Abstract
Immediately downstream from the Thermoanaerobacter ethanolicus xylAB operon, comprising genes that encode d-xylose isomerase and d-xylulose kinase, lies a 1,101-bp open reading frame that exhibits 61% amino acid sequence identity to the Escherichia coli d-xylose binding periplasmic receptor, XylF, a component of the high-affinity binding-protein-dependent d-xylose transport. The 25-residue N-terminal fragment of the deduced T. ethanolicus XylF has typical features of bacterial leader peptides. The C-terminal portion of this leader sequence matches the cleavage consensus for lipoproteins and is followed by a 22-residue putative linker sequence rich in serine, threonine, and asparagine. The putative mature 341-amino-acid-residue XylF (calculated molecular mass of 37,069 Da) appears to be a lipoprotein attached to the cell membrane via a lipid anchor covalently linked to the N-terminal cysteine, as demonstrated by metabolic labelling of the recombinant XylF with [14C]palmitate. The induced E. coli avidly bound d-[14C]xylose, yielding additional evidence that T. ethanolicus XylF is the d-xylose-binding protein. On the basis of sequence comparison of XylFs to other monosaccharide-binding proteins, we propose that the sequence signature of binding proteins specific for hexoses and pentoses be refined as (KDQ)(LIVFAG)3IX3(DN)(SGP)X3(GS)X(LIVA)2X2A. Transcription of the monocistronic 1.3-kb xylF mRNA is inducible by xylose and unaffected by glucose. Primer extension analysis indicated that xylF transcription initiates from two +1 sites, both situated within the xylAB operon. Unlike in similar transport systems in other bacteria, the genes specifying the membrane components (e.g., ATP-binding protein and permease) of the high-affinity d-xylose uptake system are not located in the vicinity of xylF in T. ethanolicus. This is the first report of a gene encoding a xylose-binding protein in a gram-positive or thermophilic bacterium.
d-Xylose is nature’s second-most-abundant carbohydrate. The initial steps of d-xylose metabolism in bacteria typically involve transport, isomerization to d-xylulose, and phosphorylation to d-xylulose-5-phosphate. The second and third steps of this pathway are catalyzed by d-xylose (d-glucose) isomerase (XylA) and xylulose kinase (XylB), respectively (4), while xylose uptake is mediated via at least two distinct transport systems: low-affinity ion-linked symport and the binding protein-dependent transport system, also referred to as ABC-type transport (ATP-binding cassette). Low-affinity xylose transport requires an electromotive gradient and is mediated by a single membrane protein, XylE, as established by genetic and molecular evidence in Escherichia coli (6, 24) and Bacillus megaterium (42). ABC-type transport of xylose, shown to function so far only in E. coli and Salmonella typhimurium (1, 43, 48), involves a periplasmic protein, XylF, which binds xylose with high affinity. Two additional components are postulated to participate in binding protein-dependent xylose uptake on the basis of DNA sequence homology with other ABC transporters of monosaccharides, i.e., a membrane-spanning permease, XylH, which receives the sugar from XylF and translocates it to the cytoplasm and an ATPase, XylG, that energizes the substrate translocation process (46).
In thermophilic anaerobic bacteria, xylose utilization has been studied mostly at the biochemical level. In the past few years, xylA gene products from several thermoanaerobes have been cloned and characterized for possible application in the production of high-fructose corn syrup (7, 25, 27, 31). However, virtually nothing is known about molecular aspects of xylose transport in these interesting organisms. We recently reported that xylA and xylB in Thermoanaerobacter ethanolicus, a gram-positive xylanolytic thermophilic anaerobe, constitute a xylose-inducible, bicistronic operon (8). In this study, we show that the T. ethanolicus xylF, encoding d-xylose-binding protein, lies downstream from the xylAB operon and that its expression is also transcriptionally regulated. In addition, we provide evidence that the T. ethanolicus XylF is a lipoprotein that has sequence similarities with other monosaccharide-binding proteins and several transcriptional regulators of carbon metabolism.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
T. ethanolicus 39E (ATCC 33223) was grown as previously described (9). Plasmids pUC18 (Life Technologies, Gaithersburg, Md.) and pBluescript II KS (Stratagene, La Jolla, Calif.) were used as cloning vectors in Escherichia coli DH5α (Life Technologies, Gaithersburg, Md.). The E. coli strains were grown in Luria-Bertani medium (39) supplemented with ampicillin (100 μg/ml) or carbenicillin (50 μg/ml) as needed.
Construction and screening of genomic library.
A T. ethanolicus genomic library was prepared as previously described (9). Probes for chromosome walking were prepared by random hexamer labelling of the appropriate restriction fragments with [α-32P]dATP (39). Colony hybridization and washes, as well as recombinant DNA manipulations, were carried out by standard techniques (39).
DNA sequencing and analysis.
Dideoxy-termination DNA sequencing was performed by following the Sequenase 2.0 kit (Amersham) protocol with double-stranded plasmid templates isolated by a boiling minipreparation procedure (39). Oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, Iowa). Sequence analyses were performed by using the Lasergene biocomputing software package (DNASTAR, Madison, Wis.). BLAST search engines were employed for sequence homology searches (2).
PCR.
Amplifications were performed as described previously (9), with the following modifications: pF-28 DNA (5 ng per reaction) was used as a template, the total number of cycles was 30, and the annealing temperature for the xylF-specific PCR product was 59°C. The oligonucleotides used for PCR of the xylF fragment were 5′AAT CTA GAT TCA AAC AAA ATT TTA GGA AGG AG and 5′ AAG AAT TCA CTA TTA GCC GAT TGA ACT AAC.
RNA isolation and analyses.
Cells were harvested during the logarithmic growth phase, and washed once in 50 mM Tris-Cl (pH 7.5), and RNA was isolated with RNeasy Total RNA miniprep kits (Qiagen, Chatsworth, Calif.). For Northern analysis, aliquots of total RNA (5 μg) were fractionated in a 1% agarose–formaldehyde gel, blotted, and hybridized to the [α-32P]dATP-labelled xylF DNA internal fragment according to standard protocols (39). The xylF probe was a PCR-amplified 323-bp fragment from xylF. Transcript sizes were determined by using the 0.24- to 9.5-kb RNA ladder (Life Technologies).
