Abstract
Objectives:
Video-assisted thoracoscopic surgery (VATS) lobectomy has been associated with improved pain, length of stay, and outcomes compared to open lobectomy. However, enhanced recovery protocols (ERP) improve outcomes after both procedures. We aimed to compare VATS and open lobectomy in the setting of a comprehensive ERP.
Methods:
All patients undergoing lobectomy for lung cancer at a single institution since the adoption of an ERP (05/2016-12/2018) were stratified by VATS vs. open status and compared. Demographics and outcomes, including length of stay, daily pain scores, and short-term operative complications, were compared using standard univariate statistics and multivariable models.
Results:
A total of 130 patients underwent lobectomy, including 71 (54.6%) VATS and 59 (45.4%) open. VATS vs. open patients exhibited similar length of stay (median 4 days for both, p=0.07), opioid requirement (33.2 vs. 30.8 mg morphine-equivalents, p=0.86) and pain scores at 0, 1, 2, and 3 days following surgery (4.3 vs. 2.8, p=0.12, 4.4 vs. 3.7, p=0.27, 3.9 vs. 3.5, p=0.83, and 3.4 vs. 3.5, p=0.98). Patients undergoing VATS lobectomy exhibited lower rates of readmission (1.4% vs. 17.0%, p<0.01), postoperative transfusion requirement (0% vs. 10.2%, p<0.01), and pneumonia (1.4% vs. 10.2%, p=0.05). After risk-adjustment, open (vs. VATS status) did not significantly impact length of stay (effect 0.18, p=0.10) or overall complication rate (OR 1.9, p=0.12).
Conclusion:
In the setting of a comprehensive enhanced recovery protocol, patients undergoing VATS versus open lobectomy exhibited similar short-term outcomes. Surgical incision may have less impact on outcomes in the setting of a comprehensive thoracic ERP.
Central Message:
Outcomes are similar between video-assisted thoracoscopic (VATS) and open lobectomy in the setting of an enhanced recovery protocol.
Introduction
For the past two decades, video-assisted thoracoscopic surgery (VATS) lobectomy has emerged as an alternative to lobectomy via the traditional open thoracotomy approach.1, 2 In multiple studies, VATS has been associated with decreased complications, a shortened length of stay (LOS), and improved pain control.1–10 VATS lobectomy and open lobectomy for early stage non-small-cell lung cancer (NSLC) have been found to have similar long-term survival outcomes though concern remains that VATS may lead to less thorough lymph node dissection.11–15 However, the majority of investigations comparing VATS and thoracotomy are retrospective, with existing randomized trials showing similar complication rates between the two operations and a potential improvement in pain and quality of life with VATS operations.8, 16 In the United States, VATS has been widely adopted, especially amongst high-volume thoracic surgeons. However, 50-78% of lobectomies are still performed via open approach.2, 11, 17, 18
Enhanced recovery protocols (ERPs) – institutionally standardized, multidisciplinary protocols for perioperative care-have been successfully adopted across many surgical specialties, including thoracic surgery.19–23 These protocols often consist of opioid-sparing, multimodal pain management strategies, patient education, early ambulation, and minimization of fluid overload and are aimed at reducing the surgical and psychological stress to achieve early recovery following surgery. 19, 24 Though thoracic ERPs are relatively new, these ERPs have been associated with shortened LOS, decreased opioid use and fewer complications following thoracic surgical procedures. 22, 24–26 Investigations of ERPs that have included VATS or open status as secondary outcomes have proposed that the operative approach may be less important in the setting of an ERP.22, 27
In 2016, our institution implemented a thoracic ERP for all patients undergoing elective pulmonary, pleural, and mediastinal surgery, which was associated with decreased opioid use, LOS, and cost. 24 We sought to specifically evaluate outcomes following VATS vs. open lobectomy for lung cancer in the setting of a comprehensive ERP. We hypothesized that in the setting of an ERP, LOS, postoperative pain, and short-term outcomes would be similar between open and VATS lobectomy.
Methods
Patient Data
All patients undergoing VATS or open lobectomy for lung cancer from May 2016 to December 2018 at a tertiary care institution were included. Data were abstracted from an institutional Society of Thoracic Surgeons (STS) database and a database of perioperative outcomes collected as part of the institutional ERP. Charts were reviewed for further perioperative information. Records were stratified by VATS (three ports, no rib spreading) or open approach. Operations that began as VATS but were converted to open thoracotomy for any reason were analyzed as open, given our goal to compare the effect of incision type on outcomes. Patients undergoing pneumonectomy, bilobectomy, chest wall, or other extended resections were excluded from analysis. Pain scores were determined as the mean of pain scores per patient per day, from patient-reported pain scores collected every four hours by bedside nursing staff per institutional protocol. Outcomes were LOS in days, mean patient-reported pain score per day, postoperative opioid use in median milligrams of morphine equivalents (MME) per hospital stay, and postoperative adverse events. The composite outcome of “Any postoperative event” was defined as incidence of reoperation, air leak greater than 5 days, atelectasis requiring bronchoscopy, pneumonia, acute respiratory distress syndrome, pleural effusion requiring a drain, respiratory failure, bronchopleural fistula, empyema, postoperative atrial fibrillation, deep venous thrombosis, ileus, urinary tract infection, surgical site infection, stroke, renal failure, readmission, or mortality within 30 days. These criteria were broader than those used in many comparative studies, in order to increase the power in a small retrospective study. The Institutional Review Board approved a waiver of consent for this study (IRB# HSR-20528) due to its retrospective nature.
