Abstract
Background
There is currently much focus on provision of general physical health advice to people with serious mental illness and there has been increasing pressure for services to take responsibility for providing this.
Objectives
To review the effects of general physical healthcare advice for people with serious mental illness.
Search methods
We searched the Cochrane Schizophrenia Group’s Trials Register (last update search October 2012) which is based on regular searches of CINAHL, BIOSIS, AMED, EMBASE, PubMed, MEDLINE, PsycINFO and registries of Clinical Trials. There is no language, date, document type, or publication status limitations for inclusion of records in the register.
Selection criteria
All randomised clinical trials focusing on general physical health advice for people with serious mental illness..
Data collection and analysis
We extracted data independently. For binary outcomes, we calculated risk ratio (RR) and its 95% confidence interval (CI), on an intention‐to‐treat basis. For continuous data, we estimated the mean difference (MD) between groups and its 95% CI. We employed a fixed‐effect model for analyses. We assessed risk of bias for included studies and created 'Summary of findings' tables using GRADE.
Main results
Seven studies are now included in this review. For the comparison of physical healthcare advice versus standard care we identified six studies (total n = 964) of limited quality. For measures of quality of life one trial found no difference (n = 54, 1 RCT, MD Lehman scale 0.20, CI ‐0.47 to 0.87, very low quality of evidence) but another two did for the Quality of Life Medical Outcomes Scale ‐ mental component (n = 487, 2 RCTs, MD 3.70, CI 1.76 to 5.64). There was no difference between groups for the outcome of death (n = 487, 2 RCTs, RR 0.98, CI 0.27 to 3.56, low quality of evidence). For service use two studies presented favourable results for health advice, uptake of ill‐health prevention services was significantly greater in the advice group (n = 363, 1 RCT, MD 36.90, CI 33.07 to 40.73) and service use: one or more primary care visit was significantly higher in the advice group (n = 80, 1 RCT, RR 1.77, CI 1.09 to 2.85). Economic data were equivocal. Attrition was large (> 30%) but similar for both groups (n = 964, 6 RCTs, RR 1.11, CI 0.92 to 1.35). Comparisons of one type of physical healthcare advice with another were grossly underpowered and equivocal.
Authors' conclusions
General physical health could lead to people with serious mental illness accessing more health services which, in turn, could mean they see longer‐term benefits such as reduced mortality or morbidity. On the other hand, it is possible clinicians are expending much effort, time and financial resources on giving ineffective advice. The main results in this review are based on low or very low quality data. There is some limited and poor quality evidence that the provision of general physical healthcare advice can improve health‐related quality of life in the mental component but not the physical component, but this evidence is based on data from one study only. This is an important area for good research reporting outcome of interest to carers and people with serious illnesses as well as researchers and fundholders.
Plain language summary
General physical health care advice for people with serious mental illness
People with serious mental illness tend to have poorer physical health than the general population with a greater risk of contracting diseases and often die at an early age. In schizophrenia, for example, life expectancy is reduced by about 10 years. People with mental health problems have higher rates of heart problems (cardiovascular disease), infectious diseases (including HIV and AIDS), diabetes, breathing and respiratory disease, and cancer.
Advising people on ways to improve their physical health is not without problems since there is often a perception, that advice offered is ineffective and will be ignored but it has been shown that healthcare professional advice can have a positive impact on behaviour. Advice can often motivate people to seek further support and treatment. Health advice could improve the quality and duration of life of people with serious mental illness. There is currently much focus on general physical health advice for people with serious mental illness with increasing pressure for health services to take responsibility for providing better advice and information.
This review focuses specifically on studies of general physical health advice and excludes more targeted health interventions.
Based on an electronic search carried out in 2012, this review now includes seven studies that randomised a total of 1113 people with serious mental illness. Six studies compared general physical health advice with standard care, one compared advice on healthy living with artistic techniques such as sketching and pottery. Information was of limited low or very low quality, there were a small number of participants and findings were ambiguous.
There is some limited evidence that the provision of physical healthcare advice can improve health‐related quality of life mentally but not physically. No studies returned results that suggest that physical healthcare advice has a powerful effect on physical healthcare behaviour or risk of ill health. More work is needed in this area. Only one adverse effect outcome was presented, death, but there were no differences between the treatment groups for this outcome.
Funders and policy makers should be aware that there may be some benefit for physical health advice for people with serious mental illness. There is an increased demand for preventative health services that involve the provision of advice and which may also reduce costs to health services.
This plain language summary has been written by a consumer, Ben Gray from RETHINK.
Summary of findings
Summary of findings for the main comparison. PHYSICAL HEALTH ADVICE versus STANDARD CARE for people with serious mental illness.
| PHYSICAL HEALTH ADVICE versus STANDARD CARE for people with serious mental illness | ||||||
| Patient or population: patients with people with serious mental illness Settings: Intervention: PHYSICAL HEALTH ADVICE versus STANDARD CARE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | PHYSICAL HEALTH ADVICE versus STANDARD CARE | |||||
| Physicl health awareness ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No studies reported on this outcome, which we had pre‐stated to be of importance. |
| Physical health behaviour moderate or vigorous physical activity Follow‐up: 6 months | The mean physical health behaviour in the control groups was 152 minutes | The mean physical health behaviour in the intervention groups was 39 higher (76.53 lower to 154.53 higher) | 80 (1 study) | ⊕⊕⊝⊝ low1,2,3 | ||
| Quality of life Lehman Quality of Life Scale. Scale from: 1 to 7 Follow‐up: 18 months | The mean quality of life in the control groups was 4.45 points4 | The mean quality of life in the intervention groups was 0.2 higher (0.47 lower to 0.87 higher) | 54 (1 study) | ⊕⊝⊝⊝ very low1,2,3,5 | ||
| Adverse effects Death of participant Follow‐up: median 6‐12 months | Low‐risk population6 | RR 0.98 (0.27 to 3.56) | 487 (2 studies) | ⊕⊕⊝⊝ low2,3,7 | ||
| 10 per 1000 | 10 per 1000 (3 to 36) | |||||
| Medium‐risk population6 | ||||||
| 15 per 1000 | 15 per 1000 (4 to 53) | |||||
| High‐risk population6 | ||||||
| 50 per 1000 | 49 per 1000 (14 to 178) | |||||
| Economic ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No studies reported on this outcome we had pre‐stated to be of importance. |
| Leaving the study early | Study population | RR 1.11 (0.92 to 1.35) | 964 (6 studies) | ⊕⊝⊝⊝ very low1,2,3,8 | ||
| 300 per 1000 | 333 per 1000 (276 to 405) | |||||
| Medium‐risk population | ||||||
| 292 per 1000 | 324 per 1000 (269 to 394) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Limitations of design: rated 'serious' (lack of allocation concealment) 2 Limitations of design: rated 'serious' (lack of blinding) 3 Imprecision: rated 'serious' (small sample size) 4 Based on seven point Likert scale 5 Indirectness: rated 'serious' (authors admit that measurement tool was difficult to interpret) 6 Range based around data from control group 7 Limitations of design: rated 'serious' (duration of study may have negative effect on motivation) 8 Inconsistency: rated 'very serious' (some of the trials were cluster trials)
Summary of findings 2. HEALTH EDUCATION versus HEALTH EMPOWERMENT EDUCATION for people with serious mental illness.
| HEALTH EDUCATION versus HEALTH EMPOWERMENT EDUCATION for people with serious mental illness | ||||||
| Patient or population: people with serious mental illness Settings: Intervention: HEALTH EDUCATION versus HEALTH EMPOWERMENT EDUCATION | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | HEALTH EDUCATION versus HEALTH EMPOWERMENT EDUCATION | |||||
| Physical health awareness ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No studies reported on this outcome we had pre‐stated to be of importance. |
| Physical health behaviour ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies reported on this outcome we had pre‐stated to be of importance. |
| Quality of Life Lehaman Quality of Life Scale. Scale from: 1 to 7. Follow‐up: 12 months | The mean quality of life in the control groups was 4.45 points | The mean Quality of Life in the intervention groups was 0.3 lower (0.99 lower to 0.39 higher) | 51 (1 study) | ⊕⊕⊝⊝ low1,2,3,4 | ||
| Adverse Effects | Study population | RR 0 (0 to 0) | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Medium‐risk population | ||||||
| Economic ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Leaving the study early Follow‐up: mean 12 months | Low‐risk population5 | RR 0.56 (0.26 to 1.19) | 78 (1 study) | ⊕⊕⊝⊝ low1,2,3,4 | ||
| 200 per 1000 | 112 per 1000 (52 to 238) | |||||
| Medium‐risk population5 | ||||||
| 300 per 1000 | 168 per 1000 (78 to 357) | |||||
| High‐risk population5 | ||||||
| 500 per 1000 | 280 per 1000 (130 to 595) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Limitations of design: rated 'serious' (lack of allocation concealment) 2 Limitations of design: rated 'serious' (lack of blinding) 3 Imprecison: rated 'serious' (small sample size) 4 Imprecision: rated 'serious' (high attrition rate) 5 Fewtrell et al. Arch Dis Child 2008; 93: 458‐461 (doi: 10.11361adc.2007.127316)
Summary of findings 3. HEALTHY LIVING STUDY CIRCLE versus AESTHETIC STUDY CIRCLE for people with serious mental illness.
| HEALTHY LIVING STUDY CIRCLE versus AESTHETIC STUDY CIRCLE for people with serious mental illness | ||||||
| Patient or population: patients with people with serious mental illness Settings: Intervention: HEALTHY LIVING STUDY CIRCLE versus AESTHETIC STUDY CIRCLE | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | HEALTHY LIVING STUDY CIRCLE versus AESTHETIC STUDY CIRCLE | |||||
| Physical health: Identification of disease state (Metabolic syndrome) | Study population | RR 1.25 (0.35 to 4.49) | 13 (1 study7) | ⊕⊕⊝⊝ low2,3,4,5,6 | ||
| 400 per 10001 | 500 per 1000 (140 to 1000)1 | |||||
| Medium‐risk population | ||||||
| 400 per 10001 | 500 per 1000 (140 to 1000)1 | |||||
| Physical health behaviour ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | No studies reported on this outcome we had pre‐stated to be of importance. |
| Quality of life ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | |
| Adverse Effects ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Economic ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | |
| Leaving the study early | Study population | RR 0 (0 to 0) | 0 (0) | See comment | ||
| See comment | See comment | |||||
| Medium‐risk population | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Cluster trial (n = 10), results subject to design effect calculation (D.E.=1.23) 2 Limitations of design: rated 'serious' (lack of allocation concealment) 3 Limitations of design: rated 'serious' (lack of blinding) 4 Duration of study may have a negative effect on motivation 5 Imprecison: rated 'serious' (small sample size) 6 Imprecision: rated 'serious' (high attrition rate) 7 National Institue of Health ‐ National Cholestrol Education Programme ‐ Adult Treatment Panel III 2001
Background
Description of the condition
The definition of serious mental illness with the widest consensus is that of the National Institute of Mental Health (NIMH) (Schinnar 1990) and is based on diagnosis, duration and disability (NIMH 1987). People with serious mental illness have conditions such as schizophrenia or bipolar disorder, over a protracted period of time, resulting in erosion of functioning in day to day life. A European survey put the total population‐based annual prevalence of serious mental illness at approximately two per thousand (Ruggeri 2000). People with serious mental illness have a higher morbidity and mortality from chronic diseases than the general population, and this results in a significantly reduced life expectancy (Robson 2007). In schizophrenia, for example, life expectancy is reduced by around 10 years (Newman 1991). Sufferers from serious mental illness have increased rates of cardiovascular disease, infectious diseases (including HIV) (Cournos 2005), non‐insulin dependent diabetes, respiratory disease and cancer (Dixon 1999; Robson 2007).
Description of the intervention
Physical health advice/promotion can take many forms, and these are highly divergent and dependent on environmental and socioeconomic factors. Physical health monitoring is the focus of a previous review (Tosh 2010a). Whereas monitoring is passive, advice is the active provision of preventative information. It has an educative component and is delivered in a gentle non‐patronising manner (Stott 1990). In the context of this review we suggest that physical health advice should not be delivered solely in the form of a structured programme or training approach. Currently, much health promotion/advice exists (Smith 2007; Smith 2007a; Solty 2009). This is often targeted at a discrete problem, such as poor diet or smoking. In this review, however, we focus on studies of general physical health advice and exclude more targeted approaches. By general physical health we mean that which is not in any way focused on any one condition, system or behaviour/intervention.
How the intervention might work
Advising people on ways to improve their physical health is not without problems since there is often a perception, from family doctors in particular, that advice offered is ineffective and patients will reject it (Sutherland 2003). This is not necessarily the case. It has been demonstrated that physician or healthcare professional advice can have a positive impact on behaviour (Kreuter 2000; Russell 1979). Advice can often act as the catalyst for motivating people to seek further support and treatment (Sutherland 2003). Given the evidence of increased rates of potentially preventable health problems in people with serious mental illness (Cournos 2005; Dixon 1999; Robson 2007), and the suggestion from a 2005 systematic review (Bradshaw 2005) that methodologically robust, healthy living interventions give "promising outcomes" in people with schizophrenia, we believe that appropriate health advice could improve the quality and duration of life for sufferers of serious mental illness. Additional benefits may include a reduction in dependence on medical services. "There are potential savings to be made on prescribing acute care budgets through prevention or early detection of serious illness in these groups of service users" (DoH 2006).
Why it is important to do this review
There is evidence to suggest that the physical health needs of people with serious mental illness are often "unrecognised, unnoticed or poorly managed" (DoH 2006). Neglecting the physical healthcare needs of people with serious mental illness adds to the already high burden placed on individuals, careers, communities and society as a whole. It is estimated that the economic and financial cost of mental health problems in the UK stands at £77 billion, mainly as a result of lost productivity (HM Government 2009). In November 2004 the UK's Department of Health published 'Choosing health: making healthy choices easier' (DoH 2005). This set out key principles to support the public to make healthier and more informed choices about lifestyles. A report by the UK's King's Fund indicated that 86% of the general public agreed that the UK Government has a responsibility to provide information and advice to prevent illness (Kings Fund 2004). Despite government policy and the public desire for more physical healthcare advice, we could not identify any systematic reviews that refer to randomised controlled trials though a "systematic review of the published and grey literature" (Bradshaw 2005) concluded that "further research is needed to assist in the development of effective interventions to help this client group" (people with serious mental illness). This is one of a series of reviews (Table 4).
