Abstract
Context
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer. Gefitinib is a first-line treatment for NSCLC. However, its effectiveness is hindered by the development of drug resistance. At present, Shenqi Fuzheng injection (SFI) is widely accepted as an adjuvant therapy in NSCLC.
Objective
This study investigates the molecular mechanism of SFI when combined with gefitinib in regulating cell progression among EGFR-TKI-resistant NSCLC.
Materials and methods
We established gefitinib-resistant PC9-GR cells by exposing gefitinib escalation from 10 nM with the indicated concentrations of SFI in PC9 cells (1, 4, and 8 mg/mL). Quantitative real-time polymerase chain reaction was performed to assess gene expression. PC9/GR and H1975 cells were treated with 50 ng/mL of interleukin (IL)-22 alone or in combination with 10 mg/mL of SFI. STAT3, p-STAT3, AKT, and p-AKT expression were evaluated using Western blot. The effects on cell proliferation, clonogenicity, and apoptosis in NSCLC cells were assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), colony formation and flow cytometry assays.
Results
SFI treatment alleviated the development of gefitinib resistance in NSCLC. PC9/GR and H1975 cells treated with SFI significantly exhibited a reduction in IL-22 protein and mRNA overexpression levels. SFI effectively counteracted the activation of the STAT3/AKT signaling pathway induced by adding exogenous IL-22 to PC9/GR and H1975 cells. Moreover, IL-22 combined with gefitinib markedly increased cell viability while reducing apoptosis. In contrast, combining SFI with gefitinib and the concurrent treatment of SFI with gefitinib and IL-22 demonstrated the opposite effect.
Discussion and Conclusion
SFI can be a valuable therapeutic option to address gefitinib resistance in NSCLC by suppressing the IL-22/STAT3/AKT pathway.
Keywords: Adjuvant therapy, EGFR-TKIs, drug resistance, cytokine pathway
Introduction
As one of the most prevalent and deadly malignancies worldwide, lung cancer remains a significant contributor to cancer-related mortality, primarily due to the widespread prevalence of smoking (Sung et al. 2021; Siegel et al. 2023). Among the various types of lung cancer, non-small cell lung cancer (NSCLC) accounts for approximately 85% of cases and is associated with a low 5-year survival rate, posing a serious threat to human health (Hirsch et al. 2017; Relli et al. 2019). Over the years, significant progress has been made in treating NSCLC, especially with personalized therapies. Targeted drugs have emerged as a more promising option than traditional chemotherapy, offering improved curative effects and fewer side effects (Friedlaender et al. 2020; Nagase et al. 2020). Among the various targeted therapies, epidermal growth factor receptor (EGFR) activating mutations have been identified as key drivers in NSCLC, with the highest incidence rates. In particular, deletions of exon 19 of EGFR and a mutation of Leu858Arg at exon 21 are among the most common mutations seen in NSCLC (Pirker et al. 2010; Sgambato et al. 2012; Wu and Shih 2018). These mutations are critical indicators for using first-generation reversible EGFR tyrosine kinase inhibitors (TKIs) as a treatment option.
However, despite advances in targeted EGFR therapeutic strategies, the emergence of significant drug resistance is an inevitable challenge faced by patients, typically occurring within a year of starting treatment, mainly due to various mechanisms, such as the mutation of exon 20 T790M (Westover et al. 2018). To address this issue, the development of alternative treatments becomes crucial. One such promising option is osimertinib, a third-generation EGFR mutant selective TKI, which has shown remarkable efficacy in patients with NSCLC who harbor the T790M mutation (Mok et al. 2017; Di Noia et al. 2021). However, even with the use of osimertinib, disease progression is inevitable due to the emergence of new mutations in the EGFR or anaplastic lymphoma kinase (ALK) domain, activation of bypass signals, phenotypic transformation, and other mechanisms (Cooper et al. 2022). Considering the challenges posed by resistance to EGFR-TKI, there is an urgent need to develop approaches to alleviate or overcome this resistance.
Traditional Chinese Medicine (TCM) healing systems have been developed through the collective experience of Chinese people in their battle against diseases. TCM attributes the cause of tumors to a weakness of healthy qi (energy) and the accumulation of cancer toxins, resulting in a complex condition of general deficiency and local manifestations (Tang et al. 2008). To address this, TCM emphasizes the need to comprehensively adjust the body’s internal environment and restore balance.
In modern cancer treatment, there is growing support for diversified therapy, with many medical practitioners recognizing the concept of supporting the body’s natural defenses while simultaneously expelling harmful elements. In this context, TCM has emerged as a vital component of the malignant tumor treatment modality within Chinese medical structures, particularly for chronic diseases, due to its proven therapeutic advantages and minimal side effects (Wang et al. 2021; Zhang et al. 2021). The Shenqi Fuzheng injection (SFI) is a pure TCM preparation made from Codonopsis pilosula Oliv. (Campanulaceae) and Astragalus membranaceus Bunge (Galegeae) as the primary raw materials. This formulation aims to eliminate pathogens, strengthen vital qi, enhance body resistance, regulate immune function, and alleviate adverse reactions during cancer treatment (Wu, Liang, et al. 2022; Wu, Liu, et al. 2022). As a result of its demonstrated efficacy, SFI has found widespread use as an adjuvant treatment for advanced cancers in clinical settings, including advanced lung cancer (Dong et al. 2010), advanced gastric cancer (Li et al. 2015) and breast cancer (Zhang et al. 2019).
In recent years, numerous studies have demonstrated the safety, reliability, and significant clinical benefits of SFI in improving the quality of life and prolonging survival (Liu et al. 2022; Meng et al. 2022). Although SFI inhibits tumor cell proliferation, limited reports have been made on its potential to alleviate EGFR-TKI resistance. Our previous research has shown promising results, revealing the synergistic efficacy of SFI when combined with gefitinib in NSCLC cells by suppressing the mitogen-activated protein kinase (MAPK)/sterol regulatory element-binding protein 1 (SREBP1) pathway (Pan et al. 2020). Furthermore, retrospective evaluations have indicated that combining SFI and EGFR-TKI could significantly extend the progression-free survival (PFS) for NSCLC patients (Wang et al. 2022).
Emerging studies have shed light on various cytokines, including interleukin (IL)-6 (Li et al. 2014; Zheng et al. 2019), IL-1β (Huang et al. 2020), IL-22 (Wang et al. 2019), IL-8 (Liu et al. 2015), and transforming growth factor (TGF)-β1 (Kuo et al. 2020) among others, and their role in promoting resistance to NSCLC EGFR-TKI by regulating multiple pathways. Therefore, targeting the cytokine pathway in tumor cells within the tumor microenvironment could be crucial in regulating EGFR-TKI resistance. However, despite the promising findings, the specific relationship between SFI and cytokine pathways in alleviating drug resistance to EGFR-TKI remains largely unknown. The primary objective of this study was to investigate the molecular mechanism underlying the synergistic effect of SFI in combination with gefitinib to regulate cell progression in NSCLC patients experiencing resistance to gefitinib treatment.
