Abstract
Background:
Socioeconomic status (SES), race, ethnicity, and medical comorbidities may contribute to Alzheimer’s disease and related disorders (ADRD) health disparities.
Objective:
Analyze effects of social and medical determinants on cognition in 374 multicultural older adults participating in a community-based dementia screening program.
Methods:
We used the Montreal Cognitive Assessment (MoCA) and AD8 as measures of cognition, and a 3-way race/ethnicity variable (White, African American, Hispanic) and SES (Hollingshead index) as predictors. Potential contributors to health disparities included: age, sex, education, total medical comorbidities, health self-ratings, and depression. We applied K-means cluster analyses to study medical and social dimension effects on cognitive outcomes.
Results:
African Americans and Hispanics had lower SES status and cognitive performance compared with similarly aged Whites. We defined three clusters based on age and SES. Cluster #1 and #3 differed by SES but not age, while cluster #2 was younger with midlevel SES. Cluster #1 experienced the worse health outcomes while cluster #3 had the best health outcomes. Within each cluster, White participants had higher SES and better health outcomes, African Americans had the worst physical performance, and Hispanics had the most depressive symptoms. In cross-cluster comparisons, higher SES led to better health outcomes for all participants.
Conclusion:
SES may contribute to disparities in access to healthcare services, while race and ethnicity may contribute to disparities in the quality and extent of services received. Our study highlights the need to critically address potential interactions between race, ethnicity, and SES which may better explain disparities in ADRD health outcomes.
Keywords: Alzheimer’s disease, brain health, cognitive impairment, dementia, ethnicity, health disparities, race, socioeconomic status
INTRODUCTION
Alzheimer’s disease and related dementias (ADRD) affect an estimated 6.2 million Americans [1], although estimates suggest nearly two-thirds of ADRD cases remain undetected until the latter stages of impairment [2, 3]. Cognitive decline may be first detected clinically as mild cognitive impairment (MCI) with approximately 32% of MCI patients going on to develop ADRD within 5 years [4]. The risk of ADRD is not equally distributed across different populations of older adults. African Americans are at a 2-fold increased risk of ADRD compared with White Americans, and Hispanic older adults have a 1.5-fold increased risk of ADRD compared with non-Hispanic White Americans [1]. Epidemiologic studies have identified low socioeconomic status (SES), often measured as years of education, as an independent risk factor for ADRD, particularly for Alzheimer’s disease [5, 6], that may help explain the lower observed performance on cognitive tests in older adults from minority backgrounds [7]. Health-related factors such as comorbid medical conditions, vascular risk factors, physical functionality, mood, and self-reported physical and mental health are not only risk factors for ADRD [5] but may also play an important role in underlying racial and ethnic differences in observed cognitive performance and risk of cognitive impairment [7–9].
Racial and ethnic disparities in risk of dementia and cognitive impairment have long been recognized, but most research efforts have focused on comparisons between White and African American older adults or between Non-Hispanic White and Hispanic older adults. Older adults from racial and ethnic minority groups tend to underperform compared to similarly aged White adults on cognitive testing and to have a higher risk of cognitive impairment and dementia [1, 8, 10]. Among older adults, those with low education perform poorer on global and specific measures of cognition such as reasoning and working memory and have an increased risk of dementia [11–14]. According to the US Census Bureau, educational attainment varies by age, sex, and race and Hispanic ethnicity, suggesting that education may help explain the observed differences in cognitive function and risk of dementia [15].
Social determinants of health represent conditions in which individuals live, work, and age and the effects of external forces that help shape the condition of every area of life, including health and health care [16]. Lower SES and less education are both associated with higher risk of ADRD while higher SES and education appear to be protective [16]. This SES effect is present for both White and African American individuals and remains significant even after removing the contribution of educational attainment [17]. There is growing body of evidence that low SES in early and mid-life may have negative effects on health and cognitive outcomes in late life [18]. Individuals with high incomes are more likely to receive an earlier ADRD diagnosis at a less severe cognitive stage than individuals with lower incomes [19]. Socioeconomic status may also impact underlying neuropathology. Compared to Whites, African Americans had greater burden of neurodegeneration measured by MRI cortical volumes and this effect was mediated by SES [20]. These efforts may also consider educational differences or distinctions in SES; however, there are fewer studies examining impact of race, ethnicity, and SES on the medical and social determinants of brain health. To address our hypothesis that social and medical determinants affect cognition and contribute to ADRD health disparities, we conducted an analysis of 374 non-Hispanic White, non-Hispanic African American, and Hispanic older adults participating in a community-based dementia screening program to study the effects of SES, race, and ethnicity on cognitive health outcomes.
METHODS
Study population
Study participants were community-dwelling adults aged 55 + years residing in Manhattan, Brooklyn, and Queens, NY, who enrolled in cognitive aging studies between 2012–2015. These studies were focused on the use of self-report and performance dementia screening tools and identification of markers of cognitive decline in community samples [21]. Exclusion criteria were limited so as to closely as possible represent a “real world” population and included: age <55 years, non-fluency in English or Spanish, and active psychiatric and neurological conditions that could impact physical and/or cognitive performance or otherwise interfere with participation. A total of 374 participants were recruited through a combination of community events, educational seminars, and word-of-mouth. The majority of participants were evaluated in their community settings (community centers, churches, public housing projects) with the remainder coming to our center for a research visit. Participants were divided into three groups based on race and ethnicity: 1) non-Hispanic Whites (hereafter referred to as “Whites”), 2) non-Hispanic African Americans (hereafter referred to as “African Americans”, and 3) Hispanics. Studies were conducted either in English or Spanish by bilingual research staff and clinicians. An additional 19 individuals were initially recruited (6 Asians, 10 American Indians, and 3 multiracial) but numbers were too few to make any meaningful conclusions and thus excluded from these analyses. Each participant provided written informed consent. This study was approved by the Institutional Review Board at New York University Grossman School of Medicine.
Outcome measures
Demographic information
Information on age, sex, years of education, primary language and occupation of participant, and head of household was collected. SES was measured using the Hollingshead two-factor index of social position [22]. The total Hollingshead score is obtained by summing weighted scores for the head of the household’s occupation and the participant’s educational attainment, both measured on 7-point scales with lower scores indicating better standing on the respective scale. The scoring algorithm used weights the occupational scale more than the educational scale and their sum is then used to create a 5-level social class variable (I: 11–17; II 18–27; III 28–43; IV 44–60; and V 61–70). Low SES was measured in this study as a social class of IV-V, while social classes of I-III were considered upper SES.
Medical history
A review of medical history information sheet was completed by participants listing 25 co-morbid medical conditions (e.g., diabetes, hypertension). A summation of comorbid medical conditions was used as measure of overall medical health. Two questions from the General Health Questionnaire [23] were used to capture self-reported physical and mental health scored on a 4-point Likert scale (Excellent, Good, Fair, Poor) with higher scores representing worse self-ratings. Vascular risk factors were assessed with the modified Cardiovascular Risk Factors, Aging, and Incidence of Dementia scale (mCAIDE) [24].
Medical evaluation
A brief physical evaluation was performed. Sitting blood pressure was measured and reported as mean arterial pressure (MAP). Anthropometric measurements by bioimpedance with the BC-558 Ironman Segmental Body Composition Monitor (Tanita Corporation, Arlington Heights, IL, USA) were used to derive body mass index, muscle mass, basal metabolic rate (BMR) and metabolic age (a comparison of the participant’s BMR against the age-predicted BMR) [25, 26]. Grip Strength Handgrip strength was measured with a handheld dynamometer (Baseline Digital Smedley Spring Dynamometer; Patterson Medical, Warrenville, IL) in each hand and expressed in kilograms (kg) and mean grip strength was calculated. The modified Mini Physical Performance Test (mPPT) was used to provide an objective rating of physical performance [27]. The mPPT includes 4 tasks (bending over, progressive Romberg, time walk, and chair rise) with a score range of 0–16, with higher scores suggesting higher physical functionality
Cognitive performance
The Montreal Cognitive Assessment (MoCA) [28] consists of 13 items tapping into six cognitive domains (memory, language, visuospatial, executive, attention/concentration, orientation) with a total range of scores from 0-30 with higher scores suggesting better global cognitive performance. Per scoring rules, an additional point was added to the total MoCA score for individuals with 12 or less years of education [28]. The Mini-Cog [29] was used as an additional cognitive screening measure with a range of scores from 0–4 with higher scores representing better performance. Animal Naming [30] was used as a test of verbal fluency where the participant is asked to name as many animals as they can in 1 min (higher scores are better). Trailmaking A [31] was used to assess psychomotor processing speed with faster times to completion representing better cognitive performance. The AD8 [32, 33] is an eight-item assessment tool designed to identify early cognitive changes as reported by the participants with a range of scores from 0–8. Higher values support greater subjective cognitive complaints. Because educational attainment can influence performance on cognitive tests, all cognitive tests were captured as continuous scores rather than applying any pre-determined cut-off for “normal” versus “abnormal”. The Hospital Anxiety and Depression Scale (HADS) [34] was completed to assess mood for orthogonal ratings of depression (HADS-D) and anxiety (HADS-A) with a range of scores from 0–21, with higher scores representing more features. HADS scores 0–7 represent no mood disturbance, score 8-10 represent borderline mood disturbance, and scores 11 or greater signify the presence of a mood disturbance.
