Abstract
The ability to append targeting biomolecules to chelators that efficiently coordinate to the diagnostic imaging radionuclide, 99mTc, and the therapeutic radionuclide, 188Re, can potentially enable receptor-targeted “theranostic” treatment of disease. Here we show that Pt(0)-catalyzed hydrophosphination reactions are well-suited to the derivatization of diphosphines with biomolecular moieties enabling the efficient synthesis of ligands of the type Ph2PCH2CH2P(CH2CH2–Glc)2 (L, where Glc = a glucose moiety) using the readily accessible Ph2PCH2CH2PH2 and acryl derivatives. It is shown that hydrophosphination of an acrylate derivative of a deprotected glucose can be carried out in aqueous media. Furthermore, the resulting glucose–chelator conjugates can be radiolabeled with either 99mTc(V) or 188Re(V) in high radiochemical yields (>95%), to furnish separable mixtures of cis- and trans-[M(O)2L2]+ (M = Tc, Re). Single photon emission computed tomography (SPECT) imaging and ex vivo biodistribution in healthy mice show that each isomer possesses favorable pharmacokinetic properties, with rapid clearance from blood circulation via a renal pathway. Both cis-[99mTc(O)2L2]+ and trans-[99mTc(O)2L2]+ exhibit high stability in serum. This new class of functionalized diphosphine chelators has the potential to provide access to receptor-targeted dual diagnostic/therapeutic pairs of radiopharmaceutical agents, for molecular 99mTc SPECT imaging and 188Re systemic radiotherapy.
Short abstract
Pt(0)−catalyzed hydrophosphination reactions are well−suited to the derivatization of diphosphines with glucose moieties. Ligands of the type Ph2PCH2CH2P(CH2CH2−Glc)2 (where Glc = a glucose moiety) have been prepared from the readily accessible Ph2PCH2CH2PH2 and acrylamide derivatives of glucosamine. The diphos-glucose conjugates are efficiently radiolabeled with 99mTc and 188Re. This work shows that hydrophosphination of acrylated biomolecules could provide a versatile route to the bioconjugation of diphos ligands.
Introduction
The radioactive, γ-emitting isotope, technetium-99m (99mTc, t1/2 = 6 h, 90% γ, 140 keV), is widely and routinely used in radiotracers for clinical diagnostic SPECT (Single Photon Emission Computed Tomography) or γ-scintigraphy imaging of disease. Currently, 99mTc imaging uses radiotracers that measure perfusion physiological processes. These 99mTc-labeled radiopharmaceuticals are based on relatively simple, low molecular weight Tc complexes, and their physicochemical properties determine the biodistribution and disease-targeting capabilities.1 By contrast, radiopharmaceuticals that have more recently entered routine clinical use target receptors that are overexpressed in diseased tissue. These radiopharmaceuticals include PET (Positron Emission Tomography) diagnostic imaging agents, as well as therapeutic systemic agents that emit cytotoxic β– or α particles. Pairs of diagnostic imaging and therapeutic radiotracers that target the same receptor are often considered “companion” or “theranostic” agents. Many of these compounds are based on radiometallic ions that are coordinated by a chelator, which in turn is attached to a biomolecule that targets receptors of diseased tissue (Scheme 1).2 For example, paired 68Ga-labeled peptides for diagnostic PET imaging and 177Lu-labeled peptides for systemic therapeutic agents have improved treatment outcomes for neuroendocrine and prostate cancer patients.3−5 New chelator platforms that enable attachment of a chelator to a biomolecule, and form stable complexes of 99mTc, have potential utility for developing novel 99mTc radiopharmaceuticals for use in receptor-targeted SPECT/γ-scintigraphy imaging of disease.
Scheme 1. General Strategy for Targeted Radiopharmaceuticals, with Amide Coupling Exemplifying a Method of Bioconjugation.
The chemistry of Re and Tc are closely similar, and, as Tc has no stable isotopes, the isostructural, naturally occurring rhenium (natRe) compounds are often used to aid chemical characterization of new 99mTc-labeled tracers.6 Significantly, there are two β–-emitting radioisotopes of Re that have suitable decay properties for systemic radiotherapy: 186Re (β–, 1.07 MeV, 92.5%; γ, 137 and 123 keV, 7.5%; t1/2 = 90 h) and 188Re (β–, 2.1 MeV, 71%; γ, 155 keV, 15%; t1/2 = 17 h). Pairs of isostructural 99mTc and 186/188Re complexes have potential applications as dual diagnostic/therapeutic radiopharmaceuticals.7 Additionally, both 99mTc and 188Re are available from benchtop generators, allowing economically viable and reliable access to these isotopes—essential criteria for many clinical applications.
Diphosphine chelators have actual and potential utility in 99mTc and 188Re radiopharmaceuticals. The radiopharmaceutical [99mTc(O)2(tetrofosmin)2]+, known as Myoview, is used routinely for imaging cardiac perfusion; it is a 99mTc(V) complex containing two tetrofosmin chelates (Chart 1).8 Tetradentate P2N2 and P2S2 ligands (Chart 1) have also previously been synthesized and radiolabeled with [99mTc(O)2]+ and [188Re(O)2]+ cores in high radiochemical yield, using simple radiosynthetic protocols suitable for clinical application.9−11 In addition, the P2N2 and P2S2 ligands have been conjugated with peptides via amidation of the carboxylic acid groups.9−11
Chart 1. Literature Examples of Phosphine-Containing Chelators for [M(O)2]+ core (M = 99mTc or 188Re)8−11.
Orthogonal azide–alkyne chemistry is frequently employed to attach chelators to biological vectors that target receptors of diseased tissue.12−15 However, this “click” approach is problematic for tertiary phosphines since they generally react rapidly with azides to form iminophosphines, R′N=PR3 (i.e., the Staudinger Reaction) which eliminates the P-binding function. The two following strategies have been previously employed to circumvent this Staudinger problem for click chemistry with phosphines (designed for applications as ligands in homogeneous catalysis), but neither is appropriate for bioconjugation. (1) The phosphine is introduced into an existing triazole group, but reactive chlorophosphine and organolithium reagents are required,16 which are incompatible with the presence of many O- and N- functional groups without extensive protection/deprotection protocols. (2) The phosphine is protected by peroxide oxidation to O=PR3 prior to undertaking a click reaction, and then after the click reaction is completed, hydridic reduction is carried out to regenerate the PR3 donor,17 but again, these manipulations involve reagents that are generally incompatible with functional groups in biomolecules.
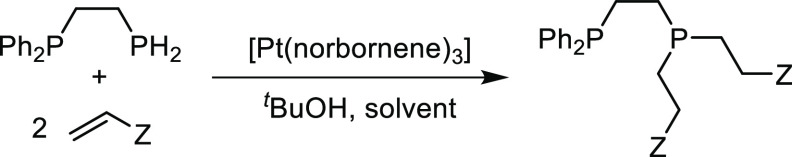
The addition of a H–PIII bond to an unsaturated C–C bond (hydrophosphination) is an atom-efficient, versatile, and generally irreversible way of making phosphine ligands.18−29 Platinum(0)-catalyzed hydrophosphination is a reliable route to phosphines that contain reactive functional groups suitable for further elaboration.30−36 We have recently demonstrated that the air-stable diphosphine Ph2PCH2CH2PR2 (where R = CH2CH2CO2Me) (L1) is readily prepared from Ph2PCH2CH2PH2 and methyl acrylate via the Pt(0)-catalyzed hydrophosphination reaction shown in Scheme 2.30 Many acryl derivatives are readily accessible synthetically, potentially paving the way for hydrophosphination to provide a simple route to diphosphines with appended biologically targeting motifs.
Scheme 2. Synthesis of Functionalized Diphosphines via Pt(0)-Catalyzed Hydrophosphination of CH2=CHZ, (Z = CO2R, CONR2, CN) by a Tertiary-Primary Diphosphine.
The protic additive t-BuOH inhibits the formation of telomeric byproducts.37
Carbohydrates and their glycoconjugates mediate a wide range of biological processes and therefore can provide highly specific glycan markers of diseased cells that can be exploited for early diagnosis and in drug development. Although carbohydrates are the most diverse and one of the most important classes of biomolecules in nature, there are relatively few carbohydrate-based drugs in clinical use.38 Glucose derivatives have been widely used to target glucose-avid diseased tissue, such as cancer, or to improve the solubility, biological stability, or other pharmacological properties of drugs.39 The radioactive 18F-labeled glucose derivative, [18F]-FDG ([18F]-2-fluoro-2-deoxyglucose), is taken up by the GLUT1 transporter which is overexpressed in cancer, and [18F]-FDG is routinely used as a diagnostic PET imaging agent in oncology.40,41 We have selected glucose derivatives as model biomolecules to assess the feasibility of bioconjugation using Pt(0)-catalyzed hydrophosphination. One attraction of glucose as the target motif is that it can be derivatized from either hydroxy-protected or unprotected precursors, allowing the versatility and scope of Pt(0)-catalyzed hydrophosphination to be gauged. Furthermore, the resulting phosphine-glucose bioconjugates are likely to be highly hydrophilic, allowing the stability of the bioconjugates and their complexes in aqueous solutions and biological media to be assessed.
