SUMMARY
Antibiotic persistence, or the ability of small subsets of bacteria to survive prolonged antibiotic treatment, is an underappreciated cause of antibiotic treatment failure. Over the past decade, researchers have discovered multiple different stress responses and mechanisms that can promote antibiotic persistence. However, many of these studies have been completed in culture-based systems that fail to truly replicate the complexities of the host environment, and it is unclear whether the mechanisms defined in in vitro studies are applicable during host infection. In this review, we focus our discussion on recent studies that utilize a mixture of ex vivo culture systems and animal models to understand what stressors in the host environment are important for inducing antibiotic persistence. Different host stressors are involved depending on the anatomical niche the bacteria reside in and whether the host immune system is primed to generate a more robust response against bacteria, which can result in differing downstream effects on antibiotic susceptibility. Bacterial pathogens can also utilize specific strategies to reprogram their metabolism, which is vital for transitioning into an antibiotic-persistent state within host tissues. Importantly, we highlight that more attention is needed to establish guidelines for in vivo work on antibiotic persistence, particularly when identifying antibiotic-persistent subpopulations and distinguishing these phenotypes from antibiotic tolerance. Studying antibiotic persistence in the context of the host environment will be crucial for developing tools and strategies to target antibiotic-persistent bacteria and increase the efficacy of antibiotic treatment.
KEYWORDS: antibiotic persistence, bacterial pathogens, mouse models
INTRODUCTION
Bacteria possess a wide variety of mechanisms that can protect them from the action of antibiotics, resulting in antibiotic treatment failure. For decades, researchers have primarily focused on the contribution of antibiotic resistance to treatment failure. Antibiotic resistance occurs when bacteria acquire genetic changes that allow them to grow in the presence of antibiotics, which increases the minimum inhibitory concentration (MIC) of antibiotic needed to prohibit growth (1). However, there are increasing numbers of cases of recurrent, relapsing infection where clinical isolates are examined in culture, and bacteria exhibit no change in MIC (2). Due to this increasing frequency, it is becoming better appreciated that antibiotic resistance is not the only reason for treatment failure. These cases can occur when bacteria develop phenotypic changes in response to stress that induce slowed growth and allow bacteria to survive antibiotic exposure longer than a susceptible population, without changing the MIC (3). When these changes occur across an entire bacterial population, this phenomenon is known as antibiotic tolerance, and when these changes occur in a subpopulation, leaving the rest of the population antibiotic susceptible, it is known as antibiotic persistence (3). These changes are typically non-inheritable and phenotypically reversible. When stress is removed, bacteria resume growth, leading to chronic and recurrent infections. It is important to note that not all tolerance mechanisms occur this way. Some mechanisms involve genetic mutations rather than induction of a stress response and some do not depend on a slowed growth phenotype (4, 5).
Recognizing this phenomenon as a concerning problem, for the past 20 years (6), researchers have garnered their attention to understanding how these antibiotic-persistent bacteria respond to different environmental stressors and what gene expression changes allow them to survive antibiotic treatment. However, most studies have been completed in bacterial culture systems that do not represent the complexity of host environment. When bacteria infect a host, they encounter a multitude of stressors, including nutrient limitation, hypoxia or oxygen limitation, acidic pH, reactive oxygen species (ROS), and reactive nitrogen species (RNS). Although these host defense mechanisms are important for controlling the replication of bacteria, they can also induce stress responses in bacteria that allow them to survive antibiotic treatment (7–9). This then causes antibiotic tolerance or persistence, depending on whether the stress impacts the entire population or a subpopulation, respectively. Some of these stress responses have been studied in isolation and induced artificially with drugs or nutrient deprivation. Although these culture-based experiments are important for building our understanding of how each of these stresses impact bacteria and predispose them to survive antibiotics, they do not recapitulate the complexity of the host environment where bacteria are likely responding to multiple stresses at once, rather than in isolation. In the following sections, we will discuss in vivo studies that shed light on how different stresses, including nutrient limitation, ROS, and RNS induce antibiotic persistence or tolerance within the host environment. Although there are many other stresses within the host environment that can induce antibiotic recalcitrance, we will focus on these stresses due to the greater attention to these in recent in vivo studies. There are also many bacterial pathogens in which antibiotic persistence has been shown to be relevant to study. However, many of these studies have been completed in vitro. To focus only on in vivo work relevant to the host environment, we will be primarily discussing Mycobacterium tuberculosis and Staphylococcus aureus, among a few other pathogens. Antibiotic persistence in Salmonella has also been studied extensively in vivo but will not be discussed in detail in this review, as it has been covered recently elsewhere (10–12).
Identification of antibiotic-persistent cells
Since persisters exhibit no change in MIC, other measurements must be used to identify antibiotic-persistent cells. To show that a fraction of bacteria survive antibiotic treatment longer than a susceptible population, bacterial viability must be assayed overtime, typically by taking multiple timepoints to enumerate for colony-forming units (CFUs). These assays are referred to as time-kill curves, which determine the minimum duration of killing (MDK99) or the amount of time it takes to kill 99% of the population (1, 13) (Fig. 1). Whereas an antibiotic-resistant population continues to grow in the presence of antibiotic treatment, antibiotic-tolerant populations are not growing and survive antibiotic treatment longer than a susceptible population (Fig. 1). These kinetics are also important for distinguishing between antibiotic persistence and tolerance. When an entire population of bacteria is exposed to a stress that induces a slowed growth phenotype, the entire population survives antibiotic exposure longer than a susceptible population, known as antibiotic tolerance (11, 13) (Fig. 1). When only a subpopulation is affected, time-kill curves resemble biphasic killing where a majority of the population is killed rapidly, but a subpopulation survives, known as antibiotic persistence (3, 14) (Fig. 1). In either case, after antibiotic exposure is removed, surviving bacteria can resume growth (Fig. 1), which is crucial to demonstrate the phenotypic reversibility of antibiotic-persistent and antibiotic-tolerant bacteria.
Fig 1.
Distinguishing between resistance, tolerance, and persistence. Time-kill curves show CFUs overtime. Resistant bacteria continue to grow in the presence of antibiotic treatment. Tolerant and persistent bacteria are not growing but survive longer than a susceptible population, resulting in an increase in the minimum duration of killing (MDK99), the minimum time it takes to kill 99% of the population. Once antibiotics wane, growth resumes.
It is important to distinguish between tolerance and persistence, since the mechanisms of surviving antibiotic exposure and resuscitation likely differ depending on whether the entire bacterial population is affected (tolerance) or only a subpopulation (persistence) (11). However, most of the current literature predates these findings, and hence, antibiotic persistence and tolerance are often discussed in the same light. To include all relevant in vivo research, we will discuss both antibiotic persistence and tolerance in this review.
