Summary
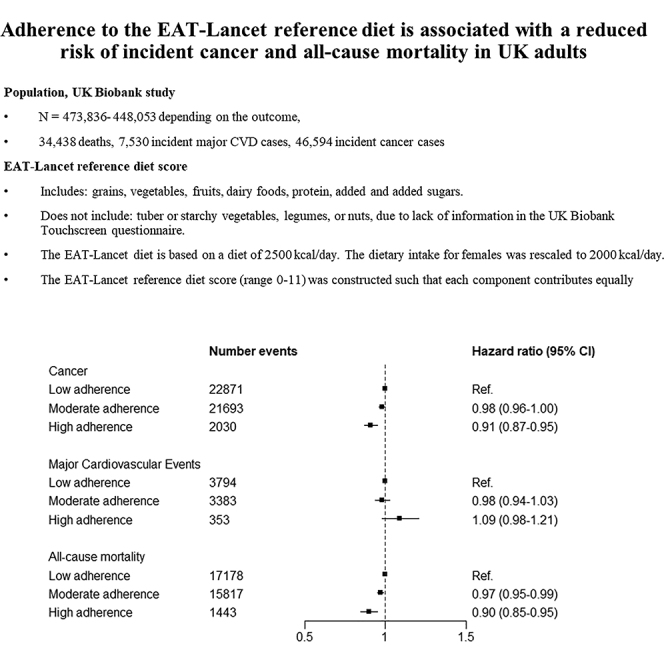
Food systems have been identified as significant contributors to the global environmental emergency. However, there is no universally agreed-upon definition of what constitutes a planetary healthy, sustainable diet. In our study, we investigated the association between the EAT-Lancet reference diet, a diet within the planetary boundaries, and incident cancer, incident major cardiovascular events, and all-cause mortality. Higher adherence to the EAT-Lancet reference diet was associated with lower incident cancer risk (hazard ratio [HR]continuous: 0.99; 95% confidence interval [CI]: 0.98–0.99]) and lower all-cause mortality (HR continuous: 0.98; 95% CI: 0.98–0.99), while mostly null associations were detected for major cardiovascular event risk (HR continuous: 1.00; 95% CI: 0.98–1.01). Stratified analyses using potentially modifiable risk factors led to similar results. Our findings, in conjunction with the existing literature, support that adoption of the EAT-Lancet reference diet could have a benefit for the prevention of non-communicable diseases.
Keywords: sustainable, diet, cancer, cardiovascular, incidence, mortality, EAT-Lancet
Graphical abstract
Highlights
-
•
Adherence to the EAT-Lancet reference diet was associated with lower cancer risk
-
•
Adherence to the reference diet was inversely associated with all-cause mortality
-
•
No association between the reference diet and cardiovascular diseases was observed
Science for society
There is an increasing interest in plant-based diets (i.e., diets rich in plant-based products that include little, if any, animal products), due to their environmental sustainability and potential health benefits. Recently, international experts recommended a mostly plant-based, sustainable diet referred to as the “planetary health diet” We investigated to find out if individuals adhering to the planetary health diet proposed by the EAT-Lancet Commission are at a lower risk for cancer, cardiovascular disease, or mortality from all causes. In our study, people who closely followed the planetary health diet had a lower risk for cancer and mortality from all causes than those who did not closely follow the planetary health diet. Our study adds to the existing literature that following the EAT-Lancet reference diet could have benefits for non-communicable disease prevention.
Food systems are significant contributors to the global environmental emergency. Therefore, we investigated the association between the EAT-Lancet reference diet, a diet within the planetary boundaries, and incident cancer, incident major cardiovascular events, and all-cause mortality. People who closely followed the planetary health diet had a lower risk for cancer and mortality from all causes than those who did not closely follow the planetary health diet. Hence, following the EAT-Lancet reference diet could have benefits for non-communicable disease prevention.
Introduction
Food systems significantly contribute to the global environmental emergency, with approximately one-third of anthropogenic greenhouse gas emissions being attributed to them,1 but there is no universally agreed-upon definition of what constitutes a planetary healthy, sustainable diet. While the Food and Agriculture Organization (FAO)2 has recently proposed a definition of sustainable diets, most scientists have long considered dietary patterns promoting a high proportion of plant-based foods in the diet as sustainable. The common characteristic of all these plant-based dietary patterns is the reduced consumption or entire exclusion of animal products from the diet. However, these patterns include a diverse range of products (both healthy and unhealthy), resulting in intakes of different diet quality.
In 2019, the EAT-Lancet Commission on Food, Planet, Health proposed the “healthy reference diet,” also referred to as the EAT-Lancet reference diet, a mainly plant-based diet that, according to the EAT-Lancet Commission, reflects a sustainable food system.3,4 The EAT-Lancet reference diet is close to most national food-based dietary or disease prevention (e.g., cancer prevention) guidelines but takes a much stricter approach regarding animal-based product consumption, in particular, meat and dairy products. According to the EAT-Lancet Commission, the reference diet addresses the diet and farming aspects of environmental changes better than most national food-based dietary guidelines.4
According to the Global Burden of Disease Study, cardiovascular disease was the leading cause of diet-related deaths globally in 2017, followed by cancer.5 Dietary factors have been associated with incident cardiovascular disease6 and incident cancer risk,7,8,9,10,11 as well as all-cause mortality.12 Studies suggest that 5%–20% of incident cardiovascular disease cases, incident cancer cases, or deaths are attributable to dietary factors13,14 (the exact proportion depends on the dietary factor, the study population, and the investigated endpoint). Furthermore, the existing evidence suggests that dietary patterns with high consumption of plant-based foods are associated with a lower risk of many non-communicable diseases.6,15,16
Despite widespread media publicity and interest in the scientific community, few studies have investigated if the EAT-Lancet reference diet is also beneficial for human health using large population-based data.17,18,19,20,21,22,23,24 Most reported an inverse association between the EAT-Lancet reference diet and their outcome of interest, despite the different methodologies they used (e.g., assessment of dietary intake, operationalization of the EAT-Lancet reference diet). To date, only one study has investigated the association between the EAT-Lancet reference diet and cancer in approximately 60,000 participants over 8 years of follow-up and reported an overall null association.18
In our study, we investigated using the largest sample size to date, the association between the EAT-Lancet reference diet and incident cancer, incident major cardiovascular events, and all-cause mortality, overall and by potential effect modifiers, in the UK Biobank cohort. Our findings showed an inverse association between higher adherence to the EAT-Lancet reference diet and cancer risk and all-cause mortality. Our study provides evidence suggesting that adherence to the diet proposed by the EAT-Lancet expert commission could not only aid in the mitigation of the climate emergency but could also be beneficial for the reduction of non-communicable diseases and all-cause mortality.