1. Series of related reviews.
| Title | Reference |
| General physical healthcare monitoring | Tosh 2010a |
| General physical healthcare advice | This review |
| Advice regarding smoking cessation | Khanna 2012 |
| Advice regarding oral health care | Khokhar 2011 |
| Advice regarding HIV/AIDs prevention | Wright 2012 |
| Advice regarding substance use | Underway |
Objectives
To review the effects of general physical healthcare advice for people with serious mental illness.
Methods
Criteria for considering studies for this review
Types of studies
We considered all relevant randomised controlled trials (RCTs) and economic evaluations conducted alongside included RCTs. We excluded quasi‐randomised studies, such as those allocating by using alternate days of the week. If we had encountered trials described in some way as to suggest or imply that the study was randomised and where the demographic details of each group's participants were similar, we intended to include them and in a sensitivity analysis of the effects of the presence or absence of these data.
Types of participants
We required that the majority of participants should be within the age range 18 to 65 years and suffering from severe mental disorder, preferably as defined by NIMH 1987 or, in the absence of this, from diagnosed illnesses such as schizophrenia, schizophrenia‐like disorders, bipolar disorder, or serious affective disorders. We did not consider substance abuse to be a severe mental disorder in its own right; however, we did feel that studies should remain eligible if they dealt with people with dual diagnoses, that is those with severe mental illness plus substance abuse. We did not include studies focusing on dementia, personality disorder and mental retardation, as they are not covered by our definition of severe mental disorder.
Types of interventions
1. General physical health advice
We have found it difficult to find a useful definition of ‘advice’. In the context of this review we define ‘advice’ as preventative information (Greenlund 2002) or counsel (Oxford English Dictionary) that leaves the recipient to make the final decision; it should have at least a suggestion of: i. an educative component; ii. a preventative aim; and iii. an ethos of self‐empowerment. Advice may be directional but not paternalistic in its delivery. It is not a programmed or training approach, focusing on the acquisition of knowledge, skills, and competencies as a result of formal teaching sessions.
We defined 'physical health' as 'soundness of body' as opposed to the World Health Organization's definition of 'health' which includes mental and social well being (WHO 1948).
‘General’ physical health advice involves the giving of advice that is not in any way focused on any one condition or system or behaviour/intervention.
2. Treatment as usual
Care in which physical health advice is not specifically emphasised above and beyond care that would be expected for people suffering from severe mental illness.
Types of outcome measures
For the purposes of this review we divided outcomes into four time periods, i. immediate (within one week) ii. short term (one week to six months) iii. medium term (six months to one year) and, iv. long term (over one year).
Primary outcomes
1. Physical health awareness
1.1 Failure to raise awareness of common physical health problems 1.2 Failure to raise awareness of behaviours which can contribute to ill‐health
2. Physical health behaviour
2.1 No substantial change in behaviour
Secondary outcomes
1. Physical health behaviour
1.1 No change in behaviour 1.2 Deterioration in physical health behaviour
2. Physical health
2.1 Failure to act on known risk factors 2.2 Failure to address disease potentially associated with psychiatric diagnosis 2.3 Failure to raise awareness of common physical health problems 2.4 Unchecked adverse effects of treatment
3. Quality of life
3.1 Loss of independence 3.2 Loss of activities of daily living (ADL) skills 3.3 Chronic pain 3.4 Immobility 3.5 Loss of social status 3.6 Healthy days 3.7 No clinically important change in general quality of life
4. Adverse event
4.1 Number of participants with at least one adverse effect 4.2 Clinically important specific adverse effects (cardiac effects, death, movement disorders, prolactin increase and associated effects, weight gain, effects on white blood cell count) 4.3 Average endpoint in specific adverse effects 4.4 Average change in specific adverse effects 4.5 Death ‐ natural or suicide
5. Service use
5.1 Hospital admission 5.2 Emergency medical treatment 5.3 Use of emergency services
6. Financial dependency
6.1 Claiming unemployment benefit 6.2 Claiming financial assistance because of a physical disability
7. Social
7.1 Unemployment/loss of earnings 7.2 Social isolation as a result of preventable incapacity 7.3 Increased burden to caregivers
8. Economic
8.1 Increased costs of health care 8.2 Days off sick from work 8.3 Reduced contribution to society 8.4 Family claiming careers’ allowance
9. Leaving the studies early (any reason, adverse events, inefficacy of treatment)
10. Global state
10.1 No clinically important change in global state (as defined by individual studies) 10.2 Relapse (as defined by the individual studies)
11. Mental state (with particular reference to the symptoms of schizophrenia)
11.1 No clinically important change in general mental state score 11.2 Average endpoint general mental score 11.3 Average change in general mental state score 11.4 No clinically important change in specific symptoms (positive/negative symptoms of schizophrenia) 11.5 Average endpoint specific symptom score 11.6 Average change in specific symptom score
12. 'Summary of findings' tables
We used the GRADE approach to interpret findings (Schünemann 2008) and used the GRADE profiler (GRADE PRO) to import data from RevMan 5 (Review Manager) to create 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes that we rated as important to patient‐care and decision making. We intended to include the following outcomes in a 'Summary of findings' table.
Physical health awareness ‐ Failure to raise awareness of common physical health problems or behaviours which can contribute to ill‐health
Physical health behaviour ‐ No substantial change in behaviour
Quality of life ‐ Loss of independence
Adverse event ‐ Clinically important specific adverse effects (cardiac effects, death, movement disorders, prolactin increase and associated effects, weight gain, effects on white blood cell count
Economic ‐ Increased costs of health care
Financial dependency ‐ Claiming financial assistance because of a physical disability
Global state ‐ No clinically important change in global state (as defined by individual studies)
Search methods for identification of studies
Electronic searches
1. Original search (2009)
The Cochrane Schizophrenia Group's Trials Register was searched (November 2009) using the phrase:
[(*physical* or *cardio* or *metabolic* or *weight* or *HIV* or *AIDS* or *Tobacc* or *Smok* or *sex* or *medical* or *dental* or *alcohol* or *oral* or *vision* or *sight*or *hearing* or *nutrition* or *advice* or *monitor* in title of REFERENCES) AND (*education* OR *health promot* OR *preventi* OR *motivate* or *advice* or *monitor* in interventions of STUDY)]
This register is compiled by systematic searches of major databases, handsearches and conference proceedings (see Group Module).
2. Update search (2012)
The Trials Search Co‐ordinator, Samantha Roberts, searched the Cochrane Schizophrenia Group's Trials Register (October 2012) using the phrase:
[(*physical* or *cardio* or *metabolic* or *weight* or *HIV* or *AIDS* or *Tobacc* or *Smok* or *sex* or *medical* or *dental* or *alcohol* or *oral* or *vision* or *sight*or *hearing* or *nutrition* or *advice* or *monitor* in title of REFERENCES) AND (*education* OR *health promot* OR *preventi* OR *motivate* or *advice* or *monitor* in interventions of STUDY)]
The Cochrane Schizophrenia Group’s Registry of Trials is compiled by systematic searches of major resources (including AMED, BIOSIS, CINAHL, EMBASE, MEDLINE, PsycINFO, PubMed, and registries of Clinical Trials) and their monthly updates, handsearches, grey literature, and conference proceedings (see Group Module). There is no language, date, document type, or publication status limitations of inclusion of records in the register.
Searching other resources
1. Reference searching
We inspected the references of all identified studies for other relevant studies.
2. Personal contact
We contacted the first author of each included trial for information regarding unpublished studies, we also contacted the first author of each ongoing study and requested information about current progress. If authors responded with relevant information we used this and noted their response in the Characteristics of included studies.
Data collection and analysis
Selection of studies
For the original review, review authors GT, AC and SM screened the results of theoriginal electronic search; to ensure reliability another review author MB inspected a random sample of the electronic search, comprising 10% of the total. GT and AC inspected all abstracts of studies identified through screening and identified potentially relevant reports. Where disagreement occurred we resolved this by discussion, and where there was still doubt, we acquired the full article for further inspection. We then requested the full articles of relevant reports for reassessment and carefully inspected them for a final decision on inclusion (see Criteria for considering studies for this review). In turn, GT and AC inspected all full reports and independently decided whether they met the inclusion criteria.
The results from the most recent 2012 electronic search were screened by JX who inspected all abstracts and identified potentially relevant reports. MW inspected full articles for final inclusion. JX, GT and AC were consulted in cases where there was uncertainty and a final decision was made when an agreement was reached by all authors. We were not blinded to the names of the authors, institutions or journal of publication.
Data extraction and management
1. Extraction
For the original review, authors GT and AC independently extracted data from included studies. Again, we discussed any disagreement, documented our decisions and, if necessary, we contacted the authors of studies for clarification. Whenever possible we only extracted data presented in graphs and figures, and we only included data if two review authors independently had the same result. We made attempts to contact authors through an open‐ended request in order to obtain any missing information or for clarification whenever necessary. Where possible, we extracted data relevant to each component centre of multi‐centre studies separately. From the 2012 update search, one of the studies previously listed as ongoing had been finished and the study was included in the review. JX and MW independently extracted data.
2. Management
2.1 Forms
For the original review, GT and AC extracted data onto standard, simple forms.
For the 2012 update, JX and MW independently extracted data from the new included study.
2.2 Data from multi‐centre trials
Where possible the authors verified independently calculated centre data against original trial reports.
3. Rating scales
A wide range of instruments are available to measure outcomes in mental and physical health studies. They vary in quality and are often not validated or are created for a particular study. It is accepted generally that measuring instruments should be both reliable and have reasonable validity (Rust 1989). For the original review, we included continuous data from rating scales only if the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000); and not those written or modified by one of the trialists for a particular trial.
4. Endpoint versus change data
We preferred to use scale endpoint data, which typically cannot have negative values and are easier to interpret from a clinical point of view. Change data are often not ordinal and are very problematic to interpret. We did not identify such data for this review update. For future updates of this review, If endpoint data are unavailable, we will use change data.
5. Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion: (a) standard deviations (SDs) and means are reported in the paper or obtainable from the authors; (b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996); (c) if a scale starts from a positive value (such as the Positive and Negative Syndrome Scale (PANSS) (Kay 1986), which can have values from 30 to 210), the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐S min), where S is the mean score and S min is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied. When continuous data are presented on a scale which includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We entered skewed data from studies of less than 200 participants in other tables within the data analyses section rather than into an statistical analysis. Skewed data pose less of a problem when looking at means if the sample size is large, and in future updates of the review, we will enter skewed data from studies with large sample sizes into syntheses, if more data are identified.
6. Common measure
To facilitate comparison between trials, we intended to convert variables that can be reported in different metrics, such as days in hospital, (mean days per year, per week or per month) to a common metric (e.g. mean days per month). Although common measure was not an issue in this update review, the above procedures will be followed in future updates.
7. Conversion of continuous to binary
We had planned to convert outcome measures to dichotomous data wherever possible, however the need did not arise. The conversion could be done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the PANSS (Kay 1986; Kay 1987), could be considered as a clinically significant response (Leucht 2005; Leucht 2005a). In future updates if data based on these thresholds are not available, we will use the primary cut‐off presented by the original authors.
8. Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for general physical health advice.
Assessment of risk of bias in included studies
Again working independently, for the original review, GT and AC and for the update review JX and MW assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome, the completeness of outcome data, selective reporting and other biases. We excluded studies where allocation was clearly not concealed. We did not include trials with high risk of bias (defined as at least three out of five domains categorised as 'No') in the meta‐analysis; we have summarised the results of our assessment of risk of bias in Figure 1. Where the raters disagreed, the final rating was made by consensus with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted the authors of the studies in order to obtain further information. We reported non‐concurrence in quality assessment.
1.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI). It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). The Number Needed to Treat/Harm (NNT/H) statistic with its confidence intervals is intuitively attractive to clinicians but is problematic both in its accurate calculation in meta‐analyses and interpretation (Hutton 2009). For binary data presented in the 'Summary of findings' tables, where possible, we calculated illustrative comparative risks.
2. Continuous data
For continuous outcomes we estimated the mean difference (MD) between groups. We preferred not to calculate effect size measures (standardised mean difference SMD). However, for future updates if scales of very considerable similarity are used, we will presume there is a small difference in measurement, and will calculate effect sizes and transform the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby P values are spuriously low, CIs unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997; Gulliford 1999).
For studies where clustering was not accounted for, we would have presented data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. If we find cluster‐randomised trial data in subsequent versions of this review, we will seek to contact first authors of studies to obtain intra class correlation co‐efficient (ICC) of their clustered data and to adjust for this by using accepted methods (Gulliford 1999). If clustering has been incorporated into the analysis of primary studies, we will present these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We sought statistical advice during the protocol state of this review, and were advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the ICC (Design effect = 1+(m‐1)*ICC) (Donner 2002). If the ICC is not reported, we will assume it to be 0.1 (Ukoumunne 1999).
If cluster studies have been appropriately analysed taking into account ICC and relevant data documented in the report, synthesis with other studies will be possible using the generic inverse variance technique.
2. Cross‐over trials
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. For the same reason cross‐over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). No cross‐over trials were identified from either search for this review, but as both effects are very likely in serious mental illness, in future updates we will only use data from the first phase of cross‐over studies.
3. Studies with multiple treatment groups
No studies with multiple treatment groups were identified for this review, but for future updates, it is planned that where a study involves more than two treatment arms, if relevant, we will present the additional treatment arms in comparisons. Where the additional treatment arms are not relevant, we will not reproduce these data.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). In the original review, for any particular outcome, if more than 50% of data were unaccounted for, we did not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we have addressed this within the 'Summary of findings' tables by down‐rating quality. Finally, we also downgraded quality within the 'Summary of findings' tables where loss was 25% to 50% in total.
2. Binary
In the original review, in the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). Those leaving the study early were assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death and adverse effects. For these outcomes, the rate of those who remained in the study ‐ in that particular arm of the trial ‐ were used for those who did not. We intended to undertake sensitivity analysis to test how prone the primary outcomes were to change when data only from people who complete the study to that point are compared to the intention‐to treat analysis using the above assumptions, but only two studies in separate comparisons reported data for the primary outcome so this was not possible. We intend to follow this procedure in future updates if new studies with data for this outcome are identified.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome was between 0% and 50%, and data only from people who completed the study to that point were reported, we reproduced these.