Herein we report the application of Pt(0)-catalyzed hydrophosphination (Scheme 2),30 in the production of diphosphine glycoconjugates with potential utility as Tc/Re radiotheranostics. It is shown that diphosphine derivatives of the type Ph2PCH2CH2P(CH2CH2CO2Z)2 (Z = CO2Me, CO2Na), coordinate to Tc(V) and Re(V), to yield complexes of stoichiometry [M(O)2(diphos)2]+ (M = natRe, 99mTc). We further show that diphosphine-glucose bioconjugates can be prepared from acrylamide derivatives of glucose and these new diphosphine-glucose derivatives can be labeled with 99mTc and 188Re in near-quantitative radiochemical yields. The resulting 99mTc radiotracers exhibit high stability in biological milieu and have favorable biodistribution properties, as exemplified by in vivo SPECT imaging of these novel 99mTc-tracers in mice.
Results
Initial Re(V) Coordination Studies
Reaction of the previously reported hydrophosphination-derived ligand L1 with [ReI(O)2(PPh3)2] yielded a mixture of geometric isomers of [Re(O)2(L1)2]+ (1c and 1t) with the trans-Re(O)2 core and the unsymmetrical diphosphines coordinated cis or trans (Scheme 3).30 The 31P{1H} NMR spectrum showed two multiplets centered at δP 8.6 and 9.0 ppm with a coordination shift Δδ of ca. 25 ppm for both isomers (Figure S1).
Scheme 3. (a) Synthesis of L2-Na2/L2-H2; (b) Preparation of [M(O)2(L1)2]+ and [M(O)2(L2-H2)2]+ (M = 99mTc, natRe).
Conditions: (i) NaOH (2 equiv), MeOH:H2O (1:1), 79%; (ii) [ReI(O)2(PPh3)2], DCM; (iii) [99mTcO4]−, Sn-containing kit. Yields given for combined cis/trans-[M(O)2(L)2]+ and as radiochemical yields for 99mTc. Note that the overall charge on complexes of L2-H2 is dependent on pH since this will determine the degree of deprotonation of the carboxylic acid groups.
Crystals of trans-[Re(O)2(L1)2]I (1t) suitable for X-ray crystallography were grown by slow diffusion of pentane into its methanol solution (Figure 1). The Re=O bond lengths (1.778(3) Å) and Re–P bond lengths (2.4651(16) and 2.4619(17) Å) are similar to the corresponding distances in the complex [Re(O)2(dmpe)2]PF6·2H2O.42
Figure 1.

Molecular structure of trans-[Re(O)2(L1)2]I from X-ray crystallographic analysis. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and bond angles (deg): Re(1)–P(1) 2.4651(16), Re(1)–P(2) 2.4619(17), Re(1)–O(1) 1.778(3), O(1)–Re(1)–O(1) 180, P(1)–Re(1)–P(2) 80.29(5), P(1)–Re(1)–O(1) 96.60(12), P(2)–Re(1)–O(1) 90.49(13).
Diester L1 was hydrolyzed with NaOH in MeOH:H2O (1:1) at ambient temperature and the product isolated as the moderately air-stable sodium salt L2-Na2 (Scheme 3(a)). Treatment of [ReI(O)2(PPh3)2] with the derived dicarboxylate ligand L2-Na2 gave cationic Re(V) complexes as a mixture of trans and cis isomers 2t and 2c (Scheme 3(b)). The two geometric isomers were separated by reverse-phase HPLC (using an acidic mobile phase containing trifluoroacetic acid) and fully characterized; under these HPLC conditions, trans-[Re(O)2(L2-H2)2][CF3CO2] (2t) eluted first.
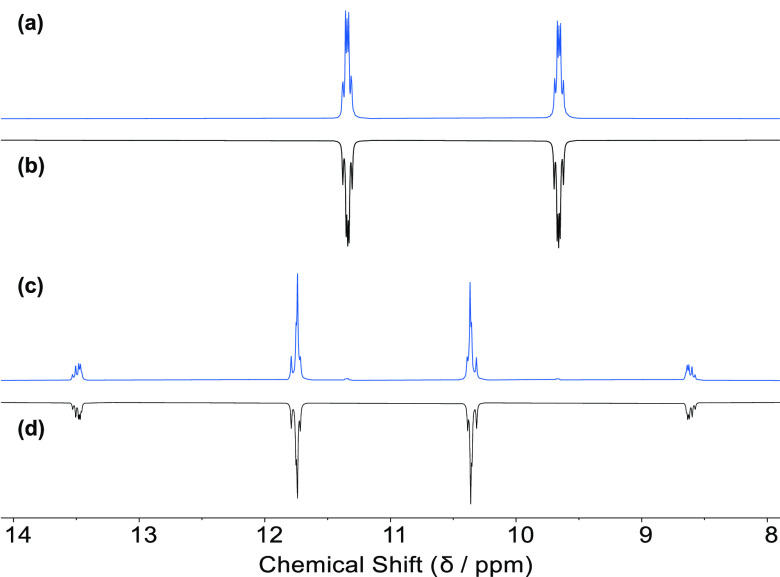
The signals observed by 31P{1H} NMR spectroscopy for 2t and 2c, were each simulated as AA′BB′ spin systems and the good agreement between the experimental and simulated spectra supports the assignment of the isomers (Figure 2).
Figure 2.
(a) Experimental and (b) simulated 31P{1H} NMR spectrum of trans-[Re(O)2(L2-H2)2]+ (2t); (c) experimental and (d) simulated 31P{1H} NMR spectrum of cis-[Re(O)2(L2-H2)2]+ (2c). See SI for the details of the simulations.
99mTc-Radiolabeling of Diester L1 and Dicarboxylate L2-Na2
99mTc-based radiopharmaceuticals are often formulated from an instant “kit” that contains all the required nonradioactive chemicals.43 These kits enable routine, simple, one-step preparations of 99mTc-labeled radiotracers in hospital radiopharmacies, simply by addition of generator-produced [99mTcO4]− dissolved in saline solution to a kit vial, followed by incubation at ambient or elevated temperatures. The ratios of the components of the lyophilized kits produced in this work were based on Myoview kits and consisted of either L1 or L2-Na2 diphosphine ligand, stannous chloride as reducing agent, sodium bicarbonate as buffer, and either sodium tartrate or sodium d-gluconate which coordinates to reduced 99mTc intermediates, to prevent their hydrolysis and formation of insoluble 99mTc-containing suspensions (see Table S1).
In an initial 99mTc-radiolabeling experiment, a solution of aqueous [99mTcO4]− was added to a lyophilized kit containing L1, and the mixture was heated at 60 °C for 30 min (Scheme 3(b)). Reverse-phase HPLC analysis indicated that numerous 99mTc-labeled species had formed in >90% radiochemical yield (see Figure S2); the complexity of the product mixture is attributed to the formation of hydrolyzed ester products as cis and trans isomers.
To obviate the difficulties of analyzing the mixture of reaction products formed from diester L1, 99mTc-radiolabeling studies were instead undertaken with dicarboxylate chelator, L2-Na2. Aqueous [99mTcO4]− was added to the kit containing L2-Na2, and the reaction mixture set aside at ambient temperature for 30 min. HPLC analysis revealed the formation of two major species, with retention times of 10 and 13 min, formed in 39% and 59% radiochemical yields, respectively (see Figure S3); these products are assigned to trans-[99mTc(O)2(L2-H2)2]+ (3t) and cis-[99mTc(O)2(L2-H2)2]+ (3c). A low intensity radioactive signal at 2 min (ca. 2% of detected 99mTc) is attributed to 99mTc intermediates or unreacted [99mTcO4]−. The 99mTc radiolabeling experiment was repeated and spiked with [99gTcO4]− (99gTc, t1/2 = 2 × 105 years) so that Tc complexes could be analyzed further. The 99gTc/99mTc-labeled products were collected and analyzed by negative ion MALDI MS and signals corresponding to [M–2H]− ([C40H48O10P499Tc]−) were observed at m/z = 909.1, consistent with the assigned stoichiometry of these complexes, and indicating that the two 99Tc-labeled species are isomeric.
Synthesis and natRe(V) Coordination Chemistry of Diphosphine Glycoconjugates
Having established that simple, unsymmetrical diphosphines coordinate to 99mTc(V), we aimed to prepare more complex diphosphine bioconjugates, and selected two glucose derivatives to demonstrate the feasibility of applying a Pt(0)-catalyzed hydrophosphination reaction to derivatize diphosphines with biologically relevant molecules.
