Abstract
Peripheral nerve regeneration is a complex physiological process. Single-function nerve scaffolds often struggle to quickly adapt to the imbalanced regenerative microenvironment, leading to slow nerve regeneration and limited functional recovery. In this study, we demonstrate a “pleiotropic gas transmitter” strategy based on endogenous reactive oxygen species (ROS), which trigger the on-demand H2S release at the defect area for transected peripheral nerve injury (PNI) repair through concurrent neuroregeneration and neuroprotection processing. This H2S delivery system consists of an H2S donor (peroxyTCM) encapsulated in a ROS-responsive polymer (mPEG-PMet) and loaded into a temperature-sensitive poly (amino acid) hydrogel (mPEG-PA-PP). This multi-effect combination strategy greatly promotes the regeneration of PNI, attributed to the physiological effects of H2S. These effects include the inhibition of inflammation and oxidative stress, protection of nerve cells, promotion of angiogenesis, and the restoration of normal mitochondrial function. The adaptive release of pleiotropic messengers to modulate the tissue regeneration microenvironment offers promising peripheral nerve repair and tissue engineering opportunities.
An ROS-triggered, on-demand H2S delivery system is introduced to quickly remodel the neuroregenerative microenvironment.
INTRODUCTION
Peripheral nerve injury (PNI) includes all damage to the peripheral nerve’s morphological structure or physiological function. The causes of injury can be divided into four categories: physical, chemical, biological, and internal factors. Physical trauma is the most common type of PNI in clinical practice. Because of the complex structure and function of the peripheral nerve, PNI often results in poor prognosis and high disability rates, remaining a big challenge for clinical medicine today. At present, nerve anastomosis, autologous nerve transplantation, and allogeneic nerve transplantation are the main treatments for PNI. However, the limited suture distance, insufficient donor sources, and potential immune rejection greatly limit the further development of this treatment method. Engineered nerve conduits to repair long nerve gaps offer an alternative approach. The basic processes of peripheral nerve regeneration using engineered nerve conduit repair include creating a regenerative microenvironment and establishing the axon regeneration pathway [cell cable formation, vasculature polarization, and Schwann cell (SC) spreading (1)], promoting axonal sprout growth and extension, and reinnervating target organs. This process involves multicellular growth, migration, proliferation, differentiation, and axon regeneration. It is susceptible to disruption by oxidative stress, excessive inflammation, ischemia, mitochondrial damage, etc. (2), leading to difficulty with revascularization and fibrous scarring. However, the local regenerative microenvironment of nerve lesions is imbalanced and complex, making it challenging to repair damaged peripheral nerves using grafts. Drug-delivered grafts have attracted attention for their potential to reconstruct the peripheral nerve microenvironment, but the appropriate regulation time and stage for regulating the microenvironment are still unclear (3, 4). Accurately administering drugs on demand is a challenge in regulating pathological microenvironments. In addition, the blood-nerve barrier at the nerve stump also restricts the effective uptake of drugs in the injured area, resulting in the inability to produce therapeutic effects on the injured nerve end in the early stage of graft implantation (5). Moreover, there is a time window for target organs to reconnect with nerves, and long-term denervation atrophy will make reinnervation difficult, resulting in permanent loss of function. Therefore, multi-effect and efficient repair strategies are urgently needed to address the bottlenecks in regulating the damaged neuropathological microenvironment and promoting slow nerve regeneration.
Hydrogen sulfide (H2S) has long been recognized as a toxic gas. However, in 1996, Abe and Kimura demonstrated that endogenous H2S plays an important role in neuromodulation, which provided a new understanding of H2S as a highly active molecule that can easily react with reactive oxygen species (ROS) and reactive nitrogen species (5, 6). Moreover, because of its ability to penetrate cell membranes and function as a signal transducer to specific cells and molecular targets, H2S has broad application prospects in anti-inflammatory, anti-apoptosis, antitumor, vasodilator, and cell protection (7). In addition, small size and diffusivity make it easy for H2S to penetrate the blood-nerve barrier and perform executive functions. Notably, the biological effect of H2S is related to the release rate, and rapid release and accumulation are likely to cause physiological toxicity (8). Therefore, the premise of H2S as a PNI microenvironment therapeutic drug is that the drug delivery system can achieve on-demand release over time. Peroxythiocarbamate (peroxyTCM) is the class of triggerable carbonyl sulfide (COS)/H2S donors that can be activated by cellular ROS (9). By loading the peroxyTCM in the affected area, a functional system can be created for efficient H2S storage and on-demand release for traumatic PNI therapy.