3.2 Standard deviations
All data used in analyses were provided in the study reports. We had planned that if standard deviations (SDs) were not reported, we would first tried to obtain the missing values from the authors. If not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and confidence intervals (CIs) available for group means, and either 'P' or 't' values available for differences in mean, we could calculate them according to the rules described in the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011): When only the SE is reported, SDs are calculated by the formula SD = SE * square root (n). Chapters 7.7.3 and 16.1.3 of the Cochrane Handbook for Systemic reviews of Interventions (Higgins 2011) present detailed formulae for estimating SDs from P, t or F values, CIs, ranges or other statistics. If these formulae do not apply, we can calculate the SDs according to a validated imputation method which is based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would be to exclude a given study’s outcome and thus to lose information. We nevertheless examined the validity of the imputations in a sensitivity analysis excluding imputed values. We will follow these procedure in future updates.
3.3 Last observation carried forward
We anticipated that in some studies the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results (Leucht 2007). Therefore, in the original review, where LOCF data were used in the trial, if less than 50% of the data had been assumed, we presented and used these data and indicated that they were the product of LOCF assumptions. This will be followed in future updates.
Assessment of heterogeneity
1. Clinical heterogeneity
To judge clinical heterogeneity, we considered all included studies, initially without seeing comparison data. We simply inspected all studies for clearly outlying situations or people which we had not predicted would arise. Where such situations or participant groups arose, we fully discussed these. The same procedure will be followed for future updates.
2. Methodological heterogeneity
For future updates, we will consider all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We will simply inspect all studies for clearly outlying methods which we had not predicted would arise. When such methodological outliers arise in updates, we will fully discuss these. This was carried out for the original review.
3. Statistical
3.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
3.2 Employing the I‐squared statistic
In the original review, we investigated heterogeneity between studies by considering the I2 method alongside the Chi2 P value. The I2 provides an estimate of the percentage of inconsistency thought to be due to chance (Higgins 2003). The importance of the observed value of I2 depends on i. magnitude and direction of effects and ii. strength of evidence for heterogeneity (e.g. ) value from Chi2 test, or a confidence interval for I2). An I2 estimate greater than or equal to around 50% accompanied by a statistically significant Chi2 statistic was interpreted as evidence of substantial levels of heterogeneity (Higgins 2011). If relevant studies are identified in updated versions of this review where substantial levels of heterogeneity are found in the primary outcome, we will explore the reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity). If the inconsistency is high and clear reasons are found, we will present data separately.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in Section 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not plan to use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar sizes. In future updates of this review, where funnel plots are possible, we will seek statistical advice in their interpretation.
Data synthesis
Where possible, we used a fixed‐effect model for analyses. We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that different studies are estimating different, yet related, intervention effects. According to our hypothesis of an existing variation across studies, to be explored further in the meta‐regression analysis, despite being cautious that random‐effects methods do put added weight onto the smaller of the studies, we will favour using the fixed‐effect model in future updates. The reader is, however, able to choose to inspect the data using the random‐effects model.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analyses
We did not conduct any subgroup analyses.
2. Investigation of heterogeneity
2.1 Unanticipated heterogeneity
For future updates, should unanticipated clinical or methodological heterogeneity be obvious, we will simply state hypotheses regarding these. We have not undertaken and do not anticipate undertaking analyses relating to these.
2.2 Anticipated heterogeneity
We are concerned that focused physical healthcare advice may have different effects than a more general approach. We therefore anticipate some heterogeneity for the primary outcomes and will propose to summate all data but also present them separately.
Sensitivity analysis
1. Implication of randomisation
We aimed to include trials in a sensitivity analysis if they were described in some way as to imply randomisation. In future updates of this review, for the primary outcomes we will include these studies and if there is no substantive difference when we add the implied randomised studies to those with better description of randomisation, we will then use all data from these studies.
2. Assumptions for lost binary data
For future updates, where assumptions will need to be made regarding people lost to follow‐up (see Dealing with missing data), we will compare the findings of the primary outcomes where we used our assumptions with completer data only. If there is a substantial difference, we will report results and discuss them, but will continue to employ our assumption.
Results
Description of studies
For substantive description of studies please see Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The initial search of the Cochrane Schizophrenia Group's register of trials in November 2009 was a combined search designed to identify studies which would be relevant to this review and to a series of sister reviews looking at more targeted advice relating to specific problems or behaviours (e.g. oral health, HIV, smoking), some of these are already underway and some are already published, seeTable 4. This search PRISMA diagram is seen in Figure 2). An additional electronic search was performed in October 2012 in order to identify recent studies relevant to this review (Figure 3).
2.

PRISMA search flow diagram ‐ 2009 search
3.

Study flow diagram ‐ updated 2012
The original search identified 2382 references (from 1558 studies). After examining search results, we identified 15 reports which were suitable for further assessment. Of these, six fulfilled criteria for inclusion, we excluded seven and confirmed that two were awaiting classification. In our most recent search in 2012, 2428 (46 additional) studies were identified, 33 of these were suitable for further evaluation, one of which ended up fulfilling criteria for inclusion and was a study that was previously listed as awaiting classification.
Included studies
For details of included studies please see Characteristics of included studies. The seven included studies randomised 1113 people. No study was double blind although Brown 2006, Brown 2009 and Danavall 2007 did attempt to maintain rater (single) blindness. Byrne 1999 and Forsberg 2008 were cluster trials.
1. Length of studies
Two of the included studies fell in to the short‐term category with a duration of six to 10 weeks. Danavall 2007 was categorised as medium term with a six‐month follow‐up, and the remaining four studies were in the long‐term category and had a duration of 12‐18 months.
2. Setting
Brown 2006, Brown 2009 and Danavall 2007 were conducted in community mental health teams, while Druss 2010 was set in primary care. Byrne 1999 and Forsberg 2008 took place in supported accomodation in the community and Chafetz 2008 was conducted in a crisis residential unit .
3. Participants
Participants in Brown 2006 and Brown 2009 were diagnosed using the International Classification of Diseases ((ICD), version 10) (WHO 2007). Byrne 1999 asked participants to self‐report what type of mental health problems they had, while Chafetz 2008, Danavall 2007 and Druss 2010 included patients who were diagnosed with a 'severe mental illness', but they did not specify any diagnostic manual. The remaining study, Forsberg 2008, used the Diagnostic and Statistical Manual of Mental Disorders IV (DSM IV 1994).
4. Study size
The largest studies were Druss 2010 (n = 407) and Chafetz 2008 (n = 309); the smallest were Brown 2006 (n = 28) and Brown 2009 (n = 26). Danavall 2007 involved 80 participants. The other two studies were cluster trials. Byrne 1999 randomised 22 clusters, with a total of 214 people therein, and Forsberg 2008 10 clusters that comprised 97 people.
5. Interventions
5.1 General physical health advice
Brown 2006 and Brown 2009 looked at semi‐structured health promotion that involved participants receiving six semi‐structured health promotion sessions, which followed the Lilly "Meaningful Day" (Lilly 2002) manual. Byrne 1999 involved a one‐year physical health educational programme consisting of an intensive 12‐week programme with less intensive follow‐up for nine months focusing on overall wellness. Chafetz 2008 promoted skills in self‐assessment, self‐monitoring, and self‐management of physical health problems. Danavall 2007 delivered six sessions to help participants become more effective managers of their chronic illnesses involving chronic disease management, exercise and physical activity, pain and fatigue management, healthy eating on a limited budget, medication management and finding and working with a regular doctor. Druss 2010 examined the effect of care management. Care managers provided "communication and advocacy with medical providers", health education and support in overcoming barriers to primary health care. This was based on standardised approaches documented in the care management literature (Druss 2010). The program was designed to help overcome patient, provider, and system‐level barriers to primary medical care experienced by persons with mental disorders. Forsberg 2008's intervention took the form of a study circle: study material comprised a book focusing on motivation, food content, stress and fitness and they also used a further comparator (aesthetic study circle) as described below. Although the trials we inspected used different methods of delivering general physical health advice, we thought these methods to be comparable on the basis that all fell under our broad definition of general physical healthcare advice.
5.2 Comparators
Comparators were largely 'standard care', which was variously described as 'treatment as usual' (Brown 2006; Brown 2009), 'control group' (Byrne 1999) and 'usual care' (Chafetz 2008; Danavall 2007; Druss 2010). Three studies, however, did not give any detailed description of their comparators (Brown 2006; Brown 2009; Byrne 1999). Both Brown studies failed to describe what 'treatment as usual' was and Byrne 1999 did not explain what treatment the 'control group' received. Chafetz 2008 described 'usual care' as basic primary care delivered by nurse practitioners and was an established part of the crisis residential unit which was the setting for the study. Danavall 2007 reported that participants should receive all medical, mental health, and peer‐based services that they were otherwise receiving prior to entry into the study. Druss 2010 described 'usual care' in which participants were given a list with contact information for local primary care medical clinics, which accepted uninsured and Medicaid patients, and these participants were allowed to obtain any type of medical care or medical service. Forsberg 2008 compared the effect of their experimental 'healthy living study circle' with a control in the form of an 'aesthetic study circle'. This was a study circle in which participants had the opportunity to learn and practice various kinds of artistic techniques such as sketching and pottery (Forsberg 2008). Additionally, because Byrne 1999 was the three‐arm study, this trial compared a one‐year health education programme not only with 'standard care' but also with an empowerment programme based on a model developed by Freire (Freire 1974; Freire 1983). This involved "group efforts identifying their problems, assessing the roots of their problems, and developing their goals" in a three‐phase process. First "the listening phase", second the "participatory dialogue" and in the final stage "group members tested out their understanding of the problem in the real world" (Byrne 1999).
6. Outcomes
6.1 General remarks
We were unable to use data from some studies (Brown 2006; Brown 2009; Chafetz 2008) because raw scores were not presented. Instead, outcomes were presented as inexact P values without means and standard deviations. We were unable to use some data in Forsberg 2008 as they were not reported by group. Byrne 1999 failed to report changes between baseline and completion of the intervention, and Druss 2010 did not reveal the distribution of individuals between the intervention arm and the control.
6.2 Outcome scales
Details of scales that provided usable data are shown below. Reasons for exclusion of data from other instruments are given under 'Outcomes' in the Characteristics of included studies.
6.2.1 Physical health behaviour
6.2.1.1 SILVA™ Pedometer plus The SILVA™ Pedometer plus was used to obtain measure of physical activity by counting the number of steps for 10 hours per day for one week. A higher score represents a higher rate of physical activity (high = good).
6.2.2.2 Physical health
6.2.2.1 Metabolic syndrome defined by the National Cholesterol Education Programme Adult Treatment Panel (NCEP 2001) This is a criterion for identifying metabolic syndrome where at least three of the following five criteria are needed: i) glucose ≥ 6.1 mmol/L, ii) blood pressure ≥ 130/85 mmHg or treatment for this, iii) triglycerides ≥ 1.7 mmol/L, iv) high‐density lipoprotein (HDL) men > 1.0 mmol/L or female > 1.3 mmol/L, and v) waist men >102 cm or female > 88 cm. A decrease in the number of people with metabolic syndrome was the desired outcome (low = good).
6.2.2.2 Incremental Shuttle Walk Test ‐ ISWT (Singh 1992) The ISWT requires participants to walk up and down a 10‐m shuttle course in a set time. It provides a direct comparison of an individual's performance (high=good).
6.2.2.3 Borg RPE (Rate of perceived exertion) Scale (Borg 1982) The Borg RPE is used to measure the perceived exertion before and after the Incremental Shuttle Walk Test was measured. The scale ranges between six and 20. Six means 'no exertion at all' and 20 means 'maximal exertion' (high=good).
6.2.3 Quality of life
6.2.3.1 Lehman Quality of Life Scale (Lehman 1988) The 127‐item questionnaire was administered in an interview format and assessed both subjective and objective indicators in eight domains: living situation daily activities and skills, family relations, social relations, finances, work and school, legal and safety issues and health. Satisfaction with life domains rated on a seven‐point scale: one is 'terrible' and seven is 'delighted' (high = good). 6.2.3.2 Medical Outcomes Study 36‐Item Short‐Form Health Survey ‐ MOS SF‐36 Health Survey (Ware 1998) The MOS SF‐36 Health Survey is a measure of health status designed for use in clinical practice, research, health policy evaluations, and general population surveys. It includes eight scales that assess the following general health concepts: physical functioning, role limitations due to physical health problems, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health. Summary scores can be constructed ranging from zero (poor health) to 100 (perfect health) (high = good).
6.2.4 Service use
6.2.4.1 U.S. Preventative Services Task Force guidelines ‐ USPSTF guidelines (AHRQ 2009) This scale is used to assess the quality of primary care. The USPSTF conducts rigorous, impartial assessments of the scientific evidence for the effectiveness of a broad range of clinical preventive services, including screening, counselling, and preventive medications. Its recommendations are considered the "gold standard" for clinical preventive services. A total of 23 indicators were included across four domains: 1) physical examination, 2) screening tests, 3) vaccination and 4) education. The primary study outcome was an aggregate preventive services score representing the proportion of services for which an individual was eligible that was obtained by the participant. The higher the value represents the percentage of recommended preventative services received (high = good).
6.2.5 Economic
6.2.4.1 Health Service Utilization Inventory (Browne 1990) The Health Service Utilization Inventory is designed to assess direct and indirect costs of health resources. A dollar value of health resource consumption is determined (low = good).
6.3 Missing outcomes
We had outlined in the first protocol for this review that we wished to find outcomes relevant to physical health awareness and behaviour, general physical health, quality of life, adverse events, service use, financial dependency, social functioning, economic implications, leaving the study early, global state and mental state. Of these outcomes, we failed to find any data at all relating to physical health awareness, financial dependency, social functioning, global state or mental state.
Excluded studies
For details of the excluded studies please see Characteristics of excluded studies. The original search strategy yielded 2382 references (from 1558 studies). From these we requested 15 studies for closer inspection. We excluded seven of these studies because their focus was on global mental well being rather than general physical health. In our most recent search from 2012 that yielded 46 studies, 18 underwent closer inspection, one of which met criteria for inclusion. An additional 14 of the studies were excluded because their focus was not on general physical health, two were not randomised and one included an education component in both arms of the trial.
1. Awaiting assessment
There are no studies awaiting assessment.
2. Ongoing studies
One study remains ongoing for the 2012 update review. For further details please see Characteristics of ongoing studies. Given the relatively small projected sample size in this study (n = 170) and considering the potential dropout rate, we do not anticipate that data from this study would significantly alter or add to the results of this review, although we look forward to them for further insights, or to be proved wrong.
Risk of bias in included studies
For details please refer to the Risk of bias in included studies tables and Figure 1.