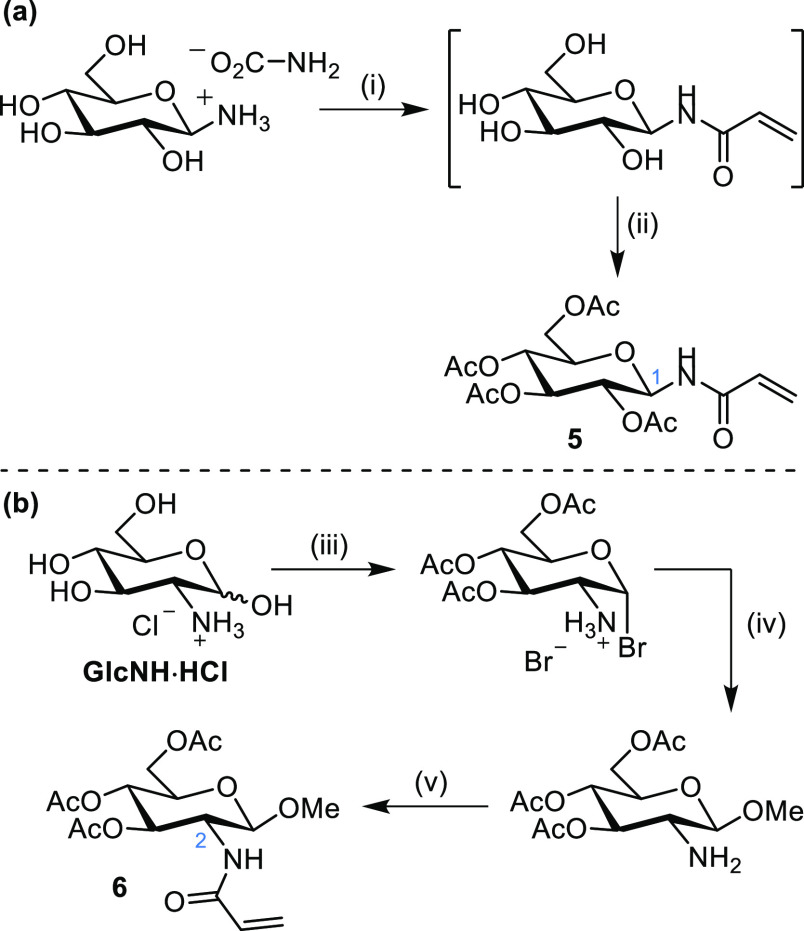
The glucose substrates 5 and 6 bearing an activated alkene at C1 and C2, respectively, were synthesized as shown in Scheme 4. Initially, 5 was synthesized via an azide derivative, as described in the SI, while the larger scale synthesis was achieved in two steps in 58% yield from acrylation and then acetylation of β-d-glucopyranosylammonium carbamate. Substrate 6 was synthesized in 3 steps and 46% overall yield from glucosamine hydrochloride (GlcNH·HCl) by installation of the β-OMe followed by acrylation.
Scheme 4. Synthesis of Glucose Substrates 5 and 6 Bearing an Activated Alkene at C1 and C2, Respectively.
Conditions: (i) Na2CO3 (5.7 equiv), MeOH:H2O (1:1) then acryloyl chloride (3.2 equiv) in THF at 0 °C; (ii) Ac2O, pyridine, 58% over two steps; (iii) acetyl bromide, 78%; (iv) MeOH, pyridine, 65%; (v) acryloyl chloride (1.2 equiv), NEt3 (1.5 equiv), DCM, 91%.
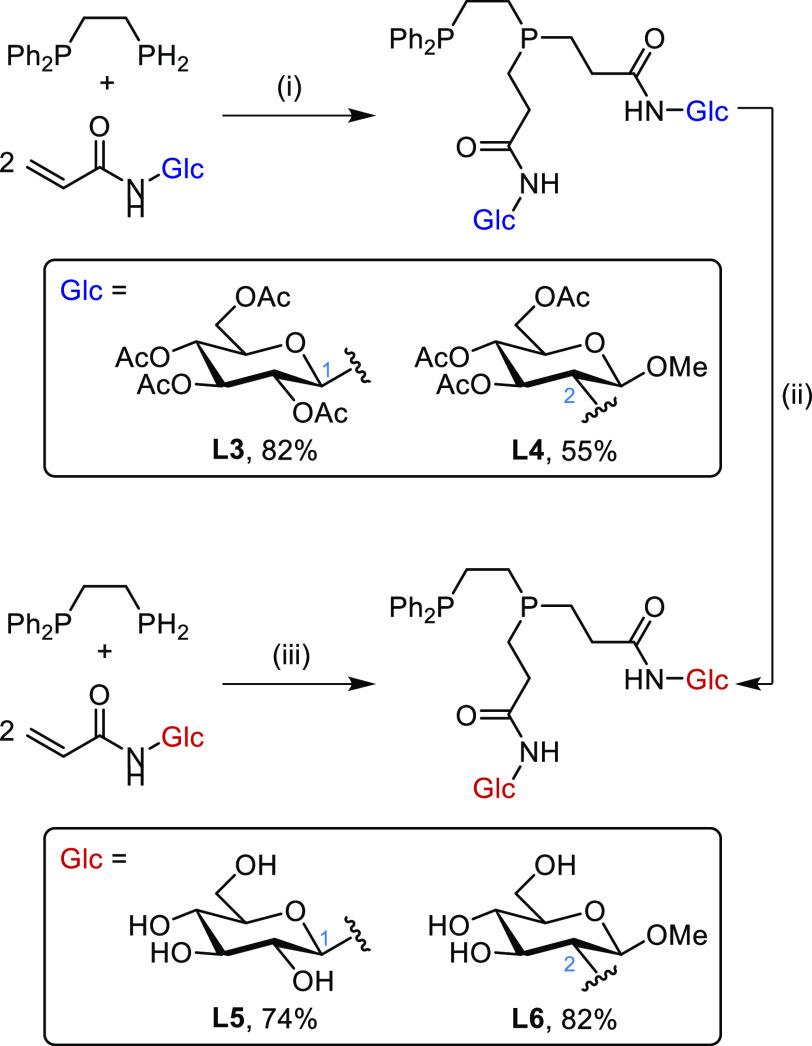
The C1-conjugate 5 was subjected to the Pt(0)-catalyzed hydrophosphination conditions, using 2.5 mol % [Pt(nbe)3] (nbe = norbornene) and i-PrOH (20 equiv) as the protic additive, to give diphosphine glycoconjugate L3 in 82% isolated yield (Scheme 5). It was found that i-PrOH was also a suitable protic additive in these reactions, and more convenient than t-BuOH which is a semisolid at room temperature. Hydrophosphination of the C2-conjugate 6 gave an unknown byproduct which was characterized by two doublet signals at δP +38.6 and −12.9 ppm (3JP,P = 44.6 Hz) in the 31P{1H} NMR spectrum of the crude material (Figure S4). Increasing the catalyst loading to 5.0 mol % reduced the amount of byproduct and pure L4 was isolated in 55% yield. Investigations into the identity of the unknown byproduct are ongoing. Acetate deprotection of the glucose motifs in both diphosphine glycoconjugates, L3 and L4, using NaOMe gave ligands L5 (74%) and L6 (82%), respectively.
Scheme 5. Synthesis of Diphosphine Glycoconjugates via Pt(0)-Catalyzed Hydrophosphination.
Conditions: (i) 2.5% or 5.0% [Pt(nbe)3], i-PrOH (20 equiv); (ii) NaOMe (0.2 mol %) in MeOH; (iii) 5.0% [Pt(nbe)3] in 20% aqueous i-PrOH gave L6 in 80% yield (determined by 31P{1H} NMR spectroscopy).
The Pt(0)-catalyzed hydrophosphination of activated alkenes is a reliable reaction, and we have now found that this reaction takes place even in aqueous media. Thus, L6 was made by Pt(0)-catalyzed hydrophosphination of the unprotected glucose acylamide precursor in 20% aqueous isopropanol (Scheme 5). Significantly, this aqueous chemistry opens up the possibility of using highly hydrophilic activated alkenes as substrates for hydrophosphination.
The diphosphine glycoconjugate ligands L5 and L6 were each reacted with [ReI(O)2(PPh3)2] (Scheme 6), furnishing cis- and trans-[Re(O)2(L)2]+ (7c/7t and 8c/8t) as expected. The cis and trans isomers of these complexes are distinguishable by 31P{1H} NMR spectroscopy based on the characteristic AA′BB′ patterns for each isomer, which are very similar to those observed for the analogous L2-H2 complexes (2c and 2t, see Figure 2). The two geometric isomers of [Re(O)2(L5)2]+ (7c and 7t) were separated by reverse-phase HPLC and characterized separately (Figure S5). After 4 days in solution, isomerization of originally pure samples of 7c and 7t was detected by 31P{1H} NMR spectroscopy (Figures S6 and S7). After 20 days, there were no further spectroscopic changes in either of the samples indicating that equilibration was complete. At equilibrium, an approximately 1:1 mixture of 7c and 7t was observed indicating that the equilibrium constant K ≈ 1. The kinetics of the cis/trans isomerization are slow enough to suggest that the individual isomers would retain their integrity on a clinical time scale.
