Abstract
Background and Objectives
Non-hepatic hyperammonemia can damage the central nervous system (CNS), and possible prognostic factors are lacking. This study aimed to investigate the prognostic and risk factors for patients admitted to the intensive care unit (ICU).
Materials and Methods
This prospective, observational, multicenter study was conducted between November and December 2019 at 11 ICUs in the Chinese Heilongjiang province. Changes in blood ammonia level during and after ICU admission were continuously monitored and expressed as the high level (H-), mean level (M-), and initial level (I-) of ammonia. The risk factors of poor prognosis were investigated by conducting univariate and multivariate logistic regression analyses. Receiver operating characteristic (ROC) curve analysis was conducted to compare the predictive ability of Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE-II) score, lactic acid, total bilirubin (TBil), and M-ammonia.
Results
A total of 1060 patients were included in this study, of which 707 (67%) had a favorable prognosis and 353 (33%) had a poor prognosis. As shown by univariate models, a poor prognosis was associated with elevated serum levels of lactic acid, TBil, and ammonia (P < 0.05) and pathologic scores from three assessments: APACHE-II, Glasgow Coma Scale (GCS), and Sequential Organ Failure Assessment (SOFA). Multivariate analysis revealed that circulating mean ammonia levels in ICU patients were independently associated with a poor prognosis (odds ratio [OR] = 1.73, 95% confidence interval [CI]: 1.07–2.80, P = 0.02). However, the APACHE-II score (area under the curve [AUC]: 0.714, sensitivity: 0.86, specificity: 0.68, P < 0.001) remained the most predictive factor for patient prognosis by ROC analysis.
Conclusion
Elevated serum levels of ammonia in the blood were independently prognostic for ICU patients without liver disease.
Key words: hyperammonemia, non-hepatic, serum ammonia, lactic acid, Acute Physiologic Assessment and Chronic Health Evaluation, prognostic factor, intensive care unit
Introduction
Ammonia is a product of protein catabolism and the syndrome, hyperammonemia, results from metabolic defects.[1] Hyperammonemia is a life-threatening condition as it leads to encephalopathy, loss of consciousness, increased intracranial pressure, and may eventually progress to cerebral herniation.[1, 2, 3, 4] Hyperammonemia is prevalent in patients with acute and chronic liver failure. However, it may also appear independently of liver disease as non-hepatic hyperammonemia (NHH).[5,6] NHH occurs in up to 73% of critically ill patients.[7] At present, solicitous and continuous monitoring of blood ammonia for NHH is not standard practice in intensive care units (ICUs).
Lactic acid is a product of the anaerobic glycolysis of glucose and it can directly reflect tissue hypoperfusion and hypoxia conditions. As demonstrated by studies of metabolism during exercise, there is a linear relationship between blood ammonia and lactic acid levels due to a shared short-term energy pathway.[8,9] During intense exercise, skeletal muscle generates adenosine triphosphate (ATP) through an adenylate kinase reaction, converting two adenosine diphosphate (ADP) molecules to one ATP molecule and one adenosine monophosphate (AMP) molecule.[10] Sustained exercise stimulates the deamidation of AMP, generating ammonia as a byproduct, in order to maintain the equilibrium states of AMP for the upstream adenylate kinase reaction. Therefore, high ammonia levels signify a state of rapid energy consumption or potential deficits in the aerobic tricarboxylic acid (TCA) cycle.[11, 12, 13] Accordingly, hyperammonemia can be representative of an insufficient energy supply or defective metabolism in critically ill patients.
Notably, increased blood ammonia levels are not solely acquired from liver diseases, and a particular attention should be paid to NHH in daily ICU practice. Patients with an underlying metabolic disorder are at particularly increased risk for NHH in the content of a critical injury or illness. A comprehensive metabolic assessment including blood ammonia, lactic acid, ketone body, and related indicators may have prognostic value in the ICU.[14, 15, 16] Two recent retrospective studies demonstrated that serum ammonia status was an independent prognostic predictor of mortality for NHH patients.[7,17] Specifically, proactive monitoring of blood ammonia levels for the prompt detection of, and early intervention against, NHH may improve the prognosis of critically ill patients.
Irrespective of etiology, delayed diagnosis and treatment of hyperammonemia can lead to significant neurologic injury. When ammonia penetrates the blood-brain barrier by facilitated transport or passive diffusion, it is processed into glutamine, an osmolyte that induces astrocyte swelling. Since hyperammonemia is most commonly associated with hepatic dysfunction, elevated ammonia levels may be overlooked during the evaluation of other critically ill patients. Therefore, this prospective, multicenter study explored the prognostic value of ammonia levels in critically ill patients without hepatic dysfunction who were hospitalized in the ICU.