Enhanced Recovery Protocol
Our institutional thoracic ERP was launched in March 2016, with the protocol, implementation, and initial results previously reported.24 The ERP includes patient education, preoperative carbohydrate load and hydration, protective ventilation, avoidance of fluid overload, early postoperative ambulation, expedient diet advancement, and removal of chest tubes as soon as possible. Central to the protocol is an opioid-sparing pain regimen that includes acetaminophen, gabapentin, celecoxib, ketamine, and both regional and neuraxial analgesia. Regional and neuraxial analgesic techniques include intercostal nerve blockade with liposomal bupivacaine and a single subarachnoid injection of preservative-free morphine, respectively. (Supplemental Table). The ERP pathway and all interventions are identical for open and VATS lobectomy.
Statistical Analysis
Demographics and outcomes are reported as median and interquartile range (IQR) for continuous variables and n, percent for categorical variables. Univariate analysis consisted of Mann-Whitney U test for continuous nonnormally distributed variables and Chi-square test or Fisher’s exact test for categorical variables. A linear regression model was created to evaluate the risk-adjusted effect of VATS vs. open status on LOS (modeled as log-transformed LOS), and a logistic regression model was created to evaluate the composite occurrence of any postoperative adverse event. Variables for these models were selected a priori based on factors expected to impact outcomes after thoracic lobectomy, including open versus VATS status. Adequacy of these models was assessed by evaluating residual plots, R-squared statistic, and C-statistic. A subgroup analysis evaluated demographics and outcomes in patients with early stage (clinical stage T1/T2, N0, M0) disease, as this population matches the population studied in many existing investigations of VATS vs. open lobectomy. Statistical analysis was performed using SAS Version 9.4 (SAS Institute, Cary, NC) using p<0.05 as the significance threshold.
Results
A total of 130 patients underwent lobectomy for a primary lung malignancy during the study period, of which 71 (54.6%) were VATS. Patients undergoing VATS and open lobectomy were similar in age (both median 68 years, p=0.53). VATS was the more frequent approach for patients with early-stage cancer (clinical T1/T2N0) (70.1% VATS vs. 29.9% open). VATS patients had no preoperative radiation (0% vs. 15.3%, p<0.01) nor chemotherapy (0% vs. 22.0%, p<0.01). Patients undergoing VATS had a higher preoperative hemoglobin concentration (13.9 vs 12.8 g/dl, p<0.01) (Table 1).
Table 1.
Demographics of patients undergoing video-assisted thoracoscopic surgery (VATS) versus open lobectomy
| Variable | VATS (n=71) | Open (n=59) | p-value |
|---|---|---|---|
| Age (years) | 68 [61-74] | 68 [58-76] | 0.53 |
| Sex (female) | 43 (60.6) | 26 (44.1) | 0.06 |
| Race (white) | 61 (85.9) | 50 (84.8) | 0.85 |
| Past/current smoker | 62 (87.3) | 49 (83.1) | 0.50 |
| Pack Years smoking | 33 [15-47] | 40 [20-60] | 0.14 |
| FEV1 | 86 [69-96] | 88 [71-96] | 0.37 |
| DLCO | 74 [64-87] | 88 [71-96] | 0.62 |
| Preoperative Creatinine | 0.80 [0.70-1.00] | 0.90 [0.80-1.00] | 0.13 |
| Preoperative Hemoglobin | 13.9 [13.1-14.4] | 12.8 [11.8-14.3] | 0.01 |
| Congestive Heart Failure | 0 (0) | 1 (1.69) | 0.45 |
| Coronary Artery Disease | 16 (22.5) | 10 (17.0) | 0.43 |
| Peripheral Arterial Disease | 1 (1.41) | 5 (8.47) | 0.09 |
| Diabetes | 18 (25.4) | 7 (11.9) | 0.05 |
| Dialysis | 0 (0) | 0 (0) | 1.00 |
| Preoperative radiation | 0 (0) | 9 (15.3) | <0.01 |
| Preoperative chemotherapy | 0 (0) | 13 (22.0) | <0.01 |
| Early Stage Cancer (T1/T2, N0, M0) | 61 (85.9) | 26 (44.1) | <0.01 |
| Clinical Stage | <0.01 | ||
| IA | 52 (73.2) | 23 (39.0) | |
| IB/IIA1 | 9 (12.7) | 3 (5.08) | |
| IIB | 9 (12.7) | 11 (18.6) | |
| III | 1 (1.41) | 18 (30.5) | |
| IV2 | 0 (0) | 4 (6.78) | |
| Pathologic Stage | <0. 01 | ||
| 0 (PCR) | 0 ( 0) | 4 (6.78) | |
| IA | 43 (60.6) | 21 (35.6) | |
| IB/II1 | 24 (33.8) | 24 (40.7) | |
| III | 3 (4.23) | 10 (17.0) | |
| IV | 1 (1.41) | 0 (0) |
Stages combined due to reporting changes from 7th to 8th edition staging systems
Patients with treated, oligometastatic diseaseFEV1, forced expiratory volume; DLCO, diffusing capacity of the lung for carbon monoxide; PCR, pathologic complete response
Results reported as median, interquartile range for continuous variables and n (percent) for categorical variables
VATS and open patients were managed with identical ERP protocols and received the same regional analgesia and anesthesia, with the majority receiving posterior intercostal nerve blockade with liposomal bupivacaine (87.3% vs. 91.5%, p=0.44) as well as subarachnoid morphine analgesia (74.3% vs. 78.0%, p=0.68). Ninety-two patients began with a VATS approach and 21 were converted to open (22.8% conversion rate), all in an elective manner. Reasons for conversion were minor vascular injury (n=1), anatomic reasons (15 patients: severe adhesions n=10, tumor related factors n=2, unfavorable fissure n=2, inability to tolerate single lung ventilation n=1), lymphadenopathy (n=4), and technical (stapler misfire n=1). Most often, standard posterolateral thoracotomy was performed at conversion (11/21); 5/21 had extension of the access port and 5/21 had muscle-sparing thoracotomy. 28 Patients undergoing VATS lobectomy had fewer total lymph nodes retrieved (median 9 vs. 13, p<0.001) and fewer nodal stations sampled (median 3 vs. 5, p<0.01). VATS operations demonstrated shorter operative time (205 vs. 225 minutes, p=0.02) (Table 2).
