Summary
Poor sleep quality is a known risk factor for Alzheimer’s disease. This longitudinal imaging study aimed to determine the acceleration in the rates of tissue loss in cognitively critical brain regions due to poor sleep in healthy elderly individuals. Cognitively-normal healthy individuals, aged ≥60 years, reported Pittsburgh Sleep Quality Index (PSQI) and underwent baseline and 2-year follow-up magnetic resonance imaging brain scans. The links between self-reported sleep quality, rates of tissue loss in cognitively-critical brain regions, and white matter hyperintensity load were assessed. A total of 48 subjects were classified into normal (n = 23; PSQI score <5) and poor sleepers (n = 25; PSQI score ≥5). The two groups were not significantly different in terms of age, gender, years of education, ethnicity, handedness, body mass index, and cognitive performance. Compared to normal sleepers, poor sleepers exhibited much faster rates of volume loss, over threefold in the right hippocampus and fivefold in the right posterior cingulate over 2 years. In contrast, there were no significant differences in the rates of volume loss in the cerebral and cerebellar grey and white matter between the two groups. Rates of volume loss in the right posterior cingulate were negatively associated with global PSQI scores. Poor sleep significantly accelerates volume loss in the right hippocampus and the right posterior cingulate cortex. These findings demonstrate that self-reported sleep quality explains inter-individual differences in the rates of volume loss in cognitively-critical brain regions in healthy older adults and provide a strong impetus to offer sleep interventions to cognitively normal older adults who are poor sleepers.
Keywords: hippocampus, longitudinal study, neurodegeneration, posterior cingulate cortex, sleep quality
1 |. INTRODUCTION
Poor sleep is one of several known life-style stressors that increase the risk of cognitive decline (Gildner, Liebert, Kowal, Chatterji, & Snodgrass, 2014) and dementia (Sterniczuk, Theou, Rusak, & Rockwood, 2013). While the mechanisms by which poor sleep contributes to cognitive decline are not fully understood, this stressor impacts the brain directly and is associated with increased deposition of beta-amyloid (Branger et al., 2016; Brown et al., 2016; Sprecher et al., 2015) and neurodegeneration (Alperin et al., 2019; Carvalho et al., 2017; Fjell et al., 2020; Lim et al., 2016; Liu et al., 2021; Sexton, Storsve, Walhovd, Johansen-Berg, & Fjell, 2014). A bidirectional relationship between poor sleep and neurodegeneration has also been suggested (Holth, Patel, & Holtzman, 2017; Ju, Lucey, & Holtzman, 2014). Although poor sleep quality is common in the general population and is prevalent in the elderly population (Cooke & Ancoli-Israel, 2011), studies of its impact on rates of global and regional brain tissue loss in healthy elderly individuals have been sporadic and inconclusive.
Cross-sectional imaging studies in cognitively-normal individuals, demonstrated that compared with normal sleepers, poor sleepers had smaller volumes of bilateral hippocampi (Alperin et al., 2019; Carvalho et al., 2017; Liu et al., 2021), left posterior cingulate (Heidbreder et al., 2017), lateral orbitofrontal cortices, inferior frontal gyri pars orbitalis (Lim et al., 2016), left amygdala (Alperin et al., 2019), and right superior frontal cortex (Sexton et al., 2014). Differences in volumes between normal and poor sleepers suggested by these studies are likely due to different rates of volume loss that cannot be determined by cross-sectional studies. To date, only three longitudinal studies have been done to determine actual rates of atrophy with conflicting results. Fjell et al. estimated the differences in the rate of hippocampal volume loss between poor and normal sleepers and demonstrated that subjects with high score of poor sleep showed 0.22% greater annual loss than low scorers (Fjell et al., 2020). The two other studies did not find associations between poor sleep and rates of volume loss in the hippocampus and posterior cingulate cortex (Lo, Loh, Zheng, Sim, & Chee, 2014; Sexton et al., 2014).
In a previous cross-sectional study in cognitively-normal elderly adults (Alperin et al., 2019), we documented significantly smaller volumes, in the order of 7%, in the bilateral hippocampi, the bilateral superior parietal lobules, and the left amygdala, and thinner thicknesses in the right superior frontal, right medial orbitofrontal, and right frontal pole of the self-reported poor sleepers. In this follow-up longitudinal study, we compared rates of tissue loss over 2 years in global and specific brain regions between cognitively-normal elderly poor and normal sleepers. The same brain regions analysed in the previous cross-sectional study (Alperin et al., 2019) were analysed in this study. Changes in volumes were tested for the hippocampi, posterior cingulate cortices, superior parietal lobules and amygdalae, which are susceptible to amnestic mild cognitive impairment (aMCI; Goerlich et al., 2017; Nickl-Jockschat et al., 2012; Yang et al., 2012). Changes in cortical thicknesses were tested for the superior frontal, rostral middle frontal, lateral and medial orbitofrontal, pars orbitalis and frontal pole, which are linked to poor sleep (Chao, Mohlenhoff, Weiner, & Neylan, 2014; Lim et al., 2016; Suh, Kim, Dang-Vu, Joo, & Shin, 2016). We hypothesised that rates of tissue loss in these cognitively-critical brain regions would be faster in poor sleepers relative to normal sleepers and would be associated with sleep quality scores in cognitively normal older adults.
2 |. METHODS
2.1 |. Subjects
This study was conducted at the University of Miami between January 2016 and December 2019, following approval by the Institutional Review Board. Subjects were healthy community dwellers aged ≥60 years who were compensated for travel-related expenses. All subjects provided informed consent. Exclusion criteria included pre-existing neurocognitive disorder, active major depression, or depressive symptoms as indicated by a Geriatric Depression Scale (GDS) score of >9 or any other neuropsychiatric disorder or major co-morbidities (e.g. obesity, untreated hypertension). Subjects with a global Clinical Dementia Rating Scale (CDR) score of 0 and memory and non-memory cognitive measures that were normal according to age- and education-related norms (<1.0 SD below normative values for all tests) were considered cognitively normal. Details of clinical assessment and cognitive tests were described previously (Alperin et al., 2019; Liu et al., 2021). A total of 48 subjects of the 69 subjects in our previous cross-sectional study (Alperin et al., 2019), had a 2-year follow-up scan and were included in the present study. Subjects provided details on their sleep habits using the Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989), which consists of seven components: subjective sleep quality, latency, duration, efficiency, disturbance, sleeping medication, and daytime sleepiness over a 1-month period. The disturbance component includes items such as sleep latency and nocturnal awakening related to insomnia, and breathing comfortably, coughing/snoring related to sleep apnea. A global PSQI score of ≥5 (out of 21) was classified as poor sleep quality (Buysse et al., 1989; Salahuddin et al., 2017). Of the 48 subjects, 23 were classified as normal sleepers (PSQI score <5) and 25 as poor sleepers (PSQI score ≥5).
2.2 |. Magnetic resonance imaging (MRI) acquisition and processing
T2-weighted fluid-attenuated inversion recovery (FLAIR) and three-dimensional T1-weighted magnetisation-prepared rapid gradient-echo (MPRAGE) sequences acquired on a 3T MR scanner (Skyra, Siemens Healthineers) were used to estimate white matter hyperintensity (WMH) load, and volumes and thicknesses of brain region of interest (ROI), respectively. The WMH load was measured to verify possible influence of sleep quality on vascular diseases. The FLAIR data had an in-plane resolution of 0.72 × 0.96 mm2 and 2.2 mm slice thickness. The MPRAGE data had a 1.0 mm isotropic resolution. The WMH volumes were segmented and measured with a previously developed and validated automated image segmentation method that is based on Gaussian modelling of the grey matter (GM) and white matter (WM) intensities (Alperin et al., 2014).
The volumes and thicknesses of cerebral and cerebellar GM and WM regions and the cognitive-critical regions were measured at baseline and at follow-up scans to assess the rates of tissue loss. Baseline and follow-up scans were first automatically processed independently with FreeSurfer 5.3 (http://surfer.nmr.mgh.harvard.edu) to perform subcortical segmentation and cortical parcellation according to the Desikan-Killiany atlas (Desikan et al., 2006). Subsequently, the same scans were automatically processed with the longitudinal processing pipeline (Reuter, Schmansky, Rosas, & Fischl, 2012). Specifically, an unbiased within-subject template was created based on the cross-sectional brain segmentation at baseline and follow-up, using robust, inverse consistent registration (Reuter, Rosas, & Fischl, 2010). Several longitudinal processing steps, such as skull stripping, Talairach transformation, atlas registration, brain segmentation and parcellation were then initialised with common information from the within-subject template (Reuter et al., 2012). Outputs of FreeSurfer were examined visually to check for segmentation errors or failures. Rates of change in global GM and WM volumes and in volume and thickness for each ROI were normalised for a period of 2 years to enable comparison between subjects.
