Abstract
The fla/che region contains more than 30 genes required for flagellar synthesis and chemotaxis in Bacillus subtilis, including the gene for the flagellum-specific ςD factor, sigD. Sequence and primer extension data demonstrate that a PA promoter immediately upstream of flgB, henceforth referred to as the fla/che PA, and the PD-3 promoter are active in vivo. Transcription from the PD-3 element is dependent on ςD activity and is regulated by the flagellum-specific negative regulator, FlgM. In a strain containing a deletion of fla/che PA (PAΔ), ςD protein was not detected, demonstrating that the fla/che PA is necessary for wild-type expression of the sigD gene. Thus, sigD is part of the >26-kb fla/che operon. Consistent with a lack of detectable ςD protein, the PAΔ strain grows as long filaments and does not express a ςD-dependent hag::lacZ reporter construct. These phenotypes are indicative of a lack of sigD expression or complete inhibition of ςD activity by FlgM. However, ςD activity is found in a double mutant containing the PAΔ and a null mutation in flgM. The double mutant no longer grows as long filaments, and expression of hag::lacZ is partially restored. These data demonstrate that a low level of ςD activity does exist in the PAΔ mutant but can be detected only in the presence of a null mutation in flgM. Therefore, normal expression of sigD may also involve another promoter(s) within the fla/che operon.
Gene expression in Bacillus subtilis is principally regulated by alternate forms of RNA polymerase differing in composition by the association of alternate ς factors, which determine the promoter specificity of the resulting holoenzyme (25, 34, 44). The ςD holoenzyme is directly responsible for transcription of the genes for flagellin (30), several hook-associated proteins (4), the MotA and MotB motor proteins (31), the CheV chemotaxis protein (7), the methyl-accepting chemotaxis proteins (12), and the FlgM negative regulator (33). The ςD regulon in B. subtilis is therefore composed of genes encoding proteins for flagellar synthesis, motility, and chemotaxis.
The structural gene for the ςD factor, sigD, is located at the distal end of the fla/che region of DNA (29). The fla/che region of DNA was originally isolated as two overlapping lambda clones, containing B. subtilis genomic DNA, that complemented chemotaxis mutations mapping between pyrD and thyA on the bacterial chromosome (39). The genes residing in this region of DNA have been sequenced, and many of the predicted protein products have been shown to be homologous to structural proteins that form the hook-basal body (HBB), as well as several chemotaxis proteins in the enteric bacteria Escherichia coli and Salmonella typhimurium (38). The homologous genes in these bacteria, however, are found in 13 different operons (21, 22).
In the enteric bacteria, a hierarchy of transcription of three classes of genes (21, 26) ensures that the expression of the gene encoding the major flagellar protein flagellin (hag) is tightly regulated and is dependent on the functional assembly of the HBB complex (14, 20). The master regulators FlhD and -C are class I gene products and are transcriptional activators that are required for expression of class II genes (24). Class II genes encode the structural proteins that form the HBB complex (36) and include the fliA locus, which encodes the alternate sigma factor ςF, a homolog of ςD (36). Class III genes possess ςF-dependent promoters and are transcribed by this form of RNA polymerase (27). Furthermore, class III gene expression is regulated by FlgM and is dependent on the expression of all class II genes (15). FlgM has been described as an anti-sigma factor that inhibits ςF binding to RNA polymerase (37). Once the HBB complex is functionally assembled, the FlgM regulator is specifically exported and ςF is able to associate with RNA polymerase (14, 20) and initiate transcription of the class III genes, including hag.
A similar hierarchy of flagellar gene expression seems to exist in B. subtilis, although homologs to the class I master regulators have not been identified. The fla/che operon appears to be primarily a class II transcription unit. Mutations within the fla/che operon that disrupt HBB open reading frames eliminate flagellin synthesis (29, 46). This morphogenetic repression of class III gene expression is controlled by the FlgM homolog in B. subtilis, since the lack of this factor restores expression of the hag gene in strains lacking an HBB complex (32). Therefore, hag gene expression in B. subtilis is dependent upon the expression of the HBB genes and on ςD RNA polymerase as it is in the enteric bacteria.
Interestingly, the genes encoding the HBB complex appear to be part of a single operon in B. subtilis. Sequence analyses of the region demonstrate the existence of consecutive open reading frames that appear to be translationally coupled (1, 38). Additionally, genetic analyses of this region suggest that it is transcribed from a single upstream promoter region, since insertions of heterologous DNA within fla/che result in decreased expression of downstream genes (29, 45, 46). Although much of the DNA that makes up the fla/che region has been cloned, sequenced, and characterized genetically, the transcription initiation signals have not been identified. Therefore, to better understand the molecular mechanisms that govern flagellar gene expression in B. subtilis, this study has centered on the characterization of the promoter region for the fla/che transcription unit. This work provides a foundation for studying the molecular machinery that governs expression of a very large transcription unit encoding flagellar and chemotaxis functions in B. subtilis which, in enteric bacteria, are encoded in more than 13 operons (21, 22).
MATERIALS AND METHODS
Bacterial strains, media, growth, and transformation.
The E. coli host for growth of recombinant plasmid was the strain Epicurian Coli XL1-Blue {recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)]} (Stratagene). For site-directed oligonucleotide mutagenesis, E. coli CJ236 [F− dut-1 ung-1 thi-1 relA1/pCJ105 (Cmr)] (Bio-Rad Laboratories), E. coli Epicurian Coli XLmutS {Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 gyrA96 relA1 lad mutS::Tn10 (Tetr)[F′ proAB lacIqZΔM15 Tn5 (Kanr)]} (Stratagene), and the M13 helper phage R408 (Promega) were used. Transformation of competent E. coli cells was performed as described in the suppliers’ protocols. Electrocompetent E. coli CJ236 (Bio-Rad Laboratories) cells were transformed by electroporation with a Gene-Pulser. E. coli cells containing plasmids were selected by growth in LB (Luria-Bertani) broth supplemented with 50 μg of ampicillin (Sigma) per ml. In cases where a blue-white screen was used to identify transformants containing the desired recombinant plasmids, transformants were plated on LB agar with 32 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Sigma) per ml, 32 μg of IPTG (isopropyl-β-d-thiogalactopyranoside; Sigma) per ml, and the appropriate antibiotic.