Materials and methods
Patients
This was a prospective, observational, multicenter research that recruited 1895 patients who were admitted to 11 general ICUs between November and December 2019. There were four centers in Harbin (the Second Affiliated Hospital of Harbin Medical University, Harbin Fifth Hospital, General Hospital of Heilongjiang Province Land Reclamation Bureau, and the First Hospital of Harbin), three centers in Daqing (the Fifth Affiliated Hospital of Harbin Medical University, Daqing Oilfield General Hospital, and the Longnan Hospital of Daqing), two centers in Jiamusi (the First Affiliated Hospital of Jiamusi University and Jamusi Central Hospital), and two centers in Mudanjiang (Hongqi Hospital affiliated with Mudanjiang Medical University and Mudanjiang City Second People’s Hospital).
We only included patients (1) older than 18 years of age and (2) who were directly admitted to the ICU and treated there for over 24 h during the study period. The exclusion criteria were as follows: (1) patients with acute liver failure (ALF), (2) patients with chronic liver diseases (CLDs), and (3) patients readmitted to the ICU. We assigned patients to a favorable prognosis group (treated or transferred out of the ICU) and a poor prognosis group (recalcitrant to treatment or experienced death as a primary endpoint).
The study protocol was approved by the ethics committee of each allied center (approval number KY2019-184), and signed informed consent forms were collected from all study subjects or their families.
Data collection
Statistical data were collected at admission, including demographic characteristics, a history of other diseases, patients’ medical status, acute physiological assessment, and the Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE-II) score.[18] Blood samples were drawn from the artery at admission and at 9 a.m. daily and were immediately stored in a refrigerator at 4℃ or wrapped in ice packs. Measurements were performed within 30 min after sample collection. At admission, we performed a basic metabolic panel for blood levels of Na+, K+, lactic acid, serum glucose, leukocyte, platelet, hematocrit (HCT), total bilirubin (TBil), creatinine (Crea), urea, albumin (ALB), and ammonia. Blood ammonia and lactic acid levels, the Glasgow Coma Scale (GCS) score, and the Sepsis-related Organ Failure Assessment (SOFA) score were examined at 9 a.m. daily during ICU hospitalization.
NHH was defined as the presence of high blood ammonia level (>35 μmol/L) in patients without a history of liver diseases and was classified as mild-NHH (36–99 μmol/L) and severe-NHH (≥100 μmol/L) according to the blood ammonia level.[19,20] I-ammonia was defined as the serum ammonia level at admission, and M- and H-ammonia were the mean and the highest serum ammonia levels during hospitalization at ICU, respectively. SOFA scoring evaluates the incidence, occurrence, and development of multiple organ dysfunction syndrome (MODS). It is a sequential assessment which assigns each organ system (respiratory, hematology, respiratory, cardiovascular, renal, and hepatic) a score from 0 to 4 for a maximum total score of 24.[21]
APACHE-II uses a point scoring system based on the initial values for 12 routine physiologic measurements, combined with age and previous health status to provide a general measure of severity of disease.[18] The APACHE-II score is used in conjunction with a patient’s disease status to predict mortality. Due to its close relationship with clinical outcomes, it is often used as a control observation to test the sensitivity and specificity of other scoring systems.
The GCS score uses a Coma Index, which sums the scores in three areas, including eye-opening response, verbal response, and body movement to evaluate the degree of coma of patients.[22,23] Healthy people have the maximum score of 15, and the scores decrease by coma severity: ≤15 indicates consciousness, 3–8 indicates a coma, and scores <3 are seen comatose patients who are unable to speak due to intubation.
Clinical outcomes
The favorable prognosis group comprised patients released from the hospital or transferred out of the ICU to recover, while the poor prognosis group was defined as the group of patients recalcitrant to treatment or who experienced death as a primary endpoint.
Statistical analysis
Data were statistically analyzed using SPSS 20.0 (IBM, Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD) and were analyzed by using the Student’s t-test. Categorical variables, presented as n (%), were compared using the chi-square test. Logistic regression analysis was employed to differentiate whether mean and initial serum ammonia levels were statistically significant for predicting the prognosis of ICU patients. Univariate logistic regression analyses were used to identify factors with a significant association to prognosis (P < 0.05). To evaluate the independence of factors, bidirectional stepwise forward selection was used for the multivariate logistic analysis of APACHE-II, GCS, lactic acid, TBil, and M-ammonia. The receiver operating characteristic (ROC) curve was drawn and the area under the curve (AUC) was calculated to assess the prognostic power of APACHE-II, M-ammonia, lactic acid, and TBil. A P-value <0.05 was considered statistically significant.
Results
Baseline characteristics and outcomes
We obtained records from 1895 patients admitted to 11 ICUs across Heilongjiang province. A total of 835 were ineligible, of which 143 were less than 18 years old (18%), 187 had CLDs (22%), 234 had ALF (28%), 67 were readmissions to the ICU (8%), and 204 failed to provide signed informed consent forms (24%) (Figure 1). A total of 1060 patients met the inclusion criteria for analysis and were divided into favorable prognosis (707 [67%]; 62.26 ± 17.15 years) and poor prognosis (353 [33%]; 63.7 ± 14.53 years) groups.
Figure 1.