2.3 |. Statistical analysis
Statistical analyses were performed using SPSS (Version 24) and Excel (Windows Version 2016). Regression models adjusting for age, gender, and ethnicity were used to compare rates of volume and thickness losses over the 2-year period between normal and poor sleepers. Regression model adjusting for age, gender and ethnicity was also used to compare WMH volumes between the two groups, after transforming absolute WMH volumes to rank. Because of a priori directional hypotheses, one-tailed partial Spearman’s correlation analyses adjusting for age, gender, and ethnicity were used to estimate associations of rates of volume and cortical thickness losses over 2 years, and WMH volumes with self-reported sleep measures based on the PSQI. These sleep measures were: global PSQI scores; scores of component 1 (subjective sleep quality); component 2 (sleep latency); component 5 (sleep disturbances), item 5b (nocturnal awakening), item 5d (breathing comfortably), item 5e (coughing/snoring); and component 7 (daytime sleepiness); as well as item 4 sleep duration in hours and component 4 sleep efficiency in percentages. All were treated as continuous variables in the analyses. As insomnia and sleep apnea are the most common types of sleep disturbances, selected items for the component 5 (sleep disturbances) such as sleep latency and nocturnal awakening related to insomnia, and breathing comfortably, coughing/snoring related to sleep apnea were further included. Given that effects of sleep medications on sleep are not well understood and do not directly reflect sleep patterns, the component 6 (sleep medications) was not included in the sleep measures. Bootstrapping techniques using 1,000 iterations with simple sampling and bias corrected and accelerated 95% confidence intervals were also employed to validate statistical findings. Multiple comparison correction was performed across the ROIs of both hemispheres and sleep measures if applicable with the false discovery rate (FDR) procedure. An FDR level of 0.05 was used to denote statistical significance in these analyses.
3 |. RESULTS
3.1 |. Subject information
The mean (SD) time interval between baseline and follow-up brain MRI scans was 2.3 (0.3) years. Subjects were classified into two groups: 23 normal sleepers (PSQI score <5) and 25 poor sleepers (PSQI score ≥5). There was no significant difference between the two groups in terms of age, gender distribution, ethnicity, education, body mass index, handedness, and cognitive performance. Global PSQI scores were not significantly associated with years of education (Spearman’s correlation coefficient rs = −0.079; p = 0.6). The mean (SD) WMH volumes between normal and poor sleepers were not significantly different, at 3.78 (3.83) versus 4.64 (2.95) cm3 (p = 0.148). Additionally, there was a trend toward a negative association between WMH volumes and sleep efficiency (rs = −0.384, p-FDR = 0.09). The demographics, subject characteristics, sleep quality measures, and scores of cognitive tests for the two groups are summarised in Table 1.
TABLE 1.
Demographics, sleep quality measures, and cognitive scores
| Normal sleeper | Poor sleeper | P | |
|---|---|---|---|
| Total number of subjects | 23 | 25 | - |
|
| |||
| Age at baseline, years, mean (SD) | 68.5 (5.7) | 70.1 (8.7) | 0.852a |
|
| |||
| Gender | 14F/9M (61% F) | 20F/5M (80% F) | 0.255b |
|
| |||
| Ethnicity, % | 0.312b | ||
| White Non-Hispanic | 43.5 | 48.0 | - |
| Hispanic | 52.2 | 36.0 | - |
| African-American | 4.3 | 16 | - |
|
| |||
| Education, years, mean (SD) | 15.1 (4.2) | 15.2 (2.1) | 0.909a |
|
| |||
| Right-handedness, % | 90.9 | 95.8 | 0.792b |
|
| |||
| Body mass index, kg/m2, mean (SD) | 27.8 (4.1) | 27.0 (4.3) | 0.415a |
|
| |||
| Sleep quality measures, mean (SD) | |||
| Global PSQI | 2.4 (1.2) | 7.1 (2.2) | <0.001a |
| PSQI component 1 subjective sleep quality | 0.6 (0.5) | 1.1 (0.7) | 0.013a |
| PSQI component 2 sleep latency | 0.3 (0.6) | 1.0 (0.8) | 0.001a |
| PSQI item 4 sleep duration (hr) | 7.3 (0.8) | 6.6 (1.2) | 0.067a |
| PSQI component 4 sleep efficiency (%) | 94.1 (6.1) | 81.8 (14.7) | 0.002a |
| PSQI component 5 sleep disturbances | 0.8 (0.5) | 1.2 (0.4) | 0.004a |
| PSQI item 5b nocturnal awakening | 0.6 (1.1) | 1.7 (1.3) | 0.005a |
| PSQI item 5d breathing comfortably | 0.2 (0.5) | 0.4 (1.0) | 0.587a |
| PSQI item 5e coughing/snoring | 0.4 (0.6) | 0.5 (1.1) | 0.557a |
| PSQI component 7 daytime sleepiness | 0.2 (0.6) | 1.0 (0.9) | 0.001a |
|
| |||
| Cognitive scores, mean (SD) | |||
| Global cognitive function z-score | −0.08 (0.70) | 0.09 (0.54) | 0.366c |
| HVLT-R – immediate total | 23.9 (4.2) | 25.6 (5.0) | 0.216c |
| HVLT-R – delayed | 9.0 (1.8) | 9.1 (1.9) | 0.827c |
| NACC delayed passage | 11.3 (3.8) | 12.8 (4.0) | 0.190c |
| WAIS-IV block design | 31.7 (10.1) | 33.6 (10.2) | 0.520c |
| Trail making test A – Time (s) | 39.9 (15.2) | 36.5 (11.2) | 0.381c |
| Trail making test B – Time (s) | 100.6 (40.1) | 90.4 (33.1) | 0.344c |
| Category fluency – TOTAL | 45.9 (12.6) | 45.8 (10.5) | 0.973c |
F, females; HVLT-R, Hopkins Verbal Learning Test-Revised; M, males; NACC, National Alzheimer’s Coordinating Center; PSQI, Pittsburgh Sleep Quality Index.
Z-scores for global cognitive function were calculated by averaging the z scores of HVLT-R Immediate Total and Delayed Recall and NACC Delayed Passage, WAIS-IV Block Design, Category Fluency, and Trail Making Test A and B.
Mann-Whitney U test.
Chi-square test with Yates correction.
Analyses of variance (ANOVAs).
3.2 |. Rates of volume and thickness loss in ROIs in normal and poor sleepers
Examples of MR images demonstrating the loss of tissue in the bilateral hippocampi and posterior cingulate cortices from two subjects, a normal sleeper and a poor sleeper, are shown in Figure 1. The segmentation results are marked by the colours superimposed on the MR images. The red and pink regions demonstrate the tissue lost from baseline to the 2-year follow-up. In all the tested cognitively critical regions, except from the superior parietal lobules, poor sleepers exhibited faster rates of volume loss compared with normal sleepers, as shown in Figure 2. However, significant differences were found only in the right hippocampus and right posterior cingulate cortex. The corresponding mean (SD) rates of volume loss in the poor and normal sleepers were −3.62 (3.02)% versus −1.10 (2.16)% (p-FDR = 0.007), and 2.66 (3.36)% versus −0.46 (1.86)% (p-FDR = 0.032), respectively. Average rate of volume loss in the poor sleepers were approximately over threefold faster in the right hippocampus, and over fivefold faster in the right posterior cingulate cortex relative to normal sleepers. The mean and SD of the measurements of the 2-year rates of volume loss are listed in Table 2. Unlike the specific cognitively critical regions, the 2-year rates of volume loss in GM and WM regions between normal and poor sleepers were not significantly different. The mean (SD) rates of volume change were −0.73 (1.76)% versus −0.72 (1.73)% (p-FDR = 0.829) for the cerebral GM, −1.40 (2.2)% versus −0.74 (1.95)% (p-FDR = 0.526) for the cerebellar GM, −1.01 (0.89)% versus −1.12 (0.79)% (p-FDR = 0.829) for the cerebral WM, and −0.83 (3.52)% versus 0.52 (3.23)% (p-FDR = 0.526) for the cerebellar WM.
FIGURE 1.
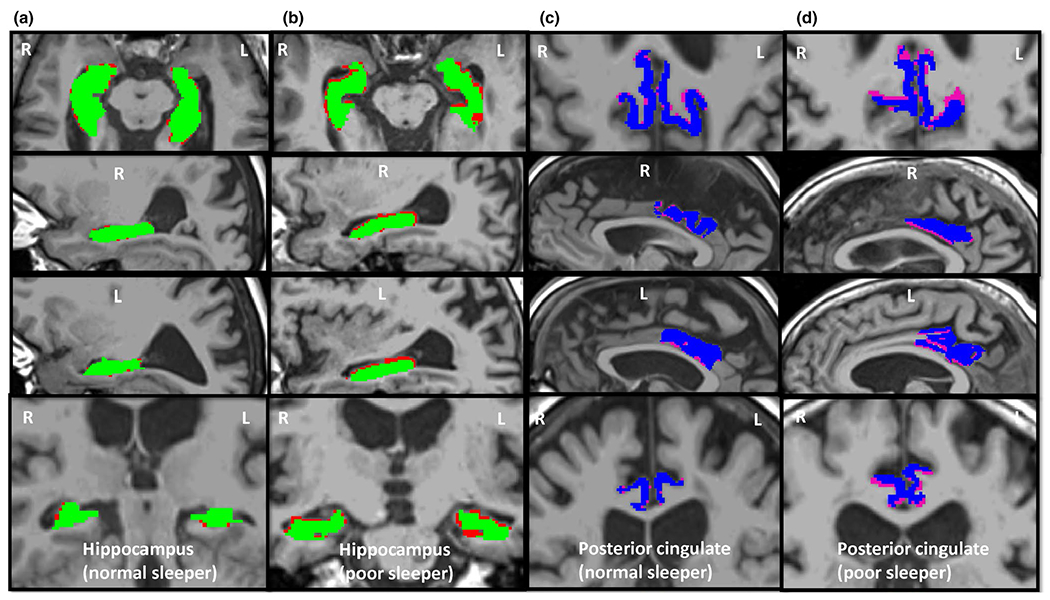
Magnetic resonance images demonstrating volume loss over 2 years in the bilateral hippocampi and posterior cingulate cortices of a normal and a poor sleeper. Red and pink regions represent the tissue loss in the hippocampi and posterior cingulate, respectively, in axial, sagittal and coronal views. Green and blue regions represent hippocampi and posterior cingulate cortices at follow-up, respectively. (a) and (c) columns are from a normal sleeper (female, aged 63 years, PSQI score = 2, global cognitive function z-score = 0.31, average rates of volume loss for hippocampus and posterior cingulate are −1.44% and −0.24%, respectively), and (b) and (d) columns are from a poor sleeper (female, aged 89 years, PSQI score = 8, global cognitive function z-score = −0.48, average rates of volume loss for hippocampus and posterior cingulate are −7.36% and −5.8%, respectively). PSQI, Pittsburgh Sleep Quality Index
FIGURE 2.