Another important measurement for identifying antibiotic-tolerant or antibiotic-persistent populations is to confirm their slowed growth. This can be done using fluorescence dilution methods, which identify slow-growing populations by the accumulation of fluorescent signal, whereas rapidly dividing cells dilute out the signal (15, 16). Importantly, this is a method that can be used at the single-cell level, which is crucial for identifying subpopulations of slow-growing bacteria and phenotypic heterogeneity.
Although these guidelines have proved valuable for research on antibiotic persistence, they have mainly been set up and used for in vitro, culture-based work (3). Fewer guidelines have been established for work in vivo from host tissues. However, it is of the utmost importance to study antibiotic persistence in vivo given how different the host environment is from in vitro culture systems.
The importance of phenotypic reversibility of persisters and studying antibiotic persistence in vivo
It is well known that stationary-phase bacteria become tolerant to antibiotic treatment as nutrients become limiting and bacteria enter a slow-growing state (17). However, a study by Pu et al. showed that E. coli grown for 24 hours become more tolerant to antibiotic treatment by entering deeper into a dormant state that is characteristically different from persisters (18). Interestingly, longer bacterial culture times correlated with increased intracellular protein aggregation. At earlier stages, these protein aggregates or “aggresomes” consist of ribosomal proteins and proteins related to carbon metabolism and oxidative phosphorylation, which correlates with the lowered metabolism and the slow-growing state of persisters. However, when bacteria enter a completely dormant state, proteins involved in DNA replication and DNA repair also begin to form aggregates (18). Without DNA replication proteins available, this likely prevents resumption of cell division.
These dormant bacteria also lacked the ability to resolve protein aggregates via the use of proteases and chaperones, rendering them completely dormant and unable to resume growth after antibiotic exposure. This directly contrasts with persisters, which are capable of growth resumption when antibiotic concentrations wane. This protein aggregation phenotype has also been reported with clinical isolates of Staphylococcus aureus from abscess pus, specifically in bacteria that were phenotypically persistent to multiple antibiotics (19). Interestingly, when Huemer et al. exposed bacteria to acidified pH to mimic abscess conditions and examined the surviving fraction of bacteria after antibiotic treatment, they found that persisters had significantly reduced ATP levels and accumulation of insoluble proteins involved in metabolism and energy production (19). Importantly, when antibiotic exposure is removed, the bacteria revert back to a normal metabolic state, with normal ATP levels and resolution of the protein aggregates (19).
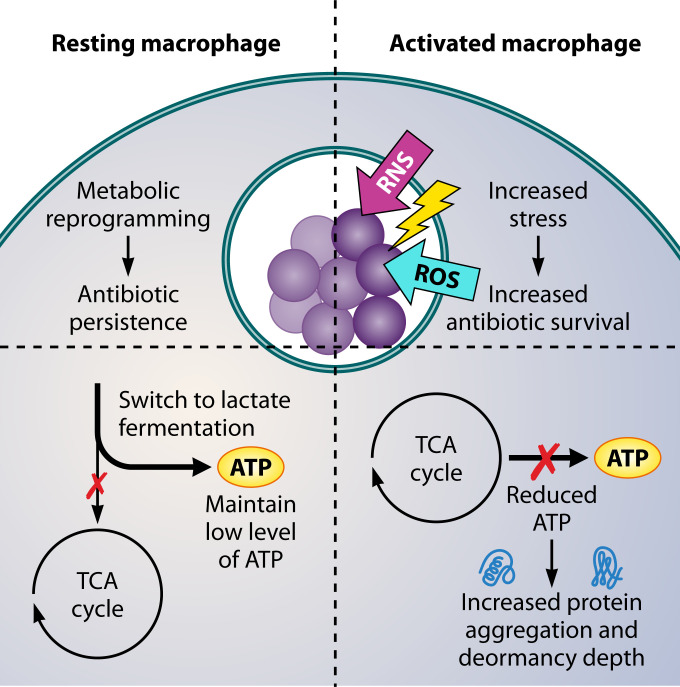
These studies reveal an important distinction between persisters and completely dormant bacteria and the importance of showing phenotypic reversibility when studying antibiotic persistence. This agrees with Helaine and colleagues studying intracellular Salmonella persisters in macrophages (4, 20). They show that antibiotic-tolerant mutants that are almost completely dormant survive better during antibiotic treatment at the population level but are unable to grow after antibiotics wane and, therefore, cannot cause infection relapse (4) or even successfully colonize a host in an animal model of infection (20). This again contrasts with persisters, which are not completely dormant and still retain a basal level of metabolic activity, including a low level of transcription and protein translation. However, it is important to note that persisters and dormant cells do not always exist as two extremes but rather a continuum referred to as “dormancy depth.” In reference (21), the authors show that intracellular S. aureus populations within macrophages can be heterogeneous and that dormancy depth is a spectrum that is influenced by host cell stress and reduction in bacterial ATP levels leading to protein aggregation (21) (Fig. 2). Thus, there are “dormant persisters” that have longer lag times but could potentially resuscitate and cause relapsing infections.
Fig 2.
Immune cell activation exposes intracellular bacteria to increased stress and increases antibiotic survival. Intracellular bacteria within resting macrophages reprogram their metabolism and enter a slow-growing and antibiotic-persistent state. When macrophages are activated, ROS and RNS are robustly produced exposing bacteria to a greater amount of stress, which promotes increased antibiotic survival. Bacteria within resting macrophages are able to alter metabolic pathways to maintain ATP levels. In contrast, robust production of ROS and RNS from activated macrophages likely do not permit bacteria time to make this metabolic switch. ROS and RNS attack tricarboxylic acid (TCA) cycle proteins resulting in a reduction in ATP levels, which correlates with increased protein aggregation and dormancy depth.
Nutrient limitation
Although bacteria can access niches in the host environment with resources to sustain them, bacteria often find themselves in nutrient-limiting conditions. Particularly in contrast to enteric bacteria that reside in the nutrient-rich environment of the gut, invasive bacteria that spread systemically to deep tissue sites, such as the spleen, liver, and kidneys or mucosal pathogens that colonize the lungs, likely encounter nutrient-limited conditions. At these sites, nutrients can be actively sequestered by immune cells, a phenomenon known as nutritional immunity (22). Interestingly, although this imposes stress on the bacteria, these conditions can induce a slow-growing state that promotes bacterial survival during antibiotic treatment (17, 23).
The stringent response is induced by nutrient starvation and is one of the most widely studied antibiotic persistence mechanisms (8). When nutrients, such as amino acids and other metabolites, are scarce, it is sensed by the cellular enzymes RelA and SpoT in E. coli (24), which synthesizes the alarmone signal, ppGpp. ppGpp can bind to RNA polymerase and downregulate transcription of genes involved in cell division and metabolism, shifting bacteria into a slow-growing state until they re-enter an environment with sufficient nutrients to resume growth (24). More in-depth discussion of the stringent response can be found elsewhere (24).