A ribonuclease protection assay was carried out by using the RPA II kit (Ambion, Austin, Tex.) according to the manufacturer’s protocol, except that RNase digestion was done at 25°C for 45 min in order to prevent excessive cleavage of breathable duplexes. The protected RNA hybrids were electrophoresed in a 5% polyacrylamide–7 M urea gel, which was dried and exposed to film with an intensifying screen. To create the size ladder, pBluescript II KS DNA was digested with Sau3A, followed by the fill-in reaction of the resulting fragments with [α-32P]dATP and Klenow fragment by following a standard protocol (39). The DNA template for the antisense riboprobe was constructed by subcloning the 3′-terminal SpeI-EcoRI fragment from pXI-10 (Fig. 1 in reference 8; the EcoRI site was from the vector) into pBluescript II KS. The SpeI-linearized template was transcribed in vitro with T3 RNA polymerase in the presence of [α-32P]CTP (39), yielding a 608-nucleotide antisense RNA.
Primer extension analysis was performed as previously described (8) by using the oligonucleotide 5′ ATG CAT ATG GAT TGT TTG ATT TTG GTC CTT C, which is complementary to the sequence beginning 57 nucleotides downstream from the xylF translation initiation site. For dot blot analysis, 1.5-μl aliquots of various dilutions of total RNA isolated from T. ethanolicus 39E cells grown in the presence of different carbon sources were spotted onto a membrane which was hybridized to the same radiolabelled xylF-specific probe used for Northern analysis. A standard hybridization procedure was used (39).
Cloning and expression of the recombinant XylF.
The xylF open reading frame (ORF) was PCR amplified by using oligonucleotides 5′ AGG AGA ATA GAC CAT GGT TAA AAA AGT CTC and 5′ GGC AAC AGT GGA TCC AGT TTT TCA AGA G, containing NcoI and BamHI sites (shown in boldface), respectively. The pET15b expression vector (Novagen, Madison, Wis.) was cut with NcoI and BamHI and dephosphorylated with calf intestine alkaline phosphatase (New England Biolabs, Beverly, Mass.) according to the manufacturer’s instructions. Both the plasmid and the NcoI-BamHI-cleaved xylF ORF PCR product were then purified with GeneClean (Bio101, Vista, Calif.), and the appropriate amounts were ligated with T4 DNA ligase. An aliquot of the ligation mix was electroporated into E. coli JM109 (Promega, Madison, Wis.) and the recombinant transformants were selected after restriction analysis of plasmid minipreparations. A positive clone, pXFe, was then transformed into the expression host, E. coli HMS174(DE3), by the CaCl2 procedure (39). E. coli cells containing pXFe were grown in Luria-Bertani medium supplemented with 50 μg of carbenicillin per ml. Expression of the recombinant protein was induced by adding isopropyl-β-thiogalactoside (IPTG [1 mM]) to liquid cultures that reached an optical density at 600 nm (OD600) of approximately 0.6.
Localization of the recombinant XylF and binding assay.
Periplasmic fractions were isolated from cultures (16 ml) by a cold osmotic shock procedure, as previously described (3). Inner (cytoplasmic) membranes were fractionated from outer membranes by a sucrose gradient procedure (32).
d-Xylose binding activity was assayed by a modified filter binding method as described by Richarme and Kepes (36). Assays (100 μl) contained 50 mM Tris-HCl (pH 7.5), 6.9 μM d-[U-14C]xylose (250 mCi/mmol), and the various isolated fractions. After incubation at 70°C for 30 s, the mixture was placed in an ice bath, and 2 ml of ice-cold 50 mM Tris (pH 7.5) saturated with ammonium sulfate was immediately added. After 10 min, the mixture was filtered through 0.45-μm-pore-size nitrocellulose filters which had been soaked in 50 mM Tris (pH 7.5). The filtered material was washed with an additional 2 ml of the Tris-ammonium sulfate solution. Filters were dried for 20 min at 110°C, and radioactivity was determined after the addition of scintillation fluid. Preliminary experiments demonstrated that less than 0.5% of the radiolabelled xylose bound to filters in the absence of cellular protein.
Metabolic radiolabelling of the recombinant XylF.
Two cultures of E. coli cells (10 ml each) carrying pXFe were grown until the OD600 reached 0.5, when [14C]palmitic acid (specific activity, 52 mCi/mmol) was added to a final concentration of 0.75 μCi/ml. Cell growth was continued until an OD600 of 0.6 was reached, when IPTG was added at 1 mM to one of the cultures. The cells were grown for an additional 90 min and then harvested by centrifugation (10,000 × g, 5 min). Cell pellets were washed twice in 1 ml of 50 mM Tris (pH 7.5), and final pellets were resuspended in 100 μl of sodium dodecyl sulfate (SDS) extraction buffer (50 mM Tris [pH 7.5], containing 2% SDS, and 0.1% 2-mercaptoethanol). Cell extracts were then incubated at 70°C for 15 min, and cell debris was removed by centrifugation. Supernatant aliquots (5 μl) were run in an SDS–10% polyacrylamide gel, which was stained with Coomassie blue, destained, dried, and exposed to film for 4 weeks at room temperature.
Nucleotide sequence accession number.
The complete xylF DNA sequence is available in GenBank under accession no. AF043466.
RESULTS
Cloning and nucleotide sequence analysis.
The ORF analysis of the T. ethanolicus genomic clone pXI-10 containing the xylAB operon, characterized in a previous study (8), revealed an N-terminal portion (93 amino acid residues) of a putative polypeptide 3′ to the xylB ORF. A homology search for its sequence was performed with combined protein sequence databases and yielded the periplasmic d-xylose-binding protein precursor, XylF, from E. coli (48) as having the sequence with the highest similarity score (data not shown). Therefore, the 3′-terminal SpeI-EcoRI fragment from pXI-10 was used to screen the T. ethanolicus 39E genomic library for overlapping clones homologous to xylF. Two positive clones were identified, one of them carrying the complete ORF.