Scheme 6. Coordination and Radiolabeling of L5 and L6 to Form [M(O)2(L)2]+, where M = natRe, 188Re, and 99mTc.
Conditions: (i) [ReI(O)2(PPh3)2], MeOH; (ii) 99mTcO4–, Sn-containing kit; (iii) 188ReO4–, SnCl2, sodium citrate. Yields given for combined cis/trans-[M(O)2(L)2]+ and as radiochemical yields for 99mTc and 188Re. The asterisk in compound numbers (e.g., 7t*) denotes a 188Re radiolabeled isotopologue.
99mTc and 188Re Radiolabeling of Diphosphine Glycoconjugates L5 and L6
The new glycoconjugate L5 was selected for more detailed radiochemical and biological evaluation, as it was thought that the fully unprotected glucose motif in L5 would be more likely to retain glucose recognition when compared with L6, which is linked via C2 and contains a OMe group at the C1 position. Lyophilized mixtures of L5, stannous chloride, sodium gluconate, and sodium bicarbonate were prepared, providing prefabricated “kits”, similar to those prepared for 99mTc-radiolabeling reactions with L1 and L2-Na2 (see above). A saline solution containing generator-produced [99mTcO4]− (220–280 MBq) was added to a kit, and the mixture was then left to react at ambient temperature (20–25 °C) for 5 min. Radio-HPLC analysis of this mixture revealed formation of trans-[99mTc(O)2(L5)2]+ (9t) (7.88 min) and cis-[99mTc(O)2(L5)2]+ (9c) (12.51 min) in exceptionally high radiochemical yields of 70% and 29%, respectively, with <1% of 99mTc present as unreacted [99mTcO4]− or other 99mTc species (Figure 3a).
Figure 3.
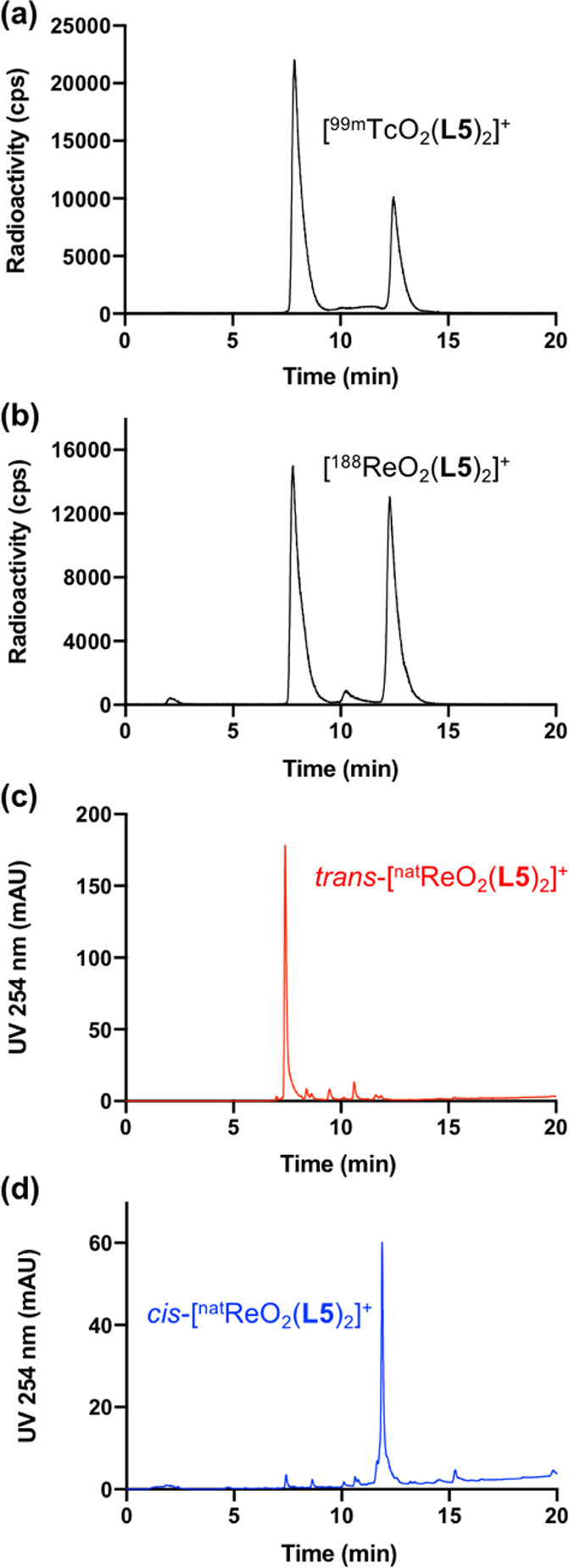
Analytical reverse-phase HPLC chromatograms of (a) [99mTc(O)2(L5)2]+ (9c and 9t); (b) [188Re(O)2(L5)2]+ (7c* and 7t*); (c) trans-[188Re(O)2(L5)2]+ (7t); (d) cis-[188Re(O)2(L5)2]+ (7c).
The radionuclide, 188Re, is available from a 188W/188Re generator. Radioactive decay of parent 188W (t1/2 = 69 days) to 188Re enables a continuous source of 188Re for 6–24 months (depending on user requirements). 188Re is eluted from the generator by saline solution, in the form of [188ReO4]−. To assess the ability of diphosphines to complex medically useful radioisotopes of Re, L5 was radiolabeled with 188Re. First, [188ReO4]− was reduced to a Re(V)-citrate precursor, by heating a saline solution of [188ReO4]− with stannous chloride at 90 °C in the presence of sodium citrate.9,11 Diphosphine L5 was then added to a solution containing the 188Re(V)-citrate precursor, and the mixture heated at 90 °C for 30 min to yield trans-[188Re(O)2(L5)2]+ (7t*) (7.80 min) and cis-[188Re(O)2(L5)2]+ (7c*) (12.31 min) in 52% and 45% radiochemical yield, respectively (Figure 3b). The very similar retention times of 99mTc and 188Re analogues are consistent with these complexes being isostructural. A third, unidentified species (10.57 min) was formed in 2% radiochemical yield.
The 188Re radiolabeling could also be accomplished at ambient temperature, with a reaction time of only 5 min; under these conditions, lower radiochemical yields of 45% for 7t* and 40% for 7c* were obtained (Figure S8). At ambient temperature, unreacted [188ReO4]−/188Re(V)-citrate precursor accounted for 7% of 188Re radioactive species and an unidentified species was also formed in 8% radiochemical yield.
To confirm the identity of these radiolabeled products, the analogous, naturally occurring Re complexes were also analyzed by HPLC: 7t eluted at 7.41 min and 7c eluted at 11.89 min (Figure 3c–d). These natRe species exhibit similar chromatographic properties to the analogous radioactive 99mTc and 188Re compounds. The small difference in retention times between natRe and 188Re isotopologues is an artifact of the configuration of the UV and radioactivity (scintillation) detectors in series.
The diphosphine-glucose conjugate, L6, was also radiolabeled with 99mTc and 188Re, using the same radiosynthetic procedures. Diphosphine L6 was similarly incorporated into a lyophilized kit mixture for radiolabeling with 99mTc to give [99mTc(O)2(L6)2]+ (10c and 10t); L6 was added to solutions of 188Re(V)-citrate for radiolabeling to give [188Re(O)2(L6)2]+ (8c* and 8t*). Similar to L5, the putative cis and trans isomers were formed in high radiochemical yields (>96% for 99mTc isomers, >99% for 188Re isomers; Figure S9).
Stability of cis- and trans-[99mTc(O)2(L5)2]+ (9c and 9t) in Serum
The stabilities of the new radiotracers, 9c and 9t, in serum were evaluated. First, each isomer was isolated from the radiolabeling reaction mixture which included their separation from unreacted L5. Then 9c and 9t were separately incubated in human serum. Radio-HPLC analysis indicated that both isomers were very stable in serum: for 9c, 99.7% remained intact after 2 h, decreasing to 95.0% after 24 h; for 9t, 98.6% of the complex remained intact after 2 h, decreasing to 93.4% after 24 h (Figure S10).
