Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) and gastroesophageal reflux disease (GERD) are both associated with obesity. However, the relationship of NAFLD with reflux esophagitis (RE) is still unclear in non-obese individuals.
Methods
Individuals with a body mass index (BMI) of 28 kg/m2 or higher, as well as waist circumference (WC) no less than 90 cm for men and no less than 85 cm for women were excluded. After controlling for other factors, 1905 eligible adult subjects were included. The components related to metabolic syndrome and the prevalence of NAFLD in the RE group as well as the non-RE group were analyzed. Risk factors for RE were determined using logistic regression.
Results
In non-obese individuals, the prevalence of RE and NAFLD increased with increasing WC and BMI (p < 0.001). Based on the results of logistic regression analysis, NAFLD was found to increase the risk of RE with statistical significance. Even after adjusting for metabolic syndrome and other related factors, NAFLD remained an independent influencing factor for the risk of RE (OR = 2.029; 95% CI 1.459–2.821, p < 0.001).
Conclusions
The prevalence of NAFLD was significantly higher in patients with RE compared to those without RE. These results indicate that NAFLD has a potential as an independent risk factor for RE, even in non-obese individuals.
Keywords: Non-alcoholic fatty liver disease, reflux esophagitis, non obese, epidemiology
Introduction
Gastroesophageal reflux disease (GERD) is a common digestive disorder caused by various factors, leading to multiple clinical manifestations and significant impairment in quality of life In addition to the typical symptoms of heartburn and reflux, patients may experience abdominal distension, belching, dysphagia, chest tightness, cough, and other symptoms [1]. This disease imposes a substantial economic burden on individuals, families, and society. GERD encompasses conditions such as Barrett’s oesophagus, reflux oesophagitis (RE), and non-erosive gastroesophageal reflux disease, with varying prevalence rates across different regions and countries. Studies have reported prevalence rates of 28% in North America, 26% in Europe, 33% in Asia, and 12% in the Middle East [2,3]. A Japanese study found that RE had a prevalence of 24.2% among severely obese patients. Similar to GERD, risk factors for RE include smoking, obesity, age, sex, alcohol consumption, Helicobacter pylori (HP) infection, and other variables [4,5].
The prevalence rate of non-alcoholic fatty liver disease (NAFLD), also known as metabolism-related fatty liver disease [6], is approximately 29.62% in Asia, and has been steadily increasing over the past two decades to 33.90% [7]. NAFLD has become a prevalent chronic liver disease, which, if left untreated, can progress to liver fibrosis, cirrhosis, and even liver cancer over time. Moreover, NAFLD is linked to lipid metabolism disorders, insulin resistance, obesity, gastrointestinal tumours, diabetes, and atrial fibrillation [8–10].
The relationship between NAFLD and GERD has garnered increasing attention from scholars. Certain studies have suggested an elevated risk of GERD in NAFLD patients in contrast with non-NAFLD populations [11,12]. However, several studies have failed to establish a definite association of NAFLD with GERD [13,14]. Numerous reports have explored the relationship between NAFLD and the risk of GERD. However, most studies have primarily focused on obesity. Consequently, there is limited research investigating the connection between NAFLD and RE, with inconsistent findings. Hence, it holds great clinical significance to examine the association between NAFLD and RE in non-obese individuals as part of the treatment for either condition. To investigate this potential association, we designed and carried out this cross-sectional study.
Subjects and methods
Subjects
The research subjects for this study were current and retired employees who underwent physical examinations at the Health Examination Centre of Ningbo Zhenhai District Refining and Chemical Hospital between March 2018 and November 2018. These individuals also underwent gastroscopy and B-ultrasound examination of the midsection and upper abdomen. The exclusion criteria were described in our previous publication [9]. Ultimately, 1905 cases in total were included in the present study. All participants were informed about the study and provided with detailed explanations of the procedures. The protocol were approved by the ethics committees of Zhenhai Lianhua Hospital. The informed consents were obtained from the objects. (no. 20120213).
