Abstract
The clonal proliferation of antigen-specific T cells during an immune response critically depends on the differential response to growth factors, such as IL-2. While activated T cells proliferate robustly in response to IL-2 stimulation, naïve (quiescent) T cells are able to ignore the potent effects of growth factors because they possess chromatin that is tightly condensed such that transcription factors, such as STAT5, cannot access DNA. Activation via the T cell receptor (TCR) induces a rapid decondensation of chromatin, permitting STAT5-DNA engagement and ultimately promoting proliferation of only antigen-specific T cells. Previous work demonstrated that the mobilization of intracellular calcium following TCR stimulation is a key event in the decondensation of chromatin. Here we examine PKC-dependent signaling mechanisms to determine their role in activation-induced chromatin decondensation and the subsequent acquisition of competence to respond to IL-2 stimulation. We found that a calcium-dependent PKC contributes to activation-induced chromatin decondensation and that the p38 MAPK and NFκB pathways downstream of PKC each contribute to regulating the proper decondensation of chromatin. Importantly, we found that p44/42 MAPK activity is required for peripheral T cells to gain competence to properly respond to IL-2 stimulation. Our findings shed light on the mechanisms that control the clonal proliferation of antigen-specific peripheral T cells during an immune response.
Keywords: chromatin, p44/42 MAPK, Cis, NFκB, p38 MAPK, STAT5
Summary Sentence
A calcium-dependent PKC induces chromatin decondensation via multiple mechanisms and through p44/42 MAPK makes T cells competent to respond to IL-2.
1. INTRODUCTION
Naïve T cells are a class of small, long-lived, metabolically inert, quiescent cells that circulate in the periphery. During an immune response, a T cell becomes activated when a cognate antigen is presented via MHC to the T cell receptor (TCR). T cell activation results in the rapid increase in metabolic rate, the hallmark ‘blasting’ of the cytosol, the acquisition of effector functions, and the ability to proliferate in response to growth factors, such as interleukin-2 (IL-2). It is imperative that only antigen-specific T cells proliferate during an immune response, requiring the existence of a mechanism that prevents the aberrant proliferation of naïve T cells that have not encountered their cognate antigen.
The signaling pathways that regulate T cell activation have been well studied. Antigen presentation to the TCR leads to the Phospholipase C (PLCγ1)-dependent hydrolysis of membrane-bound phosphatidyl 4,5-bisphosphate (PIP2) into inositol triphosphate (IP3) and diacyglycerol (DAG). IP3 is released from the membrane and can bind to the IP3R on the endoplasmic reticulum, releasing calcium from stores. Once these stores are depleted, extracellular calcium enters the cell via store-operated calcium entry (SOCE), resulting in a rapid rise in intracellular calcium [1, 2]. Calcium is a second messenger that can initiate a number of biological processes [3]. In T cells, this rise in calcium leads to the Calmodulin/Calcineurin-dependent dephosphorylation of cytosolic NFAT (Nuclear Factor of Activated T cells), permitting the translocation of NFAT proteins to the nucleus where they can regulate expression of genes required for T cell activation, differentiation, and proliferation [4].
Concurrent to calcium signaling, DAG activates Protein Kinase C (PKC), which is also critical for T cell activation. There are multiple isoforms of PKC, each classified by virtue of how they are activated: conventional PKCs (α, β1, β2, γ) require both DAG and calcium for their activation, novel PKCs (δ, ε, θ, μ, η) require only DAG, and activation of atypical PKCs (ζ, ι/λ) is independent of DAG or calcium [5]. Of these, only three PKC isoforms (α, θ, η) have been shown to positively regulate T cell activation or proliferation [6]. Analysis of PKC-α deficient mice revealed that although PKC-α is not required for T cell activation or the production of IL-2, it is required for T cell proliferation [6, 7]. Studies utilizing PKC-θ deficient mice suggest that it is required for IL-2 production and peripheral T cell proliferation [8]. More recently, it has been shown that PKC-η is required for homeostatic proliferation of peripheral T cells [9]. Of these isoforms, both PKC-θ and PKC-η have been shown to be recruited to the immunological synapse, albeit with different localization patterns, during antigen-specific T cell activation [6, 10]. Interestingly, peripheral T cells from PKC-θ and - PKC-η deficient mice have a defect in calcium flux, suggesting a mechanism of crosstalk between the calcium and PKC signaling pathways during T cell activation [8, 9].
Activation of PKC via TCR signaling ultimately leads to the activation and nuclear localization of critical transcription factors needed for T cell activation [11]. Along with DAG binding to RasGRP, PKC can activate Ras, which has been shown to activate multiple kinase cascades leading to the activation of the p44/42 (Erk1/2) and p38 Mitogen Activated Protein Kinases (MAPK). Ultimately, these signaling cascades lead to the production of AP-1 as well as the nuclear localization of NFκB. Along with NFAT, these transcription factors are required for proper T cell activation and importantly, they can regulate the production of IL-2 [11].
While TCR signaling is critical for inducing the activation of peripheral T cells, proliferation is controlled by IL-2, which signals via the JAK (Janus Kinase)/STAT (Signal Transducer and Activator of Transcription) cascade to drive clonal proliferation of activated T cells [12]. Briefly, IL-2 binds to the IL-2 receptor (IL-2R), bringing receptor-associated JAKs into proximity, resulting in their transphosphorylation. Phosphorylated JAKs can, in turn, phosphorylate STATs that are recruited to docking sites on the IL-2R. Activated STATs can dimerize, and in this form can translocate to the nucleus where they can drive expression of genes required for cell cycle progression [13, 14]. While the mammalian genome encodes a family of 7 STAT proteins, the two highly conserved members STAT5a and STAT5b (hereafter referred to as STAT5) have been shown to be indispensable for T cell proliferation [14].
In order for the clonotypic expansion of only activated T cells to occur, a mechanism must be in place to prevent naïve T cells from proliferating in response to the IL-2 produced by activated T cells as a consequence of TCR signaling. Remarkably, while naïve T cells possess all of the needed machinery to transduce the IL-2 signal, none of the known STAT5 target genes are expressed when naïve T cells are stimulated with IL-2 [13, 15]. This is achieved because naïve T cells possess a highly condensed chromatin that prevents dimerized, nuclear STAT5 from binding to the promoters of target genes. Upon TCR stimulation, chromatin decondenses, permitting STAT5-DNA engagement, resulting in the clonal proliferation of only activated cells in response to subsequent IL-2 stimulation [15].
Previously, we demonstrated that both calcium and DAG signaling can induce the decondensation of chromatin [16]. Given that calcium is known to regulate the activity of conventional PKC isoforms, including PKC-α which is required for proper T cell activation, we became interested in investigating the roles of PKC signaling in regulating chromatin decondensation and the concomitant acquisition of competence to respond to IL-2. Here, we provide data that suggests that PKC signaling is required for T cells to gain competence to properly respond to IL-2 stimulation. Furthermore, we present evidence that suggests that a calcium-dependent isoform of PKC contributes to activation-induced chromatin decondensation. Finally, we interrogated the p38 MAPK, p44/42 MAPK, and NFκB pathways downstream of PKC and determined their contribution to regulating the decondensation of chromatin. Lastly, we show that p44/42 MAPK is required for T cells to gain competence to properly respond to IL-2 stimulation.
2. METHODS
2.1. Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocol was approved by the Furman University Institutional Animal Care and Use Committee (Permit number: A3242–01).
2.2. T cell isolation
Splenocytes were processed as single cell suspensions from 6–8 week old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME, USA). For flow cytometry experiments, red blood cells were removed by ACK lysis (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM EDTA, pH 7.2). In gene expression and Western blot experiments, purified naïve (CD25-) T cells were obtained by magnetic sorting via negative depletion using the Mouse Pan T cell isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) supplemented with biotinylated CD25 antibodies (BD Biosciences, Franklin Lakes, NJ, USA). T cell purity was verified by flow cytometry and always exceeded 95% on the live cell gate.
2.3. T cell activation assays
In all experiments, cells were seeded at 2×106 cells/mL and cultured in T cell media consisting of RPMI 1640 supplemented with 10% FBS, 10mM Hepes (pH 7.0), 2mM L-glutamine, 1mM sodium pyruvate, 1x nonessential amino acids, penicillin, streptomycin (all from ThermoFisher, Waltham, MA, USA), and 50μM β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA). For flow cytometry, 2×106 splenocytes were used per sample. For all other assays 10×106 sorted T cells were used. Cells were stimulated with either 1ug/mL anti-CD3 antibodies (BD Biosciences), 10μg/mL PMA, 1μM thapsigargin (ThermoFisher), or 250ng/mL Ionomycin (Sigma) for the times indicated. In some experiments, cells were pretreated for 30 minutes with the following inhibitors: 10nM Staurosporine, 1μM CID2858522, 100nM 6-Amino-4-(4-phenoxyphenylethylamino)quinazoline (QNZ) (all from MilliporeSigma, Burlington, MA, USA), 10μM AEB071, 5μM SB203580, 0.5μM BIRB796 (all from Selleckchem, Houston, TX, USA), 10μM U0126 (Cell Signaling Technology, Danvers, MA, USA), 10μM BAPTA-AM (ThermoFisher), or DMSO (vehicle) and then activated with either 1ug/mL anti-CD3 antibodies or 10μg/mL PMA for the times indicated. For qRT-PCR experiments, following activation, samples were split in half, with one half (5×106 cells) receiving 1000 U/mL recombinant human IL-2 and the other half receiving no further treatment.