Results
Baseline study population characteristics
After an average follow-up of 11.5 years (mean: 10.49 years for cancer, 11.98 years for major cardiovascular events, 11.98 years for mortality), 46,594 incident cancer cases and 7,530 incident major cardiovascular events were diagnosed in the UK Biobank cohort, while 34,438 people died.
Participants with higher EAT-Lancet reference diet scores (i.e., higher adherence to the diet proposed by the EAT-Lancet Commission; Table 1) were more likely to be female, more physically active, and to have obtained college/university degree, while they were less likely to report being current smokers or drink alcohol.
Table 1.
Baseline characteristics of the study population, overall and by categories reflecting adherence to the EAT-Lancet reference diet
| EAT-Lancet reference diet score |
||||
|---|---|---|---|---|
| Total (n = 473,836) | Low adherence, 0–4 points (n = 229,968) | Moderate adherence, 5–7 points (n = 221,727) | High adherence, 8–11 points (n = 22,141) | |
| Age at recruitment, mean (SD) | 56.50 (8.08) | 56.33 (8.15) | 56.64 (8.02) | 57.32 (7.73) |
| Sex - Female, % | 54.52 | 50.95 | 57.15 | 65.23 |
| Highest level of attained education, % | ||||
| None of the following | 16.36 | 15.38 | 17.17 | 18.36 |
| CSEs/O-levels/GCSEsa, or equivalent | 25.55 | 25.89 | 25.34 | 24.05 |
| NVQ/HND/HNC/A-levels/AS-levels or equivalent | 17.19 | 17.38 | 17.04 | 16.74 |
| Other professional qualifications | 28.45 | 29.40 | 27.66 | 26.62 |
| College/university degree | 11.55 | 11.20 | 11.77 | 12.95 |
| Prefer not to answer/Missing | 0.90 | 0.75 | 1.02 | 1.29 |
| Smoking status, % | ||||
| Never smokers | 54.63 | 54.35 | 54.84 | 55.37 |
| Former smokers | 34.67 | 33.96 | 35.21 | 36.70 |
| Current smokers | 10.39 | 11.42 | 9.60 | 7.52 |
| Prefer not to answer/Missing | 0.32 | 0.27 | 0.35 | 0.42 |
| Body mass index, % | ||||
| ≤18.5 kg/m2 | 0.50 | 0.55 | 0.45 | 0.53 |
| 18.5–24.9 kg/m2 | 32.48 | 33.04 | 31.94 | 32.04 |
| 25.0–29.9 kg/m2 | 42.37 | 41.83 | 42.87 | 43.04 |
| ≥30 kg/m2 | 24.15 | 24.15 | 24.19 | 23.78 |
| Missing | 0.49 | 0.42 | 0.55 | 0.60 |
| Alcohol intake status, % | ||||
| Never drinkers | 4.15 | 3.40 | 4.68 | 6.59 |
| Former drinkers | 3.43 | 3.12 | 3.65 | 4.58 |
| Current drinkers | 92.34 | 93.42 | 91.59 | 88.76 |
| Prefer not to answer/Missing | 0.08 | 0.07 | 0.09 | 0.07 |
| Physical activity, % | ||||
| Less than 75 min per week | 31.85 | 32.37 | 31.58 | 29.19 |
| 75–150 min per week | 13.30 | 13.33 | 13.31 | 12.86 |
| 150 min or more per week | 49.47 | 49.16 | 49.48 | 52.52 |
| Prefer not to answer/Missing | 5.38 | 5.14 | 5.63 | 5.43 |
A-level: General Certificate of Education Advanced level; AS-levels: General Certificate of Education Advanced Supplementary level; CSE: Certificate of Secondary Education; HNC: Higher National Certificate; HND: Higher National Diploma; GCSE: General Certificate of Secondary Education; NVQ: National Vocational Qualification; O-level: General Certificate of Education Ordinary level; SD: Standard deviation.
EAT-Lancet diet and non-communicable disease risk
Higher adherence to the EAT-Lancet reference diet was associated with lower cancer risk (hazard ratio [HR]continuous: 0.99; 95% confidence interval [CI]: 0.98–0.99, per one-unit increase in the score; HRlow vs. high: 0.91; 95% CI: 0.87–0.95; Table 2). In selected cancer types, higher adherence of the EAT-Lancet reference diet was not associated with breast (HRcontinuous: 0.99; 95% CI: 0.98–1.00; HRlow vs. high: 0.94; 95% CI: 0.85–1.04), colorectal (HRcontinuous: 0.98; 95% CI: 0.98–1.00; HRlow vs. high: 0.90; 0.78–1.03), or prostate (HRcontinuous: 0.99; 95% CI: 0.98–1.01; HRlow vs. high: 0.93; 95% CI: 0.84–1.04) cancer risk. Additional adjustment for reproductive factors (for breast cancer) or first-degree family history and previous cancer screening did not substantially affect the cancer-site specific results.
