ABSTRACT
The detection rate of carbapenem-resistant nontyphoidal Salmonella (NTS) is relatively low. However, carbapenem-sensitive and carbapenem-resistant Salmonella enterica serovar Typhimurium (S. enterica serovar Typhimurium) were isolated from a clinical outpatient within a span of 10 days, named 1104–65 and 1104–75. We aimed to reveal the mechanism of carbapenem resistance in S. enterica serovar Typhimurium isolates in this case. The resistance phenotype of S. enterica serovar Typhimurium was analyzed by the VITEK-2 Compact equipment and Kirby-Bauer disc diffusion method. Whole-genome sequencing was used to analyze the serotype, ST type, genetic relationship, resistance genes, plasmid replicon, the genetic environment of blaNDM-5, and the characteristics of IncFII plasmid carrying blaNDM-5 in S. enterica serovar Typhimurium. The transferability of the IncFII plasmid carrying blaNDM-5 was verified by the conjugation assay and PCR. The serotypes of both NTS are S. enterica serovar Typhimurium, belonging to ST34. Single nucleotide polymorphisms analysis showed that they were the same clone. A conjugative IncFII plasmid pIncFII-NDM5 with transferability was detected in isolate 1104–75, which harboring the blaNDM-5 gene was the primary mechanism responsible for mediating carbapenem resistance in S. enterica serovar Typhimurium. The genetic environment of blaNDM-5 on pIncFII-NDM5 is “IS26-ΔIS3000-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-dsbC -cutA-IS26”, which was confirmed as a novel structure not previously reported in the NCBI database. Although the mechanism is rarely reported in NTS, the prevalence of IncFII plasmid pIncFII-NDM5 will pose a great threat to the clinical treatment of S. enterica serovar Typhimurium. Meanwhile, the coexistence of blaCTX-M-55, qnrS1, blaNDM-5, and mph(A) in S. enterica serovar Typhimurium ST34 warrants additional attention.
IMPORTANCE
In this study, an IncFII plasmid pIncFII-NDM5 carrying blaNDM-5 was found in carbapenem-resistant Salmonella enterica serovar Typhimurium (S. enterica serovar Typhimurium), which has conjugative transferability and carried blaNDM-5, bleMBL, mph(A), and blaTEM-1 four resistance genes that can mediate resistance to multiple antibiotics including cephalosporins, beta-lactamase inhibitor combinations, carbapenems, and macrolides. Phylogenetic analysis showed that 1104–65 and 1104–75 were closely related to other S. enterica serovar Typhimurium in this area. The above-mentioned S. enterica serovar Typhimurium chromosome carries blaCTX-M-55, qnrS1, and tet(A) genes, so the antibiotic resistance of isolates will be further enhanced after obtaining the pIncFII_NDM5-like plasmid. Meanwhile, we discovered a novel genetic structure of blaNDM-5 mediated by the IS26 composite transposon, which will expand our understanding of the emergence and spread of carbapenem-resistance genes. Altogether, the presence of the IncFII plasmid pIncFII-NDM5 further underscores the need for vigilant surveillance and appropriate infection control measures to mitigate the impact of carbapenem-resistant S. enterica serovar Typhimurium in clinical settings.
KEYWORDS: bla NDM-5 , S. enterica serovar Typhimurium, IncFII plasmid, carbapenem-resistance, IS26
INTRODUCTION
Nontyphoidal Salmonella (NTS) is a prevalent bacterial cause of gastrointestinal diseases worldwide, with over 2,600 serotypes. Salmonella Typhimurium (Salmonella enterica serovar Typhimurium) is one of the most common serotypes, infecting both humans and animals (1, 2). NTS infection is a self-limiting disease, but in immunocompromised persons, such as children and the elderly, it can progress to severe systemic disease and antibiotic therapy is also necessary (3, 4). During recent decades, as the primary antibiotic therapy choices for NTS, fluoroquinolones, and extended-spectrum cephalosporins (ESCs) were used in anti-infective therapy more frequently, which has led to an increasing resistance rate in NTS (5–7). Carbapenem antibiotics are β-lactam antibiotics with broad activity, which are specially used for the treatment of severe bacterial infections (8, 9). Therefore, carbapenem antibiotics may be the last resort for patients with aggressive, multidrug-resistant NTS infections (10).
Although carbapenem-resistant NTS is still extremely rare, it will pose a serious threat to antimicrobial therapy once it occurs (11, 12). Several carbapenemases have been reported in NTS, including KPC, IMP, NDM, VIM, and OXA-48 (11, 13–15). Here, we report an NDM-5-producing carbapenem-resistant S. enterica serovar Typhimurium from an outpatient. With two amino acid changes (Val88Leu and Met154Leu), the NDM-5 variant of NDM-1 has a higher resistance to carbapenems and ESCs than NDM-1 (16). Since it was first discovered in Escherichia coli (E. coli) in 2011, NDM-5 carbapenemase has occasionally been discovered in other Enterobacteriaceae, such as Klebsiella pneumoniae (KP) and NTS (11). In this case, a carbapenem-resistant clinical S. enterica serovar Typhimurium was discovered in the Fifth Affiliated Hospital, Southern Medical University located in Conghua District, Guangzhou in November 2021. Therefore, this study aims to investigate the mechanism of carbapenem resistance in a clinical S. enterica serovar Typhimurium isolate.
RESULTS
Isolate identification and antimicrobial susceptibility testing results
Two NTS strains were successively isolated from the stool samples of the same outpatient. In November 2021, strain 1104–65 was isolated from the patient’s stool, followed by strain 1104–75 in another stool specimen 10 days later. Unfortunately, we did not collect further details about this outpatient treatment, so it is unclear what the patient’s treatment program was during 10 days. Both isolates were typed S. enterica serovar Typhimurium (O4: Hi). The antibiotic susceptibility results are shown in Table 1. Compared with 1104–65, isolate 1104–75 had increased minimum inhibitory concentration (MIC) to ceftazidime (CAZ), cefepime (FEP), and azithromycin (AZM), among which FEP became resistant (MIC ≥32 µg/mL), and was resistant to cefoxitin (FOX), amoxicillin-clavulanic acid (AMC), piperacillin-tazobactam (TZP), imipenem (IPM), and ertapenem (ETP). Both isolates were intermediary to levofloxacin (LVX) and ciprofloxacin (CIP). The resistance profiles of 1104–65 and 1104–75 to other antibiotics were broadly similar.
TABLE 1.