2.4. Gene expression analysis by qRT-CPR
Cells were homogenized with Qiashredder (Qiagen, Hilden, Germany) and RNA was isolated using the RNeasy Plus Mini kit (Qiagen) using the manufacturer’s protocol. Quantitative RT-PCR was done with 25ng of RNA from each sample in triplicate using the TaqMan RNA-to-CT 1 Step Kit (ThermoFisher) on an Eppendorf Realplex following the manufacturer’s protocol. Probes used (all from ThermoFisher) were: Cis (Mm00599683_m1) and CD3ε (Mm00515488_m1). Each assay included a standard curve generated from a five-fold serial dilution (ranging from 250ng to 0.4ng) of total RNA from activated T cells.
Flow Cytometry
Flow cytometry was used to assess chromatin condensation by measuring histone accessibility using our previously published protocol [17]. This assay takes advantage of the fact that fluorescently labelled histone H3 antibodies cannot efficiently access their epitopes in naïve T cells, but upon activation are able to readily bind to their targets [15]. This difference in antibody-epitope binding (and therefore chromatin condensation) can be measured via the mean fluorescence intensity of the H3 stain and reflects the relative degree of chromatin condensation. Briefly, 2×106 splenocytes were loaded into the wells of a 96 well plate and washed with PBS. Dead cells were stained using the LIVE/DEAD Fixable Red Dead Cell Stain Kit (ThermoFisher) using the manufacturer’s protocol. Cells were pelleted and then resuspended in Fc Block (BD Biosciences) and surface stained with anti-CD4 and anti-CD8a (ThermoFisher) antibodies. Cells were washed twice in PBS and fixed in 4% paraformaldehyde for 5 minutes at room temperature. Following two washes in PBS, cells were permeabilized and blocked in PBS containing 2% Fetal Bovine Serum (PBS+2% FBS), 0.02% Triton X-100, and 0.2 μl/ml normal rabbit serum (Sigma) for 45 min at room temperature. Cells were incubated with H3K4me1 antibodies (Abcam, Cambridge, UK) conjugated with PE using the R-Phycoerythrin Conjugation Kit (Abcam) following the manufacturer’s protocol. Cells were washed with PBS+2% FBS and data were acquired on a FACScan flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (FlowJo, Ashland, OR, USA) using the gating strategy shown in Supplementary Figure S1. The PE-labelled Histone H3K4me1 antibody was titered each time it was conjugated and validated for use across a broad dynamic range (Supplementary Figure S2).
Western Blot
Western blot was used to assess chromatin condensation by measuring histone solubility. Previously, we demonstrated that histones from naïve T cells are relatively insoluble in whole-cell lysis buffers but become soluble as a function of activation [15]. This difference reflects chromatin condensation state and can be detected via Western blot. Briefly, 10×106 sorted T cells were lysed in 100μL buffer (1% NP40, 50 mM Tris-HCl, 100 mM NaCl, 50 mM NaF, 40 mM β-glycerophosphate, 1 mM sodium orthovanadate, 5 mM EDTA, pH 7.5) supplemented with mini-Complete protease inhibitors and phosSTOP inhibitors (Roche, Basel, Switzerland) for 30 min at 4°C. Samples were centrifuged and lysates containing soluble proteins were removed and quantified using the BCA Protein Assay (ThermoFisher). Following quantification, an equal volume of Laemmli sample buffer (BioRad, Hercules, CA, USA) was added. To detect Histone H3, 40μg of protein was resolved on a SDS-PAGE and transferred to a nitrocellulose membrane (GE Healthcare Life Sciences, Chicago, IL, USA). Blots were then washed in TBST (10mM Tris pH 8.0, 150 mM NaCl, 0.1% Tween 20) and blocked with TBST supplemented with 5% (w/v) non-fat dry milk. Blots were washed three times in TBST and incubated with primary antibodies diluted in TBST supplemented with 5% (w/v) non-fat dry milk overnight at 4 °C followed by 1 hour at room temperature. Blots were then washed 3 times in TBST and then incubated with anti-rabbit IgG HRP-linked antibody (Cell Signaling Technology) diluted 1:3000 in TBST supplemented with 5% (w/v) non-fat dry milk for 1 hour. Proteins were visualized via ECL detection using the SuperSignal West Pico PLUS kit (ThermoFisher) following the manufacturer’s protocol. Primary antibodies used were Histone H3 (1:5000, Abcam) and Pan-Actin (1:1000, Cell Signaling Technology). In some experiments, blot images were analyzed using ImageJ software to quantify the amount of Histone H3 present relative to the loading control (Actin).
Statistical analysis
Error bars indicate standard deviation. Statistical significance (p<0.05) in pairwise comparisons was determined using a Student’s t-test. All data are representative of at least three independent experiments.
3. RESULTS
3.1. DAG signaling is sufficient to make cells competent to properly respond to IL-2
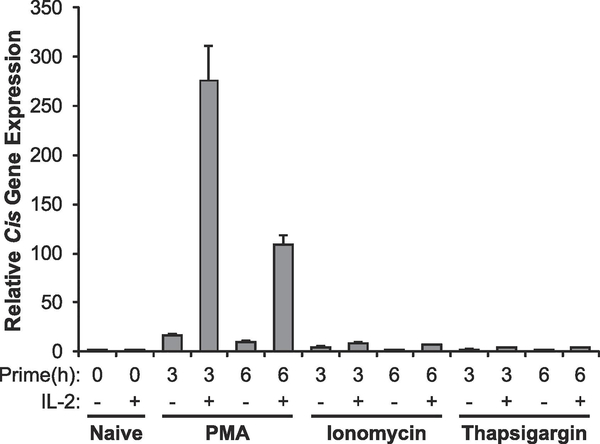
Activation via the TCR results in PLCγ-dependent hydrolysis of PIP2 into IP3 and DAG, which can then activate multiple downstream pathways resulting in the decondensation of chromatin. The phorbol-ester PMA and the calcium ionophore ionomycin can activate the DAG and IP3 signaling pathways, respectively, permitting selective activation of either of these pathways without stimulating the TCR directly [18]. Previously, we showed that either PMA or ionomycin have the ability to induce the decondensation of chromatin in peripheral T cells [16]. Therefore, to determine if the decondensation induced by these signaling pathways is sufficient to make cells competent to respond to IL-2, we primed sorted naïve (CD25-) T cells with either PMA or ionomycin and then stimulated cells with IL-2 and determined IL-2 competence by measuring expression of the well-defined IL-2/STAT5 target gene, Cis (Figure 1). We found that PMA, but not ionomycin, makes peripheral T cells competent to properly respond to IL-2, suggesting that chromatin decondensation via DAG, but not IP3 signaling, is sufficient to impart this competence. We confirmed that calcium signaling alone is unable to confer competence by priming sorted naïve peripheral T cells with thapsigargin. Thapsigargin is a Ca2+-ATPase inhibitor that induces the mobilization of calcium from intracellular stores [19, 20]. Previously, we demonstrated that thapsigargin is able to induce the decondensation of chromatin in peripheral T cells [16]. Like ionomycin, thapsigargin was unable to make cells competent to express the STAT5 target gene Cis (Figure 1). Although our previous work suggests that mobilization of intracellular calcium can decondense chromatin, the above data suggest that it is not able to make T cells competent to properly respond to IL-2 stimulation; rather DAG signaling is required for transcriptional competence.
Figure 1: DAG signaling is sufficient to make cells competent to respond to IL-2.
Naive (CD25-) T cells sorted from spleen were left untreated (naïve) or stimulated with 10ng/mL PMA, 250ng/mL ionomycin, or 1μM thapsigargin for the time indicated. Each sample was divided in half and either left untreated or stimulated with 1000U/mL of IL-2 for 1 hour. Total RNA was isolated and expression of the STAT5 target gene Cis was determined by qRT-PCR relative to the housekeeping gene CD3ε. Data are calibrated to the naïve 0 hour control. Data are representative of three independent experiments.
