ABSTRACT
The fungal pathogen Candida albicans must acquire phosphate to colonize, infect, and proliferate in the human host. C. albicans has four inorganic phosphate (Pi) transporters, Pho84 being the major high-affinity transporter; its cells can also use glycerophosphocholine (GPC) as their sole phosphate source. GPC is a lipid metabolite derived from deacylation of the lipid phosphatidylcholine. GPC is found in multiple human tissues, including the renal medulla, where it acts as an osmolyte. C. albicans imports GPC into the cell via the Git3 and Git4 transporters. Internalized GPC can be hydrolyzed to release Pi. To determine if GPC import and subsequent metabolism affect phosphate homeostasis upon Pi limitation, we monitored growth and phenotypic outputs in cells provided with either Pi or GPC. In pho84∆/∆ mutant cells that exhibit phenotypes associated with Pi limitation, GPC provision rescued sensitivity to osmotic and cell wall stresses. The glycerophosphodiesterase Gde1 was required for phenotypic rescue of osmotic stress by GPC provision. GPC provision, like Pi provision, resulted in repression of the PHO regulon and activation of TORC1 signaling. Pi uptake was similar to GPC uptake when phosphate availability was low (200 µM). While available at lower concentrations than Pi in the human host, GPC is an advantageous Pi source for the fungus because it simultaneously serves as a choline source. In summary, we find GPC is capable of substituting for Pi in C. albicans by many though not all criteria and may contribute to phosphate availability for the fungus in the human host.
IMPORTANCE
Candida albicans is the most commonly isolated species from patients suffering from invasive fungal disease. C. albicans is most commonly a commensal organism colonizing a variety of niches in the human host. The fungus must compete for resources with the host flora to acquire essential nutrients such as phosphate. Phosphate acquisition and homeostasis have been shown to play a key role in C. albicans virulence, with several genes involved in these processes being required for normal virulence and several being upregulated during infection. In addition to inorganic phosphate (Pi), C. albicans can utilize the lipid-derived metabolite glycerophosphocholine (GPC) as a phosphate source. As GPC is available within the human host, we examined the role of GPC in phosphate homeostasis in C. albicans. We find that GPC can substitute for Pi by many though not all criteria and is likely a relevant physiological phosphate source for C. albicans.
KEYWORDS: phosphate metabolism, phospholipids, Candida albicans, glycerophosphodiesters, phosphate, cell signaling
INTRODUCTION
Candida albicans is the species most commonly isolated from patients suffering from invasive fungal disease (1). It is also a commensal organism colonizing oral mucosa and the gastrointestinal and genitourinary tracts of many healthy individuals (2, 3). Within these host niches, C. albicans competes for resources with the host flora, necessitating a variety of strategies to utilize host nutrients (4, 5). Phosphate is a required nutrient and plays a key role in C. albicans survival and growth and in its ability to invade the host. Several genes involved in phosphate acquisition and homeostasis are upregulated during C. albicans infection (6–8).
The phosphate homeostatic system in C. albicans, known as the PHO regulon, is conserved among human fungal pathogens. The major transcriptional regulator of the PHO regulon is Pho4 (9, 10). Cells lacking Pho4 are unable to upregulate many genes involved in phosphate acquisition and are more sensitive to a variety of stressors, including osmotic and cell wall stresses (6, 10). Pho84, a major high-affinity inorganic H+/phosphate (Pi) symporter, a member of the major facilitator superfamily, is among the targets of Pho4. Pho84 is one of four predicted phosphate importers in C. albicans (Fig. 1). Deletion of pho84 causes increased sensitivity to external stressors, increases ROS levels, and diminishes levels of nucleotide sugars required for cell wall synthesis (11, 12). Loss of Pho84 also causes in vitro hyphal growth defects and decreases the virulence of C. albicans in a Drosophila model and in murine models of oropharyngeal and systemic candidiasis (11).
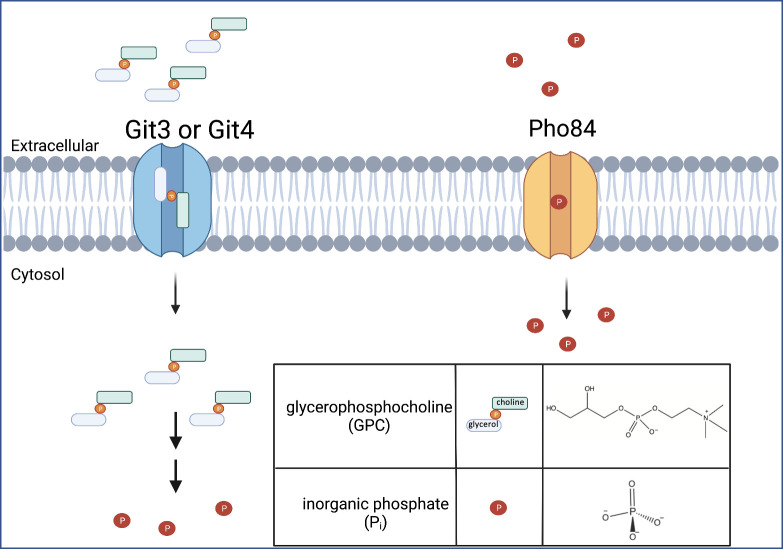
Fig 1.
Glycerophosphocholine (GPC) and Pi acquisition in Candida albicans. Representation of GPC import through Git3 or Git4 followed by its two-step catabolism to release Pi. Pi import through Pho84, a major high-affinity Pi transporter in C. albicans. Made in Biorender.
In addition to Pi transporters, Pho4 also regulates the glycerophosphodiester transporters, Git3 and Git4 (13) (Fig. 1). Glycerophosphodiesters are common lipid metabolites produced by cytosolic and secretory phospholipases of the A and B type that deacylate glycerophospholipids such as phosphatidylcholine (PC) (14–16). Importantly, phospholipase activities from both C. albicans and the human host contribute to GPC production through hydrolysis of available PC derived from their own or each other’s cellular membranes (14–18). Metabolomic studies have identified GPC in serum, in the gastrointestinal and urinary tract, and in various human tissues (19–21).
The Git transporters are homologs of the S. cerevisiae Git1 transporter, which is specific for the glycerophosphodiester, glycerophosphoinositol (GPI) (15), not to be confused with glycosylphosphatidylinositol, the lipid anchor linking proteins to the membrane. Whereas S. cerevisiae has a single GIT1 gene, C. albicans has 4 Git1 homologs, Git1–4 (22). While Git2 has yet to be characterized, Git1 is specific for GPI, and Git3 and Git4 transport GPC (Fig. 1). GPC import occurs under phosphate replete conditions and is upregulated by phosphate limitation. Of note, the rate of GPC transport in C. albicans under phosphate-limiting conditions is roughly 50× greater than that observed for S. cerevisiae, suggesting differential importance for GPC acquisition between the two organisms (13). Indeed, inhibiting GPC import by deleting git2-4 results in a decrease in the virulence of C. albicans in a mouse model of bloodstream infection (13). Once internalized, GPC can be hydrolyzed to choline and glycerol-3-phosphate by glycerophosphodiesterases. A single glycerophosphodiesterase, Gde1, has been characterized, and its expression is also upregulated by phosphate limitation (13). In order to release Pi from glycerol 3-phosphate, a phosphomonoesterase is required. Phosphomonoesterases such as Rhr2 and Dog1 have been identified in C. albicans; however, there may be other phosphomonoesterases that can hydrolyze glycerol 3-phosphate (23, 24).
Based on limited quantitative studies, GPC appears to be available at concentrations of ≥1 order of magnitude lower than those of Pi in serum (19, 25–27). Nonetheless, our previous findings led us to hypothesize that GPC import and subsequent metabolism affect phosphate homeostasis. To test this hypothesis, we examined the ability of GPC provision to affect established aspects of phosphate limitation and signaling in C. albicans. We report that provision of GPC rescued several growth defects of a pho84∆/∆ mutant. In addition, GPC provision, like Pi provision, resulted in repression of the PHO regulon though to a lesser degree. Strikingly, GPC activated TORC1 in cells lacking the major Pi transporter Pho84. Through radiolabel uptake analysis, we found that Pi uptake was roughly 2× as great as GPC uptake under low total phosphate conditions, but similar to GPC when ambient phosphate concentrations were moderate. To illustrate the nutritional utility of GPC transport and subsequent metabolism for the cell, we employed a choline auxotrophic strain to show that provision of GPC can simultaneously act as both sole phosphate and sole choline source. Overall, our studies indicate that GPC can substitute for Pi in several though not all measures of phosphate homeostasis and is likely a physiologically relevant phosphate source in vivo.
