Abstract
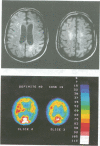
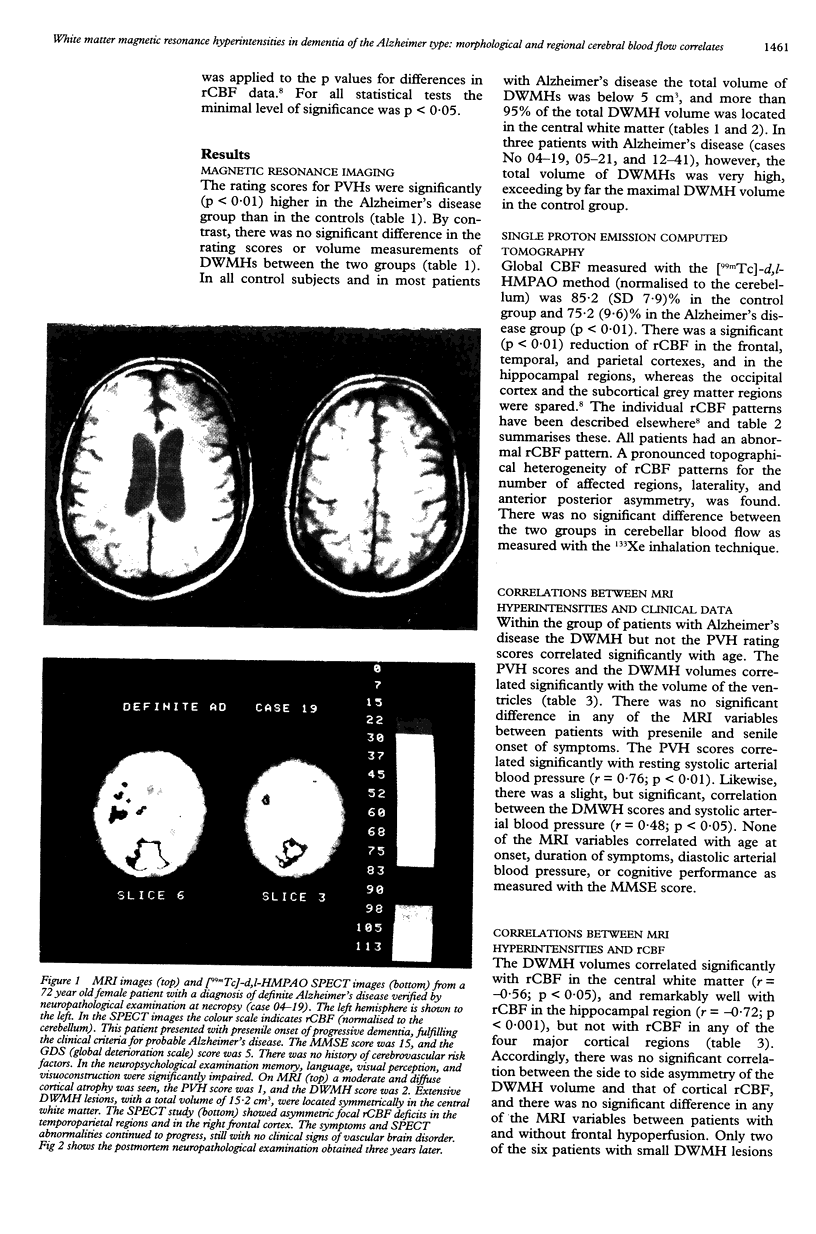
In a prospective MRI study the presence, appearance, volume, and regional cerebral blood flow (rCBF) correlates of periventricular hyperintensities (PVHs) and deep white matter hyperintensities (DWMHs) were examined in 18 patients with probable Alzheimer's disease and in 10 age matched healthy control subjects, all without major cerbrovascular risk factors. The 133Xe inhalation method and the [99mTc]-d,l-hexamethyl-propylene-amine-oxime (HMPAO) technique with single photon emission computed tomography (SPECT) were used to measure rCBF. Rating scores for PVHs were significantly higher in the Alzheimer's disease group (p < 0.01) and correlated significantly with the volume of ventricles (p < 0.05) and with systolic arterial blood pressure (p < 0.01), but not with rCBF. By contrast, there was no significant difference in the rating scores or volumes of DWMHs between the two groups, although three patients had extensive DWMH lesions in the central white matter. In the group of patients with Alzheimer's disease as a whole, the volume of DWMHs correlated well with rCBF in the hippocampal region ( r = -0.72; p < 0.001), but not with frontal, temporal, parietal, or occipital rCBF. Postmortem histopathology of extensive DWMH lesions in one patient with definite Alzheimer's disease showed a partial loss of myelin and astrocytic gliosis, but no ischaemic changes. It is concluded that DWMH lesions may be associated with reduced rCBF in the hippocampal region. The heterogenous topography of neocortical rCBF deficits in Alzheimer's disease could not be explained by deafferentation from underlying white matter hyperintensities and therefore may reflect variations in the topography of cortical abnormalities.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almkvist O., Wahlund L. O., Andersson-Lundman G., Basun H., Bäckman L. White-matter hyperintensity and neuropsychological functions in dementia and healthy aging. Arch Neurol. 1992 Jun;49(6):626–632. doi: 10.1001/archneur.1992.00530300062011. [DOI] [PubMed] [Google Scholar]
- Awad I. A., Johnson P. C., Spetzler R. F., Hodak J. A. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke. 1986 Nov-Dec;17(6):1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- Bondareff W., Raval J., Colletti P. M., Hauser D. L. Quantitative magnetic resonance imaging and the severity of dementia in Alzheimer's disease. Am J Psychiatry. 1988 Jul;145(7):853–856. doi: 10.1176/ajp.145.7.853. [DOI] [PubMed] [Google Scholar]
- Bondareff W., Raval J., Woo B., Hauser D. L., Colletti P. M. Magnetic resonance imaging and the severity of dementia in older adults. Arch Gen Psychiatry. 1990 Jan;47(1):47–51. doi: 10.1001/archpsyc.1990.01810130049007. [DOI] [PubMed] [Google Scholar]