Allocation
All included studies were stated to be randomised. Three did not describe the randomisation procedure (Brown 2006; Byrne 1999; Chafetz 2008). One randomised using a hidden computer‐generated random number programme (Brown 2009) and two using a "computerised algorithm" (Danavall 2007; Druss 2010). The final study was randomised at group level by drawing lots by a "person not in the project" (Forsberg 2008).
Blinding
Two studies failed to provide details about blinding (Byrne 1999; Forsberg 2008). One (Brown 2006) "attempted to maintain rater blindness" and, in a similar study (Brown 2009), the rater was blind to the interviewees status. In Danavall 2007 and Druss 2010 the "interviewers were blinded to subjects' randomisation status" and in the remaining study (Chafetz 2008), the "baseline severity of medical comorbidity was rated by Nurse Practitioners blind to study group". No study reported if they tested blinding.
Incomplete outcome data
The overall rate of leaving the study early was considerable (34%). In five of the studies the rate of leaving the study early was clearly above 30% (Brown 2006; Brown 2009; Byrne 1999; Chafetz 2008; Druss 2010). It is possible that reasons for this attrition were balanced across groups ‐ but there is no evidence to support this and there is also the possibility that the reasons differed for leaving early. This makes the studies vulnerable to bias. Danavall 2007 lost all but one of their participants due to being unable to locate them at follow‐up. Forsberg 2008 was a cluster trial and did not report the rate of leaving early by group.
Selective reporting
It would appear that all of the included studies reported on all of their intended outcomes. We did not, however, have access to any of the study protocols to confirm this.
Other potential sources of bias
Brown 2006 was supported by Eli Lilly (pharmaceutical industry) who supplied the Lilly "Meaningful Day" package; this package was then adapted for use in the subsequent study (Brown 2009). Danavall 2007 reported that one of the authors received royalties from the publisher of the book that was written for the intervention delivered the in the study. For Druss 2010 the lead author "received research funding from Pfizer", a pharmaceutical company which manufactures a wide range of medicines for conditions such as heart disorders, cancer, raised blood pressure, high cholesterol and sexual health. Chafetz 2008 was supported by the National Institute of Nursing Research and Forsberg 2008 received grants from five different public bodies in Sweden. The remaining study (Byrne 1999) was funded by the Ontario Ministry of Health.
Additionally, all trials were small trials that are themselves particularly associated with risks of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Comparison 1. Physical health advice versus standard care
Six studies provided data for the comparison physical health advice versus standard care. We calculated risk ratios (RR) for dichotomous data and mean differences (MD) for continuous data, with their respective 95% confidence intervals (CIs) throughout.
1.1 Physical health behaviour
Danavall 2007 reported no significant difference between groups for moderate or vigorous physical activity, the data were skewed (n = 80, 1 RCT, MD 39.00, CI ‐76.53 to 154.53, Analysis 1.1).
1.1. Analysis.

Comparison 1 PHYSICAL HEALTH ADVICE versus STANDARD CARE, Outcome 1 Physical health behaviour: Moderate or vigorous physical activity (min/week, skewed).
1.2 Quality of life
This outcome (Analysis 1.2) was reported by Byrne 1999, Danavall 2007 and Druss 2010 using different scales. Byrne 1999 (using the Lehman scale) reported no significant difference in quality of life (n = 54, 1 RCT, MD 0.20, CI ‐0.47 to 0.87). Danavall 2007 and Druss 2010 reported separately on the mental and physical components of the Quality of Life Medical Outcomes Study reporting a significant difference for the mental component (n = 487, 2 RCTs, MD 3.70, CI 1.76 to 5.64) and in the physical component (n = 487, 2 RCTs, MD 2.46, CI 0.33 to 4.59).
1.2. Analysis.
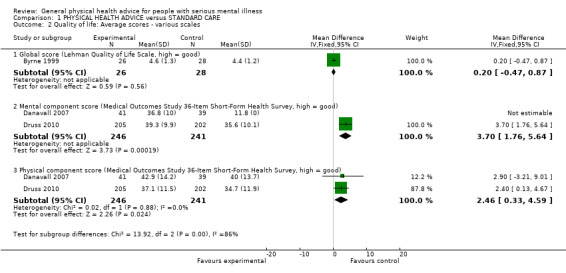
Comparison 1 PHYSICAL HEALTH ADVICE versus STANDARD CARE, Outcome 2 Quality of life: Average scores ‐ various scales.
1.3 Adverse effects: death
Danavall 2007 reported only on death and Druss 2010 reported seven deaths with "no significant difference" between treatment and control groups (n = 487, 2 RCTs, RR 0.98, CI 0.27 to 3.56, Analysis 1.3).
1.3. Analysis.

Comparison 1 PHYSICAL HEALTH ADVICE versus STANDARD CARE, Outcome 3 Adverse effects/events: Death.
1.4 Service use
One study (Druss 2010) provided data for the comparison care management versus usual care. Results significantly favoured the active treatment group (n = 363, 1 RCT, MD 36.90, CI 33.07 to 40.73, Analysis 1.4). Danavall 2007 also reported that significantly more people who received physical health advice attended primary care appointments than those receiving standard care alone (n = 80, 1 RCT, RR 1.77, CI 1.09 to 2.85).
1.4. Analysis.

Comparison 1 PHYSICAL HEALTH ADVICE versus STANDARD CARE, Outcome 4 Service use: Average percentage uptake of recommended health preventative services (US Preventative Services Task Force guidelines, high = good).
1.4 Economic
Byrne 1999 reported no significant difference between groups for general health service expenses. These data are, however, skewed and we report them in a table (Analysis 1.6).
1.6. Analysis.
Comparison 1 PHYSICAL HEALTH ADVICE versus STANDARD CARE, Outcome 6 Economic: Total value of health resource consumption (dollars, low = good, skewed data).
| Economic: Total value of health resource consumption (dollars, low = good, skewed data) | ||||
|---|---|---|---|---|
| Study | Interventions | Average consumption (US $) | SD | N |
| Byrne 1999 | Health empowerment | 1476.51 | 2191.98 | 36 |
| Byrne 1999 | Control | 956.63 | 2506.18 | 39 |
1.5 Leaving the study early
Six studies reported on participants leaving early for a variety of reasons; none identified any significant difference between experimental and control groups (Analysis 1.7).
1.7. Analysis.
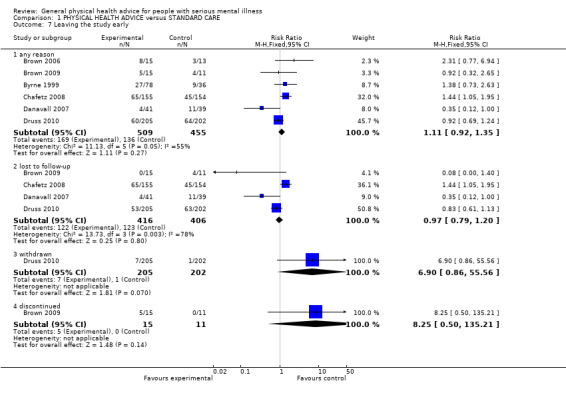
Comparison 1 PHYSICAL HEALTH ADVICE versus STANDARD CARE, Outcome 7 Leaving the study early.
1.5.1 Any reason
Six of our seven included studies provided data for the outcome of leaving the study early for any reason (n = 964, 6 RCTs, RR 1.11, CI 0.92 to 1.35). Brown 2006 and Brown 2009 reported considerable loss to follow‐up with 39% in the first study and 35% in the second. However, attrition occurred relatively evenly across intervention groups (n = 54, 2 RCTs, RR 1.49 CI 0.71 to 3.14). Byrne 1999 saw 31.6 % of participants leaving early but did not comment on the reasons for leaving (n = 114, 1 RCT, RR 1.38, CI 0.73 to 2.63). Chafetz 2008 reported 35.6% of participants leaving early (n = 309, 1 RCT, RR 1.44, CI 1.05 to 1.95) and defined these simply as "lost to follow up", citing that some had died, some had "moved on" and some were incarcerated. Further specifics were not available for these different reasons for leaving early. Danavall 2007 had the smallest percentage of participants leaving the study early of 18.8% with only one having died and the remaining being unable to locate for follow‐up (n = 80, 1 RCT, RR 0.35, CI 0.12 to 1.00). Druss 2010 only commented on "loss to follow up" (30.5%, n = 407, 1 RCT, RR 0.83, CI 0.61 to 1.13).
1.5.2 Lost to follow‐up
Brown 2009, Chafetz 2008, Danavall 2007 and Druss 2010 all reported on loss to follow‐up (n = 822, 4 RCTs, RR 0.97, CI 0.79 to 1.20).
1.5.3 Withdrawn
Druss 2010 reported on those "withdrawn" (n = 407, 1 RCT, RR 6.90, CI 0.86 to 55.56).
1.5.4 Discontinued
Brown 2009 provided data for those who 'discontinued' meaning they left for 'various personal reasons' (n = 26, 1 RCT, RR 8.25, CI 0.50 to 135.21).
Comparison 2. Health education versus empowerment education
Byrne 1999 provided data for the comparison health education versus empowerment education.
2.1 Quality of life
There was no significant difference in quality of life as assessed on the Lehman Quality of Life scale (n = 51, 1 RCT, MD ‐0.30, CI ‐0.99 to 0.39, Analysis 2.1).
2.1. Analysis.

Comparison 2 HEALTH EDUCATION versus HEALTH EMPOWERMENT EDUCATION, Outcome 1 Quality of life: Average global score (Lehman Quality of Life scale, high = good).
2.2 Economic
There was no significant difference between groups for general health education versus empowerment education; however, these data are skewed and we report them in a table (Analysis 2.2).
2.2. Analysis.
Comparison 2 HEALTH EDUCATION versus HEALTH EMPOWERMENT EDUCATION, Outcome 2 Economic: Total value of health resource consumption (dollars, low = good, skewed data).
| Economic: Total value of health resource consumption (dollars, low = good, skewed data) | ||||
|---|---|---|---|---|
| Study | Intervention | Mean (US $) | SD | N |
| Byrne 1999 | Health education | 1432.03 | 2588.67 | 39 |
| Byrne 1999 | Health empowerment | 1476.51 | 2191.98 | 36 |
2.3 Leaving early
There was no significant difference in the number of participants leaving the study early (n = 78, 1 RCT, RR 0.56, CI 0.26 to 1.19, Analysis 2.3).
2.3. Analysis.
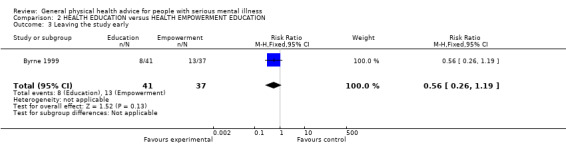
Comparison 2 HEALTH EDUCATION versus HEALTH EMPOWERMENT EDUCATION, Outcome 3 Leaving the study early.
Comparison 3. Programme of healthy living in the form of a study circle versus aesthetic study circle
Forsberg 2008 provided data for the comparison programme of healthy living in the form of a study circle versus aesthetic study circle.
3.1 Physical health behaviour
There was an increase in physical activity (steps per day) in the intervention group, but no significant difference was reported. These data, however, are skewed and we report them in a table (Analysis 3.1). Additionally, the method of measurement, the Silva pedometer, had been discredited as an "unacceptably inaccurate" activity promotion tool, due to its lack of testing.
3.1. Analysis.
Comparison 3 HEALTHY LIVING STUDY CIRCLE versus AESTHETIC STUDY CIRCLE, Outcome 1 Physical health behaviour: Average steps per day (high = good, skewed).
| Physical health behaviour: Average steps per day (high = good, skewed) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | n | Notes |
| Forsberg 2008 | Healthy living study circle | 5586 | 3313 | 9 | Clustered data ‐ but analysed as non‐clustered in report. |
| Forsberg 2008 | Aesthetic study circle | 6487 | 2743 | 8 | Clustered data ‐ but analysed as non‐clustered in report. |
3.2 Physical health ‐ metabolic syndrome
There was no significant difference in the presence of metabolic syndrome (n = 13, 1 RCT, RR 1.25, CI 0.35 to 4.49, Analysis 3.2).
3.2. Analysis.

Comparison 3 HEALTHY LIVING STUDY CIRCLE versus AESTHETIC STUDY CIRCLE, Outcome 2 Physical health: 1. Metabolic syndrome ‐ present.
3.3 Physical health ‐ physical working capacity
3.3.1 Incremental Shuttle Working Test
In the control group there was a non‐significant increase in physical working capacity measured by the Incremental Shuttle Working Test (n = 30, 1 RCT, MD ‐157.00, CI ‐321.11 to 7.11, Analysis 3.3).
3.3. Analysis.
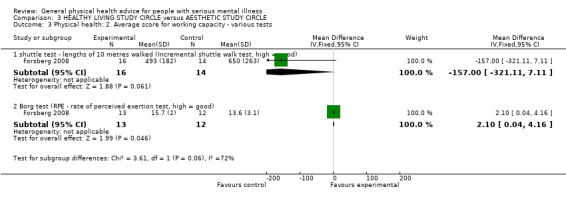
Comparison 3 HEALTHY LIVING STUDY CIRCLE versus AESTHETIC STUDY CIRCLE, Outcome 3 Physical health: 2. Average score for working capacity ‐ various tests.
3.3.2 Borg Exertion Test
In the control group there was a very slight decrease for the Borg Exertion Test (n = 25, 1 RCT, MD 2.10, CI 0.04 to 4.16).
3.4 Physical health: various continuous data
3.4.1 Metabolic criteria
Forsberg 2008 reported that at 12 months follow‐up among residents, the only significant change was a decrease in the mean number of metabolic criteria in the intervention group. Residents had decreased their mean number of metabolic criteria at the follow‐up and the number of with metabolic syndrome had decreased from 13 to 10; however, these data are skewed and are reported only as a table (Analysis 3.4).
3.4. Analysis.
Comparison 3 HEALTHY LIVING STUDY CIRCLE versus AESTHETIC STUDY CIRCLE, Outcome 4 Physical health: 3. Various continuous data (skewed).
| Physical health: 3. Various continuous data (skewed) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | n | Notes |
| metabolic syndrome ‐ average criteria score | |||||
| Forsberg 2008 | Healthy living study circle | 2.24 | 1.44 | 21 | Clustered data ‐ but analysed as non‐clustered in report. |
| average risk of fatal cardiovascular disease ‐ at present (Heart Score, high = good, skewed data) | |||||
| Forsberg 2008 | Healthy living study circle | 0.86 | 1.07 | 21 | Clustered data ‐ but analysed as non‐clustered in report. |
| average risk of fatal cardiovascular disease ‐ by 10 years (Heart Score, high = good, skewed data) | |||||
| Forsberg 2008 | Healthy living study circle | 4.67 | 3.9 | 21 | Clustered data ‐ but analysed as non‐clustered in report. |
3.4.2 Fatal cardiovascular disease
There was no significant difference in the initial risk of fatal cardiovascular disease between the intervention and the control groups; however, these data are skewed and are reported only as a table.