This study proposes a “pleiotropic messenger” strategy based on ROS-triggered on-demand H2S release at the defect area for traumatic PNI recovery. The functional system was fabricated by using peroxyTCM encapsulated with methoxy poly(ethylene glycol)-poly(l-methionine) nanoparticles (PTCM@PMet NPs) and then loaded into a thermosensitive hydrogel [methoxy poly(ethylene glycol)-polyalanine-polyphenylalanine (mPEG-PA-PP)] precursor solution. Last, the H2S functional system was injected into poly(3S-(methyl)-morpholine-2,5-dione-co-ɛ-caprolactone) [P(MMD-CL)] conduit to obtain an adaptive gas transmitter biomimetic nerve scaffold with high spatiotemporal resolution (Fig. 1). Upon implantation, the accumulation of ROS caused by ischemia and inflammation in the lesion area would disrupt the PTCM@PMet NPs and further trigger the release of H2S. In vitro studies have shown that H2S released at appropriate levels can reduce apoptosis in rat Schwann cells (RSCs), induce macrophage polarization toward the M2 phenotype, and promote human umbilical vein endothelial cells (HUVECs) migration and tube formation. H2S also exhibited cytoprotective effects in the RSCs oxidative damage model, including reducing oxidative stress, stimulating complex I and V activities, and increasing mitochondrial membrane potential (MMP) and adenosine triphosphate (ATP) production. In vivo experiments have shown that the combined treatment of PTCM@PMet/mPEG-PA-PP/P(MMD-CL) substantially facilitates the recovery of motor function in Sprague-Dawley (SD) rats with transected sciatic nerve injury, ascribable to the pleiotropic effects of H2S, such as anti-inflammatory, antioxidant, induction macrophage M2 polarization, pro-angiogenesis, mitochondrial protection, and neuroprotection. Notably, the H2S functional system can form spontaneously in the lesion area and degrade quickly. This suggests that this strategy may provide new ideas for repairing a wider range of injuries, including treating complex and difficult-to-repair traumatic spinal cord injuries.
Fig. 1. Schematic illustration of constructing an intelligently responsive multi-effect messenger biomimetic nerve scaffold for PNI repair.
Ischemia and inflammation after PNI will cause many ROS, which will trigger the release of H2S donors from the H2S functional system and further H2S production. The pleiotropic effects of H2S include anti-inflammatory, anti-oxidative, angiogenesis, and mitochondrial function repair, accelerating the reconstruction of the microenvironment in SD rats’ injured nerve regeneration, axons’ growth, and motor function recovery after trauma. IFN-γ, interferon-γ; GM-CSF, granulocyte-macrophage colony-stimulating factor.
RESULTS
Synthesis and physicochemical characterization of PTCM@PMet/mPEG-PA-PP/P(MMD-CL)
The synthesis of peroxyTCM and peroxy carbamate (peroxyCM), as well as the release mechanism in response to H2S and CO2, is shown in Fig. 2A. The boronic ester group of peroxyTCM was oxidized to a phenolic hydroxyl group under the action of H2O2. Then, COS, 4-hydroxy benzyl alcohol, and 4-fluoro aniline were generated after a 1,6-elimination reaction, and COS was then hydrolyzed into H2S by the ubiquitous carbonic anhydrase. The 1H nuclear magnetic resonance (NMR) and high-resolution mass spectrometry (HRMS) spectral characterization results of peroxyTCM and peroxyCM are shown in figs. S1 and S2, and both donors were successfully prepared. The rate of COS release from peroxyTCM under the action of H2O2 can be calculated from the peak reduction of the benzyl proton of peroxyTCM at 5.5 ppm or the peak of the benzyl proton of 4-hydroxy benzyl alcohol at 4.4 ppm in the 1H NMR spectrum (Fig. 2B).