Table 2.
The association between the EAT-Lancet reference diet score and incident cancer risk
| All incident cancer diagnoses |
Breast cancer |
Colorectal cancer |
Prostate cancer |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||||||||||||
| EAT-Lancet reference diet score | Number of cases | Model 1 a | Model 2 b | Number of cases | Model 1 a | Model 2 b | Model 3a c | Model 3b d | Number of cases | Model 1 a | Model 2 b | Model 3a | Model 3b d | Number of cases | Model 1 a | Model 2 b | Model 3a | Model 3b d |
| Continuous, 0-11 | 46,594 | 0.99 (0.98–0.99) | 0.99 (0.98–0.99) | 8,516 | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 5,120 | 0.98 (0.96–1.00) | 0.98 (0.96–1.00) | – | 0.98 (0.97–1.00) | 10,150 | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) | – | 0.99 (0.98–1.00) |
| Low adherence, 0-4 | 22,871 | Ref. | Ref. | 3,919 | Ref. | Ref. | Ref. | Ref. | 2,508 | Ref. | Ref. | – | Ref. | 5,173 | Ref. | Ref. | – | Ref. |
| Moderate adherence, 5-7 | 21,693 | 0.98 (0.96–1.00) | 0.98 (0.96–1.00) | 4,140 | 0.98 (0.93–1.02) | 0.98 (0.94–1.02) | 0.98 (0.93–1.02) | 0.98 (0.93–1.02) | 2,396 | 1.00 (0.94–1.05) | 1.00 (0.94–1.05) | – | 1.00 (0.94–1.05) | 4,595 | 1.00 (0.96–1.04) | 1.00 (0.96–1.04) | – | 0.99 (0.95–1.03) |
| High adherence, 8-11 | 2,030 | 0.90 (0.86–0.94) | 0.91 (0.87–0.95) | 457 | 0.93 (0.85–1.03) | 0.94 (0.85–1.04) | 0.94 (0.85–1.03) | 0.94 (0.85–1.04) | 216 | 0.90 (0.78–1.03) | 0.90 (0.78–1.03) | – | 0.90 (0.78–1.03) | 382 | 0.94 (0.85–1.05) | 0.94 (0.85–1.04) | – | 0.93 (0.84–1.03) |
Abbreviations: CI: confidence interval; HR: hazard ratio. In analyses with breast and prostate cancer as outcomes, only participants with reported sex as female and male, respectively, were included.
Model based on age, sex, and region.
Model based on age, sex, and region plus further adjustment for smoking status, body mass index, physical activity, highest level of attained education, Townsend deprivation index, and alcohol intake status.
Model based on age, sex, region, smoking status, body mass index, physical activity, highest level of attained education, Townsend deprivation index, and alcohol intake status (Model 2), further adjusted for reproductive factors.
Model based on age, sex, region, smoking status, body mass index, physical activity, highest level of attained education, Townsend deprivation index and alcohol intake status (Model 2), further adjusted for first-degree family history, cancer screening attendance, and time since screening attendance for the respective cancer.
Higher adherence to the EAT-Lancet reference diet was not associated with a lower risk for major cardiovascular events (HRcontinuous: 1.00; 95% CI: 0.98–1.01; HRlow vs. high: 1.09; 95% CI: 0.98–1.21: Table 3). Similar null associations were seen in analyses on total stroke (HRcontinuous: 0.99; 95% CI: 0.97–1.02; HRlow vs. high: 1.04; 95% CI: 0.87–1.24), the main subtypes of stroke, or myocardial infarction (HRcontinuous: 1.00; 95% CI: 0.99–1.02; HRlow vs. high: 1.12; 95% CI: 0.98–1.29). Upon additional adjustment for first-degree family history of cardiovascular disease, the overall association became inverse (HRcontinuous: 0.97; 95% CI: 0.96–0.98; HRlow vs. high: 0.94; 95% CI: 0.84–1.05), while the total stroke and myocardial infarction analyses did not change substantially.
Table 3.
The association between the EAT-Lancet reference diet score and incident major cardiovascular events
| Incident major cardiovascular events |
Stroke |
Ischemic stroke |
Hemorrhagic stroke |
Myocardial infarction |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||||||||||||||
| EAT-Lancet reference diet score | Number of casesa | Model 1 b | Model 2 c | Model 3c d | Number of casesa | Model 1 b | Model 2 c | Model 3c d | Number of casesa | Model 1 b | Model 2 c | Model 3c d | Number of casesa | Model 1 b | Model 2 c | Model 3c d | Number of casesa | Model 1 b | Model 2 c | Model 3c d |
| Continuous, 0-11 | 7,530 | 0.99 (0.98–1.01) | 1.00 (0.98–1.01) | 0.97 (0.96–0.98) | 2,901 | 0.99 (0.97–1.01) | 0.99 (0.97–1.02) | 0.99 (0.97–1.02) | 2,049 | 0.99 (0.96–1.02) | 1.00 (0.97–1.03) | 1.00 (0.97–1.03) | 414 | 1.02 (0.95–1.08) | 1.02 (0.95–1.08) | 1.02 (0.95–1.08) | 4,670 | 0.99 (0.97–1.01) | 1.00 (0.99–1.02) | 1.00 (0.98–1.02) |
| Low adherence, 0-4 | 3,794 | Ref. | Ref. | Ref. | 1,430 | Ref. | Ref. | Ref. | 1,000 | Ref. | Ref. | Ref. | 198 | Ref. | Ref. | Ref. | 2,384 | Ref. | Ref. | Ref. |
| Moderate adherence, 5-7 | 3,383 | 0.97 (0.93–1.02) | 0.98 (0.94–1.03) | 0.92 (0.88–0.97) | 1,336 | 0.99 (0.92–1.07) | 1.00 (0.93–1.08) | 1.00 (0.93–1.08) | 954 | 1.01 (0.93–1.11) | 1.02 (0.94–1.12) | 1.02 (0.94–1.12) | 194 | 1.03 (0.84–1.26) | 1.04 (0.85–1.27) | 1.04 (0.85–1.27) | 2,067 | 0.96 (0.90–1.02) | 0.97 (0.91–1.03) | 0.97 (0.91–1.02) |
| High adherence, 8-11 | 353 | 1.07 (0.96–1.19) | 1.09 (0.98–1.21) | 0.94 (0.84–1.05) | 135 | 1.02 (0.85–1.22) | 1.04 (0.87–1.24) | 1.03 (0.87–1.23) | 95 | 1.02 (0.83–1.27) | 1.04 (0.84–1.29) | 1.04 (0.84–1.28) | 22 | 1.17 (0.75–1.82) | 1.18 (0.76–1.84) | 1.18 (0.76–1.83) | 219 | 1.10 (0.95–1.26) | 1.12 (0.98–1.29) | 1.11 (0.97–1.28) |
Abbreviations: CI: confidence interval; HR: hazard ratio.