The B. subtilis strains used in this study are listed in Table 1. Strains were grown in 2× SG medium [2× nutrient broth, 1 mM Ca(NO3)2, 0.1 mM MnSO4, 0.1% glucose] and tryptose blood agar base (TBAB) and LB plates. Transformation of plasmid or chromosomal DNA into B. subtilis was accomplished as previously described (5). Chloramphenicol-resistant (Cmr) strains were maintained by adding 5 μg of chloramphenicol (Sigma) per ml to the appropriate medium, whereas spectinomycin-resistant (Spr) strains were maintained by adding 100 μg of spectinomycin (Sigma) per ml.
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotype | Source or derivation and referencea |
|---|---|---|
| LMB1 | trpC2 | E. Ferrari, I168b |
| LMB10 | trpC2 sigD::pLM5 (Cmr) | M. J. Chamberlin, CB100b; 13 |
| LMB24 | trpC2 phe hag::lacZ (Cmr) | M. J. Chamberlin, CB25b; 32 |
| LMB213 | trpC2 flgM::mini-Tn10 (Spr) | Transform [LMB1:HB4229, Spr]; reference 6 and this study |
| LMB214 | trpC2 fla/che PAΔ | Transform [LMB1:pWE4-int, Cmr]c; this study |
| LMB216 | trpC2 fla/che PAΔ flgM::mini-Tn10 (Spr) | Transform [LMB214:LMB213, Spr]; this study |
| LMB219 | trpC2 hag::lacZ (Cmr) | Transform [LMB1:LMB24, Cmr]; this study |
| LMB220 | trpC2 fla/che PAΔ hag::lacZ (Cmr) | Transform [LMB214:LMB24, Cmr]; this study |
| LMB221 | trpC2 flgM::mini-Tn10 (Spr) hag::lacZ (Cmr) | Transform [LMB219:LMB213, Spr]; this study |
| LMB222 | trpC2 flgM::mini-Tn10 (Spr) fla/che PAΔ hag::lacZ (Cmr) | Transform [LMB220:LMB213, Spr]; this study |
Transformation of [first (recipient) strain: with chromosomal DNA from second strain, and selecting for resistance indicated] is shown for some strains.
Previous designation of strain.
This strain was subsequently cured of the chloramphenicol resistance marker.
Manipulations of DNA.
Plasmid DNA propagated in E. coli was purified by a modification of the alkaline lysis method (41), which included isopropanol and lithium chloride precipitations. B. subtilis chromosomal DNA was isolated as described previously (28). The recovered plasmid and chromosomal DNA was resuspended in TE (10 mM Tris-Cl [pH 8.0], 1 mM EDTA), and the yield and concentration were quantified by use of a spectrophotometer (UV-1201; Shimadzu).
Restriction digests for preparatory or diagnostic purposes were performed by standard procedures (41). Agarose gels were photographed with a GDS 7500 gel documentation system (UVP) or a Polaroid camera and Polaroid 667 black-and-white film. DNA fragments of interest were gel purified with the Prep-A-Gene DNA purification system (Bio-Rad Laboratories) under the manufacturer’s recommended conditions. The 5′ phosphates were removed from the digested vector DNA by using shrimp alkaline phosphatase (United States Biochemical), and the subsequent ligation was accomplished by using the Takara ligation kit (Panvera) as recommended by the manufacturer.
DNA sequencing and analysis.
Sequencing reactions were carried out with Sequenase version 2.0 (United States Biochemical), by using the following primers: PD3 (5′-CACCCTCAATATCCTTGTCG-3′), OWE1 (5′-CATAGAAAGACTTTCAA-CCCAGG-3′), and OWE2 (5′-GAGGGTTCTTTTTTTATTTC-3′). Primers were synthesized by Cruachem, Inc. (Dulles, Va.). The nucleotide sequences obtained were compiled and analyzed by using MacVector (Kodak).
Construction of plasmids.
The 2.6-kb PstI fragment from pAZ210 (5), which contains the fla/che promoter region, was subcloned into pGEM5Zf(+) (Promega) to yield the plasmid pWE1. pWE4, a derivative of pWE1, contains a 29-bp deletion of the fla/che PA promoter (PAΔ) created by oligonucleotide-directed mutagenesis (see below). The 2.6-kb PstI fragment from pWE4 containing the PAΔ was subcloned into the B. subtilis integrational vector pJM102 (41) to generate the plasmid pWE4-int.
RNA extraction and primer extension analysis.
To determine if promoter consensus sequences identified by computer analyses are functional in vivo, total RNA was purified from the appropriate strains and subjected to primer extension analyses. B. subtilis LMB1 and LMB10 were grown to late log phase in 2× SG medium. The cells were harvested at two time points, T0, marking the point when the B. subtilis culture breaks from logarithmic-phase growth, and T0.5, marking 30 min after T0. Total RNA was extracted by using the RNeasy Maxi RNA isolation kit (Qiagen) as recommended by the manufacturer except that cells were disrupted by sonication. Total RNA was precipitated and resuspended in double-distilled water. The integrity of the total RNA was verified by resolution on a formaldehyde-agarose gel under standard conditions (41).
Primer extension reactions were performed as described previously (33). Oligonucleotides PD3 and OWE3 (5′-AATATCCGCTCTGCTCAAGGCAT-3′; Cruachem, Inc.) were end labeled with 32P to high specific activity (∼108 cpm/μg). Primer extension reactions were resolved by electrophoresis alongside sequencing reactions of pWE1 which were generated by using the same oligonucleotide used in the primer extension reactions. Autoradiography was carried out at room temperature without an intensifying screen for 5 to 22 days. Exposed X-ray film was scanned with an Epson 2.01 scanner into Adobe Photoshop 3.0 (Adobe Systems, Inc.).
Deletion of the fla/che PA.
To determine the importance of the fla/che PA promoter in initiating transcription of sigD, the promoter sequence was deleted from the B. subtilis chromosome by using oligonucleotide-directed mutagenesis as described by others (19). Oligonucleotide OWE4 (5′-GGACATTTTTTTACACGAACTTCAGAATTCAAGCATATAGTTTTACAATTC-3′) lacks the 29 bp comprising the −10 and −35 PA consensus sequences as well as the 17-bp spacer region for the fla/che PA (see Fig. 1) and in their place bears an EcoRI restriction site (underlined in the sequence above). In the in vitro synthesis reaction, OWE4 was extended with T4 DNA polymerase (Promega), and the newly synthesized strand was joined with T4 DNA ligase (Promega). The plasmid containing the PAΔ was identified by restriction map analysis and named pWE4. This plasmid was sequenced by use of an ABI Prism 377 automatic sequencer with the PD3, OWE1, and OWE2 primers; besides the PAΔ, no new mutation was introduced as a result of the mutagenesis procedure.