Materials and methods
Cell culture and reagents
Two NSCLC cell lines, PC9 and PC9/GR, were used. These cell lines carry EGFR exon 19 deletions (delE746-A750) and were obtained from Dr. Caicun Zhou at the Shanghai Pulmonary Hospital in Shanghai, China. The H1975 cell line with the EGFR L858R/T790M mutation was obtained from the National Collection of Authenticated Cell Cultures, Shanghai, China. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) or Roswell Park Memorial Institute-1640 (RPMI-1640) (KeyGEN BioTECH, Nanjing, China) and supplemented with 10% fetal bovine serum (FBS) from ExCell Bio, Shanghai, China. Cells were kept in a controlled environment with 5% CO2 at a temperature of 37 °C.
SFI was obtained from Livzon Pharmaceutics Ltd. (Zhuhai, China). Gefitinib was provided by Aladdin Industrial Corporation (Shanghai, China). IL-22 was purchased from Sino-Biological (Beijing, China).
Cell viability measurement
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to assess tumor cell viability. Tumor cells were seeded in 96-well plates at a density of 2,000 cells per well and treated with different concentrations of gefitinib for 48 h. After treatment, 5 mg/mL MTT (KeyGEN BioTECH, Nanjing, China) was added to each well and incubated at 37 °C for 2 h. Subsequently, the medium was removed, and 150 μL of dimethylsulfoxide (DMSO, KeyGEN BioTECH, Nanjing, China) was added to each well to dissolve formazan crystals.
The absorbance was measured at 570 nm using a microplate reader (Thermo Multiskan FC, USA). CompuSyn software (Biosoft, Cambridge, UK) was utilized to analyze synergistic or additive effects of drug combinations. The relevant formulas are as follows.
| (1) |
| (2) |
Note: RI is used to quantify the cell resistance to gefitinib. It is calculated as the ratio of the half-maximal inhibitory concentration (IC50) of gefitinib-resistant cells (PC9-GR) to the IC50 of the parental cells (PC9) exposed to gefitinib. IC50 represents the concentration of gefitinib required to inhibit cell growth by 50%. The relative delay rate is determined by comparing the RI of gefitinib-resistant PC9-GR cells induced jointly with SFI with the RI of gefitinib-resistant PC9-GR cells induced solely by gefitinib.
Cell cloning and wound healing assays
Cell cloning and wound healing assays were performed to compare cell proliferation and migration ability changes after combined treatment. Cells were grown in 6-well plates and treated with different combinations, including gefitinib alone (2 μM), gefitinib (2 μM) + SFI (10 mg/mL), gefitinib (2 μM) + IL-22 (50 ng/mL), or a combination of these agents. For the cell cloning assay, cells were initially seeded at a density of 700 cells per well and cultured for 15 days under designated treatment conditions. After this period, cells were fixed with methanol, stained with crystal violet (KeyGEN BioTECH, Nanjing, China) for 5 min, thoroughly washed with phosphate-buffered saline (PBS), and observed and photographed.
In the wound healing assay, a 200 μL pipette tip was used to create a scratch along a ruler on the cell monolayer until the tumor cells reached 80% confluence. The gap or wound was photographed under a microscope at 0 (immediately after creating the scratch), 24, 48, and 72 h. To quantify the wound healing progress, ImageJ software was utilized for gray value calculation.
Cell apoptosis assay
After exposing the cells to various drug treatments, they were incubated for 48 h before harvesting. The cells were then resuspended in 500 μL of binding buffer. Following the manufacturer’s instructions with the apoptosis detection kit (Vazyme, Nanjing, China), cells were stained with 5 µL of Annexin V-FITC and propidium iodide for 10 min at room temperature in the dark. Finally, the apoptosis rate was measured using flow cytometry (Becton Dickinson FACS Calibur, Becton-Dickinson, USA).
Western blot assay
After collecting the cell samples, the following experimental procedures were performed: 1) cell treatment: cell samples were treated with radioimmunoprecipitation (RIPA) lysates (KeyGEN BioTECH, Nanjing, China), along with phosphatase inhibitors and protease inhibitors (KeyGEN BioTECH, Nanjing, China); 2) protein quantification: protein concentration in cell lysates was determined using a BCA kit (Vazyme, Jiangsu, China); 3) electrophoresis: proteins were separated based on their molecular weights using either 8% or 10% SDS-PAGE gels (Vazyme, Jiangsu, China); 4) membrane transfer: after electrophoresis, the separated proteins were transferred from the gel to a PVDF membrane (Merck Millipore, Ireland); 5) antibody incubation: the PVDF membrane was then incubated with primary antibodies overnight. The primary antibodies used were: anti- AKT (Bimake, Houston, TX, USA, A5031, 1:1000 dilution), p-AKT (Ser473) (Wanleibio, Shenyang, China, WLP001a, 1:500 dilution), STAT3 (Wanleibio, WL03207, 1:500 dilution), p-STAT3 (Ser727) (Wanleibio, WLP2412, 1:500 dilution), IL-22 (Wanleibio, WL04441, 1:500 dilution), and GAPDH (Proteintech Group, Wuhan, China, 10494-1-AP, 1:10000 dilution); 6) secondary antibody incubation: after incubation with primary antibodies, the PVDF membrane was incubated with secondary antibodies for 1 h. Secondary antibodies used were HRP goat anti-rabbit IgG (H + L) (Beyotime Biotechnology, Shanghai, China, A0208, 1:10000 dilution) and HRP goat anti-mouse IgG (H + L) (Absci, WA, USA; ABL3032, 1:10000 dilution); 7) protein detection: the protein bands were visualized using ECL reagent (Tanon, Shanghai, China); and 8) data analysis: the density of the protein bands on the PVDF membrane was measured using ImageJ software.
Quantitative real-time polymerase chain reaction (qRT-PCR)
RNA was harvested from tumor cells using trizol reagent (Ambion, Shanghai, China), and cDNA was obtained using mRNA as the template by catalysis with HiScript III RT SuperMix (Vazyme, Nanjing, China). The mRNA primer sequences (GenScript, Nanjing, China) used are shown in Table 1. ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China) was used for fluorescence quantification.
Table 1.