3.2. PKC signaling is necessary for proper activation-induced chromatin decondensation and the acquisition of competence to respond to IL-2
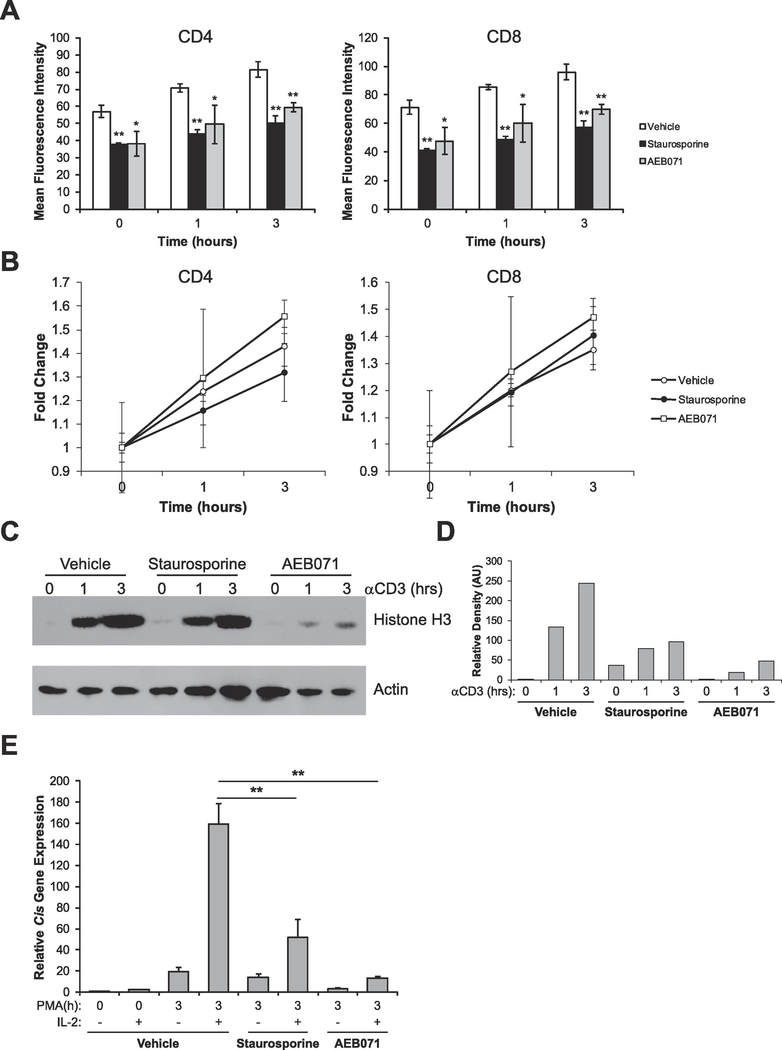
DAG is known to activate Protein Kinase C (PKC) and this event is required for T cell activation and proliferation. Previously, we demonstrated that DAG signaling is able to induce the decondensation of chromatin [16]. Since DAG-induced decondensation also led to the acquisition of competence to respond to IL-2, we wanted to confirm that DAG acts through PKC to mediate these processes. To address this, we used two well-defined PKC inhibitors, staurosporine and AEB071 (also known as sotrastaurin), both of which been shown to inhibit the isoforms of PKC required for T cell activation [21]. Splenocytes treated with these inhibitors were activated with anti-CD3 antibodies and chromatin condensation status was measured by assessing accessibility of histones via flow cytometry [17]. Consistent with our previously published report [16], activation of PKC via PMA treatment induced a significant decondensation of chromatin compared to naïve (0h) in both CD4+ and CD8+ T cells that can be observed at 1 hour (p<0.01, Student’s t-test) and at 3 hours (p<0.005, Student’s t-test) following PKC activation (Figure 2A). Upon TCR activation with anti-CD3 antibodies, inhibitor-treated cells failed to decondense chromatin to the extent of controls (Figure 2A, 1h and 3h time points). However, it should be noted that inhibition of PKC signaling did not completely prevent decondensation from occurring altogether (Figure 2B). To confirm these findings, we measured chromatin condensation by assessing the solubility of histones by Western blot. Consistent with the above, the inhibition of PKC signaling with either inhibitor prevented the full decondensation of chromatin relative to controls (Figure 2C and D). Since the above data suggest that PKC signaling is necessary for proper chromatin decondensation, we investigated the possibility that PKC might also be required to make T cells competent to respond to IL-2. To test this possibility, we treated sorted naïve T cells with the same PKC inhibitors, then primed the cells with PMA to activate PKC, and measured expression of the STAT5 target gene Cis in response to subsequent IL-2 stimulation. Consistent with the above findings, both staurosporine and AEB071 prevented T cells from expressing Cis to the same extent as controls (Figure 2E). Taken together, these data suggest that PKC signaling is necessary for the proper decondensation of chromatin and acquisition of transcriptional competence during initial T cell activation.
Figure 2: PKC signaling contributes to proper chromatin decondensation and acquisition of competence to respond to IL-2.
(A) Splenocytes were treated with vehicle (DMSO), 10nM staurosporine, or 10μM AEB071 for 30 minutes. Cells were then stimulated with 1μg/mL anti-CD3ε antibodies for the times indicated. Cells were then analyzed by flow cytometry to determine chromatin accessibility in CD4+ and CD8+ cells by intracellular staining for H3K4me1. Data are the means ± SD of triplicates and are presented as the mean fluorescence intensity of H3K4me1 staining. *p < 0.05, **p < 0.005 (Student’s t-test) compared with the vehicle control at each time point. (B) Data from A was calibrated to the 0 hour time point for each treatment group to show fold change in chromatin decondensation over time. (C) Sorted naïve (CD25-) T-cells were left untreated or stimulated with vehicle (DMSO), 10nM staurosporine, or 10μM AEB071 for the times indicated. Proteins were resolved via SDS-PAGE and histone solubility was measured via detection of histone H3 as assayed by Western blot. Detection of Actin serves as a loading control. (D) Densitometry analysis of C showing H3 relative to the Actin control (arbitrary units, AU) at each time point, calibrated to the 0h vehicle control. (E) Sorted naive (CD25-) T cells were treated with vehicle (DMSO), 10nM staurosporine, or 10μM AEB071 for 30 minutes. Cells were then stimulated with 10ng/mL PMA for the times indicated. Each sample was then divided in half and either left untreated or stimulated with 1000U/mL of IL-2 for 1 hour. Total RNA was isolated and expression of the STAT5 target gene Cis was determined by qRT-PCR relative to the housekeeping gene CD3ε. Data are calibrated to the naïve 0 hour control. **p<0.005 (Student’s t-test). All data are representative of at least 3 independent experiments.
3.3. A calcium-dependent PKC is necessary for DAG-mediated initiation of chromatin decondensation and competence to respond to IL-2
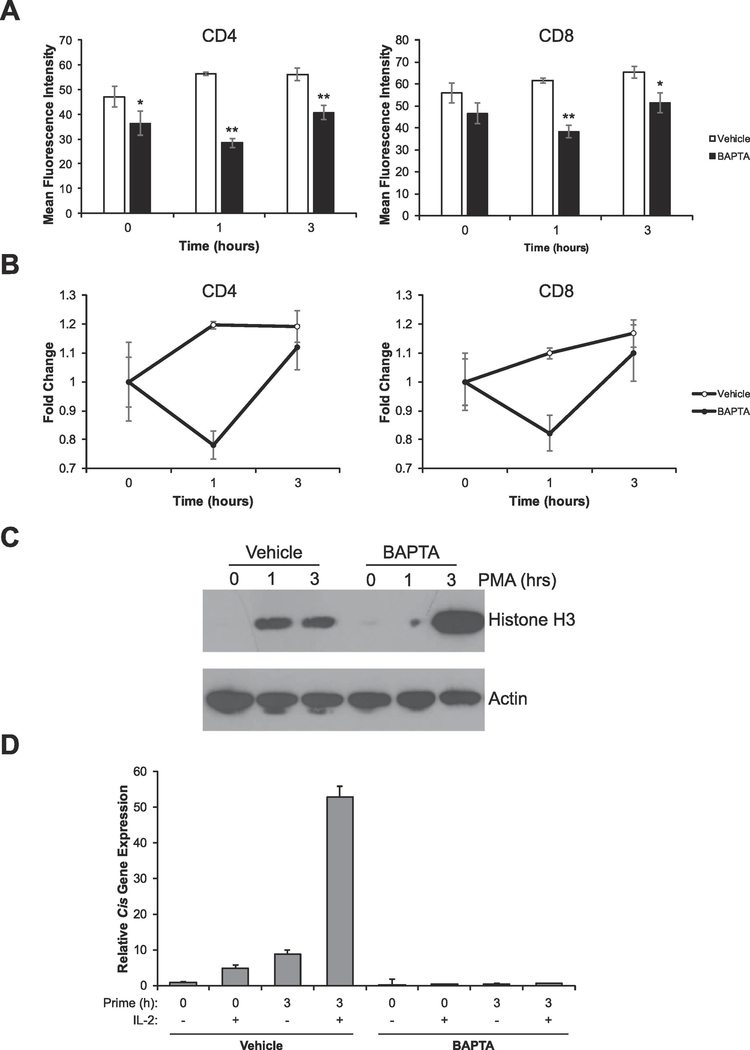
There are three PKC isoforms (PKC-α,-θ, and -η) known to positively regulate T cell activation or proliferation, one of which (PKC-α) requires calcium for its activation. Therefore, we wanted to determine if the PKC isoform responsible for initiating activation-induced chromatin decondensation is calcium-dependent. To test this hypothesis, we used BAPTA-AM to chelate intracellular calcium. BAPTA-AM has been shown to prevent the transient increase of [Ca2+]i caused by the release of calcium from intracellular stores and has also been shown to block activation-induced T cell proliferation [22–24]. Previously, we demonstrated that this reagent can block TCR-induced chromatin decondensation [16]. To test whether calcium is required for PKC-dependent initiation of chromatin decondensation, splenocytes were treated with BAPTA-AM and chromatin condensation was measured via flow cytometry in response to PKC activation via PMA. Remarkably, despite the activation of PKC signaling, the chelation of intracellular calcium caused chromatin to condense further at the 1 hour time point compared to controls (Figure 3A, 3B). However, by 3 hours, chromatin began decondensing but was still significantly less decondensed than controls (Figure 3A). To confirm these findings, we chelated intracellular calcium with BAPTA-AM in sorted naïve T cells and then activated PKC directly with PMA and assayed chromatin condensation via Western blot. Consistent with the above, chelation of intracellular calcium prevented the initial decondensation of chromatin (Figure 3C, 1h time point); however, by 3 hours, chromatin decondensed (Figure 3C, 3h time point). To provide further confirmation that calcium is required for PKC-mediated initiation of chromatin decondensation, we assessed whether intracellular calcium is required for T cells to gain competence to properly respond to IL-2 stimulation. Chelation of intracellular calcium completely prevented Cis gene expression in response to IL-2 stimulation (Figure 3D). Taken together, all of the above data suggest that a calcium-dependent PKC is necessary for the proper decondensation of chromatin and subsequent competence to respond to IL-2.