The antibiotic susceptibility results of the isolatesa
| Isolate | MIC (µg/mL) | Zone diameter (mm) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CXM | CRO | CAZ | FEP | FOX | AMC | TZP | LVX | SXT | IPM | ETP | TGC | AMP | CIP | AZM | CHL | |
| 1104–65 | ≥64 | ≥64 | 32 | 8 | ≤4 | 4 | ≤4 | 1 | ≤1 | ≤0.25 | ≤0.12 | 1 | 6 | 27 | 11 | 26 |
| 1104–75 | ≥64 | ≥64 | ≥64 | ≥32 | ≥64 | ≥32 | 128 | 1 | ≤1 | ≥16 | ≥8 | ≤0.5 | 6 | 28 | 6 | 21 |
| C600 | 16 | ≤0.25 | 0.5 | ≤0.12 | 8 | 4 | ≤4 | 0.5 | ≤1 | ≤0.25 | ≤0.12 | ≤0.5 | 14 | 29 | 25 | 24 |
| C-1104–75 | ≥64 | ≥64 | ≥64 | 16 | ≥64 | ≥32 | ≥128 | 0.5 | ≤1 | ≥16 | ≥8 | ≤0.5 | 6 | 28 | 8 | 24 |
CXM, cefuroxime; CRO, ceftriaxone; SXT, cotrimoxazole; TGC, tigecycline; AMP, ampicillin; CHL, chloramphenicol.
Plasmid and resistance gene analysis
The whole genome sequence revealed that compared with isolate 1104–65, isolate 1104–75 additionally harbored IncFII plasmid replicon and Col156_1. In addition, 1104–75 also possessed four resistant genes (blaNDM-5, bleMBL, mph(A), and blaTEM-1, which, respectively, confer resistance to carbapenems, bleomycin, and macrolides), all of which were located on the IncFII plasmid (named pIncFII-NDM5). Both chromosomes carried tet(B), blaCTX-M-55, and qnrS1 resistance genes.
Phylogenetic analysis
The bioinformatics analysis showed that the serotype 1104–65 and 1104–75 both were S. enterica serovar Typhimurium, belonging to ST34. The phylogenetic relationship of 1104–65, 1104–75, and other S. enterica serovar Typhimurium (n = 67) in this area from our previous study were assessed using S. enterica serovar Typhimurium ATCC14028 as a reference strain (see Fig. S1). Sixty-seven strains of other S. enterica serovar Typhimurium in this area were isolated from the stools of patients from May 2020 to February 2021 in the Fifth Affiliated Hospital, Southern Medical University (for details, see Table S1). Single nucleotide polymorphisms (SNPs) analysis showed that there were 17 SNPs between 1104-65 and 1104–75, indicating that they belonged to the same clone. Meanwhile, the SNPs between the two and other S. enterica serovar Typhimurium ST34 in this area ranged from 0 to 157, showing a close genetic relationship. It is worth noting that the resistance gene spectrum of 1104–65 is consistent with S24, S79, S36, S49, S34, S42, and S133. All of these strains carry blaCTX-M-55, qnrS1, and tet(B). Moreover, our previous studies have shown that these three resistance genes were located on the chromosome of S. enterica serovar Typhimurium and can be transmitted vertically.
Characterization of plasmid pIncFII-NDM5
The transconjugant was successfully obtained through the conjugation experiment and named C-1104–75. PCR and sequencing results showed that the transconjugant carried IncFII plasmid replicon and blaNDM-5 gene, which indicates that the plasmid pIncFII-NDM5 carrying the blaNDM-5 gene is a conjugative plasmid with transferability. The drug susceptibility results of C-1104–75 are shown in Table 1. Compared with the recipient strain E. coli C600, pIncFII-NDM5 confers the transconjugant with resistance to ESCs, FOX, AMP, AMC, TZP, IPM, ETP, and AZM.
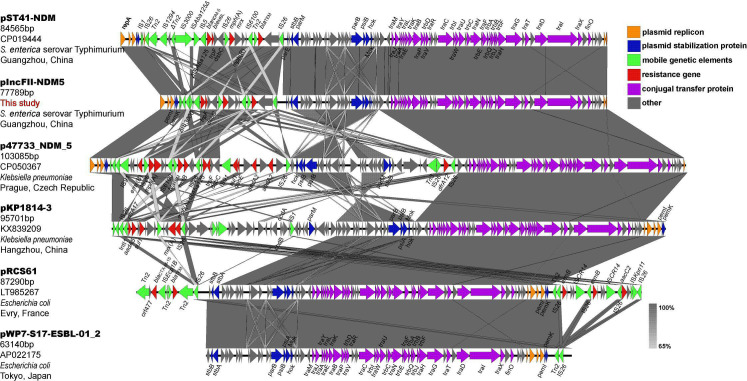
The plasmid pIncFII-NDM5 has a total length of 77,785 bp. Download the sequence of IncFII plasmids (with or without blaNDM-5) similar to pIncFII-NDM5 in other studies through NCBI: (I) Plasmid pST41-NDM (no. CP016389) was isolated from S. enterica serovar Typhimurium detected in stool samples of children with acute diarrhea in Guangzhou, China. The full length is 84,565 bp, carrying four resistance genes blaNDM-5, bleMBL, mph(A), and blaTEM-1. (II) Plasmid p47733_NDM_5 (no.CP050367) was isolated from KP and detected in rectal swab of patients in a hospital in Prague, Czech Republic. The full length is 103,085 bp, carrying nine resistance genes erm(B), mph(A), blaTEM-1, rmt(B), blaNDM-5, bleMBL, sul1, aadA2 (2 copy number), and dfrA12. (III) Plasmid pKP1814-3 (no. KX839209) was isolated from KP detected in a hospital in Hangzhou, China. The full length is 95,701 bp, carrying five resistance genes dfrA17, aadA2, sul1, erm(B), and mph(A). (IV) Plasmid pRCS61 (no. LT985267) was isolated from E. coli in Evry, France. The full length is 87,290 bp, carrying four resistance genes blaCTX-M-15, blaTEM, erm(B) (2 copy number), and aacC2. (V) Plasmid pWP7-S17-ESBL-01_2 (no. AP022175) was isolated from E. coli detected in waste water treatment plant effluent in Tokyo, Japan. The full length is 63,140 bp, with no resistance genes present. Through the comparison of pIncFII-NDM5 and the above IncFII plasmid sequence (Fig. 1), it was found that the backbone structure of IncFII plasmids was almost identical, mainly including related genes encoding proteins involved in replication, maintenance and conjugative transfer. However, most of the mobile genetic elements and resistance genes were located in the variable region of IncFII plasmids. It can be seen from Fig. 1 that the two IncFII plasmids carrying blaNDM-5 from S. enterica serovar Typhimurium are highly similar in variable regions, but the mobile genetic elements of pST41-NDM are more abundant than pIncFII-NDM5. Notably, the IS3000 upstream of blaNDM-5 of pIncFII-NDM5 was incomplete (469/3235), and one end of the ISAba125 sequence truncated by IS5 was also missing. It indicates that the IncFII plasmid carrying blaNDM-5 is still evolving in S. enterica serovar Typhimurium. Simultaneously, the gene encoding the conjugative transfer-associated protein of IncFII plasmid confers it with conjugative transferability, which will pose a significant clinical risk. In addition, IS26 appears to play an important role in the acquiring of resistant gene segments in the variable region.
Fig 1.
Linear comparison of plasmid pIncFII-NDM5 with other similar IncFII plasmids (with or without blaNDM-5). Gray shading indicates regions of shared homology among different elements. Open reading frames are marked by colored arrows, orange indicates genes encoding plasmid replicon, blue indicates genes encoding plasmid stabilizing proteins, green indicates mobile genetic elements, red indicates resistance genes, purple indicates genes encoding conjugal transfer proteins, and gray indicates other genes.