- Brun A., Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986 Mar;19(3):253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- Celsis P., Goldman T., Henriksen L., Lassen N. A. A method for calculating regional cerebral blood flow from emission computed tomography of inert gas concentrations. J Comput Assist Tomogr. 1981 Oct;5(5):641–645. doi: 10.1097/00004728-198110000-00006. [DOI] [PubMed] [Google Scholar]
- Christie J. E., Kean D. M., Douglas R. H., Engleman H. M., St Clair D., Blackburn I. M. Magnetic resonance imaging in pre-senile dementia of the Alzheimer-type, multi-infarct dementia and Korsakoff's syndrome. Psychol Med. 1988 May;18(2):319–329. doi: 10.1017/s0033291700007868. [DOI] [PubMed] [Google Scholar]
- Fazekas F., Alavi A., Chawluk J. B., Zimmerman R. A., Hackney D., Bilaniuk L., Rosen M., Alves W. M., Hurtig H. I., Jamieson D. G. Comparison of CT, MR, and PET in Alzheimer's dementia and normal aging. J Nucl Med. 1989 Oct;30(10):1607–1615. [PubMed] [Google Scholar]
- Fazekas F., Kleinert R., Offenbacher H., Payer F., Schmidt R., Kleinert G., Radner H., Lechner H. The morphologic correlate of incidental punctate white matter hyperintensities on MR images. AJNR Am J Neuroradiol. 1991 Sep-Oct;12(5):915–921. [PMC free article] [PubMed] [Google Scholar]
- Fazekas F., Niederkorn K., Schmidt R., Offenbacher H., Horner S., Bertha G., Lechner H. White matter signal abnormalities in normal individuals: correlation with carotid ultrasonography, cerebral blood flow measurements, and cerebrovascular risk factors. Stroke. 1988 Oct;19(10):1285–1288. doi: 10.1161/01.str.19.10.1285. [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- George A. E., de Leon M. J., Kalnin A., Rosner L., Goodgold A., Chase N. Leukoencephalopathy in normal and pathologic aging: 2. MRI of brain lucencies. AJNR Am J Neuroradiol. 1986 Jul-Aug;7(4):567–570. [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967 Dec;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Haxby J. V., Grady C. L., Koss E., Horwitz B., Schapiro M., Friedland R. P., Rapoport S. I. Heterogeneous anterior-posterior metabolic patterns in dementia of the Alzheimer type. Neurology. 1988 Dec;38(12):1853–1863. doi: 10.1212/wnl.38.12.1853. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Davis K. R., Buonanno F. S., Brady T. J., Rosen T. J., Growdon J. H. Comparison of magnetic resonance and roentgen ray computed tomography in dementia. Arch Neurol. 1987 Oct;44(10):1075–1080. doi: 10.1001/archneur.1987.00520220071020. [DOI] [PubMed] [Google Scholar]
- Kertesz A., Polk M., Carr T. Cognition and white matter changes on magnetic resonance imaging in dementia. Arch Neurol. 1990 Apr;47(4):387–391. doi: 10.1001/archneur.1990.00530040029015. [DOI] [PubMed] [Google Scholar]