Primers for quantitative PCR analysis.
| Gene | Primer sequences (5’-3’) |
|---|---|
| GAPDH | F: GTCTCCTCTGACTTCAACAGCG |
| R: ACCAGGCGCCCAATACGACCAA | |
| IL-22 | F: CCTCAATCTGATAGGTTCCA |
| R: TCATCACCTTCAATATGACATG | |
| IL-22RA1 | F: GCTGACCATCTTGACTGT |
| R: AGTTGCTGGACTGGAATT | |
| IL-1β | F: AGGATATGGAGCAACAAGT |
| R: GCAGGACAGGTACAGATT | |
| IL-6 | F: TGGATTCAATGAGGAGACTT |
| R: TTCTGGAGGTACTCTAGGTATA | |
| IL8 | F: TGCTAAAGAACTTAGATGTCAG |
| R: GTCCACTCTCAATCACTCT | |
| IL-17 | F: GAAGGCAGGAATCACAATC |
| R: GGTTATGGATGTTCAGGTTG | |
| IL-22 | F: CCTCAATCTGATAGGTTCCA |
| R: CATGTGCTTAGCCTGTTG | |
| TGF-β1 | F: GACTACTACGCCAAGGAG |
| R: TGTGTACTCTGCTTGAACT | |
| TNF-α | F: AGGGACCTCTCTCTAATCAG |
| R: TTTGCTACAACATGGGCTAC |
Statistical analysis
All data are presented as mean ± standard deviation (SD) and were analyzed using GraphPad Prism 8.0 software (GraphPad Software Inc., CA, USA). Two independent sample t-tests were used to compare two groups, and analysis of variance (ANOVA) was used for comparisons between multiple groups. Statistical significance was defined as p < 0.05.
Results
Establishment of induced drug resistance cell lines and detection of drug resistance index
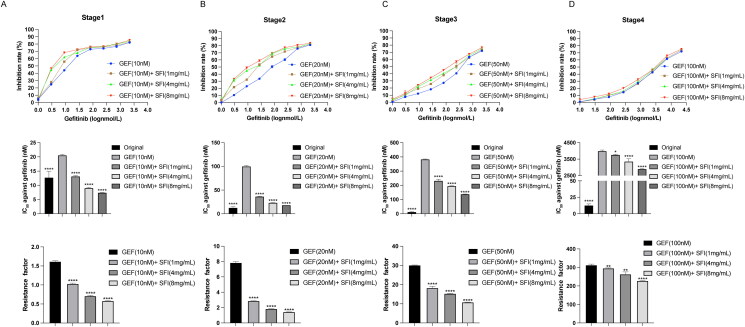
Our previous study found that combining SFI with first-generation EGFR-TKI improved PFS (Wang et al. 2022). This prompted us to investigate whether SFI could delay the development of resistance to EGFR-TKIs. To achieve this, we established gefitinib-resistant PC9-GR cells. The process involved exposing parental PC9 cells to a stepwise dose increase in gefitinib, starting at 10 nM and then gradually increasing to the indicated concentrations of SFI (1, 4, and 8 mg/mL). Furthermore, PC9 cells were treated with staged exposure to gefitinib at concentrations of 20, 50, and 100 nM every two weeks. This approach established a subline of cells resistant to the combined treatment of SFI and gefitinib within 2 months after drug exposure. The characteristics of these different cell lines, including the IC50 values of gefitinib, RI, and the relative delay rate, are summarized in Table 2.
Table 2.
IC50, RI, and relative delay rate of drug resistance induced resistant cells.
| Stage | Subgroup | IC50 (nmol/L) | Resistance index | Relative delay rate (%) |
|---|---|---|---|---|
| Parental PC9 | 12.75 ± 2.14 | |||
| Stage 1 | GEF(10 nmol/L) | 20.59 ± 0.25 | 1.63 ± 0.01 | |
| GEF(10 nmol/L)+ SFI(1 mg/mL) | 13.15 ± 0.21 | 1.04 ± 0.01 | 36.13 | |
| GEF(10 nmol/L)+ SFI(4 mg/mL) | 9.06 ± 0.23 | 0.72 ± 0.01 | 56.0 | |
| GEF(10 nmol/L)+ SFI(8 mg/mL) | 7.43 ± 0.06 | 0.58 ± 0.00 | 63.91 | |
| Stage 2 | GEF(10 nmol/L) | 100.08 ± 1.9 | 7.8 ± 0.14 | |
| GEF(10 nmol/L)+ SFI(1 mg/mL) | 36.54 ± 0.48 | 2.82 ± 0.03 | 63.49 | |
| GEF(10 nmol/L)+ SFI(4 mg/mL) | 23.41 | 1.81 ± 0.02 | 76.61 | |
| GEF(10 nmol/L)+ SFI(8 mg/mL) | 18.31 ± 0.05 | 1.44 ± 0.00 | 81.70 | |
| Stage 3 | GEF(10 nmol/L) | 383.53 ± 1.8 | 30.01 ± 0.14 | |
| GEF(10 nmol/L)+ SFI(1 mg/mL) | 232.86 ± 10.31 | 17.33 ± 0.8 | 39.29 | |
| GEF(10 nmol/L)+ SFI(4 mg/mL) | 195.46 ± 1.77 | 15.2 ± 0.13 | 49.04 | |
| GEF(10 nmol/L)+ SFI(8 mg/mL) | 138.03 ± 0.85 | 10.82 ± 0.06 | 64.01 | |
| Stage 4 | GEF(10 nmol/L) | 3988.33 ± 67.5 | 312.78 ± 5.29 | |
| GEF(10 nmol/L)+ SFI(1 mg/mL) | 3766.33 ± 30.23 | 296.31 ± 2.37 | 5.57 | |
| GEF(10 nmol/L)+ SFI(4 mg/mL) | 3357.33 ± 163.83 | 248.54 ± 12.84 | 15.82 | |
| GEF(10 nmol/L)+ SFI(8 mg/mL) | 2903 ± 17.57 | 226.11 ± 1.37 | 27.21 |
Data are expressed as mean ± SEM (n = 3).
As the induction of drug resistance in parental PC9 cells progressed with time and with increasing concentration of gefitinib, these cells demonstrated a gradual increase in gefitinib resistance and a decrease in gefitinib susceptibility. At the end of each stage of the induction process, the inhibitory effect of gefitinib on tumor growth in resistant cells weakened in direct correlation with the concentration of SFI. Consequently, RI showed a corresponding decrease, indicating a reduction in resistance level (Figure 1).
Figure 1.
The delayed drug resistance effect of SFI in NSCLC cells. (A, B, C, D) According to the MTT method, the cell proliferation inhibition rate and IC50 of gefitinib (1, 3, 9, 27, 81, 243, 729, 2187 nmol/L) and RI of each induced drug-resistant cells were measured after 48 h of treatment in four stages. Data are expressed as mean ± SEM (n = 3) *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 compared to gefitinib alone inducing gefitinib-resistant PC9-GR cells.