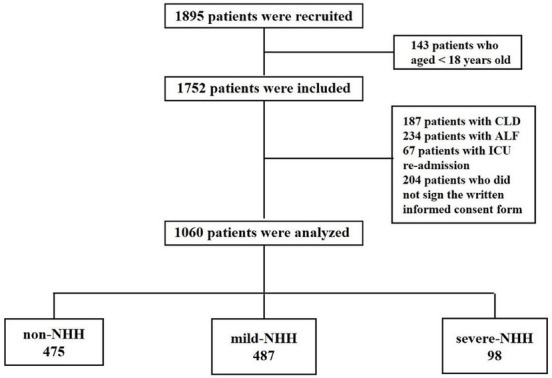
Flowchart of the patients involved in the study. NHH: non-hepatic hyperammonemia; CLD: chronic liver disease; ALF: acute liver failure; ICU: intensive care unit.
The demographic characteristics of NHH patients included in this study are presented in Table 1. Patients with a poor prognosis had a significantly faster heart rate (95.61 ± 21.85 vs. 104.87 ± 24.67 bmp, P = 0.001) and higher levels of lactic acid (1.96 ± 1.88 vs. 2.87 ± 2.80 mmol/L, P < 0.001), TBil (15.79 ± 13.70 vs. 20.31 ± 18.54, P = 0.004), Crea (126.78 ± 142.12 vs. 161.99 ± 183.95, P = 0.005), urea (9.33 ± 6.81 vs. 11.58 ± 8.97, P = 0.002), and M-ammonia (25.11 ± 21.35 vs. 30.12 ± 23.56, P = 0.001). Surprisingly, more patients with a favorable prognosis experienced respiratory failure (131 [18.53%] vs. 92 [26.06%], P = 0.005), disturbance of consciousness (175 [24.75%] vs. 122 [34.56%], P = 0.005), or underwent surgery (221 [31.26%] vs. 41 [11.61%], P < 0.001). Baseline APACHE-II (15 ± 8 vs. 21 ± 8, P < 0.001) and SOFA (7 ± 4 vs. 9 ± 4, P < 0.001) scores were higher and GCS scores (12 ± 4 vs. 10 ± 3, P < 0.001) were lower in the poor prognosis group compared to the favorable prognosis group. The length of stay in the ICU for patients with poor prognosis was significantly longer than that for patients with favorable prognosis (3.26 ± 2.33 vs. 4.25 ± 3.14 days, P = 0.008) (Table1).
Table 1.
Baseline characteristics of patients in the ICU with a favorable or poor prognosis
| Variables | Favorable prognosis (n = 707) | Poor prognosis (n = 353) | P-value |
|---|---|---|---|
| Demography | |||
| Age, years | 62.26 ± 17.15 | 63.7 ± 14.53 | 0.068 |
| Sex, M | 397 (56.15%) | 183 (51.84%) | 0.389 |
| Harbin (4 centers) | 392 (55.45%) | 208 (58.92%) | 0.293 |
| Daqing (3 centers) | 88 (12.45%) | 42 (11.90%) | 0.843 |
| Jiamusi (2 centers) | 121 (17.11%) | 53 (15.01%) | 0.429 |
| Mudanjiang (2 centers) | 106 (14.99%) | 50 (14.16%) | 0.714 |
| Vital signs | |||
| Temperature, ℃ | 37.00 ± 0.82 | 37.02 ± 0.84 | 0.807 |
| HR, bmp | 95.61 ± 21.85 | 104.87 ± 24.67 | 0.001 |
| RR, bmp | 20.57 ± 8.58 | 21.24 ± 8.41 | 0.414 |
| SBP, mmHg | 91.34 ± 45.33 | 94.10 ± 49.54 | 0.334 |
| DBP, mmHg | 63.55 ± 17.54 | 63.09 ± 16.90 | 0.257 |
| MBP, mmHg | 72.42 ± 24.03 | 74.22 ± 36.62 | 0.305 |
| Laboratory parameters | |||
| PH | 7.37 ± 0.24 | 7.37 ± 0.13 | 0.911 |
| Na+, mmol/L | 137.83 ± 10.39 | 137.43 ± 8.54 | 0.676 |
| K+, mmol/L | 3.97 ± 0.64 | 3.95 ± 0.72 | 0.721 |
| Lactic acid, mmol/L | 1.96 ± 1.88 | 2.87 ± 2.80 | <0.001 |
| Serum glucose, mmol/L | 8.91 ± 3.41 | 9.16 ± 4.23 | 0.130 |
| Leukocyte, ×109/L | 13.28 ± 8.60 | 13.96 ± 6.77 | 0.344 |
| Platelet, ×109/L | 168.26 ± 82.25 | 165.90 ± 84.05 | 0.726 |
| HCT, % | 35.03 ± 9.69 | 36.67 ± 14.68 | 0.140 |
| TBil, mg/dL | 15.79 ± 13.70 | 20.31 ± 18.54 | 0.004 |
| Crea, μmol/L | 126.78 ± 142.12 | 161.99 ± 183.95 | 0.005 |
| Urea, mmol/L | 9.33 ± 6.81 | 11.58 ± 8.97 | 0.002 |