- Kozachuk W. E., DeCarli C., Schapiro M. B., Wagner E. E., Rapoport S. I., Horwitz B. White matter hyperintensities in dementia of Alzheimer's type and in healthy subjects without cerebrovascular risk factors. A magnetic resonance imaging study. Arch Neurol. 1990 Dec;47(12):1306–1310. doi: 10.1001/archneur.1990.00530120050009. [DOI] [PubMed] [Google Scholar]
- Leys D., Soetaert G., Petit H., Fauquette A., Pruvo J. P., Steinling M. Periventricular and white matter magnetic resonance imaging hyperintensities do not differ between Alzheimer's disease and normal aging. Arch Neurol. 1990 May;47(5):524–527. doi: 10.1001/archneur.1990.00530050040010. [DOI] [PubMed] [Google Scholar]
- Magnetic resonance imaging hyperintensities in Alzheimer's disease. Arch Neurol. 1991 May;48(5):468–470. [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris J. C., Gado M., Torack R. M., McKeel D. W., Jr Binswanger's disease or artifact: a clinical, neuroimaging, and pathological study of periventricular white matter changes in Alzheimer's disease. Adv Neurol. 1990;51:47–52. [PubMed] [Google Scholar]
- Rapoport S. I. Positron emission tomography in Alzheimer's disease in relation to disease pathogenesis: a critical review. Cerebrovasc Brain Metab Rev. 1991 Winter;3(4):297–335. [PubMed] [Google Scholar]
- Reisberg B., Ferris S. H., de Leon M. J., Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982 Sep;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- Scheltens P., Barkhof F., Valk J., Algra P. R., van der Hoop R. G., Nauta J., Wolters E. C. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer's disease. Evidence for heterogeneity. Brain. 1992 Jun;115(Pt 3):735–748. doi: 10.1093/brain/115.3.735. [DOI] [PubMed] [Google Scholar]
- Stokely E. M., Sveinsdottir E., Lassen N. A., Rommer P. A single photon dynamic computer assisted tomograph (DCAT) for imaging brain function in multiple cross sections. J Comput Assist Tomogr. 1980 Apr;4(2):230–240. doi: 10.1097/00004728-198004000-00022. [DOI] [PubMed] [Google Scholar]
- Sze G., De Armond S. J., Brant-Zawadzki M., Davis R. L., Norman D., Newton T. H. Foci of MRI signal (pseudo lesions) anterior to the frontal horns: histologic correlations of a normal finding. AJR Am J Roentgenol. 1986 Aug;147(2):331–337. doi: 10.2214/ajr.147.2.331. [DOI] [PubMed] [Google Scholar]
- Waldemar G., Bruhn P., Kristensen M., Johnsen A., Paulson O. B., Lassen N. A. Heterogeneity of neocortical cerebral blood flow deficits in dementia of the Alzheimer type: a [99mTc]-d,l-HMPAO SPECT study. J Neurol Neurosurg Psychiatry. 1994 Mar;57(3):285–295. doi: 10.1136/jnnp.57.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]