Abstract
Lower extremity edema is a symptom of chronic venous disease and venous insufficiency. However, clinical evaluations rely on the qualitative evaluation of the pitting depth, which is neither well-defined nor quantitative. We created a novel three-dimensionally printed edema ruler as a quantitative method to measure pitting depth. Twenty-five patients (50 legs) with chronic venous disease were evaluated for ankle edema using the edema ruler. The results demonstrate excellent intraclass correlation for both single (0.944, P < .001) and average (0.971, P < .001) measurements. The edema ruler is a noninvasive, useful, and objective tool for the clinical measurement of lower extremity edema.
Keywords: 3D printing, Chronic venous disease, Edema, Pitting, Venous insufficiency
Chronic venous disease (CVD) develops secondary to venous insufficiency due to both primary and secondary etiologies.1,2 Patients with CVD experience a wide range of signs and symptoms, including telangiectasia, varicose veins, and lower extremity edema.3 Historically, the clinical evaluation for leg edema has been the pitting test, wherein digital pressure is applied along the tibia and an assessment is made regarding the depth of pitting induced. However, this clinical evaluation has not been standardized in terms of precisely where the pressure should be applied, the amount of pressure that should be used, nor for how long the pressure should be maintained. In addition, this method is completely qualitative in describing the depth of the pitting induced, with much interobserver variability in reporting.4 Others have proposed alternative methods to quantify pitting edema.5, 6, 7, 8 We developed a novel three-dimensionally (3D) printed edema ruler to quantitatively measure edema and validated its clinical effectiveness.
Methods
Edema ruler
The edema ruler was created to provide a noninvasive, inexpensive, and quantifiable measurement of leg edema that could be used by medical office assistants with minimal training to provide reproducible results. It was developed using computer aided design software (OnShape; PTC) and produced with polylactic acid filament using a Flashforge Creator Pro 3D Printer (Flashforge) at the University of South Florida's Advanced Visualization Center. The cost to manufacture each device was $5.
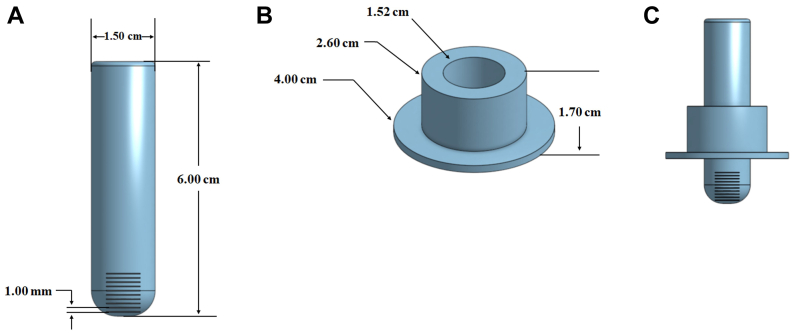
The edema ruler is composed of two units. The inner tube is 6 cm long and 1.5 cm in diameter and has a rounded end with 1-mm etched markings to measure the depth of the pitting edema (Fig 1, A). The outer slider has an inner diameter of 1.52 cm to fit over the inner tube with an outer diameter at its bottom of 4 cm (Fig 1, B). The unit is assembled before the patient examination by placing the slider on the inner tube (Fig 1, C).
Fig 1.
A, Inner tube of the ruler with 1-mm markings on its rounded tip. B, Slider portion of the ruler, which fits over the inner tube. C, Assembled edema ruler with slider positioned on inner tube.
The clinical examination begins with the patient in a seated position with their feet flat on the floor. The examiner then places their index finger on the tibia 5 cm above the medial malleolus and applies firm pressure for 20 seconds (Fig 2, A and B). The edema ruler inner tube is placed within the resultant pit (Fig 2, C), and the slider portion is slid down to become flush with the skin surrounding the indentation (Fig 2, D). The ruler is removed, and the depth of the edema is measured by visualizing the measurement markings at the edge of the slider (Fig 3).
Fig 2.
A, Finger pressure is applied on tibia 5 cm above the medial malleolus. B, The resultant pit after 20 seconds of applied pressure (arrow and circle). C, The edema ruler is placed into the pit on the skin. D, The slider is moved to come in contact with the surrounding skin surface.
Fig 3.
Depth of pitting edema is quantified by the markings in 1-mm increments on the ruler.
Clinical validation
A total of 25 patients (50 legs) with CVD were evaluated for ankle edema by physician assistants and medical assistants using both a clinical scoring grade (from ½+ to 4+) and the edema ruler. The inclusion criteria were age >18 years, and an initial office visit for venous disease. There were no exclusion criteria. After the first edema measurement, the patient underwent their office clinical evaluation. Before leaving, a second examiner, unaware of the findings of the first examiner, repeated the edema measurement. The edema ruler is not intended to be disposable and was sanitized between uses with a medical grade sanitation wipe.
All examiners were trained in using the edema ruler by a demonstration of the device and one supervised study. Medical assistants had to successfully perform the measurements under physician assistant supervision a minimum of three times to participate in the present study.
Interobserver reliability was calculated through an intraclass correlation coefficient with a two-way random effects model using SPSS Statistics, version 29.0 (IBM Corp). The College of Medicine, University of South Florida institutional review board approved the present study. The patients provided written informed consent for the report of their imaging studies.