Comparative analysis of the genetic environment of blaNDM-5
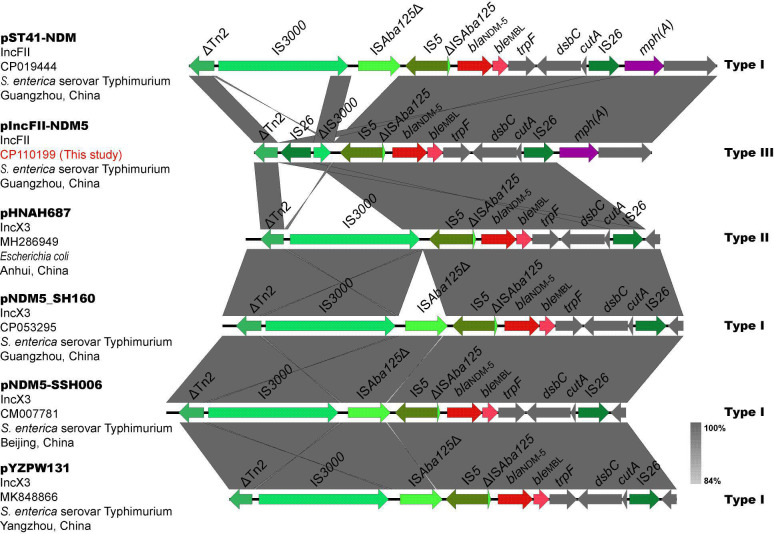
The genetic environment of blaNDM-5on pIncFII-NDM5 was intercepted for correlation analysis, about 9256 bp, compared with the BLAST database (http://www.ncbi.nlm.nhi.gov/blast/) and downloaded the plasmid sequence (GenBank accession number MH286949) of the most similar fragment. Meanwhile, literatures of S. enterica serovar Typhimurium carrying blaNDM-5 were searched through the PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/), and the plasmid sequences carrying blaNDM-5 were downloaded to compare and analyze the genetic environment of blaNDM-5 (see Fig. 2). The relevant information of the above plasmids is shown in Table 2. Three different genetic environments were found surrounding blaNDM-5.
Fig 2.
The genetic environments of blaNDM-5. Different colored arrows represent various open reading frames (ORFs), and the arrow’s direction indicates the direction of transcription. Homogeneous regions are represented by light gray shadows.
TABLE 2.
Information about plasmid carrying blaNDM-5 in Fig. 2
| Name | Isolate | Inca | Origin | Country | Year of isolation | Reference | Types of blaNDM-5 genetic structurec |
|---|---|---|---|---|---|---|---|
| pNDM5-SSH006 | S. enterica serovar Typhimurium | IncX | Patient feces | Shanghai, China | 2015 | (17) | Ⅰ |
| pNDM5_SH160 | S. enterica serovar Typhimurium | IncX | Retail pork | Shanghai, China | 2016 | (18) | Ⅰ |
| pST41-NDM | S. enterica serovar Typhimurium | IncFII | Patient feces | Guangzhou, China | 2017 | (13) | Ⅰ |
| pHNAH687 | E. coli | Inc X | Chicken feces | Anhui, China | 2018 | BLASTb | Ⅱ |
| pYZPW131 | S. enterica serovar Typhimurium | IncX | Retail pork | Jiangsu, China | 2019 | (14) | Ⅰ |
| pIncFII-NDM5 | S. enterica serovar Typhimurium | IncFII | Patient feces | Guangzhou, China | 2021 | This study | Ⅲ |
Inc, incompatibility group.
BLAST, the plasmid carrying the region most similar to the blaNDM-5 gene environment on pIncFII-NDM5 by BLAST search on the NCBI database.
Type Ⅰ was “IS3000-ISAba125Δ-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-dsbC-cutA-IS26”, type Ⅱ was “IS3000-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF -dsbC-cutA-IS26”, and type Ⅲ was “IS26-ΔIS3000-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-dsbC-cutA-IS26”.
Type I “IS3000-ISAba125Δ-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-dsbC-cutA- IS26” was the most common type of structure, discovered in the IncFII plasmid pST41-NDM and three IncX3 plasmids isolated from S. enterica serovar Typhimurium. The genetic structure of blaNDM-5 on the IncFII plasmid pIncFII-NDM5 isolated in this study was type III “IS26-ΔIS3000-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-dsbC-cutA-IS26”, while the most similar structure on the IncX3 plasmid pHNAH687 isolated from E. coli was type II “IS3000-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-dsbC-cutA-IS26”. The differences between the three types were IS3000 (complete/incomplete), IS26 (presence/absence), and one end of ISAba125 truncated by IS5 (presence/absence). Beyond comprehensive analysis, we speculate that type I evolved into type II after the loss of ISAba125Δ (1–1018/1087). After a new IS26 was inserted and truncated into IS3000 of type II, it formed a composite transposon with IS26 downstream of blaNDM-5 to mediate the transfer of blaNDM-5, and type II evolved into type III. To our knowledge, the IS26 composite transposon has never been described to mediate blaNDM-5 transfer in previous studies.
DISCUSSION
NTS is a major cause of foodborne illness in animals and humans worldwide. With the emergence and rapid development of NTS-resistance phenotype ACSSuT (defined as resistance to AMP, CHL, streptomycin, sulfamethoxazole, and tetracycline), fluoroquinolones (FQs) and ESCs are commonly used as a first-line agent for the treatment of NTS infections (17–21). However, with widespread use, the detection rate of NTS resistant to ESCs and QRs has been increasing in recent years (1, 22–25). Therefore, carbapenems may be the last resort for patients with invasive, multidrug-resistant (MDR, resistance to three or more classes of antimicrobials) NTS infection (26).
Resistance to carbapenems in Enterobacteriaceae occurs involves multiple mechanisms, such as production of carbapenemases, production of extended-spectrum β-lactamases (ESBLs) or AmpC enzymes combined with the loss of specific outer membrane porins, increased efflux pump activity (12, 27, 28). The drug resistance mechanisms mentioned above are commonly observed in E. coli and KP but are rarely reported in NTS (11, 29, 30). The first carbapenemase gene isolated in NTS was blaKPC-2, which was found in S. enterica serovar Cubana isolated from the stool of a 4-year-old boy with diarrhea in the United States in 1998 (31). Subsequently, carbapenemase genes blaIMP-4, blaNDM-1, blaNDM-5, blaVIM-2, and blaOXA-48 were successively reported in NTS (11). Carbapenem-resistant NTS has become a serious clinical problem due to limited treatment options. In this study, the mechanism that mediates the resistance of S. enterica serovar Typhimurium clinical isolate 1104–75 to carbapenems is the production of NDM-5 carbapenemase.