SPECT/CT and Biodistribution of cis- and trans-[99mTc(O)2(L5)2]+ (9c and 9t) in Healthy Mice
SPECT/CT images were acquired using 9c and 9t (Figure 4a). Each isomer was separately injected intravenously to healthy Balb/c female mice (fasted for 12–14 h prior to tracer administration), followed by SPECT scanning for 2 h. Both 9c and 9t cleared circulation rapidly, predominantly via a renal pathway. For mice that had been administered 9c, 60% of 99mTc activity was associated with the bladder/urine 30 min postinjection. Similarly, for mice that had been administered 9t, image quantification showed that by 30 min postinjection, 60% of 99mTc activity was associated with the bladder/urine. In other measured organs and tissue (including liver, muscle, kidneys, heart/blood pool, and brain) the concentration of 99mTc activity for both isomers decreased from 30 min postinjection to 2 h postinjection (Figure 4b).
Figure 4.
(a) SPECT/CT maximum intensity projections of healthy Balb/c mice administered and 9c and 9t; (b) SPECT image analysis of healthy Balb/c mice administered 9c and 9t. Regions of interest were selected on VivoQuant (inviCRO, LLC, Boston, USA), and percentage injected dose per milliliter (% ID/mL) were calculated for each of 9c (n = 1) and 9t (n = 1). (c) Biodistribution (30 min postinjection) of mice, administered either 9c or 9t intravenously (n = 4 per group).
Biodistribution studies were also undertaken, in which healthy Balb/c female mice were administered radiotracer (Figure 4c). Mice were culled 30 min postinjection, and their organs harvested for ex vivo weighing and tissue counting for radioactivity. For measured organs, the highest 99mTc radioactivity concentration was observed in the kidneys, with 12.3 ± 4.5%ID g–1 for 9c, and 8.7 ± 2.8%ID g–1 for 9t, consistent with SPECT images showing high renal excretion. Relatively low levels of 99mTc radioactivity concentration (<7%ID g–1) were observed for all other measured organs. Notably, for many measured organs, average 99mTc radioactivity concentration was higher for animals administered 9c, compared with animals administered 9t. This is possibly a result of slightly higher blood retention of 9c: blood activity measured 5.6 ± 1.3%ID g–1 for 9c, and 3.2 ± 0.6%ID g–1 for 9t, 30 min postinjection.
Urine was also collected from these mice. Radio-HPLC analysis (Figure 5) of urine showed that both 9c and 9t were excreted intact, consistent with the high serum stability observed for both radiotracers.
Figure 5.

Radio-HPLC analysis of urine administered to mice intravenously administered either (a) 9t or (b) 9c shows that both radiotracers are excreted intact.
Discussion
We have shown that Pt(0)-catalyzed hydrophosphination gives efficient access to biomolecule-functionalized diphosphines. The requisite acrylamide groups were straightforwardly introduced on two different glucose sites, namely, C1 or C2. Moreover, the ability to perform the reaction in i-PrOH (with 0–20% H2O), with highly polar biomolecular derivatives to form diphosphine bioconjugates such as L6, expands the potential substrate scope to a large range of targeted, biologically active molecules. Additionally, it negates the need for common protecting groups, which are typically used to solubilize biomolecular precursors in nonpolar organic solvents to allow for synthetic derivatization. In turn, this removes the need for a deprotection reaction, in which conditions are often incompatible with newly introduced and sensitive phosphine groups. We envisage that other classes of biomolecules, including peptides and small molecules, could also be derivatized with acryl groups, allowing many compounds of biological relevance to be functionalized with a diphosphine.
Significantly, the resulting air-stable diphosphines are capable of quantitative radiolabeling of 99mTc(V) under mild reaction conditions. We undertook 99mTc radiolabeling of L2-Na2, L5, and L6 using lyophilized kits (containing phosphine ligand and reducing agent) and generator-produced [99mTcO4]−, demonstrating the feasibility of using this diphosphine platform for rapid, one-step, kit-based 99mTc radiolabeling of receptor-targeted radiopharmaceuticals. The simple kit-based 99mTc radiolabeling methods described here are inspired by, and similar to, clinical protocols used for aseptic preparation of widely and routinely used perfusion agents. Notably, these radiolabeling methods contrast with the multistep, complicated procedures used for some newer, receptor-targeted 99mTc radiotracers currently being evaluated in clinical studies.44 The ability to prepare 99mTc-labeled receptor-targeted radiotracers using a kit could increase access to receptor-targeted radiopharmaceuticals, and increase the utility and healthcare benefits of 99mTc and SPECT/γ-scintigraphy infrastructure.
Additionally, these new diphosphine bioconjugates chelate both 99mTc(V) and 188Re(V)—the latter also in high radiochemical yields (≥85%, even with only 5 min reaction at ambient temperature)—leading to the possibility of using this diphosphine bioconjugate platform for the development of a receptor-targeted, isostructural dual diagnostic/therapeutic pair (a “theranostic” agent) for molecular 99mTc SPECT imaging and 188Re systemic radiotherapy.7,45,46
A further potential advantage of this platform is that multiple copies of a targeting motif can be introduced into a single molecule with relative ease. The hydrophosphination reaction here appends two copies of a targeting motif to each diphosphine; upon Tc(V) or Re(V) coordination, the number of copies increases to four per radiotracer molecule. Multimeric imaging agents, particularly those that incorporate peptides, have demonstrated increased in vivo accumulation at target tissue relative to their monomeric analogues, and are effective contrast agents.47−49 There are a few examples in which 99mTc coordination by two equivalents of a chelator-peptide bioconjugate yields a radiotracer containing two copies of a targeting motif.50−52 Our new diphosphine platform is eminently suited to developing molecular 99mTc/188Re radiotracers containing multiple copies of a targeting group.
The new diphosphine bioconjugates yield radiotracers consisting of cis and trans geometric isomers. This is potentially a disadvantage, as prior to clinical application of any new radiotracer based on this platform, the isomers would likely require separate assessment to determine whether or not they are biologically equivalent to each other. However, we note that the 68Ga-labeled prostate cancer radiotracer, 68Ga-HBED-PSMA, consists of at least two distinct chemical species,53,54 and yet despite this, the individual in vivo behavior of each of these species has not been assessed, and this has not prevented widespread and routine use of 68Ga-HBED-PSMA.55 Notably, the slow isomerization in solutions observed for cis- and trans-[Re(O)2(L5)2]+ (7c and 7t) was not observed for 99mTc analogues after 24 h in serum, nor in the excreted urine, suggesting that this isomerization appears to take place over a longer time scale than clinically relevant timeframes. Our initial in vivo characterization of each of cis-[99mTc(O)2(L5)2]+ and trans-[99mTc(O)2(L5)2]+ (9c and 9t) shows that the two isomers have similar pharmacokinetic profiles to each other, with both exhibiting fast blood clearance via a renal pathway.
The development of a 99mTc-labeled, GLUT1-targeted radiotracer would enable γ-scintigraphy/SPECT imaging of metabolic status, providing a SPECT-equivalent radiopharmaceutical of the widely used PET diagnostic glucose analogue, [18F]-FDG. There have been several previous attempts to label glucose or other carbohydrate derivatives with 99mTc for imaging of glucose uptake in vivo.56 Some of these derivatives have demonstrated inhibitory activity of the hexokinase enzyme, which phosphorylates glucose in glucose metabolic pathways.57−59 However, only a few of these 99mTc tracers demonstrate uptake in glucose-avid tissue.59−62 Presumably this is due to lack of molecular recognition of modified/conjugated glucose moieties by GLUT1 and other GLUT receptors, which transport glucose into cells. Furthermore, it is possible that some of the 99mTc tracers that do show tumor accumulation are taken up via nonspecific mechanisms unrelated to GLUT1 expression. Although our new radiotracers contain four glucose units per molecule, SPECT/CT scanning in fasted mice showed no evidence of 99mTc retention in glucose-avid organs, such as the heart or brain, both of which demonstrate significant accumulation of 18F in [18F]-FDG PET scans.63,64 Similar to the case of the great majority of 99mTc-labeled glucose derivatives, GLUT1 and other glucose transporters do not recognize the modified glucose moieties of L5.
Despite this absence of uptake of [99mTc(O)2(L5)2]+ (9c and 9t) in highly metabolic tissue, the high stability and rapid blood clearance of both 9c and 9t, and the lack of accumulation of 99mTc activity in healthy organs is auspicious. It suggests that other, more targeted radiotracers based on this platform will be similarly stable in vivo and have low off-target accumulation, and will hence provide high contrast SPECT/γ-scintigraphy images. In the case of any complementary 188Re derivatives, the rapid clearance of these agents from healthy tissue would minimize radiation doses to healthy organs. While the four glucose units of [99mTc(O)2(L5)2]+ no doubt contribute to the favorable clearance of [99mTc(O)2(L5)2]+, it is plausible that other radiotracers based on this platform (for example, derivatives containing hydrophilic receptor-targeted peptides) will possess similarly favorable biodistribution properties. In this regard, we recently reported the RGD-conjugated diphosphine complexes of natRe (10c/10t) and 99mTc (11c/11t) shown in Chart 2, where RGD is a cyclic peptide that targets αvβ3-integrin receptors. While complexes 10 and 11 were shown to target diseased tissue, the synthesis of the diphosphine has some limitations including the variation of the diphosphine structure. In contrast, it can readily be envisaged how RGD could be incorporated into a wide range of diphosphines by adaptation of the versatile hydrophosphination route reported here.