FIG. 1.
fla/che promoter region. The intergenic region between codY and flgB has been defined as the fla/che promoter region. Two promoter elements were found to function in vivo: a ςA-dependent promoter (fla/che PA, present study) and PD-3, a previously identified ςD-dependent promoter (11). The −10 and −35 sequences for both of these promoters are underlined, and the +1 transcription initiation sites appear in boldface type. The 29 bp that were deleted in the fla/che PAΔ strain and replaced with the EcoRI restriction site (GAATTC) are indicated by asterisks.
A fragment bearing the PAΔ was then subcloned into the B. subtilis integrational vector pJM102 (40) under standard conditions. The resultant plasmid, pWE4-int, was transformed into a wild-type B. subtilis strain, LMB1. Transformant colonies were selected by growth on LB plates containing 5 μg of chloramphenicol per ml. Transformants generated from a double-crossover event, in which two deleted copies of the fla/che PA replaced the wild-type sequence, were identified by single-colony PCR as described below. Loss of the plasmid sequences was accomplished by repeatedly growing the cells in PA (Penassay or antibiotic medium 3; Difco) broth without chloramphenicol and screening for Cms colonies by replica plating. This regimen allows for homologous recombination between directly repeated PAΔ sequences, resulting in the loss of the Cmr gene and thus antibiotic resistance. Curing of the inserted plasmid from the integrant strain was verified by single-colony PCR as described below.
Single-colony PCR.
To identify the B. subtilis cells harboring the double-crossover event and the PAΔ, DNA from single colonies was subjected to PCR analysis. Cell pellets derived from saturated 2-ml cultures were lysed in 0.1 mg of lysozyme per ml at 37°C. Cell debris was collected by centrifugation in a microcentrifuge for 1 min, and the supernatant was collected for PCR amplification. One microliter of the supernatant collected was added to a 20-μl reaction mixture containing 1× Taq buffer, 2.5 mM MgCl2, 0.18 mM deoxynucleoside triphosphates, 1 μM concentrations of oligonucleotide OWE7 (5′-GTGAGGACATTTTTTTACACG-3′) and OWE8 (5′-ACCCTCAATATCCTTGTCGAG-3′), and 0.3 U of Taq polymerase. PCRs were done in an MJR thermal cycler by using the following parameters: 1 min at 95°C, 1.5 min at 52°C, and 1 min at 72°C for 30 cycles. The PCR products were resolved on a 4% agarose gel and stained with 5 μg of ethidium bromide per ml. The gel was photographed with Polaroid 667 black-and-white film, and the photograph was scanned and the image was processed as described above.
Anti-ςD immunoblot.
The level of ςD protein was determined by immunoblot analysis. Strains to be analyzed were grown in 2× SG medium containing the appropriate antibiotic. Thirty minutes after the end of logarithmic growth (T0.5), 20 ml of cell culture was collected by centrifugation and washed in ice-cold STE (150 mM NaCl, 10 mM Tris-Cl, 100 mM EDTA). Total protein was then extracted and quantified as described previously (28), and 50 μg of protein per sample was resolved by electrophoresis on a sodium dodecyl sulfate–12.5% polyacrylamide gel. The protein was electrophoretically transferred to nitrocellulose, and the filter was blocked, incubated in hybridization buffers, and washed as described previously except that the secondary goat anti-rabbit antibody was conjugated to alkaline phosphatase (29). Reactive proteins were visualized by using BCIP-NBT (5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium) substrate (Sigma) in distilled deionized water. The developed nitrocellulose filter was scanned, and the image was processed as described above.
Light microscopy.
Strains were grown in 2× SG medium, and samples were collected 30 min after the break from logarithmic growth (T0.5). Cells were viewed by differential interference microscopy at ×1,250 with a Nikon Optiphot-2 scope with Nomarski optics and photographed with a Nikon Microflex UFX-DX attachment and Ektachrome black-and-white film for slides. Slides were subsequently scanned with a Polaroid Sprint Scan 35, and the image was processed as described above.
β-Galactosidase assay.
Flagellin gene expression was monitored by measuring the β-galactosidase activity produced in strains bearing a hag::lacZ translational fusion. Strains to be tested were grown in 2× SG sporulation medium containing 5 μg of chloramphenicol per ml when appropriate. Growth was monitored by spectrophotometer at 600 nm, and samples were collected at optical densities ranging from 0.2 to 3.0 and stored on ice. The β-galactosidase assay was conducted as described previously (8), except that β-galactosidase activity in the supernatant was measured at 420 nm. The calculation and manipulation of specific activity as Miller units, average values, and standard deviations were performed with Excel version 5.0 (Microsoft).
RESULTS
Analysis of the nucleotide sequence upstream of the fla/che region.
pAZ210 contains a 5.2-kb segment of B. subtilis DNA bearing flgB, the first gene of the fla/che region, and 1.8 kb of DNA upstream of this gene, including the region of DNA thought to be responsible for initiating transcription of the fla/che operon (46). Directed sequencing was used to obtain 620 nucleotides of sequence data upstream of flgB (Fig. 1). Computer analysis of the data generated two interesting findings.
First, the DNA sequence furthest upstream of the flgB translational start site was found to be identical to the 3′ terminus of the cod operon, an operon in B. subtilis that was recently characterized (42). The cod and fla/che operons are transcribed in the same direction and are separated by a 379-bp sequence. We have defined the intergenic region between codY (the last gene in the cod operon) and flgB (the first gene in the fla/che transcription unit) as the fla/che promoter region. It is possible, however, that one or more promoters within the cod operon, or read-through from this transcription unit, may contribute to fla/che expression.
Second, sequence analysis of this promoter region demonstrated that part of the fla/che promoter sequence is identical to a segment of B. subtilis DNA previously shown to contain a ςD-dependent promoter (11). This particular ςD-dependent promoter, originally and hereinafter called PD-3, was shown to be a sequence of DNA specifically recognized by the ςD holoenzyme in an in vitro transcription reaction (11). Whether this promoter was active in vivo, however, had not been determined; the nature of the gene(s) it potentially regulated was also unknown.