Table 2.
Operative and perioperative characteristics of patients undergoing video-assisted thoracoscopic surgery (VATS) versus open lobectomy
| Variable | VATS1 (n=71) | Open1 (n=59) | p-value |
|---|---|---|---|
| Subarachnoid morphine (250 mcg) spinal | 52 (74.3) | 46 (77.97) | 0.68 |
| Epidural | 4 (5.80) | 3 (5.08) | 1.00 |
| Liposomal Bupivacaine | 62 (87.3) | 54 (91.5) | 0.44 |
| Operative Time (minutes)2 | 205 [170-238] | 224 [187-258] | 0.03 |
| R0 Resection | 71 (100.0) | 59 (100.0) | 1.00 |
| Nodes Retrieved | 9 [6-13] | 13 [10-19] | <0.01 |
| Nodal Stations Sampled | 3 [2-4] | 5 [4-6] | <0.01 |
| Cancer Histology: | 0.05 | ||
| Adenocarcinoma | 55 (77.5) | 32 (54.2) | |
| Large Cell | 1 (1.4) | 2 (3.4) | |
| Mixed | 2 (2.8) | 1 (1.7) | |
| Neuroendocrine | 1 (1.4) | 4 (6.8) | |
| Squamous Cell | 12 (17.0) | 20 (33.9) |
92 patients started as VATS, 71 completed the procedure as VATS (conversion rate of 22.8%. The 21 patients converted are analyzed in the Open group, and are included in the n=59.
Operative time represens procedural time, beginning with bronchoscopy, to close of incision Results reported as median, interquartile range for continuous variables and n (percent) for categorical variables
Patients receiving VATS and open operations demonstrated similar LOS (median 4 days, p=0.067, Figure 1), with similar pain scores on postoperative day 0, 1, 2, and 3 and similar MME administered during hospital stay (Figure 2 and Table 3). Chest tube duration (median 3 days, p=0.21) and rates of postoperative arrhythmias (7.0 % vs. 11.9%, p=0.34), reintubation (2.8% vs. 5.1%, p=0.66) and 30-day mortality (0% vs. 1.7%, p=0.27) were similar. In the unadjusted analysis, patients undergoing VATS lobectomy had a lower rate of any postoperative complication (33.8% vs. 50.8%, p=0.05). Patients undergoing VATS lobectomy had lower rates of postoperative transfusion (0% vs 10.2%, (6/59 open patients), p<0.01) and pneumonia (1.4% vs. 10.2%, p=0.05). 6 patients were transfused postoperatively, 2 due to surgical bleeding and 4 because of low preoperative hemoglobin concentration from chronic disease or neoadjuvant treatment. 2 of these 4 patients went on to have gastrointestinal bleeding at some point during their hospitalization. We were concerned about this trend and therefore initiated routine use of a histamine-2 receptor antagonist (famotidine) as part of the protocol for any patients receiving a non-steroidal anti-inflammatory drug (NSAID). In further review of these 6 transfused thoracotomy patients, none of these resections were amenable to a VATS approach and a minimally invasive approach would not have prevented the need for transfusion.
Figure 1. Length of Stay for Patients Undergoing Video Assisted Thoracoscopic (VATS) Lobectomy and Open Lobectomy.
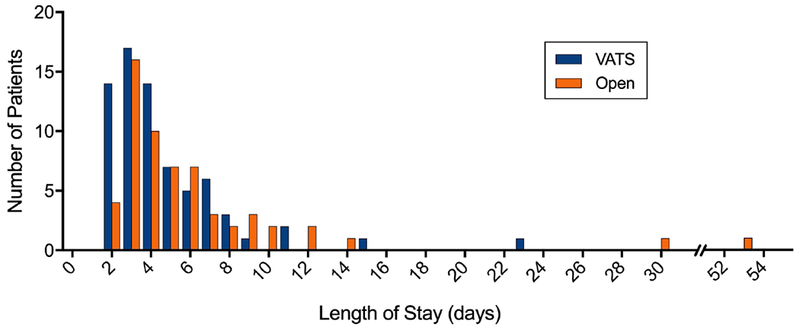
Histogram demonstrating the distribution of length of stay (in days) amongst patients undergoing Video assisted thoracoscopic (VATS) lobectomy and Open lobectomy. Patients undergoing VATS and open lobectomy demonstrated a similar distribution of length of stay, however open patients did have outliers with lengths of stays of 30 and 53 days.
Figure 2. Daily Pain Scores for Patients Undergoing Video Assisted Thoracoscopic (VATS) lobectomy and open lobectomy.