NDM-5 carbapenemase is currently primarily detected in E. coli and is still uncommon in other Enterobacteriaceae such as NTS and KP (32). The most prevalent plasmid type in Enterobacteriaceae to contain blaNDM-5 is IncX3 (33, 34). Compared with 1104–65, the MIC of 1104–75 carrying blaNDM-5 to ESCs, β-lactam/β-lactamase inhibitor, and carbapenems increased significantly, which was consistent with previous research results (13, 16, 35). The blaNDM-5 in this study is located on the IncFII plasmid pIncFII-NDM5 of S. enterica serovar Typhimurium clinical isolate 1104–75. By searching the PubMed database, it is found that blaNDM-5 is also mainly located on the IncX3 plasmid in Salmonella (14, 36–39). Only one article (13) reported that blaNDM-5 was localized on the IncFII plasmid pST41-NDM in S. enterica serovar Typhimurium isolated from a stool of a child with acute diarrhea in Guangzhou, China. The IncFII plasmid has a narrow host range was commonly found in E. coli, and has been involved in the global spread of the blaCTX-M-15 gene in the E. coli clone ST131 (40). Notably, pIncFII-NDM5 carrying the blaNDM-5 gene is a transferable conjugative plasmid that confers high levels of resistance to ESCs and carbapenems in clinical S. enterica serovar Typhimurium isolate 1104–75. Through the comparison of plasmid sequences, it was found that the backbone of pIncFII-NDM5 was very similar to other IncFII plasmids, with most genes encoding conjugative transfer proteins, which may be the main reason for the conjugative transferability of pIncFII-NDM5 (41). The IncFII plasmid pIncFII-NDM5 and pST41-NDM also carry the mph(A) gene, which confers AZM resistance. AZM is FDA-approved for the treatment of systemic Salmonella infections, particularly those caused by S. enterica serovar Typhimurium, due to increased rates of resistance to ESCs and FQs (42). Meanwhile, AZM is widely used in the treatment of various infections in children due to it is well tolerated in the presence of multiple co-morbidities and medications (43, 44). Additionally, it is worth noting that the genetic structure “IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-dsbC-cutA -IS26 -mph(A)-mrx-mph(R)-IS6100” located on the pIncFII-NDM5 and pST41-NDM plasmids are completely identical, about 9096 bp. Through the BLAST tool, it was found that this framework also exists on the IncFII plasmid pGZ_NDM5 (no. CP017981) in E. coli. Although the blaNDM-5 and mph(A) gene combinations have been reported in previous literature, it has not been noticed both are located on the same genetic framework (45, 46). This suggests that clinicians should be alert to the phenomenon of co-transfer of blaNDM-5 and mph(A).
S. enterica serovar Typhimurium has a broad host range and is one of the major NTS serotypes responsible for outbreaks of infectious diarrhea and foodborne disease worldwide (47). ST34 is the most common ST type of S. enterica serovar Typhimurium and is often associated with ACSSuT resistance patterns (48). In this study, 1104–75 and 1104–65 have a relatively close genetic relationship with other S. enterica serovar Typhimurium ST34 isolates in this area (see Fig. S1). This means that pIncFII-NDM5 will be extremely dangerous if it becomes widespread among S. enterica serovar Typhimurium ST34 in this area. In our previous studies, it has been reported that there may be a potential epidemic clone of S. enterica serovar Typhimurium ST34 in this region with blaCTX-M-55 and qnrS1 localized on the chromosome (49). The qnrS1 gene can mediate low-level resistance to FQ, and its presence can provide a selective advantage for strains exposed to FQs, thereby accelerating the development of chromosome-mediated FQs resistance in strains (50). More importantly, this study reported the emergence of NTS carrying four resistance genes (blaCTX-M-55, qnrS1, blaNDM-5, and mph(A)). Thus, the phenomenon of 1104–65-like clone S. enterica serovar Typhimurium ST34 carrying pIncFII_NDM5-like plasmid warrants additional attention because it may accelerate the development and spread of NTS coresistant to ESCs, FQs, carbapenems, and macrolide antibiotics.
The most typical blaNDM-5 genetic structure, Type I “IS3000-ISAba125Δ-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-dsbC-cutA-IS26”, is frequently observed in IncX3 plasmids (51, 52). Of note, the genetic environment (type I) of blaNDM-5 on the IncFII plasmid pST41-NDM was the same as the genetic environment of blaNDM-5 on the IncX3 plasmid carried by other Enterobacteriaceae (E. coli, KP, and Enterobacter cloacae) from the same hospital. It suggested that the genetic environment of blaNDM-5 in the variable region of pST41-NDM may be derived from the IncX3 plasmid (13). The genetic structure of blaNDM-5 on the IncFII plasmid pIncFII-NDM5 isolated in this study was type III “IS26-ΔIS3000-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-dsbC-cutA-IS26”. Compared with another IncFII plasmid pST41-NDM, pIncFII-NDM5 has a new IS26 inserted and truncated IS3000 (2754–3222/3235), and one end of ISAba125 (1–1018/1087) was missing in the genetic structure. From type I to type III, we speculate that there are two main steps. Meanwhile, it was brought to our attention that IS3000on pST41-NDM, pNDM5_SH160, pNDM5-SSH006, and pYZPW131 was complete, whereas IS3000on pHNAH687 was incomplete (1–3222/3235), which provides stronger evidence for our conjecture. Mediated by mobile elements, the genetic environment of blaNDM-5 is constantly changing during the transfer process. Currently, the genetic structure of blaNDM-5 shares two common features (33, 39): (I) the insertion sequence ISAba125 (complete or truncated) is present upstream of blaNDM-5. (II) The downstream of blaNDM-5 includes the bleMBL gene that mediates bleomycin resistance, followed by trpF (encoding phosphoribosyl anthranilate isomerase), dsbC (also known as tat, encoding a twin-arginine translocation pathway signal sequence domain protein), and cutA (also known as dct, encoding a periplasmic divalent cation tolerance protein). According to reports, trpF and dsbC play key roles in the stability, retention, or spread of blaNDM-5, or promotion of enzyme function (53). Currently, insertion sequences found in the genetic environment of blaNDM-5 include ISAba125 (complete or truncated), IS91, IS26, IS5, IS3000 (complete or truncated), and ISCR1 (54–60). IS26, a member of the IS6 insertion sequence family, promotes the spread of antibiotic-resistance genes in Gram-negative bacteria mainly through the formation of composite transposons (61). Two IS26s in the same or opposite direction often form a composite transposon to mediate the transfer of resistance genes between them (62). For example, the IS26 composite transposon in this study is involved in the mobilization of blaNDM-5. Its flanking elements are frequently deleted when IS26 is inserted (63). This may be the reason why IS3000 (2754–3222/3235) was truncated in Type III. A higher copy number of IS26 was found in the variable region of IncFII plasmids in Fig. 1, which may be involved in the recombination of plasmids MDR region, thereby endowing isolates with resistance to multiple antibacterial drugs, ultimately limiting clinical treatment options. All in all, various evidences indicated that the genetic environment of blaNDM-5 composed of the IS26 composite transposon is identified clinically for the first time in this study.