Figure 3: A calcium-dependent PKC is required for proper chromatin decondensation and acquisition of competence to respond to IL-2.
(A) Splenocytes were treated with vehicle (DMSO) or 10μM BAPTA-AM for 30 minutes. Cells were then stimulated with 10ng/mL PMA for the times indicated. Cells were then analyzed by flow cytometry to determine chromatin accessibility in CD4+ and CD8+ cells by intracellular staining for H3K4me1. Data are the means ± SD of triplicates and are presented as the mean fluorescence intensity of H3K4me1 staining. *p < 0.05, **p < 0.001 (Student’s t-test) compared with the vehicle control at each time point. (B) Data from A was calibrated to the 0 hour time point to show fold change in chromatin decondensation over time. (C) Sorted naïve (CD25-) T-cells were left untreated or stimulated with vehicle (DMSO) or 10μM BAPTA-AM for 30 minutes. Cells were then stimulated with 10ng/mL PMA for the times indicated. Proteins were resolved via SDS-PAGE and histone solubility was measured via detection of histone H3 as assayed by Western blot. Detection of Actin serves as a loading control. (D) Sorted naive (CD25-) T cells were left untreated (naïve) or treated with vehicle (DMSO) or 10μM BAPTA-AM for 30 minutes, then cells were stimulated with 10ng/mL PMA for the times indicated. Each sample was then divided in half and either left untreated or stimulated with 1000U/mL of IL-2 for 1 hour. Total RNA was isolated and expression of the STAT5 target gene Cis was determined by qRT-PCR relative to the housekeeping gene CD3ε. Data were calibrated to the naïve 0 hour control. All data are representative of at least 3 independent experiments.
3.4. p38 MAPK contributes to PKC-mediated decondensation of chromatin and the acquisition of competence to respond to IL-2
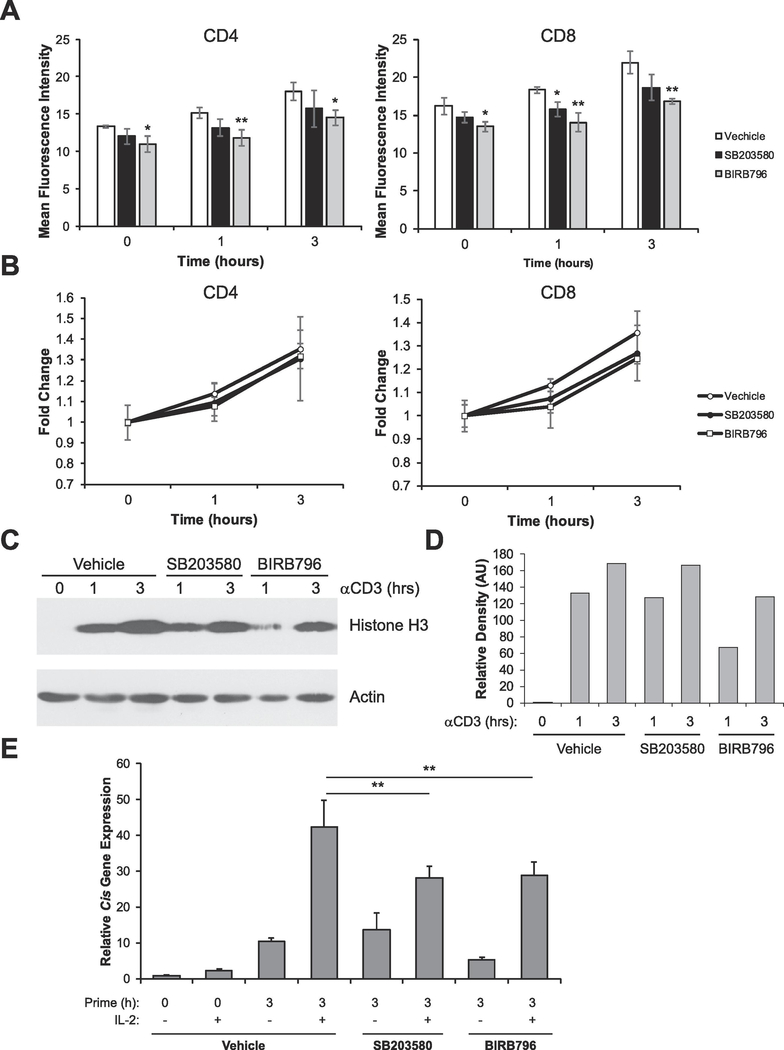
The activation of PKC leads to the subsequent activation of several signaling cascades required for T cell activation and proliferation, including p38 MAPK, NFκB and p44/42 MAPK [11]. Recently, it was shown that p38 MAPK contributed to chromatin decondensation in a mouse model of hyperglycemia [25]. Therefore, we wanted to determine if PKC-mediated chromatin decondensation was dependent on p38 MAPK. To address this question, we utilized SB203580 and BIRB796 to inhibit p38 MAPK in splenocytes and then assessed TCR-mediated chromatin decondensation. Both inhibitors have been shown to potently inhibit p38 MAPK in T cells [26–29]. We found that inhibition of p38 MAPK with BIRB796 modestly, but significantly, prevented proper chromatin decondensation, whereas SB203580 did not have much of an effect (Figure 4A and B). These results were confirmed by Western blot analysis (Figure 4C and D). Consistent with the above findings, inhibition of p38 MAPK with either inhibitor modestly, but significantly, diminished competence to respond to IL-2 (Figure 4E). Taken together, these data suggest that p38 MAPK signaling contributes to activation-induced chromatin decondensation.
Figure 4: p38 MAPK signaling contributes to activation-induced chromatin decondensation and acquisition of competence to respond to IL-2.
(A) Splenocytes were treated with vehicle (DMSO), 5μM SB203580, or 0.5μM BIRB796 for 30 minutes. Cells were then stimulated with 1μg/mL anti-CD3ε antibodies for the times indicated. Cells were then analyzed by flow cytometry to determine chromatin accessibility in CD4+ and CD8+ cells by intracellular staining for H3K4me1. Data are the means ± SD of triplicates and are presented as the mean fluorescence intensity of H3K4me1 staining. *p < 0.05, **p < 0.005 (Student’s t-test) compared with vehicle control at each time point. (B) Data from A was calibrated to the 0 hour time point to show fold change in chromatin decondensation over time. (C) Sorted naïve (CD25-) T-cells were left untreated or stimulated with vehicle (DMSO), 5μM SB203580, or BIRB796 for 30 minutes. Cells were then stimulated with 1μg/mL anti-CD3ε antibodies for the times indicated. Proteins were resolved via SDS-PAGE and histone solubility was measured via detection of histone H3 as assayed by Western blot. Detection of Actin serves as a loading control. (D) Densitometry analysis of C showing H3 relative to the Actin control (arbitrary units, AU) at each time point, calibrated to the 0h vehicle control. (E) Sorted naive (CD25-) T cells were left untreated (naïve) or treated with vehicle (DMSO), 5μM SB203580, or 0.5μM BIRB796 for 30 minutes, then cells were stimulated with 10ng/mL PMA for the times indicated. Each sample was then divided in half and either left untreated or stimulated with 1000U/mL of IL-2 for 1 hour. Total RNA was isolated and expression of the STAT5 target gene Cis was determined by qRT-PCR relative to the housekeeping gene CD3ε. Data are calibrated to the naïve 0 hour control. **p < 0.001 (Student’s t-test) compared with vehicle control. All data are representative of at least 3 independent experiments.