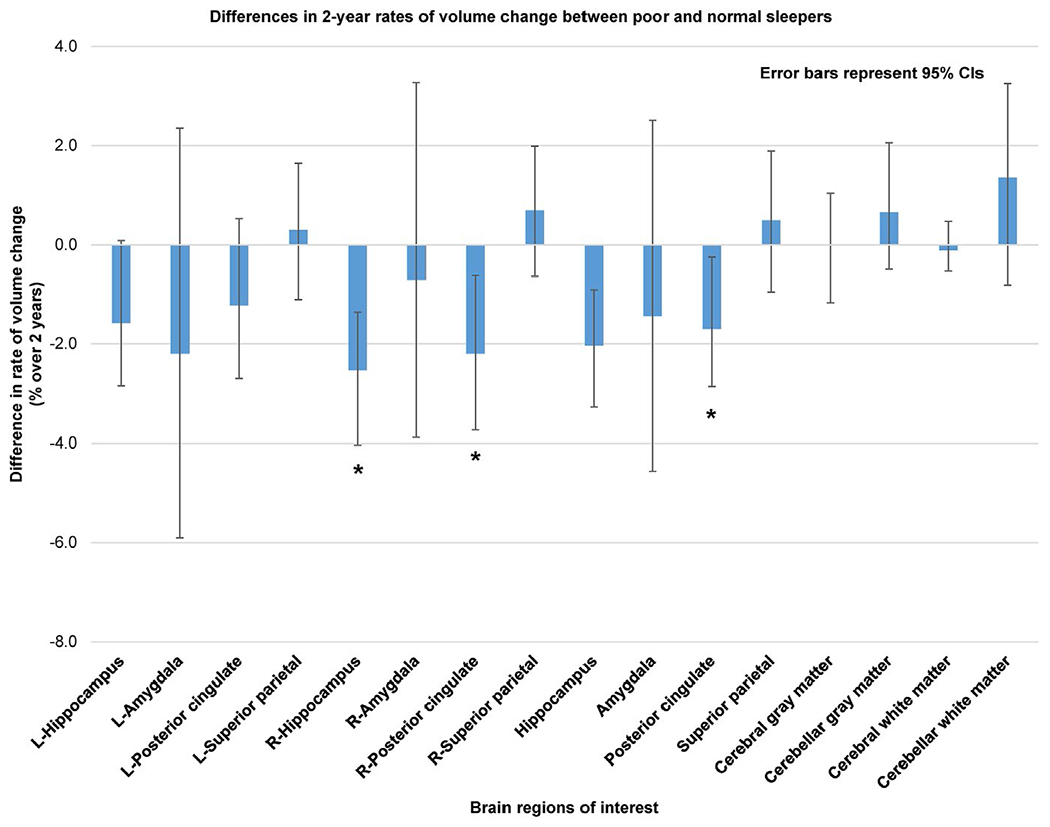
Differences in 2-year rates of volume change between poor and normal sleepers. Blue bars represent the mean differences in 2-year rates of volume change in brain regions of interest between poor and normal sleepers. Negative values indicate faster volume loss in poor sleepers compared to normal sleepers. * indicate regions of significant differences after multiple comparison correction with false discovery rate (FDR) = 0.05. Error bars represent 95% confidence intervals (CIs) estimated using bootstrapping with 1,000 iterations
TABLE 2.
Comparison of 2-year rates of volume change in ROIs between normal and poor sleepers
| Normal sleepers Rate of volume change |
Poor sleepers Rate of volume change |
p-FDR for rate in % | Ratio of poor to normal sleepers | Difference between poor and normal sleepers, % | 95% CI of the difference, % | |||
|---|---|---|---|---|---|---|---|---|
| ROIs | Mean (SD), cm3 | Mean (SD), % | Mean (SD), cm3 | Mean (SD), % | ||||
| Left | ||||||||
| Hippocampus | −0.05 (0.09) | −1.18 (2.50) | −0.10 (0.08) | −2.76 (2.38) | 0.149 | 2.34 | −1.58 | −2.84 to 0.08 |
| Amygdala | −0.008 (0.10) | −0.34 (6.64) | −0.04 (0.09) | −2.54 (6.14) | 0.444 | 7.47 | −2.20 | −5.9 to 2.36 |
| Posterior cingulate | −0.03 (0.05) | −0.96 (1.86) | −0.06 (0.09) | −2.18 (3.12) | 0.444 | 2.27 | −1.22 | −2.69 to 0.53 |
| Superior parietal | −0.13 (0.21) | −1.04 (1.82) | −0.10 (0.33) | −0.74 (2.90) | 0.752 | 0.71 | 0.30 | −1.1 to 1.64 |
|
| ||||||||
| Right | ||||||||
| Hippocampus | −0.04 (0.08) | −1.10 (2.16) | −0.13 (0.10) | −3.62 (3.02) | 0.007a | 3.29 | −2.52 | −4.04 to −1.36 |
| Amygdala | −0.03 (0.09) | −1.96 (6.00) | −0.04 (0.09) | −2.66 (5.88) | 0.909 | 1.36 | −0.70 | −3.89 to 3.27 |
| Posterior cingulate | −0.01 (0.05) | −0.46 (1.86) | −0.08 (0.08) | −2.66 (3.36) | 0.032a | 5.78 | −2.20 | −3.73 to −0.62 |
| Superior parietal | −0.16 (0.23) | −1.32 (2.02) | −0.09 (0.28) | −0.62 (2.64) | 0.444 | 0.47 | 0.70 | −0.64 to 1.99 |
|
| ||||||||
| Both hemispheres combined | ||||||||
| Hippocampus | −0.09 (0.15) | −1.14 (2.06) | −0.23 (0.15) | −3.18 (2.24) | 0.008a | 2.79 | −2.04 | −3.27 to −0.9 |
| Amygdala | −0.04 (0.16) | −1.22 (5.11) | −0.08 (0.15) | −2.66 (5.26) | 0.493 | 2.18 | −1.44 | −4.56 to 2.51 |
| Posterior cingulate | −0.04 (0.09) | −0.73 (1.56) | −0.14 (0.15) | −2.43 (2.84) | 0.056b | 3.33 | −1.70 | −2.86 to −0.25 |
| Superior parietal | −0.29 (0.41) | −1.18 (1.77) | −0.19 (0.55) | −0.68 (2.54) | 0.493 | 0.58 | 0.50 | −0.95 to 1.89 |
CI, confidence interval; FDR, false discovery rate; ROI, region of interest.
Regression models adjusting for age, gender, and ethnicity were used to compare 2-year rates of volume change in ROIs between normal and poor sleepers. Negative values of rates of volume change indicate volume loss. The 95% CIs were estimated using bootstrapping with 1,000 iterations.
Significant differences between normal and poor sleepers after multiple comparisons correction with FDR = 0.05 across all ROIs of both hemispheres.
Marginal differences between normal and poor sleepers after multiple comparisons correction with FDR = 0.1 across all ROIs of both hemispheres.
Differences between normal and poor sleepers in rates of cortical thickness changes were also not significant for all tested ROIs (i.e. superior frontal, rostral middle frontal, lateral and medial orbitofrontal, pars orbitalis, and frontal pole). The mean and SD of the measurements of the 2-year cortical thickness loss are listed in Table 3.
TABLE 3.