For Mycobacterium tuberculosis (Mtb), bacteria enter a nutrient-limiting and non-replicating state during chronic infection of the lungs. After initial intracellular replication within macrophages, bacteria can form extracellular communities within a granuloma structure, where the central bacterial community is surrounded by immune cells, including additional macrophages, neutrophils, dendritic cells, and lymphocytes (25). Bacteria are trapped by this barrier of immune cells and prevented from spreading to other sites, but immune cells are ineffective at eliminating bacteria, creating a stalemate between the bacteria and the host (25). Unable to leave in search of a new site with more nutrients, bacteria enter an almost dormant state. Hypoxia and nutrient starvation conditions cause Mtb to shift their metabolism from oxidative phosphorylation and lipid biosynthesis to synthesis of triacylglycerol (TAG) (26), which the bacteria store in lipid inclusions until the latent bacteria reactivate and utilize TAG stores for resumption of growth (27). This accumulation of lipids during nutrient starvation conditions correlates with increased dormancy and antibiotic tolerance. It is unclear whether TAG synthesis is required for antibiotic tolerance as some tsg mutants are still tolerant to antibiotic treatment (28). However, given the importance of TAG stores for Mtb resuscitation, it is possible that TAG synthesis alone may not be necessary for antibiotic survival, but instead for the phenotypic reversibility of persister cells and growth resumption, which is arguably equally as important for the success of antibiotic persisters.
It is also unclear the exact mechanisms that regulate this shift in metabolism to TAG synthesis, but it is hypothesized to be regulated by the stringent response (26). Deletion of the only known mycobacterial alarmone synthetase, Rel, abolishes the ability of bacteria to downregulate their metabolism in response to nutrient starvation cues, which causes bacteria to continue active cell division instead of entering a dormant state (29). Interestingly, the metabolic profile of Δrel more closely resembles that of bacteria grown in nutrient-rich conditions, including maintenance of TCA cycle activity, lipid metabolism, and ATP levels (29). Unable to transition into a dormant state during nutrient starvation, Δrel is more susceptible to antibiotic treatment. Whereas there is an approximately 500-fold increase in the minimum concentration of isoniazid needed to kill wild-type bacteria in nutrient starvation conditions, there is no difference in antibiotic susceptibility of Δrel regardless of nutrient conditions. However, since time-kill curves are not shown, it is unclear if the entire population is susceptible or if a subpopulation still survives, which would be informative, since mechanisms other than the stringent response can play a role in antibiotic tolerance and persistence of Mtb (5, 30). These additional experiments would aid in distinguishing between antibiotic tolerance compared to persistence.
Most importantly, though, Dutta et al. show that Δrel is more sensitive to isoniazid treatment during chronic infection of the lungs in a BALB/c mouse model (29). They also use a C3HeB/FeJ mouse model that has been shown to better mimic human lung pathology during Mtb infection, particularly in the development of necrotic granulomas, and find that without antibiotic treatment, mouse survival is significantly improved with Δrel. In the absence of antibiotic treatment, this indicates that Δrel is more efficiently cleared by the immune system. This also suggests that the stringent response and subsequent metabolic shift into dormancy are important in establishing the stalemate between Mtb and the host during granuloma formation and preventing bacterial clearance. Although this remains to be investigated, it is consistent with another study using a zebrafish infection model with Mycobacterium abscessus, another mycobacterial species that forms lung granulomas (28). Culturing bacteria in nitrogen-limited media prior to infection, which induces TAG synthesis and dormancy, led to increased bacterial load and caused increased zebrafish mortality, providing additional evidence that the ability of bacteria to become dormant correlates with their virulence.
The stringent response has also been shown to play an important role in the virulence and development of antibiotic persistence of Staphylococcus aureus. However, unlike culture-based systems that use nutrient deprivation or drug treatments to induce the stringent response (31, 32), it remains unclear how much of a role nutrient limitation has in inducing the stringent response in vivo. Clinical isolates of methicillin-resistant Staphylococcus aureus involved in an antibiotic-persistent infection have higher levels of ppGpp and virulence gene expression than isolates from an infection that was resolved by antibiotic treatment, indicating that induction of the stringent response strongly correlates with antibiotic persistence (33). This has also been observed in antibiotic-persistent isolates of Enterococcus faecium from an immunocompromised patient exposed to prolonged antibiotic treatment. Isolates acquired a relA mutation resulting in higher amounts of ppGpp, and isolates were less susceptible to antibiotic treatment specifically in a biofilm setting (34). The stringent response was also shown to be crucial for bacterial survival during antibiotic treatment of systemic S. aureus infection. In a rabbit infective endocarditis model, deletion of the alarmone synthetase, relP, renders S. aureus more sensitive to vancomycin treatment, resulting in a significant reduction in bacterial load at multiple different tissue sites, including the kidney, spleen, and heart tissue (33). It is unclear what stress or stresses are exactly responsible for the induction of the stringent response in this model. During systemic infection, S. aureus characteristically forms abscess communities, which are nutrient-limiting environments where the stringent response may be triggered (35). However, it is important to note that the stringent response can be induced by multiple different factors, including antibiotics themselves.
Interestingly, the stringent response has also been implicated to play a role in antibiotic persistence during intracellular infection with S. aureus, but it is again unclear whether nutrient limitation is the major trigger. S. aureus is not typically regarded as an intracellular pathogen, but it has been found to reside within host cells during infection, including macrophages, epithelial cells, keratinocytes, and osteoblasts. Interestingly, all these cell types harbor an increased fraction of surviving intracellular bacteria during antibiotic treatment, in comparison to exponential phase bacteria in liquid culture, and demonstrate these persister cells are slow growing using a fluorescence dilution method (36). Deleting the stringent response genes, rsh and codY, resulted in a reduction in the fraction of persisters, indicating that the stringent response plays an important role in increasing antibiotic survival. However, it is important to note that other stress responses were also induced under these conditions, including the SOS response, cell wall stress stimulus (CWSS), and heat shock responses. Further research is needed to delineate what role each of these responses plays in the antibiotic persistence of intracellular S. aureus, as it is possible that multiple mechanisms in addition to the stringent response may be responsible for the increased survival of antibiotic persisters.
Additionally, the stringent response does not seem to be induced by the host environment prior to antibiotic treatment (36). Only the other stress responses (SOS, CWSS, and heat shock) are induced in the absence of antibiotics, indicating that in this model, the stringent response may be induced by antibiotic treatment rather than nutrient limitation. In this model of intracellular infection, it is possible that bacteria are not nutrient deprived in general. Amino acid biosynthesis genes are downregulated in persisters, suggesting that intracellular bacteria have access to amino acids and potentially other metabolites. Interestingly, persisters are also still metabolically active in some regard. Persisters actively translate proteins and maintain ATP levels, likely by switching their metabolism to lactate fermentation. Interestingly, this switch of carbon sources has been shown to correspond with a growth arrest in part due to the stringent response and has been linked to antibiotic persistence (37, 38). Additionally, although Mtb persisters during chronic infection are considered to be dormant cells, they have also been found to be active in protein translation (39). This supports the idea that, although persisters can exist in a non-replicating state, they are not completely dormant.