Conclusion
In this study, an IncFII plasmid pIncFII-NDM5 carrying blaNDM-5 was isolated from S. enterica serovar Typhimurium detected from a stool sample of an outpatient in Conghua District, Guangzhou, which mediated resistance to carbapenems in S. enterica serovar Typhimurium. The genetic environment of blaNDM-5 “IS26-ΔIS3000-IS5-ΔISAba125-blaNDM-5-bleMBL-trpF-dsbC-cutA-IS26” was different from the previous typical structure, and IS26 at both ends constitutes a composite transposon to mediate the gene transfer, which is also the first report of this type of genetic environment in blaNDM-5. Currently, reports of IncFII plasmids carrying blaNDM-5 in NTS are still rare. Our results suggest that the IncFII plasmid carrying blaNDM-5 may still be evolving and this type of plasmid can mediate high levels of resistance to ESCs and carbapenem. Meanwhile, blaCTX-M-55, qnrS1, blaNDM-5, and mph(A) cotransfer warrants additional attention because it may accelerate the development and spread of NTS coresistant to ESCs, FQs, carbapenems, and macrolide antibiotics.
MATERIALS AND METHODS
Bacterial collection, culture, and identification
The carbapenem-sensitive isolate 1104–65 and carbapenem-resistant isolate 1104–75 used in this study were collected from the stool of the same outpatient in Fifth Affiliated Hospital, Southern Medical University in Conghua District, Guangzhou. Strain 1104–65 was isolated from the patient’s stool in November 2021, and 1104–75 was isolated from another stool specimen 10 days later. Extract a sufficient amount of stool sample and use an inoculation loop to inoculate it onto blood agar plates, SS medium, and MacConkey agar plates. The typical colony morphology of Salmonella on SS medium is colorless, transparent, and black in the center. After incubation at 37°C for 16–18 hours, a single colony was selected and drawn on a blood agar plate to obtain pure isolates for identification and antimicrobial susceptibility tests. Isolates were analyzed and identified by the VITEK-2 COMPACT automatic microbial identification system (bioMérieux, Marcy-l'Étoile, France). Salmonella serotyping was conducted by using the slide agglutination test with specific antisera (Tianrun, Ningbo, China) according to the manufacturer’s instructions.
Antimicrobial susceptibility testing
The MIC values for CTX, CRO, CAZ, FEP, FOX, AMC, TZP, LVX, SXT, IPM, ETP, and TGC were performed using the VITEK-2 Compact equipment. The diameter of the inhibition zone (mm) of the NTS isolates against AMP, CIP, AZM, and CHL was determined by the Kirby-Bauer disc diffusion method on Muller–Hinton (MH) agar plates. All of the procedures and results interpretation were followed by the Clinical and Laboratory Standards Institute (CLSI M100, 33th edition) guidelines.
Whole-genome sequencing (WGS) and bioinformatics analysis
Sample preparation steps and genome sequencing
Isolates 1104–65 and 1104–75 were inoculated in Luria-Bertani broth and cultured at 37°C in a 200-rpm shaker until it reached a logarithmic phase. The broth was centrifuged at 10,000 rpm for 10 min at 4°C. After centrifugation, the supernatant was removed and rinsed 3–5 times with sterile water until the supernatant were clear. Samples were placed on dry ice for transportation immediately after sampling. All library preparation and sequencing were performed by the Novogene Bioinformatics Technology (Tianjin, China).
Analysis of whole genome sequencing data
Sequence reads were assembled using Unicycler 0.4.8 (64) and annotated using Prokka 1.14.5 (65). The predicted serotype and multi-locus sequence typing (MLST) types were identified using the Salmonella in Silico Typing Resource (SISTR 1.1.1) (66), and MLST 2.18.0 (67). The antibiotic-resistance genes and plasmid replicons were predicted using ResFinder 4.1 (68) and PlasmidFinder 2.1 (69), respectively, the default parameters were applied with minimum thresholds of sequence identity (>90%) and sequence coverage (>60%). Transposon and insertion sequence (IS) elements were scanned using the ISfinder database (70). Phylogenetic analysis was performed using Parsnp (71), the phylogenetic tree was visualized using Evolview online (72), and the SNPs among the core genomes of NTS were determined by using MEGA X (73). Close relatedness of isolates was defined as <21 allele differences in cgMLST (74). The genetic environment was visualized by the EasyFig software (75) and Adobe Illustrator (AI).
Conjugation experiments
Rifampicin-resistant E. coli C600 was used as the recipient strain and imipenem-resistant isolate 1104–75 was used as donor strain to determine the transferability of carbapenem-resistance phenotype. Transconjugant was selected on Luria–Bertani plates containing 100 µg/mL rifampicin plus 2 µg/mL imipenem, and the resistance phenotype was investigated by AST. PCR and sequencing were used to confirm whether the transconjugant carried the carbapenem resistance gene (NDM-F: ATGGAATTGCCCAATATTATGCAC, NDM-R: TCAGCGCAGCTTGTCGGC) and the related plasmid replicon (FII-F: CTGATCGTTTAAGGAATTTT, FII-R: CACACCATCCTGCACTTA).
ACKNOWLEDGMENTS
This work was supported by the Guangdong Medical Science and Technology Research Fund Project (No. B2023232), President Foundation of The Fifth Affiliated Hospital, Southern Medical University (No. YZ2022Z × 01), the Guangdong Basic and Applied Basic Research Fund Provincial Enterprise Joint Fund (2021A1515220153), and Military Logistics Research Fund Project (No. CLB21J018).
Contributor Information
Xiaoyan Li, Email: xiaoyanli@gzhmu.edu.cn.
Mariana Castanheira, JMI Laboratories, North Liberty, Iowa, USA.
DATA AVAILABILITY
The nucleotide sequences of the genomes and plasmids of 1104–75 and 1104–65 have been uploaded to GenBank under the accession numbers CP110198-CP110200 and CP110201, respectively.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/msphere.00480-23.
Phylogenetic analysis between 1104-65, 1104-75, ATCC14028, and other S. enterica serovar Typhimurium in this area.