Comparison of 2-year rates of cortical thickness change in ROIs between normal and poor sleepers
| Normal sleeper Rate of thickness change |
Poor sleeper Rate of thickness change |
p-FDR for rate in % | 95% CI of the difference, % | |||
|---|---|---|---|---|---|---|
| ROIs | Mean (SD), mm | Mean (SD), % | Mean (SD), mm | Mean (SD), % | ||
| Left | ||||||
| Lateral orbitofrontal | −0.01 (0.05) | −0.44 (1.88) | −0.02 (0.06) | −0.93 (2.35) | 0.91 | −1.76 to 0.61 |
| Medial orbitofrontal | −0.04 (0.07) | −1.74 (2.84) | −0.06 (0.06) | −2.52 (2.63) | 0.91 | −2.20 to 1.23 |
| Pars orbitalis | −0.007 (0.05) | −0.21 (1.97) | −0.007 (0.09) | −0.25 (3.29) | 0.91 | −1.75 to 1.39 |
| Rostral middle frontal | −0.006 (0.05) | −0.25 (2.33) | −0.01 (0.05) | −0.55 (2.24) | 0.91 | −1.81 to 1.21 |
| Superior frontal | −0.02 (0.05) | −0.83 (1.87) | −0.02 (0.05) | −0.78 (1.99) | 0.91 | −1.22 to 1.31 |
| Frontal pole | 0.002 (0.11) | 0.12 (4.00) | 0.01 (0.09) | 0.40 (3.44) | 0.91 | −1.82 to 2.43 |
|
| ||||||
| Right | ||||||
| Lateral orbitofrontal | −0.02 (0.04) | −0.98 (1.67) | −0.04 (0.06) | −1.60 (2.37) | 0.91 | −1.79 to 0.43 |
| Medial orbitofrontal | −0.03 (0.08) | −1.04 (3.14) | −0.06 (0.06) | −2.36 (2.74) | 0.91 | −2.55 to 0.38 |
| Pars orbitalis | −0.02 (0.11) | −0.54 (4.04) | −0.02 (0.07) | −0.72 (2.51) | 0.91 | −2.66 to 1.80 |
| Rostral middle frontal | −0.001 (0.05) | −0.01 (2.15) | −0.02 (0.06) | −1.0 (2.56) | 0.528 | −2.80 to 0.08 |
| Superior frontal | −0.02 (0.04) | −0.67 (1.55) | −0.02 (0.05) | −0.70 (1.94) | 0.91 | −1.18 to 0.97 |
| Frontal pole | 0.001 (0.10) | 0.16 (3.91) | −0.02 (0.10) | −0.62 (3.76) | 0.91 | −3.26 to 1.64 |
CI, confidence interval; FDR, false discovery rate; ROI, region of interest.
Regression models adjusting for age, gender, and ethnicity were used to compare 2-year rates of thickness change in ROIs between normal and poor sleepers. Negative values of rates of thickness change indicate thickness loss. The 95% CIs were estimated using bootstrapping with 1,000 iterations. No values were significantly different between normal and poor sleepers after multiple comparisons correction with FDR = 0.05 across all ROIs of both hemispheres.
3.3 |. Associations between rates of volume and cortical thickness loss and sleep measures
Over the 2 years, the rates of volume loss of right posterior cingulate cortex were significantly negatively correlated with global PSQI scores (rs = −0.509, p-FDR = 0.017). There was a trend toward a negative correlation between rates of volume loss of the right hippocampus and global PSQI scores (rs = −0.411, p-FDR = 0.08). Scattered plots of the 2-year rates of volume loss in ROIs versus the global PSQI are shown in Figure 3. While rates of volume loss in the cerebral and cerebellar GM as well as the cerebral WM regions were not significantly associated with any of the sleep measures, rates of volume loss in the cerebellar WM regions were significantly correlated with subjective sleep quality scores (rs = 0.484, p-FDR = 0.04). Interestingly, there was a trend toward a negative correlation between the rates of volume loss in the right posterior cingulate cortex and sleep latency scores (rs = −0.463, p-FDR = 0.08). Rates of volume loss in other ROIs were not significantly associated with other sleep measures, after multiple comparison correction with FDR = 0.05. Relationships between rates of volume and cortical thickness losses in the tested brain regions and the PSQI global and sub-scores are summarised in Tables 4 and 5, respectively and can be visualised in Figure 4a,b. However, after multiple comparison correction with FDR = 0.05, the rates of cortical thickness loss were not significantly associated with any of the sleep measures.
FIGURE 3.
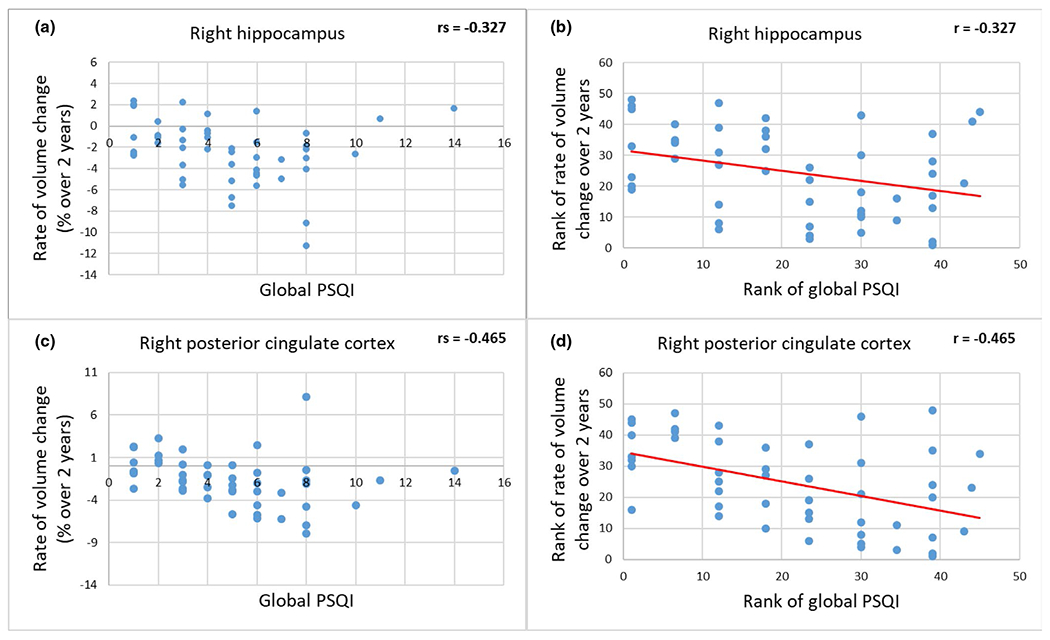
Relationships between rates of volume change in the right hippocampus and right posterior cingulate cortex and global PSQI. (a) and (b) are rates of volume change and their rank, respectively in the right hippocampus, and (c) and (d) are the corresponding rates in the posterior cingulate cortex. Rates of volume change in the right posterior cingulate cortex were significantly negatively associated with global PSQI scores (rs = −0.509, p-FDR = 0.017), after adjustment for age, gender, and ethnicity. There was a trend toward a negative association between rates of volume loss of the right hippocampus and global PSQI scores (rs = −0.411, p-FDR = 0.08). Negative values indicate volume loss, rs indicates Spearman’s correlation coefficient and r indicates Pearson’s correlation coefficient. FDR, false discovery rate; PSQI, Pittsburgh Sleep Quality Index
TABLE 4.
Relationships between rates of volume change in regions of interest (unit: % over 2 years) and Pittsburgh Sleep Quality Index measures
| Rate of volume change in ROIs | Global PSQI rs, p-FDR (95% CI) |
Subjective sleep quality rs, p-FDR (95% CI) |
Sleep latency rs, p-FDR (95% CI) |
Sleep duration rs, p-FDR (95% CI) |
Sleep efficiency rs, p-FDR (95% CI) |
|---|---|---|---|---|---|
| Left | |||||
| HP | −0.254, 0.380 (−0.54, 0.05) | 0.002, 0.50 (−0.30, 0.32) | −0.15, 0.411 (−0.48, 0.14) | 0.261, 0.380 (−0.06, 0.52) | 0.222, 0.40 (−0.08, 0.49) |
| Amygdala | −0.142, 0.411 (−0.40, 0.11) | −0.16, 0.411 (−0.49, 0.19) | −0.177, 0.411 (−0.49, 0.15) | 0.029, 0.473 (−0.31, 0.41) | 0.117, 0.421 (−0.17, 0.41) |
| Posterior cingulate | −0.14, 0.411 (−0.40, 0.18) | −0.206, 0.411 (−0.50, 0.10) | 0.092, 0.444 (−0.31, 0.51) | −0.146, 0.412 (−0.53, 0.30) | −0.115, 0.421 (−0.43, 0.26) |
| Superior parietal | 0.074, 0.467 (−0.22, 0.36) | 0.056, 0.473 (−0.28, 0.41) | 0.201, 0.411 (−0.14, 0.54) | −0.152, 0.411 (−0.46, 0.21) | −0.229, 0.40 (−0.54, 0.13) |
|
| |||||
| Right | |||||
| HP | −0.411, 0.08b (−0.65, −0.13) | −0.175, 0.411 (−0.47, 0.15) | −0.191, 0.411 (−0.51, 0.14) | 0.24, 0.40 (−0.04, 0.53) | 0.301, 0.356 (−0.02, 0.57) |
| Amygdala | 0.11, 0.421 (−0.18, 0.40) | −0.184, 0.411 (−0.49, 0.12) | 0.091, 0.444 (−0.31, 0.44) | −0.003, 0.50 (−0.34, 0.32) | 0.078, 0.467 (−0.24, 0.40) |
| Posterior cingulate | −0.509, 0.017a (−0.71, −0.29) | −0.202, 0.411 (−0.49, 0.12) | −0.463, 0.08b (−0.66, −0.22) | −0.117, 0.421 (−0.40, 0.17) | 0.052, 0.473 (−0.25, 0.36) |
| Superior parietal | 0.122, 0.421 (−0.20, 0.44) | 0.14, 0.412 (−0.19, 0.46) | 0.318, 0.356 (0.00, 0.61) | −0.114, 0.421 (−0.43, 0.19) | −0.266, 0.380 (−0.56, 0.03) |
CI, confidence interval; HP, hippocampus; PSQI, Pittsburgh Sleep Quality Index; ROI, region of interest; rs, Spearman’s correlation coefficient, FDR, false discovery rate.
One-tailed partial Spearman’s correlation analyses adjusting for age, gender and ethnicity were used to estimate associations between rates of volume loss in ROIs over 2 years and PSQI measures. The 95% CIs were estimated using bootstrapping with 1,000 iterations.