Statistical analyses
Analyses were conducted using R version 4.0.2 (r-project.org). Descriptive statistics were used to examine patient demographic characteristics, medical history and examination features, and cognitive performance. Two-sample t-tests or one-way analysis of variance (ANOVA) with post-hoc Tukey honestly significant tests were used to analyze continuous variables. Chi-square tests with post-hoc Benjamini-Hochberg correction were used for categorical variables. To evaluate the outcomes of medical and social dimensions in multicultural community samples, the MoCA was employed as an objective measure of cognition, and the AD8 as a subjective cognitive measure. We focused on the interactions between race, ethnicity, and SES to explain physical and mental health ratings and cognitive performance. For predictor variables, we used a 3-way race/ethnicity variable (White, African American, Hispanic) and SES (Hollingshead index). We considered variables that may explain race, ethnicity, and SES differences in cognitive performance including age, sex, education, self-reported medical history, vascular risk scores (mCAIDE), self-ratings of physical and mental health, and depression (HADS-D).
To address missing data, we imputed any missing values for the 374 subjects (162 Whites, 65 African American, and 147 Hispanic) for SES, years of education, HADS-D, medical history, mCAIDE, and self-reported physical and mental health using K nearest neighbors (K = 10) [35]. In order to maintain data quality, all selected variables had missing data rates less than 15%. For the imputation procedure, participants with missing values were imputed by matching sex and race/ethnicity. We then constructed two separate prediction models with MoCA and AD8 as response variables using an elastic net approach to account for the possibility that risk factors and variables may be highly correlated [36]. A convex combination of Ridge regression (L2 penalty) and Lasso regression (L1 penalty) was used in this regularized regression method. The analytic strategies were implemented with the R package “glmnet” (https://cran.r-project.org/web/packages/glmnet/glmnet.pdf). The interaction terms of racial/ethnic status (White, African American, and Hispanic) and two SES classes (upper and lower) were considered and the tuning parameter λ was obtained by default 10-fold cross validation. Next, we applied K-means clustering algorithm for the continuous variables age, years of education, medical history, self-reported physical and mental health, and HADS-D selected by the covariance test for the inference in adaptive linear modeling using R function “covTest” [37]. We then further explored the cluster characteristics with additional variables that capture dementia risk or dementia features including cognition (mini-Cog, Animal naming, Trails A), physical functionality (mPPT, mean grip, muscle mass), and medical factors (MAP, self-reported medical histories of diabetes and hypertension) in order to better understand how medical and social dimensions in a multicultural community sample can affect cognitive performance.
RESULTS
Sample characteristics
A total of 374 community-dwelling older adults were recruited. The participants had a mean age of 69.2 ± 9.8y (range: 40–100) and was 62.6% female. The sample had a mean education of 14.0 ± 4.2y (range: 0–20); 10.7% had 8 years of education or less, 7.1% had some high school, 13.4% had a high school diploma, 17.4% had some college, 20.0% had a college degree, and 31.3% had post college education. The racial and ethnic makeup of the sample was 43.3% non-Hispanic Whites, 17.4% non-Hispanic African Americans, and 39.3% Hispanic of which 73% reported White race, 14% reported Black race, and 12% reported 2 or more races. Hispanic participants reported region of origin included: 36.1% South American, 33.1% Puerto Rican, 11.3% Dominican, 10.6% Mexican and Central American, 3.8% Cuban, and 5.3% Other/Not Specified. Migration history and immigration status was not available. English was the primary language in 63.8% of the sample, while the remainder primarily spoke Spanish. The participants were largely independent (89.6%), living alone (42.5%) in a single-family residence or apartment (97.2%). The mean Hollingshead Index of Social Status was 37.8 ± 18.8 (range 11–77) supporting a wide range of SES.
The mean AD8 score was 1.8 ± 1.9 (range: 0–8), mean MoCA score was 22.7 ± 5.3 (range: 1–30), mean Mini-Cog score was 2.6 ± 1.3 (range: 0–4), mean animal naming was 16.7 ± 6.3 (range 1–36), and mean Trailmaking A was 49.1 ± 37.7 s (range: 1–300) supporting a wide range of cognitive performance. The mean mPPT score was 11.9 ± 2.8 (range: 0–16) and the mean number of medical conditions endorsed in the medical history was 5.5 ± 2.9 (range: 0–14) supporting a wide range of physical functionality and comorbidities. The mean HADS-D score was 5.3 ± 3.8 (range: 0–21) supporting a wide range of depressive symptoms with 6.0% of the sample having scores supporting a depressive disorder. The mean HADS-A score was 5.3 ± 3.6 (range 0–17) supporting a wide range of anxiety symptoms with 6.9% of the sample having scores suggesting an anxiety disorder. The participants provided self-ratings of good to excellent Physical health (72.3%) and mental health (77.3%) at the time of their research visit.
Sample characteristics by demographic groups and socioeconomic status
Table 1 provides comparison of demographic and outcome variables between White, African American, and Hispanic participants. There were more women in the African American and Hispanic groups compared with White participants (p < 0.001). Although no differences in age were seen, compared with African Americans and Hispanics, White participants had better MoCA and AD8 scores, higher SES, more education, lower vascular risk factors, better self-reported physical and mental health ratings, and less depression. These comparisons were more striking between Whites and Hispanics. Compared with African Americans, Hispanics had significantly less education, lower SES, and more depressive symptoms. We found no differences in HADS-A scores by race/ethnicity or SES. Interestingly, White older adults reported the greatest number of comorbid medical conditions. This could possibly be due to a higher SES in White participants, and consequently a greater availability of resources and access to healthcare.
Table 1.
Sample Characteristics
| Variable | White (n = 162) |
African American (n = 65) |
Hispanic (n = 147) |
ANOVA p |
Post-hoc comparison p |
||
|---|---|---|---|---|---|---|---|
| W versus AA | W versus H | H versus AA | |||||
| Age, y | 69.0 (9.4) | 69.3 (11.2) | 68.7 (9.5) | 0.903 | 0.983 | 0.944 | 0.908 |
| Education, y | 16.4 (2.6) | 13.9 (3.2) | 11.8 (4.5) | <0.001 | <0.001 | <0.001 | <0.001 |
| Sex, % Female | 51.2 | 73.8 | 77.6 | <0.001 | 0.003 | <0.001 | 0.600 |
| Hollingshead | 26.5 (12.6) | 38.3 (16.0) | 47.9 (19.3) | <0.001 | <0.001 | <0.001 | <0.001 |
| Total medical history | 6.2 (2.9) | 5.7 (3.3) | 4.9 (2.9) | 0.001 | 0.423 | <0.001 | 0.226 |
| Self-rated physical health | 2.0 (0.7) | 2.1 (0.7) | 2.3 (0.8) | <0.001 | 0.300 | <0.001 | 0.170 |
| Self-rated mental health | 1.9 (0.7) | 2.0 (0.7) | 2.2 (0.7) | 0.004 | 0.737 | 0.003 | 0.172 |
| mPPT | 12.5 (2.4) | 10.4 (3.8) | 12.1 (2.4) | <0.001 | <0.001 | 0.560 | <0.001 |
| mCAIDE | 6.6 (4.1) | 7.8 (3.7) | 7.9 (3.7) | 0.007 | 0.062 | 0.012 | 0.999 |
| MoCA | 24.6 (4.3) | 21.4 (6.1) | 21.4 (5.2) | <0.001 | <0.001 | <0.001 | 0.992 |
| AD8 | 1.5 (1.8) | 1.7 (2.0) | 2.2 (2.1) | 0.011 | 0.876 | 0.010 | 0.181 |
| HADS-Anxiety | 4.9 (3.4) | 5.2 (3.2) | 5.8 (4.0) | 0.083 | 0.788 | 0.067 | 0.517 |
| HADS-Depression | 4.8 (3.8) | 5.1 (3.4) | 7.4 (5.4) | <0.001 | 0.860 | <0.001 | 0.001 |
Mean (SD) or %, W, White; AA, African American; H, Hispanic.
One-way analysis of variance (ANOVA) with post-hoc Tukey honestly significant tests were used to analyze continuous variables. Chi-square tests with post-hoc Benjamini-Hochberg correction were used for categorical variables.
Bold indicates significant p-values.
SES, socioeconomic status; mPPT, mini-Physical Performance Test; mCAIDE, modified Cardiovascular Risk Factors, Aging, and Incidence of Dementia; HADS, Hospital Anxiety and Depression Scale; MoCA, Montreal Cognitive Assessment.
