Abstract
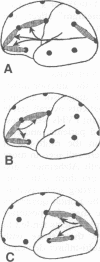
Periventricular white matter hyperintensities (PVHs) seen on T2 weighted MRI studies are common in elderly people and often represent demyelination of fibres. Damage to these fibres could lead to functional disconnection between brain regions. Electroencephalographic coherence, a measure of shared electrical activity between regions, was examined to determine if there was evidence for such disconnection. Twenty two subjects with clinically diagnosed dementia of the Alzheimer's type, 16 with multi-infarct dementia, and 18 normal controls were studied. It was hypothesised that coherence between areas presumably linked by fibres that traverse the periventricular region would be decreased in subjects with PVHs, and that PVHs would have a stronger association with decreased coherence than clinical diagnosis. It was also hypothesised that coherence between areas presumably connected by long corticocortical tracts that are neuroanatomically separated from the ventricles would be low in patients with Alzheimer's disease because of pyramidal cell death in this group, but would not be affected by the presence of PVHs. Patients with PVHs in fact had lower coherence than those without PVHs in the pre-Rolandic and post-Rolandic areas, where connecting fibres traverse the periventricular region. There was no effect of PVHs, however, on coherence between areas separated by the Rolandic fissure that were connected by long corticocortical tracts; this coherence was lowest among the patients with Alzheimer's disease. These patterns of association suggest that coherence may detect different types of neurophysiological "disconnection," and may be sensitive to selective damage to different fibre pathways.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbruzzese G., Reni L., Cocito L., Ratto S., Abbruzzese M., Favale E. Short-latency somatosensory evoked potentials in degenerative and vascular dementia. J Neurol Neurosurg Psychiatry. 1984 Sep;47(9):1034–1037. doi: 10.1136/jnnp.47.9.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrom M. G., Thompson R. F., Jr, Hendrie H. C., Norton J., Farlow M. R., Edwards M. K., Dean R. Foci of increased T2 signal intensity in MR images of healthy elderly subjects. A follow-up study. J Am Geriatr Soc. 1990 Oct;38(10):1133–1138. doi: 10.1111/j.1532-5415.1990.tb01377.x. [DOI] [PubMed] [Google Scholar]
- Awad I. A., Johnson P. C., Spetzler R. F., Hodak J. A. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke. 1986 Nov-Dec;17(6):1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- Awad I. A., Spetzler R. F., Hodak J. A., Awad C. A., Carey R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke. 1986 Nov-Dec;17(6):1084–1089. doi: 10.1161/01.str.17.6.1084. [DOI] [PubMed] [Google Scholar]
- Awad I. A., Spetzler R. F., Hodak J. A., Awad C. A., Williams F., Jr, Carey R. Incidental lesions noted on magnetic resonance imaging of the brain: prevalence and clinical significance in various age groups. Neurosurgery. 1987 Feb;20(2):222–227. doi: 10.1227/00006123-198702000-00004. [DOI] [PubMed] [Google Scholar]
- Bondareff W., Raval J., Colletti P. M., Hauser D. L. Quantitative magnetic resonance imaging and the severity of dementia in Alzheimer's disease. Am J Psychiatry. 1988 Jul;145(7):853–856. doi: 10.1176/ajp.145.7.853. [DOI] [PubMed] [Google Scholar]
- Bondareff W., Raval J., Woo B., Hauser D. L., Colletti P. M. Magnetic resonance imaging and the severity of dementia in older adults. Arch Gen Psychiatry. 1990 Jan;47(1):47–51. doi: 10.1001/archpsyc.1990.01810130049007. [DOI] [PubMed] [Google Scholar]