Fig. 2. Synthesis and physicochemical characterization of an H2S adaptive delivery system.
(A) Design and synthesis of H2S and CO2 donor and their ROS-triggered release mechanisms. (B) 1H NMR study of H2O2-induced activation of peroxyTCM. (C) a. Schematic illustration of mPEG-PMet synthesis and coassembly with peroxyTCM into NPs; b. Schematic representation and mechanism of drug release from PTCM@PMet in the presence of ROS. (D) TEM images of PTCM@PMet and PCM@PMet NPs. Scale bars, 200 nm and 100 nm. (E) Schematic diagram of mPEG-PA-PP synthesis and loading of PTCM@PMet NPs to construct an injectable H2S adaptive release system. (F) Relationship between phase transition temperature and concentration of mPEG-PA-PP hydrogel. (G and H) Rotational rheological properties of mPEG-PA-PP hydrogels and PTCM@PMet NPs/mPEG-PA-PP hydrogels (storage modulus G′ and loss modulus G″). (I) Frequency sweep measurements (0.01 to 100 rad s−1; 1% strain) of hydrogels. (J) Storage modulus (G′) curves from rotational strain sweeps (0.01 to 10,000% strain; 10 rad s−1) of hydrogels. (K) The stress-strain curves of hydrogels. (L) SEM images of hydrogels. Scale bars, 50 μm. (M) Top: Synthesis of MMD monomer using l-alanine and chloroacetyl chloride. Bottom: Ring-opening polymerization reaction of CL and MMD to P(MMD-CL) catalyzed by Sn(Oct)2. (N) Schematic diagram of electrospinning nerve conduit preparation. (O to Q) Ultimate tensile strain, stress-strain, and Young’s modulus of the PCL and P(MMD-CL) fiber membranes. (R) SEM micrographs of P(MMD-CL) fiber membranes. Scale bars, 2 μm. (S) 2D kernel density graph of the angle and diameter distribution of P(MMD-CL) fiber. (T) P(MMD-CL) fiber diameter statistical histogram and normal distribution curve. (U and V) The weight loss rate and pH change of P(MMD-CL) and PCL during degradation within 8 weeks. (W) Water contact angles of P(MMD-CL) and PCL fiber membranes. (X) The injectability of mPEG-PA-PP hydrogels. **P < 0.01, ***P < 0.001.
The amphiphilic poly(amino acid) mPEG-PMet was synthesized by ring-opening polymerization of l-methionine N-carboxy anhydride (Met NCA) initiated by mPEG-NH2 (Mn = 4000) [Fig. 2C(a)]. The mechanism of ROS response lies in the easily oxidizable thioether group in the side chain of Met, which turns into hydrophilic sulfoxide or sulfone under oxidative stress, resulting in a phase transition [Fig. 2C(b)]. The characterization results of 1H NMR spectroscopy are shown in figs. S3C and S4A. PTCM can be easily encapsulated by mPEG-PMet through self-assembly to form nanoparticles (NPs) with a loading efficiency of 4.92%. In addition, the PTCM@PMet and PCM@PMet NPs were observed as uniform rod-like structures by transmission electron microscopy (TEM; Fig. 2D). The average particle size measured by dynamic light scattering (DLS) was 54.87 and 92.54 nm, respectively (Fig. 2D). To investigate whether PTCM@PMet NPs exhibit ROS-sensitive drug release, the cumulative release rate of peroxyTCM from PTCM@PMet NPs was examined after incubation with H2O2. As shown in fig. S5, PTCM@PMet NPs exhibited a concentration-dependent, accelerated drug release in H2O2 compared to phosphate-buffered saline (PBS) after incubation. When incubated in 500 μM H2O2 PBS solution, about 37% of peroxyTCM was released from NPs within 48 hours. In contrast, about 21% of peroxyTCM was released from NPs after incubation in 50 μM H2O2 aqueous solution for 48 hours.