MATERIALS AND METHODS
Strains and media
C. albicans strains used in this study can be found in Table 1. Strains were grown aerobically at 30°C unless otherwise stated. Turbidity was monitored by measurement of absorbance at 600 nm (A600) on a BioMate 150 Thermo Scientific spectrophotometer. The medium used for this study was synthetic complete (SC) (yeast nitrogen base [YNB]) containing 2% glucose and amino acids, as described previously (28). Medium phosphate concentrations were controlled by omitting KH2PO4 (1 g/L) from the synthetic mix and replacing it with KCL (1 g/L). KH2PO4 or GPC was added back into media at high (10 mM), medium (1 mM), or low (200 µM) concentrations unless otherwise stated. Strains were maintained on YPD agar (yeast extract 10 g, peptone 20 g, and dextrose).
TABLE 1.
Strains used
| Strain | Genotype | Reference |
|---|---|---|
| JKC915, wild type (WT) | HIS1/his1::tetR-FRT | (29) |
| JKC1450, pho84∆/∆ | pho84::HIS1/pho84::ARG4 his1/his1:: tetR-FRT arg4/arg4 IRO1/iro1Δ::λimm434 URA3/ura3Δ::λimm434 | (30) |
| JKC1588, pho84∆/∆ + PHO84 | PHO84-FRT/pho84::ARG4 LEU2/leu2::C.d. HIS1 his1/his1:: tetR-FRT arg4/arg4 URA3/ura3::λimm434 IRO1/iro1Δ::λimm434 | (30) |
| JKC1659 |
HIS1/his1Δ:: tetR-FRT
PHO84/PHO84 promoter-GFP-NAT1-PHO84 |
(12) |
| git2,3,4∆/∆ + pDDB78 |
ura3Δ::λimm434 arg4::hisG his1::hisG::pHIS1 git2,3,4::ARG4 git2,3,4::URA ura3Δ::λimm434 arg4::hisG his1::hisG 3 |
(13) |
| gde1∆/∆ + pDDB78 |
ura3Δ::λimm434 arg4::hisG his1::hisG::pHIS1 gde1::ARG4 gde1::URA ura3Δ::λimm434 arg4::hisG his1::hisG 3 |
(13) |
| gde1∆/∆ + GDE1 |
ura3Δ::λimm434 arg4::hisG his1::hisG::pHIS1 gde1::ARG4 gde1::URA ura3Δ::λimm434 arg4::hisG his1::hisG 3 |
(13) |
Growth assays
Overnight cultures were used to inoculate 200 µL of media at A600 = 0.1 in a 96-well plate. Plates were incubated at 30°C, with intermittent shaking prior to each reading using a Molecular Devices SpectraMax i3 instrument. A600 readings were taken at 30-minute intervals, and time zero values were subtracted from each timepoint to reflect overall growth. Data points represent the mean and standard deviation of a minimum of three independent replicates.
Acid releasable inorganic phosphate assay
Acid-labile phosphate was measured by use of a colorimetric molybdate assay as previously described (30, 31). Briefly, overnight cultures were grown overnight in low phosphate YNB containing 200 µM Pi. Cells were washed twice, reinoculated at A600 = 0.1 in YNB containing 200 µM Pi or 200 µM GPC, and grown to a mid-log phase. Cells were then harvested and resuspended in 500 µL 0.1% Triton X-100 and lysed using zirconia/silica bead homogenization. Lysate protein concentrations were determined using a BCA Protein Assay Kit (Pierce). One hundred micrograms of whole-cell lysate was then boiled for 30 minutes in 1 M HCl before phosphate quantification using the colorimetric molybdate assay in biological triplicate.
32P-orthophosphate and 14C-choline-glycerophosphocholine uptake assays
Uptake assays were altered from references 32, 33. Cells were pregrown in low phosphate conditions (200 µM KH2PO4). Cultures were then reinoculated in the indicated media conditions and grown into log phase at 30°C. Once in a log phase, cultures were reinoculated at A600 = 0.1 and provided with either 32P-orthophosphate or 14C-choline-glycerophosphocholine at the indicated concentrations in separate cultures. After 1 hour, 1-mL aliquots were removed from each culture, centrifuged briefly, and separated into extracellular and cellular fractions. Radioactivity in each fraction was measured using liquid scintillation counting.
PHO84 promotor induction analysis
Cells of genotype PHO84/pPHO84-GFP-NAT1-PHO84 were grown in YPD liquid medium with additional 10 mM Pi for 16 hours in order to maximally repress the PHO84 promoter and washed three times with 0.9% NaCl. Cultures were adjusted to A600 = 0.01 in synthetic complete with increasing concentrations of KH2PO4 or GPC (0.05, 0.1, 0.2, 0.4, and 0.5 mM) and 50 µL/well of eight technical replicates for each condition was inoculated into a black 384-well plate with a transparent bottom. During incubation at 30°C, A600 and GFP signal (Ex 485/20 nm; Em 528/20 nm) were recorded every 30 minutes at gain 50 in a Synergy 2 BioTek Plate Reader for 18 hours. Readings were graphed in GraphPad Prism at the 16-hour timepoint.
P-S6 western blotting in Pho84 strains with GPC
Cell lysis and western blotting were performed as described in (34). Rabbit anti-P-S6 (Cell Signaling Technology, #9611L) was used as the primary antibody. The loading control was Rabbit anti-Cdc28 (PSTAIRE, Santa Cruz #sc-53). Anti-rabbit IgG (Cell Signaling Technology, #7074S) was used as the secondary antibody. For densitometry, ImageJ (imagej.net/welcome) software (opensource) was used to quantitate signals obtained from Azure biosystems c600.
RESULTS
Provision of GPC rescued hypersensitivity of pho84∆/∆ cells to osmotic and cell wall, but not peroxide, stresses
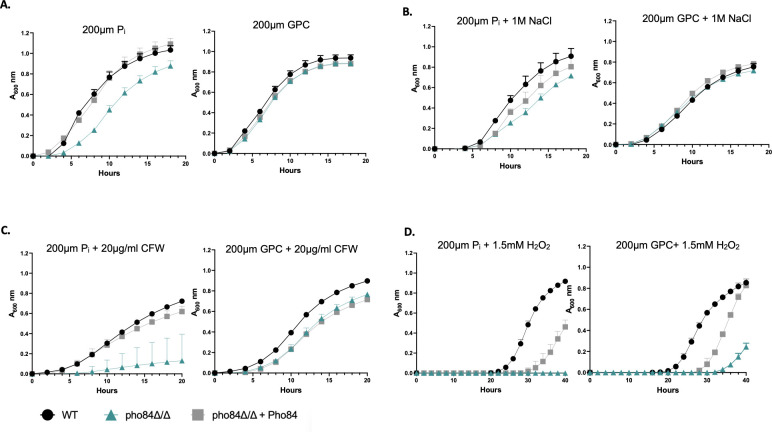
Pho84 is the major high-affinity Pi transporter in C. albicans (30), and its function can only partially be substituted by other phosphate transporters, such as Pho89 and Pho87 (Table 2). In the absence of Pho84, or of its major transcriptional regulator, Pho4, C. albicans becomes more susceptible to a variety of stresses including osmotic, cell wall, and oxidative stresses (8–12). We performed growth experiments to see if the provision of GPC can alleviate growth defects and stress hypersensitivities associated with phosphate limitation caused by loss of PHO84. As shown in Fig. 2A (top left), a pho84∆/∆ mutant displayed a growth defect in low phosphate (200 µM Pi) conditions in comparison to WT (Pho84 is primarily responsible for transport at this concentration, Table 2). Provision of 200 µM GPC instead of Pi in the media rescued this phenotype. A pho84∆/∆ mutant also showed increased sensitivity to osmotic stress caused by 1M NaCl addition when grown in media containing 200 µM Pi in comparison to WT (Fig. 2B, top right). When provided 200 µM GPC instead of Pi, this phenotype was rescued restoring growth to WT levels. Similarly, during cell wall stress induced by exposure to 20 µg/mL of the chitin-binding compound calcofluor white (CFW), pho84∆/∆ mutant cells grew poorly (Fig. 2C, bottom left), as we showed previously (12). This phenotype was rescued by provision of 200 µM GPC as phosphate source, which restored growth similar to the WT in these conditions (Fig. 2C, bottom left). Hypersensitivity of pho84∆/∆ cells to peroxide stress (11) (Fig. 2D, bottom right) induced by 1.5 mM H2O2 was not clearly rescued by provision of GPC, although some growth began to occur in pho84∆/∆ cells after 30 hours. Of note, both WT and reintegrant strains displayed long lag times (note x-axis) upon provision of either Pi or GPC in this experiment, indicating that 1.5 mM H2O2 presented the cells with a severe stress. One interpretation is that the two-step requirement to release free Pi from GPC precludes efficient rescue at this H2O2 concentration. Further experimentation will be required to test whether pho84∆/∆ cells’ elevated reactive oxygen species’ content even in the absence of exogenous oxidative stress, and their hypersensitivity to superoxide and peroxide stresses (11), can be rescued by provision of GPC.