A small number of participants was diagnosed with both stroke and myocardial infraction on the same day, thus the number of cases of the individual major cardiovascular events does not add up to the total. Similarly, a small number of participants was diagnosed with both main stroke subtypes on the same day.
Model based on age, sex, and region.
Model based on age, sex, and region plus further adjustment for smoking status, body mass index, physical activity, highest level of attained education, Townsend deprivation index and alcohol intake status.
Model based on age, sex, region, smoking status, body mass index, physical activity, highest level of attained education, Townsend deprivation index and alcohol intake status (Model 2), further adjusted for first-degree family history for heart disease and stroke.
Higher adherence to the EAT-Lancet reference diet was associated with lower mortality risk (HRcontinuous: 0.98; 95% CI: 0.98–0.99; HRlow vs. high: 0.90; 95% CI: 0.85–0.95; Table 4).
Table 4.
The association between the EAT-Lancet reference diet score and all-cause mortality
| HR (95% CI) | |||
|---|---|---|---|
| EAT-Lancet reference diet score | Number of cases | Model 1 a | Model 2 b |
| Continuous, 0–11 | 34,438 | 0.98 (0.97–0.99) | 0.98 (0.98–0.99) |
| Low adherence, 0–4 | 17,178 | Ref. | Ref. |
| Moderate adherence, 5–7 | 15,817 | 0.96 (0.94–0.98) | 0.97 (0.95–0.99) |
| High adherence, 8–11 | 1,443 | 0.88 (0.83–0.93) | 0.90 (0.85–0.95) |
Abbreviations: CI: confidence interval; HR: hazard ratio.
Model based on age, sex, and region.
Model based on age, sex, and region plus further adjustment for smoking status, body mass index, physical activity, highest level of attained education, Townsend deprivation index and alcohol intake status.
Stratified analysis
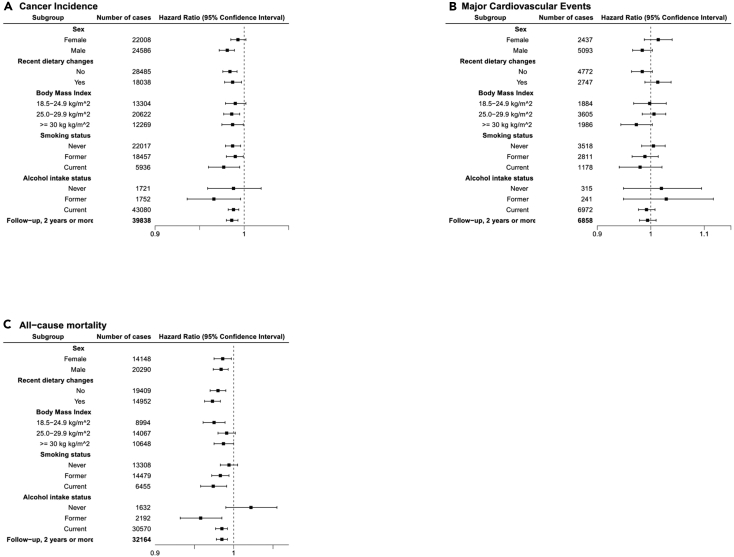
The results of stratified analysis are shown in Figure 1A (cancer incidence), Figure 1B (incident major cardiovascular events), and Figure 1C (mortality). There were no substantial differences observed between the different groups for cancer risk, major cardiovascular event risk, or all-cause mortality. The association between the EAT-Lancet reference diet and cancer was statistically significant for males, but not females. Restricting the analyses to study participants with at least 2 years of follow-up time did not alter the interpretation of the results (Figure 1).
Figure 1.
The association between the EAT-Lancet reference diet and other factors
(A) Cancer incidence, stratified by pre-selected lifestyle factors, (B) incident major cardiovascular events, stratified by pre-selected lifestyle factors, and (C) all-cause mortality, stratified by pre-selected lifestyle factors.
Discussion
In the largest study to date on the link between the EAT-Lancet reference diet and non-communicable diseases, we observed inverse associations between the EAT-Lancet reference diet and risk of all-cause mortality and incident cancer.