The putative 1,101-bp xylF ORF begins 89 bp downstream of the xylB termination codon with an ATG, preceded by a possible ribosome-binding sequence complementary to the 3′ end of the T. ethanolicus 39E 16S rRNA (35). The coding region is flanked at its 3′ terminus by the TGA triplet, specifying a translation termination codon (data not shown). A palindromic sequence was found downstream of the ORF 3′ end that may serve as a transcription termination signal (data not shown). The G + C content of this ORF was 34.9 mol%, which matches the value of 35 mol%, experimentally determined for T. ethanolicus 39E total DNA (28). The deduced peptide contains 366 amino acid residues, giving a calculated molecular mass of 39,809 Da. The database homology search against the complete ORF confirmed that its sequence was homologous to the E. coli XylF (48) and a putative XylF from Haemophilus influenzae (11), with 61 and 60% amino acid sequence identity, respectively. The next consecutive ORF was located 426 bp downstream of the xylF ORF, and it had the opposite polarity. However, given the lack of sequence homology to any known proteins, no function could be assigned to this hypothetical peptide (data not shown). Three more ORFs were identified in the contiguous sequence of pF-28, all showing high similarities with genes encoding enzymes of the histidine biosynthesis pathway (10).
Amino acid sequence analysis.
The 25-residue amino-terminal fragment of the T. ethanolicus XylF has three distinct domains: i.e., a positively charged N-terminal region, followed by a highly hydrophobic central region which ends with a stretch of short side chain neutral amino acids (data not shown). Such a sequence structure is typical of signal peptides in bacterial lipoproteins which are cleaved off at the cysteine residue (52), thus rendering Cys-26 the N terminus of the mature T. ethanolicus XylF protein, with a calculated molecular mass of 37,069 Da. The sequence LSGC, corresponding to residues 23 to 26 in T. ethanolicus XylF (data not shown), perfectly matches the four-residue sequence consensus L(S,A)(A,G)↓C defined for lipoprotein leader sequences (49, 52). Taken together, these observations strongly suggested that the processed, mature form of T. ethanolicus XylF is a lipoprotein. The putative signal peptide is followed by a 22-residue stretch, rich in polar amino acids, e.g., serine, threonine, and asparagine (7, 5, and 4 residues, respectively [Fig. 1]).
FIG. 1.
Amino acid sequence alignment of monosaccharide-binding proteins. Sequences of the mature monosaccharide-binding proteins (i.e., without their signal peptides) were aligned by the CLUSTAL method with the PAM250 residue weight table (18). Residues conserved in two or more species are boxed and shaded. Solid arrowheads indicate residues invariant in XylFs that are identical to the residues participating in protein-sugar interactions in AraF and MglB (Table 2 in reference 53). Open arrowheads denote planar polar residues invariant in XylFs, which align with isofunctional residues implicated in H-bond formation in AraF and MglB. Open circles indicate internal cysteine residues in the mature T. ethanolicus 39E XylF. The sequence region containing the consensus signature for the “cluster 2” binding proteins (50) is bracketed. Te, T. ethanolicus; Ec, E. coli; Hi, H. influenzae; At, A. tumefaciens.
To compare the T. ethanolicus XylF (Fig. 1) sequence to those of other monosaccharide-binding proteins, their primary structures were analyzed by multiple sequence alignment. Saliently, the (S,T,N)-rich region following the amino-terminal cysteine of the processed T. ethanolicus XylF was absent in other proteins. Two more cysteine residues were found in the T. ethanolicus XylF at positions 76 and 253, unlike the mesophilic d-xylose-binding proteins which lack cysteine. The T. ethanolicus XylF sequence displayed somewhat higher homology (34% sequence identity, 52% similarity) to the multiple sugar-binding periplasmic receptor ChvE from Agrobacterium tumefaciens (44), than to the E. coli ABC-transport periplasmic receptors of l-arabinose (AraF [19]), d-glucose or d-galactose (MglB [20]), and d-ribose (RbsB [16]), with 27 to 30% sequence identity and 40 to 48% similarity.
Scrutiny of the aligned sequences revealed that 4 of 14 amino acid residues implicated in binding of sugar substrates by E. coli AraF, MglB, and RbsB (53) were fully conserved in XylFs and ChvE (Fig. 1). Two of them are aromatic residues (Trp-16 in AraF and Trp-183 in MglB), which form stacking interactions with sugars and correspond to Trp-42 and Trp-194 in T. ethanolicus XylF. The other two are planar polar residues creating hydrogen bonds with sugar substrates (Asp-89 in AraF, and Asp-236 in MglB), and they align with Asp-115 and Asp-247, respectively, in T. ethanolicus XylF. The other 10 residues implicated in sugar binding correspond to residues that are (i) invariant in XylFs but not found in ChvE (Glu-14 and Asn-205 in AraF, matching Glu-40 and Asn-221, respectively, in XylF-Te), (ii) replaced by isofunctional (i.e., planar polar or charged) residues conserved in both XylFs and ChvE (Asp-90 in AraF and Asp-154 in MglB corresponding to Arg-116 and Asn-162, respectively, in T. ethanolicus XylF) or in XylFs only (Lys-10 in AraF and His-152 in MglB aligning with Asp-35 and Asp-160, respectively, in T. ethanolicus XylF), and (iii) not conserved (Met-108, Arg-151, Met-204, and Pro-254 in AraF).
The sequence signature characteristic of monosaccharide-binding proteins (50) was also identified in d-xylose receptors (Fig. 1). Except for Glu-85 in T. ethanolicus XylF and Asn-68 in mesophilic XylFs, all other residues of this sequence region perfectly matched the previously identified consensus. Additional analysis revealed that this sequence is part of a larger domain that is shared by a diverse family of proteins (51), including transcriptional regulators such as FruR and LacI in E. coli and CcpA, involved in catabolite repression regulation in Bacillus subtilis. This domain corresponds to the region comprising residues 23 to 115 in T. ethanolicus XylF and 29 to 113 in mesophilic XylFs.
Characterization of transcription.
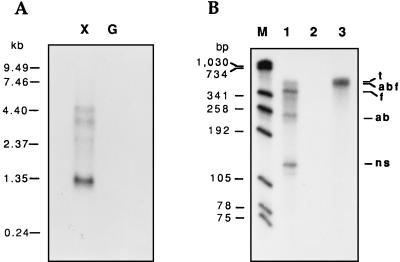
In order to perform Northern analysis, equal amounts of RNA from cells cultivated with xylose or glucose were hybridized against a 323-bp fragment from xylF. A prominent band, approximately 1.3 kb in size, was seen only in RNA from xylose-grown cells (Fig. 2A). Two other faint bands (approximately 5 and 4 kb long) were detected when xylose was the energy source. DNA sequence analysis (reference 8 and this study) showed that an inverted repeat (ΔG = −10.5 kcal/mol), followed by a poly(dT) stretch, is located in the xylB-xylF intercistronic spacer (Fig. 3B). Northern analysis suggested that this sequence is likely to function as a ρ-independent termination hairpin of xylAB transcription; therefore, the majority of xylF mRNA appeared to be monocistronic.