Chart 2. RDG-Conjugated Diphosphine Complexes.

Conclusions
New chelator platforms that enable simple and efficient labeling of receptor-targeted biomolecules with radiometals have utility in nuclear medicine. We have demonstrated that diphosphines functionalized with glucose units can be efficiently prepared using Pt(0)-catalyzed hydrophosphinations of acrylamides. Notably, it has also been demonstrated that the hydrophosphinations of hydrophilic, unprotected glucose derivatives can be accomplished in aqueous media. The resulting diphosphine-glucose bioconjugates can be radiolabeled with both 99mTc and 188Re in near-quantitative radiochemical yields. SPECT imaging studies in mice provide evidence that the new 99mTc radiotracers possess propitious pharmacokinetic properties, and the requisite high metabolic stability. Our new chemical technology therefore has significant potential for the future development of theranostic pairs of chemically analogous diagnostic 99mTc-labeled radiotracers and radiotherapeutic 188Re-labeled agents.
Acknowledgments
This research was supported by a Cancer Research UK Career Establishment Award (C63178/A24959), the EPSRC programme for Next Generation Molecular Imaging and Therapy with Radionuclides (EP/S032789/1, “MITHRAS”), Rosetrees Trust (M685, M606), the Wellcome Multiuser Equipment Radioanalytical Facility funded by Wellcome Trust (212885/Z/18/Z), the Centre for Medical Engineering funded by the Wellcome Trust and the Engineering and Physical Sciences Research Council (EPSRC) (WT088641/Z/09/Z), the European Research Council grant number ERC–COG:648239, and the Bristol Chemical Synthesis Centre for Doctoral Training, funded by EPSRC (EP/L015366/1), and the University of Bristol, for a PhD studentship (to R.E.N.).We would like to thank Dr Paul J. Gates of the University of Bristol, UK for determining the mass spectra of the technetium complexes.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.2c04008.
Experimental procedures and characterization data, including radiolabeling procedures, selected NMR spectra, supplementary figures, and X-ray crystallographic tables (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Rivas C.; Jackson J. A.; Hungnes I. N.; Ma M. T.. Radioactive Metals in Imaging and Therapy in Comprehensive Coordination Chemistry III; Elsevier, 2021; Chapter 9.21. [Google Scholar]
- Jackson J. A.; Hungnes I. N.; Ma M. T.; Rivas C. Bioconjugates of Chelators with Peptides and Proteins in Nuclear Medicine: Historical Importance, Current Innovations, and Future Challenges. Bioconjugate Chem. 2020, 31, 483–491. 10.1021/acs.bioconjchem.0c00015. [DOI] [PubMed] [Google Scholar]
- Hofman M. S.; Emmett L.; Sandhu S.; Iravani A.; Joshua A. M.; Goh J. C.; Pattison D. A.; Tan T. H.; Kirkwood I. D.; Ng S.; Francis R. J.; Gedye C.; Rutherford N. K.; Weickhardt A.; Scott A. M.; Lee S.-T.; Kwan E. M.; Azad A. A.; Ramdave S.; Redfern A. D.; Macdonald W.; Guminski A.; Hsiao E.; Chua W.; Lin P.; Zhang A. Y.; McJannett M. M.; Stockler M. R.; Violet J. A.; Williams S. G.; Martin A. J.; Davis I. D. [177Lu]Lu-PSMA-617 Versus Cabazitaxel in Patients with Metastatic Castration-Resistant Prostate Cancer (TheraP): A Randomised, Open-Label, Phase 2 Trial. Lancet 2021, 397, 797–804. 10.1016/S0140-6736(21)00237-3. [DOI] [PubMed] [Google Scholar]
- Strosberg J.; El-Haddad G.; Wolin E.; Hendifar A.; Yao J.; Chasen B.; Mittra E.; Kunz P. L.; Kulke M. H.; Jacene H.; Bushnell D.; O’Dorisio T. M.; Baum R. P.; Kulkarni H. R.; Caplin M.; Lebtahi R.; Hobday T.; Delpassand E.; Van Cutsem E.; Benson A.; Srirajaskanthan R.; Pavel M.; Mora J.; Berlin J.; Grande E.; Reed N.; Seregni E.; Öberg K.; Lopera Sierra M.; Santoro P.; Thevenet T.; Erion J. L.; Rusziewski P.; Kwekkeboom D.; Krenning E. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treglia G.; Castaldi P.; Rindi G.; Giordano A.; Rufini V. Diagnostic Performance of Gallium-68 Somatostatin Receptor PET and PET/CT in Patients with Thoracic and Gastroenteropancreatic Neuroendocrine Tumours: A Meta-Analysis. Endocrine 2012, 42, 80–87. 10.1007/s12020-012-9631-1. [DOI] [PubMed] [Google Scholar]
- Davies L. H.; Kasten B. B.; Benny P. D.; Arrowsmith R. L.; Ge H.; Pascu S. I.; Botchway S. W.; Clegg W.; Harrington R. W.; Higham L. J. Re and 99mTc Complexes of BodP3 – Multi-Modality Imaging Probes. Chem. Commun. 2014, 50, 15503–15505. 10.1039/C4CC06367H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler C. S.; Hennkens H. M.; Sisay N.; Huclier-Markai S.; Jurisson S. S. Radiometals for Combined Imaging and Therapy. Chem. Rev. 2013, 113, 858–883. 10.1021/cr3003104. [DOI] [PubMed] [Google Scholar]
- Kelly J. D.; Forster A. M.; Higley B.; Archer C. M.; Booker F. S.; Canning L. R.; Chiu K. W.; Edwards B.; Gill H. K.; McPartlin M.; Nagle K. R.; Latham I. A.; Pickett R. D.; Storey A. E.; Webbon P. M. Technetium-99m-Tetrofosmin as a New Radiopharmaceutical for Myocardial Perfusion Imaging. J. Nucl. Med. 1993, 34, 222–227. [PubMed] [Google Scholar]
- Gali H.; Hoffman T. J.; Sieckman G. L.; Owen N. K.; Katti K. V.; Volkert W. A. Synthesis, Characterization, and Labeling with 99mTc/188Re of Peptide Conjugates Containing a Dithia-Bisphosphine Chelating Agent. Bioconjugate Chem. 2001, 12, 354–363. 10.1021/bc000077c. [DOI] [PubMed] [Google Scholar]
- Karra S. R.; Schibli R.; Gali H.; Katti K. V.; Hoffman T. J.; Higginbotham C.; Sieckman G. L.; Volkert W. A. 99mTc-Labeling and in Vivo Studies of a Bombesin Analogue with a Novel Water-Soluble Dithiadiphosphine-Based Bifunctional Chelating Agent. Bioconjugate Chem. 1999, 10, 254–260. 10.1021/bc980096a. [DOI] [PubMed] [Google Scholar]
- Kothari K. K.; Gali H.; Prabhu K. R.; Pillarsetty N.; Owen N. K.; Katti K. V.; Hoffman T. J.; Volkert W. A. Synthesis and Characterization of 99mTc- and 188Re-Complexes with a Diamido-Dihydroxymethylenephosphine-Based Bifunctional Chelating Agent (N2P2-BFCA). Nucl. Med. Biol. 2002, 29, 83–89. 10.1016/S0969-8051(01)00280-3. [DOI] [PubMed] [Google Scholar]
- Zeng D.; Zeglis B. M.; Lewis J. S.; Anderson C. J. The Growing Impact of Bioorthogonal Click Chemistry on the Development of Radiopharmaceuticals. J. Nucl. Med. 2013, 54, 829–832. 10.2967/jnumed.112.115550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindt T. L.; Struthers H.; Brans L.; Anguelov T.; Schweinsberg C.; Maes V.; Tourwé D.; Schibli R. ‘Click to Chelate’: Synthesis and Installation of Metal Chelates into Biomolecules in a Single Step. J. Am. Chem. Soc. 2006, 128, 15096–15097. 10.1021/ja066779f. [DOI] [PubMed] [Google Scholar]
- Struthers H.; Spingler B.; Mindt T. L.; Schibli R. “Click-to-Chelate”: Design and Incorporation of Triazole-Containing Metal-Chelating Systems into Biomolecules of Diagnostic and Therapeutic Interest. Chem.—Eur. J. 2008, 14, 6173–6183. 10.1002/chem.200702024. [DOI] [PubMed] [Google Scholar]