To test this hypothesis, we examined the social class difference (upper SES versus lower SES) by race and ethnicity (Table 2). Although there were more women in the African American and Hispanic groups compared with White participants, there were no differences in sex by SES class. White participants with higher SES had more education (p < 0.001) and lower mCAIDE scores (p = 0.002). In African Americans, participants in the upper SES group had more education (p < 0.001). In Hispanics, there was a much greater disparity due to SES with participants in the upper SES group having more education (p < 0.001), higher rated self-reported physical health (p = 0.004), lower mCAIDE scores (p = 0.002), higher MoCA scores (p = 0.02), and less depression (p = 0.03). However, there were no differences seen in the total medical comorbidities, self-reported mental health, or AD8 scores reported by race, ethnicity, and SES class.
Table 2.
Comparison of Racial and Ethnic Groups by Socioeconomic Status
| White |
African American |
Hispanic |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Upper SES (n = 145) |
Lower SES (n = 17) |
p | Upper SES (n = 40) |
Lower SES (n = 25) |
p | Upper SES (n = 51) |
Lower SES (n = 96) |
p | |
| Age, y | 69.4 (9.4) | 66.8 (10.1) | 0.285 | 68.4 (9.8) | 70.8 (13.2) | 0.442 | 68.6 (8.2) | 68.8 (10.1) | 0.921 |
| Education, y | 16.8 (2.4) | 13.1 (2.2) | <0.001 | 15.2 (2.2) | 11.6 (3.3) | <0.001 | 15.9 (2.7) | 9.6 (3.7) | <0.001 |
| Sex, % Female | 53.1 | 35.3 | 0.257 | 77.5 | 68.0 | 0.577 | 72.6 | 80.2 | 0.394 |
| Total medical history | 6.2 (2.9) | 5.9 (3.2) | 0.711 | 5.9 (3.3) | 5.3 (3.3) | 0.532 | 4.8 (2.7) | 5.0 (3.0) | 0.628 |
| Self-rated physical health | 1.9 (0.7) | 2.2 (0.7) | 0.174 | 2.0 (0.7) | 2.3 (0.8) | 0.186 | 2.1 (0.7) | 2.4 (0.8) | 0.004 |
| Self-rated mental health | 1.9 (0.7) | 2.2 (0.6) | 0.135 | 1.9 (0.7) | 2.2 (0.7) | 0.096 | 2.1 (0.6) | 2.3 (0.8) | 0.173 |
| mPPT | 12.5 (2.4) | 11.8 (2.6) | 0.271 | 11.1 (3.5) | 9.5 (4.0) | 0.116 | 12.7 (2.2) | 11.8 (2.4) | 0.038 |
| mCAIDE | 6.2 (4.0) | 9.5 (3.8) | 0.002 | 7.2 (3.4) | 8.9 (4.0) | 0.086 | 6.5 (3.6) | 8.6 (3.6) | 0.002 |
| MoCA | 24.7 (4.4) | 23.8 (3.4) | 0.419 | 22.3 (6.3) | 19.8 (5.4) | 0.091 | 22.8 (5.0) | 20.8 (5.2) | 0.025 |
| AD8 | 1.5 (1.7) | 1.9 (2.6) | 0.414 | 1.4 (1.7) | 2.1 (2.3) | 0.222 | 1.8 (1.9) | 2.4 (2.1) | 0.089 |
| HADS-Anxiety | 4.8 (3.2) | 5.6 (4.6) | 0.329 | 4.9 (3.5) | 5.8 (2.5) | 0.254 | 5.3 (3.3) | 6.1 (4.4) | 0.236 |
| HADS-Depression | 4.7 (3.6) | 5.5 (5.1) | 0.409 | 4.9 (3.4) | 5.5 (3.5) | 0.480 | 6.3 (3.8) | 8.0 (6.0) | 0.033 |
Mean (SD) or %. One-way analysis of variance (ANOVA) with post-hoc Tukey honestly significant tests were used to analyze continuous variables. Chi-square tests with post-hoc Benjamini-Hochberg correction were used for categorical variables.
Bold indicates significant p-values.
SES, socioeconomic status; mPPT, mini-Physical Performance Test; mCAIDE, modified Cardiovascular Risk Factors, Aging, and Incidence of Dementia; HADS, Hospital Anxiety and Depression Scale; MoCA, Montreal Cognitive Assessment.
Modeling objective and subjective cognitive performance
When using MoCA (objective cognitive performance) as the response variable, the sparse matrix obtained by elastic net regression set the coefficient of the predictors mCAIDE, social class, HADS-Depression, and all interaction terms to zero for the most parsimonious model (AIC: 2217.1; λ: 0.6482). In the final fitted model, all predictors except self-reported mental health were significantly associated with MoCA scores. Sex did not contribute to the response variable. African American participants on average, had lower MoCA scores (estimated coefficient: −2.808, p < 0.001) compared with Whites. Hispanic participants, on average, had lower MoCA scores (estimated coefficient: −1.690, p = 0.023) compared with Whites. When using AD8 (subjective cognitive performance) as the response variable, the sparse matrix obtained by elastic net regression demonstrated that only self-reported mental health (estimated coefficient: 1.1469, p < 0.001), HADS-Depression (estimated coefficient: 0.1075, p < 0.001), and HADS-Anxiety (estimated coefficient: 0.2651, p < 0.001) were significantly associated with AD8 scores.
Clustering analyses
Three clusters were selected by gap statistics for determining the optimal number of clusters in K-means clustering algorithm [38]. Figure 1 shows the biplot of first two principal components with three clustering results [39]. Cluster #1 represented individual who were of older age and lower SES. Cluster #2 represented individuals who were of younger age and middle SES. Cluster #3 represented individuals who were of older age and higher SES. There are 108 subjects (23 White, 17 African American, and 68 Hispanic) in cluster #1; 137 subjects (58 White, 23 African American and 56 Hispanic) in cluster #2; and 129 subjects (81 White, 25 African American and 23 Hispanic) in cluster #3. Hispanics were best represented in cluster #1 (46.3%) while Whites were best categorized in cluster #3 (50.0%). Cluster characteristics by medical and social variables with post hoc comparisons are presented in Table 3. There were no differences in sex distribution by cluster. Cluster #1 (older age, low SES) has the lowest self-rated physical and mental health, the highest HADS-Depression and HADS-Anxiety scores, and the lowest Hollingshead SES class. This cluster had more medical comorbidities, the worst physical performance (mPPT and grip strength), and the highest mCAIDE score. Cluster #1 also had the poorest cognitive performance with the worst MoCA, Animal Naming, and Trailmaking A scores and offered the greatest subjective cognitive complaints with the highest AD8 scores.
Fig. 1.
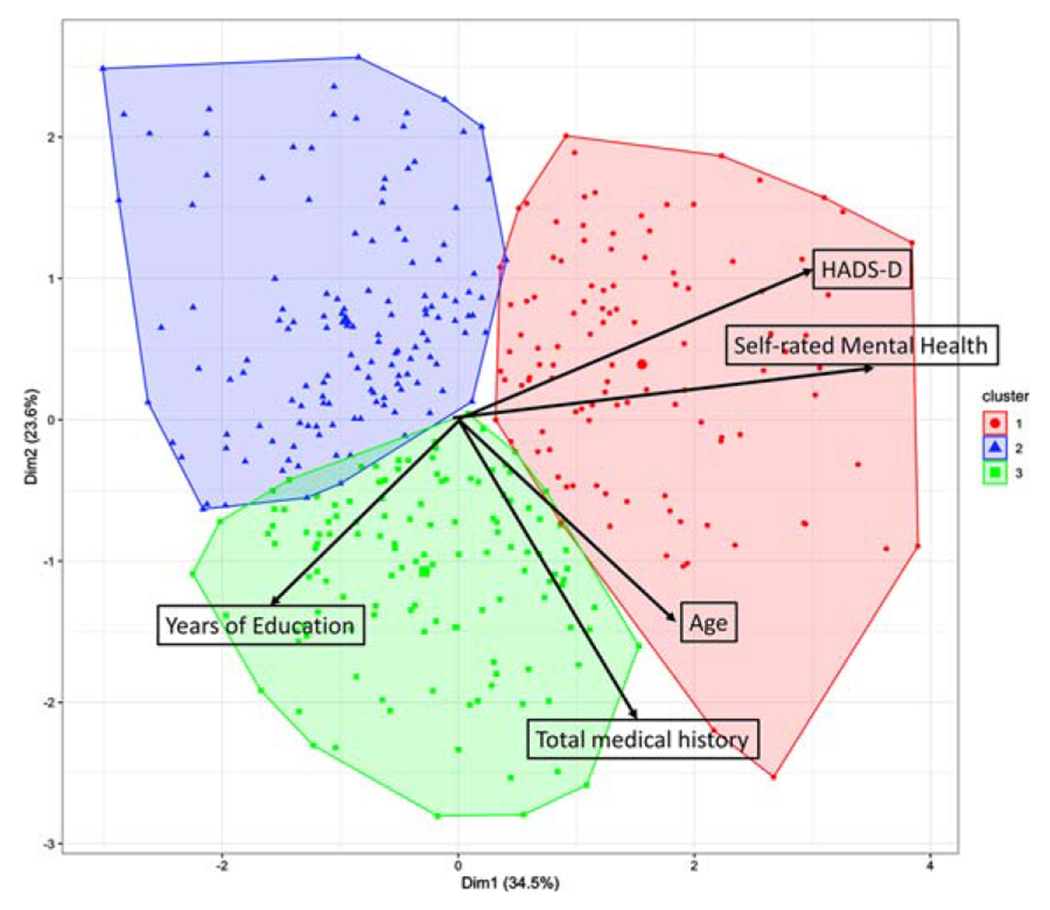
Biplot of the first two principal components (PC) with three clusters categorized from K-means algorithm. Black arrows indicate the weight of variables contributing to the first two PCs.