FIG. 2.
Analysis of xylF transcription. (A) Northern blotting. Total T. ethanolicus RNA was isolated from cells logarithmically growing with 0.4% xylose (lane X) or glucose (lane G). RNA samples (5 μg per lane) were fractionated in a 1% formaldehyde–agarose gel, blotted, and hybridized to the α-32P-labelled 323-bp PCR fragment from xylF. (B) Ribonuclease protection assay. Approximately 30 μg of T. ethanolicus RNA (lanes 1 and 3) and 10 μg of control (yeast) RNA (lane 2) were hybridized with 8 × 104 cpm of α-32P-labelled antisense 608-nucleotide transcript (t), followed by digestion with a mixture of RNases A and T1 (lanes 1 and 2). The protected fragments were denatured by heating, fractionated by 5% PAGE, and subjected to autoradiography. The detected fragments, their approximate sizes (in nucleotides), and the specific mRNAs protecting them were as follows: abf (520; read-through xylABF transcript), f (350; xylF transcript), ab (280; xylAB transcript), and ns, (140; fragment resulting from nonspecific RNase cleavage). M denotes molecular size standards.
FIG. 3.
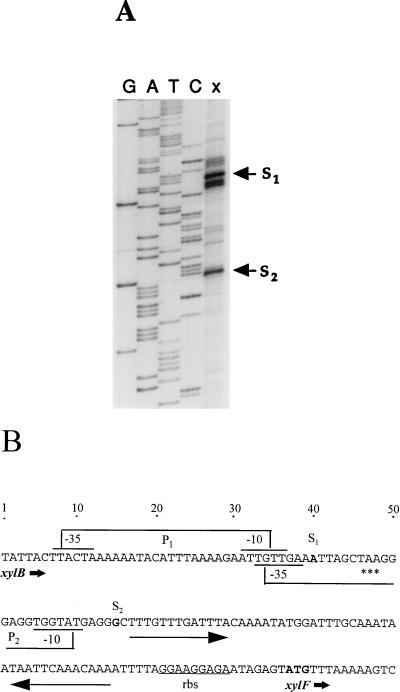
Identification of xylF transcript initiation and putative regulatory sequences. (A) Primer extension analysis. The end-labelled primer (5 × 105 cpm) was annealed to RNA (40 μg) from T. ethanolicus grown on 0.4% xylose and was extended with reverse transcriptase. An aliquot from the primer extension reaction (lane x) was electrophoresed on a denaturing 8% polyacrylamide gel along with the sequence ladder generated with the same oligonucleotide (lanes G, A, T, and C). The two cDNA products representing major transcript initiation sites (S1 and S2) are marked. (B) Regulatory elements of xylAB and xylF. A portion of the sequence including the xylB 3′ end, xylB-xylF intercistronic spacer, and xylF 5′ terminus is shown along with transcriptional and translational signals. P1 and P2 indicate the upstream and downstream xylF promoters, respectively. Long arrows depict a palindromic sequence possibly serving as the xylAB transcriptional terminator and the xylF operator (see Discussion). The potential transcription and translation start sites are shown in boldface. rbs, ribosome-binding sequence; asterisks, translation termination codon of xylB.
This observation was also supported by results of an RNase protection assay with total RNA from xylose-grown T. ethanolicus and a riboprobe overlapping the 3′ and 5′ termini of xylB and xylF, respectively. Two strong signals, corresponding in size to fragments expected from protection of xylAB and xylF mRNAs, were obtained (Fig. 2B). Also, a rather faint band, similar in length to the riboprobe, could be seen in the same lane on the original autoradiograph, which represented the protected fragment of the xylABF read-through transcript. The additional band, approximately 140 nucleotides long, that was obtained in the reaction was probably a nonspecific product of RNase cleavage, due to the AU-rich sequence. Since the RNase protection assay has higher sensitivity relative to that of Northern blotting, these observations provide evidence that the majority of xylF transcripts initiate from the xylF promoter, proximal to the xylF coding sequence.
To precisely map the 5′ terminus of the xylF transcript, primer extension analysis was performed by using an oligonucleotide complementary to the sequence region downstream of the xylF ORF 5′ end. Two major cDNA products, the longer being somewhat more prominent than the shorter (Fig. 3A), were identified in RNA samples from T. ethanolicus grown with xylose. These extension products signified two possible xylF transcription initiation sites situated within the xylAB operon (Fig. 3B). The multiple initiation sites of the longer transcript (where the major 5′ terminus is marked with S1) lie within the xylB coding region, while the downstream site, S2, is located 17 bp downstream of the xylB termination codon. Both S1 and S2 sites are preceded by properly spaced sequences (5′ TTACTA-17 bp-AATTGT 3′ and 5′ TGTTGA-16 bp-TGGTAT 3′ for S1 and S2, respectively) resembling the promoter consensus in gram-positive bacteria (15). Therefore, if one assumes that the shorter cDNA product was not the result of a premature termination of primer extension by the reverse transcriptase, it seems likely that the xylF transcription could initiate at either site. Alternatively, the transcript initiated at S2 may be a product of RNase processing, rather than of transcription initiation. The fact that the 5′ end of this transcript is adjacent to the palindromic sequence (Fig. 3B) supports this interpretation.
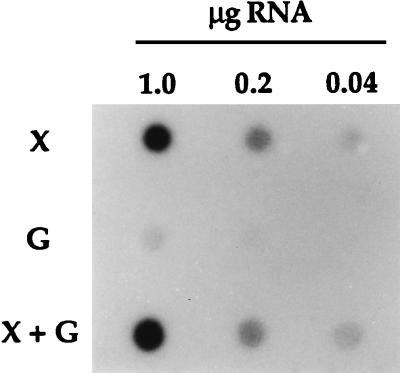
To ascertain the effect of sugar substrate on xylF expression, dot blot analysis was carried out with total RNA samples isolated from T. ethanolicus 39E grown with various carbon sources (Fig. 4). Similarly to the Northern blotting result, only a faint signal was observed in the absence of xylose. Interestingly, the presence of glucose did not have a repression effect on the xylose-induced xylF mRNA synthesis.