Despite the widespread media exposure of the diet proposed by the EAT-Lancet Commission, few studies thus far have investigated its association with non-communicable diseases. To the best of our knowledge, only one observational study exists on the association between the EAT-Lancet reference diet and cancer risk. Berthy et al., using data from the NutriNet-Santé cohort, reported a null association overall. An inverse association was seen for specific population groups (e.g., females) without adjustment for body mass index (BMI).18 These results are in slight contrast to our results, where we found that higher adherence was associated with lower risk for cancer. In our population, a statistically significant inverse association between the EAT-Lancet reference diet and cancer risk was observed for male participants in the fully adjusted model.
Studies investigating the association between the EAT-Lancet reference diet and cardiovascular diseases have reported inverse or null associations. Higher adherence to the EAT-Lancet reference diet has been inversely associated with lower risk of heart disease,17,21,23 diabetes,17,24 and stroke19 in the literature, but null associations have been reported in some studies for these endpoints.17,18,20 These inconsistencies in the findings could potentially be attributed to differences in the underlying structure of the cohorts, the way the score was constructed, the length of the follow-up, as well as analytical decisions, or they could in fact reflect differences in the true association. For all-cause mortality, an inverse association has been reported,21,22 but not in all17 studies.
In the absence of a standardized, universally accepted scoring system, it is inevitable that different groups will interpret and operationalize the EAT-Lancet reference diet differently. Despite the differences in how the scores were constructed, the follow-up time, and the different outcomes, many of the existing studies have reported a modest inverse association between the EAT-Lancet reference diet and non-communicable diseases17,19,21 or mortality.21,22 While considerable debate surrounds the potential adoption of the EAT-Lancet reference diet on a wide scale, particularly due to concerns regarding its affordability25,26 and nutrient sufficiency,27 this theoretical diet was designed, according to the EAT-Lancet Commission, to not exceed selected planetary resources. The existing evidence thus supports that adoption of a diet consistent with the EAT-Lancet reference diet cutoffs could have a dual benefit, both for the planet but also for the prevention of non-communicable diseases.
Given the complex, dynamic relationships in food systems, it seems unlikely that one single action (the widespread adoption of the EAT-Lancet reference diet or any other isolated action) will be able to effectively address the multifaceted challenges faced by food systems. This is supported by the EAT-Lancet Commission as well, which outlined five strategies for immediate food system transformation in their report.4 A wide range of targeted, interconnected, cost-effective efforts addressing factors like food production, food processing and distribution, campaigns, food labeling, and food pricing strategies28 is needed to institute meaningful changes.
The strengths of this study included the prospective study design and the substantial number of confirmed incident cases, but it also has some limitations. Dietary information was only available at baseline for the full cohort and thus may not reflect the long-term lifestyle habits of the study participants. To overcome this, we stratified the study results by self-reported dietary changes. Another limitation is an important criticism received for the EAT-Lancet reference diet, suggesting that the proposed dietary intake does not take into account the dietary needs of many population groups that are potentially susceptible to dietary deficiencies (e.g., females of reproductive age, children). To overcome this, we rescaled the diet proposed by the EAT-Lancet committee to reflect a daily intake of 2,000 kilocalories, which we used for females in our study. While we acknowledge the potential dietary deficiencies of the proposed diet for various population groups (e.g., females of reproductive age), these shortcomings seem less pertinent for our project since the inherent age inclusion criteria in the UK Biobank (mainly 40–69 years old) meant that few pregnant women are included in the study population. Nevertheless, these participants were excluded from our analyses. The dietary data collected with the touchscreen questionnaire did not include all relevant information, so some components of the EAT-Lancet reference diet could not be operationalized in our study. Due to the lack of information on first-degree family history for all cancer types, the analyses were only adjusted for first-degree family history for cancer only when information was available (i.e., breast, colorectal, and prostate cancer). As in all observational studies, the possibility of residual confounding, or the possibility that the study population might be more health-conscious than the general population cannot be excluded.
In conclusion, this large population-based study provides evidence to support the adherence to the diet proposed by the EAT-Lancet expert commission not only for mitigation of the climate emergency, but also for reduction in non-communicable diseases and all-cause mortality. Additional studies are needed, especially from non-Western countries, to further assess the link between the EAT-Lancet reference diet and non-communicable diseases in diverse populations.
Experimental procedures
Resource availability
Lead contact
Requests for further information and resources should be directed to the lead contact, Sabine Rohrmann (sabine.rohrmann@uzh.ch).
Materials availability
No materials were used in this study.
Data and code availability
This work has been conducted using the UK Biobank Resource (application number 81738). The UK Biobank is an open access resource and bona fide researchers can apply to use the UK Biobank dataset by registering and applying at http://ukbiobank.ac.uk/register-apply/. The code of this analysis has been submitted to the UK Biobank (as per contract).
Study population
The UK Biobank cohort is a large, population-based prospective study. More than 500,000 participants, mainly aged 40–69 years old, were recruited throughout the UK from 2006 to 2010. Detailed information on the study design, methods, and rationale of the cohort has been previously reported.29 The UK Biobank has ethical approval from the North West Multi-centre Research Ethics Committee. All participants provided informed consent. Briefly, participants provided medical, dietary, and lifestyle data via a Touchscreen questionnaire. Physical measurements were also taken, and participants provided blood and urine samples. Participants were followed using linkages to routinely available central registers where incident cancers, hospital admissions, and deaths were reported.
A flowchart of the study population can be seen in Figure S1. Participants were excluded from the analyses if they had a recorded prevalent cancer (excluding non-melanoma skin cancer) or major cardiovascular event (stroke, myocardial infarction) diagnosis at recruitment in the UK Biobank (based on linkage data), if they were pregnant or had unknown pregnancy status at the time of recruitment, or if they had missing information on the included dietary variables. In analyses with cancer as the outcome, participants whose sex did not match their genetic sex were excluded. Participants whose date of cancer or major cardiovascular event diagnosis coincided with their date of death were also excluded.