Table 3.
Comparison Across Three Clusters for Co-Morbid Medical Conditions
| Variable | Cluster 1 (n = 108) |
Cluster 2 (n = 137) |
Cluster 3 (n = 129) |
ANOVA |
Post-hoc comparison p |
||
|---|---|---|---|---|---|---|---|
| Older age, Low SES | Younger age, Midlevel SES | Older age, High SES | p | 1 versus 2 | 1 versus 3 | 2 versus 3 | |
| Age, y | 73.3 (8.9) | 62.5 (8.6) | 72.2 (7.8) | <0.001 | <0.001 | 0.944 | <0.001 |
| Hollingshead SES | 49.9 (19.1) | 37.0 (18.1) | 26.1 (10.9) | <0.001 | <0.001 | <0.001 | <0.001 |
| Education, y | 10.6 (4.1) | 14.5 (3.6) | 16.7 (2.3) | <0.001 | <0.001 | <0.001 | <0.001 |
| Sex, % Female | 71.3 | 65.0 | 61.2 | 0.267 | 0.507 | 0.391 | 0.524 |
| Total medical co-morbidities | 6.2 (2.7) | 3.1 (1.6) | 7.8 (2.4) | <0.001 | <0.001 | <0.001 | <0.001 |
| mCAIDE | 9.4 (3.4) | 5.4 (3.7) | 7.6 (3.6) | <0.001 | <0.001 | <0.001 | <0.001 |
| Self-reported physical health | 2.6 (0.7) | 1.9 (0.6) | 2.0 (0.6) | <0.001 | <0.001 | <0.001 | 0.189 |
| Self-reported mental health | 2.7 (0.7) | 1.8 (0.6) | 1.8 (0.5) | <0.001 | <0.001 | <0.001 | 0.818 |
| HADS-Anxiety | 7.8 (4.3) | 4.3 (3.0) | 4.6 (2.8) | <0.001 | <0.001 | <0.001 | 0.709 |
| HADS-Depression | 9.9 (5.1) | 4.0 (3.0) | 4.5 (3.3) | <0.001 | <0.001 | <0.001 | 0.599 |
| MoCA | 20.4 (5.0) | 23.3 (5.4) | 24.3 (4.5) | <0.001 | <0.001 | <0.001 | 0.290 |
| AD8 | 3.1 (2.1) | 1.2 (1.6) | 1.5 (1.7) | <0.001 | <0.001 | <0.001 | 0.448 |
| Mini Cog | 2.5 (1.2) | 2.7 (1.3) | 3.0 (1.2) | 0.024 | 0.450 | 0.018 | 0.208 |
| Animal Naming | 14.3 (4.8) | 18.6 (6.9) | 17.5 (6.2) | <0.001 | <0.001 | 0.003 | 0.435 |
| Trailmaking A, s | 60.3 (39.2) | 45.6 (33.9) | 39.8 (23.1) | 0.001 | 0.013 | <0.001 | 0.414 |
| mPPT | 10.3 (3.0) | 13.1 (2.1) | 12.0 (2.7) | <0.001 | <0.001 | <0.001 | 0.001 |
| Mean grip strength, kg | 45.9 (17.4) | 63.3 (27.0) | 57.4 (24.6) | <0.001 | <0.001 | 0.006 | 0.222 |
| Metabolic age | 64.3 (20.9) | 54.2 (20.6) | 60.5 (20.1) | 0.001 | 0.001 | 0.362 | 0.043 |
| Muscle mass, kg | 95.8 (19.6) | 102.5 (25.5) | 104.6 (21.3) | 0.014 | 0.073 | 0.012 | 0.733 |
| Mean arterial pressure | 119.3 (17.1) | 114.6 (14.3) | 118.2 (14.2) | 0.103 | |||
| Diabetes, % | 19.8 | 7.3 | 17.2 | 0.013 | 0.019 | 0.735 | 0.034 |
| Hypertension, % | 67.9 | 23.7 | 56.3 | <0.001 | <0.001 | 0.083 | <0.001 |
Mean (SD) or %. One-way analysis of variance (ANOVA) with post-hoc Tukey honestly significant tests were used to analyze continuous variables. Chi-square tests with post-hoc Benjamini-Hochberg correction were used for categorical variables.
Bold indicates significant p-values.
SES, socioeconomic status; mCAIDE, modified Cardiovascular Risk Factors, Aging, and Incidence of Dementia; HADS, Hospital Anxiety and Depression Scale; mPPT, mini-Physical Performance Test; MoCA, Montreal Cognitive Assessment.
Cluster #2 (younger age, midlevel SES) was the youngest grouping with the fewest medical comorbidities, best self-rated physical and mental health, and had mid-range SES class and educational attainment. This cluster had the lowest mCAIDE scores, the highest physical performance (mPPT, grip strength, and muscle mass), and the best metabolic age. They were less likely to have diagnoses of diabetes or hypertension than the other clusters.
Cluster #3 (older age, high SES) was similar in age to cluster #1 but had the highest SES and educational attainment. This cluster had the highest number of medical comorbidities and were equally likely as cluster #1 to have diagnoses of diabetes or hypertension. They had intermediate mCAIDE scores and intermediate physical performance and metabolic age. Interestingly, this cluster had cognitive performance scores (MoCA, AD8, Animal Naming, and Trailmaking A) similar to younger age, midlevel individuals in cluster #2, but diabetes and hypertension prevalence similar to cluster #1.
Cluster analyses by racial and ethnic groupings
We further evaluated and summarized the medical and social determinants by racial and ethnic groups within each cluster in Tables 4–6. Within each cluster, White participants had significantly better Hollingshead SES scores and tended to have higher educational attainment. Within each cluster, African American participants had the lowest mPPT scores and slowest Trailmaking A times. MoCA scores tended to be highest for White participants within each cluster, while HADS-Depression scores tended to be highest for Hispanics within each cluster. There were no differences in HADS-Anxiety scores within clusters.
Table 4.
Comparison of medical history by racial/ethnic groups in cluster 1 (Older age, Low SES)
| Variable | White (n = 23) |
African American (n = 17) |
Hispanic (n = 68) |
ANOVA p |
Post-hoc comparison p |
||
|---|---|---|---|---|---|---|---|
| W versus AA | W versus H | H versus AA | |||||
| Age, y | 72.6 (7.6) | 77.1 (8.0) | 72.7 (8.0) | 0.171 | |||
| Hollingshead SES | 35.8 (15.3) | 51.8 (15.8) | 54.1 (17.2) | <0.001 | 0.016 | <0.001 | 0.882 |
| Education, y | 14.1 (2.2) | 10.9 (3.0) | 9.4 (3.6) | <0.001 | 0.105 | <0.001 | 0.021 |
| Sex, % Female | 43.5 | 58.8 | 83.8 | <0.001 | 0.523 | 0.002 | 0.063 |
| Total medical co-morbidities | 7.3 (2.9) | 5.7 (1.7) | 5.9 (2.6) | 0.085 | |||
| mCAIDE | 9.0 (4.0) | 10.6 (2.8) | 9.3 (3.2) | 0.313 | |||
| Self-reported physical health | 2.5 (0.6) | 2.5 (0.7) | 2.7 (0.8) | 0.526 | |||
| Self-reported mental health | 2.9 (0.4) | 2.8 (0.6) | 2.6 (0.7) | 0.143 | |||
| HADS-Anxiety | 9.1 (4.3) | 7.2 (3.2) | 7.4 (4.6) | 0.249 | |||
| HADS-Depression | 10.7 (2.8) | 7.0 (3.6) | 10.4 (5.7) | 0.033 | 0.059 | 0.976 | 0.034 |
| MoCA | 23.0 (5.1) | 19.2 (5.0) | 19.8 (5.0) | 0.018 | 0.045 | 0.023 | 0.882 |
| AD8 | 3.1 (1.7) | 3.1 (2.2) | 3.1 (2.0) | 0.998 | |||
| Mini Cog | 2.7 (1.2) | 2.2 (1.4) | 2.5 (1.2) | 0.462 | |||
| Animal Naming | 16.5 (4.6) | 13.6 (4.9) | 13.7 (4.7) | 0.072 | |||
| Trailmaking A, s | 47.6 (24.8) | 81.9 (58.1) | 55.1 (28.9) | 0.038 | 0.056 | 0.824 | 0.066 |
| mPPT | 11.1 (2.8) | 8.0 (3.5) | 10.7 (2.6) | 0.001 | 0.003 | 0.813 | 0.002 |
| Mean grip strength, kg | 48.4 (19.2) | 54.4 (19.9) | 42.9 (15.5) | 0.104 | |||
| Metabolic age | 59.4 (17.5) | 70.1 (22.7) | 64.6 (21.4) | 0.347 | |||
| Muscle mass, kg | 106.5 (24.0) | 103.5 (22.8) | 90.9 (15.5) | 0.002 | 0.892 | 0.004 | 0.072 |
| Mean arterial pressure | 118.1 (19.3) | 120.1 (15.4) | 119.7 (16.8) | 0.926 | |||
| Diabetes, % | 8.7 | 29.4 | 21.2 | 0.243 | |||
| Hypertension, % | 52.2 | 76.5 | 71.2 | 0.213 | |||
Cluster 1 composition: 14.2% White (W), 26.1% African American (AA), 46.3% Hispanic (H). Mean (SD) or %. One-way analysis of variance (ANOVA) with post-hoc Tukey honestly significant tests were used to analyze continuous variables. Chi-square tests with post-hoc Benjamini-Hochberg correction were used for categorical variables.