FIG. 4.
RNA dot blot analysis. T. ethanolicus 39E was cultivated with various sugar substrates, and total RNA was purified from logarithmically grown cells. Equal aliquots of serial RNA dilutions were applied to a membrane and hybridized to [α-32P]dATP-labelled 323-bp XylF-specific PCR product, which had also served as the probe in Northern analysis. X, 0.4% xylose; G, 0.4% glucose, X + G, xylose and glucose, each at 0.2%.
Expression of the recombinant XylF.
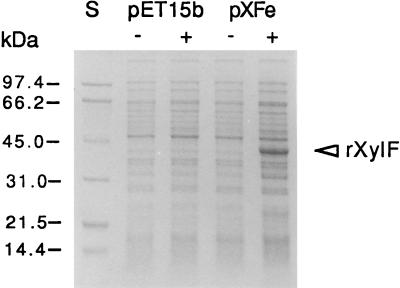
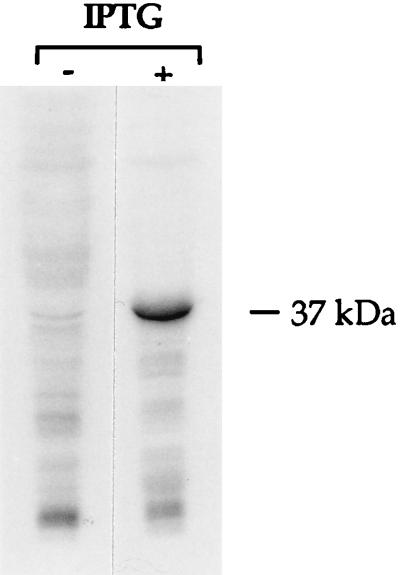
SDS-PAGE was carried out to analyze the expression of the T. ethanolicus XylF in E. coli (Fig. 5). A highly abundant protein band migrating at approximately 37 kDa was observed in cell lysates of induced cells, but not in uninduced cells or cells carrying only the vector. The isolation of the periplasmic fraction by cold osmotic shock showed that the recombinant enzyme was not present in the host periplasm (data not shown). To test the functionality of the recombinant XylF, a d-xylose binding assay was performed with crude extracts from host cells harvested 2 h after induction. The induced cells had 40-fold-higher d-xylose binding activity than the uninduced cells, e.g., 1.58 versus 0.04 nmol of d-[14C]xylose bound per mg of protein by induced versus uninduced cells. Similar specific activities of d-xylose binding were found in both inner and outer membranes (data not shown).
FIG. 5.
Expression of the recombinant XylF (rXylF). E. coli cells carrying the expression vector (pET15b) or the T. ethanolicus xylF construct (pXFe) were harvested 90 min postinduction with IPTG, and aliquots of cell lysates were fractionated by SDS-PAGE (approximately 10 μg of protein per lane). S, molecular mass protein standards; − and +, absence and presence, respectively, of IPTG in the culture.
Metabolic radiolabelling of the recombinant XylF.
The amino acid sequence analysis suggested that the native T. ethanolicus XylF is a lipoprotein. Biochemical evidence to support this hypothesis could be provided by covalently linking the recombinant XylF to a radiolabelled fatty acid. A similar approach has been used to identify lipoproteins in other gram-positive bacteria (49). In an attempt to accomplish this, the host cells harboring the recombinant clone were grown in the presence of [1-14C]palmitic acid prior to induction. Cell lysates of radiolabelled cells were then subjected to SDS-PAGE, followed by autoradiography (Fig. 6). The result clearly showed the presence of a radioactively labelled protein corresponding in size to the recombinant XylF, and this protein was absent in the control (uninduced) cells. Thus, E. coli performed posttranslational fatty acyl modification of the recombinant XylF.
FIG. 6.
Metabolically labelled lipoproteins in E. coli cells overproducing the T. ethanolicus XylF. Cells uninduced (−) or induced with IPTG (+) were grown in the presence of [14C]palmitate. Radiolabelled proteins from crude cell lysates (approximately 50 μg per lane) were run in an SDS-PAGE (10% polyacrylamide) gel, which was dried and subjected to autoradiography for 4 weeks.
DISCUSSION
To our knowledge, the molecular analysis of the T. ethanolicus xylF presented here is the first report of a gene encoding d-xylose-binding protein in a gram-positive bacterium and in a thermophilic organism. We have provided evidence strongly suggesting that the T. ethanolicus XylF is a lipoprotein: (i) the sequence begins with a putative signal peptide that contains the lipoprotein-scission consensus site (52); (ii) metabolic labelling with [14C]palmitate yielded a radiolabelled protein of the size corresponding to XylF; and (iii) the recombinant XylF was associated with cell membrane fractions, as is typical of bacterial lipoproteins (33). The last observation is particularly interesting in light of the fact that the entire xylF coding region, including the signal sequence, was present in the recombinant construct. Clearly, the cleavage sequence of T. ethanolicus XylF was recognized by the E. coli enzymatic machinery responsible for translocation, processing, and localization of lipoproteins.
There have been several reports that overproduction of heterologous lipoproteins is harmful for E. coli hosts (49). We have demonstrated that the T. ethanolicus XylF was not only highly expressed in E. coli, but also that it was functional, as could be inferred from the high d-xylose binding activity observed in induced cells. Since T. ethanolicus is gram-positive, it is likely that the native XylF is anchored to the external surface of the cell membrane by a lipid moiety thioacylated to the amino-terminal cysteine of the mature protein, as has been demonstrated in ligand-binding proteins from other gram-positive bacteria (49).
The signal peptide of the T. ethanolicus XylF is abutted by a sequence region rich in serine, threonine, and asparagine. A similar sequence was also observed in the N terminus of another mature lipoprotein, an α-amylase of a hyperthermophile, Thermotoga maritima (29). These sequences, dubbed “linkers,” are also found in immunoglobulin 1A, a pullulanase, xylanases, and cellulases, where they join different domains within an enzyme (13, 26). It has been postulated that a linker in a cell membrane-anchored lipoprotein enhances enzyme performance by serving as an extended spacer relative to the cell surface (29). The noticeable lack of linkers in periplasmic monosaccharide-binding proteins (Fig. 1) supports this view. Further work is needed to determine whether the linker facilitates interactions of XylF with membrane-associated transport proteins during d-xylose transport.