Primer extension analysis of fla/che promoter region.
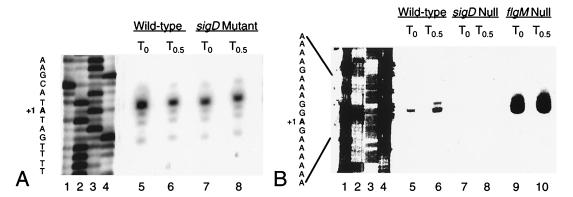
Primer extension analyses reveal that transcription of the fla/che operon initiates from a PA immediately upstream of flgB and from PD-3. The PA immediately upstream of flgB has therefore been renamed fla/che PA. Expression from this promoter is found at T0 and at T0.5 in both the wild-type and sigD mutant strains (Fig. 2A, lanes 5 to 8). Primer extension products from PD-3, however, are generated from RNA derived from the wild-type strain (Fig. 2B, lanes 5 and 6) but not from the sigD mutant (Fig. 2B, lanes 7 and 8). Therefore, transcription initiation from PD-3 is dependent on the ςD holoenzyme. Furthermore, there appears to be an increase in primer extension products produced from RNA isolated from a flgM mutant strain (Fig. 2B, lanes 9 and 10). This result demonstrates that PD-3 is subject to regulation by the FlgM negative regulator; the absence of FlgM protein may allow for increased ςD activity and thus increased transcription from PD-3 in this strain.
FIG. 2.
Primer extension products from fla/che PA and PD-3. Total RNA from B. subtilis wild-type (LMB1), sigD null (LMB10), and flgM null (LMB213) strains that was isolated at the end of logarithmic growth (T0) and 30 min later (T0.5) was subjected to primer extension analysis. (A) Lanes 1 to 4, sequencing reactions of pWE1 (G, A, T, and C, respectively); lanes 5 to 8, primer extension products from total RNA, isolated by using primer OWE3, from B. subtilis LMB1 at T0 (lane 5) and T0.5 (lane 6) and from B. subtilis LMB10 at T0 (lane 7) and T0.5 (lane 8). (B) Lanes 1 to 4, sequencing reactions of pWE1 (G, A, T, and C, respectively); lanes 5 to 10, primer extension products from total RNA, isolated by using primer PD3, from B. subtilis LMB1 at T0 (lane 5) and T0.5 (lane 6), from B. subtilis LMB10 at T0 (lane 7) and T0.5 (lane 8), and from B. subtilis LMB213 at T0 (lane 9) and T0.5 (lane 10). The sequence given to the left of each panel corresponds to the nontemplate sequence, where +1 represents the nucleotide at which transcription initiates. Reactions were performed with 98 μg of LMB1 RNA isolated at T0, 131 μg of LMB1 RNA at T0.5, 153 μg of LMB10 RNA at T0, 128 μg of LMB10 RNA at T0.5, 84 μg of LMB213 RNA at T0, and 92 μg of LMB213 RNA at T0.5.
Construction of fla/che PA deletion mutant.
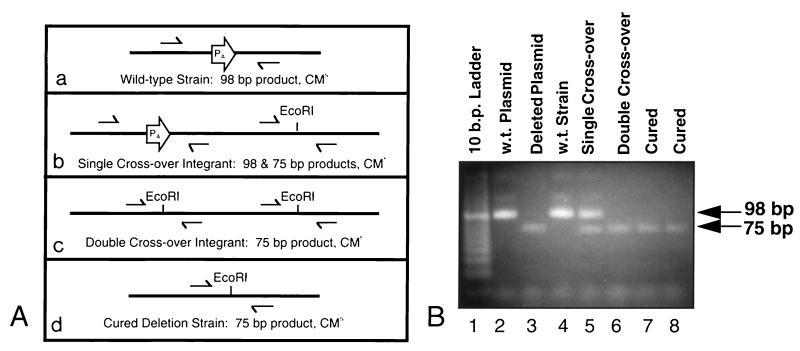
After the fla/che PA was shown to be active in vivo, it was deleted from the B. subtilis chromosome to determine its importance in promoting expression of sigD. The fla/che PA was first deleted by oligonucleotide-directed site mutagenesis of the plasmid pWE1, resulting in plasmid pWE4. To introduce this deletion into the B. subtilis chromosome, the 2.6-kb PstI fragment containing the fla/che PA deletion was subcloned into the integrational vector pJM102 (40), generating pWE4-int. pJM102 lacks a B. subtilis origin of replication and cannot be maintained as an episome. Concatemers of pWE4-int, however, can integrate into the fla/che promoter region by homologous recombination, by using either a single- or double-crossover mechanism (Fig. 3A, panels b and c). These integration events can be distinguished by the single-colony PCR method (see Materials and Methods). Only a strain bearing the double crossover can be used to obtain an exact deletion of the endogenous fla/che PA without the introduction of plasmid sequences (43). This strain was grown under nonselective conditions to allow for homologous recombination of the duplicated regions and the concomitant loss of the intervening plasmid sequence (Fig. 3A, panel d). Transformants containing a double-crossover event were identified by using a PCR-based assay (Fig. 3B, lane 6), and strains bearing the exact deletion of fla/che PA were verified by the same assay (Fig. 3B, lanes 7 and 8). Strains deleted for the endogenous fla/che PA will henceforth be referred to as PAΔ strains, and the deletion mutation will be referred to as PAΔ.
FIG. 3.
PCR-based assay for the analysis of chromosome structure at the fla/che promoter region. (A) (a) The wild-type strain is sensitive to chloramphenicol, and its chromosome contains a single fla/che PA, resulting in a 98-bp PCR product upon amplification. Introduction of pWE4-int gives rise to chloramphenicol-resistant transformants due to integration of plasmid sequences by homologous recombination. (b) DNA from a transformant generated by a single-crossover event (Campbell-like recombination) gives rise to PCR products of 98 and 75 bp. (c) DNA from a transformant generated by a double-crossover event with concatemers of pWE4-int (gene replacement) results in a single 75-bp product. (d) Once the strain bearing the double crossover is cured of chloramphenicol resistance, with a concomitant loss of the plasmid sequences, a single 75-bp fragment is produced. Half-arrows indicate primers used for PCR amplification. (B) Agarose gel of PCR products from wild-type (w.t.), transformant, and deleted strains. PCR-amplified products obtained from plasmid and chromosomal DNA with primers OWE7 and OWE8 were resolved by agarose electrophoresis. Lane 1, 10-bp ladder; lane 2, amplification of pWE1; lane 3, amplification of pWE4; lane 4, amplification of chromosomal DNA from LMB1; lane 5, amplification of chromosomal DNA from a chloramphenicol-resistant strain bearing a single crossover; lane 6, amplification of chromosomal DNA from a chloramphenicol-resistant strain bearing a double crossover; lanes 7 and 8, amplification of chromosomal DNA from chloramphenicol-sensitive strains bearing an exact deletion of fla/che PA.