Bold indicates significant p-values.
SES, socioeconomic status; mCAIDE, modified Cardiovascular Risk Factors, Aging, and Incidence of Dementia; HADS, Hospital Anxiety and Depression Scale; mPPT, mini-Physical Performance Test; MoCA, Montreal Cognitive Assessment.
Table 6.
Comparison of medical history by racial/ethnic groups in cluster 3 (Older age, High SES)
| Variable | White (n = 81) |
African American (n = 25) |
Hispanic (n = 23) |
ANOVA p |
Post-hoc comparison p |
||
|---|---|---|---|---|---|---|---|
| W versus AA | W versus H | H versus B | |||||
| Age, y | 72.9 (7.7) | 71.8 (8.1) | 70.4 (7.5) | 0.383 | |||
| Hollingshead SES | 23.4 (9.9) | 30.8 (10.9) | 30.1 (12.0) | 0.002 | 0.008 | 0.021 | 0.976 |
| Education, y | 17.1 (2.1) | 15.5 (2.1) | 16.3 (2.6) | 0.008 | 0.007 | 0.291 | 0.454 |
| Sex, % Female | 53.1 | 88.0 | 60.9 | 0.007 | 0.006 | 0.636 | 0.069 |
| Total medical co-morbidities | 7.7 (2.4) | 8.4 (2.8) | 7.2 (2.0) | 0.196 | |||
| mCAIDE | 7.5 (3.7) | 8.2 (3.5) | 7.3 (3.1) | 0.596 | |||
| Self-reported physical health | 1.9 (0.6) | 2.2 (0.6) | 2.2 (0.6) | 0.026 | 0.064 | 0.114 | 0.988 |
| Self-reported mental health | 1.7 (0.5) | 1.8 (0.4) | 1.9 (0.6) | 0.160 | |||
| HADS-Anxiety | 4.2 (2.5) | 4.9 (3.0) | 5.6 (3.1) | 0.091 | |||
| HADS-Depression | 4.1 (3.2) | 4.6 (3.5) | 5.7 (3.2) | 0.090 | |||
| MoCA | 25.3 (3.6) | 22.2 (6.5) | 23.0 (3.7) | 0.004 | 0.008 | 0.077 | 0.817 |
| AD8 | 1.3 (1.6) | 1.4 (1.8) | 2.0 (2.0) | 0.222 | |||
| Mini Cog | 3.1 (1.2) | 2.7 (1.3) | 3.1 (1.1) | 0.290 | |||
| Animal Naming | 18.2 (5.9) | 16.5 (7.4) | 17.2 (5.3) | 0.591 | |||
| Trailmaking A, s | 34.2 (10.0) | 50.1 (40.5) | 42.8 (18.8) | 0.026 | 0.024 | 0.326 | 0.554 |
| mPPT | 12.3 (2.5) | 10.3 (3.4) | 12.9 (1.6) | 0.001 | 0.003 | 0.607 | 0.002 |
| Mean grip strength, kg | 58.3 (26.1) | 55.3 (18.8) | 56.3 (26.0) | 0.899 | |||
| Metabolic age | 54.9 (19.8) | 71.1 (17.3) | 66.9 (17.5) | <0.001 | 0.001 | 0.024 | 0.718 |
| Muscle mass, kg | 108.2 (22.8) | 100.3 (15.5) | 97.5 (19.6) | 0.057 | |||
| Mean arterial pressure | 118.9 (14.4) | 119.4 (14.5) | 113.1 (12.9) | 0.412 | |||
| Diabetes, % | 5.0 | 40.0 | 34.8 | <0.001 | <0.001 | 0.001 | 0.772 |
| Hypertension, % | 47.4 | 68.0 | 74.0 | 0.019 | 0.101 | 0.058 | 0.756 |
Cluster 3 Composition: 50.0% White (W), 38.5% African American (AA) and 15.6% Hispanic (H). Mean (SD) or %. One-way analysis of variance (ANOVA) with post-hoc Tukey honestly significant tests were used to analyze continuous variables. Chi-square tests with post-hoc Benjamini-Hochberg correction were used for categorical variables.
Bold indicates significant p-values.
SES, socioeconomic status; mCAIDE, modified Cardiovascular Risk Factors, Aging, and Incidence of Dementia; HADS, Hospital Anxiety and Depression Scale; mPPT, mini-Physical Performance Test; MoCA, Montreal Cognitive Assessment.
Cross-cluster comparisons revealed interesting patterns. White participants had significantly better MoCA and AD8 scores and higher education attainment in older age, high SES cluster (#3) compared with the older age, low SES or younger age, midlevel SES clusters (#1 and #2). Similar patterns were found in African Americans and Hispanics. SES class was highest for all three groups in the older age, high SES cluster and lowest in the older age, low SES cluster. When comparing the younger group of White participants in cluster #2 with similar MoCA score, AD8 and Hollingshead SES scores to the older White participants in cluster #3, cluster #2 had significantly less total medical comorbidities, and lower mCAIDE scores. In addition, the percentage of White participants with hypertension in the younger age, midlevel SES cluster is significantly lower than in the older age, high SES cluster (17.5% versus 47.4%, p < 0.001). In the older age, low SES cluster with the worse MoCA and AD8 scores, the Hollingshead SES and MoCA scores of White participants were significantly better than African American and Hispanic participants, educational attainment and muscle mass of White participants was significantly better than Hispanics, and physical performance of White participants was significantly better than African Americans.
For the younger group of participants in cluster #2, White participants had better SES, higher educational attainment, and lower mCAIDE scores, but more total self-reported medical comorbidities compared with African American and Hispanic participants. For the older group in participants with the overall highest SES rankings in cluster #3, White participants had better MoCA scores, lower AD8 scores, lower metabolic age, and a smaller percentage of individuals diagnosed with diabetes than African American or Hispanic participants.
For the younger group of African American participants in cluster #2 compared with older African American participants in cluster #3 with similar MoCA and AD8 scores, Hollingshead SES rankings, and educational attainment, there were significant differences in total medical co-morbidities (p < 0.001), mCAIDE scores (p = 0.008), self-reported physical health (p = 0.01), and mPPT scores (p = 0.04). In addition, the younger age, midlevel SES cluster had significantly fewer African American participants diagnosed with hypertension (34.8% versus 68.0%, p = 0.04) and diabetes (4.3% versus 40.0%, p < 0.001) than the older age, high SES cluster.
Lastly, for the younger group of Hispanic participants in cluster #2 compared with older Hispanic participants in cluster #3 with similar MoCA, AD8, and mCAIDE scores, there were significant differences in total medical comorbidities (p < 0.001), miniCog scores (p < 0.04), and metabolic age (p < 0.02). In addition, cluster #2 had significantly fewer Hispanic participants diagnosed with diabetes (8.9% versus 34.8%, p = 0.01) and hypertension (25.5% versus 74.0%, p < 0.001) than cluster #3.
DISCUSSION
There is growing evidence that dementia does not affect different racial and ethnic groups equally; however, the underlying medical and social determinants of these disparities are not well understood. We found that older adults from racial and ethnic minority backgrounds had lower educational attainment and lower SES status than similarly aged White participants, and also had worse cognitive performance. Hispanic older adults had worse self-ratings of physical and mental health, more depressive symptoms and worse AD8 scores, while African American older adults had worse physical performance. Based on these analyses, lower SES contributed significantly to higher vascular risk factors in White and Hispanic older adults, but not in African Americans. Furthermore, in Hispanics, lower SES was associated with worse physical health ratings, poorer cognitive performance, and more depressive symptoms.