Information about other 67 strains of S. enterica serovar Typhimurium isolated in this area.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Liang B, Xie Y, He S, Mai J, Huang Y, Yang L, Zhong H, Deng Q, Yao S, Long Y, Yang Y, Gong S, Zhou Z. 2019. Prevalence, serotypes, and drug resistance of nontyphoidal Salmonella among paediatric patients in a tertiary hospital in Guangzhou, China, 2014-2016. J Infect Public Health 12:252–257. doi: 10.1016/j.jiph.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 2. Duong VT, The HC, Nhu TDH, Tuyen HT, Campbell JI, Minh PV, Phuc HL, Chau TTH, Ngoc NM, Vi LL, Mather AE, Baker S. 2020. Genomic serotyping clinical manifestations, and antimicrobial resistance of nontyphoidal Salmonella gastroenteritis in hospitalized children in Ho Chi Minh city Vietnam. J Clin Microbiol 58:e01465-20. doi: 10.1128/JCM.01465-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lokken KL, Walker GT, Tsolis RM. 2016. Disseminated infections with antibiotic-resistant non-typhoidal Salmonella strains: contributions of host and pathogen factors. Pathog Dis 74:ftw103. doi: 10.1093/femspd/ftw103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marks F, von Kalckreuth V, Aaby P, Adu-Sarkodie Y, El Tayeb MA, Ali M, Aseffa A, Baker S, Biggs HM, Bjerregaard-Andersen M, Breiman RF, Campbell JI, Cosmas L, Crump JA, Espinoza LMC, Deerin JF, Dekker DM, Fields BS, Gasmelseed N, Hertz JT, Van Minh Hoang N, Im J, Jaeger A, Jeon HJ, Kabore LP, Keddy KH, Konings F, Krumkamp R, Ley B, Løfberg SV, May J, Meyer CG, Mintz ED, Montgomery JM, Niang AA, Nichols C, Olack B, Pak GD, Panzner U, Park JK, Park SE, Rabezanahary H, Rakotozandrindrainy R, Raminosoa TM, Razafindrabe TJL, Sampo E, Schütt-Gerowitt H, Sow AG, Sarpong N, Seo HJ, Sooka A, Soura AB, Tall A, Teferi M, Thriemer K, Warren MR, Yeshitela B, Clemens JD, Wierzba TF. 2017. Incidence of invasive Salmonella disease in sub-Saharan Africa: a multicentre population-based surveillance study. Lancet Glob Health 5:e310–e323. doi: 10.1016/S2214-109X(17)30022-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Folster JP, Campbell D, Grass J, Brown AC, Bicknese A, Tolar B, Joseph LA, Plumblee JR, Walker C, Fedorka-Cray PJ, Whichard JM. 2015. Identification and characterization of multidrug-resistant Salmonella enterica serotype albert isolates in the United States. Antimicrob Agents Chemother 59:2774–2779. doi: 10.1128/AAC.05183-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feng Y, Chang YJ, Fang SH, Su LH, Li HC, Yang HP, Yu MJ, Chiu CH. 2019. Emergence and evolution of high-level cephalosporin-resistant Salmonella goldcoast in northern Taiwan. Open Forum Infect Dis 6:ofz447. doi: 10.1093/ofid/ofz447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma Y, Li M, Xu X, Fu Y, Xiong Z, Zhang L, Qu X, Zhang H, Wei Y, Zhan Z, Chen Z, Bai J, Liao M, Zhang J. 2018. High-levels of resistance to quinolone and cephalosporin antibiotics in MDR-ACSSuT Salmonella enterica serovar enteritidis mainly isolated from patients and foods in Shanghai, China. Int J Food Microbiol 286:190–196. doi: 10.1016/j.ijfoodmicro.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 8. Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. doi: 10.1128/AAC.00296-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oli AN, Itumo CJ, Okam PC, Ezebialu IU, Okeke KN, Ifezulike CC, Ezeobi I, Emechebe GO, Okezie UM, Adejumo SA, Okoyeh JN. 2019. Carbapenem-resistant enterobacteriaceae posing a dilemma in effective healthcare delivery. Antibiotics (Basel) 8:156. doi: 10.3390/antibiotics8040156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McKenna M. 2013. Antibiotic resistance: the last resort. Nature 499:394–396. doi: 10.1038/499394a [DOI] [PubMed] [Google Scholar]
- 11. Fernández J, Guerra B, Rodicio MR. 2018. Resistance to carbapenems in non-typhoidal Salmonella enterica serovars from humans. Vet Sci 5:40. doi: 10.3390/vetsci5020040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nordmann P, Dortet L, Poirel L. 2012. Carbapenem resistance in enterobacteriaceae: here is the storm! Trends Mol Med 18:263–272. doi: 10.1016/j.molmed.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 13. Li X, Jiang Y, Wu K, Zhou Y, Liu R, Cao Y, Wu A, Qiu Y. 2017. Whole-genome sequencing identification of a multidrug-resistant Salmonella enterica serovar typhimurium strain carrying blaNDM-5 from Guangdong, China. Infect Genet Evol 55:195–198. doi: 10.1016/j.meegid.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 14. Wang Z, He J, Li Q, Tang Y, Wang J, Pan Z, Chen X, Jiao X. 2020. First detection of NDM-5-positive Salmonella enterica serovar typhimurium isolated from retail pork in China. Microb Drug Resist 26:434–437. doi: 10.1089/mdr.2019.0323 [DOI] [PubMed] [Google Scholar]
- 15. Huang Y, Ma X, Zeng S, Fu L, Xu H, Li X. 2022. Emergence of a Salmonella rissen ST469 clinical isolate carrying bla (NDM-13) in China. Front Cell Infect Microbiol 12:936649. doi: 10.3389/fcimb.2022.936649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hornsey M, Phee L, Wareham DW. 2011. A novel variant, NDM-5, of the new Delhi metallo-beta-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob Agents Chemother 55:5952–5954. doi: 10.1128/AAC.05108-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su LH, Chiu CH, Chu C, Ou JT. 2004. Antimicrobial resistance in nontyphoid Salmonella serotypes: a global challenge. Clin Infect Dis 39:546–551. doi: 10.1086/422726 [DOI] [PubMed] [Google Scholar]
- 18. Hohmann EL. 2001. Nontyphoidal Salmonellosis. Clin Infect Dis 32:263–269. doi: 10.1086/318457 [DOI] [PubMed] [Google Scholar]
- 19. Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. 2015. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev 28:901–937. doi: 10.1128/CMR.00002-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee K-I, Kusumoto M, Sekizuka T, Kuroda M, Uchida I, Iwata T, Okamoto S, Yabe K, Inaoka T, Akiba M. 2015. Extensive amplification of GI-VII-6, a multidrug resistance genomic island of Salmonella enterica serovar typhimurium, increases resistance to extended-spectrum cephalosporins. Front Microbiol 6:78. doi: 10.3389/fmicb.2015.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Szabó M, Murányi G, Kiss J. 2021. Incc helper dependent plasmid-like replication of Salmonella genomic Island 1. Nucleic Acids Res 49:832–846. doi: 10.1093/nar/gkaa1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li L, Olsen RH, Song A, Xiao J, Wang C, Meng H, Shi L. 2021. First report of a foodborne Salmonella enterica serovar gloucester (4:i:l,w) ST34 strain harboring bla CTX-M- 55 and qnrS genes located in IS26-mediated composite transposon. Front Microbiol 12:646101. doi: 10.3389/fmicb.2021.646101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu X, Biswas S, Gu G, Elbediwi M, Li Y, Yue M. 2020. Characterization of multidrug resistance patterns of emerging Salmonella enterica serovar rissen along the food chain in China. Antibiotics 9:660. doi: 10.3390/antibiotics9100660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Na SH, Moon DC, Kang HY, Song HJ, Kim SJ, Choi JH, Yoon JW, Yoon SS, Lim SK. 2020. Molecular characteristics of extended-spectrum beta-lactamase/AmpC-producing Salmonella enterica serovar virchow isolated from food-producing animals during 2010-2017 in South Korea. Int J Food Microbiol 322:108572. doi: 10.1016/j.ijfoodmicro.2020.108572 [DOI] [PubMed] [Google Scholar]