Significant correlations after multiple comparisons correction with FDR = 0.05 across all ROIs of both hemispheres and PSQI measures.
Marginal correlations after multiple comparisons correction with FDR = 0.1 across all ROIs of both hemispheres and PSQI measures.
TABLE 5.
Relationships between rates of cortical thickness change in regions of interest (unit: % over 2 years) and Pittsburgh Sleep Quality Index measures
| Rate of thickness change in ROIs | Global PSQI rs, p-FDR (95% CI) |
Subjective sleep quality rs, p-FDR (95% CI) |
Sleep latency rs, p-FDR (95% CI) |
Sleep duration rs, p-FDR (95% CI) |
Sleep efficiency rs, p-FDR (95% CI) |
|---|---|---|---|---|---|
| Left | |||||
| Lateral orbitofrontal | −0.173, 0.393 (−0.45, 0.12) | −0.204, 0.393 (−0.49, 0.08) | 0.051, 0.443 (−0.35, 0.57) | 0.005, 0.488 (−0.40, 0.51) | −0.096, 0.393 (−0.44, 0.32) |
| Medial orbitofrontal | −0.131, 0.393 (−0.45, 0.23) | −0.184, 0.393 (−0.49, 0.20) | 0.11, 0.393 (−0.31, 0.52) | 0.205, 0.393 (−0.18, 0.58) | 0.165, 0.393 (−0.19, 0.47) |
| Pars orbitalis | −0.076, 0.393 (−0.41, 0.24) | −0.238, 0.391 (−0.51, 0.08) | −0.092, 0.393 (−0.39, 0.27) | −0.092, 0.393 (−0.48, 0.37) | −0.092, 0.393 (−0.47, 0.38) |
| Rostral middle frontal | −0.074, 0.396 (−0.39, 0.26) | −0.187, 0.393 (−0.47, 0.16) | 0.086, 0.393 (−0.32, 0.51) | −0.028, 0.465 (−0.44, 0.44) | 0.048, 0.443 (−0.35, 0.48) |
| Superior frontal | −0.121, 0.393 (−0.43, 0.17) | −0.121, 0.393 (−0.44, 0.23) | 0.078, 0.397 (−0.31, 0.45) | −0.087, 0.393 (−0.45, 0.35) | −0.137, 0.393 (−0.46, 0.24) |
| Frontal pole | −0.028, 0.465 (−0.33, 0.31) | 0.068, 0.409 (−0.28, 0.42) | −0.013, 0.482 (−0.42, 0.36) | 0.096, 0.393 (−0.22, 0.36) | 0.11, 0.393 (−0.23, 0.40) |
|
| |||||
| Right | |||||
| Lateral orbitofrontal | −0.18, 0.393 (−0.49, 0.17) | −0.088, 0.393 (−0.39, 0.22) | −0.203, 0.393 (−0.50, 0.13) | 0.195, 0.393 (−0.13, 0.56) | 0.262, 0.391 (−0.08, 0.57) |
| Medial orbitofrontal | −0.323, 0.277 (−0.54, −0.08) | −0.048, 0.443 (−0.40, 0.28) | −0.312, 0.277 (−0.62, 0.07) | −0.024, 0.472 (−0.36, 0.38) | 0.009, 0.482 (−0.31, 0.32) |
| Pars orbitalis | −0.078, 0.393 (−0.37, 0.21) | −0.067, 0.409 (−0.41, 0.29) | 0.03, 0.465 (−0.36, 0.36) | 0.096, 0.393 (−0.24, 0.41) | 0.102, 0.393 (−0.23, 0.42) |
| Rostral middle frontal | −0.403, 0.180 (−0.64, −0.14) | −0.198, 0.393 (−0.50, 0.13) | −0.09, 0.393 (−0.40, 0.24) | 0.161, 0.393 (−0.22, 0.54) | 0.308, 0.277 (−0.01, 0.65) |
| Superior frontal | −0.13, 0.393 (−0.42, 0.21) | −0.099, 0.393 (−0.40, 0.25) | 0.009, 0.482 (−0.34, 0.42) | −0.171, 0.393 (−0.53, 0.32) | −0.178, 0.393 (−0.51, 0.25) |
| Frontal pole | −0.217, 0.393 (−0.52, 0.11) | −0.083, 0.393 (−0.39, 0.25) | −0.217, 0.393 (−0.52, 0.10) | −0.31, 0.277 (−0.59, −0.01) | −0.084, 0.393 (−0.40, 0.26) |
CI, confidence interval; FDR, false discovery rate; PSQI, Pittsburgh Sleep Quality Index; ROI, region of interest; rs, Spearman’s correlation coefficient.
One-tailed partial Spearman’s correlation analyses adjusting for age, gender, and ethnicity were used to estimate associations between rates of thickness loss in ROIs over 2 years and PSQI measures. The 95% CIs were estimated using bootstrapping with 1,000 iterations. No correlations were significant after multiple comparisons correction with FDR = 0.05 across all ROIs of both hemispheres and PSQI measures.
FIGURE 4.
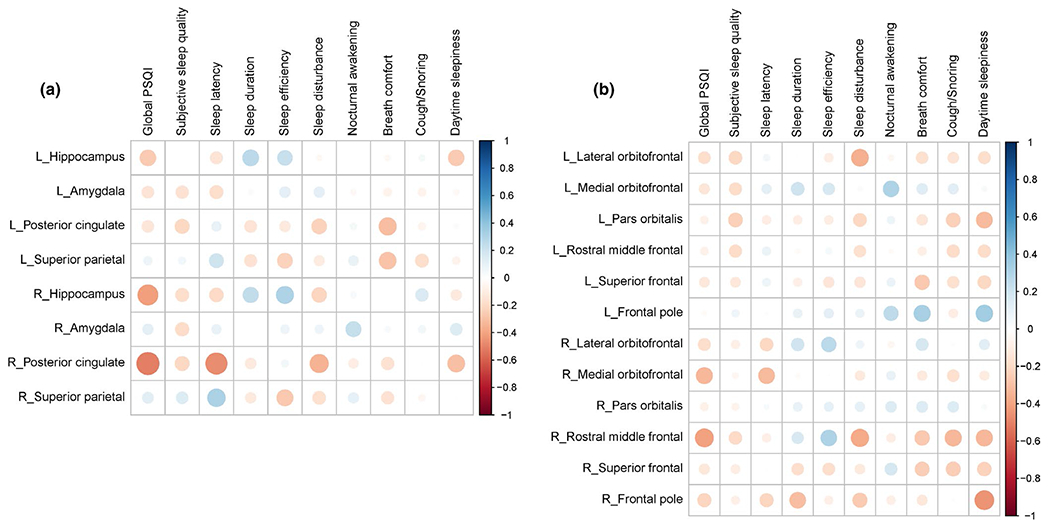
(a) Correlation matrix between rates of volume loss and sleep measures. (b) Correlation matrix between rates of cortical thickness loss and sleep measures. The areas of circles indicate the absolute values of Spearman’s correlation coefficients. Colour intensity indicates the values of Spearman’s correlation coefficients
4 |. DISCUSSION
The results of this longitudinal study in cognitively normal elderly individuals demonstrated a sizeable acceleration in the rates of tissue volume loss due to poor sleep quality in two brain regions involved in cognitive decline and dementia: the hippocampus and the posterior cingulate cortex. The mean rates of tissue volume loss in the other tested cognitively-critical regions, except the superior parietal lobules, were also faster, although differences did not reach statistical significance. Our findings indicate that the hippocampus and the posterior cingulate are most susceptible to the negative impact of poor sleep among the tested cognitively critical regions in cognitively-normal older adults. These findings provide a strong impetus to offer sleep interventions to older adults who have poor sleep quality.
In this study, as in a study by Fjell et al. (2020) with 1,299 participants, the average rate of the hippocampal volume loss was faster in the poor sleepers than in the normal sleepers, which is consistent with findings in previous cross-sectional studies demonstrating significantly smaller volumes of the hippocampus and its subfields in the poor sleepers, and significant associations between hippocampal volume and sleep quality and duration (Alperin et al., 2019; Liu et al., 2021). Yet, the ratio of the average rates of the hippocampal volume loss between the poor and normal sleepers in our cohort was ~2.8 compared with 2.0 in the Fjell et al. study (Fjell et al., 2020) for individuals in their sixth decade of life. This difference in the magnitude of the effect of poor sleep between the two studies can be related to differences in the study population and the threshold used in the classification of the subjects as either normal or poor sleepers. While these findings support causality between poor sleep and hippocampal neurodegeneration, a bidirectional relationship cannot be ruled out (Holth et al., 2017; Ju et al., 2014). However, brain areas regulating sleep and arousal cycle are primarily localised in the hypothalamus and pons (Rosenwasser & Turek, 2015). Poor sleep quality accelerated the annual hippocampal volume loss due to normal ageing by 1.0% in our study and only by 0.23% in the Fjell et al. (2020) study. Both findings establish the strong link between hippocampal volume loss and poor sleep. Yet, other longitudinal studies (Lo et al., 2014; Sexton et al., 2014) did not find significant associations between increased rates of volume loss in the hippocampus and self-reported poor sleep quality. The main differences between our study and the two studies that did not find accelerated hippocampal volume loss, were in ethnicity, field strength of MRI scanner, and in the methodologies of image analysis. The lack of consistency between studies warrants additional investigations to further explain differences in rate of hippocampal volume loss in poor sleepers.