We defined three clusters based on age and SES. Cluster #1 and #3 differed by SES but not age, while cluster #2 differed by age and was midlevel in SES. Cluster #1 with older age and lower SES had worse health outcomes, while cluster #3 with the highest SES had the best health outcomes despite being the same age range as cluster #1. This suggests that although both clusters had significant medical comorbidities, the higher SES may have afforded those in cluster #3 more resources and potentially greater access to health services. Cluster #2 was intermediate in SES but younger in age and had better outcomes than cluster #1, further suggesting that younger age and increased resources are associated with better health outcomes regardless of racial or ethnic group.
Within each cluster, however, White participants had higher educational attainment and SES rankings with better health outcomes including cognitive performance compared with similarly aged African American and Hispanic participants. Conversely, within each cluster African Americans had the worst physical performance and Hispanics had the most depressive symptoms. In a recent mediation analysis, we reported that up to 40% of the variance in cognitive performance in African American could be explained by impaired physical performance. We also found that up to 35% of the variance in cognitive performance in Hispanics could be explained by depressive symptoms [8]. These findings suggest that inherent differences in physical functionality and mood may contribute to poor health outcomes in minority populations and that any consideration of measuring cognitive performance should account for physical performance and mood [8].
In cross-cluster comparisons, higher SES led to better health outcomes for all participants, and age played a role in health disparities. Younger White participants had fewer medical co-morbidities than older Whites but more than African American or Hispanic participants at similar ages. For young African American and Hispanic participants, there were also fewer comorbidities compared with older African American and Hispanic participants. Hypertension was less common in younger White participants, and both diabetes and hypertension were less common in younger African Americans and Hispanics. Diabetes and hypertension are both known risks factor for cognitive impairment [5]. It is interesting to note that while younger participants with midlevel SES with a lower prevalence of diabetes and hypertension (cluster #2) had better cognitive performance than older participants (clusters #1 and #3), higher SES individuals (cluster #3) had better cognitive performance than lower SES individuals (cluster #1) despite being the same age range and having similar prevalence rates of diabetes and hypertension.
We previously demonstrated that multiple factors such as physical functionality, depressive symptoms, and SES help explain some of the racial, ethnic, age, and sex differences in performance on cognitive testing [8]. The poorer cognitive function observed among older adults from racial and ethnic minority groups may reflect differences in peak cognitive reserve as measured by educational and occupational attainment [12]. A clearer understanding of the factors that affect cognitive reserve including the contribution of biological, socioeconomic, and cultural promoters may help efforts to reduce racial and ethnic disparities in ADRD outcomes. The literature also suggests the higher burden of ADRD neuropathology in minority older adults, and greater pathology burden may interact with the higher prevalence of multiple chronic comorbidities [40]. Our findings highlight the importance of SES as a contributory factor to racial and ethnic disparities in cognition as well as that of physical functionality [41, 42] and depression [43] for explaining both racial and age differences in cognitive performance.
Our study differs from other studies examining SES, because the Hollingshead index takes into consideration not only the educational level of the participant but also the occupational attainment of the head of household, which in some cases may help increase the income and resources available to the participant. In addition, the number of years of formal schooling may not be representative of the quality of the educational experience, and opportunities for advanced education may not be equal across different racial, ethnic, and socioeconomic groups [44–47]. In a recent study, White participants were more likely to remain cognitively healthy compared with African American or Hispanics regardless of education; however, the benefits of education had the greatest potential effects on cognitive health in African American men and women and in Hispanic women [48]. Notably, within each cluster, we found that White participants had higher SES rankings than African American or Hispanic participants. This was most noticeable in cluster #3 with the overall highest SES. In this cluster, the occupational attainment contributed more to the differences in SES than educational attainment suggesting that studying education alone may not provide a true measure of the roots of economic and social disparities. We expanded upon the potential effects of education by including occupational attainment to establish SES, as household salary and income may be more closely related to the major occupation of the primary breadwinner than the years of education of the participant. This is consistent with recent findings that low household income and financial strain predict incident dementia in a comparable fashion to low education [49]. Another report found that lower midlife education, occupational social class, and household income were associated with worse dementia outcomes with income (a reflection of occupation) being a stronger predictor than education [50].
The relationship between SES, race/ethnicity and dementia risk is complex. Individuals with lower SES have a higher risk of vascular risk factors and stroke [51]. African Americans and Hispanics experience delays in ADRD diagnosis (~3 months in African Americans and ~12 months in Hispanics) [6], have poorer cognitive performance particularly in vocabulary and semantic memory [7], and more functional disability at time of diagnosis compared with Whites [6]. These reports are consistent with our findings that cluster #1 with the lowest SES has significantly poorer health outcomes including more comorbidities, higher vascular risk, worse self-reported physical and mental health, worse physical performance, more depressive symptoms, more subjective cognitive complaints, and worse cognitive performance than similarly aged older adults with higher SES (cluster #3).
In a recent study of electronic health records, African American patients and those patients with lower SES had less documentation of cognitive testing [52] suggesting that delivery of medical care was different, despite the fact that African American and Hispanic older adults have a higher risk of MCI and ADRD compared with Whites [1], even after correcting for age and educational differences [53]. In another study, we found that African Americans with dementia admitted to the hospital had lower physical functionality, more delirium, and more depressive symptoms than White older adults with dementia [54]. Although the risk for cognitive impairment is higher in African American and Hispanic older adults compared with White older adults, dementia prevalence has increased over the past two decades in non-Hispanic Whites from 40% in 2000 to 44% in 2016 but has remained steady in non-Hispanic African Americans during the same period (from 37% in 2000 to 38% in 2016) [55]. African American and Hispanic persons with and without dementia report lower health-related quality of life compared with Whites, particularly for self-rated health [56]. In terms of health expenditures, African American, Asian, and Hispanic Medicare beneficiaries without cognitive deficits have lower total health care expenditures than Whites without cognitive deficits, and higher total healthcare expenditures after ADRD diagnoses [57]. There were also significant differences in minority older adults with lower out-of-pocket expenditures (possibly attributable to differences in insurance coverage), lower prescription drug expenditures, and higher home health expenditures (possibly attributable to informal care by family members) [57]. Collectively, these studies and our findings suggest that SES, race, and ethnicity each contribute to disparities in health outcomes but in different fashion. While SES may contribute to disparities in access to healthcare services, race and ethnicity may instead contribute to disparities in the types and extent of healthcare services received.
Our findings should be interpreted considering study limitations. First, although our study population was racially and ethnically diverse, some racial groups such as Asian Americans and American Indians, and individuals not fluent in either English or Spanish were not included in these analyses, precluding the generalizability of our results to other populations. Second, older adults attending community dementia screening events may be more motivated than others to seek early cognitive evaluation. The number of people that participated are the number of people that approached us to participate. Other than age and primary psychiatric illnesses, there were few exclusion criteria. There is no way to know the number of people who chose not to participate or their reasons for declining, thus the threat of selection bias exists. Further, the exclusion of psychiatric Axis 1 diagnoses could have potentially excluded a group of individuals with additional risk factors for ADRD. Third, although we were able to collect information of country of origin, participants from minority backgrounds were generally unwilling to disclose their immigration status so the impact of immigration and migration could not be addressed. Fourth, the cross-sectional nature of the study precludes chronological assessment of the relationships between the study outcome (i.e., cognition) and some of the factors investigated (e.g., physical functionality, depressive symptoms). Future research could include longitudinal visits to understand the impact of these relationships over time. Although cognitive decline can and does impact an individual’s SES, in the current study SES was expected to be a precursor rather than a consequence of cognitive impairment. Lastly, cognitive test performance may be influence by race, ethnicity, culture, language, and education and the choice of screening test should be carefully considered. There are limitations associated with each of the cognitive measures chosen for inclusion in this study and this may explain the generally lower observed performance on cognitive tests in older adults from minority backgrounds. However, we selected instruments that are considered “harder” (i.e., MoCA) versus “easier” (i.e., mini-cog), “verbal” (i.e., animal naming), “visual attention” (i.e., Trailmaking A), and “subjective” (i.e., AD8) to provide a balanced assessment and considered overall performance across all the tests when considering cognitive status without using any prescribed cut-off scores.