| ALB, g/L | 32.52 ± 6.36 | 32.44 ± 7.56 | 0.893 |
| Ammonia, μmol/L | |||
| Normal | 345 (48.80%) | 130 (36.83%) | 0.004 |
| Mild-NHH | 318 (44.98%) | 169 (47.88%) | 0.395 |
| Severe-NHH | 44 (6.22%) | 54 (15.30%) | <0.001 |
| I-ammonia, μmol/L | 29.22 ± 25.10 | 32.27 ± 21.90 | 0.051 |
| H-ammonia, μmol/L | 40.65 ± 30.64 | 43.14 ± 25.31 | 0.066 |
| M-ammonia, μmol/L | 25.11 ± 21.35 | 30.12 ± 23.56 | 0.001 |
| Disease type | |||
| Gastrointestinal bleeding | 25 (3.54%) | 11 (3.12%) | 0.213 |
| Sepsis | 56 (7.92%) | 31 (8.78%) | 0.636 |
| Obesity | 45 (6.36%) | 20 (5.67%) | 0.687 |
| Shock | 90 (12.73%) | 56 (15.86%) | 0.185 |
| Respiratory failure | 131 (18.53%) | 92 (26.06%) | 0.005 |
| Heart failure | 53 (7.50%) | 37 (10.48%) | 0.103 |
| Kidney failure | 24 (3.39%) | 17 (4.82%) | 0.310 |
| Disturbance of consciousness | 175 (24.75%) | 122 (34.56%) | 0.001 |
| Poisoning | 29 (4.10%) | 13 (3.68%) | 0.867 |
| Epilepsy | 20 (2.83%) | 13 (3.68%) | 0.457 |
| Surgery | 221 (31.26%) | 41 (11.61%) | <0.001 |
| Drug type | |||
| Corticosteroids | 89 (12.59%) | 48 (7.92%) | 0.698 |
| Carbamazepine | 11 (1.56%) | 5 (1.42%) | 0.989 |
| Valproic acid | 13 (1.84%) | 6 (1.70%) | 0.939 |
| Score system | |||
| APACHE-II | 15 ± 8 | 21 ± 8 | <0.001 |
| GCS | 12 ± 4 | 10 ± 3 | <0.001 |
| SOFA | 7 ± 4 | 9 ± 4 | <0.001 |
| Length of ICU stay, days | 3.26 ± 2.33 | 4.25 ± 3.14 | 0.008 |
Data were expressed as mean ± SD and n (%). P < 0.05 was considered statistically significant.
H-, M-, and I- separately means the highest level, the mean level, the initial level; ALB: albumin; APACHE-II: Acute Physiologic Assessment and Chronic Health Evaluation II; BMI: body mass index; Crea: creatinine; DBP: diastolic blood pressure; GCS: Glasgow Coma Scale; HCT: hematocrit; HR: heart rate; ICU: intensive care unit; M: male; MBP: mean blood pressure; NHH: non-hepatic hyperammonemia; RR: respiratory rate; SBP: systolic blood pressure; SD: standard deviation; TBil: total bilirubin; SOFA: Sequential Organ Failure Assessment.
Blood ammonia levels predict patient prognosis
In univariate competing risk models shown in Table 2, serum levels of lactic acid, TBil, APACHE-II, GCS, SOFA, and serum ammonia were associated with the poor prognosis of the patients in ICUs (P < 0.05). The multivariate logistic regression analysis model found that lactic acid (odds ratio [OR] = 1.83, 95% confidence interval [CI]: 1.08–3.09, P = 0.02), TBil (OR = 2.01, 95% CI: 1.11–3.63, P = 0.02), APACHE-II (OR = 3.08, 95% CI: 1.63–5.80, P < 0.001), and SOFA (OR = 2.01, 95% CI: 1.93–4.59, P = 0.02) scores are independent factors associated with poor prognosis. M-ammonia and other serum ammonia factors were inputted into a multivariate logistic regression analysis to avoid multicollinearity. The results revealed that M-ammonia level in ICU patients was independently associated with a poor prognosis (OR = 1.73, 95% CI: 1.07–2.80, P = 0.02).
Table 2.