Data collection
The questionnaire comprised general information such as age, gender, history of liver disease, drinking and smoking habits, and use of liver protective drugs. Individual medical histories included hypertension, diabetes, hyperlipidaemia, anaemia, and liver disease. Physical examinations encompassed measurements of height, weight, waist circumference (WC), as well as blood pressure. Height, weight, along with WC were measured using standard methods in the morning on an empty stomach, while systolic/diastolic blood pressure were measured after the participant had been seated for 5 min.
Detection of blood biochemical indexes
Fasting venous blood (10 ml) were collected for the detection of serum biochemistry, and other indicators. All biochemical analyses were performed using the same Au640 automatic biochemical analyzer produced by Olympus Company in Japan.
Gastroscopy
An Olympus gastroscope system [GIF-H260, GIF-H260Z, GIF-Q260J, GIF-H290, GIF-H290I, Olympus, Japan] was used for oesophageal examination. RE lesione degree was based on the Los Angeles classification [15].
Liver B-ultrasound
The liver B-ultrasound examination was performed on an empty stomach after venous blood collection using the Philips iU22 color ultrasound diagnostic instrument with a convex array probe operating at a frequency of 2-6MHz. The imaging physician conducting the examination was blind to the patient’s medical history or the protocol of this study. NAFLD was diagnosed according to clinical diagnostic criteria [16].
Helicobacter pylori detection
Helicobacter pylori detection was carried out using the ‘C-13 urea breath test (HCBT-01, Hydway)’. A delta over baseline (DOB) value greater than 4.0 was considered positive for HP infection.
Definition of obesity
Obesity in adults refers to those with a body mass index (BMI) of 28 kg/m2 or higher as well as a WC equal to or greater than 90.0 cm for men and 85.0 cm for women [17].
Statistical analysis
SPSS 23.0 statistical software was used for data analysis. For data following a normal or approximate normal distribution, results were expressed as mean ± standard deviation (SD). T-tests and F-tests were used for group comparisons. For skewed distributed data, results were presented with median and interquartile (IQR), and Mann-Whitney U tests and Kruskal-Wallis tests were used for group comparison. Chi-square tests were employed for categorical variables. Besides, univariate and multivariate logistic regression analyses were utilized to determine risk factors, and odds ratios (ORs) with 95% confidence intervals (CIs) were reported. Graphs were created using GraphPad Prism 8.0 (GraphPad Software Inc.). A p-value < 0.05 indicated statistical significance.
Results
General information of the research objects
A total of 1905 subjects were included, comprising 1096 males as well as 809 females. Among them, there were 385 cases of RE, with a prevalence rate of 20.2%. Of these, 263 were males (24.0%) and 122 were females (15.1%). The prevalence of RE was significantly higher in men compared to women, with a statistically significant difference (χ2 = 22.945, p < 0.001).
Comparison of clinical and laboratory examination indexes between RE and control groups
The RE group had higher levels of age, WC, BMI, systolic/diastolic blood pressure, triglycerides, uric acid, total cholesterol, as well as high-density lipoprotein with statistical significance compared to the control group. The fasting blood glucose as well as the low-density lipoprotein cholesterol levels were not significantly different between groups. Additionally, there were 530 cases of HP infection, with significantly lower rates of infection in the RE group (22.9%) in contrast with the control group (29.1%) (χ2 = 5.922, p = 0.015) (Table 1).
Table 1.