- 25. Zhan Z, Xu X, Gu Z, Meng J, Wufuer X, Wang M, Huang M, Chen J, Jing C, Xiong Z, Zeng M, Liao M, Zhang J. 2019. Molecular epidemiology and antimicrobial resistance of invasive non-typhoidal Salmonella in China, 2007-2016. Infect Drug Resist 12:2885–2897. doi: 10.2147/IDR.S210961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jean SS, Lee YT, Guo SM, Hsueh PR. 2005. Recurrent infections caused by cefotaxime- and ciprofloxacin-resistant Salmonella enterica serotype choleraesuis treated successfully with imipenem. J Infect 51:e163–e165. doi: 10.1016/j.jinf.2004.12.011 [DOI] [PubMed] [Google Scholar]
- 27. Almaghrabi R, Clancy CJ, Doi Y, Hao B, Chen L, Shields RK, Press EG, Iovine NM, Townsend BM, Wagener MM, Kreiswirth B, Nguyen MH. 2014. Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother 58:4443–4451. doi: 10.1128/AAC.00099-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Queenan AM, Bush K. 2007. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 20:440–458. doi: 10.1128/CMR.00001-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Su LH, Wu TL, Chiu CH. 2012. Development of carbapenem resistance during therapy for non-typhoid Salmonella infection. Clin Microbiol Infect 18:E91–E94. doi: 10.1111/j.1469-0691.2012.03767.x [DOI] [PubMed] [Google Scholar]
- 30. Armand-Lefèvre L, Leflon-Guibout V, Bredin J, Barguellil F, Amor A, Pagès JM, Nicolas-Chanoine M-H. 2003. Imipenem resistance in Salmonella enterica serovar wien related to porin loss and CMY-4 beta-lactamase production. Antimicrob Agents Chemother 47:1165–1168. doi: 10.1128/AAC.47.3.1165-1168.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miriagou V, Tzouvelekis LS, Rossiter S, Tzelepi E, Angulo FJ, Whichard JM. 2003. Imipenem resistance in a Salmonella clinical strain due to plasmid-mediated class A carbapenemase KPC-2. Antimicrob Agents Chemother 47:1297–1300. doi: 10.1128/AAC.47.4.1297-1300.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Fu Y, Shen M, Huang D, Du X, Hu Q, Zhou Y, Wang D, Yu Y. 2018. Dissemination of blaNDM-5 gene via an IncX3-type plasmid among non-clonal Escherichia coli in China. Antimicrob Resist Infect Control 7. doi: 10.1186/s13756-018-0349-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tian D, Wang B, Zhang H, Pan F, Wang C, Shi Y, Sun Y. 2020. Dissemination of the BLA NDM-5 gene via Incx3-type plasmid among enterobacteriaceae in children. mSphere 5:e00699-00619. doi: 10.1128/mSphere.00699-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao Q, Berglund B, Zou H, Zhou Z, Xia H, Zhao L, Nilsson LE, Li X. 2021. Dissemination of blaNDM-5 via IncX3 plasmids in carbapenem-resistant enterobacteriaceae among humans and in the environment in an intensive vegetable cultivation area in Eastern China. Environ Pollut 273:116370. doi: 10.1016/j.envpol.2020.116370 [DOI] [PubMed] [Google Scholar]
- 35. Patel G, Bonomo RA. 2013. "Stormy waters ahead": global emergence of carbapenemases. Front Microbiol 4:48. doi: 10.3389/fmicb.2013.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma Y, Xu X, Gao Y, Zhan Z, Xu C, Qu X, Chen Z, Bai J, Liao M, Zhang J. 2020. Antimicrobial resistance and molecular characterization of Salmonella enterica serovar corvallis isolated from human patients and animal source foods in China. Int J Food Microbiol 335:108859. doi: 10.1016/j.ijfoodmicro.2020.108859 [DOI] [PubMed] [Google Scholar]
- 37. Gao Y, Wen J, Wang S, Xu X, Zhan Z, Chen Z, Bai J, Qu X, Zhang H, Zhang J, Liao M. 2020. Plasmid-encoded blaNDM-5 gene that confers high-level carbapenem resistance in Salmonella typhimurium of pork origin. Infect Drug Resist 13:1485–1490. doi: 10.2147/IDR.S249357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang L, Hu X, Xu X, Yang C, Xie J, Hao R, Du X, Wang L, Jia L, Li P, Qiu S, Song H. 2017. Salmonella enterica serovar typhimurium ST34 co-expressing blaNDM-5 and blaCTX-M-55 isolated in China. Emerg Microbes Infect 6. doi: 10.1038/emi.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. 2019. NDM metallo-beta-lactamases and their bacterial producers in health care settings. Clin Microbiol Rev 32:e00115–00118. doi: 10.1128/CMR.00115-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bonnin RA, Poirel L, Carattoli A, Nordmann P. 2012. Characterization of an IncFII plasmid encoding NDM-1 from Escherichia coli ST131. PLoS One 7:e34752. doi: 10.1371/journal.pone.0034752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smillie C, Garcillán-Barcia MP, Francia MV, Rocha EPC, de la Cruz F. 2010. Mobility of plasmids. Microbiol Mol Biol Rev 74:434–452. doi: 10.1128/MMBR.00020-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sjölund-Karlsson M, Joyce K, Blickenstaff K, Ball T, Haro J, Medalla FM, Fedorka-Cray P, Zhao S, Crump JA, Whichard JM. 2011. Antimicrobial susceptibility to azithromycin among Salmonella enterica isolates from the United States. Antimicrob Agents Chemother 55:3985–3989. doi: 10.1128/AAC.00590-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Salah M, Shtayeh I, Ghneim R, Al-Qass R, Sabateen A, Marzouqa H, Hindiyeh M. 2019. Evaluation of shigella species azithromycin CLSI epidemiological cutoff values and macrolide resistance genes. J Clin Microbiol 57:e01422-18. doi: 10.1128/JCM.01422-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chowdhury G, Ramamurthy T, Ghosh A, Dutta S, Takahashi E, Mukhopadhyay AK. 2019. Emergence of azithromycin resistance mediated by phosphotransferase-encoding mph(A) in diarrheagenic vibrio fluvialis. mSphere 4:e00215-19. doi: 10.1128/mSphere.00215-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vazquez-Lopez J, Navarro-Garcia F. 2020. In silico analyses of core proteins and putative effector and immunity proteins for T6SS in enterohemorrhagic E. coli. Front Cell Infect Microbiol 10:195. doi: 10.3389/fcimb.2020.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Y, Liao K, Gao H, Wang Q, Wang X, Li H, Wang R, Wang H. 2017. Decreased fitness and virulence in ST10 Escherichia coli harboring bla(NDM-5) and mcr-1 against a ST4981 strain with bla(NDM-5). Front Cell Infect Microbiol 7. doi: 10.3389/fcimb.2017.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang J, Jin H, Hu J, Yuan Z, Shi W, Ran L, Zhao S, Yang X, Meng J, Xu X. 2014. Serovars and antimicrobial resistance of non-typhoidal Salmonella from human patients in Shanghai, China, 2006-2010. Epidemiol Infect 142:826–832. doi: 10.1017/S0950268813001659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Biswas S, Li Y, Elbediwi M, Yue M. 2019. Emergence and dissemination of MCR-carrying clinically relevant Salmonella typhimurium monophasic clone ST34. Microorganisms 7:298. doi: 10.3390/microorganisms7090298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zeng S, Zhuo Z, Huang Y, Luo J, Feng Y, Gong B, Huang X, Wu A, Zhuo C, Li X. 2022. Prevalence of chromosomally located blaCTX-M-55 in Salmonella typhimurium ST34 isolates recovered from a tertiary hospital in Guangzhou, China. Microbiol Spectr 10:e0277121. doi: 10.1128/spectrum.02771-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. 