Univariate and multivariate logistic analyses of risk factors associated with a poor prognosis
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age, years | 1.56 | 0.89–2.73 | 0.13 | - | - | - |
| Male | 0.69 | 0.42–1.11 | 0.12 | - | - | - |
| MBP, mmHg | 0.60 | 0.31–1.14 | 0.13 | - | - | - |
| Laboratory parameters | ||||||
| PH | 0.98 | 0.70–1.36 | 0.89 | - | - | - |
| Na+, mmol/L | 0.75 | 0.48–1.18 | 0.21 | - | - | - |
| K+, mmol/L | 0.64 | 0.37–1.10 | 0.11 | - | - | - |
| Lactic acid, mmol/L | 1.84 | 1.13–3.00 | 0.01 | 1.83 | 1.08–3.09 | 0.02 |
| Serum glucose, mmol/L | 1.24 | 0.76–2.02 | 0.40 | - | - | - |
| Leukocyte, ×109/L | 0.83 | 0.49–1.39 | 0.48 | - | - | - |
| Platelets, ×109/L | 0.49 | 0.26–0.91 | 0.15 | - | - | - |
| HCT, % | 1.36 | 0.84–2.20 | 0.21 | - | - | - |
| TBil, mg/dL | 1.79 | 1.05–3.07 | 0.04 | 2.01 | 1.11–3.63 | 0.02 |
| Crea, μmol/L | 1.39 | 0.83–2.34 | 0.21 | - | - | - |
| Urea, mmol/L | 1.36 | 0.84–2.19 | 0.21 | - | - | - |
| ALB, g/L | 0.98 | 0.56–1.70 | 0.93 | - | - | - |
| I-Ammonia, μmol/L | 1.80 | 1.16–2.80 | 0.01 | - | - | - |
| H-Ammonia, μmol/L | 1.84 | 1.22–2.79 | <0.001 | - | - | - |
| M-Ammonia, μmol/L | 1.99 | 1.24–3.18 | <0.001 | 1.73 | 1.07–2.80 | 0.02 |
| Score system | ||||||
| APACHE-II | 4.22 | 2.50–7.12 | <0.001 | 3.08 | 1.63–5.80 | <0.001 |
| GCS | 0.31 | 0.18–0.52 | <0.001 | 0.52 | 0.28–1.00 | 0.05 |
| SOFA | 2.47 | 1.72–5.52 | 0.01 | 2.01 | 1.93–4.59 | 0.02 |
Data were expressed as OR (95% CI). P < 0.05 was considered statistically significant. H-, M-, and I- separately means the highest level, the mean level, and the initial level; ALB: albumin; APACHE-II: Acute Physiologic Assessment and Chronic Health Evaluation II; CI: confidence interval; Crea: creatinine; GCS: Glasgow Coma Scale; NHH: non-hepatic hyperammonemia; HCT: hematocrit; MBP: mean blood pressure; OR: odds ratio; SOFA: Sequential Organ Failure Assessment; TBil: total bilirubin.
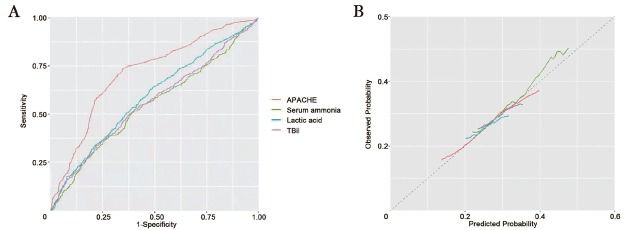
Furthermore, the ROC curve analysis for the prognostic power of APACHE-II score, ammonia, lactic acid, and TBil level revealed that the value of AUC was 0.714 (P < 0.001), 0.548 (P = 0.004), 0.589 (P = 0.002), and 0.560 ( P = 0.108). Lactic acid showed the highest sensitivity (0.86) and specificity (0.68), followed by APACHE-II (sensitivity: 0.71, specificity: 0.65) and ammonia (sensitivity: 0.67, specificity: 0.56). The cut-off point of the APACHE-II score was 15 and of ammonia was 36.95 μmol/L for the prediction of poor prognosis, respectively (Figure 2 and Table 3).
Figure 2.
ROC predicting the risk of poor prognosis in critically ill patients without liver disease admitted in ICU. ICU: intensive care unit; ROC: receiver operating characteristic; APACHE: Acute Physiologic Assessment and Chronic Health Evaluation; TBil: total bilirubin.
Table 3.
ROC for the factors to predict prognosis
| AUC | Sensitivity | Specificity | Cut-off value | P-value | |
|---|---|---|---|---|---|
| APACHE-II | 0.714 | 0.71 | 0.65 | 15 | <0.001 |
| Ammonia, μmol/L | 0.548 | 0.67 | 0.56 | 36.95 | 0.004 |
| Lactic acid, mmol/L | 0.589 | 0.86 | 0.68 | 1.35 | 0.002 |
| TBil, mg/dL | 0.560 | 0.37 | 0.23 | 18.3 | 0.108 |
APACHE-II: Acute Physiologic Assessment and Chronic Health Evaluation II; AUC: area under the curve; ROC: receiver operating characteristic; TBil: total bilirubin.
Discussion
This prospective, multicenter study reveals the prognostic biomarkers of NHH for critically ill patients who were hospitalized at ICUs, in which 1060 patients from 11 ICUs were recruited. Our findings indicated that patients with poor prognosis had higher levels of M-ammonia, and serum lactic acid, ammonia, TBil levels and APACHE-II scores on admission to ICU were independent predictors of prognosis. Moreover, SOFA score was a common risk factor for ICU patients with poor prognosis.