Results
The physician and medical assistants found no difficulty in using the edema ruler following a demonstration of its use, and no technical difficulties were encountered in measuring the edema depth. The average depth of pitting edema was 0.94 ± 1.24 mm for the 50 legs studied (Fig 4). The pitting depth for patients in this study ranged from 0 to 6 mm and their CEAP (clinical, etiology, anatomy, pathophysiology) classification ranged from C0 to C4. For pitting depth measurements, the intraclass correlation coefficient for single measures was 0.944 (P < .001) and the intraclass correlation coefficient for average measures was 0.971 (P < .001). The single measures assess the reliability of a single rater’s measurements and the average measures assess the average reliability of each rater’s measurements. We categorized the measured edema depth to correlate with the customary clinical grading scale (1/2+, >0 mm to <2 mm; 1+, ≥2 mm to <4 mm; 2+, ≥4 mm to <6 mm; 3+, ≥6 mm to <8 mm; and 4+, ≥8 mm; Table). With the clinical edema rating scale, the intraclass correlation coefficient of a single measure was 0.943 (P < .001) and the intraclass correlation coefficient of an average measure was 0.971 (P < .001).
Fig 4.
Bar graph of edema pitting depth measurements (error bars indicate 95% confidence intervals). SD, Standard deviation; SE, standard error.
Table.
Clinical rating of severity of pitting edema (50 legs; 25 patients)
| Rating | Legs, no. (%) |
|---|---|
| 0 | 14 (28) |
| ½+ | 26 (52) |
| 1+ | 7 (7) |
| 2+ | 2 (4) |
| 3+ | 1 (2) |
| 4+ | 0 (0) |
Discussion
There were several considerations when making and using the edema ruler. Because the measurement lines are in 1-mm increments on the measurement part of the ruler, it was critical to have the device printed in an orientation that would optimize the clarity of these lines. Thus, we printed the inner tube of the device vertically, building up from the flat end to the curved end. We also avoided using any support filament on the exterior of the lines. Another critical aspect in making the device is ensuring that the open channel in the center of the slider fit tightly around the inner tube and could not slide off but without being so tight that it could not be easily moved onto the skin surface. Each 3D printer has a different range in the error of measurement. Therefore, several prints with different channel sizes in the slider were required to produce the proper diameter.
Similarly, different filament resins and 3D printers are available that can be used. We also made prototypes using a Pro V1 resin filament on a Form 3B+ 3D printer (Formlabs). Using this filament was twice as expensive, and the printing process was more complicated, with washing and curing requirements. Although this resin prototype resulted in higher clarity for the measurement lines, it was far more fragile than the polylactic acid construct. The Pro V1 resin product was easily scratched and cracked if dropped from a short height. Also, the slider portion did not slide well along the inner tube.
Our results demonstrate the edema ruler to be useful in providing an objective and quantitative measurement of pitting depth. Its measurements had good reproducibility with intraclass correlation coefficients further showing excellent reliability, with all values >0.9. It was used effectively by physicians and medical assistants with minimal training in a busy office setting. One limitation of a busy clinic is that only two observers could be used for each pitting examination. Venous disease is an underdiagnosed condition, and, hopefully, quantitative methods of evaluating ankle edema will help improve the diagnosis.1,9, 10, 11
Conclusions
Our edema ruler provides a quantitative and objective measurement of leg edema with excellent reproducibility and interobserver reliability. This device can be used in the detection and measurement of edema in patients with venous disease in the office setting.
Disclosures
None.
Footnotes
M.E.C. was the recipient of a summer research grant from USF Health Morsani College of Medicine, Research, Innovation & Scholarly Endeavors.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Ortega M.A., Fraile-Martínez O., García-Montero C., et al. Understanding chronic venous disease: a critical Overview of its Pathophysiology and medical Management. J Clin Med. 2021;10:3239. doi: 10.3390/jcm10153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lurie F., Passman M., Meisner M., et al. The 2020 update of the CEAP classification system and reporting standard. J Vasc Surg: Venous and Lym Dis. 2020;8:342–352. doi: 10.1016/j.jvsv.2019.12.075. [DOI] [PubMed] [Google Scholar]
- 3.Kamel M., Blebea J. Pathophysiology of edema in patients with chronic venous insufficiency. Phlebolymphology. 2020;27:3–10. [Google Scholar]
- 4.Brodovicz K.G., McNaughton K., Uemura N., Meininger G., Girman C.J., Yale S.H. Reliability and feasibility of methods to quantitatively assess peripheral edema. Clin Med Res. 2009;7:21–31. doi: 10.3121/cmr.2009.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kogo H., Higashi T., Murata J. Reliability of a new practical evaluation method for pitting edema based on the depth of the surface imprint. J Phys Ther Sci. 2015;27:1735–1738. doi: 10.1589/jpts.27.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kogo H., Murata J., Murata S., Higashi T. Validity of a new quantitative evaluation method that Uses the depth of the surface imprint as an Indicator for pitting edema. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesarone M.R., Belcaro G., Nicolaides A.N., et al. The edema tester in the evaluation of swollen limbs in venous and lymphatic disease. Panminerva Med. 1999;41:10–14. [PubMed] [Google Scholar]
- 8.Mawdsley R.H., Hoy D.K., Erwin P.M. Criterion-related validity of the figure-of-eight method of measuring ankle edema. J Orthop Sports Phys Ther. 2000;30:149–153. doi: 10.2519/jospt.2000.30.3.149. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaides A.N., Labropoulos N. Burden and suffering in chronic venous disease. Adv Ther. 2019;36:1–4. doi: 10.1007/s12325-019-0882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies A.H. The seriousness of chronic venous disease: a Review of real-world evidence. Adv Ther. 2019;36:5–12. doi: 10.1007/s12325-019-0881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright A.M., Pittman C., Weldon S.C., Stanis S. Is edema underrecognized in superficial venous disease patients? Abstracts for the American Vein and Lymphatic Society 35th Annual Congress. Phlebology. 2022;37:3–39. [Google Scholar]