- Boone K. B., Miller B. L., Lesser I. M., Mehringer C. M., Hill-Gutierrez E., Goldberg M. A., Berman N. G. Neuropsychological correlates of white-matter lesions in healthy elderly subjects. A threshold effect. Arch Neurol. 1992 May;49(5):549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- Brant-Zawadzki M., Fein G., Van Dyke C., Kiernan R., Davenport L., de Groot J. MR imaging of the aging brain: patchy white-matter lesions and dementia. AJNR Am J Neuroradiol. 1985 Sep-Oct;6(5):675–682. [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G., Bickford R. G., Ponomareff G., Thal L. J., Mandel R., Gage F. H. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988 Nov;8(11):4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey C. E., Figiel G. S., Djang W. T., Cress M., Saunders W. B., Weiner R. D. Leukoencephalopathy in elderly depressed patients referred for ECT. Biol Psychiatry. 1988 Jun;24(2):143–161. doi: 10.1016/0006-3223(88)90270-3. [DOI] [PubMed] [Google Scholar]
- Coffey C. E., Figiel G. S., Djang W. T., Weiner R. D. Subcortical hyperintensity on magnetic resonance imaging: a comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990 Feb;147(2):187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- De Lacoste M. C., White C. L., 3rd The role of cortical connectivity in Alzheimer's disease pathogenesis: a review and model system. Neurobiol Aging. 1993 Jan-Feb;14(1):1–16. doi: 10.1016/0197-4580(93)90015-4. [DOI] [PubMed] [Google Scholar]
- De Reuck J., Crevits L., De Coster W., Sieben G., vander Eecken H. Pathogenesis of Binswanger chronic progressive subcortical encephalopathy. Neurology. 1980 Sep;30(9):920–928. doi: 10.1212/wnl.30.9.920. [DOI] [PubMed] [Google Scholar]
- Englund E., Brun A. White matter changes in dementia of Alzheimer's type: the difference in vulnerability between cell compartments. Histopathology. 1990 May;16(5):433–439. doi: 10.1111/j.1365-2559.1990.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Esiri M. M., Pearson R. C., Powell T. P. The cortex of the primary auditory area in Alzheimer's disease. Brain Res. 1986 Feb 26;366(1-2):385–387. doi: 10.1016/0006-8993(86)91324-7. [DOI] [PubMed] [Google Scholar]
- Ettlin T. M., Staehelin H. B., Kischka U., Ulrich J., Scollo-Lavizzari G., Wiggli U., Seiler W. O. Computed tomography, electroencephalography, and clinical features in the differential diagnosis of senile dementia. A prospective clinicopathologic study. Arch Neurol. 1989 Nov;46(11):1217–1220. doi: 10.1001/archneur.1989.00520470081031. [DOI] [PubMed] [Google Scholar]
- Fein G., Van Dyke C., Davenport L., Turetsky B., Brant-Zawadzki M., Zatz L., Dillon W., Valk P. Preservation of normal cognitive functioning in elderly subjects with extensive white-matter lesions of long duration. Arch Gen Psychiatry. 1990 Mar;47(3):220–223. doi: 10.1001/archpsyc.1990.01810150020004. [DOI] [PubMed] [Google Scholar]
- Gerard G., Weisberg L. A. MRI periventricular lesions in adults. Neurology. 1986 Jul;36(7):998–1001. doi: 10.1212/wnl.36.7.998. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965 Jun;88(2):237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Gloor P., Ball G., Schaul N. Brain lesions that produce delta waves in the EEG. Neurology. 1977 Apr;27(4):326–333. doi: 10.1212/wnl.27.4.326. [DOI] [PubMed] [Google Scholar]
- Grafton S. T., Sumi S. M., Stimac G. K., Alvord E. C., Jr, Shaw C. M., Nochlin D. Comparison of postmortem magnetic resonance imaging and neuropathologic findings in the cerebral white matter. Arch Neurol. 1991 Mar;48(3):293–298. doi: 10.1001/archneur.1991.00530150061019. [DOI] [PubMed] [Google Scholar]