- Kluba C. A.; Mindt T. L. Click-to-Chelate: Development of Technetium and Rhenium-Tricarbonyl Labeled Radiopharmaceuticals. Molecules 2013, 18, 3206–3226. 10.3390/molecules18033206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Van Nguyen H.; Male L.; Craven P.; Buckley B. R.; Fossey J. S. Phosphino-Triazole Ligands for Palladium-Catalyzed Cross-Coupling. Organometallics 2018, 37, 4224–4241. 10.1021/acs.organomet.8b00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde C.; Wei M. M.; Van Der Lee A.; Deydier E.; Daran J. C.; Volle J. N.; Poli R.; Pirat J. L.; Manoury E.; Virieux D. Double [3 + 2]-Dimerisation Cascade Synthesis of Bis(triazolyl)bisphosphanes, a New Scaffold for Bidentate Bisphosphanes. Dalton Trans. 2015, 44, 12539–12545. 10.1039/C5DT02197A. [DOI] [PubMed] [Google Scholar]
- Gusarova N. K.; Arbusova S. N.; Malysheva S. F.; Khil’ko M. Y.; Tatarinova A. A.; Gorokhov V. G.; Trofimov B. A. Synthesis of Primary Phosphines from Phosphine and Arylethylenes. Russ. Chem. Bull. 1995, 44, 1535–1535. 10.1007/BF00714449. [DOI] [Google Scholar]
- Wauters I.; Debrouwer W.; Stevens C. V. Preparation of Phosphines Through C–P Bond Formation. Beilstein J. Org. Chem. 2014, 10, 1064–1096. 10.3762/bjoc.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glueck D. S. Catalytic Asymmetric Synthesis of P-Stereogenic Phosphines: Beyond Precious Metals. Synlett 2021, 32, 875–884. 10.1055/s-0040-1707309. [DOI] [Google Scholar]
- Glueck D. S. Metal-Catalyzed P-C Bond Formation via P-H Oxidative Addition: Fundamentals and Recent Advances. J. Org. Chem. 2020, 85, 14276–14285. 10.1021/acs.joc.0c00667. [DOI] [PubMed] [Google Scholar]
- Delacroix O.; Gaumont A. C. Hydrophosphination of Unactivated Alkenes, Dienes and Alkynes: A Versatile and Valuable Approach for the Synthesis of Phosphines. Curr. Org. Chem. 2005, 9, 1851–1882. 10.2174/138527205774913079. [DOI] [Google Scholar]
- Moglie Y.; González-Soria M. J.; Martín-García I.; Radivoy G.; Alonso F. Catalyst- and Solvent-Free Hydrophosphination and Multicomponent Hydrothiophosphination of Alkenes and Alkynes. Green Chem. 2016, 18, 4896–4907. 10.1039/C6GC00903D. [DOI] [Google Scholar]
- Koshti V.; Gaikwad S.; Chikkali S. H. Contemporary Avenues in Catalytic PH Bond Addition Reaction: A Case Study of Hydrophosphination. Coord. Chem. Rev. 2014, 265, 52–73. 10.1016/j.ccr.2014.01.006. [DOI] [Google Scholar]
- Liu X. T.; Han X. Y.; Wu Y.; Sun Y. Y.; Gao L.; Huang Z.; Zhang Q. W. Ni-Catalyzed Asymmetric Hydrophosphination of Unactivated Alkynes. J. Am. Chem. Soc. 2021, 143, 11309–11316. 10.1021/jacs.1c05649. [DOI] [PubMed] [Google Scholar]
- Bange C. A.; Waterman R. Challenges in Catalytic Hydrophosphination. Chem.—Eur. J. 2016, 22, 12598–12605. 10.1002/chem.201602749. [DOI] [PubMed] [Google Scholar]
- Bissessar D.; Egly J.; Achard T.; Steffanut P.; Bellemin-Laponnaz S. Catalyst-Free Hydrophosphination of Alkenes in Presence of 2-Methyltetrahydrofuran: A Green and Easy Access to a Wide Range of Tertiary Phosphines. RSC Adv. 2019, 9, 27250–27256. 10.1039/C9RA04896K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinal-Viguri M.; King A. K.; Lowe J. P.; Mahon M. F.; Webster R. L. Hydrophosphination of Unactivated Alkenes and Alkynes Using Iron(II): Catalysis and Mechanistic Insight. ACS Catal. 2016, 6, 7892–7897. 10.1021/acscatal.6b02290. [DOI] [Google Scholar]
- Sharpe H. R.; Geer A. M.; Lewis W.; Blake A. J.; Kays D. L. Iron(II)-Catalyzed Hydrophosphination of Isocyanates. Angew. Chem., Int. Ed. 2017, 56, 4845–4848. 10.1002/anie.201701051. [DOI] [PubMed] [Google Scholar]
- Chadwick A. C.; Heckenast M. A.; Race J. J.; Pringle P. G.; Sparkes H. A. Self-Replication of Chelating Diphosphines via Pt(0)-Catalyzed Hydrophosphination. Organometallics 2019, 38, 3871–3879. and references therein 10.1021/acs.organomet.9b00541. [DOI] [Google Scholar]
- Pringle P. G.; Smith M. B. Platinum(0)-Catalysed Hydrophosphination of Acrylonitrile. J. Chem. Soc., Chem. Commun. 1990, 1701–1702. 10.1039/c39900001701. [DOI] [Google Scholar]
- Costa E.; Pringle P. G.; Smith M. B.; Worboys K. Self-Replication of Tris(cyanoethyl)phosphine Catalysed by Platinum Group Metal Complexes. J. Chem. Soc., Dalton Trans. 1997, 4277–4282. 10.1039/a704655c. [DOI] [Google Scholar]
- Costa E.; Pringle P. G.; Worboys K. Chemoselective Platinum(0)-Catalysed Hydrophosphination of Ethyl Acrylate. Chem. Commun. 1998, 49–50. 10.1039/a706718f. [DOI] [Google Scholar]
- Wicht D. K.; Kourkine I. V.; Kovacik I.; Glueck D. S.; Concolino T. E.; Yap G. P. A.; Incarvito C. D.; Rheingold A. L. Platinum-Catalyzed Acrylonitrile Hydrophosphination. P-C Bond Formation via Olefin Insertion into a Pt-P Bond. Organometallics 1999, 18, 5381–5394. 10.1021/om990745h. [DOI] [Google Scholar]
- Glueck D. S. Catalytic Asymmetric Synthesis of Chiral Phosphanes. Chem.—Eur. J. 2008, 14, 7108–7117. and references therein 10.1002/chem.200800267. [DOI] [PubMed] [Google Scholar]
- Sadow A. D.; Haller I.; Fadini L.; Togni A. Nickel(II)-Catalyzed Highly Enantioselective Hydrophosphination of Methacrylonitrile. J. Am. Chem. Soc. 2004, 126, 14704–14705. 10.1021/ja0449574. [DOI] [PubMed] [Google Scholar]
- Scriban C.; Kovacik I.; Glueck D. S. A Protic Additive Suppresses Formation of Byproducts in Platinum-Catalyzed Hydrophosphination of Activated Olefins. Evidence for P-C and C-C Bond Formation by Michael Addition. Organometallics 2005, 24, 4871–4874. 10.1021/om050433g. [DOI] [Google Scholar]
- Galan M. C.; Benito-Alifonso D.; Watt G. M. Carbohydrate Chemistry in Drug Discovery. Org. Biomol. Chem. 2011, 9, 3598–3610. 10.1039/c0ob01017k. [DOI] [PubMed] [Google Scholar]
- Calvaresi E. C.; Hergenrother P. J. Glucose Conjugation for the Specific Targeting and Treatment of Cancer. Chem. Sci. 2013, 4, 2319–2333. 10.1039/c3sc22205e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ido T.; Wan C.-N.; Casella V.; Fowler J. S.; Wolf A. P.; Reivich M.; Kuhl D. E. Labeled 2-Deoxy-D-Glucose Analogs. 18F-Labeled 2-Deoxy-2-Fluoro-D-Glucose, 2-Deoxy-2-Fluoro-D-Mannose and 14C-2-Deoxy-2-Fluoro-D-Glucose. J. Label. Compd. Radiopharm. 1978, 14, 175–183. 10.1002/jlcr.2580140204. [DOI] [Google Scholar]
- Mamede M.; Higashi T.; Kitaichi M.; Ishizu K.; Ishimori T.; Nakamoto Y.; Yanagihara K.; Li M.; Tanaka F.; Wada H.; Manabe T.; Saga T. [18F]FDG Uptake and PCNA, Glut-1, and Hexokinase-II Expressions in Cancers and Inflammatory Lesions of the Lung. Neoplasia 2005, 7, 369–379. 10.1593/neo.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht H. P.; Cutler C. S.; Jurisson S. S.; den Drijver L.; Roodt A. Solid State Study on Rhenium Dimethylphosphinoethane Complexes: X-ray Crystal Structures of Trans-[ReO2(Dmpe)2]PF6·2H2O, Trans-[ReO(OH)(Dmpe)2](CF3SO3)2, Trans-[ReN(Cl)(Dmpe)2]CF3SO3 and Trans-[ReCl2(Dmpe)2]ReO4. Synth. React. Inorg. M. 2005, 35, 83–99. 10.1081/SIM-200047553. [DOI] [Google Scholar]