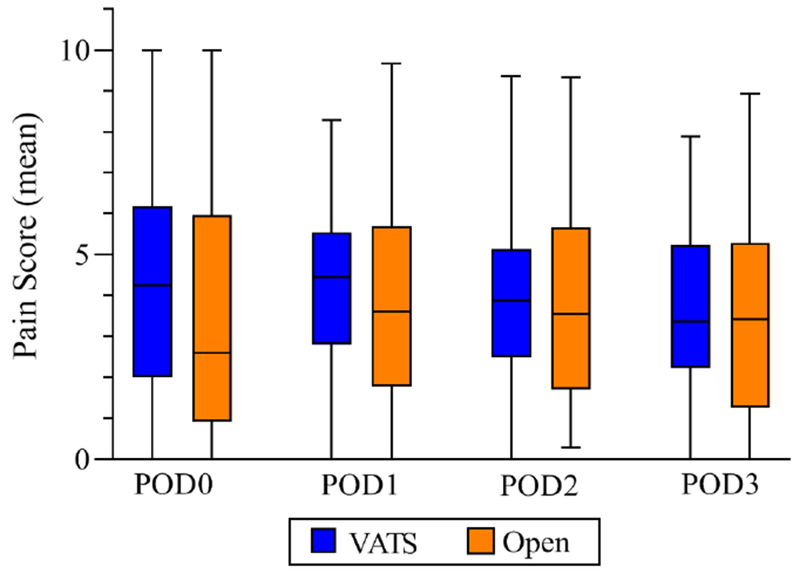
Graph shows median, interquartile range, minimum, and maximum of mean daily pain scores per patient undergoing Video Assisted Thoracoscopic (VATS) lobectomy and open lobectomy, for postoperative days 0, 1, 2, and 3. VATS is shown in blue, with open in orange. On each day, there was no statistically significant difference between daily pain scores between groups.
Table 3.
Postoperative outcomes of patients undergoing video-assisted thoracoscopic surgery (VATS) versus open lobectomy
| Variable | VATS (n=71) | Open (n=59) | p-value |
|---|---|---|---|
| Length of Stay (days) | 4 [3-6] | 4 [3-7] | 0.07 |
| POD0 Pain Score | 4.3 [2.0-6.2] | 2.8 [1.1-6.0] | 0.11 |
| POD1 Pain Score | 4.4 [2.8-5.5] | 3.7 [1.8-5.7] | 0.27 |
| POD2 Pain Score | 3.9 [2.5-5.1] | 3.5 [1.7-5.7] | 0.83 |
| POD3 Pain Score | 3.4 [2.3-5.2] | 3.53 [1.4-5.3] | 0.98 |
| Postoperative MME | 33.2 [15.4-77.5] | 30.8 [12.5-81.7] | 0.86 |
| Any postoperative complication | 24 (33.8) | 30 (50.9) | 0.05 |
| Reoperation | 1 (1.4) | 1 (1.7) | 1.00 |
| Chest tube duration (days) | 3 [2-5] | 3 [2-6] | 0.21 |
| 30-day mortality | 0 (0) | 1 (1.7) | 0.27 |
| Air Leak > 5 days | 15 (21.1) | 15 (25.4) | 0.56 |
| ARDS | 0 (0) | 1 (1.7) | 0.27 |
| Atelectasis Requiring Bronchoscopy | 4 (5.6) | 6 (10.2) | 0.51 |
| Empyema | 2 (2.8) | 2 (3.4) | 1.00 |
| Pneumonia | 1 (1.4) | 6 (10.2) | 0.05 |
| Pneumothorax | 4 (5.6) | 2 (3.4) | 0.69 |
| Myocardial Infarction | 1 (1.4) | 0 (0) | 1.00 |
| Atrial Arrhythmia | 5 (7.0) | 7 (11.9) | 0.34 |
| Postoperative Transfusion | 0 (0) | 6 (10.2) | <0.01 |
| Transfusion related to operation | 0 (0) | 2 (3.4) | 0.20 |
| Readmission within 30 days | 1 (1.4) | 10 (17.0) | <0.01 |
| Reintubation | 2 (2.8) | 3 (5.1) | 0.66 |
| Renal Failure | 0 (0) | 0 (0) | 1.00 |
| Stroke | 0 (0) | 0 (0) | 1.00 |
| Surgical Site Infection | 0 (0) | 2 (3.4) | 0.20 |
| Urinary Tract Infection | 1 (1.4) | 1 (1.7) | 1.00 |
POD, post-operative day; ARDS, acute respiratory distress syndrome; MME milligrams morphine equivalents
Results reported as median, interquartile range for continuous variables and n (percent) for categorical variables
VATS patients also demonstrated lower readmission rates (1.4% vs. 17.0%, p=<0.01). In both groups, many of the patients who were readmitted had long initial hospitalizations, (with a median LOS of 15 days (VATS) and 7.5 days (open), indicating that these were largely patients with complex postoperative courses rather than premature discharges. Process improvements were initiated in November 2017 to address the readmission rate, which led to an overall readmission rate decline from 10.4% to 5.7%. Specifically, in thoracotomy patients, readmission rates decreased from 20% (8 of the first 40 patients) to 10.5% (2 of the subsequent 19 patients) once the protocol was changed. It is also notable that four of the 11 total readmissions were either unrelated to the surgical procedure (for instance, viral illness with dehydration 22 days after discharge) or did not clearly mandate readmission to an outside facility (urinary tract infection, erythema at chest tube site), but are included in our analysis nonetheless. On risk-adjusted analysis, open vs. VATS status was not significantly associated with LOS (effect 0.18, p=0.10) or incidence of an adverse postoperative complication (OR 1.92, open vs. VATS p=0.12). The full risk-adjusted models are shown in Table 4.