Oxidative and nitrosative stress
ROS and RNS are also major stressors that can induce antibiotic persistence and tolerance. ROS and RNS are antimicrobial compounds produced in response to infection and can either aid in eliminating or restricting the growth of intracellular bacteria or be released to target extracellular bacteria. Both ROS and RNS are known to cause damage to proteins, nucleic acids, and lipids of bacteria (40–42). Bacteria possess detoxifying enzymes and utilize repair mechanisms that can alleviate the damage. These mechanisms help bacteria survive oxidative and nitrosative stress, and as a part of the stress response, bacteria can enter a slow-growing or growth-arrested state making them recalcitrant to antibiotics.
Reactive oxygen species
Interestingly, some bacteria, such as Streptococcus pneumoniae, produce their own ROS to defend themselves against host cells and compete with other microbiota in the respiratory tract. As a result, S. pneumoniae also genetically encodes for a variety of mechanisms to protect itself from oxidative damage. These mechanisms have been shown to be crucial for surviving host oxidative stress and antibiotic exposure. Hernandez-Morfa et al. showed in culture-based experiments that pre-treatment with H2O2 induces tolerance to fluoroquinolone (FQ) antibiotics that target the DNA gyrase of bacteria (43). As shown by others, exposure to the FQ antibiotic, levofloxacin, alone can increase the intracellular level of H2O2 in E. coli, which is postulated to contribute to the bactericidal activity of these antibiotics (44). Surprisingly, treatment with H2O2 before FQ exposure reduces this intracellular level of H2O2 in S. pneumoniae (43). They find that H2O2 treatment upregulates the detoxifying enzymes tpxD and sodA and, paradoxically, increases expression of the pyruvate oxidase, spxB, which is responsible for synthesizing a majority of the H2O2 produced endogenously by S. pneumoniae. Using deletion mutants, they find that each of these genes are important for levofloxacin tolerance in human cell culture models with H2O2 produced by epithelial cells (type II pneumocytes), macrophages, and neutrophils. Their data suggest that both endogenous and host H2O2 contribute to FQ tolerance of S. pneumoniae. Pre-exposure to oxidative stress in either case likely upregulates detoxifying and repair enzymes that help bacteria survive the increase in intracellular H2O2 induced by fluoroquinolones as well as inducing a slowed-growth response, which was demonstrated by Hernandez-Morfa et al. using a fluorescence dilution approach (43).
A mechanism by which ROS promotes slowed growth and antibiotic tolerance has been elucidated by Rowe et al. for S. aureus during intracellular infection of macrophages (45). They demonstrated that ROS attacks iron-sulfur clusters within bacterial TCA cycle proteins, such as aconitase, thus diminishing their activity and resulting in low levels of ATP and low levels of oxygen consumption. Rifampin tolerance could be reversed by supplementing with glucose, which allowed the bacteria to produce ATP through glycolytic fermentation and restored sensitivity to rifampin (45). It would be interesting to know if this phenotype is specific to rifampin treatment or applicable to other antibiotics as well. In references (36, 36), intracellular S. aureus maintain ATP levels likely via a metabolic reprogramming to lactate fermentation (Fig. 2) and are persistent to a variety of antibiotics (fluoroquinolones, aminoglycosides, and β-lactams), indicating that rescuing ATP levels alone may not completely restore antibiotic sensitivity depending on the antibiotic’s target (RNA polymerase vs others) and mechanism of action.
The different metabolic pathway utilized by intracellular S. aureus in reference (36) is likely due to the fact that they infected resting macrophages, whereas Rowe et al. primed macrophages with interferon-gamma (IFNγ) and lipopolysaccharide (LPS) prior to infection (36, 45). In reference (21), they do stimulate macrophages and find, similar to Rowe et al., that bacteria have reduced ATP levels and that host cell ROS is the primary driver of this (Fig. 2).
It is also important to note that in Beam et al. (46), ROS is not the only stress that induces antibiotic tolerance in stimulated macrophages. They find that ROS in the form of superoxide can react with nitric oxide (NO) to form peroxynitrite. Inhibiting either superoxide production (via Nox) or NO production (via iNos) alone significantly reduces tolerance to rifampin, indicating that both ROS and RNS together damage TCA cycle proteins and reduce ATP levels, corresponding with a significant increase in the fraction of surviving cells after antibiotic treatment as compared to unstimulated macrophages (46).
Importantly, Rowe et al. show in an animal model of systemic S. aureus infection that mice lacking the oxidative burst (Ncf1−/−) are more responsive to rifampin treatment, indicating that host oxidative stress is important for predisposing bacteria to survive antibiotic treatment (45). However, they only see this effect in the spleen of mice and not the kidneys, indicating that different host stresses play a role in antibiotic tolerance depending on the anatomical niche and, presumably, which immune cells the bacteria primarily interact with. In the spleen, S. aureus are known to interact with macrophages and are found intracellularly, but in the kidneys, S. aureus primarily interact with neutrophils that surround staphylococcal abscess communities (SACs) (47). Macrophages can be seen in the kidney, but they are blocked by the surrounding layer of neutrophils and do not interact with S. aureus as closely (48) (Fig. 3). Neutrophils also produce ROS, but the results of Rowe et al. suggest that this host stress may not play a role in the antibiotic tolerance of kidney abscesses (45).
Fig 3.
Extracellular communities of bacteria are exposed to different immune cells and host stressors. Yersinia pseudotuberculosis is surrounded by neutrophils and macrophages. Uneven diffusion of RNS results in a highly stressed subpopulation at the periphery that preferentially survives antibiotic treatment. Mycobacterium tuberculosis is surrounded by macrophages and lymphocytes. Nutrient limitation and RNS play a major role in inducing antibiotic tolerance, although further work is needed to clearly distinguish between tolerance and persistence for both of these stressors. It is unclear whether certain subpopulations within the granuloma are more exposed to stressors. Staphylococcus aureus is surrounded by neutrophils and macrophages. The exact stressors that induce antibiotic tolerance in this model are still unknown.
In an ex vivo system using a gel matrix to model the 3D structure of SACs, Hofstee et al. show that even in the absence of neutrophils, bacteria become tolerant to gentamicin during SAC maturation (49). This is in part due to the fibrin barrier surrounding the abscess that prevents diffusion of antibiotics. However, even in the absence of the fibrin barrier, SACs are not fully cleared, suggesting that there are some tolerance mechanisms that may be unique to bacteria that exist in tightly packed structures. It is possible that nutrient limitation and/or reduced oxygen diffusion play a role, but further research will be needed to understand what stresses induce tolerance in these abscess communities (Fig. 3). It is also unclear whether the phenotypes seen in this gel matrix model reflect antibiotic tolerance or persistence without assaying bacterial viability over time (49).