Increased blood lactic acid and ammonia levels are byproducts of ATP production and are associated with higher levels of anaerobic metabolic activity.[24] Critically ill patients are at a higher risk of hyperammonemia because of increased ammonia generation and decreased blood ammonia clearance. Infection by bacteria which rely on urease to hydrolyze urea to carbon dioxide and ammonia, as well as pneumonia, fever, and other stressors may ultimately result in NHH.[25] Further, gastrointestinal bleeding or steroid hormone-induced damages to the gastrointestinal tract can lead to increased protein breakdown and promote ammonia generation.[26] Moreover, several factors may hinder blood ammonia clearance: (1) acute/ chronic liver failure caused by drugs or toxins, infections, and autoimmune diseases, leading to diminished ammonia clearance by the liver; (2) salicylic acid, valproic acid, and other drugs may diminish the mitochondrial function of liver cells and inhibit the activity of rate-limiting enzymes in the urea cycle, causing urea cycle disorders and subsequently increased blood ammonia level; and (3) uremia caused by a decreased renal blood flow, glomerular filtration rate, and renal handling of urea in acute kidney injury may result in an increased blood ammonia level.[27] Therefore, a higher blood ammonia level can indicate both loss of hepatic ammonia clearance and a state of disordered energy metabolism.
Changes in energy metabolism are prevalent in critically ill patients admitted to ICU and can lead to a significant increase in blood ammonia levels.[28] However, to date, only a limited number of studies have reported the incidence of NHH. Nonetheless, a prospective study conducted by Prado et al. indicated that the incidence of NHH in patients with severe diseases was as high as 73% and blood ammonia levels were significantly correlated with the rate of mortality. Our previous research confirmed that an increased serum ammonia level is not only commonly observed in ICU patients, but also correlated to the disease severity and patient prognosis.[29] However, it is easy to overlook NHH as hyperammonemia is not related to liver disease in these patients. To date, no multicenter, prospective study on NHH patients admitted to ICU has been conducted. Thus, the current research was carried out to eliminate the mentioned clinical gap.
Patients with congestive heart failure often have high blood ammonia levels associated with reduced cardiac function. Recently, Bing et al. hypothesized that treating elevated circulating ammonia levels may prevent and reverse heart failure.[16] Elevated cardiac loads change the metabolic balance and lead to an ammonia surplus, eventually causing myocardial cell apoptosis. A therapeutic strategy targeting hyperammonemia can prevent adverse cardiac events in early stages and reverse heart failure in later stages.[13]
It is noteworthy that ammonia is involved in the functional metabolism in multiple organs such as liver, brain, kidney, muscles, and gastrointestinal tract. Therefore, hyperammonemia can indicate multiple organ dysfunction. It may also be involved in in the pathogenesis of septic shock via inflammatory responses and impaired tissue perfusion.[30,31] In the present research, we examined whether there could be differences in the different prognoses between distinct types of diseases (e.g., sepsis, kidney failure), and no significant differences were identified, consistent with previously reported results on the severity of hyperammonemia.[17] This may be attributed to the diverse and complicated etiology of NHH, which indicates the prognosis. Overall energy expenditure and severity of disease are the key factors contributing to the occurrence of NHH.
Our findings demonstrated that patients with poor prognoses had higher levels of average serum ammonia as well as a higher incidence of severe diseases (a higher APACHE-II score) and organ dysfunction (an increased SOFA score), lower level of consciousness, longer period of hospitalization, and higher costs associated with the length of stay at ICU, compared to cases with a favorable prognosis. Our previous single-center study revealed that severe NHH patients (serum ammonia level ≥100 μmol/L) required specialized treatments.[29] As for the prognosis of patients, the logistic regression and ROC curve analyses demonstrated the competitive power of disease severity (the APACHE-II score) or serum ammonia in predicting a poor prognosis of severe NHH patients. Due to the extremely fast clearance of blood ammonia in the body, about 7.7 h, an occasional increase in the blood ammonia level can only reflect a transient high metabolic state in the body.[32,33] M-ammonia is defined as continuously increased blood ammonia level and is accompanied by a high mortality rate, demonstrating that solicitous and continuous monitoring of serum ammonia level is valuable in the prediction of the prognosis of ICU patients.
The serum ammonia level has widely been used as a biomarker for the diagnosis and treatment of liver failure and for the identification of hepatic associated with hyperammonemia.[34] Treating high serum ammonia levels effectively restores consciousness, attenuates the incidence of cerebral edema, and improves overall morbidity and mortality. However, the clinical significance of NHH as a prognostic indicator has long been neglected. The present study reveals that elevated serum ammonia level is an indicator of disease severity and prognosis in patients without liver diseases. At present, the majority of commonly used ammonia-lowering drugs are targeted at hyperglycemia-related hyperammonemia and hepatic encephalopathy caused by liver diseases.[35, 36, 37] Thus, further research is warranted to indicate whether they improve outcomes in NHH patients.
Several limitations in the present study must be acknowledged. First, as our study enrolled heterogenous patients with normal or hyperammonemia, the causal relationship between NHH and poor outcome cannot be fully determined based on the present study. As we recruited patients from more than 10 centers, inter-center consistency needs to be considered. Expectedly, although NHH is a common metabolic disorder in daily ICU practice, its incidence varies among different medical centers. This may be explained by the differences in the number of participants, detection instruments and protocols, and prevalence of underlying diseases. Furthermore, since hyperammonemia was frequent in patients who died in the hospital, it is possible that early death (before ammonia measurement) could have led to further underreporting.