Table 4:
Risk-Adjusted models for length of stay and for any complication following video-assisted thoracoscopic surgery (VATS) versus open lobectomy
| Linear Regression Model for Length of Stay (log-transformed) following VATS vs. Open Lobectomy | |||
|---|---|---|---|
| Variable | Parameter Estimate | 95% Confidence Interval | p-value |
| Open vs. VATS | 0.18 | −0.04 - 0.40 | 0.10 |
| Age | 0.01 | −0.00 - 0.02 | 0.21 |
| Gender (Female) | 0.02 | −0.19 - 0.23 | 0.86 |
| Race (white) | 0.11 | −0.18 - 0.41 | 0.44 |
| FEV1 | 0.00 | −0.01 - 0.01 | 0.99 |
| DLCO | −0.01 | −0.01 - 0.00 | 0.09 |
| Stage 1a Cancer | −0.01 | −024 - 0.22 | 0.93 |
| Diabetes | −0.23 | −0.49 - 0.02 | 0.07 |
| Coronary artery disease | 0.26 | 0.00 - 0.52 | 0.048 |
| Pack Years Smoking | 0.00 | 0.000 - 0.01 | 0.09 |
| Logistic Regression Model for Any Complication following VATS vs. Open Lobectomy | |||
| Variable | Odds Ratio | 95% Confidence Interval | p-value |
| Open vs. VATS | 1.92 | 0.85 - 4.34 | 0.12 |
| Age | 1.01 | 0.97 - 1.06 | 0.51 |
| Stage Ia Cancer | 0.38 | 0.16 - 0.91 | 0.03 |
| Coronary artery disease | 4.04 | 1.50 - 10.84 | 0.01 |
| FEV1 | 0.99 | 0.97 - 1.01 | 0.36 |
| Past/current smoker | 2.72 | 0.76 - 9.68 | 0.12 |
R-squared (for linear model)= 0.204, C-statistic (for logistic model)= 0.725
FEV1, forced expiratory volume; DLCO, diffusing capacity of the lung for carbon monoxide
A subgroup analysis of patients with early stage cancer (clinical T1/T2, N0, M0, the inclusion criteria for many similar investigations) compared 61 patients who underwent VATS lobectomy (70.1%) with 26 (29.9%) who underwent open thoracotomy. Seventy-six early stage patients began as VATS, and 15 required conversion (19.7% conversion rate) and were subsequently analyzed with the other 11 patients in the open cohort. Compared to patients undergoing VATS lobectomy, patients undergoing open operations had increased rates of peripheral arterial disease (15.4% vs. 0%, p<0.01) and increased smoking burden (median 55 pack years vs. 33, p=0.02). Pain scores and MMEs were again similar between open and VATS patients in this subset (all p<0.05.). Even in this early stage group undergoing open lobectomy, there were increased rates of pneumonia (19.2% vs. 1.6%, p<0.01) and readmission (23.1% vs. 1.6%, p<0.01), as well as an increased LOS (median 5 vs. 4 days, p=0.02). Further demographics and outcomes are shown in Table 5.
Table 5.
Demographics and outcomes for patients with early stage cancer (T1/T2, N0, M0) undergoing video-assisted thoracoscopic surgery (VATS) versus open lobectomy
| Demographics: Variable | VATS (n=61) | Open (n=26) | p-value |
|---|---|---|---|
|
| |||
| Age (years) | 68 [61-74] | 68 [58-70] | 0.32 |
| Sex (female) | 39 (63.9) | 12 (46.2) | 0.16 |
| Race (white) | 52 (85.3) | 22 (84.5) | 1.00 |
| Past/current smoker | 55 (90.2) | 23 (88.5) | 1.00 |
| Pack Years smoking | 33 [15-45] | 55 [30-75] | 0.02 |
| FEV1 | 85 [70-92] | 72 [64-90] | 0.32 |
| DLCO | 75 [64-88] | 68 [62-87] | 0.18 |
| Coronary Artery Disease | 11 (18.0) | 6 (23.1) | 0.59 |
| Peripheral Arterial Disease | 0 (0) | 4 (15.4) | 0.01 |
| Diabetes | 13 (21.3) | 4 (15.4) | 0.77 |
| Outcomes: Variable | VATS (n=61) | Open (n=26) | p-value |
|
| |||
| Operative Time (minutes) | 202 [170-237] | 217 [180-244] | 0.20 |
| Converted to Open Surgery1 | 15/76 (19.7) | n/a | |
| Nodes Retrieved | 9 [6-13] | 11 [7-15] | 0.01 |
| Length of Stay (days) | 4 [3-5] | 5 [4-9] | 0.02 |
| POD0 Pain Score | 4.3 [2.0-6.1] | 2.5 [0.1-6.4] | 0.34 |
| POD1 Pain Score | 4.4 [2.9-5.5] | 4.0 [0.8 - 6.0] | 0.56 |
| POD2 Pain Score | 3.6 [2.5-5.0] | 3.6 [1.7 - 5.9] | 0.99 |
| POD3 Pain Score | 3.4 [2.2-5.3] | 3.5 [2.0 - 4.6] | 0.99 |
| Postoperative MME | 32.3 [14.7 - 80.0] | 51.3 [20.0-137.7] | 0.23 |
| Reoperation | 1 (1.6) | 0 (0) | 1.00 |
| 30-day mortality | 0 (0) | 1 (3.9) | 0.30 |
| Atelectasis Requiring Bronchoscopy | 2 (3.3) | 3 (11.5) | 0.16 |
| Empyema | 2 (3.3) | 2 (7.7) | 0.58 |
| Pneumonia | 1 (1.6) | 5 (19.2) | 0.01 |
| Myocardial Infarction | 1 (1.6) | 0 (0) | 1.00 |
| Atrial Arrhythmia | 5 (8.2) | 3 (11.5) | 0.69 |
| Readmission within 30 days | 1 (1.6) | 6 (23.1) | <0.01 |
| Reintubation | 2 (3.3) | 2 (7.7) | 0.58 |
| Surgical Site Infection | 0 (0) | 1 (3.9) | 0.30 |
FEV1, forced expiratory volume; DLCO, diffusing capacity of the lung for carbon monoxide; POD, post-operative day; MME milligrams morphine equivalents
Results reported as median, interquartile range for continuous variables and n (percent) for categorical variables
76 patients started with VATS; 15 were converted; these were analyzed in the open group and included in n=26
Discussion
The present study compares VATS versus open technique under a contemporary, comprehensive, thoracic enhanced recovery protocol. In this population, the two operations resulted in similar lengths of stay, pain scores, and in-hospital opioid use. Patients undergoing open lobectomy were generally higher risk with later stage malignancies (56% stage IIB or higher; 22% received neoadjuvant therapy), and exhibited increased rates of readmission, postoperative transfusion, and pneumonia, which persisted in a subanalysis of only early-stage cancer. However, after risk adjustment in a multivariable analysis, neither LOS nor overall complication rate were significantly different between operative approaches for lobectomy. Furthermore, readmission and transfusion rates improved over time with adjustments to the ERP protocol. Further discussion and description of the results can be seen in Video 1.