TABLE 2.
Comparison of Pi and GPC importa
| Strain | Concentration (Pi + GPC) |
*GPC uptake (per ODU) | *Pi uptake (per ODU) |
|---|---|---|---|
| WT | 100 µM + 100 µM | 7.43 ± 0.66 nmol | 10.6 ± 1.27 nmol |
| git2-4∆/∆, PHO84+/+ | 0.00 ± 0.72 nmol | ND | |
| pho84∆/∆, GIT2-4+/+ | ND | 0.00 ± 0.45 nmol | |
| WT | 250 µM + 250 µM | 11.6 ± 1.26 nmol | 23.25 ± 3.31 nmol |
| git2-4∆/∆, PHO84+/+ | 0.00 ± 0.65 nmol | ND | |
| pho84∆/∆, GIT2-4+/+ | ND | 0.00 ± .07 nmol |
Cells were grown to log phase in YNB containing equivalent amounts of Pi and GPC at two concentrations: very low phosphate (100 µM Pi + 100 µM GPC) or low phosphate (250 µM Pi + 250 µM GPC) conditions. Cells were then reinoculated into the same nutrient conditions at an A600 of 0.1, but in media in which only one of the compounds was radiolabeled (14C-choline-GPC or 32P-orthophosphate, indicated by an asterisk at the top of the data column). Samples were incubated for 1 hour at which point cells were harvested and cellular radioactivity was determined per 1 ODU. Data represent the mean and standard deviation of biological triplicates. ND, not done.
Fig 2.
GPC rescued phosphate starvation phenotypes. (A) Cells were grown overnight in YNB containing 200 µM Pi. Cells were then reinoculated into phosphate-free YNB containing either 200 µM Pi or 200 µM GPC at an A600 of 0.1, monitored every 30 minutes. (B) Grown as in panel A with the addition of 1 M NaCl to induce osmotic stress. (C) Grown as in panel A with the addition of 20 µg/mL calcofluor white to induce cell wall stress. (D) Grown as in panel A with the addition of 1.5 mM H2O2 to induce oxidative stress. Data are displayed as the mean ± standard deviation of at least three replicates.
Overall, these results suggest that GPC is transported into the cell and enters cellular metabolism efficiently enough to counter major phenotypes associated with severe Pi limitation as experienced by cells lacking PHO84 in low ambient phosphate.
Gde1 was required for GPC utilization as a phosphate source
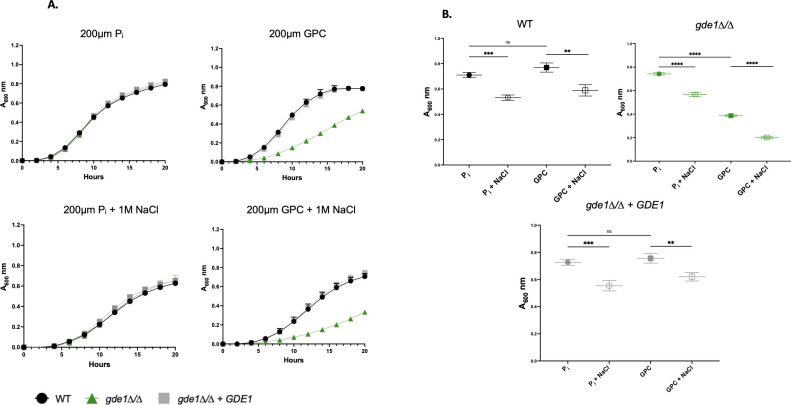
Uptake of GPC requires the Git3 and Git4 transporters (13). Once internalized, GPC is hydrolyzed by the glycerophosphodiesterase Gde1 into choline and glycerol-3 phosphate (13). To verify that GPC must be hydrolyzed prior to its utilization as a phosphate source under stressed and non-stressed conditions, we employed a gde1∆/∆ mutant subjected to NaCl stress. As shown in Fig. 3A, WT and gde1∆/∆ grew identically when Pi was the phosphate source, both in the presence and in the absence of 1 M NaCl. However, when GPC was provided as the phosphate source, gde1∆/∆ showed a growth defect in the absence of stress, and that defect increased in the presence of 1 M NaCl. The growth defect of gde1∆/∆ is shown in Fig. 3B, where only the 16-hour timepoint is presented. Of note, loss of Gde1 does not completely inhibit growth on GPC, indicating that there are other, as yet uncharacterized, enzymes that can hydrolyze GPC (13).
Fig 3.
A gde1∆/∆ mutant displayed defective growth on GPC. (A) Cells were grown overnight in YNB containing 200 µM Pi. Cells were then reinoculated into phosphate-free YNB containing either 200 µM Pi or 200 µM GPC at an A600 of 0.1 with or without 1 M NaCl. A600 was monitored every 30 minutes using a plate reader. Data are displayed as the mean and standard deviation of at least three replicates. (B) The 16-hour timepoint from the data in A. A one-way ANOVA was performed. **, P < 0.001; ***, P < 0.0005; ****, P < 0.0001.
GPC and Pi provision supported similar levels of intracellular acid-labile phosphate
To directly determine how provision of GPC compares with provision of Pi in terms of internal phosphate stores, we quantified acid-labile phosphate within cells. Boiling acid treatment releases phosphate from polyphosphates and has been used to determine estimates of free Pi arising from polyphosphates in yeast (30, 35).
As shown in Fig. 4, GPC and Pi provision supported similar levels of total free and acid-labile internal phosphate in a wild-type strain. This implies that despite the two steps of catabolism needed to release free phosphate from GPC, internal phosphate stores are not negatively impacted. Previous studies have shown that in the absence of Pho84, there is a significant decrease in the amount of acid-labile phosphate, interpreted to be representative of polyphosphate storage (30, 35). We have repeated those findings and report that provision with GPC was not able to rescue this phenotype (Fig. 4). Given the predicted lower Pi content of pho84∆/∆ cells at the beginning of the experiment (30), the provided GPC may have only been sufficient to enable biomass addition but not replenishment of polyphosphate stores in these cells.
Fig 4.