Comparison of clinical and laboratory examination indexes of RE and normal groups.
| Characteristic | All (n = 1905) | RE(n = 385) | Controls (n = 1520) | T value | p value |
|---|---|---|---|---|---|
| Gender (n) (male/female) | 1096/809 | 263/122 | 833/687 | 22.945* | <0.001 |
| Age (year) | 56.0 ± 14.0 | 57.8 ± 14.0 | 55.5 ± 14.1 | 2.866 | =0.004 |
| HP infection | 530 | 88 | 442 | 5.922 * | =0.015 |
| Waist circumference (cm) | 78.2 ± 6.5 | 79.9 ± 6.1 | 77.8 ± 6.5 | 5.854 | <0.001 |
| body mass index (kg/m2) | 21.9 ± 2.2 | 22.2 ± 2.1 | 21.8 ± 2.2 | 2.797 | =0.005 |
| systolic pressure (mmHg) | 126.3 ± 16.5 | 130.0 ± 16.6 | 125.4 ± 16.3 | 4.914 | <0.001 |
| diastolic pressure (mmHg) | 76.0 ± 10.3 | 77.6 ± 10.6 | 75.6 ± 10.2 | 3.355 | =0.001 |
| total cholesterol (mmol/L) | 4.9 ± 0.9 | 4.7 ± 0.9 | 4.9 ± 0.9 | −2.996 | =0.003 |
| triglyceride (mmol/L) | 1.2(0.9,1.6) | 1.3(0.9,1.8) | 1.2(0.9,1.6) | 1.576† | =0.014 |
| high density lipoprotein (mmol/L) | 1.4 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.4 | −5.223 | <0.001 |
| low density lipoprotein (mmol/L) | 2.9 ± 0.8 | 2.9 ± 0.8 | 3.0 ± 0.8 | −0.867 | =0.386 |
| fasting blood glucose (mmol/L) | 5.3 ± 0.9 | 5.2 ± 0.6 | 5.3 ± 0.9 | −0.925 | =0.355 |
| uric acid (μmol/L) | 300.5 ± 73.0 | 315.8 ± 76.9 | 296.6 ± 71.5 | 4.612 | <0.001 |
| hs-CRP (mg/L) | 0.5(0.3,1.0) | 0.7(0.4,1.2) | 0.5(0.3,0.9) | 2.177† | <0.001 |
Note: 1 mmHg = 0.133 kPa;Data are expressed as mean (SD) or median (IQR). *, χ2 value;†, Z value; HP: Helicobacter pylori; hs-CRP: high-sensitivity C-reactive protein.
Relationship between WC, BMI and prevalence of RE and NAFLD
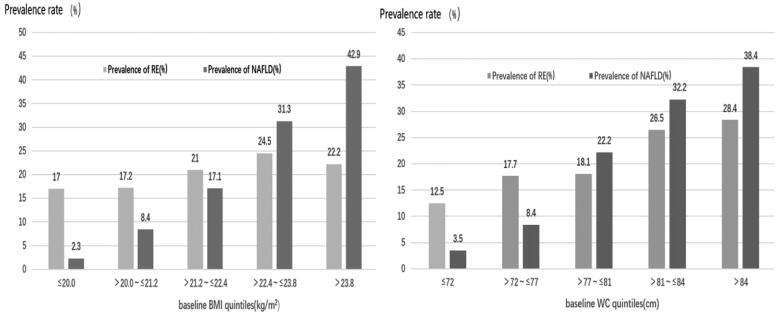
To investigate the association of obesity indicators with the prevalence of RE and NAFLD, we divided the participants into five groups based on quintiles of WC (≤ 72 cm, 72–77 cm, 78-81 cm, 82–84 cm, >84cm) and BMI(≤20kg/m2, 20.1–21.2 kg/m2, 21.3–22.4 kg/m2, 22.5–23.8 kg/m2, > 23.8 kg/m2). The results shown in Figure 1 indicate that both RE and NAFLD prevalence increased with higher WC (χ2 = 40.309, p < 0.001; χ2 = 208.557, p < 0.001) and BMI (χ2 = 9.511, p = 0.05; χ2 = 214.034, p < 0.001).
Figure 1.
Prevalence of RE and NAFLD at different BMI and WC levels.