Strengths of our study include the recruitment of a large community-based sample into brain health and dementia screening program rather than being recruited from a tertiary clinic cohort. Our K-means clustering procedure allowed us to examine how race, ethnicity, social class, and medical co-morbidities associated with disparities in health outcomes are associated with cognitive performance and ADRD risk. Further, as part of the study from which these analyses were derived, we studied the consequences of participating in a dementia screening program [21]. After 60 days, participants were re-contacted to determine what they did with results of their screening visit. We found that 54% of participants shared results with family, 33% shared results with health care providers (HCPs), and 52% initiated behavioral change. Among participants sharing results with HCPs, 51% reported HCPs did not follow-up on the results, and 18% that HCPs did not show any interest in the screening visit or its results. Only 25% of HCPs ordered further testing and evaluation. The tremendous diversity of the sample reflects the multicultural population of New York City, which increases the generalizability of the findings. Our study highlights the need to critically address the relationship between race, ethnicity, SES, and medical and social determinants of brain health and how older adults from different backgrounds can better engage their primary care providers and health systems to improve delivery of care and medical decision-making. Future studies taking account of potential interactions between race, ethnicity, and SES, rather than focusing on educational attainment alone, may help to better explain disparities in ADRD health outcomes.
Table 5.
Comparison of medical history by racial/ethnic groups in cluster 2 (Younger age, Midlevel SES)
| Variable | White (n = 58) |
African American (n = 23) |
Hispanic (n = 56) |
ANOVA p |
Post-hoc comparison p |
||
|---|---|---|---|---|---|---|---|
| W versus AA | W versus H | H versus AA | |||||
| Age, y | 62.4 (7.1) | 61.0 (10.4) | 63.3 (9.3) | 0.567 | |||
| Hollingshead SES | 27.0 (13.3) | 36.5 (12.9) | 47.6 (18.3) | <0.001 | 0.037 | <0.001 | 0.013 |
| Education, y | 16.3 (2.7) | 14.2 (2.4) | 12.8 (3.9) | <0.001 | 0.022 | <0.001 | 0.211 |
| Sex, % Female | 51.7 | 69.6 | 76.8 | 0.017 | 0.320 | 0.019 | 0.573 |
| Total medical co-morbidities | 3.6 (1.5) | 2.6 (1.5) | 2.8 (1.5) | 0.004 | 0.022 | 0.010 | 0.904 |
| mCAIDE | 4.4 (3.9) | 5.5 (3.1) | 6.4 (3.6) | 0.017 | 0.413 | 0.012 | 0.625 |
| Self-reported physical health | 1.9 (0.6) | 1.7 (0.6) | 1.9 (0.6) | 0.526 | |||
| Self-reported mental health | 1.9 (0.7) | 1.6 (0.6) | 1.8 (0.4) | 0.146 | |||
| HADS-Anxiety | 4.2 (2.9) | 4.2 (2.8) | 4.4 (3.2) | 0.932 | |||
| HADS-Depression | 3.4 (2.5) | 4.2 (2.8) | 4.6 (3.5) | 0.110 | |||
| MoCA | 24.4 (5.1) | 22.0 (5.8) | 22.8 (5.3) | 0.114 | |||
| AD8 | 1.3 (1.7) | 0.9 (1.5) | 1.2 (1.4) | 0.661 | |||
| Mini Cog | 2.9 (1.4) | 2.6 (1.3) | 2.6 (1.3) | 0.569 | |||
| Animal Naming | 20.7 (8.0) | 18.1 (6.6) | 17.2 (5.8) | 0.058 | |||
| Trailmaking A, s | 46.8 (45.0) | 36.7 (16.9) | 47.9 (25.4) | 0.470 | |||
| mPPT | 13.2 (2.0) | 12.4 (3.5) | 13.4 (1.3) | 0.150 | |||
| Mean grip strength, kg | 66.9 (25.2) | 68.0 (27.0) | 57.2 (28.4) | 0.214 | |||
| Metabolic age | 50.1 (20.5) | 62.2 (23.5) | 55.7 (18.8) | 0.060 | |||
| Muscle mass, kg | 112.4 (26.5) | 102.1 (24.0) | 91.5 (20.2) | <0.001 | 0.211 | <0.001 | 0.204 |
| Mean arterial pressure | 115.4 (13.5) | 116.5 (18.0) | 113.0 (13.9) | 0.650 | |||
| Diabetes, % | 6.9 | 4.3 | 8.9 | 0.767 | |||
| Hypertension, % | 17.5 | 34.8 | 25.5 | 0.226 | |||
Cluster 2 composition: 35.8% White (W), 35.4% African American (AA) and 38.1% Hispanic (H). Mean (SD) or %. One-way analysis of variance (ANOVA) with post-hoc Tukey honestly significant tests were used to analyze continuous variables. Chi-square tests with post-hoc Benjamini-Hochberg correction were used for categorical variables.
Bold indicates significant p-values.
SES, socioeconomic status; mCAIDE, modified Cardiovascular Risk Factors, Aging, and Incidence of Dementia; HADS, Hospital Anxiety and Depression Scale; mPPT, mini-Physical Performance Test; MoCA, Montreal Cognitive Assessment.
ACKNOWLEDGMENTS
This study was supported by grants to JEG from the National Institute on Aging (R01AG040211, R01 AG071514, R01 AG069765, and R01 NS101483), the Harry T. Mangurian Foundation, and the Leo and Anne Albert Charitable Trust. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/21-5020r1).
REFERENCES
- [1].Alzheimer’s Association (2021) Alzheimer’s disease facts and figures 2021. Alzheimers Dement 17, 327–406. [DOI] [PubMed] [Google Scholar]
- [2].Lang L, Clifford A, Wei L, Zhang D, Leung D, Augustine G, Danat IM, Zhou W, Copeland JR, Anstey KJ, Chen R (2017) Prevalence and determinants of undetected dementia in the community: A systematic literature review and a meta-analysis. BMJ Open 7, e011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Savva GM, Arthur A (2015) Who has undiagnosed dementia? A cross-sectional analysis of participants of the Aging, Demographics and Memory Study. Age Ageing 44, 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ward A, Tardiff S, Dye C, Arrighi H (2013) Rate of conversion from prodromal Alzheimer’s disease to Alzheimer’s dementia: A systematic review of the literature. Dement Geriatr Cogn Dis Extra 3, 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Galvin JE (2017) Prevention of Alzheimer’s disease: Lessons learned and applied. J Am Geriatr Soc 65, 2128–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lin PJ, Daly AT, Olchanski N, Cohen JT, Neumann PJ, Faul JD, Fillit HM, Freund KM (2021) Dementia diagnosis disparities by race and ethnicity. Med Care 59, 679–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hernandez Saucedo H, Whitmer RA, Glymour M, DeCarli C, Mayeda ER, Gilsanz P, Miles SQ, Bhulani N, Farias ST, Olichney J, Mungas D (2021) Measuring cognitive health in ethnically diverse older adults. J Gerontol B Psychol Sci Soc Sci, doi: 10.1093/geronb/gbab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tolea MI, Chrisphonte S, Galvin JE (2020) The effect of sociodemographics, physical function, and mood on dementia screening in a multicultural cohort. Clin Interv Aging 15, 2249–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xiong C, Luo J, Coble D, Agboola F, Kukull W, Morris JC (2020) Complex interactions underlie racial disparity in the risk of developing Alzheimer’s disease dementia. Alzheimers Dement 16, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Diaz-Venegas C, Downer B, Langa KM, Wong R (2016) Racial and ethnic differences in cognitive function among older adults in the USA. Int J Geriatr Psychiatry 31, 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ritchie SJ, Bates TC, Deary IJ (2015) Is education associated with improvements in general cognitive ability, or in specific skills? Dev Psychol 51, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R (1994) Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 271, 1004–1010. [PubMed] [Google Scholar]
- [13].Evans DA, Hebert LE, Beckett LA, Scherr PA, Albert MS, Chown MJ, Pilgrim DM, Taylor JO (1997) Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol 54, 1399–1405. [DOI] [PubMed] [Google Scholar]
- [14].Prince M, Acosta D, Ferri CP, Guerra M, Huang Y, Llibre Rodriguez JJ, Salas A, Sosa AL, Williams JD, Dewey ME, Acoista I, Jotheeswaran AT, Liu Z (2012) Dementia incidence and mortality in middle-income countries, and associations with indicators of cognitive reserve: A 10/66 dementia research group population-based cohort study. Lancet 380, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ryan CL, Bauman K (2016) Educational attainment in the United States: 2015. population characteristics: Current population reports. US Census Bureau. Department of Commerce, Economics and Statistics Administration. Available from: https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Accessed on 24 June 2021. [Google Scholar]
- [16].Majoka MA, Schimming C (2021) Effect of social determinants of health on cognition and risk of Alzheimer disease and related dementias. Clin Ther 43, 922–929. [DOI] [PubMed] [Google Scholar]