3.4.3 10‐year risk Heart Score
There was no significant difference in the 10‐year risk Heart Score between the intervention and the control groups; however, these data are skewed and are reported only as a table.
Discussion
Summary of main results
We included seven studies with a total number of 1113 participants. Only Comparison 1 included more than one study. Across the six studies which reported data for leaving early, the attrition rate was 32%. Some studies had significant potential for influence from industry (Brown 2006; Brown 2009; Druss 2010). Much data were often reported in such a way as to make comparative analyses impossible and we were unable to report data for many outcomes These factors must be a threat to the validity, or at the very least, the credibility of results (Xia 2009).
1. Comparison 1: Physical health advice versus standard care
Most studies we identified were included in this comparison (6 RCTs, n = 964). There was, however, an attrition of 32% (Table 1).
1.1 Physical health behaviour
Danavall 2007 reported an increase in the time spent doing moderate or vigorous exercise per week for participants receiving physical health advice but it was not statistically significant when compared to standard care. At the six‐month follow‐up, the intervention group reported spending an additional 40 minutes per week undertaking moderate or vigorous exercise which represents a 20% increase from baseline. It should be kept in mind that these findings are from a single small trial (n = 80), so although it appears that physical health advice has influenced the increase in moderate or vigorous exercise this should be investigated further before strong conclusions can be drawn.
1.2 Quality of life
Only three studies provided data for this important outcome and only two used the same rating scales, making interpretation difficult. Byrne 1999 reported no significance difference in quality of life, while Danavall 2007 and Druss 2010 reported separately on the mental and physical components of quality of life and both said that, at follow‐up, the intervention group had a "significantly higher" score than controls on the mental component summary score and a "nearly significant" difference in the physical component summary score. These differences are in the range of three and two points and we are not clear about their meaning to carers or participants. The meaning is not explained in the original papers. It is possible that this rating does represent a good improvement, but the trialists have left us unclear if this is so.
1.3 Adverse effects: death
Only two studies reported on adverse effects with no statistically significant difference reported for this outcome (Danavall 2007; Druss 2010). Danavall 2007 reported only one participant had died in the standard care group. In Druss 2010 about 2% died in each group by one year. There is no indication of any effect physical health advice may have on this important outcome. Certainly, much larger studies are needed if this is to be investigated within the context of trials.
1.4 Service use: average percentage uptake of recommended health preventative services
Druss 2010 compared medical care management versus standard care and showed a statistically significant effect on service use. At 12‐month follow‐up, the average proportion of indicated preventive services more than doubled in the intervention group but remained constant in the usual care group. This suggests that there are benefits for physical healthcare advice (care management) in the primary care setting. Care managers did not provide any medical interventions; however, they did facilitate improved primary care through a combination of "advocacy, education, and helping patients overcome logistical barriers to care" (Druss 2010). Results are only available from one study and should be interpreted with caution, but do seem encouraging.
1.5 Service use: one or more primary care visit
Danavall 2007 reported that significantly more people who received physical health advice had attended one or more primary care appointments than those receiving standard care alone. There was not a dramatic increase from baseline for those receiving physical health advice (baseline = 24 people, six‐month follow‐up 26 people), in fact, there was a decrease for those receiving standard care (baseline = 23 people, six‐month follow‐up = 14 people). Results do seem to suggest that receiving physical health advice encouraged people to continue to visit primary care more than those who did not, but the reason for the drop in visits for those who received standard care is unclear.
1.6 Economic: health service utilisation
A total (US) dollar value of health resource consumption was determined. These data were skewed but trial authors did not report a significant statistical difference between groups (Byrne 1999).
1.7 Leaving the study early
Six of the seven studies reported on 'leaving the study early', an outcome which can be considered as a composite measure of acceptability of treatment. There was no difference in premature discontinuations due to leaving early for any reason ‐ but over 30% of people left these trials. This has to leave us with an issue of credibility (Xia 2009), as 30% losses are not what would be expected in clinical life and simply ignoring this attrition in analyses is not the best option. It is reassuring that there is not imbalance in numbers lost to follow‐up ‐ but it remains a worry that there may be imbalance in reasons for attrition.
2. Comparison 2: Health education versus health empowerment
Byrne 1999 is the only included study (n = 214, Table 2).
2.1 All outcomes
There were no differences apparent for measures of quality of life, economic outcomes or attrition. Byrne 1999 was a small study and there may be real differences to be seen by use of a larger trial. However, comparing different types of health advice would seem inadvisable until more data were supporting its use overall.
3. Comparison 3. Healthy living study circle versus aesthetic study circle
Only Forsberg 2008 (97 participants in 10 clusters) was included in this comparison. The attrition rate was not reported (Table 3).
3.1 All outcomes
Forsberg 2008 measured both behaviour and health indicators. It found no clear differences in physical activity, but that residents in the intervention group did have a decrease rate of metabolic syndrome compared with an increase in the control group. Once differences were calculated in these data using the Design Effect (see Unit of analysis issues), no clear difference was apparent. Physical working capacity measures and risk of physical disease data were difficult to interpret with confidence. Again, it seems advisable that more data be created on the first comparison (physical healthcare advice versus standard care) before different ways of delivering this advice are investigated.
Overall completeness and applicability of evidence
1. Completeness
1.1 Duration of follow‐up
Four of the seven included studies presented long‐term data (over one year of follow‐up). This is a good length of time to assess any difference in the intervention effects. One study had a six‐month follow‐up and two studies presented short‐term data, a duration of six to 10 weeks, which is probably too short a time to assess any difference in the intervention effects.
1.2 Coverage of outcomes
The was a range of outcomes reported including quality of life, health behaviour, service use and economic impact. However, even for these outcomes, there are very few and poorly reported data. Much more robust data are needed in this important area that relate directly to clinicians, policymakers and consumers of care. It would not be difficult to generate better data on other outcomes such as service use (use of primary care, Accident & Emergency (A&E)), general state, adverse event or costs.
2. Applicability
2.1 Origin
In this review, 50% of the included studies were completed in Europe and the other half in North America. The great majority of people with serious mental illnesses such as schizophrenia live in low‐ or middle‐income countries where advice regarding malaria, tuberculosis, sexually transmitted diseases and accident avoidance may be more pertinent than advice regarding cholesterol monitoring. More relevant studies need to be undertaken.
2.2 Interventions
Experimental interventions were provided by nurses and key workers who had training or experience of providing care for people with serious mental illness. These are healthcare personnel who are widely accessible in many settings. However, it may also be possible to delegate the intervention role to volunteer workers within a health system.
Quality of the evidence
Overall quality was poor (Figure 1). All studies report that they were randomised; however, further details on how randomisation was achieved were provided by only four studies. Brown 2009 used a "hidden computer‐generated random number programme", Danavall 2007 and Druss 2010 used "a computerised algorithm" and Forsberg 2008 randomised on a group level by "drawing lots". No further details are given on any randomisation techniques. Blindness was attempted in Brown 2006, Brown 2009 and Druss 2010, but there was no investigation as to whether this had been successful. In most of the studies it is unclear if randomisation and blinding were done appropriately. There were high rates of participants leaving the study prematurely and three studies were supported by the pharmaceutical industry. These factors limit the overall quality of the evidence (Cohen 2010).
Potential biases in the review process
The search criteria on the Cochrane Schizophrenia Group Trials Register (October 2012) should have been robust enough to detect relevant studies. It is possible that we have failed to identify small studies, but we think it unlikely that we would have missed large trials.
Studies published in languages other than English, and those with equivocal results, are often difficult to find (Egger 1997). Our search was biased by use of English phrases. However, given that the Cochrane Schizophrenia Group’s register covers many languages but is indexed in English, we feel that this would not have missed many studies within the register. For example, the search uncovered 101 studies for which the title was only available in Chinese characters. These were checked for relevance by a Chinese‐speaking colleague (Jun Xia) and we identified three as possibly relevant to this review. These had to be excluded after closer inspection. We did not perform a funnel plot analysis.
Agreements and disagreements with other studies or reviews
The only other similar systematic review that we are aware of is Bradshaw 2005. This reports on efficacy of healthy living interventions for people with schizophrenia. Our findings do agree with Bradshaw 2005, in that we too feel that data point to the need for rigorous studies.
Authors' conclusions
Implications for practice.
1. For people with serious mental illness
There is some limited and poor quality evidence that the provision of general physical healthcare advice can improve health‐related quality of life in the mental component but not the physical component. This evidence comes from one study, which only looked specifically at benefits in the primary care setting. Otherwise, no studies returned results that suggest that physical healthcare advice has a powerful effect on physical healthcare behaviour or risk of ill health. More work is needed in this area and people with serious mental illness could best contribute by becoming involved in research that is meaningful to their interests and needs.
2. For clinicians
Clinicians should know there is some randomised evidence that the provision of general physical healthcare advice to people with serious mental illness may improve health‐related quality of life. There is little current evidence that providing physical healthcare advice is an effective way of improving the physical health of people with serious mental illness. It is possible clinicians are expending much effort, time and financial expenditure on giving ineffective advice. Clinicians should therefore attempt to initiate or get involved with any studies which could provide an evidence base for this practice.
3. Funders and policy makers
Funders and policy makers should be aware that there may be some benefit for physical health advice for people with serious mental illness. It is equally possible clinicians are expending much effort, time and financial expenditure on giving ineffective advice. There is an increased demand for preventative health services through provision of advice, so there may be a requirement for short‐term speculative investment in services in order to make long‐term savings. This is a ripe area for good real‐world research.
Implications for research.
1. General
Strict adherence to the CONSORT statement (Moher 2001) would have provided us with more useable data. We were unable to use data from some studies because raw scores were not presented. Instead, outcomes were presented as inexact P values without means and standard deviations. Randomisation techniques were not always made clear and blinding was untested ‐ although, of course, difficult to achieve for this type of study. Nevertheless, pioneering researchers have shown that this type of work is possible. We hope that future trialists will sign up for ensuring that all data are publicly available (ALLTRIALS).
2. Specific
There is an obvious lack of research in this area and the small number of included studies fails to reflect the huge amount of healthcare advice given to people with serious mental illness. We realise that much thought and care goes into the design of randomised studies. We have, however, also given this issue some consideration and suggest the outline of a feasible design (see Table 5).
2. Suggested design for future study.
| Methods | Allocation: randomised, clearly described. Blinding: single ‐ tested. Duration: 10 years. |
| Participants | Diagnosis: schizophrenia or any serious mental illness. N = 900. Age: 18‐65. Sex: both. History: any. |
| Interventions | 1. Physical health assessment: volunteer worker encouraging an acceptable form of physical healthcare advice including information, advice regarding access to services to reduce barriers to interventions and provide sustained encouragement for engagement/behavioural change. 2. Care as usual: no change to current practice. |
| Outcomes | Adverse health events: death, major illness ‐ recorded by type (open list). Quality of life ‐ social relations, family relations, financial situation (EuroQol). Physical health ‐ healthy days. Service use ‐ physical healthcare admission, days in hospital due to physical illness, visit to healthcare practitioner. Mental state ‐ no clinically important change in general mental state (CGI). Leaving the study early ‐ why. Economic outcomes. |
| Notes | For 20% difference between groups for a binary outcome to be highlighted with reasonable degree of confidence, 150 people are needed per group. |
CG‐I: Clinical Global Impression
What's new
| Date | Event | Description |
|---|---|---|
| 4 March 2014 | New citation required but conclusions have not changed | New data added to review but no overall change to conclusions |
| 9 September 2013 | New search has been performed | Results of 2012 update search added to review. A previous ongoing study is now complete and added to the included studies. |
History
Protocol first published: Issue 7, 2010 Review first published: Issue 2, 2011
| Date | Event | Description |
|---|---|---|
| 17 October 2012 | Amended | Update search of Cochrane Schizophrenia Group's Trial Register (see Search methods for identification of studies), 43 studies added to awaiting classification. |
| 17 October 2012 | Amended | Contact details updated. |
| 5 October 2011 | Amended | Contact details updated. |
Acknowledgements
Thanks to Professor Clive Adams, Claire Irving and the editorial team at the Nottingham University Cochrane Schizophrenia Group for their unwavering support in the writing of this review. To Sheeren Mala for her contributions in helping write the review and screening results from the searches. The Cochrane Schizophrenia Group Editorial Base in Nottingham produces and maintains standard text for use in the Methods sections of their reviews. We have used this text as the basis of what appears here and adapted it as required.
We would also like to thank and acknowledge the contribution of Mick Bachner a co‐reviewer from previous versions of this review who helped screen the results of 2010 electronic search.
Appendices
Appendix 1. Previous methods text
Data extraction and managment
5. Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we aimed to apply the following standards to all data before inclusion: (a) standard deviations and means are reported in the paper or obtainable from the authors; (b) when a scale starts from the finite number zero, the standard deviation, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996); (c) if a scale starts from a positive value (such as PANSS which can have values from 30 to 210) the calculation described above will be modified to take the scale starting point into account. In these cases skew is present if 2SD>(S‐S min), where S is the mean score and S min is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied. When continuous data are presented on a scale which includes a possibility of negative values (such as change data), it is difficult to tell whether data are skewed or not. We entered skewed data from studies of less than 200 participants in additional tables rather than into an analysis. Skewed data pose less of a problem when looking at means if the sample size is large, and we entered skewed data from large sample sizes into syntheses.
Measures of treatment effect
1. Binary data
For binary outcomes we calculated a standard estimation of the random‐effects RR and its 95% CI. It has been shown that RR is more intuitive (Boissel 1999) than odds ratios (OR) and that ORs tend to be interpreted as RR by clinicians (Deeks 2000). Within the 'Summary of findings' table we assumed for calculation of the low risk groups that the lowest control risk applied to all data. We did the same for the assumption of the highest risk groups. We used the 'Summary of findings' table to calculate absolute risk reduction for primary outcomes.
Dealing with missing data
1. Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). For any particular outcome, should more than 50% of data be unaccounted for, we did not reproduce these data or use them within analyses. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we marked such data with '*' to indicate that such a result may well be prone to bias.