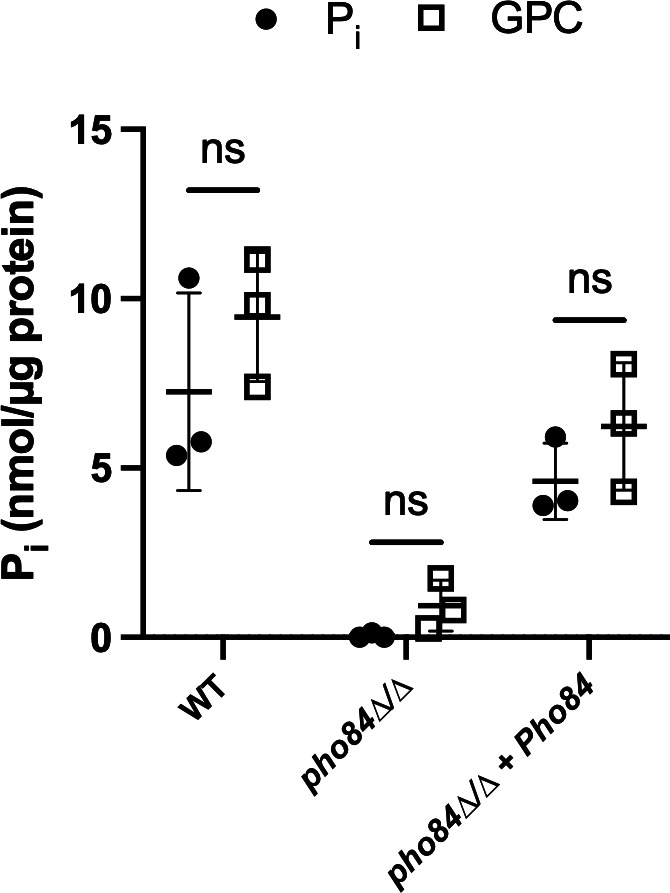
Internal phosphate levels were similar when cells were provided with either GPC or Pi. Cells were grown overnight in YNB containing 200 µM Pi. Cells were then reinoculated into phosphate-free YNB containing either 200 µM Pi or 200 µM GPC at an A600 of 0.2 and grown to mid-log phase. Cells were then harvested and assayed for acid-labile phosphate. Data represent the mean and standard deviation of biological triplicates. A one-way ANOVA was performed.
GPC repressed PHO84 promoter activation
The PHO regulon is the key homeostatic mechanism for phosphate import and intracellular phosphate distribution. Pho4 is the primary transcriptional activator of the PHO regulon, upregulating the expression of PHO84, the genes encoding the GPC importers Git3 and Git4, and the glycerophosphodiesterase Gde1 among many other genes (9, 10). Pho4 activity increases as the Pi available within the cell decreases. To assess how provision of GPC impacts PHO regulon signaling, we measured the fluorescence produced through activation of the PHO84 promoter driving GFP in a strain containing two functional PHO84 alleles (12) as a proxy for Pho4 transcriptional activity.
As expected and previously shown (12), fluorescence from pPHO84-GFP decreased as Pi concentration in the medium was increased from 0.05 mM to 0.5 mM (Fig. 5). When GPC was provided as the phosphate source, a comparable decrease in pPHO84-GFP fluorescence occurred (Fig. 5). Thus, GPC repressed the PHO regulon in a qualitatively similar manner to Pi. At 0.05 mM and 0.1 mM Pi, we observed a statistically significant but small increase in repression (roughly 110%) of pPHO84-GFP by GPC as compared with Pi, a result for which we have no obvious explanation. At 0.2 mM, repression by both phosphorus sources appeared equivalent. A larger quantitative difference was noted at the 0.4 mM and 0.5 mM levels, where Pi repressed expression to a greater extent (roughly 150%) than GPC. Pho4 also regulates the expression of Gde1, the enzyme needed for the first step in hydrolysis to release Pi by GPC hydrolysis. It may be that at high GPC concentrations, less Pi is released from GPC due to insufficient Gde1 production, leading to decreased repression of the PHO regulon.
Fig 5.
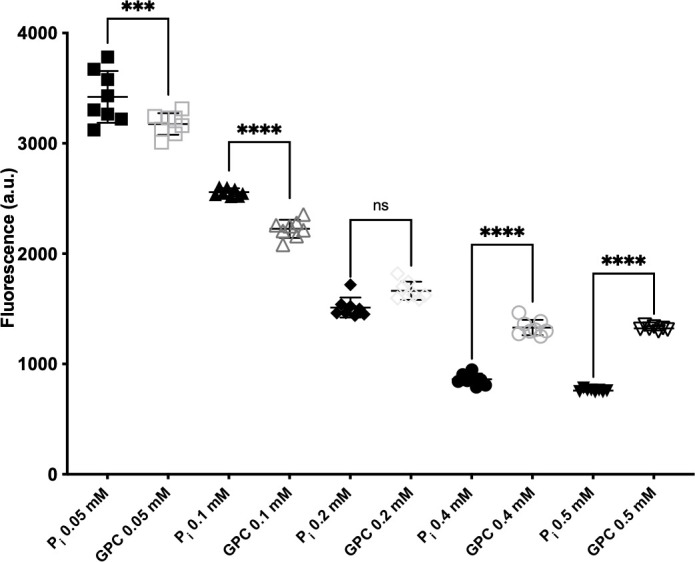
GPC repressed the PHO84 promoter. Cells expressing GFP under the control of the PHO84 promoter (JKC1659) were pregrown in YPD with an additional 10 mM Pi overnight. Cells were then inoculated at an OD600 = 0.01 into media without Pi with indicated Pi or GPC concentrations. The fluorescent signal and A600 were followed over 18 hours. The 16-hour timepoint is shown. Data represent the mean and standard deviation of eight biological replicates. A one-way ANOVA was performed. a.u., arbitrary unit ***, P < 0.0005; ****, P < 0.0001.
GPC activates TORC1 signaling independently of Pho84
The mechanisms by which the cell senses cellular phosphate is an active area of research.
However, it has been established that phosphate, in addition to nitrogen and carbon, is one of the nutrients sensed by the C. albicans and S. cerevisiae TOR (target of rapamycin) complex 1 (TORC1) signaling pathway (12, 30). TORC1 signaling is highly conserved within eukaryotes and controls cellular growth and proliferation in dependence on nutrient availability. During phosphate starvation or upon PHO84 deletion, TORC1 signaling is decreased. TORC1 signaling is activated by the upstream GTPase, Gtr1 (12, 30). In a recent study, Pho84 is hypothesized to have transceptor activity, affecting TORC1 signaling in combination with Pi import (36). To examine if the provision of phosphorus as GPC is able to activate TORC1 similarly to the provision of Pi, we monitored the phosphorylation of ribosomal protein S6, a known downstream target of TORC1 (34). In a wild-type strain, provision of GPC activated TORC1 signaling similarly to Pi provision. Consistent with previous studies, we found that TORC1 signaling was decreased in pho84∆/∆ compared with wild-type cells when they were provided with Pi (Fig. 6). When GPC was provided to pho84∆/∆ cells at concentrations of 0.1 and 10 mM, TORC1 signaling was restored. Thus, GPC activated TORC1 independently of Pho84 to return signaling to wild-type levels (Fig. 6).
Fig 6.
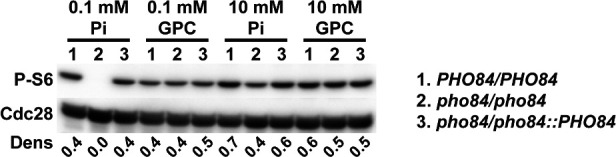
GPC activated TORC signaling. Cells pregrown in YPD overnight were washed three times in 0.9% NaCl and then inoculated into synthetic complete medium with 0.1 mM or 10 mM inorganic phosphate or glycerophosphocholine, at an A600 of 0.2. Cells were collected after 2 hours of incubation (200 rpm) at 30°C. Total protein extracts were probed with antibody to phosphorylated Rps6 (P-S6) and Cdc28 as loading control. Dens, signal intensity ratio of P-S6 to Cdc28. (1, PHO84/PHO84, JKC915; 2, pho84/pho84, JKC1450; 3, pho84/pho84::PHO84, JKC1588.) Representative of three biological replicates.
Comparison of GPC and Pi transport
In the experiments represented in Fig. 2–6, either GPC or Pi was provided as the sole phosphate source. In the human host, GPC and Pi are both available albeit at different concentrations and compartment distributions (37). Human serum Pi is measured routinely as part of standard electrolyte panels and ranges from 0.8 mM to 1.3 mM (26, 27). GPC concentrations in serum have been measured much less frequently and range from 3 mM to 35 mM (19, 25). However, the relative concentrations of GPC and Pi in host microenvironments have not been established. Further, increased GPC transport capacity of C. albicans compared with S. cerevisiae, regulation of its transporters Git3 and Git4 by the PHO regulon, and their contribution to C. albicans virulence argue for a significant role of GPC in the fungus’ nutritional repertoire in the host.