- [17].George KM, Lutsey PL, Kucharska-Newton A, Palta P, Heiss G, Osypuk T, Folsom AR (2020) Life-course individual and neighborhood socioeconomic status and risk of dementia in the Atherosclerosis Risk in Communities Neurocognitive Study. Am J Epidemiol 189, 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weissberger GH, Han SD, Yu L, Barnes LL, Lamar M, Bennett DA, Boyle PA (2021) Impact of early life socioeconomic status on decision making in older adults without dementia. Arch Gerontol Geriatr 95, 104432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Petersen JD, Wehberg S, Packness A, Svensson NH, Hyldig N, Raunsgaard S, Andersen MK, Ryg J, Mercer SW, Søndergaard J, Waldorff FB (2021) Association of socioeconomic status with dementia diagnosis among older adults in Denmark. JAMA Netw Open 4, e2110432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Meeker KL, Wisch JK, Hudson D, Coble D, Xiong C, Babulal GM, Gordon BA, Schindler SE, Cruchaga C, Flores S, Dincer A, Benzinger TL, Morris JC, Ances BM (2021) Socioeconomic status mediates racial differences seen using the AT(N) framework. Ann Neurol 89, 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Galvin JE, Tolea MI, Chrisphonte SC (2020) What do older adults do with results from dementia screening. PLoS One 15, e0235534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hollingshead AB (1958) Two factor index of social position. In: Social Class and Mental Illness: A Community Study, Hollingshead AB, Redlich FC, eds. John Wiley, New York. [Google Scholar]
- [23].Goldberg D (1978) Manual of the general health questionnaire. NFER Publishing, Windsor, England. [Google Scholar]
- [24].Tolea MI, Heo J, Chrisphonte S, Galvin JE (2021) A modified CAIDE risk score as a screening tool for cognitive impairment in older adults. J Alzheimers Dis 82, 1755–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Garcia-Rubira JC, Cano-Garcia FJ, Bullon B, Seoane T, Villar PV, Cordero MD, Bullon P (2018) Body fat and metabolic age as indicators of inflammation and cardiovascular risk. Eur J Prev Cardiol 25, 233–234. [DOI] [PubMed] [Google Scholar]
- [26].Wilczyński J, Pedrycz A, Mucha D, Ambroży T, Mucha D (2017) Body posture, postural stability, and metabolic age in patients with Parkinson’s disease. Biomed Res Int 2017, 3975417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wilkins CH, Roe CM, Morris JC (2010)A brief clinical tool to assess physical function: The mini-physical performance test. Arch Gerontol Geriatr 50, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal Cognitive Assessment, MoCA: Abrief screening tool for mild cognitive impairment. J Am Geriatr Soc 53, 695–699. [DOI] [PubMed] [Google Scholar]
- [29].Seitz DP, Chan CC, Newton HT, Gill SS, Herrmann N, Smailagic N, Nikolaou V, Fage BA (2018) Mini-Cog for the diagnosis of Alzheimer’s disease dementia and other dementias within a primary care setting. Cochrane Database Syst Rev 2, CD011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Canning SJ, Leach L, Stuss D, Ngo L, Black SE (2004) Diagnostic utility of abbreviated fluency measures in Alzheimer disease and vascular dementia. Neurology 62, 556–562. [DOI] [PubMed] [Google Scholar]
- [31].Reitan RM (1958) Validity of the trail making test as an indication of organic brain damage. Percept Mot Skills 8, 271–276. [Google Scholar]
- [32].Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, Miller JP, Storandt M, Morris JC (2005) The AD8: A brief informant interview to detect dementia. Neurology 65, 559–594. [DOI] [PubMed] [Google Scholar]
- [33].Galvin JE, Roe CM, Coats MA, Morris JC (2007) Patient’s rating of cognitive ability: Using the AD8, a brief informant interview, as a self-rating tool to detect dementia. Arch Neurol 64, 725–730 [DOI] [PubMed] [Google Scholar]
- [34].Snaith RP (2003) The Hospital Anxiety and Depression Scale. Health Qual Life Outcomes 1, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Troyanskaya O, Cantor M, Sherlock G, Brown P, Hastie T, Tibshirani R, Botstein D, Altman RB (2001) Missing value estimation methods for DNA microarrays. Bioinformatics 17, 520–525. [DOI] [PubMed] [Google Scholar]
- [36].Zou H, Hastie T (2005) Regularization and variable selection via the elastic net. J R Statc Soc B Stat Method 67, 301–320. [Google Scholar]
- [37].Lockhart R, Taylor J, Tibshirani RJ, Tibshirani R (2014) A significance test of the Lasso. Ann Stat 42, 413–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tibshirani R, Walther G, Hastie T (2001) Estimating the number of clusters in a data set via the gap statistic. J R Stat Soc B Stat Method 63, 411–423. [Google Scholar]
- [39].Pittelkow YE, Wilson SR (2003) Visualisation of gene expression data - the GE-biplot, the Chip-plot and the Gene-plot. Stat Appl Genet Mol Biol 2, 6. [DOI] [PubMed] [Google Scholar]
- [40].Quiñones AR, Kaye J, Allore HG, Botoseneanu A, Thielke SM (2020) An agenda for addressing multimorbidity and racial and ethnic disparities in Alzheimer’s disease and related dementia. Am J Alzheimers Dis Other Demen 35, 1533317520960874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tolea MI, Chrisphonte S, Galvin JE (2018) Sarcopenic obesity and cognitive performance. Clin Interv Aging 13, 1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tolea MI, Morris JC, Galvin JE (2015) Longitudinal associations between physical and cognitive function among community-dwelling older adults. PLoS One 10, e0122878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Byers AL, Yaffe K (2011)Depression and risk of developing dementia. Nat Rev Neurol 7, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Avila JF, Renteria MA, Jones RN, Vonk JMJ, Turney I, Sol K, Seblova D, Arias F, Hill-Jarrett T, Levy SA, Meyer O, Racine AM, Tom SE, Melrose RJ, Deters K, Medina LD, Carrion CI, Diaz-Santos M, Byrd DR, Chesebro A, Colon J, Igwe KC, Maas B, Brickman AM, Schupf N, Mayeuz R, Manly JJ (2021) Education differentially contributes to cognitive reserve across racial/ethnic groups. Alzheimers Dement 17, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zahodne LB, Mayeda ER, Hohman TJ, Fletcher E, Racine AM, Gavett B, Manly JJ, Schupf N, Mayeux R, Brickman AM, Mungas D (2019) The role of education in a vascular pathway to episodic memory: Brain maintenance or cognitive reserve? Neurobiol Aging 84, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zahodne LB, Manly JJ, Smith J, Seeman T, Lachman ME (2017) Socioeconomic, health, and psychosocial mediator of racial disparities in cognition in early, middle, and late adulthood. Psychol Aging 32, 118–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zahodne LB, Stern Y, Manly JJ (2015) Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology 29, 649–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Garcia MA, Downer B, Chiu CT, Saenz JL, Ortiz K, Wong R (2021) Educational benefits and cognitive health life expectancies: Racial/ethnic, nativity, and gender disparities. Gerontologist 61, 330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Samuel LJ, Szanton SL, Wolff JL, Ornstein KA, Parker LJ, Gitlin LN (2020) Socioeconomic disparities in six-year incident dementia in a nationally representative cohort of U.S. older adults: An examination of financial resources. BMC Geriatr 20, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Korhonen K, Einiö E, Leinonen T, Tarkiainen L, Martikainen P (2020) Midlife socioeconomic position and old-age dementia mortality: A large prospective register-based study from Finland. BMJ Open 10, e033234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Maalouf M, Fearon M, Lipa MC, Chow-Johnson H, Tayeh L, Lipa D (2021) Neurologic complications of poverty: The associations between poverty as a social determinant of health and adverse neurologic outcomes. Curr Neurol Neurosci Rep 21, 29. [DOI] [PubMed] [Google Scholar]
- [52].Maserejian N, Krzywy H, Eaton S, Galvin JE (2021) Cognitive measures lacking in EHR prior to dementia or Alzheimer’s disease diagnosis. Alzheimers Dement 17, 1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wright CB, DeRosa JT, Moon MP, Strobino K, DeCarli C, Cheung YK, Assuras S, Levin B, Stern Y, Sun X, Rundek T, Elkind MSV, Sacco RL (2021) Race/ethnic disparities in mild cognitive impairment and dementia: The Northern Manhattan Study. J Alzheimers Dis 80, 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Boltz M, BeLue R, Resnick B, Kuzmik A, Galik E, Jones JR, Arendacs R, Sinvani L, Mogle J, Galvin JE (2021) Disparities in physical and psychological symptoms in hospitalized African American and white persons with dementia. J Aging Health 33, 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Power MC, Bennett EE, Turner RW, Dowling NM, Ciarleglio A, Glymour MM, Gianattasio KZ (2021) Trends in relative incidence and prevalence of dementia across non-Hispanic black and white individuals in the United States, 2000-2016. JAMA Neurol 78, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hayes-Larson E, Mobley TM, Gilmore-Bykovskyi A, Shaw C, Karlamangla A, Manly JJ, Mayeda ER (2021) Racial/ethnic differences in health-related quality of life in persons with and without dementia. J Am Geriatr Soc 69, 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Park S, Chen J (2020) Racial and ethnic patterns and differences in health care expenditures among Medicare beneficiaries with and without cognitive deficits or Alzheimer’s disease and related dementias. BMC Geriatr 20, 482. [DOI] [PMC free article] [PubMed] [Google Scholar]