| Sleep disturbance r, p-FDR (95% CI) |
Nocturnal awakening rs, p-FDR (95% CI) |
Breath comfort rs, p-FDR (95% CI) |
Cough/Snoring rs, p-FDR (95% CI) |
Daytime sleepiness rs, p-FDR (95% CI) |
|---|---|---|---|---|
| −0.031, 0.473 (−0.38, 0.28) | −0.001, 0.50 (−0.37, 0.36) | −0.034, 0.473 (−0.42, 0.33) | 0.04, 0.473 (−0.32, 0.37) | −0.253, 0.382 (−0.59, 0.05) |
| 0.11, 0.421 (−0.26, 0.41) | −0.033, 0.473 (−0.35, 0.27) | −0.069, 0.473 (−0.34, 0.20) | −0.066, 0.473 (−0.38, 0.26) | −0.033, 0.473 (−0.33, 0.29) |
| −0.226, 0.40 (−0.53, 0.11) | 0.046, 0.473 (−0.30, 0.38) | −0.307, 0.356 (−0.59, 0.03) | −0.058, 0.473 (−0.40, 0.33) | 0.023, 0.483 (−0.37, 0.43) |
| −0.109, 0.421 (−0.44, 0.30) | 0.098, 0.444 (−0.28, 0.47) | −0.288, 0.356 (−0.62, 0.15) | −0.174, 0.411 (−0.56, 0.24) | −0.061, 0.473 (−0.41, 0.37) |
|
| ||||
| −0.217, 0.404 (−0.51, 0.07) | 0.037, 0.473 (−0.27, 0.35) | <−0.001, 0.50 (−0.27, 0.24) | 0.16, 0.411 (−0.13, 0.42) | −0.113, 0.421 (−0.40, 0.17) |
| 0.073, 0.473 (−0.26, 0.38) | 0.235, 0.40 (−0.09, 0.49) | 0.033, 0.473 (−0.25, 0.28) | 0.058, 0.473 (−0.25, 0.35) | 0.141, 0.412 (−0.19, 0.42) |
| −0.34, 0.356 (−0.59, −0.05) | −0.092, 0.444 (−0.41, 0.21) | −0.159, 0.411 (−0.44, 0.16) | −0.002, 0.50 (−0.32, 0.32) | −0.292, 0.356 (−0.57, −0.02) |
| −0.17, 0.411 (−0.46, 0.17) | 0.109, 0.421 (−0.23, 0.43) | −0.155, 0.411 (−0.46, 0.14) | −0.046, 0.473 (−0.45, 0.29) | −0.008, 0.50 (−0.35, 0.33) |
Accelerated rate of hippocampal atrophy is a strong risk factor for development of Alzheimer’s disease (AD; Barnes et al., 2009; Driscoll et al., 2009). A longitudinal study in non-demented individuals aged 64–86 years found a 2.5-times faster rate of volume loss in the hippocampus in subjects who converted to MCI or AD during a 10-year follow-up period relative to those who remained non-demented (Driscoll et al., 2009). While that study did not assess the possible cause for the accelerated hippocampal volume loss, it points to the considerable risk of cognitive decline for subjects in whom rates of hippocampal volume loss are 2.5-fold faster than normal ageing. The ratio of hippocampal volume loss between MCI and normal subjects in that study (Driscoll et al., 2009) is similar to the one we found between poor and normal sleepers in this study. Thus, it is important to slow down hippocampal volume loss in order to reduce the risk of conversion to MCI or AD. While the present study did not include sleep intervention nor was designed to elucidate the exact mechanism of AD, assuming poor sleep quality doubles the rate of the hippocampal volume loss every 2 years above the rate associated with normal ageing, interventions that effectively improve sleep quality could delay the disease onset by ~2 years. Delay of onset of AD by as few as 2 years could reduce the number of projected new cases by 1.94 million (Braak & Braak, 1991).
In the present longitudinal study, we found a large difference in the rates of volume loss between poor and normal sleepers in the right posterior cingulate cortex. Additionally, we found significant negative association of rates of volume loss in this region with global sleep quality scores. The link between sleep and atrophy and dysfunction of the cingulate cortex has been reported by others. Heidbreder et al. showed that in young adults (mean [SD] age of 29 [4.2] years), non-rapid eye movement parasomnias was associated with lower volume in the left dorsal posterior cingulate cortex and posterior midcingulate cortex compared to age-matched normal controls (Heidbreder et al., 2017). Suh et al. (2016) demonstrated that sleep quality was related to structural connectivity between posterior cingulate cortex and precuneus in patients with persistent insomnia symptoms (mean [SD] age of 51.2 [8.1] years). Kay et al. reported that patients with primary insomnia (mean [SD] age of 37 [10] years) had abnormally high glucose metabolism in the posterior cingulate cortex during sleep compared to age-matched normal-sleeper controls (Kay et al., 2016). It is also interesting to note that the posterior cingulate cortex is one of the brain regions that exhibits the earliest accumulation of beta-amyloid in non-demented adults (mean [SD] age of 71 [7] years; Palmqvist et al., 2017). The finding of faster rates of volume loss in the posterior cingulate cortex reinforces the plausibility of causality between poor sleep quality and progression to AD.
Accelerated volume loss associated with poor sleep were found in the hippocampus and the posterior cingulate cortex in this study. Interestingly, these regions are the main hubs of the default mode network (DMN; Buckner, Andrews-Hanna, & Schacter, 2008), functional connectivity of which is affected by the pathophysiology of AD (Beason-Held, 2011; Grajski, Bressler, & Initia, 2019; Pasquini et al., 2019). A study in healthy young adults (mean [SD] age of 22.5 [2.0] years) showed that resting-state functional connectivity of the DMN was reduced following sleep deprivation compared to connectivity after a night of normal sleep (De Havas, Parimal, Soon, & Chee, 2012). Another study of adults aged ≥55 years demonstrated that MCIs with nocturnal awakenings had reductions in DMN functional connectivity relative to MCIs with intact sleep (McKinnon et al., 2017). These findings of DMN and sleep further strengthen associations of hippocampus and posterior cingulate cortex with sleep. The link between sleep and DMN and the important role of DMN in pathophysiology of AD (Beason-Held, 2011; Grajski et al., 2019; Pasquini et al., 2019) together may suggest a connection between poor sleep quality and increased risk of AD.
In this longitudinal study, differences in rates of volume loss in the bilateral superior parietal lobules between normal and poor sleepers were not significant, and there were no significant associations of these rates with sleep quality measures. Yet, in a previous cross-sectional study, volumetric differences of these regions between normal and poor sleepers were significant (Alperin et al., 2019), most likely because these volumetric differences evolved over a period longer than the 2-year period used in this study. Another possible explanation for our finding that the rates of volume loss in superior parietal lobules were slower in poor sleepers, although not significantly, is in part due to brain plasticity as part of possible compensation for the rapid volume loss in the hippocampus and posterior cingulate.
It is important to note that the rates of volume loss in the measured GM and WM regions were not significantly different between normal and poor sleepers. This is most likely because these regions are impacted by multiple factors and therefore rates of loss are associated with larger variability that could mask the effect of poor sleep, if it exists. The association between the cerebellar WM region and the objective sleep quality could have been a spurious finding and thus is too premature to associate a link between the cerebellar WM region in the brain and poor sleep.