The marked binding of d-xylose by E. coli carrying the recombinant XylF suggested that the protein also functions in vivo in T. ethanolicus. Our preliminary transport studies indicate that a high-affinity d-xylose uptake system does operate in T. ethanolicus (10). These observations imply the existence of additional components needed for binding protein-dependent transport of d-xylose in T. ethanolicus, i.e., a permease, and an ATP-binding protein. In E. coli, genes encoding these two enzymes (XylH and XylG, respectively) are clustered together with xylF in the xylFGHR operon (48), where xylR codes for a transcriptional regulator of xylose utilization (47). In H. influenzae, d-xylose transport genes also constitute an operon, xylFGH (11). The operon organization is characteristic of genes encoding ABC transporters of other mono- and disaccharides (12), metals, ions, and peptides (12, 17). However, our finding that no sequences homologous to either xylG or xylH lie in the same genomic locus with xylF in T. ethanolicus represents an exception to this paradigm. Similarly, it was recently reported that genes encoding ATP-binding proteins which mediate maltose transport in Streptomyces reticuli (41) and Thermoanaerobacterium thermosulfurigenes (38) are not situated within operons. It remains to be elucidated how the regulation of expression of these remote cistrons is coordinated.
The solitary location of xylF on the T. ethanolicus chromosome relative to putative xylG and xylH is also reflected in the monocistronic nature of the xylF mRNA. Our RNA analyses indicated that the inverted repeat, located in the xylB-xylF intercistronic spacer (Fig. 3B), is likely to function as an efficient transcriptional terminator, yielding barely detectable xylABF read-through transcripts. Both xylF +1 sites are situated within the xylAB operon. The question as to how the transcription of xylAB is coupled with xylF expression, with respect to the two internal xylF promoters, awaits further study.
As the RNA dot blot data demonstrated, transcription of T. ethanolicus xylF is inducible by xylose and is not repressed by glucose. Such a phenomenon, also observed in regulation of xylAB transcription in T. ethanolicus (8), is markedly different from those in other xylose-utilizing bacteria, where glucose exerts strong inhibition of xylose utilization via several distinct mechanisms, most frequently, catabolite repression (21). Previously, a palindromic sequence was identified in the vicinity of the xylAB +1 site (8) and was postulated to serve as a T. ethanolicus xylose operator (xylO) on the basis of its striking sequence similarity to xylO palindromes, with the sequence consensus TTNGTTTNNNNNNNAAACNAA (5), mediating negative regulation of xylose catabolism in bacilli, Staphylococcus xylosus, and Lactobacillus pentosus (23, 30, 37, 45). In this respect, it was intriguing to find that an inverted repeat, TTTGTTTGATTT-23 bp-AATTCAAACAAA, with half-sites perfectly matching corresponding portions in the xylO consensus sequence, lies adjacent to the downstream transcription initiation site of xylF (Fig. 3B). In B. subtilis, an oligomeric xylose repressor, XylR, was shown to bind two tandemly repeated overlapping palindromes spaced 4 bp apart (5). Notably, the xylO sites do not have to overlap for binding to occur (i.e., XylR can bind isolated palindromes as single sites) (5). However, without experimental evidence, it is difficult to explain how an XylR homolog in T. ethanolicus would achieve simultaneous binding of the xylF palindromes spaced by the 23-bp loop. Future studies should show whether a looping mechanism, analogous to that observed in l-arabinose operon regulation by AraC (40), may be involved in regulation of xylF expression in T. ethanolicus. Nevertheless, the presence of conserved palindromes adjacent to both xylAB and xylF promoters and similar expression patterns of the two operons with respect to the carbon sources suggest that both xylAB and xylF operons may be controlled by a similar mechanism of negative regulation. Interestingly, the inverted repeat implicated in the xylAB transcription termination coincides with the putative xylF operator sequence. Hence, this might serve as an example of versatility of cis-acting elements in transcriptional regulation of bacterial carbon metabolism.
A recent study showed that XylFs from E. coli and H. influenzae are more closely related to the A. tumefaciens multiple-sugar-binding protein ChvE than to the E. coli periplasmic receptors of l-arabinose, d-glucose or d-galactose, or d-ribose (22). Our inclusion of T. ethanolicus XylF in the multiple sequence comparison confirmed this observation. Significantly, our sequence analysis indicated that most residues involved in protein-sugar interactions in E. coli monosaccharide-binding transporters (i.e., AraF, MglB, and RbsB) appear to be either fully conserved or replaced by isofunctional residues in d-xylose-binding proteins. In E. coli AraF and MglB, they include residues that sandwich sugar molecules inside the protein cleft by stacking interactions and those forming hydrogen bonds with sugars, which confer substrate specificity (34).
We also noted, for the first time, that XylF sequences possess a region closely matching a sequence pattern of binding proteins specific for hexoses and pentoses, the so-called cluster 2 signature. This consensus was defined as K(LIVFAG)3IX3D(SGP)X3(GS)X(LIVA)2X2A, where boldface letters indicate invariant residues in all of the relevant sequences available at the time (50). Our inclusion of XylFs in multiple sequence alignment revealed two residues in XylFs that deviate from this consensus (Fig. 1). Thus, we propose that the cluster 2 sequence signature be refined as (KDQ)(LIVFAG)3IX3(DN)(SGP)X3(GS)X(LIVA)2X2A, where boldface letters reflect the changes introduced. This may facilitate future sequence analyses of sugar-binding proteins.
At present, it is not clear which residues and/or interactions are responsible for the presumed thermostability of XylF in the thermophilic bacterium T. ethanolicus. The amino acid composition analysis of its predicted sequence (data not shown) compared to that of mesophilic XylFs did not reveal any obvious differences, with one exception: there are three cysteines in the peptide chain of the T. ethanolicus XylF, unlike in the other two XylFs, which completely lack cysteine residues. Now that the recombinant T. ethanolicus XylF is available, it will be possible to examine if a disulfide bridge is present in the mature, properly folded protein, and, if so, whether such a conformation is required for the protein thermostability, as has been documented for numerous other thermostable enzymes (14).
ACKNOWLEDGMENT
This work was supported by the U.S. Department of Agriculture under agreement 95-37500-1793.
Footnotes
Published with the approval of the Director of the Kentucky Agricultural Experiment Station as journal article no. 98-07-80.