2. Binary
In the case where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat analysis). Those lost to follow‐up were all assumed to have the same rates of negative outcome as those who completed, with the exception of the outcome of death. We undertook a sensitivity analysis testing how prone the primary outcomes were to change when 'completed' data only were compared to the intention‐to‐treat analysis using the above assumption.
3. Continuous
3.1 Attrition
In the case where attrition for a continuous outcome is between 0% and 50% and completer‐only data were reported, we have reproduced these.
3.2 Standard deviations
Where there are missing measures of variance for continuous data but exact standard error and CI are available for group means, either P value or T value are available for differences in mean, we calculated standard deviation value according to method described in Section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). If standard deviations were not reported and could not be calculated from available data, we asked authors to supply the data. In the absence of data from authors, we used the mean standard deviation from other studies.
3.3 Last observation carried forward
We anticipated that in some studies the method of last observation carried forward (LOCF) would be employed within the study report. As with all methods of imputation to deal with missing data, LOCF introduces uncertainty about the reliability of the results. Therefore, where LOCF data have been used in the trial, if less than 50% of the data had been assumed, we reproduced these data, and indicated that they are the product of LOCF assumptions.
Data and analyses
Comparison 1. PHYSICAL HEALTH ADVICE versus STANDARD CARE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Physical health behaviour: Moderate or vigorous physical activity (min/week, skewed) | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 39.0 [‐76.53, 154.53] |
| 2 Quality of life: Average scores ‐ various scales | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Global score (Lehman Quality of Life Scale, high = good) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.47, 0.87] |
| 2.2 Mental component score (Medical Outcomes Study 36‐Item Short‐Form Health Survey, high = good) | 2 | 487 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [1.76, 5.64] |
| 2.3 Physical component score (Medical Outcomes Study 36‐Item Short‐Form Health Survey, high = good) | 2 | 487 | Mean Difference (IV, Fixed, 95% CI) | 2.46 [0.33, 4.59] |
| 3 Adverse effects/events: Death | 2 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.27, 3.56] |
| 4 Service use: Average percentage uptake of recommended health preventative services (US Preventative Services Task Force guidelines, high = good) | 1 | 363 | Mean Difference (IV, Fixed, 95% CI) | 36.90 [33.07, 40.73] |
| 5 Service use: One or more primary care visit | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.09, 2.85] |
| 6 Economic: Total value of health resource consumption (dollars, low = good, skewed data) | Other data | No numeric data | ||
| 7 Leaving the study early | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 any reason | 6 | 964 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.92, 1.35] |
| 7.2 lost to follow‐up | 4 | 822 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.79, 1.20] |
| 7.3 withdrawn | 1 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.90 [0.86, 55.56] |
| 7.4 discontinued | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.25 [0.50, 135.21] |
1.5. Analysis.
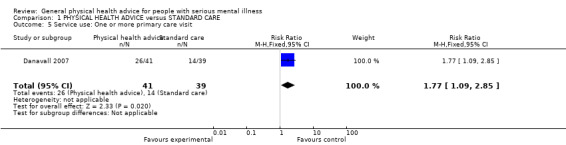
Comparison 1 PHYSICAL HEALTH ADVICE versus STANDARD CARE, Outcome 5 Service use: One or more primary care visit.
Comparison 2. HEALTH EDUCATION versus HEALTH EMPOWERMENT EDUCATION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life: Average global score (Lehman Quality of Life scale, high = good) | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.99, 0.39] |
| 2 Economic: Total value of health resource consumption (dollars, low = good, skewed data) | Other data | No numeric data | ||
| 3 Leaving the study early | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.26, 1.19] |
Comparison 3. HEALTHY LIVING STUDY CIRCLE versus AESTHETIC STUDY CIRCLE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Physical health behaviour: Average steps per day (high = good, skewed) | Other data | No numeric data | ||
| 2 Physical health: 1. Metabolic syndrome ‐ present | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.35, 4.49] |
| 3 Physical health: 2. Average score for working capacity ‐ various tests | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 shuttle test ‐ lengths of 10 metres walked (Incremental shuttle walk test, high = good) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐157.0 [‐321.11, 7.11] |
| 3.2 Borg test (RPE ‐ rate of perceived exertion test, high = good) | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [0.04, 4.16] |
| 4 Physical health: 3. Various continuous data (skewed) | Other data | No numeric data | ||
| 4.1 metabolic syndrome ‐ average criteria score | Other data | No numeric data | ||
| 4.2 average risk of fatal cardiovascular disease ‐ at present (Heart Score, high = good, skewed data) | Other data | No numeric data | ||
| 4.3 average risk of fatal cardiovascular disease ‐ by 10 years (Heart Score, high = good, skewed data) | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brown 2006.
| Methods | Allocation: random. Blinding: attempted to maintain rater blindness. Duration: 6 weeks. | |
| Participants | Diagnosis: severe and enduring mental Illness (ICD ‐10 diagnosis of psychosis, major affective illness, or severe personality disorder). N = 28. Age: range 18‐65 years. Sex: 4 men, 24 women. History: excluded if screening doctor thought that anyone with health problems, such as uncontrolled hypertension, severe cardiac disease, or any other medical condition, which might have worsened by unaccustomed exercise. | |
| Interventions | 1. Semi‐structured health promotion sessions: based on the Lilly "Meaningful Day"* manual which draws on extensive experience of best practice in delivering health promotion interventions. The six sessions covered weight control, healthy eating, exercise, structured daily activity and substance misuse. N = 15. 2. Treatment as usual. N = 13. |
|
| Outcomes | Leaving early. Unable to use ‐ Diet: Dietary Instrument for Nutrition Education Questionnaire (mean change, no SD, impossible to calculate lost data).** Exercise: Godin Leisure‐Time Exercise Questionnaire (mean change, no SD, impossible to calculate lost data).** Psychological health: Hospital Anxiety and Depression scale (mean change, no SD, impossible to calculate lost data).** Subjective well being: Likert rating scale (mean change, no SD, impossible to calculate lost data).** |
|
| Notes | * (Lilly 2002) ** Sought statistical advice. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" ‐ no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded but "... attempted to maintain rater blindness but in many cases this was not possible". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11 of 28 included patients were missing at outcome. "Subjects failed to attend or cancelled at short notice a total of 73 (out of 199) appointments" ‐ described but not addressed. |
| Selective reporting (reporting bias) | Low risk | Four rating scales were listed in the methods, all four reported. |
| Other bias | Unclear risk | Supported by Eli Lilly (pharmaceutical industry) who supplied the Lilly "Meaningful Day" package. |
Brown 2009.
| Methods | Allocation: random. Blinding: rater was blind to interviewee status. Duration:10 weeks. | |
| Participants | Diagnosis: severe and enduring mental Illness (ICD ‐10 diagnosis of schizophrenia, major affective disorder, neurotic or personality disorder). N = 26. Age: range 18‐65 years. Sex: 8 men, 18 women. History: excluded if anyone had "significant health problems" ‐ none were. | |
| Interventions | 1. Semi‐structured health promotion session: based on the Lilly "Meaningful Day"* manual which draws on extensive experience of best practice in delivering health promotion interventions. The six sessions covered weight control, healthy eating, exercise, structured daily activity and substance misuse. N = 15. 2. Treatment as usual. N = 11. |
|
| Outcomes | Leaving early. Unable to use ‐ Diet: Dietary Instrument for Nutrition Education Questionnaire (mean change, no SD, impossible to calculate lost data).** Exercise: Godin Leisure‐Time Exercise Questionnaire (mean change, no SD, impossible to calculate lost data).** Psychological health: Hospital Anxiety and Depression scale (mean change, no SD, impossible to calculate lost data).** Substance use: direct enquiry (mean change, no SD, impossible to calculate lost data).** |
|
| Notes | * (Lilly 2002) ** Sought statistical advice. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised, using a hidden computer‐generated random number programme" ‐ no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded but "pre and post intervention measurements were made by the same rater who was blind to the interviewees' status in the study". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Five subjects (33%) did not complete the programme, most deciding not to continue with the programme after just one session" ‐ described but not addressed. |
| Selective reporting (reporting bias) | Low risk | Four rating scales were listed in the methods, all four reported. |
| Other bias | Unclear risk | Health promotion operating manual was adapted from the Lilly "Meaningful Day" package (Lilly 2002). |
Byrne 1999.
| Methods | Allocation: random, clustered by home. Blinding: no. Duration: 18 months. | |
| Participants | Diagnosis: chronic psychiatric illness.* N = 22 homes (214 people). Age: mean 49.9 years. Sex: 140 men, 74 women. History: excluded if less than 50% of residents in the home agreed to attend sessions and if the majority of the residents in a home did not speak English. | |
| Interventions | 1. Health Education: intensive 12‐week educational session focusing on enhancing overall wellness, reducing smoking, and increasing activity facilitated by public health nurses. N = 7 homes (77 individuals). 2. Health Empowerment: a three‐phase process, first "the listening phase", second the "participatory dialogue" and finally in the final stage "group members tested out their understanding of the problem in the real world". N = 7 homes (69 individuals).** 3. Control group. N = 8 homes (68 individuals). |
|
| Outcomes | Leaving early.
Quality of Life: Lehman Quality of Life Scale.
Health service utilization: resource consumption quantified according to their dollar value (using Ontario Health Insurance Plan schedule of fees). "A total dollar value of health resource consumption was determined in all groups" using the Health Service Utilization Inventory similar to Browne 1990. Unable to use ‐ Life satisfaction: Cantril Self‐Anchoring Ladder (did not report changes between baseline and completion of intervention). |
|
| Notes | * participants asked to report what type of mental health problem they had ‐ 31% schizophrenia, 14.1% affective disorders, 16.4% "other mental health problems", 25.8% "did not know", 12.2% "said they had no problem of this type". ** for the purposes of this review we considered both health empowerment and health education as 'general healthcare advice'. We calculated the design effects for the health education versus health empowerment education (D.E.=1.873) and health education versus control (D.E.=1.418); both were applied accordingly. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The homes in each strata were then randomly assigned to one of the three study groups" ‐ no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "By time 3 only 53% of the original sample remained in the study, and those actually participating in the groups (completing more than 20% of the sessions) were 40% of the original sample". |
| Selective reporting (reporting bias) | Low risk | Three rating scales were listed in the methods, all three reported. |
| Other bias | Low risk | State funded (Ontario Ministry of Health, Canada), no evidence of other bias. |
Chafetz 2008.
| Methods | Allocation: random. Blinding: no. Duration: 18 months. | |
| Participants | Diagnosis: severe mental illness. N = 309. Age: mean 38.2 years. Sex: 210 men, 99 women. History: excluded if did not speak English, unable to provide informed consent, diagnosed with cognitive/adjustment disorder. | |
| Interventions | 1. Wellness training + basic primary care: promote skills in self‐assessment, self‐monitoring, and self‐management of physical health problems, including use of health services........... + basic primary care (see below). N = 154. 2. Basic primary care: provide health assessments, immediate or short‐term care, health education, and referrals. N = 155. |
|
| Outcomes | Leaving early. Unable to use ‐ Physical functioning: Medical Outcomes Health Survey Short Form 36 (no mean change, no SD, impossible to calculate lost data).* Health‐related self‐efficacy: assessed using a method adapted by MacDonald 1988 (no mean change, no SD, impossible to calculate lost data).* Psychosocial function: Global Assessment of Function (no mean change, no SD, impossible to calculate lost data).* |
|
| Notes | * Sought statistical advice. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation" ‐ no further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Baseline severity of medical comorbidity was rated by NPs [Nurse Practitioners] blind to study group......" ‐ no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "... we are confident that results for outcomes reported here are not biased by differences between study groups in number of interviews completed" ‐ described and addressed. |
| Selective reporting (reporting bias) | Low risk | Three rating scales were listed in the methods, all three reported. |
| Other bias | Unclear risk | Supported by the National Institute of Nursing Research |
Danavall 2007.
| Methods | Allocation: randomised. Blinding: no. Duration: six months. | |
| Participants | Diagnosis: people who are receiving care at a community mental health centre and who suffer from chronic mental illness.
N = 80.
Sex: both.
Age: 18 and older. History: active patient roster at the CMHC, have a severe mental illness (National Advisory Mental Health Council, 1993) have one or more chronic medical condition, and have the capacity to provide informed consent. |
|
| Interventions | 1. Peer‐led medical illness self‐management group sessions. N = 41. 2. Standard care. N = 39. |
|
| Outcomes | Physical health behaviour ‐ minutes of moderate/vigorous exercise.
Health‐related quality of life. Leaving the study early. Adverse events. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computerised algorithm, patients were randomised to the intervention or usual care group by the project manager. |
| Allocation concealment (selection bias) | Unclear risk | Allocation "by the project group manager" ‐ no further details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Interviewers were blinded to subjects' randomization status" ‐ no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 participants were lost to follow‐up. Stated that "all analyses were conducted as intent‐to‐treat". |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the method appear to have been reported. |
| Other bias | Unclear risk | Dr. Lorig receives royalties from Bull Publisher for being an author of Living a Healthy Life with Chronic Conditions. This book was written for the self‐management course and is used in this study. |
Druss 2010.
| Methods | Allocation: random. Blinding: interviewers blinded to participants' randomisation status. Duration: 12 months. | |
| Participants | Diagnosis: severe mental illness. N = 407. Age: mean age 47 (intervention), mean age 46.3 (usual care). Sex: 210 men, 197 women. History: excluded if not on active patient roster at community mental health centres, could not provide informed consent and did not have a severe mental illness. | |
| Interventions | 1. Care management intervention: a manualised protocol for care based on standardised approaches documented in the care management literature. "The program was designed to help overcome patient, provider, and system‐level barriers to primary medical care experienced by persons with mental disorders". N = 205. 2. Usual care: individuals were given a list with contact information for local primary care medical clinics that accept uninsured and Medicaid patients. N = 202. |
|
| Outcomes | Leaving early.
Death.
Quality of preventative services: U.S. Preventative Services Task Force guidelines.