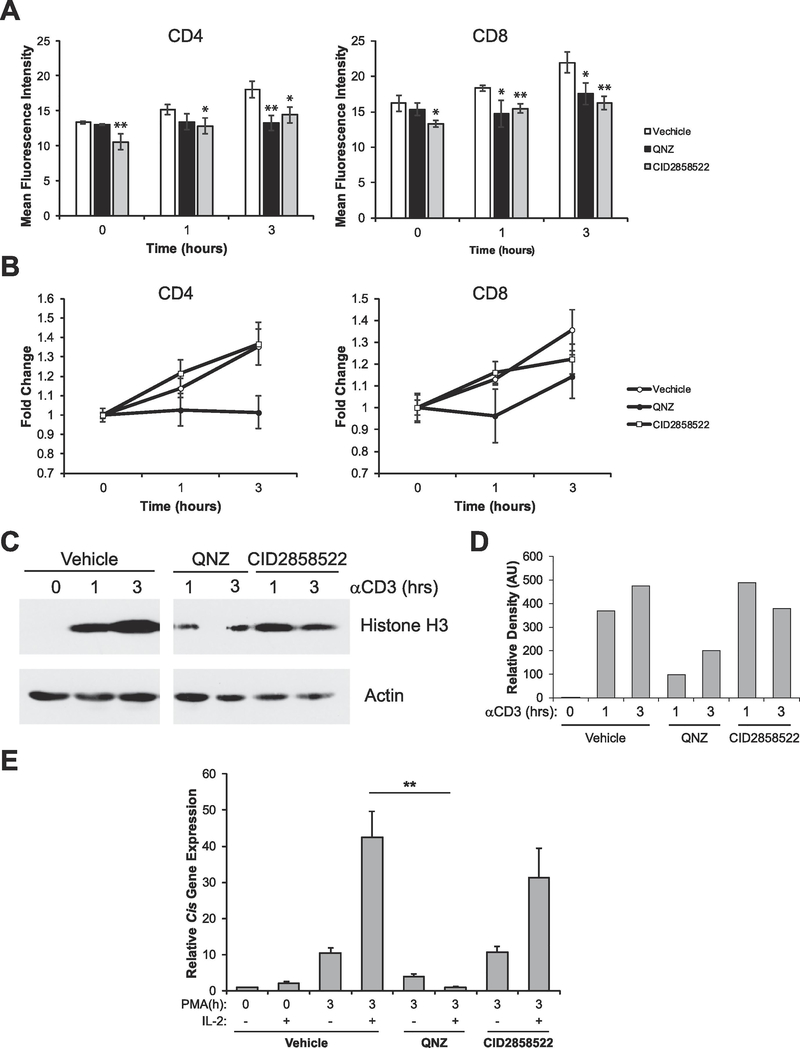
3.5. NFκB contributes to PKC-mediated chromatin decondensation and the acquisition of competence to respond to IL-2
PKC activation initiates a signaling cascade that ultimately leads to the dissociation of NFκB from IκB, permitting its translocation from the cytosol to the nucleus where it can modulate expression of genes required for T cell activation and proliferation [11]. To determine if PKC acts through NFκB to decondense chromatin, we utilized two potent NFκB inhibitors, 6-Amino-4-(4-phenoxyphenylethylamino)quinazoline (QNZ, also known as EVP4593) and CID2858522, both of which have been shown to inhibit NFκB activity in lymphocytes [30–33]. Inhibition of NFκB with either inhibitor significantly prevented the full decondensation of chromatin following TCR stimulation compared to controls as assessed by flow cytometry (Figure 5A, 3h time points). Furthermore, QNZ appeared to completely block PKC-induced decondensation in CD4+ T cells (Figure 5B). To confirm the above, we assessed chromatin decondensation by Western blot on sorted naïve (CD25-) T cells. Inhibition with CID2858522 did not appear to have much of an effect on chromatin decondensation, whereas inhibition with QNZ partially inhibited activation-induced chromatin decondensation. Finally, we assessed the ability of the NFκB inhibitors to prevent the acquisition of competence to respond to IL-2. Consistent with the above data, inhibition with QNZ but not CID2858522, prevented the cells from gaining competence to respond to IL-2 (Figure 5E). The discordant results obtained with QNZ and CID2858522 can be explained by the fact that it has been shown that QNZ can block Ca2+ flux [34]. As CID2858522 showed some effect on decondensation (Figure 5A), our data suggest that NFκB may contribute to activation-induced chromatin decondensation.
Fig. 5.
NFκB signaling contributes to activation-induced chromatin decondensation but does not contribute to acquisition of competence to respond to IL-2. (A) Splenocytes were treated with vehicle (DMSO), 100 nM QNZ, or 1 mM CID2858522 for 30 min. Cells were then stimulated with 1 μg/mL anti-CD3ε antibodies for the times indicated. Cells were then analyzed by flow cytometry to determine chromatin accessibility in CD4+ and CD8+ cells by intracellular staining for H3K4me1. Data are the means ± SD of triplicates and are presented as the mean fluorescence intensity of H3K4me1 staining. (B) Data from A was calibrated to the 0 h time point to show fold change in chromatin decondensation over time. (C) Sorted naïve (CD25-) T-cells were left untreated or stimulated with vehicle (DMSO), 100 nM QNZ, or 1 mM CID2858522 for 30 min. Cells were then stimulated with 1 μg/mL anti-CD3ε antibodies for the times indicated. Proteins were resolved via SDS-PAGE and histone solubility was measured via detection of histone H3 as assayed by Western blot. Detection of Actin serves as a loading control. (D) Sorted naive (CD25-) T cells were left untreated (naïve) or treated with vehicle (DMSO), 100 nM QNZ, or 1 mM CID2858522 for 30 min, then cells were stimulated with 10 ng/mL PMA for the times indicated. Each sample was then divided in half and either left untreated or stimulated with 1000U/mL of IL-2 for 1 h. Total RNA was isolated and expression of the STAT5 target gene Cis was determined by qRT-PCR relative to the housekeeping gene CD3ε. All data are calibrated to the naïve 0 h control. **p < 0.001 (Student’s t-test) compared with vehicle control. All data are representative of at least 3 independent experiments.
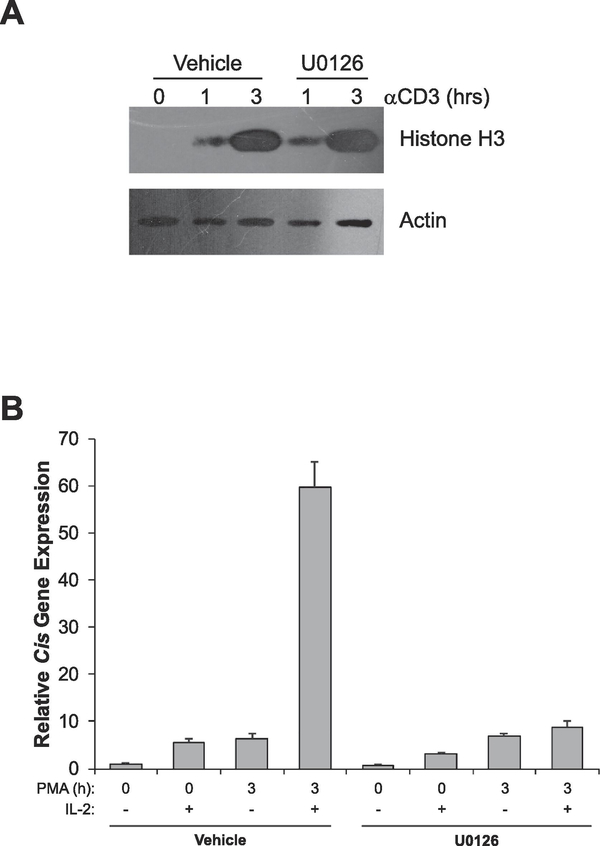
3.6. p44/42 MAPK does not play a role in chromatin decondensation but is required for competence to respond properly to IL-2 signaling
The p44/42 MAPK (ERK1/2) signaling cascade is activated downstream of PKC during T cell activation. Activation of this pathway ultimately assembles the AP-1 transcription factor which is required for T cell activation and proliferation [11]. Therefore, we sought to determine whether p44/42 MAPK signaling plays a role in chromatin decondensation and/or the acquisition of competence to respond to IL-2 signaling. To test this possibility, we used U0126, a well-defined inhibitor of p44/42 MAPK that has been shown to block T cell activation [35]. In sorted naïve T cells pretreated with U0126, chromatin decondensed similar to controls when activated with anti-CD3 antibodies, suggesting that MAPK signaling plays no role in activation-induced decondensation of chromatin (Figure 6A). However, inhibition of MAPK signaling almost completely blocked the ability of these cells to respond to IL-2 stimulation (Figure 6B), suggesting that p44/42 MAPK signaling is essential for T cells to properly respond to IL-2 stimulation.