The experimental findings showed that with prolonged administration of gefitinib, the RI of the sensitive cells gradually increased, indicating an increase in their resistance to the drug. However, during induction of resistance to gefitinib, the combination of SFI and gefitinib played a crucial role in significantly slowing the growth of NSCLC cells. The combination treatment demonstrated a significant reduction in cell resistance index, indicating increased sensitivity to gefitinib, and effectively delayed the emergence of drug resistance.
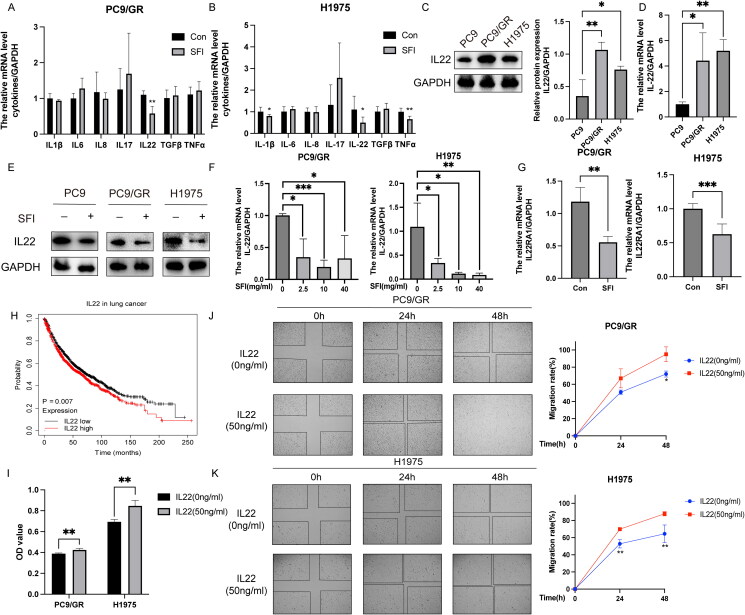
SFI reverses high IL-22 levels in EGFR-TKI-resistant NSCLC cells
Following a 6 h treatment with SFI (10 mg/mL), we assessed the mRNA levels of various cytokines in PC9/GR and H1975 cells. The results revealed that SFI significantly reduced the mRNA expression of IL-1β, IL-22, and tumor necrosis factor (TNF)-α in PC9/GR cells, the most prominent effect observed in suppressing IL-22 expression (Figure 2A). Among the measured cytokines, IL-22 expression in H1975 cells showed a noticeable decrease (Figure 2B). Furthermore, IL-22 protein and mRNA expression were elevated in gefitinib-resistant PC9/GR and H1975 cells compared to gefitinib-sensitive PC9 cells (Figure 2C and D). Furthermore, IL-22 overexpression in both gefitinib-sensitive and -resistant cell lines was reversed after SFI administration (Figure 2E). When H1975 cells were treated with SFI for 6 h, IL-22 expression gradually decreased with increasing SFI concentration. In contrast, in PC9/GR cells, the reduction in IL-22 expression did not depend on SFI concentration (Figure 2F). Similarly, SFI also attenuated the mRNA level of the IL-22 receptor IL-22RA1 (Figure 2G).
Figure 2.
SFI relieves gefitinib resistance by reducing IL-22 levels. (A, B) After treating PC9/GR and H1975 cells with SFI (10 mg/mL) for 6 h, the mRNA levels of various cytokines were evaluated. Compared to the control group, * p < 0.05, ** p < 0.01. (C) IL-22 protein expression of cellular protein extracted from indicated cells was assessed by Western blot. *p < 0.05 or **p < 0.01 compared to PC9 cells. (D) Results of qRT-PCR presented IL-22 mRNA levels in PC9/GR and H1975 cells. *p < 0.05 or **p < 0.01 compared to PC9 cells. (E) IL-22 protein expression was verified by Western blot in SFI (10 mg/mL) treated or untreated indicated cells. (F) Cells were treated or untreated with 2.5, 10, or 40 mg/mL SFI for 6 h, then relative IL-22 mRNA level in indicated cells was measured by qRT-PCR. (G) The mRNA expression levels of IL-22RA1 in PC9/GR and H1975 cells after a 6 h treatment of 10 mg/mL SFI were determined using qRT-PCR. (H) The data on the relationship between IL-22 (221165_s_at) expression and overall survival for lung cancer patients and p-values were obtained from the Kaplan-Meier plotter database (http://kmplot.com/analysis/index.php?p=background). (I) After treating PC9/GR and H1975 cells with or without recombinant IL-22 (50 ng/mL) for 48 h, cell proliferation was assessed by MTT assay. **p < 0.01 compared to the control without exogenous addition of IL-22. (J, K) PC9/GR and H1975 cells were incubated with IL-22 (50 ng/mL) at the indicated time points. The relative migration rate was measured. **p < 0.01, ***p < 0.001 compared to the control without exogenous addition of IL-22 at a 48 h time point.
The Kaplan-Meier survival analysis indicated that high IL-22 expression was correlated with reduced overall survival (Figure 2H). Furthermore, the addition of exogenous IL-22 consistently promoted cell proliferation compared to the control group (Figure 2I). Wound healing assays performed on gefitinib-resistant PC9/GR and H1975 cells treated with recombinant IL-22 demonstrated enhanced cell migration (Figure 2J and K). These findings collectively suggest that the high levels of IL-22 in gefitinib-tolerant cells were effectively reversed by SFI treatment.
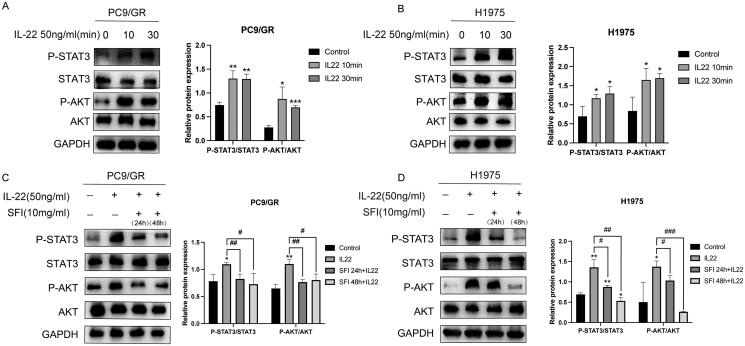
SFI reduces STAT3/AKT stimulated by IL-22 to overcome NSCLC resistance
Previous studies have shown that IL-22 plays a role in activating the JAK-STAT3/MAPK/Akt signaling pathway in various types of cancer, both in vitro and in vivo (Bi et al. 2016; Su et al. 2021; Yao et al. 2022). To investigate whether IL-22 is involved in signal transduction in gefitinib-resistant PC9/GR and H1975 cells, we stimulated these NSCLC cells with exogenously added IL-22, at a 50 ng/mL concentration and measured the p-STAT3 and p-AKT protein levels 10 and 30 min after stimulation. Western blot analysis revealed that STAT3 and AKT activation was enhanced in gefitinib-tolerant cells after stimulation with recombinant human IL-22 (Figure 3A and B).