Reactive nitrogen species
Nitrosative stress has been shown to play a role in the antibiotic tolerance of intracellular Mtb. Interestingly, the activation state of immune cells prior to infection also had a significant effect on antibiotic tolerance, similar to the findings of Beam et al. (46, 50). In Liu et al. (50), mice that are immunized with heat-killed Mtb before infection have less bacterial burden but are less sensitive to isoniazid treatment. They further test this in cell culture and show that bacteria internalized in activated macrophages become more tolerant to multiple different antibiotics. Looking at bacterial transcriptional changes, they find that intracellular bacteria in activated macrophages (primed with IFNγ and LPS) exhibit gene expression changes indicative of increased exposure to host stressors including, nutrient deprivation, acidification, oxidative stress, and nitrosative stress. They test the role of RNS and ROS specifically using bone marrow-derived macrophages isolated from Nos2 (RNS)-deficient mice and p47phox (ROS)-deficient mice. The authors found that Nos2 deficiency had the greatest impact on isoniazid and rifampin tolerance, restoring sensitivity to that of resting macrophages, indicating that immune cell activation strongly induces tolerance due to nitrosative stress (50).
Interestingly, in resting macrophages during antibiotic treatment, bacteria also exhibit gene expression changes that resemble increased host stress with activated macrophages, when compared to antibiotic treatment alone in broth culture, or infection of resting macrophages without antibiotic treatment (50). This suggests that when bacteria are inhibited by antibiotics, they are unable to suppress host defense mechanisms and become more susceptible to host-derived stressors. When Mtb are phagocytosed, they typically inhibit phagolysosome maturation and prevent acidification, allowing them to either reside within vacuoles or escape the phagosome into the cytosol (51). However, these expression changes during antibiotic treatment indicate that Mtb are likely unable to escape or inhibit these host defenses as expertly when they are inhibited by antibiotics. The host stress indirectly imposed by the antibiotics may then explain why there are still surviving bacteria in resting macrophages after antibiotic treatment.
However, it is difficult to distinguish whether these phenotypes are tolerance or persistence in this model. Using a murine macrophage cell line (J774), time-kill curves clearly show biphasic killing of intracellular Mtb by rifampin when macrophages are resting and monophasic killing when macrophages are activated (50). However, this phenotype was less clear for Mtb infection of other cell types and for other antibiotics. In general, it may be necessary to include other measurements in addition to time-kill curves to distinguish between tolerance and persistence more clearly. For example, using fluorescent reporter strategies or RNA-fluorescent in situ hybridization to label which bacterial cells within a population are specifically responding to stress would clarify whether there is phenotypic heterogeneity or if the entire population is exposed to the stress.
Nitric oxide has also been determined to be an important host stress that predisposes Yersinia pseudotuberculosis (Yptb) to survive antibiotic treatment in vivo. Yptb is an enteric pathogen that typically presents as a self-limiting gastrointestinal infection. In immunocompromised individuals, bacteria can spread systemically where they seed deep tissue sites, such as the spleen, and form microcolonies surrounded by immune cells, including neutrophils and macrophages (52–54) (Fig. 3). In a systemic mouse model of infection, Raneses et al. showed that doxycycline treatment reduces the bacterial load of Yptb in the spleen, but a subpopulation of bacteria survives and can resume growth after antibiotic concentrations wane (55). Using a fluorescent reporter for hmp, a detoxifying gene expressed in response to NO, Raneses et al. find that hmp-expressing bacteria make up a significant percentage of the surviving bacteria after doxycycline treatment (55). This indicates that NO-stressed bacteria preferentially survive antibiotic treatment. The bacteria specifically at the periphery of the microcolony respond to NO stress, indicating that NO does not diffuse through evenly and reach the entire bacterial population (52) (Fig. 3). Using a fluorescence dilution approach, Liu et al. find that heightened hmp expression at the periphery of microcolonies correlates with a subpopulation of slowly dividing Yptb cells (56). These findings are consistent with the idea that increased NO exposure predisposes bacteria to survive antibiotic treatment by inducing a slowed-growth phenotype and antibiotic persistence.
Therapeutic options and strategies for combating antibiotic persistence
Concerningly, there is no broadly established strategy for targeting antibiotic persisters. Interestingly, the in vivo studies discussed in the sections above highlight a few potential options.
One strategy is to inhibit the bacterial stress response that leads to slowed growth and antibiotic persistence. Given the prominent role that the stringent response plays in the antibiotic persistence of Mtb, this could be a promising strategy. In Dutta et al., they test a small molecule inhibitor of the alarmone synthetase, Rel (29). In culture, the inhibitor increased the efficacy of isoniazid treatment against nutrient-starved Mtb. Further testing in vivo, with animal models that recapitulate human tuberculosis pathology, will be informative for how efficacious this treatment strategy could be clinically. Notably, the findings of Liu et al. suggest that nitrosative stress plays a prominent role during intracellular Mtb infection (50), indicating that the stringent response may not be the only contributor to antibiotic persistence or tolerance for Mtb. Oxidative stress is known to induce the stringent response in Mtb (57), but it is unclear whether this applies to nitrosative stress as well. Based on studies that have showed that nitric oxide can disrupt bacterial cell division, RNS may contribute more to growth arrest compared to slowed growth (56, 58).
It is also unclear whether this strategy of targeting specific stress responses will be applicable for all bacterial pathogens. The stringent response may play an important role in S. aureus antibiotic persistence, particularly during systemic infection where bacteria are found in nutrient-limiting environments within deep tissue sites. However, the results of reference (36) demonstrated that while the stringent response is involved, other stress responses also play a role, indicating that inhibiting the stringent response would not completely eradicate persisters (36).
Another strategy is to target the metabolic reprogramming of antibiotic persisters. A commonality among all bacterial persisters is that they reprogram their metabolism when they switch into a slow-growing and antibiotic-recalcitrant state. There are several studies showing that supplementing with nutrients can prevent downregulation and reprogramming of metabolism and consequently sensitize persisters to antibiotics. For Mtb, bacteria switch from oxidative phosphorylation to synthesis of TAG during nutrient-limiting and hypoxic conditions. Supplementing with the TCA cycle substrate, phosphoenol-pyruvate, is sufficient to restore growth and antibiotic susceptibility in vitro and seems to correlate with in vivo studies (26, 28). Interestingly, a recent study has discovered that commensal microbiota within the gut of mice produce certain metabolites that can sensitize E. coli and S. aureus bacteria to different bactericidal antibiotics (59). Treatment with the metabolite, indole-3-acetic acid, increased the ATP production and intracellular ROS levels of bacteria. These studies showcase that targeting the metabolism of persisters is a promising strategy to target these antibiotic-recalcitrant populations.