We therefore examined the transport of GPC and Pi when both metabolites were available. Equimolar amounts of Pi and GPC were provided in experiments in which one of the two was radiolabeled (14C-choline-GPC or 32P-orthophosphate). We used low and moderate total phosphate concentrations, either 200 µM total phosphate (100 µM Pi plus 100 µM GPC) or 500 µM total phosphate (250 µM Pi plus 250 µM GPC). In this way, we compared two conditions in which the PHO regulon was induced, and therefore, the transporters of both GPC and Pi were expected to be expressed, albeit at higher levels in lower phosphate concentrations. To control for a background level of GPC transport, we used a git2-4∆/∆ strain that showed negligible GPC transport under the conditions examined. We also observed negligible Pi transport in pho84∆/∆ cells (Table 2). At a total ambient phosphate concentration of 200 µM, Pi and GPC were imported at similar rates in the course of the 1-hour assay: roughly 10 nanomoles of Pi as compared with 7 nanomoles of GPC per mL of cell suspension at an OD600 of 1 (ODU) (Table 2). At 500 µM ambient phosphate, Pi import was roughly 2× greater than that of GPC: 23 nanomoles of Pi as compared with 11 nanomoles of GPC (Table 2). We concluded that when both sources of phosphate were available at the same concentration, GPC was imported at a substantial rate, especially when Pi was limiting. GPC transport may be more quickly saturable since Pi import increased more with increasing ambient Pi availability than GPC import.
GPC was able to act as both a phosphate and a choline source
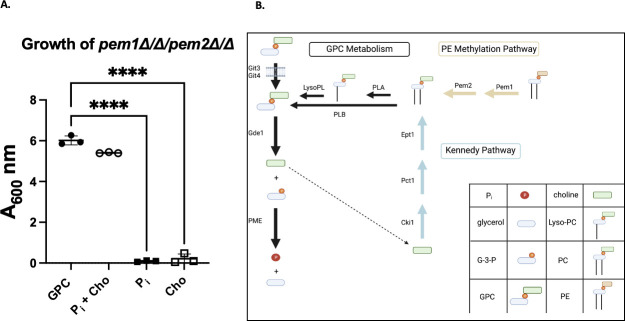
We used a choline auxotrophic strain to test the possibility that GPC can simultaneously act as the sole phosphate and the sole choline source (Fig. 7A). The pem1∆/∆ pem2∆/∆ strain is a choline auxotroph because it lacks the phosphatidylethanolamine methylation pathway, leaving the Kennedy pathway as the sole biosynthetic pathway for phosphatidylcholine biosynthesis in C. albicans (38). When this strain was grown without exogenous Pi or choline, provision of GPC was able to support growth similarly to media containing both Pi and choline (Fig. 7B). We concluded that GPC hydrolysis sufficed to simultaneously provide choline and phosphate to C. albicans.
Fig 7.
GPC served simultaneously as a Pi and choline source. (A) pem1∆/∆pem2∆/∆ strain, a choline auxotroph, was grown overnight in YNB supplemented with 200 µM choline. Cells were then starved of phosphate and choline for 8 hours before restarting in phosphate-free YNB supplemented with either 200 µM GPC, 200 µM Pi, 200 µM choline (cho), or 200 µM Pi plus 200 µM choline as indicated. Cells were started at an A600 of 0.2 and allowed to grow for 24 hours. Data represent the mean and standard deviation of biological triplicates. (B) Schematic of GPC and PC metabolism. GPC production and catabolism is indicated by black arrows. The Kennedy pathway for PC biosynthesis is shown in blue, and the PE methylation pathway for PC biosynthesis is shown in yellow. Shown in black is GPC production through the deacylation of PC by phospholipase of the b type or through a single deacylation of PC by a phospholipase of the A type to produce Lyso-PC followed by another deacylation by a lysophospholipase. GPC, glycerophosphocholine; G-3-P, glycerol-3-phosphate; Pi, inorganic phosphate; LPC, lyso-phosphatidylcholine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PLB, phospholipase B; PLA, phospholipase A; LysoPl, lysophospholipase; PME, phosphomonoesterase . A one-way ANOVA was performed. ****, P < 0.0001. Made in Biorender.
DISCUSSION
GPC is a ubiquitous lipid metabolite produced by phospholipases in bacteria (39), fungi (15, 40), and mammalian cells (17). GPC is found in human tissues and fluids including serum, prostate, breast tissue, renal cells, saliva, blood, breast milk, and cerebrospinal fluid (17, 19, 41–43). Based on data contained in the human metabolome database (https://hmdb.ca), GPC levels that have been reported thus far have ranged roughly from 1 µM in saliva to 3–35 µM in serum to 30–500 µM in breast milk (19, 25, 41, 44, 45), with the concentrations varying widely depending upon the study and the methodology employed. Although quantitative metabolomic data on GPC are limited, it is clear that serum Pi (at roughly 1 mM [26, 27]) is more abundant than serum GPC (19, 25). However, the relative concentrations of GPC and Pi in host microenvironments have not been established and are undoubtedly dynamic, as GPC is liberated through phospholipase-mediated PC hydrolysis, during which it may reach higher concentrations than those found in serum at a steady state, while serum and cytosolic Pi concentrations are highly regulated (17, 46, 47). Both phospholipase B1 and B5 are required for full virulence of C. albicans. One potential mechanism for these phospholipases in pathogenesis is the release of GPC from host cells’ plasma membranes that provides phosphorus as well as choline for the fungus (14, 48–51). GPC is one of four major renal osmolytes found in tubular cells of the renal medulla during mammalian dehydration states, along with glycine betaine, myo-inositol, and sorbitol (52). In renal cells, GPC has been quantified by mass instead of volume and has been reported to be roughly 18 nmol/mg protein (53, 54). In some human candidiasis syndromes and in the murine intravenous infection model, the kidney is a major target for disseminated candidiasis (55), though typically, the renal cortex and medulla are equally infected. Further work, especially regarding the contribution of fungal phospholipases to GPC release, should shed more light on these questions.
C. albicans upregulates high-affinity phosphate transporters during invasive infection suggesting that the host environment mimics Pi starvation conditions (56–62). At the human serum Pi concentration of 0.8–1.3 mM (26, 27), C. albicans would be expected to experience Pi sufficiency, but given the acidic optima of all but one of its Pi importers (our unpublished data), their activity may be inefficient at the normal pH of human serum (pH 7.35–7.45). An analogous phenomenon was observed in the opportunistic fungal pathogen Cryptococcus neoformans and called “alkaline pH-simulated nutrient deprivation” (63, 64). We examined the possibility that GPC is among the sources of phosphorus used by C. albicans in the host.
Previous studies have established that C. albicans can utilize GPC efficiently as a phosphate source (13). Importantly, loss of the Git3 transporter results in decreased virulence in a mouse model of bloodstream infection (13). We demonstrate here that provision of equimolar GPC can rescue several growth phenotypes associated with phosphate limitation imposed by the lack of PHO84 (Fig. 2). Further, provision of either Pi or GPC at equimolar concentrations results in similar levels of total intracellular phosphate in a WT strain, despite the need for a two-step catabolic process to release Pi from GPC. Provision of GPC did not rescue Pi stores of pho84∆/∆ cells (Fig. 4). One interpretation could be that the amount of GPC provided was not enough to support growth of pho84∆/∆ cells which requires incorporation of phosphorus into many macromolecules and to simultaneously restore depleted internal phosphate stores over the time span of the assay.
To test whether GPC is used in conditions where both Pi and GPC are available at equimolar concentrations, we examined the import of each phosphorus source in the presence of the other. GPC import is similar to Pi under low ambient phosphate conditions and measures roughly half as much as Pi import in moderate ambient phosphate (Table 2). Both import and hydrolysis of GPC are regulated by the PHO regulon; therefore, Pi is the most readily usable form of phosphorus (8, 13). In moderate phosphate conditions, Pi appears to be preferentially selected over GPC and the molecular mechanism of this selectivity remains to be discovered. Future work will examine whether the GPC transporters, Git3/4, are saturable at lower transport rates than Pho84, whether their expression is downregulated earlier than that of Pho84 in rising ambient Pi concentrations, and whether mechanisms beyond the PHO regulon determine their expression and activity. GPC transporters Git3/4 compensate for loss of PHO84 under conditions where the other Pi transporters which are present in the pho84∆/∆ mutant cannot (Fig. 2 and 3), so that 200 µM GPC rescues phenotypes in a pho84∆/∆ mutant that cannot be rescued by 200 µM Pi.
GPC has the potential to provide the cell with choline and glycerol in addition to phosphate. Strains lacking a functional PE methylation pathway are choline auxotrophs as they require choline to make PC via the CDP-choline pathway. It has been shown previously that GPC can act as a choline source (38). Here, we further demonstrate the robustness of GPC import and catabolism by showing that GPC can simultaneously act as both a choline and phosphate source in a pem1∆/∆ pem2∆/∆ mutant (Fig. 7). This variety of metabolic uses for GPC may be one of the reasons that the loss of the major GPC transporter, Git3, leads to decrease in virulence in a mouse model (13).
Induction of the PHO84 promoter is a readout of PHO regulon activity in C. albicans (12) as in S. cerevisiae (65). We observed repression of the PHO84 promoter with increasing provision of both Pi and GPC in the medium, though Pi was the more potent repressor at equimolar concentrations. Why Pi has a stronger effect on the PHO regulon than GPC, when equal amounts of phosphate are delivered intracellularly, e.g., whether Pho84 has transceptor activity toward the PHO regulon, remains to be determined.
The ability of GPC to activate TORC1 signaling in cells lacking PHO84 (Fig. 6) suggests that it is intracellular Pi, and not simply a direct signal from Pho84, that provides a crucial stimulus to TORC1. While Pho84 may also have a transceptor activity in addition to its role providing Pi to the cytoplasm (30, 36), our current findings indicate that in the presence of sufficient intracellular Pi provided by GPC, this activity is not required for TORC1 activation. How intracellular Pi availability is signaled to TORC1 remains an area of active investigation.
An open area of inquiry is the complete identification of gene products involved in GPC catabolism. As shown in Fig. 3, growth on GPC as a phosphate source is delayed in a gde1∆/∆ mutant, but there are undoubtedly other glycerophophodiesterases involved as well. Secondly, the phosphomonoesterase(s) responsible for the release of free phosphate from glycerol-3 phosphate have yet to be identified. While phosphomonoesterases, like Rhr2, Dog1, and multiple others are known, their role in GPC catabolism has not been established, and there are likely other phosphomonoesterase-encoding genes in the C. albicans genome (7, 8, 23, 24).
Several aspects of the phosphate deprivation response are conserved among pathogenic and nonpathogenic fungi, including the Pho4 transcriptional regulator (10, 66, 67). However, others have noted that pathogenic fungi have an expanded range of Pho4 targets that include lipid metabolism (63). Lipid metabolism, both synthesis and turnover, is an ongoing process in pathogenic fungi and the human host. Our results show that C. albicans has adapted to use GPC, the product of PC metabolism, as part of its phosphate deprivation response, and that the choline released in the process can feed into PC biosynthesis. C. albicans GPC importers Git3/4 hence have significant roles in both phosphate homeostasis and lipid biosynthesis. Ongoing studies are exploring the possibility that GPC can be converted to PC through direct acylation, as recently shown in S. cerevisiae, plants, and mitis group streptococci (28, 68, 69). Interconvertibility of membrane organic phosphates with free cellular Pi via GPC may contribute to C. albicans adaptation to insufficient Pi access in the host.
ACKNOWLEDGMENTS
We thank Dr. Todd Reynolds for the pem1∆/∆pem2∆/∆ strain.
This work was supported by National Institute of Health Grant NIH R15 GM104876 to J.P.-V. and Boston Children’s Hospital Support to J.R.K.
Contributor Information
Jana Patton-Vogt, Email: pattonvogt@duq.edu.
Michael Lorenz, The University of Texas Health Science Center at Houston, Houston, Texas, USA.
REFERENCES
- 1. Köhler JR, Casadevall A, Perfect J. 2015. The spectrum of fungi that Infects humans. Cold Spring Harb Perspect Med 5:a019273. doi: 10.1101/cshperspect.a019273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wijnants S, Riedelberger M, Penninger P, Kuchler K, Van Dijck P. 2020. Sugar phosphorylation controls carbon source utilization and virulence of Candida albicans. Front Microbiol 11:1274. doi: 10.3389/fmicb.2020.01274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ganguly S, Mitchell AP. 2011. Mucosal Biofilms of Candida albicans. Curr Opin Microbiol 14:380–385. doi: 10.1016/j.mib.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moran GP, Coleman DC, Sullivan DJ. 2011. Comparative genomics and the evolution of pathogenicity in human pathogenic fungi. Eukaryot Cell 10:34–42. doi: 10.1128/EC.00242-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bäumler AJ, Sperandio V. 2016. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535:85–93. doi: 10.1038/nature18849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ikeh M, Ahmed Y, Quinn J. 2017. Phosphate acquisition and virulence in human fungal pathogens. Microorganisms 5:48. doi: 10.3390/microorganisms5030048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. MacCallum DM, Castillo L, Nather K, Munro CA, Brown AJP, Gow NAR, Odds FC. 2009. Property differences among the four major Candida albicans strain clades. Eukaryot Cell 8:373–387. doi: 10.1128/EC.00387-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Köhler JR, Acosta-Zaldívar M, Qi W. 2020. Phosphate in virulence of Candida albicans and Candida glabrata. JoF 6:40. doi: 10.3390/jof6020040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Urrialde V, Prieto D, Pla J, Alonso-Monge R. 2016. The Candida albicans Pho4 transcription factor mediates susceptibility to stress and influences fitness in a mouse commensalism model. Front Microbiol 7:1062. doi: 10.3389/fmicb.2016.01062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ikeh MAC, Kastora SL, Day AM, Herrero-de-Dios CM, Tarrant E, Waldron KJ, Banks AP, Bain JM, Lydall D, Veal EA, MacCallum DM, Erwig LP, Brown AJP, Quinn J. 2016. Pho4 mediates phosphate acquisition in Candida albicans and is vital for stress resistance and metal homeostasis. Mol Biol Cell 27:2784–2801. doi: 10.1091/mbc.E16-05-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu N-N, Uppuluri P, Broggi A, Besold A, Ryman K, Kambara H, Solis N, Lorenz V, Qi W, Acosta-Zaldívar M, Emami SN, Bao B, An D, Bonilla FA, Sola-Visner M, Filler SG, Luo HR, Engström Y, Ljungdahl PO, Culotta VC, Zanoni I, Lopez-Ribot JL, Köhler JR. 2018. Intersection of phosphate transport, oxidative stress and TOR signalling in Candida albicans virulence. PLoS Pathog 14:e1007076. doi: 10.1371/journal.ppat.1007076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu N-N, Acosta-Zaldívar M, Qi W, Diray-Arce J, Walker LA, Kottom TJ, Kelly R, Yuan M, Asara JM, Lasky-Su JA, Levy O, Limper AH, Gow NAR, Köhler JR. 2020. Phosphoric metabolites link phosphate import and polysaccharide biosynthesis for Candida albicans cell wall maintenance. mBio 11:e03225-19. doi: 10.1128/mBio.03225-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bishop AC, Ganguly S, Solis NV, Cooley BM, Jensen-Seaman MI, Filler SG, Mitchell AP, Patton-Vogt J. 2013. Glycerophosphocholine utilization by Candida albicans: role of the git3 transporter in virulence. J Biol Chem 288:33939–33952. doi: 10.1074/jbc.M113.505735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghannoum MA. 2000. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev 13:122–143. doi: 10.1128/CMR.13.1.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patton-Vogt J. 2007. Transport and metabolism of glycerophosphodiesters produced through phospholipid deacylation. Biochim Biophys Acta 1771:337–342. doi: 10.1016/j.bbalip.2006.04.013 [DOI] [PubMed] [Google Scholar]
- 16. Murakami M, Nakatani Y, Atsumi G-I, Inoue K, Kudo I. 2017. Regulatory functions of phospholipase A2. Crit Rev Immunol 37:121–180. doi: 10.1615/CritRevImmunol.v37.i2-6.20 [DOI] [PubMed] [Google Scholar]
- 17. Sonkar K, Ayyappan V, Tressler CM, Adelaja O, Cai R, Cheng M, Glunde K. 2019. Focus on the glycerophosphocholine pathway in choline phospholipid metabolism of cancer. NMR Biomed 32:e4112. doi: 10.1002/nbm.4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gallazzini M, Burg MB. 2009. What's new about osmotic regulation of glycerophosphocholine. Physiology 24:245–249. doi: 10.1152/physiol.00009.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ilcol YO, Ozbek R, Hamurtekin E, Ulus IH. 2005. Choline status in newborns, infants, children, breast-feeding women, breast-fed infants and human breast milk. J Nutr Biochem 16:489–499. doi: 10.1016/j.jnutbio.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Holmes E, Comelli EM, Fotopoulos G, Dorta G, Tang H, Rantalainen MJ, Lindon JC, Corthésy-Theulaz IE, Fay LB, Kochhar S, Nicholson JK. 2007. Topographical variation in metabolic signatures of human gastrointestinal biopsies revealed by high-resolution magic-angle spinning 1H NMR spectroscopy. J Proteome Res 6:3944–3951. doi: 10.1021/pr0702565 [DOI] [PubMed] [Google Scholar]
- 21. Teruya T, Goga H, Yanagida M. 2020. Aging markers in human urine: a comprehensive, non-targeted LC-MS study. FASEB Bioadv 2:720–733. doi: 10.1096/fba.2020-00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bishop AC, Sun T, Johnson ME, Bruno VM, Patton-Vogt J. 2011. Robust utilization of phospholipase-generated metabolites, glycerophosphodiesters, by Candida albicans: role of the CaGit1 permease. Eukaryot Cell 10:1618–1627. doi: 10.1128/EC.05160-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Desai JV, Bruno VM, Ganguly S, Stamper RJ, Mitchell KF, Solis N, Hill EM, Xu W, Filler SG, Andes DR, Fanning S, Lanni F, Mitchell AP. 2013. Regulatory role of glycerol in Candida albicans Biofilm formation. mBio 4:e00637–12. doi: 10.1128/mBio.00637-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fan J, Whiteway M, Shen S-H. 2005. Disruption of a gene encoding glycerol 3-phosphatase from Candida albicans impairs intracellular glycerol accumulation-mediated salt-tolerance. FEMS Microbiol Lett 245:107–116. doi: 10.1016/j.femsle.2005.02.031 [DOI] [PubMed] [Google Scholar]
- 25. Guasch-Ferré M, Hu FB, Ruiz-Canela M, Bulló M, Toledo E, Wang DD, Corella D, Gómez-Gracia E, Fiol M, Estruch R, Lapetra J, Fitó M, Arós F, Serra-Majem L, Ros E, Dennis C, Liang L, Clish CB, Martínez-González MA, Salas-Salvadó J. 2017. Plasma metabolites from choline pathway and risk of cardiovascular disease in the PREDIMED (prevention with mediterranean diet) study. J Am Heart Assoc 6:e006524. doi: 10.1161/JAHA.117.006524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bansal VK. 1990. Serum inorganic phosphorus. Clin Methods Hist Phys Lab Exam [Google Scholar]
- 27. Crook M, Swaminathan R. 1996. Disorders of plasma phosphate and indications for its measurement. Ann Clin Biochem 33:376–396. doi: 10.1177/000456329603300502 [DOI] [PubMed] [Google Scholar]
- 28. Anaokar S, Kodali R, Jonik B, Renne MF, Brouwers J, Lager I, de Kroon A, Patton-Vogt J. 2019. The glycerophosphocholine acyltransferase Gpc1 is part of a phosphatidylcholine (PC)-remodeling pathway that alters PC species in yeast. J Biol Chem 294:1189–1201. doi: 10.1074/jbc.RA118.005232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen J, Cowen LE, Griffin AM, Chan L, Köhler JR. 2008. The Candida albicans pescadillo homolog is required for normal hypha-to-yeast morphogenesis and yeast proliferation. Proc Natl Acad Sci U S A 105:20918–20923. doi: 10.1073/pnas.0809147105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu NN, Flanagan PR, Zeng J, Jani NM, Cardenas ME, Moran GP, Köhler JR. 2017. Phosphate is the third nutrient monitored by TOR in Candida Albicans and provides a target for fungal-specific indirect TOR inhibition. Proc Natl Acad Sci U S A 114:6346–6351. doi: 10.1073/pnas.1617799114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ames BN. 1966. [10] assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8:115–118. [Google Scholar]
- 32. Surlow BA, Cooley BM, Needham PG, Brodsky JL, Patton-Vogt J. 2014. Loss of Ypk1, the yeast homolog to the human Serum- and glucocorticoid-induced protein kinase, accelerates phospholipase B1-mediated phosphatidylcholine deacylation. J Biol Chem 289:31591–31604. doi: 10.1074/jbc.M114.581157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patton JL, Pessoa-Brandao L, Henry SA. 1995. Production and reutilization of an extracellular phosphatidylinositol catabolite, glycerophosphoinositol, by Saccharomyces cerevisiae. J Bacteriol 177:3379–3385. doi: 10.1128/jb.177.12.3379-3385.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chowdhury T, Köhler JR. 2015. Ribosomal protein S6 phosphorylation is controlled by TOR and modulated by PKA in Candida albicans. Mol Microbiol 98:384–402. doi: 10.1111/mmi.13130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. YANAGITA T. 1964. Successive determinations of the free, acid-labile and residual phosphates in biological systems. J Biochem 55:260–268. doi: 10.1093/oxfordjournals.jbchem.a127879 [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Zhou J, Zou Y, Chen X, Liu L, Qi W, Huang X, Chen C, Liu N-N. 2022. Fungal Commensalism modulated by a dual-action phosphate transceptor. Cell Rep 38:110293. doi: 10.1016/j.celrep.2021.110293 [DOI] [PubMed] [Google Scholar]
- 37. Zablocki K, Miller SP, Garcia-Perez A, Burg MB. 1991. Accumulation of glycerophosphocholine (GPC) by renal cells: osmotic regulation of GPC:choline phosphodiesterase. Proc Natl Acad Sci U S A 88:7820–7824. doi: 10.1073/pnas.88.17.7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tams RN, Cassilly CD, Anaokar S, Brewer WT, Dinsmore JT, Chen Y-L, Patton-Vogt J, Reynolds TB. 2019. Overproduction of phospholipids by the kennedy pathway leads to hypervirulence in Candida albicans. Front Microbiol 10:86. doi: 10.3389/fmicb.2019.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jiang F, Huang S, Imadad K, Li C. 2012. Cloning and expression of a gene with phospholipase B activity from Pseudomonas fluorescens in Escherichia coli. Bioresour Technol 104:518–522. doi: 10.1016/j.biortech.2011.09.112 [DOI] [PubMed] [Google Scholar]
- 40. Köhler GA, Brenot A, Haas-Stapleton E, Agabian N, Deva R, Nigam S. 2006. Phospholipase A2 and phospholipase B activities in fungi. Biochim Biophys Acta 1761:1391–1399. doi: 10.1016/j.bbalip.2006.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smilowitz JT, O’Sullivan A, Barile D, German JB, Lönnerdal B, Slupsky CM. 2013. The human milk metabolome reveals diverse oligosaccharide profiles. J Nutr 143:1709–1718. doi: 10.3945/jn.113.178772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walter A, Korth U, Hilgert M, Hartmann J, Weichel O, Hilgert M, Fassbender K, Schmitt A, Klein J. 2004. Glycerophosphocholine is elevated in cerebrospinal fluid of alzheimer patients. Neurobiol Aging 25:1299–1303. doi: 10.1016/j.neurobiolaging.2004.02.016 [DOI] [PubMed] [Google Scholar]
- 43. Dame ZT, Aziat F, Mandal R, Krishnamurthy R, Bouatra S, Borzouie S, Guo AC, Sajed T, Deng L, Lin H, Liu P, Dong E, Wishart DS. 2015. The human saliva metabolome. Metabolomics 11:1864–1883. doi: 10.1007/s11306-015-0840-5 [DOI] [Google Scholar]