Figure 3.
SFI inhibits the STAT3/AKT branch activated by IL-22 treatment in PC9/GR and H1975 cells. (A, B) Western blot of PC9/GR and H1975 cells determining p-STAT3 and p-AKT following stimulation with IL-22 (50 ng/mL) for 10 or 30 min. (C, D) Western blot analysis of PC9/GR and H1975 cells was stimulated with IL-22 (50 ng/mL) for 20 min after pre-treated or not with 10 mg/mL SFI for 24 h or 48 h. Results are expressed as mean ± SEM (n = 3) *p < 0.05, **p < 0.01, ***p < 0.001 when compared with control group; #p < 0.05, ##p < 0.01, ###p < 0.001 when compared with IL-22 group in A, B, C, and D.
Given that SFI was effective in reducing the expression of IL-22, we investigated whether the mechanism of action of SFI was related to the downstream signaling pathway induced by IL-22. To assess this, we pre-treated PC9/GR and H1975 cells with 10 mg/mL SFI for 24 h or 48 h and then administered exogenous IL-22 for 20 min.
To investigate the effect of SFI on IL-22-mediated signaling in gefitinib-tolerant cells, Western blot analysis was performed to examine the phosphorylation of STAT3 and AKT. The results revealed a down-regulation of STAT3 and AKT phosphorylation in response to pre-treatment with SFI (Figure 3C and D). In particular, as the duration of SFI pre-treatment increased, the expression levels of p-STAT3 showed a time-dependent decrease (Figure 3D). These findings demonstrate that SFI can antagonize IL-22-mediated activation of STAT3 and AKT in gefitinib-tolerant cells.
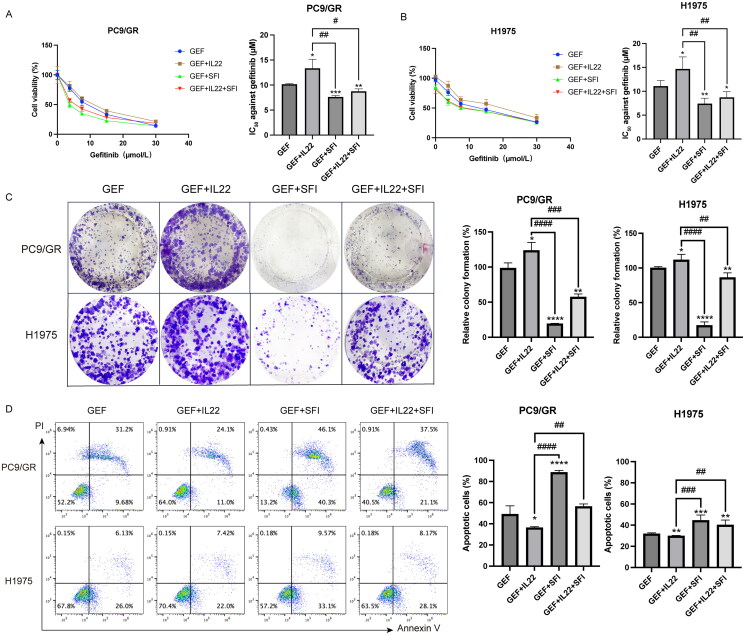
SFI plus gefitinib restores gefitinib sensitivity by regulating IL-22
We previously demonstrated that combining SFI and gefitinib synergized NSCLC by involving the MAPK/SREBP1 pathway (Pan et al. 2020). Based on these findings, we investigated whether the sensitization effect of SFI was dependent on the reduction in IL-22 expression. To confirm this hypothesis, we performed an MTT assay to assess cell viability of PC9/GR and H1975 cells under various conditions: with or without supplementation with IL-22 (50 ng/mL), with or without SFI (10 mg/mL), and with different concentrations of gefitinib. As expected, IL-22 supplementation significantly increased cell survival compared to gefitinib treatment alone. In contrast, combining SFI and gefitinib effectively inhibited the proliferation of PC9/GR and H1975 cells. In particular, the group treated with SFI combined with gefitinib exhibited the lowest cell viability among all groups. Cell viability was even lower in the group treated with SFI combined with gefitinib and IL-22 than in the group treated with gefitinib combined with IL-22 group (Figure 4A and B).
Figure 4.
SFI facilitates the gefitinib cytotoxicity of PC9/GR and H1975 cells. (A, B) Cell proliferation inhibition profiles were measured after 48 h of different concentrations of gefitinib (30, 15, 7.5, 3.25 μmol/L), respectively co-administered IL-22 (50 ng/mL) alone or in combination with SFI (10 mg/mL) exposure to PC9/GR and H1975 cells. (C) Clonogenic assays of PC9/GR and H1975 cells after gefitinib alone, gefitinib + IL-22, gefitinib + SFI, and gefitinib + IL-22 + SFI treatment for 13 days. (D) After 48 h incubation with different schemes, including gefitinib alone, gefitinib + IL-22, gefitinib + SFI, and gefitinib + IL-22 + SFI, flow cytometric analysis of apoptosis was detected, and the data were processed by FlowJo VX software. Results are expressed as mean ± SEM (n = 3) *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, when compared with gefitinib; ##p < 0.01, ###p < 0.001, ####p < 0.001 when compared with gefitinib + IL-22.
Compared to gefitinib monotherapy or the combination of gefitinib and IL-22, the combination treatment of SFI with gefitinib, as well as SFI with gefitinib and IL-22, resulted in a significant reduction in the ability to form cell clones, as observed in the clonogenic assay. Furthermore, combination treatments decreased suppression of apoptosis rates, as examined by flow cytometry (Figure 4C and D). These results demonstrate that SFI effectively alleviates resistance of NSCLC cells to gefitinib by regulating IL-22 expression.
Discussion
NSCLC is a prevalent form of lung cancer associated with high incidence and mortality rates, leading to a low 5-year survival (Ganti et al. 2021; Wang et al. 2021). EGFR mutations, a key therapeutic target, are common in NSCLC (da Cunha Santos et al. 2011). Significant advances have been made in the clinical management of EGFR mutations through targeted therapies, including first-generation EGFR-TKI gefitinib and third-generation EGFR-TKI osimertinib. These TKIs in cancer cells block EGFR tyrosine kinase phosphorylation and downstream signaling pathways, such as MAPK and AKT. This results in the inhibition of proliferation, promotion of apoptosis, and anti-tumor angiogenesis, ultimately leading to the attenuation of tumor growth (Zhao et al. 2022). However, resistance to EGFR-TKI remains a significant challenge in EGFR-targeted treatment, and patients experience disease progression within approximately one year of therapy (Vaid et al. 2021). SFI, as an adjunctive drug, has been reported to enhance both immunity and drug efficacy (Yang et al. 2017). However, the precise molecular mechanism through which SFI collaborates with EGFR-TKIs to regulate the occurrence and development of NSCLC has not been fully elucidated and has received limited investigation. Our study shows that SFI synergizes with gefitinib by inhibiting the IL-22/STAT3/AKT axis in NSCLC.
