Abstract
On September 7 and 8, 2022, Healthy Environment and Endocrine Disruptors Strategies, an Environmental Health Sciences program, convened a scientific workshop of relevant stakeholders involved in obesity, toxicology, or obesogen research to review the state of the science regarding the role of obesogenic chemicals that might be contributing to the obesity pandemic. The workshop’s objectives were to examine the evidence supporting the hypothesis that obesogens contribute to the etiology of human obesity; to discuss opportunities for improved understanding, acceptance, and dissemination of obesogens as contributors to the obesity pandemic; and to consider the need for future research and potential mitigation strategies. This report details the discussions, key areas of agreement, and future opportunities to prevent obesity. The attendees agreed that environmental obesogens are real, significant, and a contributor at some degree to weight gain at the individual level and to the global obesity and metabolic disease pandemic at a societal level; moreover, it is at least, in theory, remediable.
Keywords: obesity, obesogens, adipose tissue, metabolism, adipocytes
Workshop objectives
Despite unprecedented awareness of its negative effects, the prevalence of obesity worldwide continues to grow. For example, 42% of United States adults manifest obesity on clinical grounds [body mass index (kg/m2) >30] [1]. Although energy balance is the most widely accepted cause, obesity is a multifactorial disease with multiple contributing factors [2]. In addition, evidence supporting the influence of endocrine-disrupting chemical obesogens in obesity is substantial [3,4]. Therefore, we developed a workshop to bring together some of the leading scientists in the clinical obesity field, in the biology of adipose tissue and mechanisms underlying obesity-related science, and in obesogen research so that obesity could be discussed more holistically. It was held at Wingspread, Wind Point, WI, on 7 and 8 September, 2022. The workshop was sponsored by Healthy Environment and Endocrine Disruptor Strategies (www.HEEDS.org), an Environmental Health Sciences program, and endorsed by The Endocrine Society and The Obesity Society.
This was a unique workshop during which obesity researchers from various backgrounds discussed the data supporting or refuting obesogens as etiologic agents in the pathophysiology of clinical obesity. In addition, they identified research gaps in the field and how to obtain wider acceptance of the importance of obesogens as contributors to the obesity pandemic. The overall aim was to forge a path forward, incorporating obesogens into comprehensive assessments of the origins of the obesity pandemic and possible interventions to ameliorate it (Table 1).
TABLE 1.
The objectives of the obesity workshop
|
|
|
|
|
Workshop overview
Healthy Environment and Endocrine Disruptor Strategies brought together a total of 30 prominent scientists (10 obesogen researchers, 10 basic scientists specializing in aspects of obesity and adipose biology, and 10 clinical obesity and nutrition scientists) to review and discuss the data supporting the role of obesogens in the etiology of obesity. Participants were selected by the Planning Committee [consisting of Drs Heindel, Blumberg, Ziegler (Obesity Society), Kahan (Endocrine Society), Stevens (Basic Science), and Lustig] based on their standing within the obesity research community, paying attention to geographic, age, sex, and racial diversity. In addition, participants were selected based on varied basic and clinical obesity backgrounds, with no formal interest in obesogens but a general interest in learning about them as possible players in the obesity pandemic. Twenty-six attendees were from the United States, 2 from Canada, and 2 from Europe.
The workshop encompassed 9 sessions over 2 d, with each including speakers and designated discussants. The workshop began by setting the stage with reviews of 3 main models of obesity: the energy balance model, which considers overeating as the cause of obesity, reviewed in [5]; the carbohydrate-insulin model, which considers overeating a consequence of increasing adiposity driven in part by increased insulin secretion [6]; and the reduction-oxidation (redox) network model, which posits that a disruption in electron redox signaling promotes metabolic dysfunction [7]. Specifically, the redox network model hypothesizes that environmental agents and food additives disrupt the normal redox signaling network by generating reactive oxygen species, which induces inappropriate responses, such as elevated insulin concentrations, pathological fat storage, and alterations in hunger or satiety unrelated to nutrient status.
This session also discussed physiological factors distinguishing increased adiposity from metabolically deleterious obesity and metabolic syndrome [8].
The discussion focused on the fact that there are competing models to explain the pathogenesis of obesity, which highlights the potential lack of consensus in the scientific community. Despite this uncertainty and complexity, most publications and guidance on obesity prevention and treatment within populations are based on the concept that obesity can be successfully treated through behavioral interventions intended to produce a negative energy balance. However, individual-level treatments have instead focused on specific diets, drugs, or surgery. Since the prevalence of obesity continues to increase despite evidence that EI has not increased [9], attendees agreed that it is reasonable to infer that other contributing factors may also be important, including obesogenic agents. None of the proposed models of obesity preclude a role for obesogens. Prevention of obesogen exposures and mitigation of the effects of obesogen exposures offer novel strategies to improve current efforts for obesity prevention.
The workshop then turned to obesogens specifically, including several presentations focusing on a discussion of the obesogen hypothesis/model of obesity. Three recent reviews that discussed the data linking obesogen exposures to adipocyte development in vitro, the development of obesity in animal studies, and human epidemiological cohorts [3,5,10] were used as the starting point for the obesogen workshop and presentations.