Prevalence of NAFLD in RE and non-RE patients
As depicted in Table 2, the prevalence of NAFLD was 30.1% (116/385) in patients with RE and 17.5% (266/1520) in patients without RE. The prevalence of NAFLD between these two groups were different with statistical significance (χ2 = 30.566, p < 0.001).
Table 2.
Prevalence of NAFLD in patients with and without RE.
| NAFLD | Controls | Total | |
|---|---|---|---|
| RE | 116 | 269 | 385 |
| Non- RE | 266 | 1254 | 1520 |
| total | 382 | 1523 | 1905 |
Increased the risk of RE in non-obese individuals with NAFLD
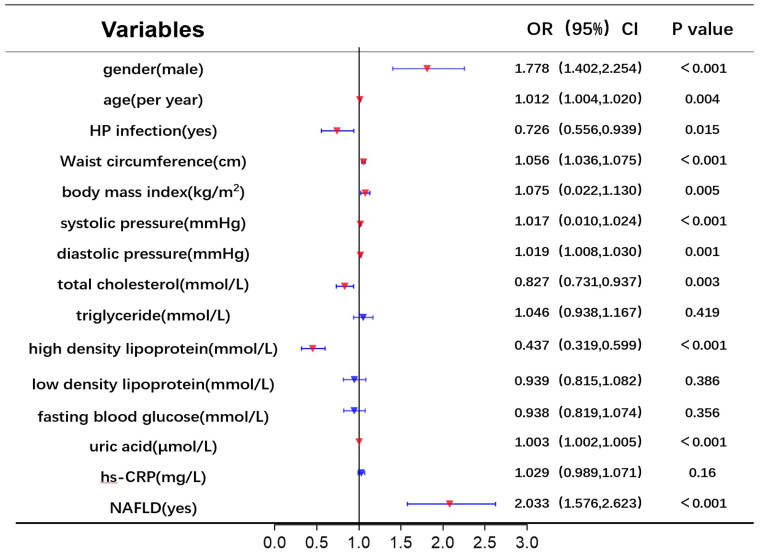
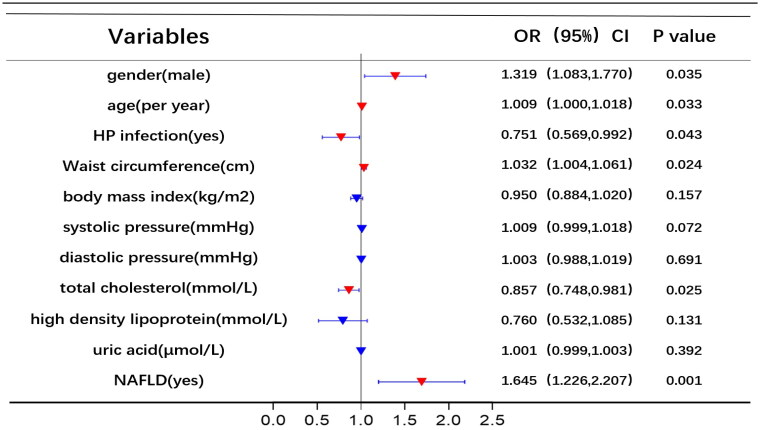
Univariate and multivariate logistic regression analyses were utilized to identify risk factors for RE. Figure 2 displays the results of univariate logistic analysis, which identified NAFLD, uric acid, high-density lipoprotein, total cholesterol, diastolic pressure, systolic pressure, BMI, WC, HP infection, age, and gender as independent factors associated with RE. Figure 3 shows the results of multivariable logistic analysis, revealing that gender, age, HP infection, WC, total cholesterol, and NAFLD were correlated with an increased risk of RE.
Figure 2.
Univariate logistic regression analysis of risk factors for RE.
Figure 3.
Multivariate logistic regression analysis of risk factors for RE.