SFI, a TCM preparation primarily composed of Codonopsis pilosula and Radix Astragalus, is traditionally used to replenish qi and nourish the body (Liu et al. 2022). It is widely used as an adjunctive treatment for various cancers characterized by vital energy deficiency (Li et al. 2015; Liu et al. 2019). For example, studies have demonstrated the efficacy of SFI in alleviating cancer-related fatigue through experiments conducted on fatigue models in tumor-bearing mice (Zhu et al. 2019). In addition, SFI enhances cell cycle arrest and mitofusin-2-mediated mitochondrial apoptosis, leading to increased chemosensitivity of NSCLC to cisplatin resistance (Xiong et al. 2018). In clinical practice, a correlation has been observed between the combination of SFI and EGFR-TKI regimens and the extension of PFS in NSCLC patients (Wang et al. 2022).
We used a concentration-increasing induction method to study drug resistance. Prolonged administration of gefitinib resulted in a gradual loss of inhibition in the proliferation of the sensitive cell line PC9. As the concentration of gefitinib increased and the administration time was extended, the efficacy of gefitinib decreased in inhibiting cell proliferation. However, when SFI was included in the process of inducing drug resistance, a reduction in RI was observed, indicating a slowdown in the drug resistance process. The degree of enhancement in RI was negatively correlated with the concentration of SFI, implying that higher concentrations of SFI were more effective in delaying the development of gefitinib drug resistance.
A growing body of evidence suggests that cytokines contribute significantly to EGFR-TKI resistance in NSCLC. For example, IL-6 could suppress T- and natural killer (NK)-cell function in NSCLC patients with acquired resistance to EGFR-TKI (Patel et al. 2023). Additionally, IL-1β has been implicated in promoting EGFR-TKI resistance in NSCLC. IL-1β increased the expression of H-domain containing protein 1 (EHD1) and tubulin β-3 class III (TUBB3), two proteins involved in the epithelial-mesenchymal transition (EMT) process, a crucial mechanism to promote drug resistance in cancer (Huang et al. 2020).
SFI, known for its immunomodulatory effects, has shown promise in combating cytokine resistance mechanisms. SFI significantly reduced the levels of IL-6 and C-C motif chemokine ligand 1 (CCL1), both cytokines involved in tumor growth and immune modulation. Additionally, SFI inhibited the activation of p-PI3K, a key signaling molecule in cancer cell survival and growth (Yan et al. 2023).
We analyzed cytokine changes in NSCLC EGFR-TKI-resistant cell lines after SFI administration and observed a significant down-regulation of IL-22 compared to other cytokines. This led us to focus on IL-22 for further investigation, as it has been reported to have carcinogenic effects in various cancers and is significantly upregulated in lung cancer tissues. Previous studies have demonstrated that inhibiting IL-22 can suppress cell proliferation and promote cell apoptosis (Wang et al. 2019). Consistent with these findings, our observations revealed that IL-22 could promote proliferation and migration in NSCLC cells. In contrast, SFI administration resulted in a reduction in IL-22 expression, as well as its receptor subunit IL-22RA1. Subsequently, we explored the mechanisms by which SFI exerts a significant inhibitory effect on IL-22 overexpression.
The involvement of IL-22 in tumor proliferation and migration has been attributed to the reactivation of the STAT3 signaling pathway, which subsequently triggers AKT phosphorylation (Khare et al. 2015). Furthermore, IL-22 has been shown to alleviate gefitinib-induced injury by up-regulating the expression of p-AKT, p-EGFR, and phosphorylated extracellular signal-regulated kinase (p-ERK) (Wang et al. 2019). In particular, IL-22 has been reported to induce the overexpression of p-STAT3 and p-AKT through IL-22RA1 in lung cancer (Bi et al. 2016). This finding highlights the potential cross talk between the phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR)/AKT pathways and resistance to EGFR-TKI (Fang et al. 2020). To further investigate the effects IL-22 on cells resistant to EGFR-TKI, we conducted experiments to measure STAT3 and AKT phosphorylation levels after adding exogenous IL-22. The results showed a notable elevation in phosphorylation, indicating that, similarly to previous studies, IL-22 functions to activate the STAT3/AKT signaling pathway.
We have confirmed that excessive activation of the STAT3/AKT signaling pathway is responsible for the enhanced proliferation capacity of EGFR-TKI-resistant NSCLC cells. Given that SFI can reduce the expression of IL-22 and exert an auxiliary anti-tumor proliferation effect, we hypothesized that its impact might be achieved by suppressing the increase in p-STAT3 and p-AKT levels induced by exogenous IL-22. Our experiments supported this hypothesis, as pre-treatment with SFI for 24 or 48 h effectively reversed IL-22-induced phosphorylation of STAT3 and AKT in EGFR-TKI-resistant NSCLC cells. Furthermore, when we combined gefitinib with SFI or with SFI and IL-22, we observed enhanced effects on inhibiting cell viability, suppressing colony formation efficiency, and promoting drug-resistant cell apoptosis compared to gefitinib monotherapy or gefitinib combined with IL-22. This finding suggests that co-treatment of NSCLC cells with gefitinib and SFI promotes sensitivity to gefitinib even in cases where resistance to EGFR-TKI has occurred.
Conclusions
SFI enhances the effect of gefitinib through the IL-22/STAT3/AKT axis, providing a solid theoretical basis for developing SFI-assisted clinical formulations targeted for NSCLC. The combination of gefitinib and SFI emerges as a promising therapeutic regimen for NSCLC patients who have acquired resistance to EGFR-TKIs, offering a potential treatment approach to overcome drug resistance. More research and clinical trials are warranted to explore this combination therapy’s full potential in managing EGFR-TKI-resistant NSCLC patients.
Funding Statement
This study was financially supported by the China Pharmaceutical University Double First-Rate Construction Plan (CPU2022QZ19).
Authors’ contributions
J-lW and X-sD designed the experiments. J-lW and X-hH edited the manuscript and participated in major experiments. A-wY and KX conducted cell experiments. Z-rJ, SL, M-jH, Y-lL, and X-sD revised the manuscript accordingly. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data sets used in the current study are available from the corresponding author upon reasonable request.