High blood ammonia level is frequent among patients who have been admitted to ICU and is an independent predictor of the patients’ clinical prognosis. Evaluating the levels of M-ammonia in the blood may improve prediction of disease prognosis in ICU patients.
Acknowledgment
The authors thank all the ICU staffs for their commitment and significant effort.
Footnotes
Source of Funding
This study was financially supported by the Scientific Research Project of Heilongjiang Health and Family Planning Commission (No. 2019045).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Ethics Approval
The study protocol was approved by the ethics committee of each allied center (approval number KY2019-184), and signed informed consent forms were collected from all study subjects or their families.
Data Availability Statement
Data sharing is not applicable to this article as no additional datasets were generated or analyzed during the current study.
Author Contributions
Li Yue, Yao Z, and Wang H designed the research; Li Yue, Yao Z and Wang H performed the research; Yao Z analyzed the data; Li Yue wrote the paper; Li Yunlong, Yang Z, Li M, Chen Z, Liu S, Gong J, Huang L, Xu P, Li Yan, Li H, Liu X, Zhang L, and Zhang G collected data. All authors have read and approved the final manuscript.
References
- 1.Dabrowska K, Skowronska K, Popek M, Obara-Michlewska M, Albrecht J, Zielinska M. Roles of Glutamate and Glutamine Transport in Ammonia Neurotoxicity: State of the Art and Question Marks. Endocr Metab Immune Disord Drug Targets. 2018;18:306–15. doi: 10.2174/1871520618666171219124427. [DOI] [PubMed] [Google Scholar]
- 2.Monfort P, Cauli O, Montoliu C, Rodrigo R, Llansola M, Piedrafita B. et al. Mechanisms of cognitive alterations in hyperammonemia and hepatic encephalopathy: therapeutical implications. Neurochem Int. 2009;55:106–12. doi: 10.1016/j.neuint.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Prado FA, Delfino VD, Grion CM, de Oliveira JA. Hyperammonemia in ICU patients: a frequent finding associated with high mortality. J Hepatol. 2015;62:1216–8. doi: 10.1016/j.jhep.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Walker V. Severe hyperammonaemia in adults not explained by liver disease. Ann Clin Biochem. 2012;49:214–28. doi: 10.1258/acb.2011.011206. [DOI] [PubMed] [Google Scholar]
- 5.Sakusic A, Sabov M, McCambridge AJ, Rabinstein AA, Singh TD, Mukesh K. et al. Features of Adult Hyperammonemia Not Due to Liver Failure in the ICU. Crit Care Med. 2018;46:e897–e903. doi: 10.1097/CCM.0000000000003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asrani SK, Simonetto DA, Kamath PS. Acute-on-Chronic Liver Failure. Clin Gastroenterol Hepatol. 2015;13:2128–39. doi: 10.1016/j.cgh.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Zhou Q, Song JN, Chen XZ, Zhang XZ, Sun Y. Analysis of clinical prognosis in patients with non-hepatic hyperammonemia. Medicine (Baltimore) 2021;100:e24157. doi: 10.1097/MD.0000000000024157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson AK, Adermark L, Persson M, Westerlund A, Olsson T, Hansson E. Lactate contributes to ammonia-mediated astroglial dysfunction during hyperammonemia. Neurochem Res. 2009;34:556–65. doi: 10.1007/s11064-008-9819-1. [DOI] [PubMed] [Google Scholar]
- 9.Shin WK, Jang YE, Lee H, Min SH, Ryu HG. Sudden severe hyperammonemia and status epilepticus -a case report. Korean J Anesthesiol. 2013;65:262–5. doi: 10.4097/kjae.2013.65.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natesan V, Mani R, Arumugam R. Clinical aspects of urea cycle dysfunction and altered brain energy metabolism on modulation of glutamate receptors and transporters in acute and chronic hyperammonemia. Biomed Pharmacother. 2016;81:192–202. doi: 10.1016/j.biopha.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Fuster-Cabré M, Ezquerro-Sáenz S, Requena-Calleja M, Medrano-Peña J, Lapetra-Labé AM. Acute hyperammonemic encephalopathy due to a portosystemic shunt in a non-cirrhotic adult patient. J Crit Care. 2019;53:59–61. doi: 10.1016/j.jcrc.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Gropman A. Brain imaging in urea cycle disorders. Mol Genet Metab. 2010;1:S20–30. doi: 10.1016/j.ymgme.2010.01.017. 100 Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medeiros WM, Carvalho AC, Peres P, De Luca FA, Gun C. The dysfunction of ammonia in heart failure increases with an increase in the intensity of resistance exercise, even with the use of appropriate drug therapy. Eur J Prev Cardiol. 2014;21:135–44. doi: 10.1177/2047487312460520. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman JE, Wagner DP, Seneff MG, Becker RB, Sun X, Knaus WA. Intensive care unit admissions with cirrhosis: risk-stratifying patient groups and predicting individual survival. Hepatology. 1996;23:1393–401. doi: 10.1002/hep.510230615. [DOI] [PubMed] [Google Scholar]