- Liu S.; Chakraborty S. 99mTc-Centered One-Pot Synthesis for Preparation of 99mTc Radiotracers. Dalton Trans. 2011, 40, 6077–6086. 10.1039/c0dt01462a. [DOI] [PubMed] [Google Scholar]
- Hillier S. M.; Maresca K. P.; Lu G.; Merkin R. D.; Marquis J. C.; Zimmerman C. N.; Eckelman W. C.; Joyal J. L.; Babich J. W. 99mTc-Labeled Small-Molecule Inhibitors of Prostate-Specific Membrane Antigen for Molecular Imaging of Prostate Cancer. J. Nucl. Med. 2013, 54, 1369–1376. 10.2967/jnumed.112.116624. [DOI] [PubMed] [Google Scholar]
- Lepareur N.; Lacœuille F.; Bouvry C.; Hindré F.; Garcion E.; Chérel M.; Noiret N.; Garin E.; Knapp F. F. R. Jr. Rhenium-188 Labeled Radiopharmaceuticals: Current Clinical Applications in Oncology and Promising Perspectives. Front. Med. 2019, 6, 132. 10.3389/fmed.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blower P. J. A Nuclear Chocolate Box: The Periodic Table of Nuclear Medicine. Dalton Trans. 2015, 44, 4819–4844. 10.1039/C4DT02846E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia N. A.; Cullinane C.; Van Zuylekom J. K.; Waldeck K.; McInnes L. E.; Buncic G.; Haskali M. B.; Roselt P. D.; Hicks R. J.; Donnelly P. S. A Bivalent Inhibitor of Prostate Specific Membrane Antigen Radiolabeled with Copper-64 with High Tumor Uptake and Retention. Angew. Chem. 2019, 131, 15133–15136. 10.1002/ange.201908964. [DOI] [PubMed] [Google Scholar]
- Imberti C.; Terry S. Y. A.; Cullinane C.; Clarke F.; Cornish G. H.; Ramakrishnan N. K.; Roselt P.; Cope A. P.; Hicks R. J.; Blower P. J.; Ma M. T. Enhancing PET Signal at Target Tissue in Vivo: Dendritic and Multimeric Tris(hydroxypyridinone) Conjugates for Molecular Imaging of Αvβ3 Integrin Expression with Gallium-68. Bioconjugate Chem. 2017, 28, 481–495. 10.1021/acs.bioconjchem.6b00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.-B.; Cai W.; Cao Q.; Chen K.; Wu Z.; He L.; Chen X. 64Cu-Labeled Tetrameric and Octameric RGD Peptides for Small-Animal PET of Tumor αvβ3 Integrin Expression. J. Nucl. Med. 2007, 48, 1162–1171. 10.2967/jnumed.107.039859. [DOI] [PubMed] [Google Scholar]
- North A. J.; Karas J. A.; Ma M. T.; Blower P. J.; Ackermann U.; White J. M.; Donnelly P. S. Rhenium and Technetium-Oxo Complexes with Thioamide Derivatives of Pyridylhydrazine Bifunctional Chelators Conjugated to the Tumour Targeting Peptides Octreotate and Cyclic-RGDfK. Inorg. Chem. 2017, 56, 9725–9741. 10.1021/acs.inorgchem.7b01247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoloi J. K.; Berry D.; Khan I. U.; Sunassee K.; de Rosales R. T. M.; Shanahan C.; Blower P. J. Technetium-99m and Rhenium-188 Complexes with One and Two Pendant Bisphosphonate Groups for Imaging Arterial Calcification. Dalton Trans. 2015, 44, 4963–4975. 10.1039/C4DT02965H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungnes I. N.; Al-Salemee F.; Gawne P. J.; Eykyn T.; Atkinson R. A.; Terry S. Y. A.; Clarke F.; Blower P. J.; Pringle P. G.; Ma M. T. One-Step, Kit-Based Radiopharmaceuticals for Molecular SPECT Imaging: A Versatile Diphosphine Chelator for 99mTc Radiolabelling of Peptides. Dalton Trans. 2021, 50, 16156–16165. 10.1039/D1DT03177E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder M.; Neels O.; Müller M.; Bauder-Wüst U.; Remde Y.; Schäfer M.; Hennrich U.; Eisenhut M.; Afshar-Oromieh A.; Haberkorn U.; Kopka K. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals 2014, 7, 779–796. 10.3390/ph7070779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsionou M. I.; Knapp C. E.; Foley C. A.; Munteanu C. R.; Cakebread A.; Imberti C.; Eykyn T. R.; Young J. D.; Paterson B. M.; Blower P. J.; Ma M. T. Comparison of Macrocyclic and Acyclic Chelators for Gallium-68 Radiolabelling. RSC Adv. 2017, 7, 49586–49599. 10.1039/C7RA09076E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennrich U.; Eder M. [68Ga]Ga-PSMA-11: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging of Prostate Cancer. Pharmaceuticals 2021, 14, 713. 10.3390/ph14080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen M. L.; Orvig C. 99m-Technetium Carbohydrate Conjugates as Potential Agents in Molecular Imaging. Chem. Commun. 2008, 5077–5091. 10.1039/b809365b. [DOI] [PubMed] [Google Scholar]
- Schibli R.; Dumas C.; Petrig J.; Spadola L.; Scapozza L.; Garcia-Garayoa E.; Schubiger P. A. Synthesis and In Vitro Characterization of Organometallic Rhenium and Technetium Glucose Complexes Against Glut 1 and Hexokinase. Bioconjugate Chem. 2005, 16, 105–112. 10.1021/bc049774l. [DOI] [PubMed] [Google Scholar]
- Ferreira C. L.; Ewart C. B.; Bayly S. R.; Patrick B. O.; Steele J.; Adam M. J.; Orvig C. Glucosamine Conjugates of Tricarbonylcyclopentadienyl Rhenium(I) and Technetium(I) Cores. Inorg. Chem. 2006, 45, 6979–6987. 10.1021/ic0605672. [DOI] [PubMed] [Google Scholar]
- Yang D. J.; Kim C.-G.; Schechter N. R.; Azhdarinia A.; Yu D.-F.; Oh C.-S.; Bryant J. L.; Won J.-J.; Kim E. E.; Podoloff D. A. Imaging with 99mTc ECDG Targeted at the Multifunctional Glucose Transport System: Feasibility Study with Rodents. Radiology 2003, 226, 465–473. 10.1148/radiol.2262011811. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Ruan Q.; Duan X.; Gan Q.; Song X.; Fang S.; Lin X.; Du J.; Zhang J. Novel 99mTc-Labeled Glucose Derivative for Single Photon Emission Computed Tomography: A Promising Tumor Imaging Agent. Mol. Pharmaceutics 2018, 15, 3417–3424. 10.1021/acs.molpharmaceut.8b00415. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Wen Huang Z.; He L.; Long Zheng S.; Lian Li J.; Lian Qin D. Synthesis and Evaluation of a Technetium-99m-Labeled Diethylenetriaminepentaacetate–Deoxyglucose Complex ([99mTc]–DTPA–DG) as a Potential Imaging Modality for Tumors. Appl. Radiat. Isot. 2006, 64, 342–347. 10.1016/j.apradiso.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Xiong Q.; Yang X.; Huang Z.; Zhao Y.; He L. Noninvasive Scintigraphic Detection of Tumor with 99mTc-DTPA-Deoxyglucose: An Experimental Study. Cancer Biother. Radiopharm. 2007, 22, 403–405. 10.1089/cbr.2006.327. [DOI] [PubMed] [Google Scholar]
- Toyama H.; Ichise M.; Liow J.-S.; Modell K. J.; Vines D. C.; Esaki T.; Cook M.; Seidel J.; Sokoloff L.; Green M. V.; Innis R. B. Absolute Quantification of Regional Cerebral Glucose Utilization in Mice by 18F-FDG Small Animal PET Scanning and 2-14C-DG Autoradiography. J. Nucl. Med. 2004, 45, 1398–1405. [PubMed] [Google Scholar]
- Fueger B. J.; Czernin J.; Hildebrandt I.; Tran C.; Halpern B. S.; Stout D.; Phelps M. E.; Weber W. A. Impact of Animal Handling on the Results of 18F-FDG PET Studies in Mice. J. Nucl. Med. 2006, 47, 999–1006. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.