Characterization of fla/che PA deletion strain. (i) Anti-ςD immunoblot.
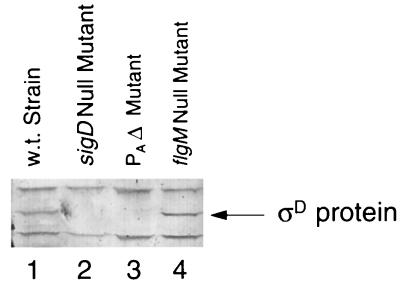
Computer analyses of sequences 5′ and 3′ of the sigD gene suggest that sigD is the penultimate gene in the fla/che region, >26 kb downstream of the fla/che PA (4a). To determine the effect of the PAΔ on sigD expression, total protein was isolated from the appropriate strains and analyzed by anti-ςD immunoblot. While ςD protein was found in a wild-type and an flgM null mutant strain, there was no detectable ςD protein in either the fla/che PAΔ strain or the sigD null mutant (Fig. 4, lanes 2 and 3). Interestingly, the level of ςD protein found in the flgM null mutant is comparable to the level found in the wild-type strain, even though transcription from the PD-3 promoter elements is dramatically increased in the mutant lacking the negative regulator (Fig. 2B, lanes 9 and 10).
FIG. 4.
Anti-ςD immunoblot. The reactive ςD protein was visualized in total protein extracts isolated from a wild-type and three mutant strains. Lane 1, wild-type strain (LMB1); lane 2, sigD null mutant (LMB10); lane 3, fla/che PAΔ strain (LMB214); lane 4, flgM null mutant (LMB213). Fifty micrograms of total protein was loaded into each lane.
(ii) Microscopic observation.
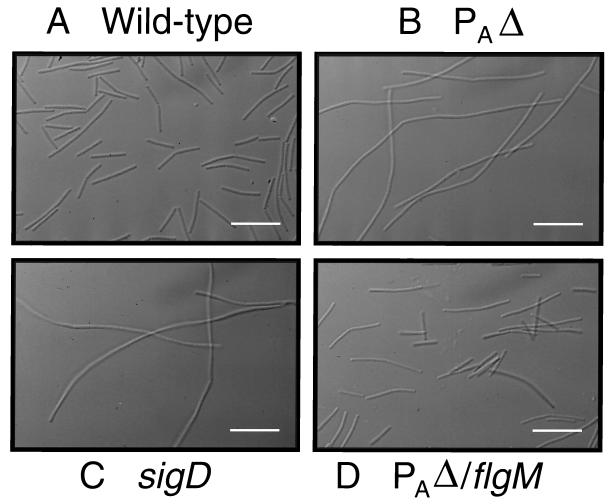
Having demonstrated that there is no detectable ςD protein in the PAΔ strain, we examined cell morphology since the lack of ςD has been shown to result in a filamentous phenotype (28). This is due to the fact that the genes encoding the major autolysins in B. subtilis responsible for hydrolyzing the cell wall after chromosomal replication are preceded by ςD-dependent promoters (23). The lack of ςD protein results in a significant decrease in autolysin production and thus a filamentous phenotype. The results obtained by microscopic observation are shown in Fig. 5. The wild-type strain grows as short rods (Fig. 5A), whereas the sigD null mutant grows as long filaments (Fig. 5B). The PAΔ strain also grows as long filaments (Fig. 5C), which is consistent with a lack of detectable ςD protein in this strain (Fig. 4, lane 3). This phenotype can also be explained by a complete inhibition of ςD activity by FlgM (33). A double mutant bearing the PAΔ and a null mutation in flgM (PAΔ/flgM null strain) no longer grows as long filaments (Fig. 5D) but as short rods like the wild-type cells.
FIG. 5.
Microscopic observation of wild-type and mutant strains. (A) Wild-type strain (LMB1); (B) fla/che PAΔ mutant (LMB214); (C) sigD null mutant (LMB10); (D) fla/che PAΔ/flgM double mutant (LMB216). White scale bar = 5 μm.
(iii) hag::lacZ expression.
To further assess ςD activity, we examined the expression of a hag::lacZ reporter construct in the wild-type, PAΔ, and PAΔ/flgM null strains. The hag gene, which encodes the flagellin protein in B. subtilis, is preceded by a strong ςD-dependent promoter element that is at least partially responsible for its high level of expression (30). In fact, 7% of the normal amount of ςD protein is sufficient to produce nearly 70% of the wild-type levels of flagellin protein (28). Expression of the hag::lacZ fusion, as measured by β-galactosidase assay, is therefore a sensitive measure of ςD activity in the cell.
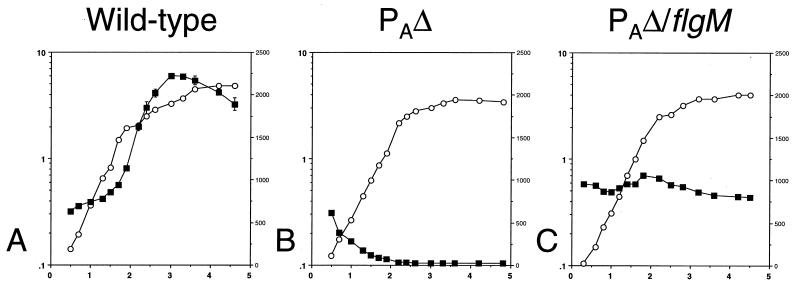
We found that expression of the hag::lacZ reporter is greatly reduced in a strain bearing the PAΔ (Fig. 6B). The pattern of expression is nearly identical to that found for an isogenic strain lacking the hag::lacZ fusion and for a strain bearing the fusion as well as a null mutation in sigD (data not shown). The low levels of expression found early in logarithmic growth appears to result from an error introduced by very low cell density during this time. In the PAΔ/flgM null double mutant, hag::lacZ expression is restored to approximately 50% of the wild-type level and exhibits an altered pattern (Fig. 6C). Specifically, hag::lacZ expression in the double mutant appears higher earlier in vegetative growth yet never displays the significant increase at the end of logarithmic growth that is found in the wild-type strain (Fig. 6A).