The amphiphilic poly(amino acid) mPEG-PA-PP was synthesized by the ring-opening polymerization of l-alanine (Ala) NCA and l-phenylalanine (Phe) NCA initiated by mPEG-NH2 (number-average molecular weight (Mn) = 2000) (Fig. 2E). The characterization results of 1H NMR spectroscopy are shown in figs. S3 (A and B) and S4B. Figure 2X illustrates that the mPEG-PA-PP hydrogel precursor can write “WUT” through a syringe and undergo a phase transition to form a hydrogel at 37°C. The pore structure and phase transition temperature of hydrogels can be regulated by adjusting the concentration of precursors. In Fig. 2F, the phase diagram indicates that a concentration exceeding 2 wt % of mPEG-PA-PP hydrogel is necessary for gel formation, and at 4% concentration, the phase transition temperature surpasses 37°C. Elevated polymer concentrations in thermosensitive hydrogels tend to reduce pore size, leading to a denser network structure after gelation. Previous studies have shown that the basic structure of a 10-mm sciatic nerve defect can be rebuilt within 4 weeks (10). Therefore, the hydrogel’s degradation traits must be considered to prevent hindrance to developing the essential nerve structure. In vitro degradation results indicate that 6 wt % mPEG-PA-PP hydrogel degrades more rapidly than the 8 wt % hydrogels (fig. S6). This suggests that the 6 wt % mPEG-PA-PP hydrogel would have a lesser impact on nerve growth.
According to the rheological characteristics (Fig. 2, G and H), the mPEG-PA-PP hydrogel exhibited an elastic, solid-like behavior (storage modulus G′ > loss modulus G″) after heating up to around 30°C, and the addition of PTCM@PMet NPs did not change the rheological properties of the hydrogel. The frequency scan storage modulus (G′) measurement curve (0.01 to 10 rad s−1, 1% strain) shows that the G′ values of mPEG-PA-PP and PTCM@PMet/mPEG-PA-PP hydrogels are correlated with the frequency of 0.01 to 10 rad s−1, which may be related to the progressive increase in cross-linking of the hydrogels at 37°C (Fig. 2I). The strain sweep results (0.01 to 1000% strain, 10 rad s−1) (Fig. 2J) revealed that the loading of drug-loaded NPs did not affect storage modulus or fracture strain of the hydrogel. The compressive stress-strain curves (Fig. 2K) indicated that the loading of NPs had no notable effect on the hydrogel’s compressive strength and final deformation rate. Scanning electron microscopy (SEM) imaging (Fig. 2L) showed that the mPEG-PA-PP and PTCM@PMet/mPEG-PA-PP exhibited a sheet-like structure with many 50-μm pores interconnected like a “highway system.”
P(MMD-CL) were obtained by melting ring-opening polymerization of MMD and CL catalyzed by Sn(Oct)2 (Fig. 2M). The resulting polymer had an average molecular weight (Mn) of 153,261, a polydispersity index of 2.27, and a monomer ratio of MMD to CL of 1:12.68 (figs. S7 and S8). Electrospinning prepared a P(MMD-CL) fiber membrane, which was uniform and nearly bead-free (Fig. 2, N and R). The tensile strength of the P(MMD-CL) electrospun film was 2.47 ± 0.14 MPa (Fig. 2P), with an elongation at a break of 291.47 ± 15.34% (Fig. 2O) and Young’s modulus of 9.09 ± 0.59 MPa (Fig. 2Q), which were close to the mechanical properties of the polycaprolactone (PCL) electrospun film (1.97 ± 0.16 MPa, 597.11 ± 37.26%, and 6.74 ± 0.54 MPa). The two-dimensional (2D) kernel density graph showed that the P(MMD-CL)–oriented fibers had a high consistency of the orientation direction, with most fibers deviating from the orientation direction by less than 10° (Fig. 2S). The average diameters of P(MMD-CL)–oriented fibers and random fibers were 1413.10 ± 180.02 nm and 924.50 ± 139.33 nm, with a normal distribution of fiber diameter (Fig. 2T). The water contact angle of P(MMD-CL) was 127°, revealing an improvement in hydrophilicity compared to that of PCL (141°) (Fig. 2W). After 8 weeks of degradation, the weight loss rate of PCL was only 2.53% while that of P(MMD-CL) reached 6.72% (Fig. 2U). Although P(MMD-CL) degraded faster than PCL, its pH remained above 7.0 (Fig. 2V). Overall, P(MMD-CL) generally maintained favorable mechanical properties and improved hydrophilicity and degradation properties compared to PCL, which provided a new choice for peripheral nerve repair materials.