These data highlight the potential for ERPs to act as an equalizer between surgical approaches. Two often-reported advantages of VATS are improved pain control and decreased LOS, both of which are targeted in ERP’s.1, 3, 5, 9 As multimodal pain control and strategies to promote shorter hospital stay are implemented in both groups, the difference conferred by operative strategy may become less pronounced. Additionally, the magnitude of effect of ERAS on open operations may be larger, as has previously been suggested, further aligning outcomes between the two operations.22 It is notable that the median LOS for both groups in this study (4 days) is shorter than many of the previously reported lengths of stay for both VATS and open operations (4 vs 6, 5 vs 6, and 6 vs 7.4). 5, 11, 16, 17 Van Haren et al. also reported a median LOS of 4 days for both thoracotomy and VATS for lung cancer in the context of a robust thoracic ERP, further substantiating the ability of ERP to reduce the influence of incision on outcomes.22
Postoperative opioid use in both study groups (median 33 and 30 MMEs, or the equivalent of 4 - 4.5 oxycodone 5 mg tablets) is a substantial reduction from our previously reported pre-ERAS opioid use for either group (86 MME VATS, 130 MME open, a 61% and 77% reduction respectively).24 Despite this radical decrease in opioid use, pain scores were acceptably low in both groups and interestingly, equivalent between the two groups. This is a departure from multiple prior reports indicating lower pain scores with VATS.8, 29, 30 Similar to our findings, the MD Anderson Cancer Center? study also did not find a difference in pain between the two incision types.31, 32 These findings highlight the importance of comparing VATS and open operations in the setting of a uniform ERP. In existing retrospective studies, VATS cohorts are often more recent, introducing potential bias due to changes in perioperative care between study periods.3, 7 Furthermore, as academic centers are more likely to adopt VATS techniques, multicenter evaluations of VATS vs. open lobectomy may be biased by differences in institutional perioperative protocols between institutions.17 One potential opportunity to track and evaluate perioperative interventions in thoracic surgery patients lies in large, multi-institutional databases such as the STS General Thoracic Surgery Database. It has been previously noted that risk-prediction models may be impacted by ERPs, By either tracking ERP’s in large databases, or by pooling institutional data, future investigations could discern the impact of both operative technique and perioperative care protocols on outcomes.33
Patients undergoing open lobectomy in this study did exhibit higher rates of readmission, pneumonia, and postoperative blood transfusion compared to those undergoing VATS lobectomy. Higher pneumonia and transfusion rates have previously been reported in open lobectomy.3, 10 It is notable that the patients undergoing open lobectomy were at baseline higher risk, with higher rates of comorbidities, higher stage malignancies, increased rates of preoperative chemotherapy and radiation, and lower baseline hemoglobin. In the risk-adjusted models for LOS and overall complication rate, some of these variables significantly impacted outcomes, while incision type did not. These comorbidities likely also contributed to the higher complication rates, though a full risk-adjusted analysis for individual complications was not possible in this sample size. These findings did persist in the subanalysis of patients with early-stage disease, implying that cancer stage alone is likely not responsible for the differing complication rates between groups. In our institution, quarterly data review associated with the ERP program prompted evaluation of all readmitted patients in real time and again for the purposes of this study. Areas for improvement have been considered and implemented; we have not found a reason to alter our discharge timing or criteria. We did initiate steps to prevent readmission in high-risk patients, including improved education for those discharged with a chest tube, post-discharge phone calls, and close follow-up visits. After these changes were initiated in November 2017, combined readmission rate declined from 10.4% to 5.7%. It is further notable that within the subgroup analysis of early stage cancer, there was a statistically longer length of stay for patients undergoing open operations- it is not readily apparent why the smaller subgroup exhibits this difference while the larger cohort does not.
Complete resection with negative margins (R0) was achieved in all cases. Interestingly, fewer lymph nodes were retrieved and fewer nodal stations were sampled in patients undergoing VATS lobectomy (9 median total nodes by VATS, and 13 by thoracotomy, and fewer nodal stations (3 vs 5) p<0.01 for both).. Previous studies have also noted concern regarding fewer nodes retrieved after VATS operations, with evidence of frequent nodal upstaging by thoracotomy.13–15, 34 It is not clear if these findings translate to long-term survival differences, as recent multi-institutional investigations of long-term cancer survival demonstrate similar outcomes for VATS vs. open lobectomy.11, 12 With reduction of perioperative outcome differences, improved nodal staging achieved by thoracotomy may have a meaningful impact. Similar LOS and pain between VATS and open operations in the setting of an ERP may also support earlier conversion from VATS to open for technically difficult lymph node dissections or when there is high clinical suspicion for nodal metastasis. As recent evidence associated an ERP with increased compliance with adjuvant chemotherapy for NSCLC, the impact of ERP, rather than incision type, on long-term oncologic outcomes may be the more interesting question to explore.35
The ability of an ERP to align outcomes between VATS and open lobectomy may be particularly relevant in situations where a thoracotomy provides a safer, more complete oncologic resection due to a patient’ anatomy, tumor characteristics, or clinical condition. In fact, one major benefit noted by our group is the seamlessness of converting from VATS to open on an ERP. As the protocol is identical for VATS and open cases, there is no need for a preoperative decision regarding placing an epidural or giving certain preoperative medications based on likelihood of intraoperative conversion. These findings are also relevant to surgeons less experienced with VATS technique. Despite rapid rise at large-volume medical centers, it is estimated that fewer than half of lobectomies in the United States are performed via VATS, with lower rates for non-thoracic surgeons, non-academic hospitals, and in less-densely populated regions of the country.2, 17, 18 For surgeons with low lobectomy volume practicing in areas with limited access to tertiary care centers, it may be simpler to implement an ERP than to adopt VATS technique. In all of these settings, surgeons may perform lobectomy via either technique and ensure satisfactory patients outcomes when following an ERP.