FIG. 6.
hag::lacZ expression in wild-type and mutant strains. β-Galactosidase activity was monitored in strains bearing a hag::lacZ reporter construct throughout growth in sporulation medium. For each panel, the left y axis is absorbance at 600 nm, the right y axis is β-galactosidase activity as expressed in Miller units, and the x axis is time expressed in hours. Symbols: ○, growth; ▪, β-galactosidase activity. (A) Wild-type strain (LMB219); (B) fla/che PAΔ strain (LMB220); (C) fla/che PAΔ/flgM double mutant (LMB222).
DISCUSSION
The 620-nucleotide fragment upstream of flgB, the first gene in the fla/che transcription unit, has been sequenced and characterized by primer extension and genetic analyses. Computer analysis of the sequence data obtained has allowed for (i) the demarcation of the putative limits of the fla/che promoter region as the intergenic region between the cod operon and flgB and (ii) the identification of a ςD promoter consensus sequence, PD-3, a previously described sequence of DNA shown to be recognized by the ςD holoenzyme in vitro (11). However, whether this promoter was functional in vivo and the function of the gene(s) dependent on this element for transcription initiation were unknown. Primer extension data demonstrate that the PA immediately upstream of flgB (fla/che PA) and the PD-3 promoter are active in vivo (Fig. 2A, lanes 5 to 8, and 2B, lanes 5 to 6). Expression from the PD-3 element is absent in a sigD null mutant and is increased in a flgM null mutant (Fig. 2B, lanes 7 to 10), demonstrating that this promoter is ςD dependent and is regulated by the flagellum-specific regulator, FlgM. The presence of two functional promoters upstream of a single transcription unit, recognized by different forms of the holoenzyme, is common in B. subtilis and allows for precise control of gene expression depending upon the availability and activation of the alternate sigma factors (25). Interestingly, the structural gene for ςF, the enteric homolog of ςD, has been shown to be transcribed from two overlapping promoters, one recognized by the major form of the holoenzyme and a second recognized by the ςF holoenzyme (35). The spacing of the fla/che PA and PD-3 elements, however, differs from the enteric system in that the PD-3 is 133 bp upstream of fla/che PA.
The functional PA promoter, identified above and referred to as fla/che PA, was deleted to analyze its role in sigD expression and ςD activity. In B. subtilis cells lacking the fla/che PA (PAΔ), no ςD protein was detected, in contrast to ςD levels in wild-type or flgM null mutant backgrounds (Fig. 4, lanes 1 and 4). Microscopic observation of the strain bearing the PAΔ demonstrates that this mutant grows as long filaments, which is consistent with a lack of ςD expression or activity (Fig. 5B). However, a mutant strain containing both the PAΔ and a null mutation in flgM no longer grows as long filaments. This suggests that a low level of ςD protein is present in the PAΔ strain (which is not detectable by immunoblot) but activity of the remaining sigma factor is inhibited by the FlgM regulator. It is likely that deletion of the fla/che PA results in a lack of HBB gene expression, preventing the functional assembly of the HBB complex, which would allow FlgM to inhibit ςD activity. This inference is further supported by the lack of expression of a hag::lacZ reporter construct in the PAΔ mutant, whereas expression is partially restored in a PAΔ/flgM null double mutant (Fig. 6B and C).
These data demonstrate that the sigD structural gene, located >26 kb downstream of the fla/che PA, is part of the fla/che operon, since the fla/che PA is necessary for wild-type sigD expression. While the fla/che PA is necessary for the production of detectable ςD protein, expression of sigD may also involve another promoter(s) within the fla/che operon, since strains lacking the fla/che PA and bearing a flgM null mutation have the ςD function partially restored. In the absence of the FlgM regulator, low levels of ςD protein produced from an internal promoter(s) could bind to RNA polymerase and initiate transcription from the PD-3 promoter. Expression from the PD-3 promoter would then result in increased expression of the fla/che transcription unit, including sigD. A minor ςA-dependent promoter immediately upstream of sigD, PsigD, has been identified recently, and deletion of this element results in a modest decrease in fla/che expression (2). Studies are in progress to determine the relative importance of PD-3, fla/che PA, and a putative promoter(s) within the fla/che operon in initiating transcription of sigD.
In this work, we have demonstrated that dual promoters recognized by the ςA and ςD holoenzymes initiate transcription of the fla/che operon, including sigD. In a previous study, however, it was concluded that the fla/che transcription unit (and sigD in particular) is not expressed from a ςD-dependent promoter (3). This inference was based on the observation that ςD protein levels in a flgM mutant are not elevated with respect to a wild-type control (3), since a lack of the FlgM regulator has been shown to increase the expression of ςD-dependent genes 10- to 15-fold (6). We have also found the level of ςD protein in the flgM mutant (Fig. 4, lane 4) to be comparable to the amount found in a wild-type strain, despite the significant increase in mRNA production from PD-3 found in this strain (Fig. 2B, lanes 9 and 10). Our results suggest that either (i) PD-3 drives transcription of fla/che including sigD and there is posttranscriptional regulation of ςD expression, (ii) the PD-3 transcript does not extend 26 kb and does not encode ςD protein, or (iii) PD-3 is a very weak promoter, in comparison to fla/che PA, such that even increased ςD activity cannot increase overall fla/che gene expression. We favor the first hypothesis and postulate that there is a general mechanism for posttranscriptional regulation of flagellar gene expression. As shown for sigD mRNA and ςD protein in this study, it has been demonstrated that hag mRNA production in a flgM null mutant is significantly increased but flagellin protein levels in the cell are nearly identical to flagellin protein levels found in a wild-type strain (9).
Our working model for transcriptional regulation of fla/che is diagrammed in Fig. 7. Transcription of the fla/che operon, including sigD, is initiated from the fla/che PA. The importance of PsigD, the minor PA immediately upstream of sigD (2), in allowing expression of ςD protein under normal conditions is unclear. Expression of ςD protein and its association with RNA polymerase allows for transcription from PD-3, which may account for the rapid accumulation of ςD protein during logarithmic growth (29a) and the rapid increase in ςD activity found during this period of growth (10, 30). In fact, several alternate sigma factors are encoded in transcription units preceded by a cognate promoter sequence, allowing for a rapid accumulation of the factor (16–18). The dependence of wild-type fla/che expression on the PD-3 element would also allow for a sensitive monitor of HBB assembly, since ςD activity is intimately tied to FlgM regulation (32).
FIG. 7.
Model for fla/che expression. Transcription of the fla/che operon, including sigD, is initiated from the fla/che PA. Once ςD protein is synthesized, expression from PD-3 is initiated and may account for the rapid accumulation of this transcription factor. The importance of the minor PA immediately upstream of sigD under normal conditions is unclear.
Although the flagellar regulons in both B. subtilis and the enteric bacteria are highly homologous (38), the physical organizations of the genes that comprise the HBB complex and the alternate sigma factors are very different. The genes encoding the HBB and ςD in B. subtilis are found within the fla/che operon (38). In contrast, the genes encoding the HBB in the enteric bacteria are found in 13 operons located throughout the bacterial chromosome (21, 22) and the gene for the ςD homolog, ςF, is found in yet another transcription unit (27). Our studies demonstrate that the precise expression of fla/che operon gene products is dependent on at least two forms of the holoenzyme and morphogenetic control by FlgM. Moreover, our data suggests that posttranscriptional regulation of the operon occurs.
ACKNOWLEDGMENTS
We are grateful to Michael Chamberlin and John Helmann for providing strains, reagents, and technical advice. We thank Daniel Mirel for his assistance in identifying the PD-3 promoter element, both Mary Mathieu and Charlyn Primous for their efforts in constructing and characterizing flagellin expression in several B. subtilis strains, Joyce West for critically reading the manuscript, and Dennis Desjardin for use of his microscopy platform.
This work was supported by an NIH-Bridges to the Future grant GM48972 to W.E., NIH-MBRS support grant GM52588-03 to M.A., and an NSF-CAREER grant MCB-9600932 to L.M.-M.
REFERENCES
- 1.Albertini A M, Caramori T, Crabb W D, Scoffone F, Galizzi A. The flaA locus of Bacillus subtilis is part of a large operon coding for flagellar structures, motility functions, and an ATPase-like polypeptide. J Bacteriol. 1991;173:3573–3579. doi: 10.1128/jb.173.11.3573-3579.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allmansberger R. Temporal regulation of sigD from Bacillus subtilis depends on a minor promoter in front of the gene. J Bacteriol. 1997;179:6531–6535. doi: 10.1128/jb.179.20.6531-6535.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caramori T, Barillá D, Nessi C, Sacchi L, Galizzi A. Role of FlgM in ςD-dependent gene expression in Bacillus subtilis. J Bacteriol. 1996;178:3113–3118. doi: 10.1128/jb.178.11.3113-3118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y F, Helmann J D. Restoration of motility in an Escherichia coli fliA flagellar mutant by a Bacillus subtilis sigma factor. Proc Natl Acad Sci USA. 1992;89:5123–5127. doi: 10.1073/pnas.89.11.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Cunningham, H. L., and L. M. Márquez-Magaña. Unpublished results.
- 5.Cutting S M, VanderHorn P B. Genetic analysis. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley & Sons, Ltd.; 1990. pp. 27–74. [Google Scholar]
- 6.Fredrick K, Helmann J D. FlgM is a primary regulator of ςD activity, and its absence restores motility to a sinR mutant. J Bacteriol. 1996;178:7010–7013. doi: 10.1128/jb.178.23.7010-7013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredrick K L, Helmann J D. Dual chemotaxis signaling pathways in Bacillus subtilis: a ςD-dependent gene encodes a novel protein with both CheW and CheY homologous domains. J Bacteriol. 1994;176:2727–2735. doi: 10.1128/jb.176.9.2727-2735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredrick K, Caramori T, Chen Y F, Galizzi A, Helmann J D. Promoter architecture in the flagellar regulon of Bacillus subtilis: high-level expression of flagellin by the ςD RNA polymerase requires an upstream promoter element. Proc Natl Acad Sci USA. 1995;92:2528–2586. doi: 10.1073/pnas.92.7.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galizzi A. Abstracts of lectures of the 9th International Conference on Bacilli. Lausanne, Switzerland: Université de Lausanne; 1997. Regulation of flagellar gene expression; p. 9. [Google Scholar]
- 10.Gilman M Z, Chamberlin M J. Developmental and genetic regulation of Bacillus subtilis genes transcribed by ς28-RNA polymerase. Cell. 1983;35:285–293. doi: 10.1016/0092-8674(83)90231-3. [DOI] [PubMed] [Google Scholar]
- 11.Gilman M Z, Glenn J S, Singer V L, Chamberlin M J. Isolation of sigma-28-specific promoters from Bacillus subtilis DNA. Gene. 1984;32:11–20. doi: 10.1016/0378-1119(84)90027-1. [DOI] [PubMed] [Google Scholar]
- 12.Hanlon D W, Ordal G W. Cloning and characterization of genes encoding methyl-accepting chemotaxis protein in Bacillus subtilis. J Biol Chem. 1994;269:14038–14046. [PubMed] [Google Scholar]
- 13.Helmann J D, Márquez L M, Chamberlin M J. Cloning, sequencing, and disruption of the Bacillus subtilis ς28 gene. J Bacteriol. 1988;170:1568–1574. doi: 10.1128/jb.170.4.1568-1574.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes K T, Gillen K L, Simon M I, Karlinsey J F. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 15.Jones C L, Macnab R M. Flagellar assembly in Salmonella typhimurium: analysis with temperature-sensitive mutants. J Bacteriol. 1990;172:1327–1339. doi: 10.1128/jb.172.3.1327-1339.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalman S, Duncan M L, Thomas S M, Price C W. Similar organization of the sigB and spoIIA operons encoding alternate sigma factors of Bacillus subtilis RNA polymerase. J Bacteriol. 1990;172:5575–5585. doi: 10.1128/jb.172.10.5575-5585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karamazyn-Campelli C, Bonamy C, Savelli B, Stragier P. Tandem genes encoding sigma factors for consecutive steps of development in Bacillus subtilis. Genes Dev. 1989;3:150–157. doi: 10.1101/gad.3.2.150. [DOI] [PubMed] [Google Scholar]