As noted in the presentations, most obesogens are a subset of endocrine-disrupting chemicals that alter energy metabolism, reviewed in [3]. These agents disrupt signaling pathways (eg, hormone receptors and transcription factors) in various cell types and tissues that regulate EI and expenditure, nutrient handling, and adipose biology. Obesogens can be naturally occurring (eg, heavy metals, viruses, and particulate matter), anthropogenic (prescription drugs, insecticides, plastics, household chemicals, personal care products, flame retardants, and water/oil repellants), or food components (non-nutritive sweeteners, fructose, trans-fats, preservatives, additives, and emulsifiers), any of which can increase weight via endocrine or nonendocrine mechanisms [3,4,11,12]. Exposures can occur via food, air, water, dermal contact, or inhalation [13,14]. Table 2 shows a current list of environmental chemical obesogens discussed at the workshop. Chemicals in this table have multiple in vitro, animal models and [except tributyltin (TBT)] supporting epidemiology studies. These chemicals can stimulate adipose tissue development and/or lead to weight gain in animal models and are also associated with excess adiposity in human epidemiological studies. Table 3 lists the potential obesogens. These chemicals have only been tested in vitro using, for example, human mesenchymal stem cells or 3T3-L1 preadipocytes and have shown the capacity to promote adipocyte differentiation [10].
TABLE 2.
Environmental chemical obesogens
| General chemicals |
| Maternal smoking (nicotine) |
| Air Pollution (PAHs) and fine particulate matter (PM2.5)] |
| DDT |
| Bisphenols (bisphenol A, S, F, and AF) |
| Phthalates (DEHP, DBP, DISBP, BBzP) |
| Tributyltin and dibutyltin |
| PFAS |
| Flame Retardants, PBDEs, OPFRs |
| PCBs |
| Arsenic |
| Cadmium |
| Some microplastics |
| Non-nutritive sweeteners |
| Aspartame |
| Sucralose |
| Saccharin |
| Agricultural chemicals |
| Chlorpyrifos |
| Diazinon |
| Neonicotinoid pesticides |
| Permethrin |
| Tolyfluanid |
| Food preservatives/additives/emulsifiers |
| Parabens |
| Benzoates |
| Propionic acid |
| CMC |
| MSG |
| DOSS |
| 3-BHA |
| Fructose/high fructose corn syrup |
3-BHA, 3-tertbutyl-hydroxyanisole; BBzP, butyl benzyl phthalate; CMC, Carboxymethylcellulose; DBP, dibutyl phthalate; DEHP, diethyl hexyl phthalate; DISBP, diisobutyl phthalate; DOSS, Dioctyl sodium sulfosuccinate; MSG, Monosodium glutamate; OPFR, organophosphate flame retardants; PAHS, polyaromatic hydrocarbons; PBDE, polybrominated diphenol ether; PCB, Dioxins and polychlorinated biphenyls; PFAS, per- and poly-fluoroalkyl substances.
TABLE 3.
Chemicals that are potential obesogens as evidenced by in vitro studies
| Glyphosate |
| Diazinon |
| Eldrin |
| Strobilurin pesticides |
| Triclosan |
| BADGE |
| Atrazine |
| Acetamiprid |
| Fludioxonil |
| Triflumizole |
| Quinoxyfen |
| Triphenyltin |
| House Dust Extracts |
| Alkylphenols and alcohols |
| Ethyl butyrate |
| Methyl salicylate |
| Red coloring 40 |
| Yellow coloring 5 |
| Yellow coloring 6 |
Since the term obesogen was coined in 2006 [15], the obesogen field has grown steadily [16]. There are now 3 categories of evidence supporting the obesogen hypothesis: 1) in vitro cellular screening assays; 2) in vivo animal studies revealing dose responses and mechanisms of obesogen action; and 3) epidemiological studies of various human cohorts show associations between obesogen exposure and body fat increases. Clinical studies in humans have been limited to studies of obesogen exposures on only a few outcomes, such as resting metabolic rate, weight gain, weight loss, and weight regain after dieting [17,18]. Because of ethical concerns, it is not possible to produce evidence via randomized controlled clinical trials exposing humans to varying doses of obesogens, the usually accepted “gold standard” evidence for determining the effects of pharmaceutical agents.
Basic science and preclinical models show that obesogens can disrupt adipose tissue development by increasing the number of fat cells or altering the function of adipocytes. Four obesogens have been shown to produce dysfunctional adipocytes [bisphenol A (BPA), TBT, di ethyl-hexyl phthalate (DEHP), and triphenyl phosphate(TPhP)]. Correlative data exist for humans for BPA and DEHP [19,20], organophosphate flame retardants [21], polybrominated diphenyl ethers (flame retardants) [22], DDT [23], and air pollution [24]. Animal models show that obesogens can act directly on the brain to increase food intake; or via metabolic mechanisms; including increasing TG deposition into adipose tissue, altering the metabolic rate, and disrupting energy metabolism through effects on the endocrine pancreas, liver, gastrointestinal tract, skeletal muscle, and by altering the microbiome and gut-derived hormones, (as reviewed in [3,11]). Obesogens can act via multiple mechanisms and on multiple tissues. Table 4 lists some of the known mechanisms of obesogen effects on various tissues. Virtually all the tissues and mechanisms known to regulate metabolic physiology and control adipogenic weight gain have been shown to be modified by obesogens.