Fig. 6.
p44/42 MAPK signaling does not contribute to activation-induced chromatin decondensation but is required for peripheral T cells to acquire competence to respond to growth factors. (A) Sorted naïve (CD25-) T-cells were left untreated or stimulated with vehicle (DMSO) or 10 μM U0126 for 30 min. Cells were then stimulated with 1 μg/mL anti-CD3ε antibodies for the times indicated. Proteins were resolved via SDS-PAGE and histone solubility was measured via detection of histone H3 as assayed by Western blot. Detection of Actin serves as a loading control. (D) Sorted naive (CD25-) T cells were left untreated (naïve) or treated with vehicle (DMSO) or 10 μM U0126 for 30 min, then cells were stimulated with 10 ng/mL PMA for the times indicated. Each sample was then divided in half and either left untreated or stimulated with 1000U/mL of IL-2 for 1 h. Total RNA was isolated and expression of the STAT5 target gene Cis was determined by qRT-PCR relative to the housekeeping gene CD3ε. Data are calibrated to the naïve 0 h control. All data are representative of at least 3 independent experiments.
4. DISCUSSION
A proper immune response critically depends on the clonal proliferation of only those T cells that bear a TCR specific for antigen. Activation-induced exit from quiescence is achieved through the reconfiguration of chromatin as a consequence of TCR signaling. Specifically, naïve T cells possess a condensed chromatin that is generally inaccessible to transcription factors, such as STAT5. Upon activation, the chromatin decondenses, permitting STAT5 to bind to promoters of genes required for cell cycle progression. This mechanism provides a simple, yet efficient means to regulate proliferation during an immune response in such a way that only those cells that have been activated will proliferate when exposed to growth factors.
The mechanism by which TCR signaling induces the decondensation of chromatin is not clear. Previously, we demonstrated that mobilization of intracellular calcium is both required and sufficient to induce the decondensation of chromatin in peripheral T cells [16]. In that report, we also noted that DAG signaling is also capable of inducing the decondensation of chromatin. Here, we show that DAG, but not calcium, is required to make T cells competent to properly respond to IL-2 stimulation (Figure 1), as assessed by examining expression of the well-defined STAT5 target gene Cis [13, 14, 36]. This finding suggests that both calcium and DAG signaling could have distinct roles in regulating chromatin decondensation. Alternatively, it is possible that once chromatin is decondensed via a calcium flux-dependent mechanism, additional mechanisms, dependent on the DAG pathway, may be required for subsequent competence to express STAT5 target genes. We confirmed the above by demonstrating that DAG induced decondensation is dependent on PKC (Figure 2A–D) and that PKC is required to make T cells competent to respond to IL-2 (Figure 2E).
We then investigated the possibility that calcium and PKC signaling might work cooperatively to facilitate the decondensation of chromatin, as PKC-α is known to both positively regulate T cell activation and is dependent on calcium for its function [5]. Using the calcium chelator BAPTA-AM to sequester intracellular calcium, we demonstrated that calcium is required for the initial decondensation of chromatin induced by PKC (Figure 3A, B, C, 1 hour time points). Furthermore, we noted that the addition of BAPTA-AM caused chromatin to condense further than controls, consistent with our previous observation [16], suggesting that chromatin status is extremely sensitive to changes in intracellular calcium concentration. We also noted that by 3 hours, chromatin began decondensing. This result was expected as while it is known that BAPTA-AM can sequester calcium released from stores, the subsequent influx of extracellular calcium (via store-operated calcium entry, SOCE) overwhelms the buffering capacity of this reagent [22]. This result is also consistent with our previous study in which BAPTA-AM initially prevented chromatin decondensation in T cells that were activated with anti-CD3 antibodies [16]. Taken together, the above observations suggest that a calcium-dependent PKC (most likely PKC-α) is required for the proper initiation of chromatin decondensation following T cell activation. To confirm this finding we showed that intracellular calcium also is required to make cells competent to properly respond to IL-2 signaling (Figure 3D).
Given that PKC signaling is important for chromatin decondensation and subsequent competence to respond to IL-2 signaling, we investigated the downstream pathways to determine how PKC is able to regulate the initiation of the decondensation event. In this report, we analyzed p38 MAPK, NFκB, and p44/42 MAPK signaling pathways (Figures 4, 5, 6) and found that inhibition of NFκB and p38 MAPK individually had a modest effect on TCR-induced chromatin decondensation. These findings are similar to our previous study where we examined the role of NFAT activation and found that it too had a modest effect on activation-induced chromatin decondensation [16]. Collectively, these findings could suggest that it is the combinatorial effect of all of these pathways that is required to properly induce the complete decondensation of chromatin observed during T cell activation. This model could predict that each pathway might decondense a distinct domain of chromatin, which we cannot discern using our assays because they are designed to measure global changes in chromatin status, not to observe decondensation at specific loci. Alternatively, PKC could decondense chromatin independently of the classical downstream signaling cascades, as there is growing evidence implicating multiple PKC isoforms in the direct regulation of chromatin [5].
Throughout our studies we noted that T cell chromatin appears to be exquisitely sensitive to perturbations in signaling cascades. For example, the inhibition of PKC signaling (Figure 2A) or the simple chelation of intracellular calcium (Figure 3A) caused chromatin to adopt an even more condensed state. This level of condensation is reminiscent of what is observed during thymocyte development, where chromatin is more condensed in single positive thymocytes than it is in mature peripheral T cells [15]. We hypothesize that this sensitivity reflects the ability of T cells to rapidly change their chromatin configuration in response to stimuli. In support of this hypothesis, decondensation of chromatin can be observed in as little as 15 minutes following TCR stimulation [15].
In our studies, we noted that while inhibition of PKC greatly diminished the ability of TCR signaling to decondense chromatin, some decondensation still occurred (Figure 2), suggesting that calcium may have additional PKC-independent means to regulate chromatin architecture. One way this could be accomplished is via the action of Cohesin, a SMC (Structural Maintenance of Chromosomes) protein complex that is a known regulator of chromatin condensation [37]. It has been shown that calcium can regulate the activity of the Cohesin complex by at least two distinct mechanisms [38–40]. Functional Cohesin can regulate chromatin architecture through its interactions with CTCF, which has been implicated as an insulator for “super-enhancers” [41–44]. Intriguingly, the Cis gene possesses a potent STAT5 super-enhancer [36]. As an alternative mechanism, calcium could regulate the activity of the SWI/SNF-like BAF (Brahma-related gene/Brahma associated factor) complex, as it has been shown that the addition of calcium is sufficient to induce the rapid association of this complex with chromatin in T cell nuclei [45]. It has since been shown that the BAF chromatin remodeling complex can regulate gene expression in T cells in a number of contexts, including during activation [46].
Here we demonstrate that DAG acts via a calcium-dependent PKC to make cells competent to properly respond to IL-2/STAT5 signaling as assessed by measuring Cis gene expression. Consistent with our findings, PKC signaling has been implicated in the regulation of other known STAT5 target genes, including Cyclin D2, Cyclin D3, Cdk6, and Bcl-XL [47–51]. More specifically, we demonstrate that p44/42 MAPK activity, presumably via AP-1, is required for Cis expression in response to IL-2 stimulation. A number of studies have implicated p44/42 MAPK activity in the regulation of other known STAT5 target genes, including Ifng, Socs-1, and Socs-3 [8, 52–54]. Interestingly, although several of these genes, along with Cis, contain a STAT5 super-enhancer [36], our data indicate that additional factors (e.g. AP-1) may be required for STAT5 target gene expression in T cells. Consistent with these observations, it has been shown that AP-1 can promote chromatin accessibility in multiple contexts [55, 56], by acting as a pioneer transcription factor that can recruit the BAF chromatin remodeling complex to displace nucleosomes at enhancers permitting their function [57].
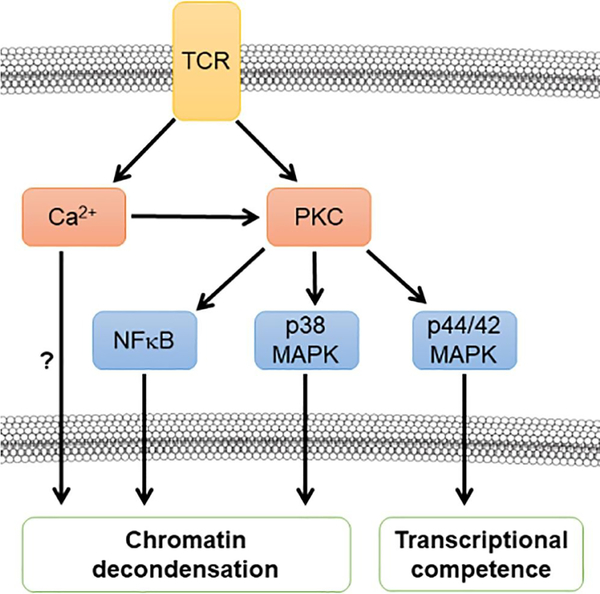
Taken into context with published literature, our data suggest a model for regulation of IL-2/STAT5 target genes in peripheral T cells, whereby TCR signaling triggers multiple pathways that can initiate chromatin decondensation (Figure 7). Our findings suggest that both calcium- and PKC-dependent changes to chromatin architecture may be necessary to permit subsequent STAT5-DNA engagement in response to IL-2 stimulation. This model is in line with a recent report that demonstrated that the combinatorial effect of calcium and TCR-dependent kinase signaling act cooperatively to induce chromatin remodeling at approximately 2100 distinct regions during T cell activation, whereas far fewer sites were remodeled when these signals were provided separately [58]. Understanding how the integration of multiple signaling cascades can regulate chromatin architecture to control gene expression is key to understanding how cells can differentially respond to stimuli, such as what occurs during the clonal proliferation of antigen-specific lymphocytes during an immune response.