There are noted limitations to this investigation. First, the small sample size was driven by the duration of the institutional ERP program, and the study is underpowered. Furthermore, given the sample size, we were only able to risk-adjust for the composite outcome of any complication and for length of stay, with the remainder of the outcomes presented in an unadjusted fashion. However, despite the study’s small size, it is the first to specifically investigate open vs. VATS outcomes in this setting, and should inform design of future comparative studies. Additionally, in order to increase sample size, the study includes higher-stage cancers, primarily in the open group, whereas most studies of VATS technique compare only early stage cancers. Though not necessarily a limitation, it is notable that patients who had a VATS converted to open lobectomy are counted as open lobectomy for purposes of this study, and often were not able to undergo complete VATS operation for technical reasons such as adhesions, inability to tolerate single lung ventilation, difficult hilar nodes, and tumor size or invasiveness. Finally, it is possible the true difference between open and VATS pulmonary resection is less than previously thought and the present study’s findings are not necessarily due to the ERP itself. Given the retrospective nature of the study, it is inherently subject to selection bias and precludes demonstration of causation.
Conclusion
In the setting of a comprehensive thoracic ERP, patients undergoing VATS and open lobectomy exhibited similar length of stay, pain scores, and opioid use, with low opioid use and short length of stay in both groups. There were increased readmissions, pneumonia, and blood transfusion in open lobectomy patients, though these patients were generally at higher baseline risk; interventions based on regular review of ERP results led to improvements in these parameters during the study period. The number of lymph nodes and nodal stations evaluated were significantly higher with open lobectomy. In the setting of an ERP, differences between VATS and open lobectomy may not be as pronounced as previously demonstrated. Future studies comparing surgical approach should account for the impact of perioperative care on outcomes. Enhanced recovery protocols provide a continued opportunity to improve care for patients undergoing both open and VATS operations.
Supplementary Material
The author discusses the study and the clinical relevance of this research
Perspective Statement:
Video-assisted thoracoscopic (VATS) lobectomy has been associated with decreased length of stay, pain, and complications compared to open lobectomy. Enhanced recovery protocols have been shown to decrease pain, complications, and length of stay as well. We sought to compare outcomes between VATS and open lobectomy in the setting of a comprehensive thoracic enhanced recovery protocol.
Acknowledgments
Funding:
The National Institutes of Health supported research in this publication under award number UM1 HL088925 (EDK), and NCI Cancer Center Support Grant 5P30CA044579-27 (LWM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
All authors have nothing to disclose
Presentation:
Presented at American Association for Thoracic Surgery 99th Annual Meeting, Toronto, ON, May 5, 2019
References
- 1.Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. The Annals of thoracic surgery. 2008;86:2008–2016; discussion 2016–2008. [DOI] [PubMed] [Google Scholar]
- 2.Berfield KS, Farjah F, Mulligan MS. Video-Assisted Thoracoscopic Lobectomy for Lung Cancer. The Annals of thoracic surgery. 2019;107:603–609. [DOI] [PubMed] [Google Scholar]
- 3.Paul S, Altorki NK, Sheng S, Lee PC, Harpole DH, Onaitis MW, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139:366–378. [DOI] [PubMed] [Google Scholar]
- 4.Scott WJ, Allen MS, Darling G, Meyers B, Decker PA, Putnam JB, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg. 2010;139:976–981; discussion 981–973. [DOI] [PubMed] [Google Scholar]
- 5.Paul S, Sedrakyan A, Chiu YL, Nasar A, Port JL, Lee PC, et al. Outcomes after lobectomy using thoracoscopy vs thoracotomy: a comparative effectiveness analysis utilizing the Nationwide Inpatient Sample database. Eur J Cardiothorac Surg. 2013;43:813–817. [DOI] [PubMed] [Google Scholar]
- 6.Falcoz PE, Puyraveau M, Thomas PA, Decaluwe H, Hurtgen M, Petersen RH, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg. 2016;49:602–609. [DOI] [PubMed] [Google Scholar]
- 7.Boffa DJ, Dhamija A, Kosinski AS, Kim AW, Detterbeck FC, Mitchell JD, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg. 2014;148:637–643. [DOI] [PubMed] [Google Scholar]
- 8.Bendixen M, Jorgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17:836–844. [DOI] [PubMed] [Google Scholar]
- 9.Nwogu CE, D’Cunha J, Pang H, Gu L, Wang X, Richards WG, et al. VATS lobectomy has better perioperative outcomes than open lobectomy: CALGB 31001, an ancillary analysis of CALGB 140202 (Alliance). The Annals of thoracic surgery. 2015;99:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pages PB, Delpy JP, Orsini B, Gossot D, Baste JM, Thomas P, et al. Propensity Score Analysis Comparing Videothoracoscopic Lobectomy With Thoracotomy: A French Nationwide Study. The Annals of thoracic surgery. 2016;101:1370–1378. [DOI] [PubMed] [Google Scholar]