- Hachinski V. C., Potter P., Merskey H. Leuko-araiosis. Arch Neurol. 1987 Jan;44(1):21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- Inzitari D., Diaz F., Fox A., Hachinski V. C., Steingart A., Lau C., Donald A., Wade J., Mulic H., Merskey H. Vascular risk factors and leuko-araiosis. Arch Neurol. 1987 Jan;44(1):42–47. doi: 10.1001/archneur.1987.00520130034014. [DOI] [PubMed] [Google Scholar]
- Jernigan T. L., Press G. A., Hesselink J. R. Methods for measuring brain morphologic features on magnetic resonance images. Validation and normal aging. Arch Neurol. 1990 Jan;47(1):27–32. doi: 10.1001/archneur.1990.00530010035015. [DOI] [PubMed] [Google Scholar]
- Junqué C., Pujol J., Vendrell P., Bruna O., Jódar M., Ribas J. C., Viñas J., Capdevila A., Marti-Vilalta J. L. Leuko-araiosis on magnetic resonance imaging and speed of mental processing. Arch Neurol. 1990 Feb;47(2):151–156. doi: 10.1001/archneur.1990.00530020047013. [DOI] [PubMed] [Google Scholar]
- Kato H., Sugawara Y., Ito H., Kogure K. White matter lucencies in multi-infarct dementia: a somatosensory evoked potentials and CT study. Acta Neurol Scand. 1990 Feb;81(2):181–183. doi: 10.1111/j.1600-0404.1990.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Kawamura J., Meyer J. S., Terayama Y., Weathers S. Leuko-araiosis and cerebral hypoperfusion compared in elderly normals and Alzheimer's dementia. J Am Geriatr Soc. 1992 Apr;40(4):375–380. doi: 10.1111/j.1532-5415.1992.tb02138.x. [DOI] [PubMed] [Google Scholar]
- Kinkel W. R., Jacobs L., Polachini I., Bates V., Heffner R. R., Jr Subcortical arteriosclerotic encephalopathy (Binswanger's disease). Computed tomographic, nuclear magnetic resonance, and clinical correlations. Arch Neurol. 1985 Oct;42(10):951–959. doi: 10.1001/archneur.1985.04060090033010. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick J. B., Hayman L. A. White-matter lesions in MR imaging of clinically healthy brains of elderly subjects: possible pathologic basis. Radiology. 1987 Feb;162(2):509–511. doi: 10.1148/radiology.162.2.3797666. [DOI] [PubMed] [Google Scholar]
- Kobari M., Meyer J. S., Ichijo M. Leuko-araiosis, cerebral atrophy, and cerebral perfusion in normal aging. Arch Neurol. 1990 Feb;47(2):161–165. doi: 10.1001/archneur.1990.00530020061017. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Yamaguchi S., Okada K., Yamashita K. Primitive reflexes and MRI findings, cerebral blood flow in normal elderly. Gerontology. 1990;36(4):199–205. doi: 10.1159/000213200. [DOI] [PubMed] [Google Scholar]
- Leuchter A. F., Newton T. F., Cook I. A., Walter D. O., Rosenberg-Thompson S., Lachenbruch P. A. Changes in brain functional connectivity in Alzheimer-type and multi-infarct dementia. Brain. 1992 Oct;115(Pt 5):1543–1561. doi: 10.1093/brain/115.5.1543. [DOI] [PubMed] [Google Scholar]
- Leuchter A. F., Spar J. E., Walter D. O., Weiner H. Electroencephalographic spectra and coherence in the diagnosis of Alzheimer's-type and multi-infarct dementia. A pilot study. Arch Gen Psychiatry. 1987 Nov;44(11):993–998. doi: 10.1001/archpsyc.1987.01800230073012. [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Campbell M. J., Terry R. D., Morrison J. H. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer's disease: a quantitative study of visual and auditory cortices. J Neurosci. 1987 Jun;7(6):1799–1808. doi: 10.1523/JNEUROSCI.07-06-01799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro K., Hatazawa J., Yamaguchi T., Itoh M., Matsuzawa T., Ono S., Miyazawa H., Hishinuma T., Yanai K., Sekita Y. Cerebral circulation and oxygen metabolism associated with subclinical periventricular hyperintensity as shown by magnetic resonance imaging. Ann Neurol. 1990 Sep;28(3):378–383. doi: 10.1002/ana.410280313. [DOI] [PubMed] [Google Scholar]