References
- Bi Y, Cao J, Jin S, Lv L, Qi L, Liu F, Geng J, Yu Y.. 2016. Interleukin-22 promotes lung cancer cell proliferation and migration via the IL-22R1/STAT3 and IL-22R1/AKT signaling pathways. Mol Cell Biochem. 415(1–2):1–11. doi: 10.1007/s11010-016-2663-8. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Sequist LV, Lin JJ.. 2022. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat Rev Clin Oncol. 19(8):499–514. doi: 10.1038/s41571-022-00639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cunha Santos G, Shepherd FA, Tsao MS.. 2011. EGFR mutations and lung cancer. Annu Rev Pathol. 6(1):49–69. doi: 10.1146/annurev-pathol-011110-130206. [DOI] [PubMed] [Google Scholar]
- Di Noia V, D’Aveni A, D’Argento E, Rossi S, Ghirardelli P, Bortolotti L, Vavassori V, Bria E, Ceresoli GL.. 2021. Treating disease progression with osimertinib in EGFR-mutated non-small-cell lung cancer: novel targeted agents and combination strategies. ESMO Open. 6(6):100280. doi: 10.1016/j.esmoop.2021.100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Su SY, Wang MY, Zhan Z.. 2010. Shenqi Fuzheng, an injection concocted from Chinese medicinal herbs, combined with platinum-based chemotherapy for advanced non-small cell lung cancer: a systematic review. J Exp Clin Cancer Res. 29(1):137. doi: 10.1186/1756-9966-29-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Huang Y, Gu W, Gan J, Wang W, Zhang S, Wang K, Zhan J, Yang Y, Huang Y, et al. . 2020. PI3K-AKT-mTOR pathway alterations in advanced NSCLC patients after progression on EGFR-TKI and clinical response to EGFR-TKI plus everolimus combination therapy. Transl Lung Cancer Res. 9(4):1258–1267. doi: 10.21037/tlcr-20-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlaender A, Addeo A, Russo A, Gregorc V, Cortinovis D, Rolfo CD.. 2020. Targeted therapies in early stage NSCLC: hype or hope? Int J Mol Sci. 21(17):6329. doi: 10.3390/ijms21176329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganti AK, Klein AB, Cotarla I, Seal B, Chou E.. 2021. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 7(12):1824–1832. doi: 10.1001/jamaoncol.2021.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Jr., Wu YL, Paz-Ares L.. 2017. Lung cancer: current therapies and new targeted treatments. Lancet. 389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- Huang J, Lan X, Wang T, Lu H, Cao M, Yan S, Cui Y, Jia D, Cai L, Xing Y.. 2020. Targeting the IL-1beta/EHD1/TUBB3 axis overcomes resistance to EGFR-TKI in NSCLC. Oncogene. 39(8):1739–1755. doi: 10.1038/s41388-019-1099-5. [DOI] [PubMed] [Google Scholar]
- Khare V, Paul G, Movadat O, Frick A, Jambrich M, Krnjic A, Marian B, Wrba F, Gasche C.. 2015. IL10R2 overexpression promotes IL22/STAT3 signaling in colorectal carcinogenesis. Cancer Immunol Res. 3(11):1227–1235. doi: 10.1158/2326-6066.CIR-15-0031. [DOI] [PubMed] [Google Scholar]
- Kuo MH, Lee AC, Hsiao SH, Lin SE, Chiu YF, Yang LH, Yu CC, Chiou SH, Huang HN, Ko JC, et al. . 2020. Cross-talk between SOX2 and TGFbeta signaling regulates EGFR-TKI tolerance and lung cancer dissemination. Cancer Res. 80(20):4426–4438. doi: 10.1158/0008-5472.CAN-19-3228. [DOI] [PubMed] [Google Scholar]
- Li J, Wang JC, Ma B, Gao W, Chen P, Sun R, Yang KH.. 2015. Shenqi Fuzheng injection for advanced gastric cancer: a systematic review of randomized controlled trials. Chin J Integr Med. 21(1):71–79. doi: 10.1007/s11655-014-1768-8. [DOI] [PubMed] [Google Scholar]
- Li L, Han R, Xiao H, Lin C, Wang Y, Liu H, Li K, Chen H, Sun F, Yang Z, et al. . 2014. Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res. 20(10):2714–2726. doi: 10.1158/1078-0432.CCR-13-2613. [DOI] [PubMed] [Google Scholar]
- Liu Q, Lou Y, Li L, Yang G, Cui H, Cheng Z, Li Y, Liu M, Deng C, Wan D, et al. . 2022. A single-arm phase II study to evaluate efficacy and safety of first-line treatment with DCVAC/LuCa, standard of care chemotherapy and Shenqi Fuzheng injection in advanced (Stage IIIB/IV) non-small cell lung cancer patients. Integr Cancer Ther. 21:15347354221083968. 15347354221083968. doi: 10.1177/15347354221083968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhang D, Wu J, Wang K, Zhao Y, Ni M, Meng Z, Zhang X.. 2019. Shenqi Fuzheng injection in the treatment of breast cancer: a meta-analysis of randomized controlled trials. Integr Cancer Ther. 18:1534735418816824. 1534735418816824. doi: 10.1177/1534735418816824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YN, Chang TH, Tsai MF, Wu SG, Tsai TH, Chen HY, Yu SL, Yang JC, Shih JY.. 2015. IL-8 confers resistance to EGFR inhibitors by inducing stem cell properties in lung cancer. Oncotarget. 6(12):10415–10431. doi: 10.18632/oncotarget.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng FX, Yang X, Li ML.. 2022. Shenqi Fuzheng injection combined with chemotherapy for acute leukemia: a meta-analysis. Chin J Integr Med. 28(1):81–87. doi: 10.1007/s11655-020-3264-7. [DOI] [PubMed] [Google Scholar]
- Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et al. . 2017. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase M, Aksenov S, Yan H, Dunyak J, Al-Huniti N.. 2020. Modeling tumor growth and treatment resistance dynamics characterizes different response to gefitinib or chemotherapy in non-small cell lung cancer. CPT Pharmacometrics Syst Pharmacol. 9(3):143–152. doi: 10.1002/psp4.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Wang K, Chen Q, Zheng X, Song Z, Ding X.. 2020. SFI enhances therapeutic efficiency of gefitinib: an insight into reversal of resistance to targeted therapy in non-small cell lung cancer cells. J Cancer. 11(2):334–344. doi: 10.7150/jca.32989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Nilsson MB, Yang Y, Le X, Tran HT, Elamin YY, Yu X, Zhang F, Poteete A, Ren X, et al. . 2023. IL6 mediates suppression of T- and NK-cell function in EMT-associated TKI-resistant EGFR-mutant NSCLC. Clin Cancer Res. 29(7):1292–1304. doi: 10.1158/1078-0432.CCR-22-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker R, Herth FJ, Kerr KM, Filipits M, Taron M, Gandara D, Hirsch FR, Grunenwald D, Popper H, Smit E, et al. . 2010. Consensus for EGFR mutation testing in non-small cell lung cancer: results from a European workshop. J Thorac Oncol. 5(10):1706–1713. doi: 10.1097/JTO.0b013e3181f1c8de. [DOI] [PubMed] [Google Scholar]