REFERENCES
- 1.Ahlem C, Huisman W, Neslund G, Dahms S. Purification and properties of a periplasmic D-xylose-binding protein from Escherichia coli K-12. J Biol Chem. 1982;257:2926–2931. [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F A, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing; 1996. [Google Scholar]
- 4.Bhosale S H, Rao M B, Deshpande V V. Molecular and industrial aspects of glucose isomerase. Microbiol Rev. 1996;60:280–300. doi: 10.1128/mr.60.2.280-300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahl M K, Degenkolb J, Hillen W. Transcription of the xyl operon is controlled in Bacillus subtilis by tandem overlapping operators spaced by four base-pairs. J Mol Biol. 1994;243:413–424. doi: 10.1006/jmbi.1994.1669. [DOI] [PubMed] [Google Scholar]
- 6.Davis E O, Henderson P J F. The cloning and DNA sequence of the gene xylE for xylose-proton symport in Escherichia coli K12. J Biol Chem. 1987;262:13928–13932. [PubMed] [Google Scholar]
- 7.Dekker K, Yamagata H, Sakaguchi K, Udaka S. Xylose (glucose) isomerase gene from the thermophilic Clostridium thermohydrosulfuricum; cloning, sequencing, and expression in Escherichia coli. Agric Biol Chem. 1991;55:221–227. [PubMed] [Google Scholar]
- 8.Erbeznik M, Dawson K A, Strobel H J. Cloning and characterization of transcription of the xylAB operon from Thermoanaerobacter ethanolicus. J Bacteriol. 1998;180:1103–1109. doi: 10.1128/jb.180.5.1103-1109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erbeznik M, Jones C R, Dawson K A, Strobel H J. Clostridium thermocellum JW20 (ATCC 31549) is a coculture with Thermoanaerobacter ethanolicus. Appl Environ Microbiol. 1997;63:2949–2951. doi: 10.1128/aem.63.7.2949-2951.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erbeznik, M., H. J. Strobel, and K. A. Dawson. Unpublished data.
- 11.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C A, Gocayne J D, Scott J D, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 12.Furlong C E. Osmotic-shock-sensitive transport systems. In: Neidhart F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C: American Society for Microbiology; 1987. pp. 768–796. [Google Scholar]
- 13.Gilkes N R, Henrissat B, Kilburn D G, Miller R C, Jr, Warren R A J. Domains in microbial β-1,4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev. 1991;55:303–315. doi: 10.1128/mr.55.2.303-315.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodenough P W. A review of protein engineering for the food industry. Mol Biotechnol. 1995;4:151–166. doi: 10.1007/BF02921609. [DOI] [PubMed] [Google Scholar]
- 15.Graves M C, Rabinowitz J C. In vivo and in vitro transcription of the Clostridium pasteurianum ferredoxin gene. J Biol Chem. 1986;261:11409–11415. [PubMed] [Google Scholar]
- 16.Groarke J M, Mahoney W C, Hope J N, Furlong C E, Robb F T, Zalkin H, Hermodson M A. The amino acid sequence of D-ribose-binding protein from Escherichia coli K12. J Biol Chem. 1983;258:12952–12956. [PubMed] [Google Scholar]
- 17.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 18.Higgins D G, Sharp P M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989;5:151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- 19.Hogg R W, Hermodson M A. Amino acid sequence of the L-arabinose-binding protein from Escherichia coli B/r. J Biol Chem. 1977;252:5135–5141. [PubMed] [Google Scholar]
- 20.Hogg R W, Voelker C, Von Carlowitz I. Nucleotide sequence and analysis of the mgl operon of Escherichia coli K12. Mol Gen Genet. 1991;229:453–459. doi: 10.1007/BF00267469. [DOI] [PubMed] [Google Scholar]
- 21.Hueck C J, Hillen W. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the gram-positive bacteria? Mol Microbiol. 1995;15:395–401. doi: 10.1111/j.1365-2958.1995.tb02252.x. [DOI] [PubMed] [Google Scholar]
- 22.Kemner J K, Liang X, Nester E W. The Agrobacterium tumefaciens virulence gene chvE is part of a putative ABC-type sugar transport operon. J Bacteriol. 1997;179:2452–2458. doi: 10.1128/jb.179.7.2452-2458.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreuzer P, Gärtner D, Allmansberger R, Hillen W. Identification and sequence analysis of the Bacillus subtilis W23 xylR gene and xyl operator. J Bacteriol. 1989;171:3840–3845. doi: 10.1128/jb.171.7.3840-3845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lam V M S, Daruwalla K R, Henderson P J F, Jones-Mortimer M C. Proton-linked d-xylose transport in Escherichia coli. J Bacteriol. 1980;143:396–402. doi: 10.1128/jb.143.1.396-402.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C, Ramesh M V, Zeikus J G. Cloning, sequencing and biochemical characterization of xylose isomerase from Thermoanaerobacterium saccharolyticum strain B6A-RI. J Gen Microbiol. 1994;139:1227–1234. doi: 10.1099/00221287-139-6-1227. [DOI] [PubMed] [Google Scholar]
- 26.Lee S-P, Morikawa M, Takagi M, Imanaka T. Cloning of the aapT gene and characterization of its product, α-amylase-pullulanase (AapT) from thermophilic and alkaliphilic Bacillus sp. strain XAL601. Appl Environ Microbiol. 1994;60:3764–3773. doi: 10.1128/aem.60.10.3764-3773.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee Y-E, Bagdasarian M, Meng M, Zeikus J G. Catalytic mechanism of xylose (glucose) isomerase from Clostridium thermosulfurogenes. J Biol Chem. 1990;265:19082–19090. [PubMed] [Google Scholar]
- 28.Lee Y-E, Jain M K, Lee C, Lowe S E, Zeikus J G. Taxonomic distinction of saccharolytic thermophilic anaerobes: description of Thermoanaerobacterium xylanolyticum gen. nov., sp. nov., and Thermoanaerobacterium saccharolyticum gen. nov., sp. nov.; reclassification of Thermoanaerobium brockii, Clostridium thermosulfurogenes, and Clostridium thermohydrosulfuricum E100-69 as Thermoanaerobacter brockii comb. nov., Thermoanaerobacterium thermosulfurigenes comb. nov., and Thermoanaerobacter thermohydrosulfuricus comb. nov., respectively; and transfer of Clostridium thermohydrosulfuricum 39E to Thermoanaerobacter ethanolicus. Int J Syst Bacteriol. 1993;43:41–51. [Google Scholar]