Cell imaging of H2O2-induced H2S release from PeroxyTCM
To investigate the biological effects of peroxyTCM, we initially determined whether H2O2 in the cellular environment could trigger the release of H2S from peroxyTCM. 7-Azido-4-methylcoumarin (AzMC), a fluorescent probe, can be reduced by H2S to generate fluorescent 7-amino-4-methylcoumarin (AMC), thereby allowing for monitoring the accumulation of H2S. No AMC fluorescence was observed in the control (CON) group, indicating that peroxyTCM was stable in the normal cellular environment. In contrast, RAW264.7 cells and RSCs were observed to emit AMC fluorescence upon the addition of H2O2, and this fluorescence intensity increased dose-dependently with rising H2O2 levels (Fig. 3E and fig. S9), suggesting that ROS can activate peroxyTCM in the cellular environment, leading to the release of H2S. In general, the phenylboronic acid pinacol ester in the molecular structure of peroxyTCM acted as a ROS sensor to enable the precise temporal and spatial release of H2S, which could be used for precise treatment of inflammatory sites in vivo without causing systemic side effects.
Fig. 3. H2S regulates macrophage inflammation and polarization.
(A) The visualized PCA plot is based on the relative quantitative values of the samples, with the horizontal and vertical axes representing the degree of interpretation of PC1 and PC2. (B) Differential gene expression volcano plot. (C) Top 30 differential protein radar graph. The first circle of the diagram indicates the differential proteins, according to the P value from small to large along the orange arrows in clockwise order; the second circle indicates the ratio value in log2 format, where pink denotes up-regulation and light blue signifies down-regulation, with larger points indicating greater fold changes (FC); the third circle indicates the average quantification level of the two groups, with spikes indicating high expression of differential proteins. (D) The proteins were divided into four fractions based on differential expression ploidy, called Q1, Q2, Q3, and Q4. (E) The cellular uptake of peroxyTCM by RAW264.7 and the release of H2S in response to H2O2. Scale bars, 25 μm. (F) Representative images of CD206 immunofluorescence staining of RAW 264.7 after 3 days of treatment with PTCM@PMet NPs at an effective H2S concentration of 50 μM. Scale bar, 20 μm and 50 μm. (G) Schematic diagram of macrophage polarization induced by H2S. (H) Western blot results of CD206 protein expression in macrophages cultured with PTCM@PMet NPs for 3 days. (I) Schematic diagram of the calculation of macrophage elongation. (J) Statistical results of elongation rate of macrophage cells cultured with PTCM@PMet NPs for 3 days. (K) ELISA detection results of TNF-α and IL-10 factors expression in macrophages cultured with PTCM@PMet NPs for 3 days. *P < 0.05, **P < 0.01, ***P < 0.001. GAPDH, glyceraldehyde phosphate dehydrogenase.
The cytocompatibility of PTCM@PMet NPs, mPEG-PA-PP hydrogels, and P(MMD-CL) membrane
Drugs that modulate the neuroregenerative microenvironment should not significantly affect cell viability. As a result, RSCs were chosen to evaluate the cytocompatibility of PTCM@PMet NPs. Although peroxyTCM cultured directly with RSCs at a concentration of 50 μM exhibited noticeable cytotoxicity, peroxyTCM encapsulated in mPEG-PMet had negligible effect on cell viability (fig. S10A). To determine whether PTCM@PMet NPs remain at safe concentrations under H2O2 stimulation, LIVE/DEAD cell staining experiments were performed. