Abstract
Background
Excessive weight gain affects some persons with HIV after switching to integrase strand transfer inhibitor (INSTI)-containing antiretroviral therapy (ART). We studied associations between CYP2B6 genotype and weight gain after ART switch among ACTG A5001 and A5322 participants.
Methods
Eligible participants switched from efavirenz- to INSTI-containing ART, had genotype data, and had weight data at least once from 4 weeks to 2 years post-switch. Multivariable linear mixed effects models adjusted for race/ethnicity, CD4, age, BMI and INSTI type assessed relationships between CYP2B6 genotype and estimated differences in weight change.
Results
A total of 159 eligible participants switched ART from 2007 to 2019, of whom 138 had plasma HIV-1 RNA < 200 copies/mL (65 CYP2B6 normal, 56 intermediate, 17 poor metabolizers). Among participants with switch HIV-1 RNA < 200 copies/mL, weight increased in all 3 CYP2B6 groups. The rate of weight gain was greater in CYP2B6 poor than in CYP2B6 normal metabolizers overall, and within 9 subgroups (male, female, White, Black, Hispanic, dolutegravir, elvitegravir, raltegravir, and TDF in the pre-switch regimen); only in Hispanic and elvitegravir subgroups were these associations statistically significant (P < 0.05). Compared to normal metabolizers, CYP2B6 intermediate status was not consistently associated with weight gain.
Conclusion
CYP2B6 poor metabolizer genotype was associated with greater weight gain after switch from efavirenz- to INSTI-containing ART, but results were inconsistent. Weight gain in this setting is likely complex and multifactorial.
Keywords: efavirenz, HIV-1, integrase strand transfer inhibitors, pharmacogenetics, weight gain
Introduction
Initiation of antiretroviral therapy (ART) in treatment-naive people living with HIV (PWH) is typically associated with an early period of weight gain as health improves with immunologic recovery [1,2]. Through at least the first 96 weeks of ART, PWH may have greater lean mass and total, trunk, and limb fat gain compared with HIV-negative controls [3]. In recent years, it has been repeatedly observed that some PWH with ART-suppressed HIV gain unwanted weight following a switch from non-integrase strand transfer inhibitor (INSTI)-containing regimens to INSTI-containing regimens [4–6]. The magnitude of such weight gain appears to vary by specific INSTI, being greater with dolutegravir and bictegravir, and less with elvitegravir/cobicistat. Rather than a class-specific weight-promoting effect of INSTIs, at least some observed differences in weight gain may reflect a weight-suppressive effect of the pre-switch ART. Understanding these distinctions is important, as recommended initial ART for HIV-1 includes an INSTI plus nucleos(t)ide reverse transcriptase inhibitors (NRTIs) [7–9], and as millions of individuals worldwide are switching from non-INSTI- to INSTI-containing ART.
Among individuals who switch regimens while virologically suppressed on ART, selected pre-switch antiretrovirals have been reported to predict greater post-switch weight gain. In AIDS Clinical Trials Group (ACTG) observational cohort studies A5001 and A5322, weight gain following switch to INSTI-containing ART was most pronounced in participants who switched from efavirenz-containing regimens [5]. In a pooled analysis of data from 12 active-controlled clinical trials sponsored by Gilead Sciences, weight gain was greatest in those who switched from efavirenz to either rilpivirine or the INSTI elvitegravir/cobicistat, and also in those who switched from tenofovir disoproxil fumarate (TDF) to tenofovir alafenamide (TAF) [6]. Switching from abacavir to TAF was associated with less weight gain than switching from TDF to TAF [6]. These studies support inhibitory effects of efavirenz and TDF on pre-switch weight gain, with greater weight gain post-switch after this inhibition is no longer present.
Studies of treatment-naive PWH who initiate ART have also demonstrated that the magnitude of weight gain differs with specific antiretrovirals. In a pooled analysis of data from 8 active-controlled clinical trials sponsored by Gilead Sciences, efavirenz was associated with less weight gain than rilpivirine. In addition, TDF, abacavir, and zidovudine were associated with less weight gain than TAF [1]. Similarly, in the South African ADVANCE study, a randomized trial of ART-naive PWH which compared efavirenz/TDF/3TC to dolutegravir with TAF or TDF, the efavirenz-containing arm experienced less weight gain, while in the dolutegravir-containing arms, TDF was associated with lesser weight gain than TAF, especially in females [10]. These studies further support suppressive effects of efavirenz and TDF on weight gain.
Frequent genetic polymorphisms affect plasma efavirenz exposure. Efavirenz is metabolized by cytochrome P450 (CYP) 2B6 [11]. Three CYP2B6 polymorphisms in combination (516G→T (rs3745274), 983T→C (rs28399499), and 15582C→T (rs4803419)) explain approximately 30% of interindividual variability in plasma efavirenz exposure [12–16], and are associated with increased likelihood of suicidality on efavirenz [17], and discontinuation for central nervous system side effects [18]. Among 462 ART-naive individuals who initiated efavirenz-containing ART in ACTG clinical trials, CYP2B6 poor metabolizers had a lesser degree of weight gain at week 48 compared to CYP2B6 normal and intermediate metabolizers [19]. This was seen in participants receiving efavirenz with TDF, but not those receiving efavirenz with abacavir. Among 61 individuals in an observational cohort from a single clinic in the Southeast USA [4], CYP2B6 poor metabolizers had greater weight gain after the switch, but only when the switch was to elvitegravir or raltegravir, not to dolutegravir. These observations are consistent with a concentration-dependent suppressive effect of efavirenz on weight gain, with the greater efavirenz exposure in poor metabolizers contributing to greater weight suppression.
The present analysis focused on individuals in prospective follow-up in ACTG studies to test the hypothesis that, among PWH who switched from efavirenz- to INSTI-containing ART, CYP2B6 poor metabolizer genotypes would be associated with greater weight gain. We further sought to characterize this relationship in analyses stratified by sex, race/ethnicity, specific INSTI, and pre-switch TDF use.
Methods
Participants
Participants were from ACTG longitudinal, observational cohort studies A5001 and A5322. In 2000, A5001 (ACTG Longitudinal Linked Randomized Trials, ALLRT) [20] began enrolling participants who had previously participated in ACTG randomized interventional trials. Parent trials included both ART initiation in treatment-naive individuals and change to salvage therapy. A5001 followed participants every 4 months. In 2013, A5001 follow-up ended, and participants 40 years of age or older from treatment-naive parent trials were offered enrollment into A5322 (the HIV Infection, Aging, Immune Function Long-Term Observational Study, HAILO). HAILO involved prospective observational evaluations every 24 weeks from 2013 to 2019, and then every 48 weeks from 2020 to 2022 [21].
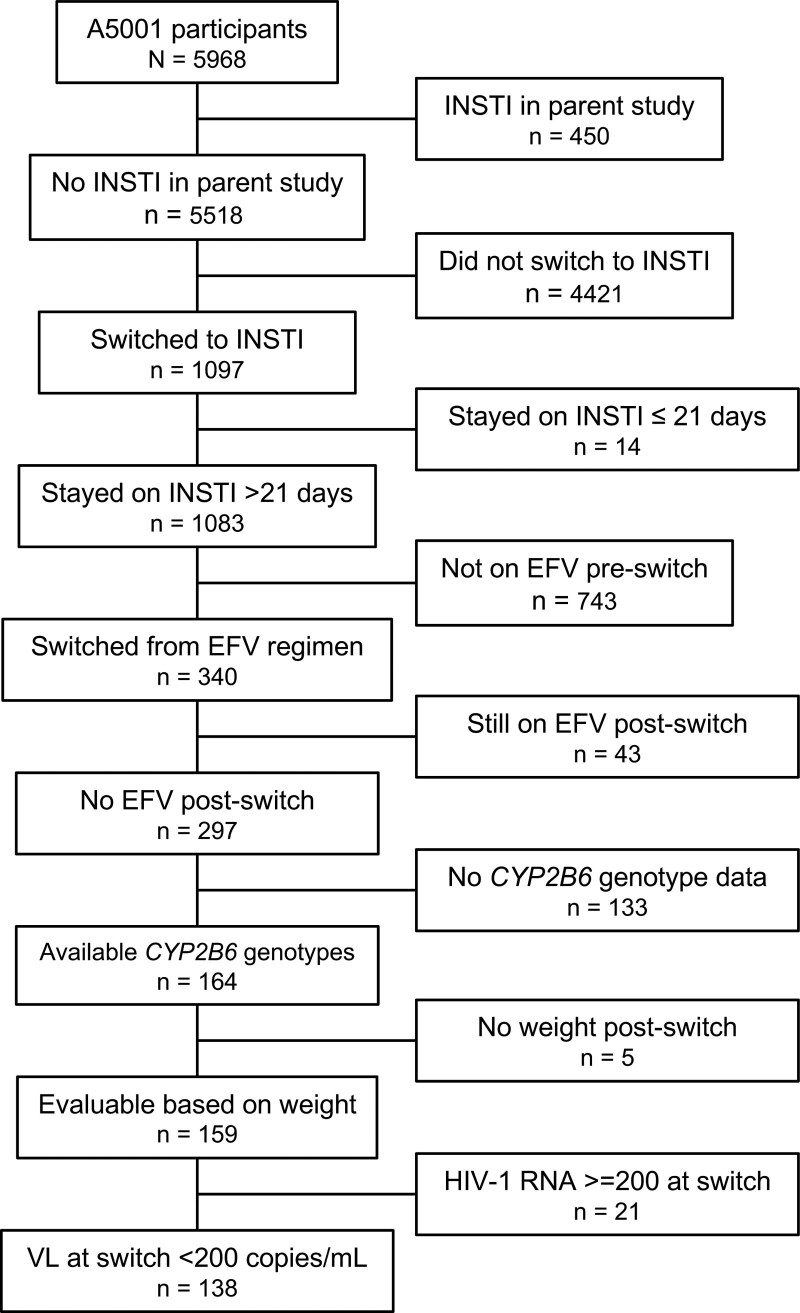
The present analyses include all A5001 and A5322 participants who had CYP2B6 genotype data available from previous analyses [15], who switched from an efavirenz-containing regimen to an INSTI-containing regimen during A5001/A5322 follow-up, had at least one weight or waist circumference measurement from 4 weeks to 2 years after switch, and who had an HIV-1 RNA < 200 copies/mL at the time of switch. Participants were excluded if the post-switch INSTI-containing regimen still included efavirenz. All participants provided written informed consent including for genetic research. Race/ethnicity was by self-report. This study was approved by the Institutional Review Boards at the participating institutions. Cohort derivation is shown in Fig. 1.
Fig. 1.
Derivation of the study cohort. ACTG, AIDS Clinical Trials Group; EFV, efavirenz; INSTI, integrase strand transfer inhibitor; VL, viral load (HIV-1 RNA).
Outcomes
Outcomes that we evaluated included change in weight (in kilograms) from the time of INSTI switch to 2 years after switch, and change in waist circumference (in centimeters) from the time of INSTI switch to 2 years, both collected using a standardized protocol.
Genotyping
Genotypes for CYP2B6 were generated for previous analyses as described elsewhere [15,19,22]. Three CYP2B6 polymorphisms that define 10 ordinal composite genotype levels and predict progressively greater efavirenz exposure were collapsed into 3 metabolizer levels (normal, intermediate, and poor) [18].
Statistical analyses
Basic summary statistics for demographics and baseline covariates at time of entry into the parent ACTG protocol, and at time of INSTI switch, were calculated by CYP2B6 metabolizer genotype. Linear mixed-effects models were fit to assess the relationship between CYP2B6 metabolizer genotype, weight change, and waist circumference change after the first switch to INSTI. First, unadjusted linear mixed-effects models were fit for weight (or waist circumference) and CYP2B6 metabolizer group on all participants who had at least one weight measurement (or waist circumference) within 2 years after switch. Models were also fit for the subset of participants who had weight (or waist circumference) measured both at time of INSTI switch and also within 2 years after this switch. The above models were also fit by subgroups: self-ascertained sex (separate models for male and female); race/ethnicity (non-Hispanic Black, non-Hispanic White, Hispanic); and specific INSTI (raltegravir, elvitegravir, and dolutegravir). Bictegravir was excluded from this analysis due to small sample size. Separate models were also fit only among participants who switched from regimens that included TDF.
For all of the above, multivariable models were fit adjusting for potential confounding variables, including age at switch, sex, race/ethnicity, parent study, BMI at switch, specific INSTI, nadir and current CD4 + T-cell count, history of smoking, history of diabetes, years of stavudine (D4T)/didanosine (DDI)/zidovudine (ZDV) prescribing before switch, percent follow-up time with HIV-1 RNA < 200 copies/mL, and use of psychiatric medications at switch. These covariates were evaluated individually by adding them to the unadjusted model. Factors that changed unadjusted effect estimates by at least 10% were retained in multivariable models.
Results
Participant characteristics
A total of 159 individuals switched from efavirenz- to INSTI-containing regimens (between 2007 and 2019), had CYP2B6 genotype data, and had weight data within 2 years after switch. Of these individuals, 138 had plasma HIV-1 RNA < 200 copies/mL at time of switch and were included in these analyses. Participant characteristics are shown in Table 1. Of the switches, 76 (55%) occurred in the years 2015 through 2017. The INSTI prescribed at switch was dolutegravir in 67 (49%), elvitegravir in 38 (28%), raltegravir in 26 (19%), and bictegravir in 7 (5%). Concomitant NRTIs in the first INSTI regimen included abacavir in 49 (36%), TAF in 40 (29%), and TDF in 39 (28%). Of the participants who had weight data after switch, 81 had weight data at the time of switch (defined as from 12 weeks prior to switch to up to 4 weeks after switch).
Table 1.
Participant characteristics at first INSTI switch by CYP2B6 metabolizer genotype among participants with viral suppression at time of switch
| Characteristic | CYP2B6 metabolizer group | ||||
|---|---|---|---|---|---|
| Total (N = 138) |
Normal (N = 65) |
Intermediate (N = 56) |
Poor (N = 17) |
||
| Sexa | M | 115 (83%) | 58 (89%) | 45 (80%) | 12 (71%) |
| F | 23 (17%) | 7 (11%) | 11 (20%) | 5 (29%) | |
| Race/ethnicity | White non-Hispanic | 74 (54%) | 36 (55%) | 31 (55%) | 7 (41%) |
| Black non-Hispanic | 37 (27%) | 15 (23%) | 15 (27%) | 7 (41%) | |
| Hispanic | 25 (18%) | 12 (18%) | 10 (18%) | 3 (18%) | |
| Other | 2 (1%) | 2 (3%) | 0 (0%) | 0 (0%) | |
| First post-switch INSTI | RAL | 26 (19%) | 13 (20%) | 10 (18%) | 3 (18%) |
| EVG | 38 (28%) | 20 (31%) | 13 (23%) | 5 (29%) | |
| DTG | 67 (49%) | 30 (46%) | 29 (52%) | 8 (47%) | |
| BIC | 7 (5%) | 2 (3%) | 4 (7%) | 1 (6%) | |
| Age at first INSTI switch | Median (Q1, Q3) | 55 (48, 61) | 53 (47, 60) | 57 (49, 64) | 54 (52, 60) |
| TDF in regimen before switch | No | 49 (36%) | 24 (37%) | 20 (36%) | 5 (29%) |
| Yes | 89 (64%) | 41 (63%) | 36 (64%) | 12 (71%) | |
| Selected NRTIs in first post-switch regimen | ABC | 49 (36%) | 25 (38%) | 19 (34%) | 5 (29%) |
| TDF | 39 (28%) | 19 (29%) | 15 (27%) | 5 (29%) | |
| TAF | 40 (29%) | 16 (25%) | 19 (34%) | 5 (29%) | |
| OTHER | 10 (7%) | 5 (8%) | 3 (5%) | 2 (12%) | |
| Years prior D4T exposure | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Years prior DDI exposure | Median (Q1, Q3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Years prior ZDV exposure | Median (Q1, Q3) | 2.0 (0.0, 6.6) | 2.7 (0.0, 6.0) | 1.2 (0.0, 6.8) | 0.0 (0.0, 6.8) |
| Years prior D4T/DDI/ZDV exposure | Median (Q1, Q3) | 3.3 (0.0, 7.2) | 3.3 (0.0, 6.6) | 3.4 (0.0, 9.1) | 0.0 (0.0, 8.3) |
| Most recent CD4 count before switch | Median (Q1, Q3) | 709 (512, 916) | 717 (553, 933) | 640 (487, 818) | 878 (622, 1042) |
| Nadir CD4 before switch | Median (Q1, Q3) | 164 (39, 275) | 200 (55, 297) | 157 (38, 271) | 43 (20, 149) |
| Most recent BMI on or before switch | Median (Q1, Q3) | 26.9 (24.0, 30.0) | 27.1 (23.7, 29.3) | 26.5 (24.3, 29.8) | 28.1 (25.2, 31.4) |
| Weight at INSTI switch in kg | N | 81 | 36 | 33 | 12 |
| Median (Q1, Q3) | 82.2 (72.2, 94.0) | 81.8 (74.0, 93.6) | 79.0 (70.2, 91.8) | 88.8 (74.7, 104.5) | |
| History of diabetes at switch | 20 (14%) | 6 (9%) | 11 (20%) | 3 (18%) | |
| History of smoking at switch | 76 (55%) | 34 (52%) | 34 (61%) | 8 (47%) | |
| Week of first INSTI regimen from baseline | Median (Q1, Q3) | 687 (501, 837) | 651 (450, 817) | 716 (535, 858) | 687 (582, 807) |
| Duration of first INSTI regimen (weeks) | Median (Q1, Q3) | 143 (78, 203) | 152 (78, 209) | 134 (76, 194) | 141 (94, 163) |
| Study | A5001 | 22 (16%) | 13 (20%) | 6 (11%) | 3 (18%) |
| A5001+A5322 | 116 (84%) | 52 (80%) | 50 (89%) | 14 (82%) | |
| Psychiatric medication at switch | No | 135 (98%) | 64 (98%) | 55 (98%) | 16 (94%) |
| Yes | 3 (2%) | 1 (2%) | 1 (2%) | 1 (6%) | |
D4T, stavudine; DDI, didanosine; INSTI, integrase strand transfer inhibitor; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
Information regarding gender identity is not available from study participants.
Weight gain after switch to INSTI-containing regimens
In unadjusted models of estimates of weight change, there was post-switch weight gain among all participants combined, and among CYP2B6 normal, intermediate, and poor metabolizers analyzed separately. The post-switch weight gain was greatest among CYP2B6 poor metabolizers (estimate 3.5 kg/year; 95% C.I., 2.1, 5.0 kg/year) and normal metabolizers (2.2 kg/year; 95% C.I., 1.5, 3.0 kg/year), and least among intermediate metabolizers (1.0 kg/year; 95% C.I., 0.2, 1.8 kg/year) (Table 2, columns to left).
Table 2.
Estimates of weight change by CYP2B6 metabolizer group, and difference in weight change between groups, among participants with viral suppression at time of switch, time points within 2 years after INSTI switch
| Variable | Unadjusted models | Adjusted modelsa | ||
|---|---|---|---|---|
| Estimate [95% CI] | P-value | Estimate [95% CI] | P-value | |
| All (n = 138) | ||||
| Weight change in normal metabolizers (kg/yr) | 2.23 [1.45 to 3.02]b | < 0.001 | 2.29 [1.51 to 3.07] | < 0.001 |
| Weight change in intermediate metabolizers (kg/yr) | 0.99 [0.15 to 1.83] | 0.02 | 0.93 [0.09 to 1.77] | 0.03 |
| Weight change in poor metabolizers (kg/yr) | 3.53 [2.06 to 5.01] | < 0.001 | 3.67 [2.21 to 5.14] | < 0.001 |
| Difference in weight change (Inter. vs. Normal) | −1.25 [−2.40 to −0.10] | 0.03 | −1.36 [−2.50 to −0.22] | 0.02 |
| Difference in weight change (Poor vs. Normal) | 1.30 [−0.37 to 2.97] | 0.1 | 1.39 [−0.27 to 3.04] | 0.1 |
| Male (n = 115) | ||||
| Weight change in normal metabolizers (kg/yr) | 2.55 [1.67 to 3.42] | < 0.001 | 2.64 [1.77 to 3.51] | < 0.001 |
| Weight change in intermediate metabolizers (kg/yr) | 0.57 [−0.40 to 1.54] | 0.3 | 0.53 [−0.43 to 1.49] | 0.3 |
| Weight change in poor metabolizers (kg/yr) | 3.73 [2.03 to 5.42] | < 0.001 | 3.66 [1.98 to 5.34] | < 0.001 |
| Difference in weight change (Inter. vs. Normal) | −1.98 [−3.29 to −0.68] | 0.003 | −2.10 [−3.40 to −0.81] | 0.002 |
| Difference in weight change (Poor vs. Normal) | 1.18 [−0.73 to 3.09] | 0.2 | 1.03 [−0.87 to 2.92] | 0.3 |
| Female (n = 23) | ||||
| Weight change in normal metabolizers (kg/yr) | 0.80 [−0.91 to 2.50] | 0.4 | 0.80 [−0.90 to 2.51] | 0.4 |
| Weight change in intermediate metabolizers (kg/yr) | 2.50 [0.89 to 4.10] | 0.003 | 2.32 [0.63 to 4.01] | 0.008 |
| Weight change in poor metabolizers (kg/yr) | 2.46 [−0.40 to 5.32] | 0.09 | 2.62 [−0.24 to 5.48] | 0.07 |
| Difference in weight change (Inter. vs. Normal) | 1.70 [−0.64 to 4.04] | 0.2 | 1.52 [−0.88 to 3.91] | 0.2 |
| Difference in weight change (Poor vs. Normal) | 1.66 [−1.66 to 4.99] | 0.3 | 1.82 [−1.51 to 5.15] | 0.3 |
| White (n = 74) | ||||
| Weight change in normal metabolizers (kg/yr) | 2.71 [1.60 to 3.82] | < 0.001 | 2.64 [1.53 to 3.75] | < 0.001 |
| Weight change in intermediate metabolizers (kg/yr) | 0.90 [−0.35 to 2.16] | 0.2 | 0.86 [−0.38 to 2.10] | 0.2 |
| Weight change in poor metabolizers (kg/yr) | 3.10 [0.46 to 5.74] | 0.02 | 3.42 [0.82 to 6.02] | 0.01 |
| Difference in weight change (Inter. vs. Normal) | −1.81 [−3.49 to −0.13] | 0.03 | −1.78 [−3.44 to −0.11] | 0.04 |
| Difference in weight change (Poor vs. Normal) | 0.38 [−2.48 to 3.25] | 0.8 | 0.78 [−2.05 to 3.61] | 0.6 |
| Black (n = 37) | ||||
| Weight change in normal metabolizers (kg/yr) | 1.21 [−0.50 to 2.92] | 0.2 | 1.30 [−0.42 to 3.02] | 0.1 |
| Weight change in intermediate metabolizers (kg/yr) | 0.61 [−1.12 to 2.34] | 0.5 | 0.85 [−0.95 to 2.64] | 0.4 |
| Weight change in poor metabolizers (kg/yr) | 2.81 [0.27 to 5.34] | 0.03 | 2.81 [0.26 to 5.36] | 0.03 |
| Difference in weight change (Inter. vs. Normal) | −0.59 [−3.03 to 1.84] | 0.6 | −0.46 [−2.96 to 2.05] | 0.7 |
| Difference in weight change (Poor vs. Normal) | 1.60 [−1.46 to 4.65] | 0.3 | 1.51 [−1.57 to 4.58] | 0.3 |
| Hispanic (n = 25) | ||||
| Weight change in normal metabolizers (kg/yr) | 2.16 [0.71 to 3.61] | 0.004 | 2.26 [0.76 to 3.76] | 0.004 |
| Weight change in intermediate metabolizers (kg/yr) | 1.74 [0.47 to 3.01] | 0.008 | 1.75 [0.47 to 3.03] | 0.008 |
| Weight change in poor metabolizers (kg/yr) | 5.61 [3.55 to 7.67] | < 0.001 | 5.63 [3.49 to 7.76] | < 0.001 |
| Difference in weight change (Inter. vs. Normal) | −0.42 [−2.35 to 1.51] | 0.7 | −0.51 [−2.47 to 1.45] | 0.6 |
| Difference in weight change (Poor vs. Normal) | 3.45 [0.93 to 5.97] | 0.008 | 3.37 [0.82 to 5.91] | 0.01 |
| Raltegravir (n = 26) | ||||
| Weight change in normal metabolizers (kg/yr) | 2.32 [0.67 to 3.97] | 0.006 | 2.52 [0.88 to 4.16] | 0.003 |
| Weight change in intermediate metabolizers (kg/yr) | −2.18 [−3.82 to −0.53] | 0.01 | −2.21 [−3.84 to −0.57] | 0.009 |
| Weight change in poor metabolizers (kg/yr) | 3.50 [0.04 to 6.97] | 0.05 | 3.53 [0.09 to 6.97] | 0.04 |
| Difference in weight change (Inter. vs. Normal) | −4.50 [−6.83 to −2.17] | < 0.001 | −4.73 [−7.04 to −2.41] | < 0.001 |
| Difference in weight change (Poor vs. Normal) | 1.18 [−2.66 to 5.02] | 0.5 | 1.01 [−2.80 to 4.82] | 0.6 |
| Elvitegravir (n = 38) | ||||
| Weight change in normal metabolizers (kg/yr) | 2.74 [1.86 to 3.63] | < 0.001 | 2.73 [1.83 to 3.63] | < 0.001 |
| Weight change in intermediate metabolizers (kg/yr) | 1.41 [0.35 to 2.46] | 0.009 | 1.40 [0.33 to 2.46] | 0.01 |
| Weight change in poor metabolizers (kg/yr) | 5.73 [4.20 to 7.25] | < 0.001 | 5.75 [4.19 to 7.31] | < 0.001 |
| Difference in weight change (Inter. vs. Normal) | −1.33 [−2.71 to 0.04] | 0.06 | −1.33 [−2.72 to 0.05] | 0.06 |
| Difference in weight change (Poor vs. Normal) | 2.98 [1.22 to 4.74] | 0.001 | 3.02 [1.24 to 4.80] | 0.001 |
| Dolutegravir (n = 67) | ||||
| Weight change in normal metabolizers (kg/yr) | 1.91 [0.70 to 3.12] | 0.002 | 1.93 [0.72 to 3.14] | 0.002 |
| Weight change in intermediate metabolizers (kg/yr) | 2.43 [1.14 to 3.73] | < 0.001 | 2.34 [1.05 to 3.63] | < 0.001 |
| Weight change in poor metabolizers (kg/yr) | 1.87 [−0.47 to 4.20] | 0.1 | 2.06 [−0.27 to 4.38] | 0.08 |
| Difference in weight change (Inter. vs. Normal) | 0.52 [−1.25 to 2.29] | 0.6 | 0.41 [−1.35 to 2.17] | 0.6 |
| Difference in weight change (Poor vs. Normal) | −0.05 [−2.67 to 2.58] | 0.9 | 0.12 [−2.50 to 2.75] | 0.9 |
| Pre-switch regimen included TDF (n = 89) | ||||
| Weight change in normal metabolizers (kg/yr) | 2.70 [1.52 to 3.88] | < 0.001 | 2.69 [1.50 to 3.87] | < 0.001 |
| Weight change in intermediate metabolizers (kg/yr) | 1.33 [0.04 to 2.61] | 0.04 | 1.37 [0.07 to 2.67] | 0.04 |
| Weight change in poor metabolizers (kg/yr) | 3.61 [1.58 to 5.64] | < 0.001 | 3.86 [1.84 to 5.89] | < 0.001 |
| Difference in weight change (Inter. vs. Normal) | −1.37 [−3.11 to 0.38] | 0.1 | −1.31 [−3.07 to 0.44] | 0.1 |
| Difference in weight change (Poor vs. Normal) | 0.92 [−1.43 to 3.26] | 0.4 | 1.18 [−1.17 to 3.52] | 0.3 |
Models were adjusted for race/ethnicity, CD4, age, BMI and INSTI type when they were not stratification factors.
Positive values for estimates indicate that participants gained weight post-switch. Positive values for estimates of difference on weight gain indicate that weight gain post-switch was greater than weight gain in CYP2B6 normal metabolizers.
In models adjusting for race/ethnicity, CD4 + T-cell count at switch, age, BMI at switch, and specific INSTI, similar to unadjusted models, there was similar post-switch weight gain among all participants analyzed together, and among CYP2B6 normal, intermediate, and poor metabolizers analyzed separately. The weight gain was again greatest among CYP2B6 poor metabolizers (3.7 kg/year; 95% C.I., 2.2, 5.1 kg/year) and normal metabolizers (2.3 kg/year; 95% C.I., 1.5, 3.1 kg/year), and least among intermediate metabolizers (1.0 kg/year; 95% C.I., 0.1, 1.8 kg/year) (Table 2, columns to right).
Stratified analyses of weight gain after switch to INSTI-containing regimens
We repeated the above analyses stratified by sex (115 males, 23 females), by race/ethnicity (74 White, 37 Black, and 25 Hispanic participants), by INSTI type (26 raltegravir, 38 elvitegravir, 67 dolutegravir), and restricted to those with TDF in pre-switch regimen (n = 89). These stratifications provided nine subgroups for analysis. In unadjusted models, post-switch weight gain was greater in CYP2B6 poor metabolizers than in normal metabolizers in 8 of 9 subgroups (only among dolutegravir recipients was this not so). Only among Hispanic participants, and those receiving elvitegravir, was the difference in weight gain among CYP2B6 poor metabolizers compared to normal metabolizers statistically significant. In adjusted models, post-switch weight gain was greater in CYP2B6 poor metabolizers than in normal metabolizers in all 9 subgroups, including among dolutegravir recipients. Again, only among Hispanic participants, and those receiving elvitegravir, was the difference in weight gain among CYP2B6 poor metabolizers compared to normal metabolizers statistically significant.
With regard to CYP2B6 intermediate metabolizers, in most subgroup analyses both unadjusted and adjusted, the change in weight post-switch was less in intermediate metabolizers than in CYP2B6 normal metabolizers. The exceptions were among females, and among dolutegravir recipients, in whom change in weight post-switch was greater in CYP2B6 intermediate metabolizers.
The above analyses were limited to 138 participants with plasma HIV-1 RNA < 200 copies/ml at time of switch. In analyses that included all 159 evaluable individuals regardless of plasma HIV-1 RNA at time of switch, results were generally consistent, including the finding that only among Hispanic participants, and those receiving elvitegravir, was the difference in weight gain among CYP2B6 poor metabolizers compared to normal metabolizers statistically significant (data not shown).
Analyses of waist circumference after switch to INSTI-containing regimens
The above analyses were repeated with change in waist circumference as the outcome of interest. Briefly, results of both unadjusted and adjusted models of estimates of slope of waist circumference among 126 evaluable participants were somewhat consistent with results based on change in weight. In adjusted mixed-effects models of estimates of slope of waist circumference among participants with plasma HIV-1 RNA < 200 copies/ml at time of switch, there was greater post-switch waist circumference gain with all CYP2B6 groups combined, and among CYP2B6 normal, intermediate, and poor metabolizers analyzed separately, with the greatest gain in waist circumference among CYP2B6 poor metabolizers. The increase in waist circumference among intermediate metabolizers was less than among either CYP2B6 normal or poor metabolizers (data not shown).
Additional stratified analyses found post-switch increases in waist circumference were greatest in CYP2B6 poor metabolizers among the 104 males, the 21 Hispanic participants, the 24 raltegravir recipients, the 36 elvitegravir recipients, and the 81 participants whose pre-switch regimen included TDF. In comparing CYP2B6 poor vs. normal metabolizers, none of these differences were statistically significant. Post-switch increase in waist circumference was greatest in groups other than CYP2B6 poor metabolizers among the 22 females, 68 White participants, 36 Black participants, and 63 dolutegravir recipients.
Discussion
Among virologically suppressed PWH on ART, unwanted weight gain has been reported following a switch to INSTI-containing regimens [4]. The objective of the present study was to determine whether, following a switch from efavirenz-containing regimens to INSTI-containing regimens, post-switch weight gain was explained by CYP2B6 metabolizer status. We show that, among 138 individuals who switched from efavirenz-containing regimens to INSTI-containing regimens, and with plasma HIV-1 RNA < 200 copies/ml at time of switch, post-switch weight gain was greatest among CYP2B6 poor metabolizers. In subgroup analyses, there was also evidence of greater weight gain post-switch among CYP2B6 poor metabolizers in nearly all of our stratified analyses. The differences were only statistically significant in selected subgroup analyses.
This finding is generally consistent with a previous observational study of 61 individuals who switched from efavirenz- to INSTI-containing regimens at a clinic in the Southeastern USA [19]. In that study, CYP2B6 poor metabolizers had overall greater weight gain after switch. It was hypothesized that the association between CYP2B6 genotype and weight gain might reflect withdrawal of an effect of higher efavirenz concentrations (i.e. poor metabolizers) on weight gain. This hypothesis is supported by ART-naive studies showing that, following initiation of efavirenz-containing regimens, weight gain may be less among CYP2B6 poor metabolizers than among CYP2B6 intermediate and normal metabolizers. Among 168 partici-pants randomly assigned to initiate efavirenz/TDF/3TC in the South African ADVANCE study, weight gain from baseline to week 48 was the least among CYP2B6 poor metabolizers, especially among women [23]. Similarly, among 462 individuals who initiated efavirenz-containing ART in ACTG clinical trials, CYP2B6 poor metabolizers had the least weight gain at week 48 [19].
Multiple studies have associated CYP2B6 poor metabolizer genotypes with efavirenz adverse events including central nervous system symptoms, suicidality, hepatic injury, and QTc prolongation [16,18,24,25]. Our study lends evidence to the hypothesis that an additional effect of CYP2B6 poor metabolizer genotypes may be unwanted weight gain following switch from efavirenz- to INSTI-containing regimens.
Cellular mechanisms of efavirenz adverse effects may include altered energy metabolism, mitochondrial function, or other processes involved in stress responses such as inflammation [26]. In ACTG study A5170, which involved asymptomatic participants with chronic ART exposure, neurocognition improved after treatment interruption, particularly with efavirenz-containing regimens [27]. Improvements in everyday life following switch from efavirenz-containing regimens have also been reported in asymptomatic persons [28]. Subjective improvement after discontinuing efavirenz may include increased appetite, especially among individuals with higher efavirenz concentrations.
There is evidence that TDF has a weight-suppressive effect. Weight gain has been reported in ART-experienced individuals following switch from TDF- to TAF-containing ART [29]. Among ADVANCE study participants who had been randomized to receive dolutegravir plus either TAF/FTC or TDF/FTC, weight gain was less with TDF than with TAF, especially in females [10]. In a placebo-controlled trial of preexposure prophylaxis for HIV-negative individuals, TDF/emtricitabine was associated with a lesser increase in weight through 72 weeks than with placebo [30]. Among 462 individuals randomly assigned to initiate efavirenz-containing ART in ACTG trials, the association of CYP2B6 poor metabolizer status with lesser weight gain was seen among those receiving TDF but not those receiving abacavir [19], suggesting that TDF exposure in the presence of higher efavirenz concentrations may particularly interfere with expected weight gain [19].
The present study did not find a strong association between CYP2B6 poor metabolizer status and weight change after switch to dolutegravir, which is surprising considering that dolutegravir is often associated with greater weight gain than raltegravir or elvitegravir. Greater weight gain has also been reported in ART-naive participants initiating a dolutegravir-containing regimen [2]. This finding is generally consistent with the previous report based on an observational cohort of 61 participants [19], in which the association of CYP2B6 poor metabolizer status with greater weight gain was only apparent following switch to elvitegravir or raltegravir, but not dolutegravir. In the South African ADVANCE study, dolutegravir-containing arms experienced greater weight gain than the control efavirenz/TDF/3TC arm, especially with TAF [10]. We cannot explain why we did not see a strong association with dolutegravir in our observational cohort.
Our study had limitations. The sample size for participants who met criteria for evaluation was relatively small, particularly for participants with CYP2B6 poor metabolizer genotypes. Because the cohort was not specifically designed to study weight gain at time of switch, not all participants had weight data at the time of switch. Findings should be replicated in larger cohorts. Such future cohort analyses should ideally be used for meta-analyses, together with results from the present study and from the prior study [19].
In summary, among participants who switched from efavirenz- to INSTI-containing therapy during observational follow-up in A5001 and A5322, participants with CYP2B6 poor metabolizer genotypes had greater increase in weight following switch when considering all 138 participants, and in 8 of 9 stratified analysis, although the difference in change in weight between CYP2B6 poor metabolizers and CYP2B6 normal metabolizers was statistically significant only among Hispanic participants and among elvitegravir recipients. Weight gain in this setting is likely complex and multifactorial. This is particularly relevant given the global transition to INSTI-containing ART regimens for HIV-1 infection [31].
Acknowledgements
The authors are grateful to the many persons living with HIV who volunteered for ACTG protocols A5001 and A5322, and who consented for genetic testing under protocol A5128. In addition, they acknowledge the contributions of study teams and site staff for these protocols. We thank Paul J. McLaren, PhD (Public Health Agency of Canada, Winnipeg, Canada) for prior involvement and collaborations that used these genome-wide genotype data.
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1 AI068634, UM1 AI068636 and UM1 AI106701. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Supported in part by grants funded by the National Center for Research Resources and the National Center for Advancing Translational Sciences.
Grant support included AI110527, AI077505, TR000445, and AI069439 (to D.W.H.). This work was supported by the Tennessee Center for AIDS Research (P30) AI110527.
Clinical research sites that participated in ACTG protocols A5095, A5142 and/or A5202, and collected DNA under protocol A5128 were supported by the following grants from the National Institutes of Health (NIH): A1069412, A1069423, A1069424, A1069503, AI025859, AI025868, AI027658, AI027666, AI027675, AI032782, AI034853, AI038858, AI045008, AI046370, AI046376, AI050409, AI050410, AI058740, AI060354, AI069412, AI069415, AI069418, AI069419, AI069423, AI069424, AI069428, AI069432, AI069434, AI069439, AI069447, AI069450, AI069452, AI069465, AI069467, AI069470, AI069471, AI069472, AI069474, AI069477, AI069481, AI069484, AI069494, AI069495, AI069496, AI069501, AI069502, AI069503, AI069511, AI069513, AI069532, AI069534, AI069556, AI072626, AI073961, RR000046, RR000425, RR023561, RR024156, RR024160, RR024996, RR025008, RR025747, RR025777, RR025780, TR000004, TR000058, TR000124, TR000170, TR000439, TR000457, TR001079, TR001082, TR001111, and TR024160.
Conflicts of interest
JK has served as a consultant to Gilead, Merck, ViiV Healthcare, Theratechnologies and Janssen, and has received research support from Gilead Sciences and Merck. KE has received grant funding from Gilead and provided consultation for Gilead, ViiV, and Merck. SHB reports research grants to her institution from ViiV Health Care and Janssen, and scientific advisory to Gilead Sciences. TB has served as consultant to Gilead Sciences, Merck, ViiV Healthcare, and Janssen. JL has received research grants from Gilead and has consulted to Theratechnologies.
References
- 1.Sax PE, Erlandson KM, Lake JE, McComsey GA, Orkin C, Esser S, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourgi K, Rebeiro PF, Turner M, Castilho JL, Hulgan T, Raffanti SP, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020; 70:1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant PM, Kitch D, McComsey GA, Collier AC, Bartali B, Koletar SL, et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. AIDS 2016; 30:2805–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, Bebawy S, et al. Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 2017; 76:527–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lake JE, Wu K, Bares SH, Debroy P, Godfrey C, Koethe JR, et al. Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis 2020; 71:e471–e477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erlandson KM, Carter CC, Melbourne K, Brown TT, Cohen C, Das M, et al. Weight change following antiretroviral therapy switch in people with viral suppression: pooled data from randomized clinical trials. Clin Infect Dis 2021; 73:1440–1451. [DOI] [PubMed] [Google Scholar]
- 7.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. (Updated March 23, 2023). 2023. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf. [Accessed 20 June 2018].
- 8.Raffi F, Rachlis A, Stellbrink HJ, Hardy WD, Torti C, Orkin C, et al.; SPRING-2 Study Group. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013; 381:735–743. [DOI] [PubMed] [Google Scholar]
- 9.Clotet B, Feinberg J, van Lunzen J, Khuong-Josses MA, Antinori A, Dumitru I, et al.; ING114915 Study Team. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–2231. [DOI] [PubMed] [Google Scholar]
- 10.Venter WDF, Moorhouse M, Sokhela S, Fairlie L, Mashabane N, Masenya M, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–815. [DOI] [PubMed] [Google Scholar]
- 11.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther 2003; 306:287–300. [DOI] [PubMed] [Google Scholar]
- 12.Rotger M, Tegude H, Colombo S, Cavassini M, Furrer H, Decosterd L, et al. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin Pharmacol Ther 2007; 81:557–566. [DOI] [PubMed] [Google Scholar]
- 13.Wyen C, Hendra H, Vogel M, Hoffmann C, Knechten H, Brockmeyer NH, et al.; German Competence Network for HIV/AIDS. Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J Antimicrob Chemother 2008; 61:914–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an adult AIDS clinical trials group study. AIDS 2004; 18:2391–2400. [PubMed] [Google Scholar]
- 15.Holzinger ER, Grady B, Ritchie MD, Ribaudo HJ, Acosta EP, Morse GD, et al. Genome-wide association study of plasma efavirenz pharmacokinetics in AIDS clinical trials group protocols implicates several CYP2B6 variants. Pharmacogenet Genomics 2012; 22:858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desta Z, Gammal RS, Gong L, Whirl-Carrillo M, Gaur AH, Sukasem C, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2B6 and efavirenz-containing antiretroviral therapy. Clin Pharm Therap 2019;. 106:726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mollan KR, Tierney C, Hellwege JN, Eron JJ, Hudgens MG, Gulick RM, et al.; AIDS Clinical Trials Group. Race/ethnicity and the pharmacogenetics of reported suicidality with efavirenz among clinical trials participants. J Infect Dis 2017; 216:554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leger P, Chirwa S, Turner M, Richardson DM, Baker P, Leonard M, et al. Pharmacogenetics of efavirenz discontinuation for reported central nervous system symptoms appears to differ by race. Pharmacogenet Genomics 2016; 26:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard MA, Cindi Z, Bradford Y, Bourgi K, Koethe J, Turner M, et al. Efavirenz Pharmacogenetics and weight gain following switch to integrase inhibitor-containing regimens. Clin Infect Dis 2021; 73:e2153–e2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smurzynski M, Collier AC, Koletar SL, Bosch RJ, Wu K, Bastow B, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials 2008; 9:269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlandson KM, Wu K, Koletar SL, Kalayjian RC, Ellis RJ, Taiwo B, et al. Association between frailty and components of the frailty phenotype with modifiable risk factors and antiretroviral therapy. J Infect Dis 2017; 215:933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson DH, Venuto C, Ritchie MD, Morse GD, Daar ES, McLaren PJ, et al. Genomewide association study of atazanavir pharmacokinetics and hyperbilirubinemia in AIDS Clinical Trials Group protocol A5202. Pharmacogenet Genomics 2014; 24:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griesel R, Maartens G, Chirehwa M, Sokhela S, Akpomiemie G, Moorhouse M, et al. CYP2B6 genotype and weight gain differences between dolutegravir and Efavirenz. Clin Infect Dis 2021; 73:e3902–e3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelhady AM, Shugg T, Thong N, Lu JB, Kreutz Y, Jaynes HA, et al. Efavirenz inhibits the Human Ether-A-Go-Go Related Current (hERG) and Induces QT Interval Prolongation in CYP2B6*6*6 allele carriers. J Cardiovasc Electrophysiol 2016; 27:1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desta Z, Saussele T, Ward B, Blievernicht J, Li L, Klein K, et al. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics 2007; 8:547–558. [DOI] [PubMed] [Google Scholar]
- 26.Apostolova N, Blas-Garcia A, Galindo MJ, Esplugues JV. Efavirenz: What is known about the cellular mechanisms responsible for its adverse effects. Eur J Pharmacol 2017; 812:163–173. [DOI] [PubMed] [Google Scholar]
- 27.Robertson KR, Su Z, Margolis DM, Krambrink A, Havlir DV, Evans S, et al.; A5170 Study Team. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology 2010; 74:1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hakkers CS, Arends JE, van den Berk GE, Ensing MHM, Hooijenga I, Vink M, et al. Objective and subjective improvement of cognition after discontinuing efavirenz in asymptomatic patients: a randomized controlled trial. J Acquir Immune Defic Syndr 2019; 80:e14–e22. [DOI] [PubMed] [Google Scholar]
- 29.Gomez M, Seybold U, Roider J, Harter G, Bogner JR. A retrospective analysis of weight changes in HIV-positive patients switching from a tenofovir disoproxil fumarate (TDF)- to a tenofovir alafenamide fumarate (TAF)-containing treatment regimen in one German university hospital in 2015-2017. Infection 2019; 47:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glidden DV, Mulligan K, McMahan V, Anderson PL, Guanira J, Chariyalertsak S, et al. Metabolic effects of preexposure prophylaxis with coformulated tenofovir disoproxil fumarate and emtricitabine. Clin Infect Dis 2018; 67:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach.; 2021. https://www.who.int/publications/i/item/9789240031593. [Accessed 21 March 2023]. [PubMed]