CONCLUDING REMARKS
Improving antibiotic efficacy is a complex problem that involves tackling not only antibiotic resistance but also antibiotic tolerance and persistence. It is extremely important to apply findings from culture-based experiments to host infection models to determine the role of individual bacterial stress response pathways in the complexity of the host environment. As discussed here, in vivo models are becoming more of a focus in recent years and will be critical in identifying pathways that can be targeted to improve antibiotic efficacy and eliminate all bacterial subpopulations from host tissues.
The use of fluorescent reporters in vivo provides further insight into how differential exposure to host stress within certain environments can result in phenotypic heterogeneity. These tools are valuable for systems such as SACs and Mycobacterium tuberculosis granulomas, where extracellular bacteria are surrounded by immune cell populations. In these systems, it is less clear if specific subpopulations of bacteria are preferentially exposed to host stress and thus predisposed to survive antibiotic treatment. Whether host stress responses impact subpopulations of bacteria or the entire bacterial population is critical in determining whether antibiotic persistence or tolerance occurs downstream of these stresses. Determining the extent of bacterial heterogeneity is also critical in devising effective treatment approaches, which will need to utilize combination therapeutic approaches to specifically target all relevant bacterial subpopulations.
ACKNOWLEDGMENTS
This work was supported by NIAID grant 1R21AI154116-01A1 to K.M.D. K.L.C. is also supported by training grant 2T32AI007417-26 through NIAID. The funders had no role in the content, interpretation of results, or decision to submit this work for publication.
Conceptualization, K.L.C. and K.M.D.; writing, the original draft preparation, K.L.C.; writing, review, and editing, K.L.C. and K.M.D.
The authors of this manuscript declare no conflicts of interest.
Biographies

Katherine L. Cotten received her B.S. in Biology from the State University of New York at Geneseo. At SUNY Geneseo she studied the sexual development of Neurospora crassa in the laboratory of Dr. Elizabeth Hutchison. During her undergraduate career she also had the opportunity to do summer research at the New York State Department of Health at Wadsworth Center, one summer in the laboratory of Dr. Todd Gray and Dr. Keith Derbyshire, identifying small proteins in Mycobacterium tuberculosis, and another summer in the laboratory of Dr. Pallavi Ghosh, elucidating a mechanism of antibiotic resistance in Mycobacterium abscessus. She is now pursuing her Ph.D. at the Johns Hopkins Bloomberg School of Public Health. In the lab of Dr. Kimberly Davis, she studies how bacteria phenotypically respond to antibiotic treatment using Yersinia pseudotuberculosis as a model bacterium with the goal of identifying gene expression changes that are necessary to survive antibiotic treatment.

Kimberly M. Davis received her B.S. and M.S. in Cell and Molecular Biology from the University of Michigan. She completed her Ph.D. in Cell and Molecular Biology at the University of Pennsylvania in the laboratory of Dr. Jeffrey Weiser, and studied the contribution of host sensing of peptidoglycan to clearance of pneumococcal colonization. She completed her post-doctoral research in the laboratory of Dr. Ralph Isberg at Tufts University, where her research utilized Yersinia pseudotuberculosis mouse models of infection to identify the spatial location of phenotypically distinct bacterial cells. She is currently an Assistant Professor at the Johns Hopkins Bloomberg School of Public Health, and has continued to use Y. pseudotuberculosis mouse models to understand how subsets of bacteria cooperate to establish infection within deep tissues, and to determine how immune cells differentially impact the growth rates and antibiotic susceptibility of subpopulations of bacteria.
Contributor Information
Kimberly Michele Davis, Email: kdavi140@jhu.edu.
Kumaran S. Ramamurthi, National Cancer Institute, Bethesda, Maryland, USA
REFERENCES
- 1. Brauner A, Fridman O, Gefen O, Balaban NQ. 2016. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 14:320–330. doi: 10.1038/nrmicro.2016.34 [DOI] [PubMed] [Google Scholar]
- 2. Fauvart M, De Groote VN, Michiels J. 2011. Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies. J Med Microbiol 60:699–709. doi: 10.1099/jmm.0.030932-0 [DOI] [PubMed] [Google Scholar]
- 3. Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ, Dehio C, Fortune S, Ghigo J-M, Hardt W-D, Harms A, Heinemann M, Hung DT, Jenal U, Levin BR, Michiels J, Storz G, Tan M-W, Tenson T, Van Melderen L, Zinkernagel A. 2019. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17:441–448. doi: 10.1038/s41579-019-0207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hill PWS, Moldoveanu AL, Sargen M, Ronneau S, Glegola-Madejska I, Beetham C, Fisher RA, Helaine S. 2021. The vulnerable versatility of Salmonella antibiotic persisters during infection. Cell Host Microbe 29:1757–1773. doi: 10.1016/j.chom.2021.10.002 [DOI] [PubMed] [Google Scholar]
- 5. Kreutzfeldt KM, Jansen RS, Hartman TE, Gouzy A, Wang R, Krieger IV, Zimmerman MD, Gengenbacher M, Sarathy JP, Xie M, Dartois V, Sacchettini JC, Rhee KY, Schnappinger D, Ehrt S. 2022. CinA mediates multidrug tolerance in Mycobacterium tuberculosis. Nat Commun 13:2203. doi: 10.1038/s41467-022-29832-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. 2004. Bacterial persistence as a phenotypic switch. Science 305:1622–1625. doi: 10.1126/science.1099390 [DOI] [PubMed] [Google Scholar]
- 7. Fang FC, Frawley ER, Tapscott T, Vázquez-Torres A. 2016. Bacterial stress responses during host infection. Cell Host Microbe 20:133–143. doi: 10.1016/j.chom.2016.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maisonneuve E, Gerdes K. 2014. Molecular mechanisms underlying bacterial persisters. Cell 157:539–548. doi: 10.1016/j.cell.2014.02.050 [DOI] [PubMed] [Google Scholar]
- 9. Helaine S, Cheverton AM, Watson KG, Faure LM, Matthews SA, Holden DW. 2014. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 343:204–208. doi: 10.1126/science.1244705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newson JP, Gaissmaier MS, McHugh SC, Hardt W-D. 2022. Studying antibiotic persistence in vivo using the model organism Salmonella typhimurium. Curr Opin Microbiol 70:102224. doi: 10.1016/j.mib.2022.102224 [DOI] [PubMed] [Google Scholar]