- 11.Yang CJ, Kumar A, Klapper JA, Hartwig MG, Tong BC, Harpole DH Jr., et al. A National Analysis of Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann Surg. 2019;269:163–171. [DOI] [PubMed] [Google Scholar]
- 12.Su S, Scott WJ, Allen MS, Darling GE, Decker PA, McKenna RJ, et al. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J Thorac Cardiovasc Surg. 2014;147:747–752: Discussion 752–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medbery RL, Gillespie TW, Liu Y, Nickleach DC, Lipscomb J, Sancheti MS, et al. Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol. 2016;11:222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boffa DJ, Kosinski AS, Paul S, Mitchell JD, Onaitis M. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. The Annals of thoracic surgery. 2012;94:347–353; discussion 353. [DOI] [PubMed] [Google Scholar]
- 15.Licht PB, Jorgensen OD, Ladegaard L, Jakobsen E. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. The Annals of thoracic surgery. 2013;96:943–949; discussion 949–950. [DOI] [PubMed] [Google Scholar]
- 16.Long H, Tan Q, Luo Q, Wang Z, Jiang G, Situ D, et al. Thoracoscopic Surgery Versus Thoracotomy for Lung Cancer: Short-Term Outcomes of a Randomized Trial. The Annals of thoracic surgery. 2018;105:386–392. [DOI] [PubMed] [Google Scholar]
- 17.Blasberg JD, Seder CW, Leverson G, Shan Y, Maloney JD, Macke RA. Video-Assisted Thoracoscopic Lobectomy for Lung Cancer: Current Practice Patterns and Predictors of Adoption. The Annals of thoracic surgery. 2016;102:1854–1862. [DOI] [PubMed] [Google Scholar]
- 18.Mehta H, Osasona A, Shan Y, Goodwin JS, Okereke IC. Trends and Outcomes of Thoracoscopic Lobectomy or Segmentectomy: A National Surgical Quality Improvement Project Analysis. Semin Thorac Cardiovasc Surg. 2018;30:350–359. [DOI] [PubMed] [Google Scholar]
- 19.Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA surgery. 2017;152:292–298. [DOI] [PubMed] [Google Scholar]
- 20.Group EC. The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection: Results From an International Registry. Ann Surg. 2015;261:1153–1159. [DOI] [PubMed] [Google Scholar]
- 21.Thiele RH, Rea KM, Turrentine FE, Friel CM, Hassinger TE, McMurry TL, et al. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. Journal of the American College of Surgeons. 2015;220:430–443. [DOI] [PubMed] [Google Scholar]
- 22.Van Haren RM, Mehran RJ, Mena GE, Correa AM, Antonoff MB, Baker CM, et al. Enhanced Recovery Decreases Pulmonary and Cardiac Complications After Thoracotomy for Lung Cancer. The Annals of thoracic surgery. 2018;106:272–279. [DOI] [PubMed] [Google Scholar]
- 23.Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS(R)) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019;55:91–115. [DOI] [PubMed] [Google Scholar]
- 24.Martin LW, Sarosiek BM, Harrison MA, Hedrick T, Isbell JM, Krupnick AS, et al. Implementing a Thoracic Enhanced Recovery Program: Lessons Learned in the First Year. The Annals of thoracic surgery. 2018;105:1597–1604. [DOI] [PubMed] [Google Scholar]
- 25.Madani A, Fiore JF Jr., Wang Y, Bejjani J, Sivakumaran L, Mata J, et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery. 2015;158:899–908; discussion 908–810. [DOI] [PubMed] [Google Scholar]
- 26.Paci P, Madani A, Lee L, Mata J, Mulder DS, Spicer J, et al. Economic Impact of an Enhanced Recovery Pathway for Lung Resection. The Annals of thoracic surgery. 2017;104:950–957. [DOI] [PubMed] [Google Scholar]
- 27.Rogers LJ, Bleetman D, Messenger DE, Joshi NA, Wood L, Rasburn NJ, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg. 2018;155:1843–1852. [DOI] [PubMed] [Google Scholar]
- 28.Gazala S, Hunt I, Valji A, Stewart K, Bedard ER. A method of assessing reasons for conversion during video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg. 2011;12:962–964. [DOI] [PubMed] [Google Scholar]
- 29.Walker WS. Video-assisted thoracic surgery (VATS) lobectomy: the Edinburgh experience. Semin Thorac Cardiovasc Surg. 1998;10:291–299. [DOI] [PubMed] [Google Scholar]
- 30.Kwon ST, Zhao L, Reddy RM, Chang AC, Orringer MB, Brummett CM, et al. Evaluation of acute and chronic pain outcomes after robotic, video-assisted thoracoscopic surgery, or open anatomic pulmonary resection. J Thorac Cardiovasc Surg. 2017;154:652–659 e651. [DOI] [PubMed] [Google Scholar]
- 31.Rice DC, Cata JP, Mena GE, Rodriguez-Restrepo A, Correa AM, Mehran RJ. Posterior Intercostal Nerve Block With Liposomal Bupivacaine: An Alternative to Thoracic Epidural Analgesia. The Annals of thoracic surgery. 2015;99:1953–1960. [DOI] [PubMed] [Google Scholar]
- 32.Rizk NP, Ghanie A, Hsu M, Bains MS, Downey RJ, Sarkaria IS, et al. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. The Annals of thoracic surgery. 2014;98:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonoff MB. Commentary: Predicting patients at risk for complications after thoracic surgery-Application in the era of enhanced recovery. J Thorac Cardiovasc Surg. 2019;157:2502–2503. [DOI] [PubMed] [Google Scholar]
- 34.Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. The Annals of thoracic surgery. 2013;96:1171–1177. [DOI] [PubMed] [Google Scholar]
- 35.Nelson DB, Mehran RJ, Mitchell KG, Correa AM, Sepesi B, Antonoff MB, et al. Enhanced recovery after thoracic surgery is associated with improved adjuvant chemotherapy completion for non-small cell lung cancer. J Thorac Cardiovasc Sing. 2019;158:279–286 e271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The author discusses the study and the clinical relevance of this research