Figure 7: Model for TCR induced chromatin decondensation and subsequent acquisition of competence to respond to IL-2 signaling.
Antigen presentation to the TCR induces the activation of Ca2+-dependent and PKC-dependent signaling cascades. Our data suggest that a calcium-dependent PKC acts via NFκB and p38 MAPK to contribute to chromatin decondensation. It is likely that there are additional calcium-dependent, PKC-independent mechanisms contributing to chromatin decondensation. Following chromatin decondensation, our data suggest that PKC acts via p44/42 MAPK to impart competence to respond to IL-2 signaling.
Supplementary Material
HIGHLIGHTS.
Chromatin decondensation is required for IL-2 dependent T cell proliferation
DAG but not IP3 signaling makes naïve T cells competent to respond to IL-2
A calcium-dependent PKC is necessary for proper chromatin decondensation
p38 MAPK and NFκB pathways contribute to chromatin decondensation
p44/42 MAPK signaling is required for IL-2 competence
ACKNOWLEDGEMENTS
This project was supported by grants from the National Institutes of Health (5 P20 RR016461) and the National Science Foundation (EPS-0903795). Further support provided by Furman University’s Research and Professional Growth (RPG), Furman Advantage awards, and Furman’s Office for Integrative Research in the Sciences.
ABBREVIATIONS
- Cis
Cytokine Inducible SH2 containing protein
- DAG
Diacylglycerol
- IL-2
Interleukin-2
- IP3
Inositol Triphosphate
- JAK
Janus Kinase
- MAPK
Mitogen Acdtivated Protein Kinase
- MHC
Major Histocompatibility Complex
- NFAT
Nuclear Factor of Activated T cells
- NFκB
Nuclear Factor Kappa B
- PKC
Protein Kinase C
- PLCγ
Phospholipase C gamma
- PMA
Phorbol 12-myristate 13-acetate
- QNZ
6-Amino-4-(4-phenoxyphenylethylamino)quinazoline
- SOCE
Store Operated Calcium Entry
- STAT5
Signal Transducer and Activator of Transcription 5
- TCR
T Cell Receptor
Footnotes
DISCLOSURES
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Feske S, Calcium signalling in lymphocyte activation and disease, Nat Rev Immunol, 7 (2007) 690–702. [DOI] [PubMed] [Google Scholar]
- [2].Hogan PG, Lewis RS, Rao A, Molecular basis of calcium signaling in lymphocytes: STIM and ORAI, Annu Rev Immunol, 28 (2010) 491–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Berridge MJ, Lipp P, Bootman MD, The versatility and universality of calcium signalling, Nat Rev Mol Cell Biol, 1 (2000) 11–21. [DOI] [PubMed] [Google Scholar]
- [4].Macian F, NFAT proteins: key regulators of T-cell development and function, Nat Rev Immunol, 5 (2005) 472–484. [DOI] [PubMed] [Google Scholar]
- [5].Lim PS, Sutton CR, Rao S, Protein kinase C in the immune system: from signalling to chromatin regulation, Immunology, 146 (2015) 508–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fu G, Gascoigne NR, The role of protein kinase ceta in T cell biology, Front Immunol, 3 (2012) 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maly K, Strese K, Kampfer S, Ueberall F, Baier G, Ghaffari-Tabrizi N, Grunicke HH, Leitges M, Critical role of protein kinase C alpha and calcium in growth factor induced activation of the Na(+)/H(+) exchanger NHE1, FEBS Lett, 521 (2002) 205–210. [DOI] [PubMed] [Google Scholar]
- [8].Pfeifhofer C, Kofler K, Gruber T, Tabrizi NG, Lutz C, Maly K, Leitges M, Baier G, Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells, J Exp Med, 197 (2003) 1525–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fu G, Hu J, Niederberger-Magnenat N, Rybakin V, Casas J, Yachi PP, Feldstein S, Ma B, Hoerter JA, Ampudia J, Rigaud S, Lambolez F, Gavin AL, Sauer K, Cheroutre H, Gascoigne NR, Protein kinase C eta is required for T cell activation and homeostatic proliferation, Sci Signal, 4 (2011) ra84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Isakov N, Altman A, Protein kinase C(theta) in T cell activation, Annu Rev Immunol, 20 (2002) 761–794. [DOI] [PubMed] [Google Scholar]
- [11].Lin J, Weiss A, T cell receptor signalling, J Cell Sci, 114 (2001) 243–244. [DOI] [PubMed] [Google Scholar]
- [12].Rawlings JS, Rosler KM, Harrison DA, The JAK/STAT signaling pathway, J Cell Sci, 117 (2004) 1281–1283. [DOI] [PubMed] [Google Scholar]
- [13].Gatzka M, Piekorz R, Moriggl R, Rawlings J, Ihle JN, A role for STAT5A/B in protection of peripheral T-lymphocytes from postactivation apoptosis: insights from gene expression profiling, Cytokine, 34 (2006) 143–154. [DOI] [PubMed] [Google Scholar]
- [14].Moriggl R, Topham DJ, Teglund S, Sexl V, McKay C, Wang D, Hoffmeyer A, van Deursen J, Sangster MY, Bunting KD, Grosveld GC, Ihle JN, Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells, Immunity, 10 (1999) 249–259. [DOI] [PubMed] [Google Scholar]
- [15].Rawlings JS, Gatzka M, Thomas PG, Ihle JN, Chromatin condensation via the condensin II complex is required for peripheral T-cell quiescence, EMBO J, 30 (2011) 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee MD, Bingham KN, Mitchell TY, Meredith JL, Rawlings JS, Calcium mobilization is both required and sufficient for initiating chromatin decondensation during activation of peripheral T-cells, Mol Immunol, 63 (2015) 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bingham KN, Lee MD, Rawlings JS, The Use of Flow Cytometry to Assess the State of Chromatin in T Cells, J Vis Exp, (2015) e53533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nobrega AF, Maldonado MS, Dos Reis GA, Analysis of isolated and combined effects of calcium ionophore and phorbol ester on T lymphocyte activation, Clin Exp Immunol, 65 (1986) 559–569. [PMC free article] [PubMed] [Google Scholar]
- [19].Lytton J, Westlin M, Hanley MR, Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps, J Biol Chem, 266 (1991) 17067–17071. [PubMed] [Google Scholar]
- [20].Zweifach A, Lewis RS, Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores, Proc Natl Acad Sci U S A, 90 (1993) 6295–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Baier G, Wagner J, PKC inhibitors: potential in T cell-dependent immune diseases, Curr Opin Cell Biol, 21 (2009) 262–267. [DOI] [PubMed] [Google Scholar]
- [22].Gelfand EW, Cheung RK, Mills GB, Grinstein S, Uptake of extracellular Ca2+ and not recruitment from internal stores is essential for T lymphocyte proliferation, Eur J Immunol, 18 (1988) 917–922. [DOI] [PubMed] [Google Scholar]
- [23].Saxena HM, Dikshit M, Abrogation of DTH response and mitogenic lectin- and alloantigen-induced activation of lymphocytes by calcium inhibitors TMB-8 and BAPTA-AM, Immunol Lett, 101 (2005) 60–64. [DOI] [PubMed] [Google Scholar]
- [24].Tsien RY, New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures, Biochemistry, 19 (1980) 2396–2404. [DOI] [PubMed] [Google Scholar]
- [25].Martinez N, Vallerskog T, West K, Nunes-Alves C, Lee J, Martens GW, Behar SM, Kornfeld H, Chromatin decondensation and T cell hyperresponsiveness in diabetes-associated hyperglycemia, Journal of immunology, 193 (2014) 4457–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Crawley JB, Rawlinson L, Lali FV, Page TH, Saklatvala J, Foxwell BM, T cell proliferation in response to interleukins 2 and 7 requires p38MAP kinase activation, The Journal of biological chemistry, 272 (1997) 15023–15027. [DOI] [PubMed] [Google Scholar]
- [27].Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC, SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1, FEBS Lett, 364 (1995) 229–233. [DOI] [PubMed] [Google Scholar]
- [28].Kuma Y, Sabio G, Bain J, Shpiro N, Marquez R, Cuenda A, BIRB796 inhibits all p38 MAPK isoforms in vitro and in vivo, The Journal of biological chemistry, 280 (2005) 19472–19479. [DOI] [PubMed] [Google Scholar]
- [29].Di Mitri D, Azevedo RI, Henson SM, Libri V, Riddell NE, Macaulay R, Kipling D, Soares MV, Battistini L, Akbar AN, Reversible senescence in human CD4+CD45RA+CD27-memory T cells, Journal of immunology, 187 (2011) 2093–2100. [DOI] [PubMed] [Google Scholar]