TABLE 4.
Obesogens affect multiple tissues and endpoints in animal studies
| Increased inflammation | Dysfunctional adipocytes | NAFLD | Altered microbiome | Altered satiety and appetite neurons | Stimulate food intake | Stimulate weight gain with no change in food intake | Larger effect on weight gain with a high-fat diet | Insulin resistance | Leptin resistance | Transgenerational epigenetic inheritance |
|---|---|---|---|---|---|---|---|---|---|---|
| TBT | TBT | TBT | BPA, BPS | TBT | BPA | TBT | TBT | Tolyfluanid | Chlorpyrifos | TBT |
| BPA | BPA | BPA | DEHP | BPA | DEHP | Chlorpyrifos | BPA | BPA | BPA | BPA |
| DEHP | DEHP | DEHP | TCDD | OPFRs | DES | BBzP | PM2.5 | PM2.5 | DEHP, DBP | |
| PBDEs | TPhP | DDT | PBDEs | DDT | PFOS | PFOA | DDT | |||
| Triclosan | PCBs | PCBs | Permethrin | Atrazine | OPFRs | Jet fuel mixture | ||||
| PM2.5 (air pollution) | BaP | PAHs (air pollution) | Atrazine | Cadmium | Cadmium | |||||
| CMC | Triclosan | Triclosan | Chlorpyrifos | Permethrin | ||||||
| Diazinon | PFAS | Methyl Paraben | Chlorpyrifos | |||||||
| PCBs | OPFRs |
In addition to increasing the number and size of adipocytes, obesogens also affect other tissues and mechanisms in animal studies. Several main effects of obesogens are shown here to indicate the diversity of the effects. Although the chemicals shown here are reported to show that effect, this does not mean that other obesogens may not also show similar effects. In many cases, specific obesogens have not been examined for effects on tissues and mechanisms shown. Data for this table comes from [3].
BaP, benzo[a]pyrene; BBzP, butyl benzyl phthalate; BPA, bisphenol A; BPS, bisphenol S; CMC, carboxymethylcellulose; DBP, dibutyl phthalate; DDT, dichlorodiphenyltrichloroethane; DEHP, diethyl hexyl phthalate; DES, diethylstilbestrol; NAFLD, nonalcoholic fatty liver disease; OPFRs, organophosphate flame retardants; PAHs, poly aromatic hydrocarbons; PBDEs, polybrominated diethyl ethers; PCBs, polychlorinated biphenyls; PFAS, per- and poly-fluoroalkyl substances; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PM2.5, particulate matter; TBT, tributyltin; TCDD, 2,3,7,8 -tetrachlorodibenzo-p-dioxin; TPhP, triphenyl phosphate.
Discussions included the importance of sensitive periods across the lifespan during which obesogen exposures are most likely to induce permanent alterations in adipose development, function, and metabolic physiology. Like other noncommunicable diseases, obesity can originate in utero and during early development because of under or overnutrition or exposure to obesogens. In addition, obesogens can alter epigenetic programming in early life, resulting in tissues with altered gene expression and physiologic function, which can start accumulating adipose tissue in utero and across the lifespan. These changes can lead to increased sensitivity to weight gain later in life because of enhanced adipocyte development, limited central control of body weight by the brain, the altered set point for weight, and impaired function of metabolic tissues, such as the endocrine pancreas and liver .[[25], [26], [27], [28]] Although food intake and exercise are direct inputs to body weight control, obesogens, via epigenetic reprogramming, can alter how much food is required to gain weight versus how much energy expenditure or caloric restriction is needed to lose weight.
Obesogen exposures during development can also induce epigenetic changes that persist across generations through the process of transgenerational epigenetic inheritance [29,30]. Transgenerational epigenetic inheritance is defined as the inheritance of an effect in generations that were not directly exposed to the agent in question. TBT, DDT, BPA, mixtures of BPA, DEHP, and dibutyl phthalate, and a mixture of hydrocarbons (jet fuel) can elicit transgenerational epigenetic inheritance of obesity. In a rodent model, transgenerational inheritance of weight gain because of TBT exposure sensitized the fourth-generation males with normal weight to weight gain on a high-fat diet, and to resist weight loss on calorie restriction, mimicking the human situation [31]. The presentation on transgenerational effects produced the most interest and discussion at the workshop. Attendees were intrigued by the possibility of epigenetic inheritance of obesity across generations because of obesogen exposures. Although there are no human cohorts available where transgenerational epigenetic inheritance of obesity can be studied, there is a publication showing multigenerational inheritance .(2 generations) Exposure of grandmothers to DDT during pregnancy resulted in increased weight gain in the grandchildren at age 26 [32]. If transgenerational inheritance could be confirmed in humans, then the current obesity pandemic could be partly because of obesogen exposures experienced by our parents, grandparents, or great-grandparents.