- 29.Liebl W, Stemplinger I, Ruile P. Properties and gene structure of the Thermotoga maritima α-amylase AmyA, a putative lipoprotein of a hyperthermophilic bacterium. JBacteriol. 1997;179:941–948. doi: 10.1128/jb.179.3.941-948.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lokman B C, Leer R J, van Sorge R, Pouwels P H. Promoter analysis and transcriptional regulation of Lactobacillus pentosus genes involved in xylose catabolism. Mol Gen Genet. 1994;245:117–125. doi: 10.1007/BF00279757. [DOI] [PubMed] [Google Scholar]
- 31.Meaden P G, Adose-Opoku J, Reizer J, Reizer A, Lanceman Y A, Martin M F, Mitchell W J. The xylose isomerase-encoding gene (xylA) of Clostridium thermosaccharolyticum: cloning, sequencing and phylogeny of XylA enzymes. Gene. 1994;141:97–101. doi: 10.1016/0378-1119(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 32.Osborn M J, Munson R. Separation of the inner (cytoplasmic) and outer membranes of gram negative bacteria. Methods Enzymol. 1974;31A:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- 33.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quiocho F A. Atomic structures of periplasmic binding proteins and the high affinity active transport systems in bacteria. Phil Trans R Soc Lond B. 1990;326:341–351. doi: 10.1098/rstb.1990.0016. [DOI] [PubMed] [Google Scholar]
- 35.Rainey F A, Ward N L, Morgan H W, Toalster R, Stackebrandt E. Phylogenetic analysis of anaerobic thermophilic bacteria: aid for their reclassification. J Bacteriol. 1993;175:4772–4779. doi: 10.1128/jb.175.15.4772-4779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richarme G, Kepes A. Study of binding protein-ligand interactions by ammonium sulfate-assisted adsorption on cellulose esters filters. Biochim Biophys Acta. 1983;742:16–24. doi: 10.1016/0167-4838(83)90353-9. [DOI] [PubMed] [Google Scholar]
- 37.Rygus T, Scheler A, Allmansberger R, Hillen W. Molecular cloning, structure, promoters and regulatory elements for transcription of the Bacillus megaterium encoded regulon for xylose utilization. Arch Microbiol. 1991;155:535–542. doi: 10.1007/BF00245346. [DOI] [PubMed] [Google Scholar]
- 38.Sahm K, Matuschek M, Müller H, Mitchell W J, Bahl H. Molecular analysis of the amy gene locus of Thermoanaerobacterium thermosulfurigenes EM1 encoding starch-degrading enzymes and a binding protein-dependent maltose transport system. J Bacteriol. 1996;178:1039–1046. doi: 10.1128/jb.178.4.1039-1046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 40.Schleif R. The l-arabinose operon. In: Neidhart F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 2. Washington, D.C: American Society for Microbiology; 1987. pp. 1473–1481. [Google Scholar]
- 41.Schlösser A, Kampers T, Schrempf H. The Streptomyces ATP-binding component MsiK assists in cellobiose and maltose transport. J Bacteriol. 1997;179:2092–2095. doi: 10.1128/jb.179.6.2092-2095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmiedel D, Kintrup M, Küster E, Hillen W. Regulation of expression, genetic organization and substrate specificity of xylose uptake in Bacillus megaterium. Mol Microbiol. 1997;23:1053–1062. doi: 10.1046/j.1365-2958.1997.2881654.x. [DOI] [PubMed] [Google Scholar]
- 43.Shamanna D K, Sanderson K E. Genetics and regulation of xylose utilization in Salmonella typhimurium LT2. J Bacteriol. 1979;139:71–79. doi: 10.1128/jb.139.1.71-79.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimoda N, Toyoda-Yamamoto A, Aoki S, Machida Y. Genetic evidence for an interaction between the VirA sensor protein and the ChvE sugar-binding protein of Agrobacterium. J Biol Chem. 1993;268:26552–26558. [PubMed] [Google Scholar]
- 45.Sizemore C, Wieland B, Götz F, Hillen W. Regulation of Staphylococcus xylosus xylose utilization genes at the molecular level. J Bacteriol. 1992;174:3042–3048. doi: 10.1128/jb.174.9.3042-3048.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sofia H J, Burland V, Daniels D L, Plunket III G, Blattner F R. Analysis of the Escherichia coli genome. V. DNA sequence region from 76.0 to 81.5 minutes. Nucleic Acids Res. 1994;22:2576–2586. doi: 10.1093/nar/22.13.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song S, Park C. Organization and regulation of the d-xylose operons in Escherichia coli K-12: XylR acts as a transcriptional activator. J Bacteriol. 1997;179:7025–7032. doi: 10.1128/jb.179.22.7025-7032.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sumiya M, Davis E O, Packman L C, McDonald T P, Henderson P J F. Molecular genetics of a receptor protein for D-xylose, encoded by the gene xylF in Escherichia coli. Recept Channels. 1995;3:117–128. [PubMed] [Google Scholar]
- 49.Sutcliffe I C, Russell R R B. Lipoproteins of gram-positive bacteria. J Bacteriol. 1995;177:1123–1128. doi: 10.1128/jb.177.5.1123-1128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tam R, Saier M H., Jr Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev. 1993;57:320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vartak N B, Reizer J, Reizer A, Gripp J T, Groisman E A, Wu L-F, Tomich J M, Saier M H., Jr Sequence and evolution of the FruR protein of Salmonella typhimurium: a pleiotropic transcriptional regulatory protein possessing both activator and repressor functions which is homologous to the periplasmic ribose-binding protein. Res Microbiol. 1991;142:951–963. doi: 10.1016/0923-2508(91)90005-u. [DOI] [PubMed] [Google Scholar]
- 52.von Heijne G. The structure of signal peptides from bacterial lipoproteins. Protein Eng. 1989;2:531–534. doi: 10.1093/protein/2.7.531. [DOI] [PubMed] [Google Scholar]
- 53.Vyas N K, Vyas M N, Quiocho F A. Comparison of the periplasmic receptors for L-arabinose, D-glucose/D-galactose, and D-ribose. J Biol Chem. 1991;266:5226–5237. [PubMed] [Google Scholar]