Using RE (1 = yes, 0 = no) as the dependent variable, the independent variables in Table 1 were gradually included for analysis, multivariate logistic regression models were constructed based on different conditions. In Model 1, without adjusting for other parameters, NAFLD significantly increased the risk of RE (OR = 2.033; 95% CI 1.576–2.623, p < 0.001). ORs of RE remained statistically significant even after adjusting for age, sex, and HP infection (Model 2). Moreover, NAFLD remained a risk factor for RE when adjusting for BMI, WC, and metabolic syndrome (Model 5) (Table 3).
Table 3.
Logistic regression analysis of risk factors for RE.
| β | S.E | Wald x2 value | p value | OR 95.0%CI | |
|---|---|---|---|---|---|
| Modle 1 | 0.709 | 0.130 | 29.793 | <0.001 | 2.033(1.576,2.623) |
| Modle 2 | 0.619 | 0.132 | 21.988 | <0.001 | 1.717(1.349,2.186) |
| Modle 3 | 0.760 | 0.144 | 27.680 | <0.001 | 2.138(1.611,2.838) |
| Modle 4 | 0.692 | 0.157 | 19.517 | <0.001 | 1.998(1.470,2.717) |
| Modle 5 | 0.707 | 0.168 | 17.702 | <0.001 | 2.029(1.459,2.821) |
Note: Model 1 is unadjusted, Model 2 is adjusted for age, sex, and HP infection, Model 3 adds hs-CRP to Model 2, Model 4 adjusts for model 3 and includes variables such as BMI and WC, Model 5 adjusts for model 4 and adds variables such as systolic blood pressure, diastolic blood pressure, total cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, serum uric acid, and fasting blood glucose.
Discussion
This study found a higher prevalence of RE in men compared to women, and a higher prevalence of NAFLD in the RE group compared to the control group. It may be due to the frequent occurrence of weak acid reflux in male patients, which may aggravate the damage of oesophageal mucosa [18]. In the RE group, various factors including age, WC, BMI, systolic and diastolic blood pressure, triglyceride levels, as well as uric acid levels were obviously higher in contrast with the control group. Logistic regression analysis confirmed NAFLD as an independent risk factor for RE, even after adjusting for related variables. These findings suggest that NAFLD can independently increase the risk of RE in non-obese individuals, highlighting the need for further research on the relationship between NAFLD and RE.
A cross-sectional study by Chung et al. [19] conducted in South Korea on 7078 subjects revealed metabolic syndrome to be a risk factor for RE during upper gastrointestinal endoscopy. Prospective studies have also indicated that abdominal obesity, particularly visceral obesity, is a risk factor for reflux oesophagitis [5,20]. Possible explanations for this association include increased abdominal pressure due to obesity, more frequent relaxation of the lower oesophageal sphincter leading to acid reflux, and fat accumulation at the gastroesophageal junction[21]. Additionally, studies have suggested that conditions like hyperlipidaemia, diabetes, and hypertension can lead to reduced oesophageal peristalsis, inhibited contraction of the lower oesophageal sphincter [22,23], and ultimately contribute to the development of RE [24,25]. The present study also observed a higher prevalence of metabolic syndrome components among the RE group in contrast with the control group, suggesting that actively managing these components may benefit patients with RE[26].
NAFLD and RE are both common clinical conditions that may share certain associations. Literature on their relationship have yielded inconsistent results [11–14,27,28]. Both NAFLD and RE are linked to obesity. Therefore, it remains unclear whether NAFLD is an independent risk factor for RE or if the two diseases are simply connected through obesity. This study found that the prevalence of NAFLD and RE increased gradually with increasing BMI and WC even among non-obese individuals. Moreover, the prevalence of RE was higher in NAFLD patients compared to non-NAFLD patients. After adjusting for factors related to metabolic syndrome, the risk of RE was 2.029 times higher in NAFLD patients than in non-NAFLD patients. Several mechanisms have been proposed to explain how NAFLD may contribute to RE: (1) the abnormal movement of neurogenic oesophageal smooth muscle, resulting in decreased gastric acid clearance ability of the oesophagus and increased reflux attacks [28,29]; (2) the increased oxidative stress, leading to inflammation and ulceration of the oesophageal mucosa; (3) the reduced antioxidant function, reducing the repair ability of oesophageal mucosa while correspondingly increasing the severity of GERD [30]; (4) the pH-lowering effect of leptin released by adipose tissue of NAFLD patients on the oesophageal cavity, resulting in the damage to the oesophageal mucosa [31].
This study had a few limitations. Firstly, the diagnosis of fatty liver was based on B-ultrasound, which did not provide an assessment of the severity of fatty liver. Similarly, RE severity was not graded in this study. Secondly, the cross-sectional design prevented the establishment of a causal relationship between NAFLD and RE. Thirdly, hypoglycaemic, hypertensive, lipid-lowering, PPI, and glucocorticoid medications, as well as other medications that may affect metabolic profiles, NAFLD, and/or reflux oesophagitis risk, were not evaluated. Prospective research would be needed to confirm this relationship. Despite these limitations, this study contributed valuable insights into the association between NAFLD and RE in non-obese individuals, potentially aiding future prevention and treatment strategies for both conditions.
Conclusion
In conclusion, our study demonstrated a strong association between NAFLD and RE in non-obese individuals. Further investigation into the underlying causes of this relationship will enhance our understanding of the characteristics and mechanisms of RE.
Ethics approval and consent to participate
This study was conducted following the principles of the Declaration of Helsinki and approved by the ethics committees of Zhenhai Lianhua Hospital (No.20120213).
Consent for publication
Not applicable.
Acknowledgements
Not applicable.
Funding Statement
The work was supported by the Zhejiang Medical and Health Science and Technology Plan Project (2022KY1182); Ningbo Natural Science Foundation (2017A610276); Science and Technology Project of Agriculture and Social Science of Ningbo Yinzhou District (2021AS0053).
Authors’ contributions
Study concept and design, Peihong Qiu and Changxi Chen; Data curation, Juan Du, Cheng Zhang and Mengting Li; Formal analysis, Changxi Chen and Hongliang Li;Funding acquisition, Changxi Chen and Hongliang Li; Writing–original draft, Peihong Qiu and Changxi Chen; Writing–review and editing,All author.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and analysed during the current study are not publicly available (Because the datasets contain personal privacy) but are available from the corresponding author on reasonable request.
References
- 1.Broderick R, Fuchs KH, Breithaupt W, et al. Clinical presentation of gastroesophageal reflux disease: a prospective study on symptom diversity and modification of questionnaire application. Dig Dis. 2020;38(3):1–7. Epub 2019 Sep 12. PMID: 31514190. doi: 10.1159/000502796. [DOI] [PubMed] [Google Scholar]
- 2.Boeckxstaens G, El-Serag HB, Smout AJ, et al. Symptomatic reflux disease: the present, the past and the future. Gut. 2014;63(7):1185–1193. Epub 2014 Mar 7. PMID: 24607936; PMCID: PMC4078752. doi: 10.1136/gutjnl-2013-306393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eusebi LH, Ratnakumaran R, Yuan Y, et al. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67(3):430–440. Epub 2017 Feb 23. PMID: 28232473. doi: 10.1136/gutjnl-2016-313589. [DOI] [PubMed] [Google Scholar]
- 4.Taraszewska A. Risk factors for gastroesophageal reflux disease symptoms related to lifestyle and diet. Rocz Panstw Zakl Hig. 2021;72(1):21–28. PMID: 33882662. doi: 10.32394/rpzh.2021.0145. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Seki Y, Kasama K, et al. Prevalence of reflux esophagitis in obese Japanese undergoing bariatric surgery. JGH Open. 2019;4(3):519–524. PMID: 32514464; PMCID: PMC7273729. doi: 10.1002/jgh3.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martín-Domínguez V, González-Casas R, Mendoza-Jiménez-Ridruejo J, et al. Pathogenesis, diagnosis and treatment of non-alcoholic fatty liver disease. Rev Esp Enferm Dig. 2013;105(7):409–420. PMID: 24206551. doi: 10.4321/s1130-01082013000700006. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Zou B, Yeo YH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4(5):389–398. doi: 10.1016/S2468-1253(19)30039-1.Epub 2019 Mar 20. PMID: 30902670. [DOI] [PubMed] [Google Scholar]
- 8.Ishido S, Tamaki N, Takahashi Y, et al. Risk of cardiovascular disease in lean patients with nonalcoholic fatty liver disease. BMC Gastroenterol. 2023;23(1):211. PMID: 37330485; PMCID: PMC10276489. doi: 10.1186/s12876-023-02848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Zhu Z, Mao Y, et al. HbA1c may contribute to the development of non-alcoholic fatty liver disease even at normal-range levels. Biosci Rep. 2020;40(1):BSR20193996. PMID: 31940026; PMCID: PMC6997109. doi: 10.1042/BSR20193996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani A, Petracca G, Beatrice G, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: a meta-analysis of observational cohort studies. Gut. 2022;71(4):778–788. Epub 2021 Mar 8. PMID: 33685968. doi: 10.1136/gutjnl-2021-324191. [DOI] [PubMed] [Google Scholar]
- 11.Hung WC, Wu JS, Yang YC, et al. Nonalcoholic fatty liver disease vs. obesity on the risk of erosive oesophagitis. Eur J Clin Invest. 2014;44(12):1143–1149. Epub 2014 Nov 3. PMID: 25293867. doi: 10.1111/eci.12348. [DOI] [PubMed] [Google Scholar]
- 12.Yang HJ, Chang Y, Park SK, et al. Nonalcoholic fatty liver disease is associated with increased risk of reflux esophagitis. Dig Dis Sci. 2017;62(12):3605–3613. Epub 2017 Oct 23. PMID: 29063416. doi: 10.1007/s10620-017-4805-6. [DOI] [PubMed] [Google Scholar]
- 13.Min YW, Kim Y, Gwak GY, et al. Non-alcoholic fatty liver disease and the development of reflux esophagitis: a cohort study. J Gastroenterol Hepatol. 2018;33(5):1053–1058. Epub 2018 Feb 15. PMID: 29131401. doi: 10.1111/jgh.14042. [DOI] [PubMed] [Google Scholar]
- 14.Bang KB, Shin JE, Shin HD, et al. Non-obese non- alcoholic fatty liver disease is associted with erosive esophagitis. Gastroenterology. 2018;154(6):S-241. doi: 10.1016/S0016-5085(18)31184-3. [DOI] [Google Scholar]
- 15.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45(2):172–180. PMID: 10403727; PMCID: PMC1727604. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan JG, Jia JD, Li YM, et al. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese on Chinese Journal of hepatology 2010:18:163–166). J Dig Dis. 2011;12(1):38–44. doi: 10.1111/j.1751-2980.2010.00476.x. [DOI] [PubMed] [Google Scholar]
- 17.Bao Y, Lu J, Wang C, et al. Optimal waist circumference cutoffs for abdominal obesity in chinese. Atherosclerosis. 2008;201(2):378–384. Epub 2008 Mar 18. PMID: 18417137. doi: 10.1016/j.atherosclerosis.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Krigel A, Lebwohl B, Yadlapati R, et al. Association of patient gender and gastroenterologists’ diagnosis and management choices in gastroesophageal reflux disease. Dis Esophagus. 2021;34(9):doab019. PMID: 33870435. doi: 10.1093/dote/doab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung SJ, Kim D, Park MJ, et al. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut. 2008;57(10):1360–1365. Epub 2008 Apr 25. PMID: 18441006. doi: 10.1136/gut.2007.147090. [DOI] [PubMed] [Google Scholar]
- 20.Chung TH, Lee J, Jeong ID, et al. Effect of weight changes on the development of erosive esophagitis. Korean J Fam Med. 2020;41(1):14–19. Epub 2020 Jan 9. PMID: 31914725; PMCID: PMC6987027. doi: 10.4082/kjfm.19.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McColl KEL. What is causing the rising incidence of esophageal adenocarcinoma in the west and will it also happen in the east? J Gastroenterol. 2019; 54(8):669–673. Epub 2019 Jun 6. PMID: 31172291; PMCID: PMC6647360. doi: 10.1007/s00535-019-01593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida K, Furuta K, Adachi K, et al. Effects of anti-hypertensive drugs on esophageal body contraction. World J Gastroenterol. 2010;16(8):987–991. 8 PMID: 20180238; PMCID: PMC2828604. doi: 10.3748/wjg.v16.i8.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirata A, Kishida K, Nakatsuji H, et al. High prevalence of gastroesophageal reflux symptoms in type 2 diabetics with hypoadiponectinemia and metabolic syndrome. Nutr Metab. 2012;9(1):4 PMID: 22277344; PMCID: PMC3293023. doi: 10.1186/1743-7075-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toki Y, Yamauchi R, Kayashima E, et al. Predictive factors for future onset of reflux esophagitis: a longitudinal case-control study using health checkup records. J Neurogastroenterol Motil. 2022;28(1):86–94. PMID: 34980691; PMCID: PMC8748855. doi: 10.5056/jnm20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng SM, Hung KL, Wang YJ, et al. Influence of gastric morphology on gastroesophageal reflux in adults: an observational study. Medicine . 2021;100(38):e27241.). PMID: 34559123; PMCID: PMC8462620. doi: 10.1097/MD.0000000000027241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh YH, Wu MF, Yang PY, et al. What is the impact of metabolic syndrome and its components on reflux esophagitis? A cross-sectional study. BMC Gastroenterol. 2019;19(1):33. PMID: 30782138; PMCID: PMC6381695. doi: 10.1186/s12876-019-0950-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wijarnpreecha K, Panjawatanan P, Thongprayoon C, et al. Association between gastroesophageal reflux disease and nonalcoholic fatty liver disease: a meta-analysis. Saudi J Gastroenterol. 2017;23(6):311–317. PMID: 29205182; PMCID: PMC5738791. doi: 10.4103/sjg.SJG_161_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi JS, Kim HM, Yang YJ, et al. Fatty liver disease and the risk of erosive oesophagitis in the Korean population: a cross-sectional study. BMJ Open. 2019;9(1):e023585. PMID: 30705240; PMCID: PMC6359731. doi: 10.1136/bmjopen-2018-023585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieder F, Biancani P, Harnett K, et al. Inflammatory mediators in gastroesophageal reflux disease: impact on esophageal motility, fibrosis, and carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2010;298(5):G571–81. Epub 2010 Mar 18. PMID: 20299604; PMCID: PMC286741 doi: 10.1152/ajpgi.00454.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han D, Zhang C.. The oxidative damage and inflammation mechanisms in GERD-induced Barrett’s esophagus. Front Cell Dev Biol. 2022;10:885537. PMID: 35721515; PMCID: PMC9199966. doi: 10.3389/fcell.2022.885537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pardak P, Filip R, Woliński J, et al. Associations of obstructive sleep apnea, obestatin, leptin, and ghrelin with gastroesophageal reflux. J Clin Med. 2021;10(21):5195. PMID: 34768715; PMCID: PMC8584398. doi: 10.3390/jcm10215195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available (Because the datasets contain personal privacy) but are available from the corresponding author on reasonable request.