- [30].Okolotowicz KJ, Shi R, Zheng X, MacDonald M, Reed JC, Cashman JR, Selective benzimidazole inhibitors of the antigen receptor-mediated NF-kappaB activation pathway, Bioorganic & medicinal chemistry, 18 (2010) 1918–1924. [DOI] [PubMed] [Google Scholar]
- [31].Peddibhotla S, Shi R, Khan P, Smith LH, Mangravita-Novo A, Vicchiarelli M, Su Y, Okolotowicz KJ, Cashman JR, Reed JC, Roth GP, Inhibition of protein kinase C-driven nuclear factor-kappaB activation: synthesis, structure-activity relationship, and pharmacological profiling of pathway specific benzimidazole probe molecules, J Med Chem, 53 (2010) 4793–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shi R, Re D, Dudl E, Cuddy M, Okolotowicz KJ, Dahl R, Su Y, Hurder A, Kitada S, Peddibhotla S, Roth GP, Smith LH, Kipps TJ, Cosford N, Cashman J, Reed JC, Chemical biology strategy reveals pathway-selective inhibitor of NF-kappaB activation induced by protein kinase C, ACS chemical biology, 5 (2010) 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tobe M, Isobe Y, Tomizawa H, Nagasaki T, Takahashi H, Fukazawa T, Hayashi H, Discovery of quinazolines as a novel structural class of potent inhibitors of NF-kappa B activation, Bioorganic & medicinal chemistry, 11 (2003) 383–391. [DOI] [PubMed] [Google Scholar]
- [34].Vigont V, Nekrasov E, Shalygin A, Gusev K, Klushnikov S, Illarioshkin S, Lagarkova M, Kiselev SL, Kaznacheyeva E, Patient-Specific iPSC-Based Models of Huntington’s Disease as a Tool to Study Store-Operated Calcium Entry Drug Targeting, Front Pharmacol, 9 (2018) 696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].DeSilva DR, Jones EA, Favata MF, Jaffee BD, Magolda RL, Trzaskos JM, Scherle PA, Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy, Journal of immunology, 160 (1998) 4175–4181. [PubMed] [Google Scholar]
- [36].Li P, Mitra S, Spolski R, Oh J, Liao W, Tang Z, Mo F, Li X, West EE, Gromer D, Lin JX, Liu C, Ruan Y, Leonard WJ, STAT5-mediated chromatin interactions in superenhancers activate IL-2 highly inducible genes: Functional dissection of the Il2ra gene locus, P Natl Acad Sci USA, 114 (2017) 12111–12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rawlings JS, Roles of SMC Complexes During T Lymphocyte Development and Function, Advances in protein chemistry and structural biology, 106 (2017) 17–42. [DOI] [PubMed] [Google Scholar]
- [38].Liu J, Maller JL, Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor, Curr Biol, 15 (2005) 1458–1468. [DOI] [PubMed] [Google Scholar]
- [39].Panigrahi AK, Zhang N, Mao Q, Pati D, Calpain-1 cleaves Rad21 to promote sister chromatid separation, Mol Cell Biol, 31 (2011) 4335–4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU, Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation, Nature, 437 (2005) 1048–1052. [DOI] [PubMed] [Google Scholar]
- [41].Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA, Master transcription factors and mediator establish super-enhancers at key cell identity genes, Cell, 153 (2013) 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Willi M, Yoo KH, Reinisch F, Kuhns TM, Lee HK, Wang C, Hennighausen L, Facultative CTCF sites moderate mammary super-enhancer activity and regulate juxtaposed gene in non-mammary cells, Nature communications, 8 (2017) 16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gong Y, Lazaris C, Sakellaropoulos T, Lozano A, Kambadur P, Ntziachristos P, Aifantis I, Tsirigos A, Stratification of TAD boundaries reveals preferential insulation of super-enhancers by strong boundaries, Nature communications, 9 (2018) 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Huang J, Li K, Cai W, Liu X, Zhang Y, Orkin SH, Xu J, Yuan GC, Dissecting super-enhancer hierarchy based on chromatin interactions, Nature communications, 9 (2018) 943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhao K, Wang W, Rando OJ, Xue Y, Swiderek K, Kuo A, Crabtree GR, Rapid and phosphoinositol-dependent binding of the SWI/SNF-like BAF complex to chromatin after T lymphocyte receptor signaling, Cell, 95 (1998) 625–636. [DOI] [PubMed] [Google Scholar]
- [46].Chi T, A BAF-centred view of the immune system, Nat Rev Immunol, 4 (2004) 965–977. [DOI] [PubMed] [Google Scholar]
- [47].Afrasiabi E, Ahlgren J, Bergelin N, Tornquist K, Phorbol 12-myristate 13-acetate inhibits FRO anaplastic human thyroid cancer cell proliferation by inducing cell cycle arrest in G1/S phase: evidence for an effect mediated by PKCdelta, Mol Cell Endocrinol, 292 (2008) 26–35. [DOI] [PubMed] [Google Scholar]
- [48].Glassford J, Soeiro I, Skarell SM, Banerji L, Holman M, Klaus GG, Kadowaki T, Koyasu S, Lam EW, BCR targets cyclin D2 via Btk and the p85alpha subunit of PI3-K to induce cell cycle progression in primary mouse B cells, Oncogene, 22 (2003) 2248–2259. [DOI] [PubMed] [Google Scholar]
- [49].Kim YH, Lim JH, Lee TJ, Park JW, Kwon TK, Expression of cyclin D3 through Sp1 sites by histone deacetylase inhibitors is mediated with protein kinase C-delta (PKC-delta) signal pathway, Journal of cellular biochemistry, 101 (2007) 987–995. [DOI] [PubMed] [Google Scholar]
- [50].Manicassamy S, Sun Z, The critical role of protein kinase C-theta in Fas/Fas ligand-mediated apoptosis, Journal of immunology, 178 (2007) 312–319. [DOI] [PubMed] [Google Scholar]
- [51].Saibil SD, Jones RG, Deenick EK, Liadis N, Elford AR, Vainberg MG, Baerg H, Woodgett JR, Gerondakis S, Ohashi PS, CD4+ and CD8+ T cell survival is regulated differentially by protein kinase Ctheta, c-Rel, and protein kinase B, Journal of immunology, 178 (2007) 2932–2939. [DOI] [PubMed] [Google Scholar]
- [52].Hayashi K, Ishizuka S, Yokoyama C, Hatae T, Attenuation of interferon-gamma mRNA expression in activated Jurkat T cells by exogenous zinc via down-regulation of the calcium-independent PKC-AP-1 signaling pathway, Life Sci, 83 (2008) 6–11. [DOI] [PubMed] [Google Scholar]
- [53].Khalaf H, Demirel I, Bengtsson T, Suppression of inflammatory gene expression in T cells by Porphyromonas gingivalis is mediated by targeting MAPK signaling, Cellular & molecular immunology, 10 (2013) 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sugimoto N, Nakahira M, Ahn HJ, Micallef M, Hamaoka T, Kurimoto M, Fujiwara H, Differential requirements for JAK2 and TYK2 in T cell proliferation and IFN-gamma production induced by IL-12 alone or together with IL-18, European journal of immunology, 33 (2003) 243–251. [DOI] [PubMed] [Google Scholar]
- [55].Biddie SC, John S, Sabo PJ, Thurman RE, Johnson TA, Schiltz RL, Miranda TB, Sung MH, Trump S, Lightman SL, Vinson C, Stamatoyannopoulos JA, Hager GL, Transcription factor AP1 potentiates chromatin accessibility and glucocorticoid receptor binding, Molecular cell, 43 (2011) 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Su Y, Shin J, Zhong C, Wang S, Roychowdhury P, Lim J, Kim D, Ming GL, Song H, Neuronal activity modifies the chromatin accessibility landscape in the adult brain, Nature neuroscience, 20 (2017) 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vierbuchen T, Ling E, Cowley CJ, Couch CH, Wang X, Harmin DA, Roberts CWM, Greenberg ME, AP-1 Transcription Factors and the BAF Complex Mediate Signal-Dependent Enhancer Selection, Molecular cell, 68 (2017) 1067–1082 e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Brignall R, Cauchy P, Bevington SL, Gorman B, Pisco AO, Bagnall J, Boddington C, Rowe W, England H, Rich K, Schmidt L, Dyer NP, Travis MA, Ott S, Jackson DA, Cockerill PN, Paszek P, Integration of Kinase and Calcium Signaling at the Level of Chromatin Underlies Inducible Gene Activation in T Cells, Journal of immunology, 199 (2017) 2652–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.