Health‐related quality of life: Medical Outcomes Study 36‐Item Short‐Form Health Survey. Unable to use ‐ Quality and outcomes of cardio‐metabolic care: RAND Community Quality Index study*, Framingham Cardiovascular Risk Index score.** |
|
| Notes | *The RAND Community Quality Index study was completed for individuals who had one or more cardio‐metabolic conditions (n = 202) the distribution of these individuals is unknown. **The Framingham Cardiovascular Risk Index score was only completed for individuals with complete blood test results available (n = 100) the distribution of these individuals is unknown. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Using a computerized algorithm, patients were randomly assigned to a care management intervention group or a usual care group" ‐ no further details. |
| Allocation concealment (selection bias) | Unclear risk | Allocation "by the project group manager" ‐ no further details. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Interviewers were blinded to subjects' randomisation status" ‐ no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Of those randomly assigned, 73% completed 6‐month follow‐up interviews and 68% completed 12‐month follow‐up interviews". Lost to follow‐up was "unable to locate", "deceased", and "withdrawn" ‐ described but not addressed. |
| Selective reporting (reporting bias) | Low risk | Four rating scales were listed in the methods, all four reported. |
| Other bias | Unclear risk | Lead author "Dr Druss received research funding from Pfizer", who manufacture a wide range of medicines for conditions such as heart disorders, cancer, raised blood pressure, high cholesterol and sexual health. |
Forsberg 2008.
| Methods | Allocation: random, clustered by "supported housing facilities".* Blinding: no. Duration: 12 months. | |
| Participants | Diagnosis: psychiatric diagnosis in accordance with DSM‐IV. N = 49 residents, 48 staff members. Age: range 22‐71 (residents), range 27‐62 (staff). Sex: 28 men (residents), 21 women (residents), 16 men (staff) 25 women (staff). History: people with psychiatric disability and their staff working with housing support or in supported housing facilities. | |
| Interventions | 1. A programme of healthy living in the form of a study circle: study material comprised of a book focusing on motivation, food content, stress and fitness. N = 24 (residents), 22 (staff). 2. Aesthetic study circle: participants had an opportunity to learn and practice various kinds of artistic techniques. N = 17 (residents), 19 (staff). |
|
| Outcomes | Physical working capacity: i) Incremental Shuttle Walk Test ii) Borg RPE (Rate of perceived exertion) Scale.
Rate of metabolic syndrome:NCEP ATP 2001.
Physical activity: SILVA™ "Pedometer plus".
Heart score: "estimates the present and 10‐year risk of fatal Coronary Vascular Disease".** Unable to use ‐ Leaving early (not reported by group). Satisfaction of programme: "Satisfaction in participating in the study" questionnaire (not applicable to outcomes). |
|
| Notes | * Author kindly clarified that suggestion that people within housing facilities were randomised (page 489 of report) is incorrect. **This is done "by using factors of age, sex, cholesterol level, systolic blood pressure and smoking habits". We calculated the design effect for the healthy living circle versus the aesthetic living circle as 1.23 and applied it accordingly. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation was conducted on group level by the drawing of lots" ‐ no further details. |
| Allocation concealment (selection bias) | Low risk | Allocation "by a person not involved in the project". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Clients leaving early: "no informed reasons were mentioned", or "informed reasons were studies, health reasons", or "dissatisfaction of their study circle", "health problem" and "job" ‐ described but not addressed. Staff leaving early: "no informed reasons", "new job", dissatisfaction of study circle" and "sick list" ‐ described but not addressed. |
| Selective reporting (reporting bias) | Unclear risk | Five rating scales were listed in the methods, all five reported. Leaving the study early ‐ not reported by group. Study reported as if not clustered ‐ no intra‐class correlation coefficient. |
| Other bias | Unclear risk | Supported by grants from "The Vasterbotten County Council, The Swedish Institute for Health Sciences,The Swedish Council for Working Life and Social Research, Stiftelsen J C Kempes Minnes Stipendiefond and The Foundation of Medical Research in Skelleftea". |
DSM IV: Diagnostic and Statistical Manual, 4th edition ICD 10 ‐ International Classification of Diseases SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almomani 2009 | Allocation: randomised Participants: schizophrenia, bipolar, depression Intervention: education + mechanical toothbrush vs mechanical toothbrush alone. Not focused on general physical health. |
| Baker 2009 | Allocation: randomised. Participants: severe mental disorder ‐ schizophrenia or bipolar disorder. Intervention: multi‐component intervention for smoking cessation and CVD risk reduction vs telephone‐based minimal intervention focusing on smoking cessation. Not focused on general physical health. |
| Berti 2011 | Allocation: non‐randomised for phase 1 of study, phase 2 to be randomised (has not begun). Participants: affective and non‐affective functional psychotic disorders. Intervention: none in phase 1, phase 2 reported to focus on weight loss vs standard care; not focused on general physical health. |
| Brown 2011 | Allocation: randomised. Participants: serious mental illness (not specified). Intervention: six months of special meal schedule and education. Not focused on general physical health. |
| Eberhard 2009 | Allocation: randomised. Participants: non‐psychotic psychiatric patients. |
| Gao 2001 | Allocation: unclear ‐ people "divided" into groups. Participants: people with schizophrenia. Intervention: health education vs standard care, health education refers to mental health rather than general physical health. |
| Goetz 2011 | Allocation: randomised. Participants: 50% schizophrenia, 26% bipolar, 24% major depression. Intervention: 12‐week program of education/skills training aimed at improved nutrition vs control (unclear what control was), increased activity and weight loss. Does not focus on general physical health. |
| Huang 2005 | Allocation: unclear ‐ people "divided" into groups. Participants: convalescent psychotic patients. Intervention: health education vs standard care, health education refers to mental health rather than general physical health. |
| Jiang 2006 | Allocation: randomised. Participants: people with schizophrenia. Intervention: health education + routine care vs routine care. Focused on mental health rather than general health. |
| Jones 2001 | Allocation: randomised. Participants: people with schizophrenia. Intervention: education by community mental health nurse vs computer‐assisted Instruction vs standard care. Focused on mental health rather than general physical health. |
| Kuosmanen 2009 | Allocation: randomised. Participants: schizophrenia. Intervention: needs‐based computerised patient education on deprivation of liberty vs oral sessions and written material education on deprivation of liberty vs standard treatment. Not focused on general physical health. |
| Leutwyler 2009 | Allocation: randomised. Participants: schizophrenia and schizoaffective disorder. Intervention: 24‐week diabetes education program vs usual care plus information. Focus is on diabetes, not on general physical health. |
| Li 2005 | Allocation: unclear ‐ people "divided" into groups. Participants: Outpatients with schizophrenia Intervention: health education vs standard care. Not focused on general physical health. |
| Lothringer 2009 | Allocation: randomised. Participants: severe mental illness, 50% schizophrenia. Intervention: HIV prevention vs control. Not focused on general physical health advice. |
| NCT00902694 | Allocation: randomised. Participants: overweight psychiatric patients. Intervention: group and individual weight counselling and group physical activity classes vs group health classes quarterly with topics not related to weight. Intervention not focused on general physical health. |
| NCT00990925 | Allocation: randomised. Participants: schizophrenia, schizoaffective disorder, obesity. Intervention: lifestyle Modification for Weight Loss in Schizophrenia. Not focused on general physical health. |
| NCT01324973 | Allocation: randomised. Participants: serious mental illness, overweight. Intervention: a web‐based weight management program that includes computerised delivery of evidence‐based education regarding diet and physical activity vs care as usual. Not focused on general physical health. |
| NCT01547026 | Allocation: randomised. Participants: schizophrenia and schizo‐affective disorder. Intervention: structured procedure to build up implementation intentions to participate in the sports therapy vs individual 10‐minute psycho‐education session on the helpfulness of sports to improve the health. Focused on weight gain and improving sports uptake, not focused on general physical health. |
| Osborn 2010 | Allocation: randomised. Participants: serious mental illness, no details provided. Those "too unwell" were excluded. Intervention: nurse‐led intervention + education vs education alone, outcome limited to numbers that received screening for cardiovascular risk factors and not the impact of the screening on physical health. |
| Stockings 2011 | Allocation: randomised. Participants: acute mental illness without severe distress at time of interview. Intervention: smoking cessation program vs regular hospital smoking program. Not focusing on general health advice. |
| Subramaniam 2010 | Allocation: randomised. Participants: schizophrenia vs healthy controls. Intervention: computerised targeted cognitive training. Not focusing on general physical health advice. |
| Walker 2005 | Allocation: not randomised, feasibility study for conducting RCT. |
| Zhou 2007 | Allocation: randomised. Participants: people with first episode schizophrenia. Intervention: systematic healthcare education vs standard care, not focused on general physical health. |
| 林素兰 2010 | Allocation: randomised. Participants: schizophrenia. Intervention: education in both study and control group. |
CVD: cardiovascular disease RCT: randomised controlled trial vs: versus
Characteristics of ongoing studies [ordered by study ID]
NCT00137267.
| Trial name or title | A brief community linkage intervention for dually diagnosed individuals. |
| Methods | Allocation: randomised. Blinding: open label. Duration: 8 weeks. |
| Participants | Diagnosis: people who have a substance abuse disorder and a diagnosis of schizophrenia, schizoaffective disorder, or bipolar I disorder. N = 170 (estimated enrolment). Sex: both. Age: 18 and older. |
| Interventions | 1.Time‐Limited Case (TLC) Management. 2.Treatment as usual. |
| Outcomes | Rate at outpatient day treatment centre within one week post–hospitalisation. Differences in TLC group completion at 2 months. Number of days treatment attended at 6 months and 12 months. Number days re‐hospitalised at 6 months and 12 months. Global Level of Functioning at 2 months, 6 months and 12 months. Number of days alcohol use at 2 months, 6 months, 12 months. Number of days drug use at 2 months, 6 months, 12 months. |
| Starting date | 06/08/2007 completed 6/2009 |
| Contact information | Selvija Gjonbalaj‐Marovic * ‐ selvija.gjonbalaj‐marovic@va.gov, |
| Notes | * We have emailed project lead for further details. New PI David Smelson PSYD, Edith Nourse Rogers Memorial Veterans Hospital, Bedford. |
Differences between protocol and review
Minor corrections to outcomes.
Correction of wording to ensure that we are referring to general physical health.
For the 2013 update, we amended some sections of Methods to reflect the latest changes to the Cochrane Schizophrenia Group Template for methods. To see previous published versions of the amended section see Appendix 1.
Contributions of authors
Graeme Tosh ‐ project initiation, protocol writing, primary reviewer, results and discussion writing. Andrew Clifton ‐ co‐reviewer protocol writing, primary reviewer, results and discussion writing. Jun Xia ‐ co‐reviewer, screened results of review 2012 update search. Margueritte White ‐ co‐reviewer, screened results of 2012 update search.
Sources of support
Internal sources
Nottinghamshire CLAHRC (Collaboration for Leadership in Applied Health Research and Care), UK.
National Institute of Health Research, UK.
Nottinghamshire Healthcare NHS Trust, UK.
NHS Nottingham City, UK.
NHS Nottinghamshire County, UK.
Nottingham University Hospitals NHS Trust, UK.
NHS Derby City, UK.
Derbyshire County PCT, UK.
Derbyshire Mental Health NHS Trust, UK.
Lincolnshire Partnership NHS Foundation Trust, UK.
Bassetlaw PCT, UK.
NHS East Midlands, UK.
University of Nottingham, UK.
Nottingham City Council, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Brown 2006 {published data only}
- Brown AS. An evaluation of the effectiveness of delivery of health promotion interventions to people with serious mental illness by their key workers. http://www.controlled‐trials.com/ISRCTN95265680. United Kingdom, (accessed 04 March 2014).
Brown 2009 {published data only}
- Brown S, Smith E. Can a brief health promotion intervention delivered by mental health key workers improve clients' physical health: a randomized controlled trial. Journal of Mental Health 2009;18(5):372‐8 . [Google Scholar]
Byrne 1999 {published data only}
- Byrne C, Brown B, Voorberg N, Schofield R, Browne G, Gafni A, et al. Health education or empowerment education with individuals with a serious persistent psychiatric disability. Psychiatric Rehabilitation Journal 1999;22(4):368‐80. [Google Scholar]
Chafetz 2008 {published data only}
- Chafetz L, White M, Collins‐Bride G, Cooper BA, Nickens J. Clinical trial of wellness training: health promotion for severely mentally ill adults. Journal of Nervous and Mental Disease 2008;196(6):475‐83. [PUBMED: 18552625] [DOI] [PubMed] [Google Scholar]
Danavall 2007 {published data only}
- Danavall L, Druss B. Medical self‐management for improving health behaviour among individuals in community mental health settings. National Institue of Mental Health (NIMH) 2007.
Druss 2010 {published data only}
- Druss BG, Esenwein SA, Compton MT, Rask KJ, Zhao L, Parker RM. A randomized trial of medical care management for community mental health settings: the primary care access, referral, and evaluation (PCARE) study. American Journal of Psychiatry 2010;167:151‐9. [PUBMED: 20008945] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forsberg 2008 {published data only}
- Forsberg KA, Bjorkman T, Sandman PO, Sandlund M. Physical health ‐ a cluster randomized controlled lifestyle intervention among persons with a psychiatric disability and their staff. Nordic Journal of Psychiatry 2008;62(6):486‐95. [PUBMED: 18843564] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Almomani 2009 {published data only}
- Almomani F, Williams K, Catley D, Brown C. Effects of an oral health promotion program in people with mental illness. Journal of Dental Research 2009;88:648‐52. [DOI] [PubMed] [Google Scholar]
Baker 2009 {published data only}
- Baker A, Geddes J. Healthy lifestyle intervention for cardiovascular disease risk reduction among smokers with psychotic disorders. Australian New Zealand Clinical Trials Registry 2009.