- 18.Kroos L, Kunkel B, Losick R. Switch protein alters specificity of Bacillus subtilis RNA polymerase containing a compartment-specific sigma factor. Science. 1989;243:526–529. doi: 10.1126/science.2492118. [DOI] [PubMed] [Google Scholar]
- 19.Kunkel T A. Oligonucleotide-directed mutagenesis without phenotypic selection. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons Inc.; 1992. pp. 8.2–8.5. [Google Scholar]
- 20.Kutsukake K. Excretion of the anti-sigma factor through a flagellar substructure couples flagellar gene expression with flagellar assembly in Salmonella typhimurium. Mol Gen Genet. 1994;243:605–612. doi: 10.1007/BF00279569. [DOI] [PubMed] [Google Scholar]
- 21.Kutsukake K, Ohya Y, Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J Bacteriol. 1990;172:741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kutsukake K, Ohya Y, Yamaguchi S, Iino T. Operon structure of flagellar genes in Salmonella typhimurium. Mol Gen Genet. 1988;214:11–15. doi: 10.1007/BF00340172. [DOI] [PubMed] [Google Scholar]
- 23.Lazarevic V, Margot P, Soldo B, Karamata D. Sequencing and analysis of the Bacillus subtilis lytRABC divergon: a regulatory unit encompassing the structural genes of the N-acetylmuramoyl-l-alanine amidase and its modifier. J Gen Microbiol. 1992;138:1949–1961. doi: 10.1099/00221287-138-9-1949. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Matsumura P. The FlhD/FlhC complex, a transcriptional activator of the Escherichia coli flagellar class II operons. J Bacteriol. 1994;176:7345–7351. doi: 10.1128/jb.176.23.7345-7351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Losick R, Youngman P, Piggot P J. Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet. 1986;20:625–669. doi: 10.1146/annurev.ge.20.120186.003205. [DOI] [PubMed] [Google Scholar]
- 26.Macnab R M. Flagella. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 70–83. [Google Scholar]
- 27.Macnab R M. Genetics and biogenesis of bacterial flagella. Annu Rev Genet. 1992;26:131–158. doi: 10.1146/annurev.ge.26.120192.001023. [DOI] [PubMed] [Google Scholar]
- 28.Márquez L M, Helmann J, Ferrari E, Parker H M, Ordal G W, Chamberlin M J. Studies of ςD-dependent functions in Bacillus subtilis. J Bacteriol. 1990;172:3435–3443. doi: 10.1128/jb.172.6.3435-3443.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Márquez-Magaña L M, Chamberlin M J. Characterization of the sigD transcription unit of Bacillus subtilis. J Bacteriol. 1994;176:2427–2434. doi: 10.1128/jb.176.8.2427-2434.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Márquez-Magaña, L. M. Unpublished results.
- 30.Mirel D B, Chamberlin M J. The Bacillus subtilis flagellin gene (hag) is transcribed by the ς28 form of RNA polymerase. J Bacteriol. 1989;174:3095–3101. doi: 10.1128/jb.171.6.3095-3101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirel D B, Lustre V M, Chamberlin M J. An operon of motility genes in Bacillus subtilis is transcribed by the ςD form of RNA polymerase. J Bacteriol. 1992;174:4197–4204. doi: 10.1128/jb.174.13.4197-4204.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirel D B, Lauer P, Chamberlin M J. Identification of flagellar synthesis regulatory and structural genes in a ςD-dependent operon of Bacillus subtilis. J Bacteriol. 1995;176:4492–4500. doi: 10.1128/jb.176.15.4492-4500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moran C P., Jr . Measuring gene expression in Bacillus. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley & Sons, Ltd.; 1990. pp. 262–282. [Google Scholar]
- 34.Moran C P., Jr . RNA polymerase and transcription factors. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 653–665. [Google Scholar]
- 35.Mytelka D S, Chamberlin M J. Escherichia coli fliAZY operon. J Bacteriol. 1996;178:24–34. doi: 10.1128/jb.178.1.24-34.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohnishi K, Kutsukake K, Suzuki H, Iino T. Gene fliA encodes an alternative sigma factor specific for flagellar operons in Salmonella typhimurium. Mol Gen Genet. 1990;221:139–147. doi: 10.1007/BF00261713. [DOI] [PubMed] [Google Scholar]
- 37.Ohnishi K, Kutsukake K, Suzuki H, Iino T. A novel transcriptional regulation mechanism in the flagellar regulon of Salmonella typhimurium: an anti-sigma factor inhibits the activity of the flagellum-specific sigma factor, ςF. Mol Microbiol. 1992;6:3149–3157. doi: 10.1111/j.1365-2958.1992.tb01771.x. [DOI] [PubMed] [Google Scholar]
- 38.Ordal G W, Márquez-Magaña L, Chamberlin M J. Motility and chemotaxis. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 765–784. [Google Scholar]
- 39.Ordal G W, Nettleton D O, Hoch J A. Genetics of Bacillus subtilis chemotaxis: isolation and mapping of mutations and cloning of chemotaxis genes. J Bacteriol. 1983;154:1088–1097. doi: 10.1128/jb.154.3.1088-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perego M. Integrational vectors for genetic manipulation in Bacillus subtilis. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 615–624. [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Slack F J, Serror P, Joyce E, Sonenshein A L. A gene required for nutritional repression of the Bacillus subtilis dipeptide permease operon. Mol Microbiol. 1995;15:689–702. doi: 10.1111/j.1365-2958.1995.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 43.Stahl M L, Ferrari E. Replacement of the Bacillus subtilis subtilisin gene with an in vitro-derived deletion mutant. J Bacteriol. 1984;158:411–418. doi: 10.1128/jb.158.2.411-418.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stragier P, Losick R. Molecular genetics of sporulation of Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 45.Zuberi A R, Ying C, Parker H M, Ordal G W. Transposon Tn917lacZ mutagenesis of Bacillus subtilis: identification of two new loci required for motility and chemotaxis. J Bacteriol. 1990;172:6841–6848. doi: 10.1128/jb.172.12.6841-6848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuberi A R, Ying C, Weinreich M R, Ordal G W. Transcriptional organization of a cloned chemotaxis locus of Bacillus subtilis. J Bacteriol. 1990;172:1870–1876. doi: 10.1128/jb.172.4.1870-1876.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]