- Relli V, Trerotola M, Guerra E, Alberti S.. 2019. Abandoning the notion of non-small cell lung cancer. Trends Mol Med. 25(7):585–594. doi: 10.1016/j.molmed.2019.04.012. [DOI] [PubMed] [Google Scholar]
- Sgambato A, Casaluce F, Maione P, Rossi A, Rossi E, Napolitano A, Palazzolo G, Bareschino MA, Schettino C, Sacco PC, et al. . 2012. The role of EGFR tyrosine kinase inhibitors in the first-line treatment of advanced non-small cell lung cancer patients harboring EGFR mutation. Curr Med Chem. 19(20):3337–3352. doi: 10.2174/092986712801215973. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Wagle NS, Jemal A.. 2023. Cancer statistics, 2023. CA Cancer J Clin. 73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- Su SB, Qin SY, Xian XL, Huang FF, Huang QL, ZhangDi HJ, Jiang HX.. 2021. Interleukin-22 regulating Kupffer cell polarization through STAT3/Erk/Akt crosstalk pathways to extenuate liver fibrosis. Life Sci. 264:118677. doi: 10.1016/j.lfs.2020.118677. [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, Bray F.. 2021. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tang J-L, Liu B-Y, Ma K-W.. 2008. Traditional Chinese medicine. Lancet. 372(9654):1938–1940. doi: 10.1016/S0140-6736(08)61354-9. [DOI] [PubMed] [Google Scholar]
- Vaid AK, Gupta A, Momi G.. 2021. Overall survival in stage IV EGFR mutation‑positive NSCLC: comparing first‑, second‑ and third‑generation EGFR‑TKIs (Review). Int J Oncol. 58(2):171–184. doi: 10.3892/ijo.2021.5168. [DOI] [PubMed] [Google Scholar]
- Wang JL, Chen CS, Jia ZR, Miao LY, Xie J, Pan ZZ, Duan YL, Liu S, Hou MJ, Ding XS.. 2022. Efficacy and safety of EGFR‑TKIs plus Shenqi Fuzheng injection for non-small cell lung cancer patients with EGFR-sensitive mutations. J Cancer Res Clin Oncol. 149(7):3895–3903. doi: 10.1007/s00432-022-04297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Herbst RS, Boshoff C.. 2021. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 27(8):1345–1356. doi: 10.1038/s41591-021-01450-2. [DOI] [PubMed] [Google Scholar]
- Wang S, Fu JL, Hao HF, Jiao YN, Li PP, Han SY.. 2021. Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol Res. 170:105728. doi: 10.1016/j.phrs.2021.105728. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu J, Chen J, Jin S, Yao J, Yu T, Wang W, Guo R.. 2019. IL-22 confers EGFR-TKI resistance in NSCLC via the AKT and ERK signaling pathways. Front Oncol. 9:1167. doi: 10.3389/fonc.2019.01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westover D, Zugazagoitia J, Cho BC, Lovly CM, Paz-Ares L.. 2018. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 29(suppl_1):i10–i19. doi: 10.1093/annonc/mdx703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Liang Y, Fu F.. 2022. Effect of Shenqi Fuzheng injection on leukopenia and T-cell subsets in patients with non-small cell lung cancer undergoing radiotherapy. Evid Based Complement Alternat Med. 2022:2832739. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu SG, Shih JY.. 2018. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 17(1):38. doi: 10.1186/s12943-018-0777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Liu Y, Zhang Z, Ou Z, Wang G, Zhang T, Long H, Lei M, Liu L, Huang W, et al. . 2022. Authentication of Shenqi Fuzheng injection via UPLC-coupled ion mobility-mass spectrometry and chemometrics with kendrick mass defect filter data mining. Molecules. 27(15):4734. doi: 10.3390/molecules27154734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Zhao Q, Gu L, Liu C, Wang C.. 2018. Shenqi Fuzheng injection reverses cisplatin resistance through mitofusin-2-mediated cell cycle arrest and apoptosis in A549/DDP cells. Evid Based Complement Alternat Med. 2018:8258246–8258212. doi: 10.1155/2018/8258246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Shi R, Lu YY, Fang DD, Ye MN, Zhou QM.. 2023. Shenqi Fuzheng injection reverses M2 macrophage-mediated cisplatin resistance through the PI3K pathway in breast cancer. PLoS One. 18(1):e0279752. doi: 10.1371/journal.pone.0279752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ting W, Xiao L, Shufei F, Wangxiao T, Xiaoying W, Xiumei G, Boli Z.. 2017. Immunoregulation of Shenqi Fuzheng injection combined with chemotherapy in cancer patients: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2017:5121538. doi: 10.1155/2017/5121538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Yang G, Lu G, Ye J, Cui L, Zeng Z, Chen J, Zhou J.. 2022. Th22 cells/IL-22 serves as a protumor regulator to drive poor prognosis through the JAK-STAT3/MAPK/AKT signaling pathway in non-small-cell lung cancer. J Immunol Res. 2022:8071234–8071212. doi: 10.1155/2022/8071234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Chen T, Shan L.. 2019. Shenqi Fuzheng injection as an adjunctive treatment to chemotherapy in breast cancer patients: a meta-analysis. Pharm Biol. 57(1):612–624. doi: 10.1080/13880209.2019.1660383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Qiu H, Li C, Cai P, Qi F.. 2021. The positive role of traditional Chinese medicine as an adjunctive therapy for cancer. Biosci Trends. 15(5):283–298. doi: 10.5582/bst.2021.01318. [DOI] [PubMed] [Google Scholar]
- Zhao P, Zhen H, Zhao H, Zhao L, Cao B.. 2022. Efficacy and safety of adjuvant EGFR-TKIs for resected non-small cell lung cancer: a systematic review and meta-analysis based on randomized control trials. BMC Cancer. 22(1):328. doi: 10.1186/s12885-022-09444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Lu G, Yao Y, Gu W.. 2019. An autocrine IL-6/IGF-1R loop mediates EMT and promotes tumor growth in non-small cell lung cancer. Int J Biol Sci. 15(9):1882–1891. doi: 10.7150/ijbs.31999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Zhang B, Jiang F, Zhao L, Liu F.. 2019. Shenqi Fuzheng injection ameliorates fatigue-like behavior in mouse models of cancer-related fatigue. Biomed Pharmacother. 111:1376–1382. doi: 10.1016/j.biopha.2019.01.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used in the current study are available from the corresponding author upon reasonable request.