Dietary variables
Dietary information collected via the UK Biobank Touchscreen questionnaire was used to develop a score that reflects adherence to the EAT-Lancet reference diet. The Touchscreen questionnaire inquired about the consumption frequency over the past year (29 questions). The items assessed were cooked vegetables, salad/raw vegetables, fresh fruit, dried fruit, oily fish, other fish, processed meats, poultry, beef, lamb, pork, cheese, salt added to food, tea, and water. Additionally, questions on the type of milk and spread most commonly consumed, number of slices and type of bread most commonly consumed, number of bowls and type of breakfast cereal most commonly consumed, cups of coffee and type most commonly consumed were asked. Furthermore, the avoidance of specific food groups (eggs or foods containing eggs, dairy products, wheat products, sugar or foods/drinks containing sugar), the age when the participant last ate meat, the preferred temperature of hot drinks, changes in diet in the past 5 years, and variation in diet were also assessed.
Food items whose consumption was asked at a weekly level were transformed into daily consumption (in grams per day). Standard portion sizes reported for the United Kingdom30 were used to transform consumption to the necessary level of daily consumption.
Adherence to the EAT-Lancet reference diet
Participants received a score based on whether each component of their diets (e.g., daily fruit intake; Table S1) was within the cutoffs of the EAT-Lancet reference diet. The components we were able to use were grains, vegetables, fruits, dairy foods, protein sources (i.e., beef, lamb, and pork, chicken and other poultry, eggs, fish), added fats (unsaturated oils, saturated oils), and added sugars. Due to lack of information in the UK Biobank Touchscreen questionnaire, we were not able to use the components on tuber or starchy vegetables, legumes, or nuts. Our scoring approach closely resembles the one described by Knuppel et al.17 When the UK Biobank Touchscreen information did not sufficiently capture the food group, different scoring was used (Table S1).
The cutoffs for the EAT-Lancet diet are based on a diet of 2,500 kcal/day.4 Since females have been known to consume less than males, the dietary intake for females was rescaled to reflect a diet of 2,000 kcal/day, rounded to the nearest whole number. For each component, participants received one point if their consumption was within the cutoff values. Zero points were awarded if the consumption was outside of the cutoff values. The sum of all components resulted in each participant’s overall EAT-Lancet reference diet score. The overall score was constructed such that each component contributes equally to the EAT-Lancet reference diet score.
Due to the lack of information on the exact consumption of certain EAT-Lancet reference diet components in the UK Biobank Touchscreen questionnaire, the self-reported avoidance of these food groups was used (Table S1). Based on the question “Which of the following do you never eat?” with possible answers including eggs or foods containing eggs, dairy products, wheat products, sugar or foods/drinks containing sugar, participants received one point if they avoided these components. The score ranged from 0 to 11, with higher scores reflecting greater adherence to the EAT-Lancet reference diet. The score was additionally categorized into three groups: low adherence (0–4 points), moderate adherence (5–7 points), and high adherence (8–11 points).
Case ascertainment
Participants diagnosed with first primary incident cancer, based on cancer registry information, between recruitment and the latest date of complete information were considered as incident cancer cases. The latest dates of complete information for cancer incidence varied among countries (February 29, 2020, for England and Wales; January 31, 2021, for Scotland). Cancer cases were coded according to the 10th Revision of the International Statistical Classification of Diseases, Injuries, and Causes of Death. Cases recorded as C00-C97, as well as D320, D321, D329, D330, D332, D333, D334, D339, D352, D420, D429, D430, D431, D432, D439, D443, D444, D445, excluding C44, were considered as incident cancer cases.
Participants diagnosed with first primary incident major cardiovascular events, based on hospital admissions, between recruitment and the latest date of complete information were considered as incident cardiovascular event cases. The latest dates of complete information for hospital admissions varied among countries (September 30, 2021, for England; March 31, 2016, for Wales; July 31, 2021, for Scotland). Cases were coded according to the 10th Revision of the International Statistical Classification of Diseases, Injuries, and Causes of Death. Cases recorded as I60, I61, I63, I64, I21, I22, I23, I241, and I252 were considered major cardiovascular event cases.
Participants who were reported to be dead, based on central registers, between recruitment and the latest date of complete information (September 30, 2021, for England and Wales; October 31, 2021, for Scotland) were considered as mortality cases.
Adjusting variables
Upon recruitment, medical, dietary, anthropometric, and lifestyle data were collected, including information on alcohol use, smoking status, physical activity, education, reproductive history, and previous illnesses. Body size measurements were also taken. The baseline questionnaire also asked participants to estimate the number of days in a typical week they engage in moderate and vigorous physical activity, as well as the typical duration of these activities (in minutes; separate questions for moderate and vigorous activities) on a typical day.
Statistical analyses
Categorical variables were presented by percentages and continuous variables by arithmetic means and standard deviations (SDs) for descriptive purposes.
Cox proportional hazards regression models were used to assess the association of the EAT-Lancet reference diet score with incident cancer (overall and for selected cancer sites), incident major cardiovascular events, and all-cause mortality. Entry time was defined as a participant’s age at study recruitment and exit time as a participant’s age at the first incident cancer diagnosis, incident cardiovascular event or death (depending on the analytical subsample), loss to follow-up, or end of follow-up, whichever came first.
The EAT-Lancet reference diet score was assessed as continuous and as categorical variables. Categories, as mentioned above, were created to reflect low, moderate, or high adherence to the EAT-Lancet reference diet.
Confounding adjustment followed a tiered approach. Model 1 was stratified for age (5-year intervals), region at study assessment (10 regions), and sex. Model 2 was further adjusted for education level, Townsend deprivation index (quintiles; as an indicator for socio-economic status), smoking status (never, former, current, prefer not to answer/missing), BMI categories (≤18.5 kg/m2, 18.5–24.9 kg/m2, 25.0–29.9 kg/m2, ≥30 kg/m2, missing; according to the World Health Organization classification31), physical activity (<75 min per week, 75–150 min per week, >150 min per week, prefer not to answer/missing) and alcohol intake status (never, former, current drinker, prefer not to answer/missing). Model 2 is considered the main model in our analyses. In analyses with breast cancer as the outcome, reproductive factors (i.e., parity, age at menopause, menopausal status, use of oral contraceptives, or menopausal hormone therapy) were included in an additional model (Model 3a). Analyses with breast, colorectal, and prostate cancers as outcomes were also adjusted for first-degree family history for each respective cancer, previous cancer screening attendance (breast, colorectal, prostate-specific antigen test), and time since the last screening in an additional model (Model 3b). Analyses with major cardiovascular events as the outcome were additionally adjusted for first-degree family history of heart disease and stroke (Model 3c).
Pre-planned stratified analyses by potential effect modifiers were conducted including by sex, BMI, smoking status, alcohol intake status, and self-reported changes in diet in the past 5 years. Confounding adjustment in these analyses followed Model 2, as described above. When one of the adjustment variables was used as a stratifying variable, it was not included in the model. Sensitivity analyses were conducted restricting the analyses to participants with at least 2 years of follow-up time. Analyses were conducted using Stata version 13 (StataCorp, Texas). All statistical tests were two-sided, and p values <0.05 were considered statistically significant.
Acknowledgments
This work was conducted using the UK Biobank Resource under Application Number 81738. Funding for grant IIG_FULL_2021_012 was obtained from the World Cancer Research Fund (WCRF UK), as part of the World Cancer Research Fund International grant program. The funder had no role in study design, data analysis, decision to publish, or preparation of the manuscript. Dr. Papier was supported by the Wellcome Trust, Our Planet Our Health (Livestock, Environment and People—LEAP) [grant number 205212/Z/16/Z] and Cancer Research UK [grant number C570/A16491 and C16077/A29186]. At the time of publication, Dr. Karavasiloglou is an EFSA staff member. At the time of preparation of this work, Dr. Karavasiloglou was affiliated with the University of Zurich and the University Hospital Zurich. Where authors are identified as personnel of the European Food Safety Authority, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the European Food Safety Authority.
Author contributions
Conception and design: N.K., A.T., G.P., A.K., K.P., A.C., T.K., S.R. Data acquisition: N.K., S.R. Analyzing the data: N.K., A.T., T.K., S.R. Interpretation of the data: N.K., A.T., G.P., A.C., T.K., S.R. Verifying the data: A.T., A.K., K.P. Drafting the manuscript: N.K. Critically revising the manuscript: N.K., A.T., G.P., A.K., K.P., A.C., T.K., S.R. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published: November 21, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.oneear.2023.11.002.
Supplemental information
References
- 1.Vermeulen S.J., Campbell B.M., Ingram J.S. Climate Change and Food Systems. Annu. Rev. Environ. Resour. 2012;37:195–222. [Google Scholar]
- 2.Burlingame B., Dernini S. 2012. Sustainable Diets and Biodiversity Directions and Solutions for Policy, Research and Action. [Google Scholar]
- 3.Willett W., Rockström J., Loken B., Springmann M., Lang T., Vermeulen S., Garnett T., Tilman D., DeClerck F., Wood A., et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393:447–492. doi: 10.1016/S0140-6736(18)31788-4. [DOI] [PubMed] [Google Scholar]
- 4.EAT-Lancet Commission . Food Planet Health. Healthy Diets From Sustainable Food Systems; 2019. Summary Report of the EAT-Lancet Commission. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2017 Diet Collaborators Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Lond. Engl. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bechthold A., Boeing H., Schwedhelm C., Hoffmann G., Knüppel S., Iqbal K., De Henauw S., Michels N., Devleesschauwer B., Schlesinger S., Schwingshackl L. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2019;59:1071–1090. doi: 10.1080/10408398.2017.1392288. [DOI] [PubMed] [Google Scholar]
- 7.Schwedhelm C., Boeing H., Hoffmann G., Aleksandrova K., Schwingshackl L. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta-analysis of cohort studies. Nutr. Rev. 2016;74:737–748. doi: 10.1093/nutrit/nuw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vineis P., Wild C.P. Global cancer patterns: causes and prevention. Lancet Lond. Engl. 2014;383:549–557. doi: 10.1016/S0140-6736(13)62224-2. [DOI] [PubMed] [Google Scholar]
- 9.Schüz J., Espina C., Villain P., Herrero R., Leon M.E., Minozzi S., Romieu I., Segnan N., Wardle J., Wiseman M., et al. European Code against Cancer 4th Edition: 12 ways to reduce your cancer risk. Cancer Epidemiol. 2015;39(Suppl 1):S1–S10. doi: 10.1016/j.canep.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Behrens G., Gredner T., Stock C., Leitzmann M.F., Brenner H., Mons U. Cancers Due to Excess Weight, Low Physical Activity, and Unhealthy Diet. Dtsch. Arztebl. Int. 2018;115:578–585. doi: 10.3238/arztebl.2018.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Cancer Research Fund/American Institute for Cancer Research . 2018. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective.dietandcancerreport.org Continuous Update Project Expert Report. [Google Scholar]
- 12.Schwingshackl L., Schwedhelm C., Hoffmann G., Lampousi A.-M., Knüppel S., Iqbal K., Bechthold A., Schlesinger S., Boeing H. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017;105:1462–1473. doi: 10.3945/ajcn.117.153148. [DOI] [PubMed] [Google Scholar]
- 13.Islami F., Goding Sauer A., Miller K.D., Siegel R.L., Fedewa S.A., Jacobs E.J., McCullough M.L., Patel A.V., Ma J., Soerjomataram I., et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA. Cancer J. Clin. 2018;68:31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 14.Petermann-Rocha F., Ho F.K., Foster H., Boopor J., Parra-Soto S., Gray S.R., Mathers J.C., Celis-Morales C., Pell J.P. Nonlinear Associations Between Cumulative Dietary Risk Factors and Cardiovascular Diseases, Cancer, and All-Cause Mortality: A Prospective Cohort Study From UK Biobank. Mayo Clin. Proc. 2021;96:2418–2431. doi: 10.1016/j.mayocp.2021.01.036. [DOI] [PubMed] [Google Scholar]
- 15.Schwingshackl L., Schwedhelm C., Hoffmann G., Knüppel S., Laure Preterre A., Iqbal K., Bechthold A., De Henauw S., Michels N., Devleesschauwer B., et al. Food groups and risk of colorectal cancer. Int. J. Cancer. 2018;142:1748–1758. doi: 10.1002/ijc.31198. [DOI] [PubMed] [Google Scholar]
- 16.Schwingshackl L., Hoffmann G., Lampousi A.-M., Knüppel S., Iqbal K., Schwedhelm C., Bechthold A., Schlesinger S., Boeing H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017;32:363–375. doi: 10.1007/s10654-017-0246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knuppel A., Papier K., Key T.J., Travis R.C. EAT-Lancet score and major health outcomes: the EPIC-Oxford study. Lancet. 2019;394:213–214. doi: 10.1016/S0140-6736(19)31236-X. [DOI] [PubMed] [Google Scholar]
- 18.Berthy F., Brunin J., Allès B., Fézeu L.K., Touvier M., Hercberg S., Galan P., Pointereau P., Lairon D., Baudry J., Kesse-Guyot E. Association Between Adherence to the EAT-Lancet Diet and Risk of Cancer and Cardiovascular Outcomes in the Prospective NutriNet-Santé Cohort. Am. J. Clin. Nutr. 2022;116:980–991. doi: 10.1093/ajcn/nqac208. [DOI] [PubMed] [Google Scholar]
- 19.Ibsen D.B., Christiansen A.H., Olsen A., Tjønneland A., Overvad K., Wolk A., Mortensen J.K., Dahm C.C. Adherence to the EAT-Lancet Diet and Risk of Stroke and Stroke Subtypes: A Cohort Study. Stroke. 2022;53:154–163. doi: 10.1161/STROKEAHA.121.036738. [DOI] [PubMed] [Google Scholar]
- 20.Xu C., Cao Z., Yang H., Hou Y., Wang X., Wang Y. Association Between the EAT-Lancet Diet Pattern and Risk of Type 2 Diabetes: A Prospective Cohort Study. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.784018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colizzi C., Harbers M.C., Vellinga R.E., Verschuren W.M.M., Boer J.M.A., Biesbroek S., Temme E.H.M., van der Schouw Y.T. Adherence to the EAT-Lancet Healthy Reference Diet in relation to Coronary Heart Disease, All-Cause Mortality Risk and Environmental Impact: Results from the EPIC-NL Cohort. J. Am. Heart Assoc. 2023;12:e026318. doi: 10.1161/JAHA.122.026318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stubbendorff A., Sonestedt E., Ramne S., Drake I., Hallström E., Ericson U. Development of an EAT-Lancet index and its relation to mortality in a Swedish population. Am. J. Clin. Nutr. 2022;115:705–716. doi: 10.1093/ajcn/nqab369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S., Dukuzimana J., Stubbendorff A., Ericson U., Borné Y., Sonestedt E. Adherence to the EAT-Lancet diet and risk of coronary events in the Malmö Diet and Cancer cohort study. Am. J. Clin. Nutr. 2023;117:903–909. doi: 10.1016/j.ajcnut.2023.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S., Stubbendorff A., Olsson K., Ericson U., Niu K., Qi L., Borné Y., Sonestedt E. Adherence to the EAT-Lancet diet, genetic susceptibility, and risk of type 2 diabetes in Swedish adults. Metabolism. 2023;141 doi: 10.1016/j.metabol.2023.155401. [DOI] [PubMed] [Google Scholar]
- 25.Goulding T., Lindberg R., Russell C.G. The affordability of a healthy and sustainable diet: an Australian case study. Nutr. J. 2020;19:109. doi: 10.1186/s12937-020-00606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirvonen K., Bai Y., Headey D., Masters W.A. Affordability of the EAT-Lancet reference diet: a global analysis. Lancet Global Health. 2020;8:e59–e66. doi: 10.1016/S2214-109X(19)30447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beal T., Ortenzi F., Fanzo J. Estimated micronutrient shortfalls of the EAT-Lancet planetary health diet. Lancet Planet. Health. 2023;7:e233–e237. doi: 10.1016/S2542-5196(23)00006-2. [DOI] [PubMed] [Google Scholar]
- 28.Webb P., Benton T.G., Beddington J., Flynn D., Kelly N.M., Thomas S.M. The urgency of food system transformation is now irrefutable. Nat. Food. 2020;1:584–585. doi: 10.1038/s43016-020-00161-0. [DOI] [PubMed] [Google Scholar]
- 29.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., Downey P., Elliott P., Green J., Landray M., et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.British Dietetic Association Portion sizes: Food Fact Sheet. 2022. [Google Scholar]
- 31.World Health Organization . World Health Organization; 2000. Obesity: Preventing and Managing the Global Epidemic. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This work has been conducted using the UK Biobank Resource (application number 81738). The UK Biobank is an open access resource and bona fide researchers can apply to use the UK Biobank dataset by registering and applying at http://ukbiobank.ac.uk/register-apply/. The code of this analysis has been submitted to the UK Biobank (as per contract).