2009. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev 22:664–689. doi: 10.1128/CMR.00016-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Z, Xiao X, Li Y, Liu Y, Li R, Wang Z. 2019. Emergence of IncX3 plasmid-harboring bla (NDM-) (5) dominated by Escherichia coli ST48 in a goose farm in Jiangsu, China. Front Microbiol 10:2002. doi: 10.3389/fmicb.2019.02002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weng R, Gu Y, Zhang W, Hou X, Wang H, Tao J, Deng M, Zhou M, Zhao Y. 2022. Corrigendum: whole-genome sequencing provides insight into antimicrobial resistance and molecular characteristics of Salmonella from livestock meat and diarrhea patient in Hanzhong, China. Front Microbiol 13:981414. doi: 10.3389/fmicb.2022.981414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brinkac LM, White R, D’Souza R, Nguyen K, Obaro SK, Fouts DE. 2019. Emergence of new Delhi metallo-beta-lactamase (NDM-5) in Klebsiella quasipneumoniae from neonates in a Nigerian hospital. mSphere 4:e00685-18. doi: 10.1128/mSphere.00685-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Prah I, Nukui Y, Yamaoka S, Saito R. 2022. Emergence of a high-risk Klebsiella michiganensis clone disseminating carbapenemase genes. Front Microbiol 13:880248. doi: 10.3389/fmicb.2022.880248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dong H, Li Y, Cheng J, Xia Z, Liu W, Yan T, Chen F, Wang Z, Li R, Shi J, Qin S. 2022. Genomic epidemiology insights on NDM-producing pathogens revealed the pivotal role of plasmids on blaNDM transmission. Microbiol Spectr 10:e0215621. doi: 10.1128/spectrum.02156-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhao QY, Zhu JH, Cai RM, Zheng XR, Zhang LJ, Chang MX, Lu YW, Fang LX, Sun J, Jiang HX. 2021. IS26 is responsible for the evolution and transmission of blaNDM-harboring plasmids in Escherichia coli of poultry origin in China. mSystems 6. doi: 10.1128/mSystems.00646-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chudejova K, Kraftova L, Mattioni Marchetti V, Hrabak J, Papagiannitsis CC, Bitar I. 2021. Genetic plurality of OXA/NDM-encoding features characterized from enterobacterales recovered from Czech hospitals. Front Microbiol 12:641415. doi: 10.3389/fmicb.2021.641415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hong JS, Song W, Jeong SH. 2020. Molecular characteristics of NDM-5-producing Escherichia coli from a cat and a dog in South Korea. Microb Drug Resist 26:1005–1008. doi: 10.1089/mdr.2019.0382 [DOI] [PubMed] [Google Scholar]
- 59. Pitart C, Solé M, Roca I, Román A, Moreno A, Vila J, Marco F. 2015. Molecular characterization of blaNDM-5 carried on an IncFII plasmid in an Escherichia coli isolate from a nontraveler patient in Spain. Antimicrob Agents Chemother 59:659–662. doi: 10.1128/AAC.04040-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ahmad N, Ali SM, Khan AU. 2018. Detection of new Delhi metallo-beta-lactamase variants NDM-4, NDM-5, and NDM-7 in enterobacter aerogenes isolated from a neonatal intensive care unit of a north India hospital: a first report. Microb Drug Resist 24:161–165. doi: 10.1089/mdr.2017.0038 [DOI] [PubMed] [Google Scholar]
- 61. Sun YW, Liu YY, Wu H, Wang LF, Liu JH, Yuan L, Pan YS, He DD, Hu GZ. 2018. IS26-flanked composite transposon Tn6539 carrying the tet(M) gene in IncHI2-type conjugative plasmids from Escherichia coli isolated from ducks in China. Front Microbiol 9:3168. doi: 10.3389/fmicb.2018.03168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Acman M, Wang R, van Dorp L, Shaw LP, Wang Q, Luhmann N, Yin Y, Sun S, Chen H, Wang H, Balloux F. 2022. Role of mobile genetic elements in the global dissemination of the carbapenem resistance gene bla(NDM) Nat Commun 13:1131. doi: 10.1038/s41467-022-28819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Partridge SR, Kwong SM, Firth N, Jensen SO. 2018. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev 31:e00088-00017. doi: 10.1128/CMR.00088-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595. doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 66. Yoshida CE, Kruczkiewicz P, Laing CR, Lingohr EJ, Gannon VPJ, Nash JHE, Taboada EN. 2016. The Salmonella in silico typing resource (SISTR): an open web-accessible tool for rapidly typing and subtyping draft Salmonella genome assemblies. PLoS One 11:e0147101. doi: 10.1371/journal.pone.0147101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Pontén T, Ussery DW, Aarestrup FM, Lund O. 2012. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 50:1355–1361. doi: 10.1128/JCM.06094-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, Cattoir V, Philippon A, Allesoe RL, Rebelo AR, Florensa AF, Fagelhauer L, Chakraborty T, Neumann B, Werner G, Bender JK, Stingl K, Nguyen M, Coppens J, Xavier BB, Malhotra-Kumar S, Westh H, Pinholt M, Anjum MF, Duggett NA, Kempf I, Nykäsenoja S, Olkkola S, Wieczorek K, Amaro A, Clemente L, Mossong J, Losch S, Ragimbeau C, Lund O, Aarestrup FM. 2020. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother 75:3491–3500. doi: 10.1093/jac/dkaa345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, Møller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using plasmidfinder and plasmid multilocus sequence typing . Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34:D32–D36. doi: 10.1093/nar/gkj014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Treangen TJ, Ondov BD, Koren S, Phillippy AM. 2014. The harvest suite for rapid core-genome alignment and visualization of thousands of Intraspecific microbial genomes. Genome Biol 15:524. doi: 10.1186/s13059-014-0524-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Subramanian B, Gao S, Lercher MJ, Hu S, Chen WH. 2019. Evolview v3: a webserver for visualization, annotation, and management of Phylogenetic trees. Nucleic Acids Res 47:W270–W275. doi: 10.1093/nar/gkz357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pightling AW, Pettengill JB, Luo Y, Baugher JD, Rand H, Strain E. 2018. Interpreting whole-genome sequence analyses of foodborne bacteria for regulatory applications and outbreak investigations. Front Microbiol 9:1482. doi: 10.3389/fmicb.2018.01482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic analysis between 1104-65, 1104-75, ATCC14028, and other S. enterica serovar Typhimurium in this area.
Information about other 67 strains of S. enterica serovar Typhimurium isolated in this area.
Data Availability Statement
The nucleotide sequences of the genomes and plasmids of 1104–75 and 1104–65 have been uploaded to GenBank under the accession numbers CP110198-CP110200 and CP110201, respectively.