- Baker A, Kay‐Lambkin FJ, Richmond R, Filia S, Castle D, Williams J, et al. Study protocol: a randomised controlled trial investigating the effect of a healthy lifestyle intervention for people with severe mental disorders. BMC Public Health 2011;11(1):10. [DOI: 10.1186/1471-2458-11-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berti 2011 {published data only}
- Berti L, Bonfioli E, Castellazzi M, Fiorini I, Mazzi M, Muraro F, et al. Physico‐physical comorbidity, poor health behaviour and health promotion in south verona patients with functional psychoses. Psychiatrische Praxis 2011;38:OP02_TP. [Google Scholar]
Brown 2011 {published data only}
- Brown C, Goetz J, Hamera E. Weight loss intervention for people with serious mental illness: a randomized controlled trial of the RENEW program. Psychiatric Services 2011;62:800‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eberhard 2009 {published data only}
- Eberhard S, Nordstrom G, Hoglund P, Ojehagen A. Secondary prevention of hazardous alcohol consumption in psychiatric out‐patients: a randomised controlled study. Social Psychiatry and Psychiatric Epidemiology 2009;44(12):1013‐21. [DOI] [PubMed] [Google Scholar]
Gao 2001 {published data only}
- Gao XC, Wu CJ, Kong Y. An investigation about the rehabilitation of schizophrenics insight by health education. Journal of Qilu Nursing 2001;7(9):641‐3. [Google Scholar]
Goetz 2011 {published data only}
- Goetz J, Brown C, Hamera E. Preliminary 1‐year results of the renew program for weight loss. Schizophrenia Bulletin 2011;1:304. [Google Scholar]
Huang 2005 {published data only}
- Huang R‐E, Chen K‐J, Zheng L, Zheng A‐Q. Study on the practice of health education in convalescent psychotic patients. Journal of Nursing Administration 2005;5(8):18‐20. [Google Scholar]
Jiang 2006 {published data only}
- Jiang J, Shen X, Jiang C. The study of the application of the clinical pathway during health education for schizophrenics. Journal of Nurses Training 2006;21(10):887‐9. [Google Scholar]
Jones 2001 {published data only}
- Jones RB, Atkinson JM, Coia DA, Paterson L, Morton AR, McKenna K, et al. Randomised trial of personalised computer based information for patients with schizophrenia. BMJ 2001;322(7290):835‐40. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuosmanen 2009 {published data only}
- Kuosmanen L, Valimaki M, Joffe G, Pitkanen A, Hatonen H, Patel A, et al. The effectiveness of technology‐based patient education on self‐reported deprivation of liberty among people with severe mental illness: a randomized controlled trial. Nordic Journal of Psychiatry 2009;63:383‐9. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Valimaki M, Kuosmanen L, Katajisto J, Koivunen M, Hatonen H, et al. Patient education methods to support quality of life and functional ability among patients with schizophrenia: A randomised clinical trial. Quality of Life Research 2012;21(2):247‐56. [DOI] [PubMed] [Google Scholar]
Leutwyler 2009 {published data only}
Li 2005 {published data only}
- Li S, Zhu Y, Zhao X. Study of health education on improving and maintaining treatment compliance of schizophrenia. Health Psychology Journal 2005;13(1):33‐5. [Google Scholar]
Lothringer 2009 {published data only}
- Lothringer L, Borges C, Elkington K, Mann CG, Wainberg M. A Brazilian HIV prevention intervention for adults with severe mental illness: a randomized clinical trial feasibility study. Proceedings of the 162nd Annual Meeting of the American Psychiatric Association; 2009 May 16‐21; San Francisco, CA 2009.
NCT00902694 {published data only}
- Casagrande SS, Jerome GJ, Dalcin AT, Dickerson FB, Anderson CA, Appel LJ, et al. Randomized trial of achieving healthy lifestyles in psychiatric rehabilitation: The achieve trial. BMC Psychiatry 2010;10:108. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00902694. Examining an exercise and nutrition program for reducing weight in people with severe mental illnesses (the achieve study). http://ClinicalTrials.gov/show/NCT00902694 2009.
NCT00990925 {published data only}
- NCT00990925. Lifestyle modification for weight loss in schizophrenia. http://www.clinicaltrials.gov 2009.
NCT01324973 {published data only}
- NCT01324973. Web‐based weight management for individuals with mental illness. http://ClinicalTrials.gov/show/NCT01324973 2011.
NCT01547026 {published data only}
- NCT01547026. Self‐regulation strategies to improve exercise behavior among schizophrenic patients. http://ClinicalTrials.gov/show/NCT01547026 2012.
Osborn 2010 {published data only}
- Osborn DPJ, Nazareth I, Wright CA, King MB. Impact of a nurse‐led intervention to improve screening for cardiovascular risk factors in people with severe mental illnesses. Phase‐two cluster randomised feasibility trial of community mental health teams. BMC Health Services Research 2010;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stockings 2011 {published data only}
- Stockings EA, Bowman JA, Wiggers J, Baker AL, Terry M, Clancy R, et al. A randomised controlled trial linking mental health inpatients to community smoking cessation supports: A study protocol. BMC Public Health 2011;11(1):570. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Subramaniam 2010 {published data only}
- Subramaniam K, Luks T, Aldebot S, Hearst A, Thangavel A, Fisher M, et al. Neuroplasticity‐based cognitive training improves reality monitoring in schizophrenia patients: Behavioral and fMRI assessments. Schizophrenia Research 2010;117(2‐3):471. [MEDLINE: ] [Google Scholar]
Walker 2005 {published data only}
- Walker L, Doody G, Arthur T, Abbott M. Engaging people with schizophrenia in health promotion in the UK: a challenge for healthcare professionals. Schizophrenia Bulletin 2005;31:554. [Google Scholar]
Zhou 2007 {published data only}
- Zhou W‐D, Li S‐F, Liu X‐L, Zheng G‐Z, Zeng D‐Z, Luo J‐W. Effect of systematic health education on home rehabilitation of first episode schizophrenics. Nursing Journal of Chinese People's Liberation Army 2007;24(5):1‐3. [Google Scholar]
林素兰 2010 {published data only}
- 林素兰, 张实秋, 林贵兰. health education intervention on the impact of schizophrenia patients with constipation [Google Translate] [健康教育干预对精神分裂症病人便秘影响的研究]. 全科护理 2010; Vol. 8, issue 7C:1891‐3.
References to ongoing studies
NCT00137267 {published data only}
- NCT00137267. A brief community linkage intervention for dually diagnosed individuals. http://clinicaltrials.gov/ct2/show/NCT00137267 2005.
Additional references
AHRQ 2009
- AHRQ ‐ Agency for Healthcare Research and Quality. 2009 guide to clinical preventive services. http://www.ahrq.gov/Clinic/pocketgd.htm (accessed 12th February 2010). [PubMed]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite.]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] [PubMed] [Google Scholar]
Borg 1982
- Borg GA. Psychophysical bases of perceived exertion. Medicine & Science in Sports & Exercise 1982;14(5):377‐81. [PubMed] [Google Scholar]
Bradshaw 2005
- Bradshaw T, Lovell K, Harris N. Healthy living interventions and schizophrenia: a systematic review. Journal of Advanced Nursing 2005;49(6):634‐54. [PUBMED: 15737224] [DOI] [PubMed] [Google Scholar]
Browne 1990
- Browne GB, Arpin K, Corey P, Fitch M, Gafni A. Individual correlates of health service utilization and the cost of poor adjustment to chronic illness. Medical Care 1990;28:43‐58. [DOI] [PubMed] [Google Scholar]
Cohen 2010
- Cohen D, Carter P. Conflicts of interest. WHO and the pandemic flu "conspiracies". BMJ 2010;340:c2912. [PUBMED: 20525679] [DOI] [PubMed] [Google Scholar]
Cournos 2005
- Cournos F, McKinnon K, Sullivan G. Schizophrenia and comorbid human immunodeficiency virus or hepatitis C virus. Journal of Clinical Psychiatry 2005;66(Suppl 6):27‐33. [PUBMED: 16107181] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape town. Cape Town: The Cochrane Collaboration, 2000.
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Dixon 1999
- Dixon L, Postrado L, Delahanty J, Fischer PJ, Lehman A. The association of medical comorbidity in schizophrenia with poor physical and mental health. Journal of Nervous and Mental Disease 1999;187(8):496‐502. [PUBMED: 10463067] [DOI] [PubMed] [Google Scholar]
DoH 2005
- Department of Health. Choosing health: making healthy choices easier. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/Browsable/DH_4097491 2005 (accessed 14 Dec 2009).
DoH 2006
- Department of Health. Choosing Health: Supporting the physical needs of people with severe mental illness. www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4138290.pdf (accessed 5 Nov 2009).
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
DSM IV 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Health Disorders. 4th Edition. Washington DC: American Psychiatric Association, 1994. [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder CSO. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;13:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Freire 1974
- Freire P. Pedagogy of the Oppressed. New York: Seabury Press, 1974. [Google Scholar]
Freire 1983
- Freire P. Education for Critical Consciousness. New York: Seabury Press (1973); Continuum Press, 1983. [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. [DOI] [PubMed] [Google Scholar]
Greenlund 2002
- Greenlund KJ, Giles WH, Keenan NL, Croft JB, Mensah GA. Physician advice, patient actions, and health‐related quality of life in secondary prevention of stroke through diet and exercise. Stroke 2002;33(2):565‐70. [PUBMED: 11823671] [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the health survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org..
HM Government 2009
- HM Government. New horizons: a shared vision for mental health. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_109708.pdf (accessed 7 December 2009).
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. [DOI] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) manual. North Tonawanda, NY: Multi‐Health Systems, 1986. [Google Scholar]
Kay 1987
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13(2):261‐76. [PUBMED: 3616518] [DOI] [PubMed] [Google Scholar]
Khanna 2012
- Khanna P, Clifton A, Banks D, Tosh G. Smoking cessation advice for people with serious mental illness. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009704] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khokhar 2011
- Khokhar WA, Clifton A, Jones H, Tosh G. Oral health advice for people with serious mental illness. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD008802.pub2] [DOI] [PubMed] [Google Scholar]
Kings Fund 2004
- Kings Fund. Public attitudes to public health policy. www.kingsfund.org.uk/free (accessed December 2009).
Kreuter 2000
- Kreuter MW, Chheda SG, Bull FC. How does physician advice influence patient behavior? Evidence for a priming effect. Archives of Family Medicine 2000;9(5):426‐33. [PUBMED: 10810947] [DOI] [PubMed] [Google Scholar]
Lehman 1988
- Lehman AF. A quality of life interview for the chronically mentally ill. Evaluation and Program Planning 1988;11 (1):51‐62. [Google Scholar]
Leucht 2005
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] [DOI] [PubMed] [Google Scholar]
Leucht 2007
- Leucht S, Engel RR, Bauml J, Davis JM. Is the superior efficacy of new generation antipsychotics an artifact of LOCF?. Schizophrenia Bulletin 2007;33(1):183‐91. [PUBMED: 16905632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lilly 2002
- Lilly. A meaningful day [multimedia package]. Eli Lilly Pharmaceuticals 2002.
MacDonald 1988
- Macdonald EM, Pica S, Mcdonald S, Hayes R, Baglioni AJ. Stress and coping in early psychosis. Role of symptoms, self‐efficacy, and social support in coping with stress. British Journal of Psychiatry 1998;172(33):122‐7. [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [PUBMED: 10755072] [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357:1191‐4. [PubMed] [Google Scholar]
NCEP 2001
- National Institute of Health. Third report of national cholesterol education programme expert panel on detection, evaluation and treatment of high blood cholesterol in adults. Adult Treatment Panel III 2001. [NIH Publication 01‐3670]
Newman 1991
- Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Canadian Journal of Psychiatry 1991;36(4):239‐45. [PUBMED: 1868416] [DOI] [PubMed] [Google Scholar]
NIMH 1987
- National Institute of Mental Health. Towards a Model for a Comprehensive Community‐Based Mental Health System. National Institute of Mental Health Washington, DC: NIMH, 1987. [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Robson 2007
- Robson D, Gray R. Serious mental illness and physical health problems: a discussion paper. International Journal of Nursing Studies 2007;44(3):457‐66. [PUBMED: 17007859] [DOI] [PubMed] [Google Scholar]
Ruggeri 2000
- Ruggeri M, Leese M, Thornicroft G, Bisoffi G, Tansella M. Definition and prevalence of severe and persistent mental illness. British Journal of Psychiatry 2000;177:149‐55. [PUBMED: 11026955] [DOI] [PubMed] [Google Scholar]
Russell 1979
- Russell MA, Wilson C, Taylor C, Baker CD. Effect of general practitioners' advice against smoking. British Medical Journal 1979;2(6184):231‐5. [PUBMED: 476401] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rust 1989
- Rust J, Golonbok S. Modern Psychometrics. London: Routledge, 1989. [Google Scholar]
Schinnar 1990
- Schinnar AP, Rothbard AB, Kanter R, Jung YS. An empirical literature review of definitions of severe and persistent mental illness. American Journal of Psychiatry 1990;147(12):1602‐8. [PUBMED: 2244636] [DOI] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83. [Google Scholar]
Singh 1992
- Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992;47:1019‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2007
- Smith S, Yeomans D, Bushe CJ, Eriksson C, Harrison T, Holmes R, et al. A well‐being programme in severe mental illness. Baseline findings in a UK cohort. International Journal of Clinical Practice 2007;61(12):1971‐8. [PUBMED: 17997803] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2007a
- Smith S, Yeomans D, Bushe CJ, Eriksson C, Harrison T, Holmes R, et al. A well‐being programme in severe mental illness. Reducing risk for physical ill‐health: a post‐programme service evaluation at 2 years. European Psychiatry 2007;22(7):413‐8. [PUBMED: 17765483] [DOI] [PubMed] [Google Scholar]
Solty 2009
- Solty H, Crockford D, White WD, Currie S. Cigarette smoking, nicotine dependence, and motivation for smoking cessation in psychiatric inpatients. Canadian Journal of Psychiatry 2009;54(1):36‐45. [PUBMED: 19175978] [DOI] [PubMed] [Google Scholar]
Stott 1990
- Stott NC, Pill RM. 'Advise yes, dictate no'. Patients' views on health promotion in the consultation. Family Practice 1990;7(2):125‐31. [PUBMED: 2369980] [DOI] [PubMed] [Google Scholar]
Sutherland 2003
- Sutherland G. Smoking: can we really make a difference?. Heart 2003;89(Suppl 2):ii25‐7; discussion ii35‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tosh 2010a
- Tosh G, Clifton A, Mala S, Bachner M. Physical health care monitoring for people with serious mental illness. Cochrane Database of Systematic Reviews 2010;3:CD008298. [DOI: 10.1002/14651858.CD008298.pub2; PUBMED: 20238365] [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. [PubMed] [Google Scholar]
Ware 1998
- Ware JE, Gandek B. Overview of the SF‐36 health survey and the international quality of life assessment (IQOLA) project. Journal of Clinical Epidemiology 1998;51(11):903‐12. [DOI] [PubMed] [Google Scholar]
WHO 1948
- World Health Organization. Definition of health. Preamble to the constitution of the world health organization as adopted by the international health conference, New York. Official Records of the World Health Organization. New York, 1948; Vol. 2:100.
WHO 2007
- World Health Organization. International statistical classification of diseases and related health problems. http://apps.who.int/classifications/apps/icd/icd10online/ 2007; Vol. (accessed 20th June 2010).
Wright 2012
- Wright N, Akhtar A, Tosh G, Clifton A. HIV prevention advice for people with serious mental illness. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009639] [DOI] [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7. [Google Scholar]
References to other published versions of this review
Tosh 2011
- Tosh G, Clifton A, Bachner M. General physical health advice for people with serious mental illness. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008567.pub2] [DOI] [PubMed] [Google Scholar]