- 11. Ronneau S, Hill PW, Helaine S. 2021. Antibiotic persistence and tolerance: not just one and the same. Curr Opin Microbiol 64:76–81. doi: 10.1016/j.mib.2021.09.017 [DOI] [PubMed] [Google Scholar]
- 12. Bumann D. 2019. Salmonella single-cell metabolism and stress responses in complex host tissues. Microbiol Spectr 7. doi: 10.1128/microbiolspec.BAI-0009-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brauner A, Shoresh N, Fridman O, Balaban NQ. 2017. An experimental framework for quantifying bacterial tolerance. Biophys J 112:2664–2671. doi: 10.1016/j.bpj.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gollan B, Grabe G, Michaux C, Helaine S. 2019. Bacterial persisters and infection: past, present, and progressing. Annu Rev Microbiol 73:359–385. doi: 10.1146/annurev-micro-020518-115650 [DOI] [PubMed] [Google Scholar]
- 15. Helaine S, Holden DW. 2013. Heterogeneity of intracellular replication of bacterial pathogens. Curr Opin Microbiol 16:184–191. doi: 10.1016/j.mib.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 16. Helaine S, Thompson JA, Watson KG, Liu M, Boyle C, Holden DW. 2010. Dynamics of intracellular bacterial replication at the single cell level. Proc Natl Acad Sci U S A 107:3746–3751. doi: 10.1073/pnas.1000041107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, McKay G, Siehnel R, Schafhauser J, Wang Y, Britigan BE, Singh PK. 2011. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334:982–986. doi: 10.1126/science.1211037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pu Y, Li Y, Jin X, Tian T, Ma Q, Zhao Z, Lin S-Y, Chen Z, Li B, Yao G, Leake MC, Lo C-J, Bai F. 2019. ATP-dependent dynamic protein aggregation regulates bacterial dormancy depth critical for antibiotic tolerance. Mol Cell 73:143–156. doi: 10.1016/j.molcel.2018.10.022 [DOI] [PubMed] [Google Scholar]
- 19. Huemer M, Mairpady Shambat S, Bergada-Pijuan J, Söderholm S, Boumasmoud M, Vulin C, Gómez-Mejia A, Antelo Varela M, Tripathi V, Götschi S, Marques Maggio E, Hasse B, Brugger SD, Bumann D, Schuepbach RA, Zinkernagel AS. 2021. Molecular reprogramming and phenotype switching in Staphylococcus aureus lead to high antibiotic persistence and affect therapy success. Proc Natl Acad Sci U S A 118:e2014920118. doi: 10.1073/pnas.2014920118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michaux C, Ronneau S, Giorgio RT, Helaine S. 2022. Antibiotic tolerance and persistence have distinct fitness trade-offs. PLoS Pathog 18:e1010963. doi: 10.1371/journal.ppat.1010963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peyrusson F, Nguyen TK, Najdovski T, Van Bambeke F. 2022. Host cell oxidative stress induces dormant Staphylococcus aureus persisters. Microbiol Spectr 10:e0231321. doi: 10.1128/spectrum.02313-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skaar EP, Raffatellu M. 2015. Metals in infectious diseases and nutritional immunity. Metallomics 7:926–928. doi: 10.1039/c5mt90021b [DOI] [PubMed] [Google Scholar]
- 23. Eng RH, Padberg FT, Smith SM, Tan EN, Cherubin CE. 1991. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob Agents Chemother 35:1824–1828. doi: 10.1128/AAC.35.9.1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Irving SE, Choudhury NR, Corrigan RM. 2021. The stringent response and physiological roles of (pp)pGpp in bacteria. Nat Rev Microbiol 19:256–271. doi: 10.1038/s41579-020-00470-y [DOI] [PubMed] [Google Scholar]
- 25. Bussi C, Gutierrez MG. 2019. Mycobacterium tuberculosis infection of host cells in space and time. FEMS Microbiol Rev 43:341–361. doi: 10.1093/femsre/fuz006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim J, Lee JJ, Lee S-K, Kim S, Eum S-Y, Eoh H. 2021. Phosphoenolpyruvate depletion mediates both growth arrest and drug tolerance of Mycobacterium tuberculosis in hypoxia. Proc Natl Acad Sci U S A 118:e2105800118. doi: 10.1073/pnas.2105800118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kapoor N, Pawar S, Sirakova TD, Deb C, Warren WL, Kolattukudy PE. 2013. Human granuloma in vitro model, for TB dormancy and resuscitation. PLoS One 8:e53657. doi: 10.1371/journal.pone.0053657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santucci P, Johansen MD, Point V, Poncin I, Viljoen A, Cavalier J-F, Kremer L, Canaan S. 2019. Nitrogen deprivation induces triacylglycerol accumulation, drug tolerance and hypervirulence in mycobacteria. Sci Rep 9:8667. doi: 10.1038/s41598-019-45164-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dutta NK, Klinkenberg LG, Vazquez M-J, Segura-Carro D, Colmenarejo G, Ramon F, Rodriguez-Miquel B, Mata-Cantero L, Porras-De Francisco E, Chuang Y-M, Rubin H, Lee JJ, Eoh H, Bader JS, Perez-Herran E, Mendoza-Losana A, Karakousis PC. 2019. Inhibiting the stringent response blocks Mycobacterium tuberculosis entry into quiescence and reduces persistence. Sci Adv 5:eaav2104. doi: 10.1126/sciadv.aav2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vilchèze C, Jacobs WR. 2019. The isoniazid paradigm of killing, resistance, and persistence in Mycobacterium tuberculosis. J Mol Biol 431:3450–3461. doi: 10.1016/j.jmb.2019.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao W, Chua K, Davies JK, Newton HJ, Seemann T, Harrison PF, Holmes NE, Rhee H-W, Hong J-I, Hartland EL, Stinear TP, Howden BP, Cheung A. 2010. Two novel point mutations in clinical Staphylococcus aureus reduce linezolid susceptibility and switch on the stringent response to promote persistent infection. PLoS Pathog 6:e1000944. doi: 10.1371/journal.ppat.1000944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson KL, Roberts C, Disz T, Vonstein V, Hwang K, Overbeek R, Olson PD, Projan SJ, Dunman PM. 2006. Characterization of the Staphylococcus aureus heat shock, cold shock, stringent, and SOS responses and their effects on log-phase mRNA turnover. J Bacteriol 188:6739–6756. doi: 10.1128/JB.00609-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li L, Bayer AS, Cheung A, Lu L, Abdelhady W, Donegan NP, Hong J-I, Yeaman MR, Xiong YQ. 2020. The stringent response contributes to persistent methicillin-resistant Staphylococcus aureus endovascular infection through the purine biosynthetic pathway. J Infect Dis 222:1188–1198. doi: 10.1093/infdis/jiaa202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Honsa ES, Cooper VS, Mhaissen MN, Frank M, Shaker J, Iverson A, Rubnitz J, Hayden RT, Lee RE, Rock CO, Tuomanen EI, Wolf J, Rosch JW. 2017. RelA mutant Enterococcus faecium with multiantibiotic tolerance arising in an immunocompromised host. mBio 8:e02124-16. doi: 10.1128/mBio.02124-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng AG, DeDent AC, Schneewind O, Missiakas D. 2011. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol 19:225–232. doi: 10.1016/j.tim.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peyrusson F, Varet H, Nguyen TK, Legendre R, Sismeiro O, Coppée J-Y, Wolz C, Tenson T, Van Bambeke F. 2020. Intracellular Staphylococcus aureus persisters upon antibiotic exposure. Nat Commun 11:2200. doi: 10.1038/s41467-020-15966-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Radzikowski JL, Vedelaar S, Siegel D, Ortega ÁD, Schmidt A, Heinemann M. 2016. Bacterial persistence is an active σs stress response to metabolic flux limitation. Mol Syst Biol 12:882. doi: 10.15252/msb.20166998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Traxler MF, Chang D-E, Conway T. 2006. Guanosine 3',5'-bispyrophosphate coordinates global gene expression during glucose-lactose diauxie in Escherichia coli. Proc Natl Acad Sci U S A 103:2374–2379. doi: 10.1073/pnas.0510995103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manina G, Dhar N, McKinney JD. 2015. Stress and host immunity amplify Mycobacterium tuberculosis phenotypic heterogeneity and induce nongrowing metabolically active forms. Cell Host Microbe 17:32–46. doi: 10.1016/j.chom.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 40. Crack JC, Green J, Thomson AJ, Le Brun NE. 2014. Iron-sulfur clusters as biological sensors: the chemistry of reactions with molecular oxygen and nitric oxide. Acc Chem Res 47:3196–3205. doi: 10.1021/ar5002507 [DOI] [PubMed] [Google Scholar]
- 41. Fang FC, Vázquez-Torres A. 2019. Reactive nitrogen species in host-bacterial interactions. Curr Opin Immunol 60:96–102. doi: 10.1016/j.coi.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ezraty B, Gennaris A, Barras F, Collet J-F. 2017. Oxidative stress, protein damage and repair in bacteria. Nat Rev Microbiol 15:385–396. doi: 10.1038/nrmicro.2017.26 [DOI] [PubMed] [Google Scholar]
- 43. Hernandez-Morfa M, Reinoso-Vizcaíno NM, Olivero NB, Zappia VE, Cortes PR, Jaime A, Echenique J. 2022. Host cell oxidative stress promotes intracellular fluoroquinolone persisters of Streptococcus pneumoniae. Microbiol Spectr 10:e0436422. doi: 10.1128/spectrum.04364-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dwyer DJ, Kohanski MA, Hayete B, Collins JJ. 2007. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol Syst Biol 3:91. doi: 10.1038/msb4100135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rowe SE, Wagner NJ, Li L, Beam JE, Wilkinson AD, Radlinski LC, Zhang Q, Miao EA, Conlon BP. 2020. Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection. Nat Microbiol 5:526. doi: 10.1038/s41564-020-0679-z [DOI] [PubMed] [Google Scholar]
- 46. Beam JE, Wagner NJ, Shook JC, Bahnson ESM, Fowler VG, Rowe SE, Conlon BP, Torres VJ. 2021. Macrophage-produced peroxynitrite induces antibiotic tolerance and supersedes intrinsic mechanisms of persister formation. Infect Immun 89:e0028621. doi: 10.1128/IAI.00286-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thammavongsa V, Missiakas DM, Schneewind O. 2013. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 342:863–866. doi: 10.1126/science.1242255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Winstel V, Schneewind O, Missiakas D. 2019. Staphylococcus aureus exploits the host apoptotic pathway to persist during infection. mBio 10:e02270-19. doi: 10.1128/mBio.02270-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hofstee MI, Riool M, Terjajevs I, Thompson K, Stoddart MJ, Richards RG, Zaat SAJ, Moriarty TF. 2020. Three-dimensional in vitro Staphylococcus aureus abscess communities display antibiotic tolerance and protection from neutrophil clearance. Infect Immun 88:e00293-20. doi: 10.1128/IAI.00293-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu Y, Tan S, Huang L, Abramovitch RB, Rohde KH, Zimmerman MD, Chen C, Dartois V, VanderVen BC, Russell DG. 2016. Immune activation of the host cell induces drug tolerance in Mycobacterium tuberculosis both in vitro and in vivo. J Exp Med 213:809–825. doi: 10.1084/jem.20151248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chandra P, Grigsby SJ, Philips JA. 2022. Immune evasion and provocation by Mycobacterium tuberculosis. Nat Rev Microbiol 20:750–766. doi: 10.1038/s41579-022-00763-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Davis KM, Mohammadi S, Isberg RR. 2015. Community behavior and spatial regulation within a bacterial microcolony in deep tissue sites serves to protect against host attack. Cell Host Microbe 17:21–31. doi: 10.1016/j.chom.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peterson LW, Philip NH, DeLaney A, Wynosky-Dolfi MA, Asklof K, Gray F, Choa R, Bjanes E, Buza EL, Hu B, Dillon CP, Green DR, Berger SB, Gough PJ, Bertin J, Brodsky IE. 2017. RIPK1-dependent apoptosis bypasses pathogen blockage of innate signaling to promote immune defense. J Exp Med 214:3171–3182. doi: 10.1084/jem.20170347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang Y, Khairallah C, Sheridan BS, van der Velden AWM, Bliska JB, Raffatellu M. 2018. CCR2+ inflammatory monocytes are recruited to Yersinia pseudotuberculosis pyogranulomas and dictate adaptive responses at the expense of innate immunity during oral infection. Infect Immun 86:e00782-17. doi: 10.1128/IAI.00782-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Raneses JR, Ellison AL, Liu B, Davis KM. 2020. Subpopulations of stressed Yersinia pseudotuberculosis preferentially survive doxycycline treatment within host tissues. mBio 11:e00901-20. doi: 10.1128/mBio.00901-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu B, Braza RED, Cotten KL, Davidson RK, Davis KM. 2022. NO-stressed Y. pseudotuberculosis have decreased cell division rates in the mouse spleen. Infect Immun 90:e0016722. doi: 10.1128/iai.00167-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stallings CL, Stephanou NC, Chu L, Hochschild A, Nickels BE, Glickman MS. 2009. CarD is an essential regulator of rRNA transcription required for Mycobacterium tuberculosis persistence. Cell 138:146–159. doi: 10.1016/j.cell.2009.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jones-Carson J, Yahashiri A, Kim J-S, Liu L, Fitzsimmons LF, Weiss DS, Vázquez-Torres A. 2020. Nitric oxide disrupts bacterial cytokinesis by poisoning purine metabolism. Sci Adv 6:eaaz0260. doi: 10.1126/sciadv.aaz0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu Y, Yang K, Jia Y, Shi J, Tong Z, Fang D, Yang B, Su C, Li R, Xiao X, Wang Z. 2021. Gut microbiome alterations in high-fat-diet-fed mice are associated with antibiotic tolerance. Nat Microbiol 6:874–884. doi: 10.1038/s41564-021-00912-0 [DOI] [PubMed] [Google Scholar]