| Sleep disturbance rs, p-FDR (95% CI) |
Nocturnal awakening rs, p-FDR (95% CI) |
Breath comfort rs, p-FDR (95% CI) |
Cough/Snoring rs, p-FDR (95% CI) |
Daytime sleepiness rs, p-FDR (95% CI) |
|---|---|---|---|---|
| −0.352, 0.277 (−0.61, −0.01) | −0.043, 0.452 (−0.36, 0.25) | −0.168, 0.393 (−0.48, 0.33) | −0.145, 0.393 (−0.49, 0.37) | −0.179, 0.393 (−0.50, 0.24) |
| 0.028, 0.465 (−0.27, 0.36) | 0.302, 0.283 (0.01, 0.57) | 0.139, 0.393 (−0.08, 0.39) | 0.126, 0.393 (−0.19, 0.50) | 0.033, 0.465 (−0.29, 0.37) |
| −0.208, 0.393 (−0.49, 0.13) | 0.072, 0.407 (−0.28, 0.40) | −0.139, 0.393 (−0.45, 0.24) | −0.238, 0.391 (−0.53, 0.13) | −0.315, 0.277 (−0.61, 0.07) |
| −0.167, 0.393 (−0.49, 0.23) | −0.022, 0.472 (−0.33, 0.31) | −0.088, 0.393 (−0.42, 0.32) | −0.189, 0.393 (−0.52, 0.31) | −0.188, 0.393 (−0.54, 0.28) |
| −0.137, 0.393 (−0.48, 0.27) | 0.083, 0.393 (−0.28, 0.47) | −0.262, 0.391 (−0.61, 0.26) | −0.165, 0.393 (−0.52, 0.24) | −0.209, 0.393 (−0.55, 0.24) |
| 0.087, 0.393 (−0.30, 0.44) | 0.251, 0.391 (−0.14, 0.58) | 0.328, 0.277 (−0.17, 0.66) | −0.096, 0.393 (−0.39, 0.22) | 0.342, 0.277 (0.04, 0.59) |
| 0.071, 0.407 (−0.24, 0.35) | −0.049, 0.443 (−0.36, 0.25) | 0.179, 0.393 (−0.09, 0.43) | −0.012, 0.482 (−0.31, 0.32) | 0.126, 0.393 (−0.22, 0.42) |
|
| ||||
| −0.113, 0.393 (−0.45, 0.30) | 0.095, 0.393 (−0.24, 0.44) | −0.112, 0.393 (−0.37, 0.20) | −0.168, 0.393 (−0.47, 0.19) | −0.107, 0.393 (−0.44, 0.29) |
| 0.108, 0.393 (−0.30, 0.50) | 0.141, 0.393 (−0.18, 0.45) | 0.15, 0.393 (−0.21, 0.51) | 0.141, 0.393 (−0.17, 0.40) | 0.031, 0.465 (−0.36, 0.40) |
| −0.376, 0.277 (−0.61, −0.04) | −0.093, 0.393 (−0.40, 0.22) | −0.263, 0.391 (−0.53, 0.05) | −0.329, 0.277 (−0.60, 0.02) | −0.326, 0.277 (−0.63, 0.04) |
| −0.117, 0.393 (−0.46, 0.32) | 0.179, 0.393 (−0.20, 0.59) | −0.243, 0.391 (−0.58, 0.26) | −0.246, 0.391 (−0.58, 0.21) | −0.23, 0.393 (−0.58, 0.26) |
| −0.251, 0.391 (−0.53, 0.11) | −0.09, 0.393 (−0.43, 0.24) | −0.124, 0.393 (−0.44, 0.28) | 0.01, 0.482 (−0.35, 0.37) | −0.443, 0.180 (−0.70, −0.11) |
The mechanism by which poor sleep quality accelerates brain tissue volume loss is not known, but evidence suggests that poor sleep impedes removal of neurotoxins from the brain thereby accelerating their deposition (Xie et al., 2013), which in turn leads to tissue volume loss in the hippocampus and the posterior cingulate cortex (Mormino et al., 2009; Shima et al., 2012). Several studies demonstrated a strong link between poor sleep and accumulation of neurotoxins. A single night of sleep deprivation was found to increase hippocampal interstitial fluid tau by 100% (Holth et al., 2019), cerebrospinal fluid (CSF) tau by >50% (Holth et al., 2019) and CSF beta-amyloid by 30% (Lucey et al., 2018). Reduced non-rapid eye movement sleep was associated with accumulation of tau and beta-amyloid in the brain (Lucey et al., 2019). Beta-amyloid deposition in the brain measured by positron emission tomography (PET) or by CSF were negatively associated with sleep quality (Sprecher et al., 2015, 2017) and sleep efficiency (Ju et al., 2013), and positively associated with sleep latency (Branger et al., 2016; Brown et al., 2016). Higher amyloid deposition and higher rate of increase in tau protein aggregation in the right posterior cingulate cortex were related to obstructive sleep apnea (Bubu et al., 2019; Yun et al., 2017). Moreover, back-and-forth movement of CSF between the cranium and spinal canal with every heartbeat is over 2.5-fold larger in the supine position compared to the sitting position (Alperin, Burman, & Lee, 2021), which suggests a better mixture between the cranial and spinal CSF, and perhaps a more efficient removal of neurotoxins. Consequently, these studies provide a link between poor sleep, accumulation of neurotoxins, and accelerated tissue loss in specific brain regions.
Over a period of 1-year, poor sleepers lost −1.6% of the hippocampal volume compared with a normal rate of −0.57% in our study. The corresponding rates in the posterior cingulate cortex were −1.2% versus −0.36%, respectively. It has been shown that other life-style factors also impact rates of tissue volume change in these two regions. Aerobic exercise was found to increase hippocampal volume 2%–3.6% over 1 year (Erickson et al., 2011; Niemann, Godde, & Voelcker-Rehage, 2014), and exercise prevented tissue loss in the posterior cingulate (Ji et al., 2017; Suo et al., 2016). Dietary diversity was negatively associated with rates of hippocampal volume loss, between 0.66% and 0.41% among Japanese community-dwelling middle-aged and older adults (Otsuka et al., 2020). This may imply that these regions are susceptible to both negative stressors and positive interventions.
The main limitation of the present investigation is the small number of subjects in each cohort. The bootstrapping technique using 1,000 iterations was employed to further test the validity of the statistical findings based on the present sample size. Regardless of this limitation, our study was able to demonstrate the accelerated hippocampal and posterior cingulate volume loss in the self-reported poor sleepers, as well as significant correlations between rates of posterior cingulate volume loss and global sleep quality scores. Another limitation is the lack of amyloid and tau PET imaging that would enable the identification of a direct link between deposition of neurotoxins and accelerated tissue loss, if such a link exists in regions known to be affected by poor sleep quality. The third limitation is not complementing the subjective measures of sleep quality with actigraphy. Yet, the reliability of the PSQI has been previously validated (Buysse et al., 1989; Salahuddin et al., 2017). Furthermore, findings in this study imply that older adults who have poor sleep quality based on the PSQI alone should be considered to receive sleep interventions in an effort to slow down the accelerated brain tissue loss. The relatively short follow-up period used in this study is another limitation. Finally, the apolipoprotein E4 (ApoE4) carrier status for 25% of our subjects was not available (17.4% versus 32.0% for normal and poor sleepers, respectively) due to refusal to give a blood sample. Although based on available information about subjects’ ApoE4 carrier statuses, there were no significant difference in number of ApoE4 carriers between normal and poor sleepers (26.3% versus 5.9%, p = 0.232), status of ApoE4 carrier may be a potential confounding factor. Despite these limitations, our study identified potential associations between rates of brain tissue loss in regions associated with cognitive performances and poor sleep to inform future larger scale studies.
In conclusion, we demonstrated substantial acceleration in the rates of volume loss in two cognitively-critical brain regions of cognitively-normal elderly self-reported poor sleepers. Their rates of volume loss were three- and five-times faster in the right hippocampus and right posterior cingulate cortex, respectively. Large inter-study differences in the magnitude of the effect of poor sleep quality on the rate of tissue loss warrant further investigations to establish the degree of acceleration in tissue loss due to poor sleep. This study demonstrates the importance of good sleep quality in preserving the health and integrity of the cognitively-critical brain regions in healthy older adults. These findings provide a strong impetus to offer sleep interventions to older adults who have poor sleep quality.
Funding information
Research for this study was supported by State of Florida Department of Health [grants 8AZ22 and 20A19] (PI: Alperin), and NIH [grant R01 AG047649] (PI: Loewenstein).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- Alperin N, Burman R, & Lee SH (2021). Role of the spinal canal compliance in regulating posture-related cerebrospinal fluid hydrodynamics in humans. Journal of Magnetic Resonance Imaging, 54(1), 206–214. 10.1002/jmri.27505 [DOI] [PubMed] [Google Scholar]
- Alperin N, Oliu CJ, Bagci AM, Lee SH, Kovanlikaya I, Adams D, … Relkin N (2014). Low-dose acetazolamide reverses periventricular white matter hyperintensities in iNPH. Neurology, 82(15), 1347–1351. 10.1212/WNL.0000000000000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alperin N, Wiltshire J, Lee SH, Ramos AR, Hernandez-Cardenache R, Rundek T, … Loewenstein D (2019). Effect of sleep quality on amnestic mild cognitive impairment vulnerable brain regions in cognitively normal elderly individuals. Sleep, 42(3), zsy254. 10.1093/sleep/zsy254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Bartlett JW, van de Pol LA, Loy CT, Scahill RI, Frost C, … Fox NC (2009). A meta-analysis of hippocampal atrophy rates in Alzheimer’s disease. Neurobiology of Aging, 30(11), 1711–1723. 10.1016/j.neurobiolaging.2008.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beason-Held LL (2011). Dementia and the default mode. Current Alzheimer Research, 8(4), 361–365. 10.2174/156720511795745294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, & Braak E (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica, 82(4), 239–259. 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- Branger P, Arenaza-Urquijo EM, Tomadesso C, Mézenge F, André C, de Flores R, & Rauchs G (2016). Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiology of Aging, 41, 107–114. 10.1016/j.neurobiolaging.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Brown BM, Rainey-Smith SR, Villemagne VL, Weinborn M, Bucks RS, Sohrabi HR, … Martins RN (2016). The relationship between sleep quality and brain amyloid burden. Sleep, 39(5), 1063–1068. 10.5665/sleep.5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubu OM, Pirraglia E, Andrade AG, Sharma RA, Gimenez-Badia S, Umasabor-Bubu OQ, … Osorio RS (2019). Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes. Sleep, 42(6), zsz048. 10.1093/sleep/zsz048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index - A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Carvalho DZ, Louis EK, Boeve BF, Mielke MM, Przybelski SA, Knopman DS, … Vemuri P (2017). Excessive daytime sleepiness and fatigue may indicate accelerated brain aging in cognitively normal late middle-aged and older adults. Sleep Medicine, 32, 236–243. 10.1016/j.sleep.2016.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Mohlenhoff BS, Weiner MW, & Neylan TC (2014). Associations between subjective sleep quality and brain volume in gulf war veterans. Sleep, 37(3), 445–452. 