- 44. Sugimoto M, Saruta J, Matsuki C, To M, Onuma H, Kaneko M, Soga T, Tomita M, Tsukinoki K. 2013. Physiological and environmental parameters associated with mass spectrometry-based salivary metabolomic profiles. Metabolomics 9:454–463. doi: 10.1007/s11306-012-0464-y [DOI] [Google Scholar]
- 45. Wishart DS, Guo A, Oler E, Wang F, Anjum A, Peters H, Dizon R, Sayeeda Z, Tian S, Lee BL, Berjanskii M, Mah R, Yamamoto M, Jovel J, Torres-Calzada C, Hiebert-Giesbrecht M, Lui VW, Varshavi D, Varshavi D, Allen D, Arndt D, Khetarpal N, Sivakumaran A, Harford K, Sanford S, Yee K, Cao X, Budinski Z, Liigand J, Zhang L, Zheng J, Mandal R, Karu N, Dambrova M, Schiöth HB, Greiner R, Gautam V. 2022. HMDB 5.0: the human metabolome database for 2022. Nucleic Acids Res 50:D622–D631. doi: 10.1093/nar/gkab1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harada S, Taketomi Y, Aiba T, Kawaguchi M, Hirabayashi T, Uranbileg B, Kurano M, Yatomi Y, Murakami M. 2023. The lysophospholipase PNPLA7 controls hepatic choline and methionine metabolism. Biomolecules 13:471. doi: 10.3390/biom13030471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Austin S, Mayer A. 2020. Phosphate homeostasis - A vital metabolic equilibrium maintained through the INPHORS signaling pathway. Front Microbiol 11:1367. doi: 10.3389/fmicb.2020.01367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mukherjee PK, Seshan KR, Leidich SD, Chandra J, Cole GT, Ghannoum MA. 2001. Reintroduction of the PLB1 gene into Candida albicans restores virulence in vivo. Microbiology (Reading) 147:2585–2597. doi: 10.1099/00221287-147-9-2585 [DOI] [PubMed] [Google Scholar]
- 49. Leidich SD, Ibrahim AS, Fu Y, Koul A, Jessup C, Vitullo J, Fonzi W, Mirbod F, Nakashima S, Nozawa Y, Ghannoum MA. 1998. Cloning and disruption of caPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans. J Biol Chem 273:26078–26086. doi: 10.1074/jbc.273.40.26078 [DOI] [PubMed] [Google Scholar]
- 50. Djordjevic JT. 2010. Role of phospholipases in fungal fitness, pathogenicity, and drug development - lessons from cryptococcus neoformans. Front Microbiol 1:125. doi: 10.3389/fmicb.2010.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Theiss S, Ishdorj G, Brenot A, Kretschmar M, Lan C-Y, Nichterlein T, Hacker J, Nigam S, Agabian N, Köhler GA. 2006. Inactivation of the phospholipase B gene Plb5 in wild-type Candida albicans reduces cell-associated phospholipase A2 activity and attenuates virulence. Int J Med Microbiol 296:405–420. doi: 10.1016/j.ijmm.2006.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sizeland PCB, Chambers ST, Lever M, Bason LM, Robson RA. 1993. Organic osmolytes in human and other mammalian kidneys. Kidney Int 43:448–453. doi: 10.1038/ki.1993.66 [DOI] [PubMed] [Google Scholar]
- 53. Gallazzini M, Ferraris JD, Kunin M, Morris RG, Burg MB. 2006. Neuropathy target esterase catalyzes osmoprotective renal synthesis of Glycerophosphocholine in response to high NaCI. Proc Natl Acad Sci U S A 103:15260–15265. doi: 10.1073/pnas.0607133103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gallazzini M, Ferraris JD, Burg MB. 2008. GDPD5 is a glycerophosphocholine phosphodiesterase that osmotically regulates the osmoprotective organic osmolyte GPC. Proc Natl Acad Sci U S A 105:11026–11031. doi: 10.1073/pnas.0805496105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jawale CV, Biswas PS. 2021. Local antifungal immunity in the kidney in disseminated Candidiasis. Curr Opin Microbiol 62:1–7. doi: 10.1016/j.mib.2021.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fradin C, De Groot P, MacCallum D, Schaller M, Klis F, Odds FC, Hube B. 2005. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol Microbiol 56:397–415. doi: 10.1111/j.1365-2958.2005.04557.x [DOI] [PubMed] [Google Scholar]
- 57. Thewes S, Kretschmar M, Park H, Schaller M, Filler SG, Hube B. 2007. In vivo and ex vivo comparative transcriptional profiling of invasive and non-invasive Candida albicans isolates identifies genes associated with tissue invasion. Mol Microbiol 63:1606–1628. doi: 10.1111/j.1365-2958.2007.05614.x [DOI] [PubMed] [Google Scholar]
- 58. Zakikhany K, Naglik JR, Schmidt-Westhausen A, Holland G, Schaller M, Hube B. 2007. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol 9:2938–2954. doi: 10.1111/j.1462-5822.2007.01009.x [DOI] [PubMed] [Google Scholar]
- 59. Walker LA, Maccallum DM, Bertram G, Gow NAR, Odds FC, Brown AJP. 2009. Genome-wide analysis of Candida albicans gene expression patterns during infection of the mammalian kidney. Fungal Genet Biol 46:210–219. doi: 10.1016/j.fgb.2008.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hebecker B, Vlaic S, Conrad T, Bauer M, Brunke S, Kapitan M, Linde J, Hube B, Jacobsen ID. 2016. Corrigendum: Dual-species transcriptional profiling during systemic Candidiasis reveals organ-specific host-pathogen interactions. Nat Publ Gr 6:39423. doi: 10.1038/srep39423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Muñoz JF, Delorey T, Ford CB, Li BY, Thompson DA, Rao RP, Cuomo CA. 2019. Coordinated host-pathogen transcriptional dynamics revealed using sorted subpopulations and single Macrophages infected with Candida albicans. Nat Commun 10:1607. doi: 10.1038/s41467-019-09599-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Urrialde V, Prieto D, Pla J, Alonso-Monge R. 2016. The Candida albicans Pho4 transcription factor mediates susceptibility to stress and influences fitness in a mouse Commensalism model. Front Microbiol 7:1062. doi: 10.3389/fmicb.2016.01062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lev S, Djordjevic JT. 2018. Why is a functional PHO pathway required by fungal pathogens to disseminate within a phosphate-rich host: a paradox explained by alkaline pH-simulated nutrient deprivation and expanded PHO pathway function. PLoS Pathog 14:e1007021. doi: 10.1371/journal.ppat.1007021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lev S, Kaufman-Francis K, Desmarini D, Juillard PG, Li C, Stifter SA, Feng CG, Sorrell TC, Grau GER, Bahn Y-S, Djordjevic JT. 2017. Pho4 is essential for dissemination of Cryptococcus neoformans to the host brain by promoting phosphate uptake and growth at alkaline pH. mSphere 2:e00381-16. doi: 10.1128/mSphere.00381-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lam FH, Steger DJ, O’Shea EK. 2008. Chromatin decouples promoter threshold from dynamic range. Nature 453:246–250. doi: 10.1038/nature06867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Toh-e A, Ohkusu M, Li H-M, Shimizu K, Takahashi-Nakaguchi A, Gonoi T, Kawamoto S, Kanesaki Y, Yoshikawa H, Nishizawa M. 2015. Identification of genes involved in the phosphate metabolism in Cryptococcus neoformans. Fungal Genet Biol 80:19–30. doi: 10.1016/j.fgb.2015.04.019 [DOI] [PubMed] [Google Scholar]
- 67. He BZ, Zhou X, O’Shea EK. 2017. Evolution of reduced co-activator dependence led to target expansion of a starvation response pathway. Elife 6:e25157. doi: 10.7554/eLife.25157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Głąb B, Beganovic M, Anaokar S, Hao M-S, Rasmusson AG, Patton-Vogt J, Banaś A, Stymne S, Lager I. 2016. Cloning of glycerophosphocholine acyltransferase (GPCAT) from fungi and plants: a novel enzyme in phosphatidylcholine synthesis. J Biol Chem 291:25066–25076. doi: 10.1074/jbc.M116.743062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Joyce LR, Guan Z, Palmer KL. 2019. Phosphatidylcholine biosynthesis in mitis group Streptococci via host metabolite scavenging. J Bacteriol 201:e00495-19. doi: 10.1128/JB.00495-19 [DOI] [PMC free article] [PubMed] [Google Scholar]