The workshop then focused on integrating known mechanisms for obesity development with data on obesogens affecting those mechanisms and critical tissues, such as the endocrine pancreas and liver. Table 4 shows the result of those presentations. Obesogens can alter a variety of endpoints known to be involved in weight gain. Indeed, some obesogens, like BPA, for example, affect multiple tissues and endpoints.
Social inequities in obesogen exposures as contributors to the racial/ethnic and socioeconomic disparities in obesity risk were the next focus of discussion. This discussion highlighted the higher exposure to obesogens in Black, Hispanic/Latinx, low-income, and other disadvantaged communities that are disproportionately burdened by the obesity pandemic. Exposures from food and air pollution because of proximity to polluting industries contribute to this problem [33,34]. The note was made of the co-localization of greater obesogen exposures with other adverse social and structural determinants of health, raising the possibility of synergy between obesogens and other factors in the pathogenesis of metabolically deleterious obesity.
The discussion then expanded from what is currently known to what needs to be known, including examples of possible clinical experiments where the human impact of obesogen exposures could be dissected through targeted addition or subtraction. These data could identify the causal relationship between exposures and metabolically deleterious human obesity. Examples of potential research include leveraging studies in which human metabolic physiology has been extensively examined and dichotomizing participants based on the amounts of known obesogen exposure; interrogating energy metabolism following adipose tissue release of lipophilic obesogens after extreme weight loss; as well as other studies that could help infer causation, and the eventual aggregation and synthesis of data sufficient for triggering policy change to reduce and/or eliminate exposure to obesogens [35].
Indeed, there was a session focused on what data and approaches are needed to stimulate policy and regulatory action on obesogens [36]. The discussion focused on the need for a robust assessment of the fraction of obesity arising from obesogen exposures that is attributable compared with other obesity risk factors. Additional issues discussed included identifying the most potent and widespread obesogens and the greatest risks associated with obesogen exposures. It was noted that policy change could also be stimulated by stronger and more frequent statements from professional bodies supporting the obesogen concept, citizen demands labeling products containing obesogens, and legal action targeting producers of obesogenic chemicals.
The final session dealt with ideas for expanding the acceptance of the obesogen model of obesity, including future directions for the field. It was clear to participants that there are sufficient data supporting the obesogen model of obesity, so it should gain wider acceptance by the obesity research and clinical communities. The discussion focused on what obesogen scientists should/could do to stimulate wider acceptance.
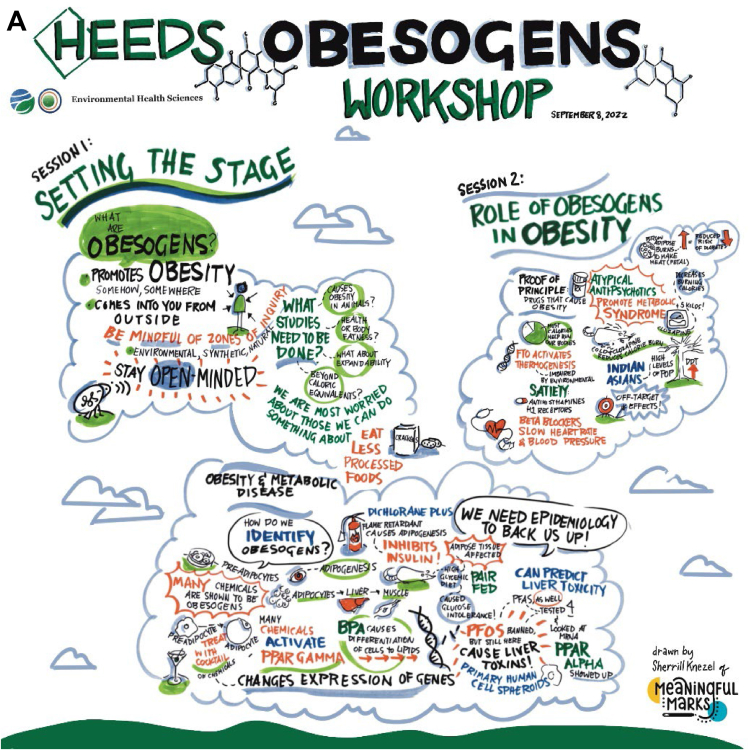
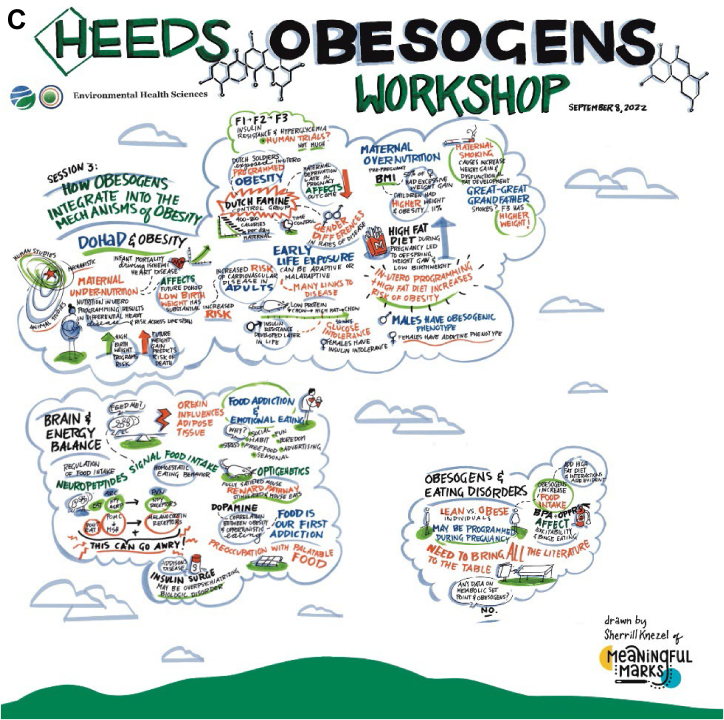
Figure 1 provides a graphic overview of some of the workshop sessions and discussions.