10.5665/sleep.3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke JR, & Ancoli-Israel S (2011). Normal and abnormal sleep in the elderly. Handbook of Clinical Neurology, 98, 653–665. 10.1016/B978-0-444-52006-7.00041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Havas JA, Parimal S, Soon CS, & Chee MWL (2012). Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. NeuroImage, 59(2), 1745–1751. 10.1016/j.neuroimage.2011.08.026 [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, & Resnick SM (2009). Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology, 72(22), 1906–1913. 10.1212/WNL.0b013e3181a82634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, … Kramer AF (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences of United States of America, 108(7), 3017–3022. 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Sørensen Ø, Amlien IK, Bartrés-Faz D, Bros DM, Buchmann N, … Walhovd KB. (2020). Self-reported sleep relates to hippocampal atrophy across the adult lifespan: results from the Lifebrain consortium.Sleep, 43(5), zsz280. 10.1093/sleep/zsz280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildner TE, Liebert MA, Kowal P, Chatterji S, & Snodgrass JJ (2014). Associations between sleep duration, sleep quality, and cognitive test performance among older adults from six middle income countries: Results from the Study on Global Ageing and Adult Health (SAGE). Journal of Clinical Sleep Medicine, 10(6), 613–621. 10.5664/jcsm.3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerlich KS, Votinov M, Dicks E, Ellendt S, Csukly G, & Habel U (2017). Neuroanatomical and neuropsychological markers of amnestic MCI: A three-year longitudinal study in individuals unaware of cognitive decline. Frontiers in Aging Neuroscience, 9, 1–12. 10.3389/fnagi.2017.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grajski KA, Bressler SL, & Initia ADN (2019). Differential medial temporal lobe and default-mode network functional connectivity and morphometric changes in Alzheimer’s disease. Neuroimage: Clinical, 23, 101860. 10.1016/j.nicl.2019.101860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder A, Stefani A, Brandauer E, Steiger R, Kremser C, Gizewski ER, … Scherfler C (2017). Gray matter abnormalities of the dorsal posterior cingulate in sleep walking. Sleep Medicine, 36, 152–155. 10.1016/j.sleep.2017.05.007 [DOI] [PubMed] [Google Scholar]
- Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, … Holtzman DM (2019). The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science, 363(6429), 880–883. 10.1126/science.aav2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holth JK, Patel T, & Holtzman DM (2017). Sleep in Alzheimer’s disease – Beyond amyloid. Neurobiol Sleep Circadian Rhythms, 2, 4–14. 10.1016/j.nbscr.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Zhang H, Potter GG, Zang YF, Steffens DC, Guo H, & Wang L (2017). Multiple neuroimaging measures for examining exercise-induced neuroplasticity in older adults: A quasi-experimental study. Frontiers in Aging Neuroscience, 9, 102. 10.3389/fnagi.2017.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YE, Lucey BP, & Holtzman DM (2014). Sleep and Alzheimer disease pathology-a bidirectional relationship. Nature Reviews Neurology, 10(2), 115–119. 10.1038/nrneurol.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YE, McLeland JS, Toedebusch CD, Xiong CJ, Fagan AM, Duntley SP, … Holtzman DM (2013). Sleep quality and preclinical Alzheimer disease. JAMA Neurology, 70(5), 587–593. 10.100l/jamaneurol.2013.2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay DB, Karim HT, Soehner AM, Hasler BP, Wilckens KA, James JA, … Buysse DJ (2016). Sleep-wake differences in relative regional cerebral metabolic rate for glucose among patients with insomnia compared with good sleepers. Sleep, 39(10), 1779–1794. 10.5665/sleep.6154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ASP, Fleischman DA, Dawe RJ, Yu L, Arfanakis K, Buchman AS, & Bennett DA (2016). Regional neocortical gray matter structure and sleep fragmentation in older adults. Sleep, 39(1), 227–235. 10.5665/sleep.5354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Lee SH, Hernandez-Cardenache R, Loewenstein D, Kather J, & Alperin N (2021). Poor sleep is associated with small hippocampal subfields in cognitively normal elderly individuals. Journal of Sleep Research, 30(5), e13362. 10.1111/jsr.13362 [DOI] [PubMed] [Google Scholar]
- Lo JC, Loh KK, Zheng H, Sim SK, & Chee MW (2014). Sleep duration and age-related changes in brain structure and cognitive performance. Sleep, 37(7), 1171–1178. 10.5665/sleep.3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey BP, Hicks TJ, McLeland JS, Toedebusch CD, Boyd J, Elbert DL, … Bateman RJ (2018). Effect of sleep on overnight cerebrospinal fluid amyloid beta kinetics. Annals of Neurology, 83(1), 197–204. 10.1002/ana.25117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey BP, McCullough A, Landsness EC, Toedebusch CD, McLeland JS, Zaza AM, … Holtzman DM (2019). Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Science Translational Medicine, 11(474), eaau6550. 10.1126/scitranslmed.aau6550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon AC, Duffy SL, Cross NE, Terpening Z, Grunstein RR, Lagopoulos J, … Naismith SL (2017). Functional connectivity in the default mode network is reduced in association with nocturnal awakening in mild cognitive impairment. Journal of Alzheimers Disease, 56(4), 1373–1384. 10.3233/Jad-160922 [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, … Jagust WJ (2009). Episodic memory loss is related to hippocampal-mediated -amyloid deposition in elderly subjects. Brain, 132, 1310–1323. 10.1093/brain/awn320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Kleiman A, Schulz JB, Schneider F, Laird AR, Fox PT, … Reetz K (2012). Neuroanatomic changes and their association with cognitive decline in mild cognitive impairment: a meta-analysis. Brain Structure & Function, 217(1), 115–125. 10.1007/s00429-011-0333-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann C, Godde B, & Voelcker-Rehage C (2014). Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Frontiers in Aging Neuroscience, 6, 170. 10.3389/fnagi.2014.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka R, Nishita Y, Nakamura A, Kato T, Iwata K, Tange C, … Arai H (2020). Dietary diversity is associated with longitudinal changes in hippocampal volume among Japanese community dwellers. European Journal of Clinical Nutrition, 75(6), 946–953. 10.1038/s41430-020-00734-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Scholl M, Strandberg O, Mattsson N, Stomrud E, Zetterberg H, … Hansson O (2017). Earliest accumulation of beta-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nature Communications, 8, 1214. 10.1038/s41467-017-01150-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini L, Rahmani F, Maleki-Balajoo S, La Joie R, Zarei M, Sorg C, … Tahmasian M (2019). Medial temporal lobe disconnection and hyperexcitability across Alzheimer’s disease stages. Journal of Alzheimers Disease Reports, 3(1), 103–112. 10.3233/Adr-190121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, & Fischl B (2010). Highly accurate inverse consistent registration: A robust approach. NeuroImage, 53(4), 1181–1196. 10.1016/j.neuroimage.2010.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, & Fischl B (2012). Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage, 61(4), 1402–1418. 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, & Turek FW (2015). Neurobiology of circadian rhythm regulation. Sleep Medicine Clinics, 10(4), 403–412. 10.1016/j.jsmc.2015.08.003 [DOI] [PubMed] [Google Scholar]
- Salahuddin M, Maru TT, Kumalo A, Pandi-Perumal SR, Bahammam AS, & Manzar D (2017). Validation of the Pittsburgh sleep quality index in community dwelling Ethiopian adults. Health and Quality of Life Outcomes, 15, 58. 10.1186/sl2955-017-0637-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Storsve AB, Walhovd KB, Johansen-Berg H, & Fjell AM (2014). Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology, 83(11), 967–973. 10.1212/Wnl.0000000000000774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Matsunari I, Samuraki M, Chen WP, Yanase D, Noguchi-Shinohara M, … Yamada M (2012). Posterior cingulate atrophy and metabolic decline in early stage Alzheimer’s disease. Neurobiology of Aging, 33(9), 2006–2017. 10.1016/j.neurobiolaging.2011.07.009 [DOI] [PubMed] [Google Scholar]
- Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, … Benca RM (2015). Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiology of Aging, 36(9), 2568–2576. 10.1016/j.neurobiolaging.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Koscik RL, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, … Bendlin BB (2017). Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology, 89(5), 445–453. 10.1212/Wnl.0000000000004171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterniczuk R, Theou O, Rusak B, & Rockwood K (2013). Sleep disturbance is associated with incident dementia and mortality. Current Alzheimer Research, 10(7), 767–775. 10.2174/15672050113109990134 [DOI] [PubMed] [Google Scholar]
- Suh S, Kim H, Dang-Vu TT, Joo E, & Shin C (2016). Cortical thinning and altered cortico-cortical structural covariance of the default mode network in patients with persistent insomnia symptoms. Sleep, 39(1), 161–171. 10.5665/sleep.5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo C, Singh MF, Gates N, Wen W, Sachdev P, Brodaty H, … Valenzuela MJ (2016). Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise (vol 21, pg 1633, 2016). Molecular Psychiatry, 21(11), 1645. 10.1038/mp.2016.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LL, Kang HY, Xu QW, Chen MJ, Liao YH, Thiyagarajan M, … Nedergaard M (2013). Sleep drives metabolite clearance from the adult brain. Science, 342(6156), 373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Pan PL, Song W, Huang R, Li JP, Chen K, … Shang HF (2012). Voxelwise meta-analysis of gray matter anomalies in Alzheimer’s disease and mild cognitive impairment using anatomic likelihood estimation. Journal of the Neurological Sciences, 316(1–2), 21–29. 10.1016/j.jns.2012.02.010 [DOI] [PubMed] [Google Scholar]
- Yun CH, Lee HY, Lee SK, Kim H, Seo HS, Bang SA, … Thomas RJ (2017). Amyloid burden in obstructive sleep apnea. Journal of Alzheimers Disease, 59(1), 21–29. 10.3233/Jad-161047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