- 15.Tchan M. Hyperammonemia and lactic acidosis in adults: Differential diagnoses with a focus on inborn errors of metabolism. Rev Endocr Metab Disord. 2018;19:69–79. doi: 10.1007/s11154-018-9444-5. [DOI] [PubMed] [Google Scholar]
- 16.Bing OHL. Hypothesis: role for ammonia neutralization in the prevention and reversal of heart failure. Am J Physiol Heart Circ Physiol. 2018;314:H1049–h52. doi: 10.1152/ajpheart.00003.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao L, Walline JH, Gao Y, Lu X, Yu S, Ge Z. et al. Prognostic Role of Ammonia in Critical Care Patients Without Known Hepatic Disease. Front Med (Lausanne) 2020;7:589825. doi: 10.3389/fmed.2020.589825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 19.Alfadhel M, Mutairi FA, Makhseed N, Jasmi FA, Al-Thihli K, Al-Jishi E. et al. Guidelines for acute management of hyperammonemia in the Middle East region. Ther Clin Risk Manag. 2016;12:479–87. doi: 10.2147/TCRM.S93144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernal W, Hall C, Karvellas CJ, Auzinger G, Sizer E, Wendon J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46:1844–52. doi: 10.1002/hep.21838. [DOI] [PubMed] [Google Scholar]
- 21.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H. et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 22.Reith FC, Van den Brande R, Synnot A, Gruen R, Maas AI. The reliability of the Glasgow Coma Scale: a systematic review. Intensive Care Med. 2016;42:3–15. doi: 10.1007/s00134-015-4124-3. [DOI] [PubMed] [Google Scholar]
- 23.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 24.Clay AS, Hainline BE. Hyperammonemia in the ICU. Chest. 2007;132:1368–78. doi: 10.1378/chest.06-2940. [DOI] [PubMed] [Google Scholar]
- 25.Wijdicks EF. Hepatic Encephalopathy. N Engl J Med. 2016;375:1660–70. doi: 10.1056/NEJMra1600561. [DOI] [PubMed] [Google Scholar]
- 26.Olde Damink SW, Dejong CH, Jalan R. Review article: hyperammonaemic and catabolic consequences of upper gastrointestinal bleeding in cirrhosis. Aliment Pharmacol Ther. 2009;29:801–10. doi: 10.1111/j.1365-2036.2009.03938.x. [DOI] [PubMed] [Google Scholar]
- 27.Auron A, Brophy PD. Hyperammonemia in review: pathophysiology, diagnosis, and treatment. Pediatr Nephrol. 2012;27:207–22. doi: 10.1007/s00467-011-1838-5. [DOI] [PubMed] [Google Scholar]
- 28.Häberle J. Clinical practice: the management of hyperammonemia. Eur J Pediatr. 2011;170:21–34. doi: 10.1007/s00431-010-1369-2. [DOI] [PubMed] [Google Scholar]
- 29.Yao ZP, Li Y, Liu Y, Wang HL. Relationship between the incidence of non-hepatic hyperammonemia and the prognosis of patients in the intensive care unit. World J Gastroenterol. 2020;26:7222–31. doi: 10.3748/wjg.v26.i45.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woźnica EA, Inglot M, Woźnica RK, Łysenko L. Liver dysfunction in sepsis. Adv Clin Exp Med. 2018;27:547–51. doi: 10.17219/acem/68363. [DOI] [PubMed] [Google Scholar]
- 31.Yohei K, Takehiro O, Ryusuke D. Hyperammonemic encephalopathy with septic shock caused by obstructive urinary tract infection. IDCases. 2018;14:e00436. doi: 10.1016/j.idcr.2018.e00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naorungroj T, Yanase F, Eastwood GM, Baldwin I, Bellomo R. Extracorporeal Ammonia Clearance for Hyperammonemia in Critically Ill Patients: A Scoping Review. Blood Purif. 2021;50:453–61. doi: 10.1159/000512100. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, Eid T, Hassel B, Danbolt NC. Novel aspects of glutamine synthetase in ammonia homeostasis. Neurochem Int. 2020;140:104809. doi: 10.1016/j.neuint.2020.104809. [DOI] [PubMed] [Google Scholar]
- 34.Hu C, Huang K, Zhao L, Zhang F, Wu Z, Li L. Serum ammonia is a strong prognostic factor for patients with acute-on-chronic liver failure. Sci Rep. 2020;10:16970. doi: 10.1038/s41598-020-73603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masini A, Efrati C, Merli M, Nicolao F, Amodio P, Del Piccolo F. et al. Effect of blood ammonia elevation following oral glutamine load on the psychometric performance of cirrhotic patients. Metab Brain Dis. 2003;18:27–35. doi: 10.1023/a:1021926601907. [DOI] [PubMed] [Google Scholar]
- 36.Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437–47. doi: 10.1016/j.jhep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Ridola L, Faccioli J, Nardelli S, Gioia S, Riggio O. Hepatic Encephalopathy: Diagnosis and Management. J Transl Int Med. 2020;8:210–9. doi: 10.2478/jtim-2020-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]