- Newton T. F., Leuchter A. F., Walter D. O., Van Gorp W. C., Stern C. E., Mandelkern M., Weiner H. EEG coherence in men with AIDS: association with subcortical metabolic activity. J Neuropsychiatry Clin Neurosci. 1993 Summer;5(3):316–321. doi: 10.1176/jnp.5.3.316. [DOI] [PubMed] [Google Scholar]
- O'Connor K. P., Shaw J. C., Ongley C. O. The EEG and differential diagnosis in psychogeriatrics. Br J Psychiatry. 1979 Aug;135:156–162. doi: 10.1192/bjp.135.2.156. [DOI] [PubMed] [Google Scholar]
- Oken B. S., Kaye J. A. Electrophysiologic function in the healthy, extremely old. Neurology. 1992 Mar;42(3 Pt 1):519–526. doi: 10.1212/wnl.42.3.519. [DOI] [PubMed] [Google Scholar]
- Pearson R. C., Esiri M. M., Hiorns R. W., Wilcock G. K., Powell T. P. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S. M., Mittenberg W., Bernardin L., Haughton V., Leo G. J. Neuropsychological test findings in subjects with leukoaraiosis. Arch Neurol. 1989 Jan;46(1):40–44. doi: 10.1001/archneur.1989.00520370042017. [DOI] [PubMed] [Google Scholar]
- Román G. C. Senile dementia of the Binswanger type. A vascular form of dementia in the elderly. JAMA. 1987 Oct 2;258(13):1782–1788. doi: 10.1001/jama.1987.03400130096040. [DOI] [PubMed] [Google Scholar]
- Román G. C. The identity of lacunar dementia and Binswanger disease. Med Hypotheses. 1985 Apr;16(4):389–391. doi: 10.1016/0306-9877(85)90059-3. [DOI] [PubMed] [Google Scholar]
- Schmidt R., Fazekas F., Offenbacher H., Lytwyn H., Blematl B., Niederkorn K., Horner S., Payer F., Freidl W. Magnetic resonance imaging white matter lesions and cognitive impairment in hypertensive individuals. Arch Neurol. 1991 Apr;48(4):417–420. doi: 10.1001/archneur.1991.00530160087019. [DOI] [PubMed] [Google Scholar]
- Steingart A., Hachinski V. C., Lau C., Fox A. J., Fox H., Lee D., Inzitari D., Merskey H. Cognitive and neurologic findings in demented patients with diffuse white matter lucencies on computed tomographic scan (leuko-araiosis). Arch Neurol. 1987 Jan;44(1):36–39. doi: 10.1001/archneur.1987.00520130028013. [DOI] [PubMed] [Google Scholar]
- Steriade M., Gloor P., Llinás R. R., Lopes de Silva F. H., Mesulam M. M. Report of IFCN Committee on Basic Mechanisms. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr Clin Neurophysiol. 1990 Dec;76(6):481–508. doi: 10.1016/0013-4694(90)90001-z. [DOI] [PubMed] [Google Scholar]
- Thatcher R. W., Krause P. J., Hrybyk M. Cortico-cortical associations and EEG coherence: a two-compartmental model. Electroencephalogr Clin Neurophysiol. 1986 Aug;64(2):123–143. doi: 10.1016/0013-4694(86)90107-0. [DOI] [PubMed] [Google Scholar]
- Tucker D. M., Roth D. L., Bair T. B. Functional connections among cortical regions: topography of EEG coherence. Electroencephalogr Clin Neurophysiol. 1986 Mar;63(3):242–250. doi: 10.1016/0013-4694(86)90092-1. [DOI] [PubMed] [Google Scholar]
- Tupler L. A., Coffey C. E., Logue P. E., Djang W. T., Fagan S. M. Neuropsychological importance of subcortical white matter hyperintensity. Arch Neurol. 1992 Dec;49(12):1248–1252. doi: 10.1001/archneur.1992.00530360046016. [DOI] [PubMed] [Google Scholar]
- Wade J. P., Mirsen T. R., Hachinski V. C., Fisman M., Lau C., Merskey H. The clinical diagnosis of Alzheimer's disease. Arch Neurol. 1987 Jan;44(1):24–29. doi: 10.1001/archneur.1987.00520130016010. [DOI] [PubMed] [Google Scholar]
- Wahlund L. O., Agartz I., Almqvist O., Basun H., Forssell L., Säf J., Wetterberg L. The brain in healthy aged individuals: MR imaging. Radiology. 1990 Mar;174(3 Pt 1):675–679. doi: 10.1148/radiology.174.3.2305048. [DOI] [PubMed] [Google Scholar]