FIGURE 1.
(A) Session I, graphic overview of obesogen workshop, (B) Session II, graphic overview of obesogen workshop, and (C) Session III, graphic overview of obesogen workshop.
Results
Although the workshop’s purpose was not to develop a consensus statement, the discussion led to statements of agreement among the participants. These included:
The key statement of the agreement
-
•
Based on the robust nature of the data from in vitro and animal models characterizing obesogens, the obesogen hypothesis/model of obesity should receive greater attention from the broader scientific community as a potential contributor to the obesity pandemic.
Additional statements of agreement
-
•
The weakest link for extrapolation to policy is the limited human studies, which rely on epidemiological analyses that broadly demonstrate correlation. Human studies specifically designed to show clinical relevance and possible causation are needed.
-
•
The current models of obesity do not preclude a role for obesogens. Indeed, obesogens have been shown to act via mechanisms that play critical roles in the currently accepted models of obesity, including mechanisms for stimulating food intake.
-
•
Some effects of obesogen exposures can be transmitted across generations, posing a long-term threat to human health.
-
•
Improving metabolic health and achieving health equity requires addressing social and environmental drivers of obesity, including obesogens.
-
•
Knowledge of obesogens and interventions to reduce exposures add another tool and focus to clinical efforts to reduce the incidence of obesity that supplement traditional interventions, such as diets, drugs, and surgery.
-
•
Obesogens also offer a focus on prevention instead of current intervention after a person is obese.
-
•
A role for obesogens in the etiology of obesity moves the focus away from personal behavior as the sole or critical cause of obesity.
-
•
Data demonstrating that some obesogens affect specific adipose depots whereas others lead to abnormal adipocyte function opens the door to an improved understanding of the pathophysiology of metabolically healthy compared with unhealthy obesity.
-
•
Some obesogens act through specific mechanisms leading only to obesity. In contrast, others may affect additional endpoints leading to other metabolic disorders, including type 2 diabetes, cardiovascular disease, and nonalcoholic fatty liver disease.
-
•
Addressing the obesity pandemic and its disproportionate impact on vulnerable communities necessitates addressing the environmental drivers of obesity, including exposure to obesogens.
Data needed
The workshop participants identified knowledge and data gaps that, if filled, would lead to broader acceptance of the role of obesogens in the etiology of obesity.
-
•
Human data show that decreased obesogen exposure can improve metabolic health.
-
•
Determination of the risk of obesity attributable to obesogens compared to diet, genetics, and other factors.
-
•
More transgenerational and intergenerational data, especially in humans, including umbilical cord stem cell studies.
-
•
More experiments to understand the mechanism of obesogen action on the brain satiety and appetite centers and the hedonic, emotional eating center.
-
•
Determination of the role and extent of obesogen action during other periods of energy accumulation, such as puberty, pregnancy, and menopause.
-
•
Development of high-throughput assay method(s) to screen chemical compounds for effects on hepatocytes, adipocytes, and other cells/tissues regulating metabolism to determine their obesogenic, with follow-up studies of active compounds in animal models.
-
•
Development of an ethical framework for handling the burden of proof needed for action to reduce exposures, especially as it relates to the needs of vulnerable communities and within the context of exposures to mixtures of obesogens.
-
•
Leveraging clinical studies to establish causality, including identification of well-designed human studies (eg, in patients undergoing bariatric surgery as 1 example) for which biospecimens or tissues are available to measure obesogen amounts that can be linked to critical body composition and metabolic outcomes. Of particular importance are analyses of obesogen effects in studies examining traditional, well-accepted obesity risk factors, such as physical activity and dietary factors (eg, macronutrient composition, energy density, meal timing, etc).
-
•
Because exposure to some obesogens is influenced by diet and other lifestyle factors, examining how clinical interventions to mitigate metabolic disease risk alter obesogen amounts further delineates the role of obesogens in modulating metabolic health.
-
•
Numerous potential obesogens are present in our environment. Screen the adipose tissue and plasma of people with and without obesity to identify potential obesogens and determine their physiologic concentration ranges.
-
•
Leverage the power and scale of clinical trials for pharmaceutical agents developed to treat metabolic disorders with secondary analysis of obesogen exposures in clinical trial subjects. This would offer an opportunity to: 1) understand how obesogen exposures may modulate drug efficacy, 2) interrogate the mechanisms by which obesogens disrupt metabolism, and 3) determine whether pharmacological agents improve metabolic health in part through reductions in the amounts of obesogenic compounds.
-
•
The biomedical infrastructure exists to ascertain the contribution of the ambient environment, in general, and environmental exposures, to metabolic health and disease; we simply need to leverage these resources in a way that robustly investigates the obesogen hypothesis.
-
•
Since it is not likely that robust causal evidence for most obesogenic chemicals can be generated in humans, strategies are needed to affect change, including policy and regulatory changes that can be implemented despite incomplete knowledge.
Future directions
Participants indicated that the following actions would help gain wider knowledge and acceptance of the obesogen model of obesity.
-
•
Formalize collaborations among basic obesogen researchers, epidemiologists, and basic and clinical obesity and nutrition researchers, although globally widening the scope of participation.
-
•
Develop training and mentorship programs in metabolism research incorporating environmental health principles and knowledge. Such programs should include individuals from groups currently underrepresented in science and medicine.
-
•
Develop position statements on the importance of the obesogen model by The Endocrine Society, The Obesity Society, and The World Obesity Federation, as well as other medical specialty societies, such as the American Diabetes Association, the ASN, and those focused on Obstetrics and Gynecology and Pediatrics.
-
•
Expand NIEHS, NIDDK, NICHD, and National Institute on Minority Health and Health Disparities research on the obesogen model.
-
•
Take advantage of the current cadre of NIEHS Environmental Health Centers and NIDDK Nutrition and Obesity Centers to spur investigation in this area and specifically to fund pilot projects.
-
•
Develop support documents to attract federal funding for multi-disciplinary grants (eg, NIH program project grants).
-
•
Develop strategies for discussion with patients to help them reduce their exposure to obesogens.
-
•
Engage communities disproportionately burdened by obesogen exposures in science and advocacy efforts.
-
•
Incorporate environmental health and toxicant exposures into concepts, discussions, and interventions regarding the social/structural determinants of health. Identify interactions between exposures and other social determinants.
-
•
Translate findings into regulations. Work with policymakers and regulatory agencies to reduce the production of and subsequent exposure to obesogens.
In conclusion, the Wingspread Obesogen Workshop helped focus the attention of basic and clinical obesity researchers on the obesogen model. There was intense but cordial discussion of the obesogen model among experts with diverse perspectives. Workable solutions and future directions were developed, providing tangible next steps to advance the field. The participants agreed to continue to work together to expand understanding and acceptance of the role obesogens play in the pathogenesis of metabolic disease, including among basic and clinical obesity researchers, clinicians, funding agencies, policymakers, regulatory authorities, and consumers. The workshop was a great start. Now the work begins as we aim to fill in the data gaps while continuing to expand communication and outreach. We hope the results of the workshop will lead to changes in the direction of obesity research, and a wider acceptance of the obesogen model of obesity by researchers, clinicians, policy makers, regulatory agencies, and the public, which may reduce obesity across the lifespan and generations. Critically, the focus on reducing obesogen exposures prioritizes prevention as a key focus of the research and clinical toolbox for addressing the obesity pandemic. We believe that this unique workshop focused on developing improved knowledge and acceptance among disparate scientific subdisciplines could serve as a model for the future with the lessons learned from this experiment helping to generate new collaborative workshops to improve human health.
Acknowledgments
We thank Healthy Environment and Endocrine Disruptor Strategies (www.HEEDS.org), an Environmental Health Sciences program www.ehsciences.org, for supporting and organizing this workshop. We also thank The Endocrine Society, The Obesity Society, and The World Obesity Federation for their endorsement and help with the workshop. Finally, we thank the venue, The Wingspread Conference Center, for their assistance with the workshop.
DSL received royalties for books that recommend a carbohydrate-modified diet. He is an Associate Editor of the American Journal of Clinical Nutrition but played no role in evaluating this manuscript. SK is consulting for Lilly, Novo Nordisk, Vivus, textbook royalties: Johns Hopkins University Press, Lippincott Williams & Wilkins. BB is a named inventor on patents related to PPARγ. RMS, honoraria from SVS/Health, and the American Medical Forum.
Author contribution
The authors’ responsibilities were as follows–JJH: composed a rough draft and all authors: contributed to the interpretation of the meeting results, agreed to be accountable for all aspects of the work, and read and approved the final manuscript.
Conflicts of interest
All other authors report no conflicts of interest.
Funding
The workshop was funded by Healthy Environment and Endocrine Disruptor Strategies (HEEDS), which is a program and website for the endocrine disruptor field that focuses on training the next generation of endocrine disruptor-focused scientists and improving the impact of endocrine disruptor research on human, wildlife, and global health. HEEDS is a program of Environmental Health Sciences, a nonpartisan, nonprofit news and scientific organization that aims to drive good science into public policy. HEEDS received funding from The Passport, Forsythia, and Maine Community Foundations, private grant making organizations that promote healthier people by reducing exposure to harmful chemicals.
References
- 1.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;360(360):1–8. [PubMed] [Google Scholar]
- 2.Lustig R.H., Fennoy I. The history of obesity research. Horm. Res. Paediatr. 2022;95(6):638–648. doi: 10.1159/000526520. [DOI] [PubMed] [Google Scholar]
- 3.Heindel J.J., Howard S., Agay-Shay K., Arrebola J.P., Audouze K., Babin P.J., et al. Obesity II: establishing causal links between chemical exposures and obesity. Biochem. Pharmacol. 2022;199:115015. doi: 10.1016/j.bcp.2022.115015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egusquiza R.J., Blumberg B. Environmental obesogens and their impact on susceptibility to obesity: new mechanisms and chemicals. Endocrinology. 2020;161(3) doi: 10.1210/endocr/bqaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lustig R.H., Collier D., Kassotis C., Roepke T.A., Kim M.J., Blanc E., et al. Obesity I: overview and molecular and biochemical mechanisms. Biochem. Pharmacol. 2022;199:115012. doi: 10.1016/j.bcp.2022.115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig D.S., Aronne L.J., Astrup A., de Cabo R., Cantley L.C., Friedman M.I., et al. The carbohydrate-insulin model: a physiological perspective on the obesity pandemic. Am. J. Clin. Nutr. 2021;114(6):1873–1885. doi: 10.1093/ajcn/nqab270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corkey B.E., Deeney J.T. The redox communication network as a regulator of metabolism. Front Physiol. 2020;11:567796. doi: 10.3389/fphys.2020.567796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blüher M. Metabolically healthy obesity. Endocr. Rev. 2020;41(3) doi: 10.1210/endrev/bnaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mozaffarian D. Perspective: obesity-an unexplained epidemic. Am. J. Clin. Nutr. 2022;115(6):1445–1450. doi: 10.1093/ajcn/nqac075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kassotis C.D., Vom Saal F.S., Babin P.J., Lagadic-Gossmann D., Le Mentec H., Blumberg B., et al. Obesity III: obesogen assays: limitations, strengths, and new directions. Biochem. Pharmacol. 2022;199:115014. doi: 10.1016/j.bcp.2022.115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heindel J.J., Blumberg B. Environmental obesogens: mechanisms and controversies. Annu. Rev. Pharmacol. Toxicol. 2019;59:89–106. doi: 10.1146/annurev-pharmtox-010818-021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lind L., Lind P.M., Lejonklou M.H., Dunder L., Bergman Å., Guerrero-Bosagna C., et al. Uppsala consensus statement on environmental contaminants and the global obesity epidemic. Environ. Health Perspect. 2016;124(5):A81. doi: 10.1289/ehp.1511115. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertram M.G., Gore A.C., Tyler C.R., Brodin T. Endocrine-disrupting chemicals. Curr. Biol. 2022;32(13):R727–R730. doi: 10.1016/j.cub.2022.05.063. [DOI] [PubMed] [Google Scholar]
- 14.Metcalfe C.D., Bayen S., Desrosiers M., Muñoz G., Sauvé S., Yargeau V. An introduction to the sources, fate, occurrence and effects of endocrine disrupting chemicals released into the environment. Environ. Res. 2022;207:112658. doi: 10.1016/j.envres.2021.112658. [DOI] [PubMed] [Google Scholar]
- 15.Grün F., Watanabe H., Zamanian Z., Maeda L., Arima K., Cubacha R., et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 2006;20(9):2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- 16.Heindel J.J. History of the obesogen field: looking back to look forward. Front Endocrinol. 2019;10(14):14. doi: 10.3389/fendo.2019.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G., Dhana K., Furtado J.D., Rood J., Zong G., Liang L., et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLOS Med. 2018;15(2) doi: 10.1371/journal.pmed.1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandjean P., Meddis A., Nielsen F., Sjödin A., Hjorth M.F., Astrup A., et al. Weight loss relapse associated with exposure to perfluorinated alkylate substances. Obesity (Silver Spring). 2023;31(6):1686–1696. doi: 10.1002/oby.23755. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro C.M., Beserra B.T.S., Silva N.G., Lima C.L., Rocha P.R.S., Coelho M.S., et al. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: a systematic review and meta-analysis. BMJ, (Open). 2020;10(6) doi: 10.1136/bmjopen-2019-033509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckley J.P., Engel S.M., Braun J.M., Whyatt R.M., Daniels J.L., Mendez M.A., et al. Prenatal phthalate exposures and body mass index among 4- to 7-year-old children: A pooled analysis. Epidemiology. 2016;27(3):449–458. doi: 10.1097/EDE.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuiper J.R., Stapleton H.M., Wills-Karp M., Wang X., Burd I., Buckley J.P. Predictors and reproducibility of urinary organophosphate ester metabolite concentrations during pregnancy and associations with birth outcomes in an urban population. Environ. Health. 2020;19(1):55. doi: 10.1186/s12940-020-00610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vuong A.M., Braun J.M., Wang Z., Yolton K., Xie C., Sjodin A., et al. Exposure to polybrominated diphenyl ethers (PBDEs) during childhood and adiposity measures at age 8 years. Environ. Int. 2019;123:148–155. doi: 10.1016/j.envint.2018.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cano-Sancho G., Salmon A.G., La Merrill M.A. Association between exposure to p,p'-DDT and its metabolite p,p'-DDE with obesity: integrated systematic review and meta-analysis. Environ. Health Perspect. 2017;125(9) doi: 10.1289/EHP527. 096002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parasin N., Amnuaylojaroen T., Saokaew S. Effect of Air pollution on obesity in children: A systematic review and meta-analysis. Children (Basel). 2021;8(5) doi: 10.3390/children8050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleming T.P., Watkins A.J., Velazquez M.A., Mathers J.C., Prentice A.M., Stephenson J., et al. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391(10132):1842–1852. doi: 10.1016/S0140-6736(18)30312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanson M.A., Gluckman P.D. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol. Rev. 2014;94(4):1027–1076. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heindel J.J., Skalla L.A., Joubert B.R., Dilworth C.H., Gray K.A. Review of developmental origins of health and disease publications in environmental epidemiology, Reprod. Toxicol. 2017;68:34–48. doi: 10.1016/j.reprotox.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Heindel J.J., Blumberg B., Cave M., Machtinger R., Mantovani A., Mendez M.A., et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee M.K., Blumberg B. Transgenerational effects of obesogens. Basic Clin. Pharmacol. Toxicol. 2019;125(Suppl 3):44–57. doi: 10.1111/bcpt.13214. Suppl 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohajer N., Joloya E.M., Seo J., Shioda T., Blumberg B. Epigenetic transgenerational inheritance of the effects of obesogen exposure. Front Endocrinol. (Lausanne). 2021;12:787580. doi: 10.3389/fendo.2021.787580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamorro-Garcia R., Diaz-Castillo C., Shoucri B.M., Käch H., Leavitt R., Shioda T., et al. Ancestral perinatal obesogen exposure results in a transgenerational thrifty phenotype in mice. Nat. Commun. 2017;8(1):2012. doi: 10.1038/s41467-017-01944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cirillo P.M., La Merrill M.A., Krigbaum N.Y., Cohn B.A. Grandmaternal perinatal serum DDT in relation to granddaughter early menarche and adult obesity: three generations in the child health and development studies cohort. Cancer Epidemiol. Biomarkers. Prev. 2021;30(8):1480–1488. doi: 10.1158/1055-9965.EPI-20-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz D., Becerra M., Jagai J.S., Ard K., Sargis R.M. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care. 2018;41(1):193–205. doi: 10.2337/dc16-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McConnell R., Gilliland F.D., Goran M., Allayee H., Hricko A., Mittelman S. Does near-roadway air pollution contribute to childhood obesity? Pediatr. Obes. 2016;11(1):1–3. doi: 10.1111/ijpo.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sargis R.M., Heindel J.J., Padmanabhan V. Interventions to address environmental metabolism-disrupting chemicals: changing the narrative to empower action to restore metabolic health. Front Endocrinol. 2019;10(33):33. doi: 10.3389/fendo.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lobstein T., Brownell K.D. Endocrine-disrupting chemicals and obesity risk: a review of recommendations for obesity prevention policies. Obes. Rev. 2021;22(11) doi: 10.1111/obr.13332. [DOI] [PubMed] [Google Scholar]
- 37.Veiga-Lopez A., Pu Y., Gingrich J., Padmanabhan V. Obesogenic endocrine disrupting chemicals: identifying knowledge gaps. Trends Endocrinol. Metab. 2018;29(9):607–625. doi: 10.1016/j.tem.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auerbach S., Filer D., Reif D., Walker V., Holloway A.C., Schlezinger J., et al. Prioritizing environmental chemicals for obesity and diabetes outcomes research: A screening approach using ToxCast™ high-throughput data. Environ. Health Perspect. 2016;124(8):1141–1154. doi: 10.1289/ehp.1510456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filer D.L., Hoffman K., Sargis R.M., Trasande L., Kassotis C.D. On the utility of ToxCast-based predictive models to evaluate potential metabolic disruption by environmental chemicals. Environ. Health Perspect. 2022;130(5):57005. doi: 10.1289/EHP6779. [DOI] [PMC free article] [PubMed] [Google Scholar]