, Broadfoot CK, Johnson S, Connor NP, Rogus‐Pulia N. Patient adherence to dysphagia recommendations: a systematic review. Dysphagia. 2018;33:173‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wall LR, Ward EC, Cartmill B, Hill AJ, Porceddu SV. Adherence to a prophylactic swallowing therapy program during (chemo) radiotherapy: impact of service‐delivery model and patient factors. Dysphagia. 2017;32:279‐292. [DOI] [PubMed] [Google Scholar]
- 31. Schindler A, Denaro N, Russi EG. Dysphagia in head and neck cancer patients treated with radiotherapy and systemic therapies: literature review and consensus. Crit Rev Oncol/Hematol. 2015;96:372‐384. [DOI] [PubMed] [Google Scholar]
- 32. van der Molen L, van Rossum MA, Burkhead LM, Smeele LE, Rasch CR, Hilgers FJ. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemoradiotherapy: feasibility, compliance, and short‐term effects. Dysphagia. 2011;26:155‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kotz T, Federman AD, Kao J, et al. Prophylactic swallowing exercises in patients with head and neck cancer undergoing chemoradiation: a randomized trial. Arch Otolaryngol Head Neck Surg. 2012;138:376‐382. [DOI] [PubMed] [Google Scholar]
- 34. Virani A, Kunduk M, Fink DS, McWhorter AJ. Effects of 2 different swallowing exercise regimens during organ‐preservation therapies for head and neck cancers on swallowing function. Head Neck. 2015;37:162‐170. [DOI] [PubMed] [Google Scholar]
- 35. Shinn EH, Basen‐Engquist K, Baum G, et al. Adherence to preventive exercises and self‐reported swallowing outcomes in post‐radiation head and neck cancer patients. Head Neck. 2013;35:1707‐1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhen Y, Wang JG, Tao D, Wang HJ, Chen WL. Efficacy survey of swallowing function and quality of life in response to therapeutic intervention following rehabilitation treatment in dysphagic tongue cancer patients. Eur J Oncol Nurs. 2012;16:54‐58. [DOI] [PubMed] [Google Scholar]
- 37. Tong MC, Lo PS, Wong KH, et al. Development and validation of the functional assessment of cancer therapy nasopharyngeal cancer subscale. Head Neck. 2009;31:738‐747. [DOI] [PubMed] [Google Scholar]
- 38. Cichero JA, Lam P, Steele CM, et al. Development of international terminology and definitions for texture‐modified foods and thickened fluids used in dysphagia management: the IDDSI framework. Dysphagia. 2017;32:293‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosenbek J, Robbins J, Roecker E, et al. A penetration aspiration scale. Dysphagia. 1996;11:93‐98. [DOI] [PubMed] [Google Scholar]
- 40. Wijting Y, Freed M. VitalStim Therapy Training Manual – Electrode Placement Abstract. Chattanooga Group; 2003:4. https://www.djoglobal.com/sites/default/files/vitalstim/VitalStim_Therapy_Electrode_Placement_On_The_Neck.pdf [Google Scholar]
- 41. Murray T, Hegland KW. Nonsurgical therapeutic intervention for swallowing disorders. In: Carrau RL, ed. Comprehensive Management of Swallowing Disorders. Plural Publishing, Inc; 2017:337‐346. [Google Scholar]
- 42. Starmer HM, Arrese L, Langmore S, et al. Adaptation and validation of the dynamic imaging grade of swallowing toxicity for flexible endoscopic evaluation of swallowing: DIGEST‐FEES. J Speech Lang Hear Res. 2021;64:1802‐1810. [DOI] [PubMed] [Google Scholar]
- 43. Salama JK, Stenson KM, List MA, et al. Characteristics associated with swallowing changes after concurrent chemotherapy and radiotherapy in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134:1060‐1065. [DOI] [PubMed] [Google Scholar]
- 44. Crary MA, Carnaby Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. 2005;86:1516‐1520. [DOI] [PubMed] [Google Scholar]
- 45. Mortensen HR, Jensen K, Aksglæde K, Lambertsen K, Eriksen E, Grau C. Prophylactic swallowing exercises in head and neck cancer radiotherapy. Dysphagia. 2015;30:304‐314. [DOI] [PubMed] [Google Scholar]
- 46. Messing BP, Ward EC, Lazarus CL, et al. Prophylactic swallow therapy for patients with head and neck cancer undergoing chemoradiotherapy: a randomized trial. Dysphagia. 2017;32(4):487‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang W, Nie W, Zhou X, et al. Review of prophylactic swallowing interventions for head and neck cancer. Int J Nurs Stud. 2021;123:104074. [DOI] [PubMed] [Google Scholar]
- 48. Starmer HM. Dysphagia in head and neck cancer: prevention and treatment. Curr Opin Otolaryngol Head Neck Surg. 2014;22:195‐200. [DOI] [PubMed] [Google Scholar]
- 49. Wang R, Kang M. Guidelines for radiotherapy of nasopharyngeal carcinoma. Prec Radiat Oncol. 2021;5:122‐159. [Google Scholar]
- 50. Ku PK, Yuen EH, Cheung DM, et al. Early swallowing problems in a cohort of patients with nasopharyngeal carcinoma: symptomatology and videofluoroscopic findings. Laryngoscope. 2007;117:142‐146. [DOI] [PubMed] [Google Scholar]
- 51. Ng LK, Lee KY, Chiu SN, Ku PK, van Hasselt CA, Tong MC. Silent aspiration and swallowing physiology after radiotherapy in patients with nasopharyngeal carcinoma. Head Neck. 2011;33:1335‐1339. [DOI] [PubMed] [Google Scholar]
- 52. Ku PK, Vlantis AC, Leung SF, et al. Laryngopharyngeal sensory deficits and impaired pharyngeal motor function predict aspiration in patients irradiated for nasopharyngeal carcinoma. Laryngoscope. 2010;120:223‐228. [DOI] [PubMed] [Google Scholar]
- 53. Law T, Lee KY, Wong RW, et al. Effects of electrical stimulation on vocal functions in patients with nasopharyngeal carcinoma. Laryngoscope. 2017;127:1119‐1124. [DOI] [PubMed] [Google Scholar]
- 54. Labeit B, Ahring S, Boehmer M, et al. Comparison of simultaneous swallowing endoscopy and videofluoroscopy in neurogenic dysphagia. J Am Med Dir Assoc. 2021;23(8):1360‐1366. [DOI] [PubMed] [Google Scholar]
- 55. Tong MC, Lee KY, Yuen MT, Lo PS. Perceptions and experiences of post‐irradiation swallowing difficulties in nasopharyngeal cancer survivors. Eur J Cancer Care (Engl). 2011;20:170‐178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1: Rosenbek's penetration and aspiration scale (PAS).
TABLE S2: Swallowing Performance and Severity Scale (SPSS).
TABLE S3: Functional Oral Intake Scale (FOIS).


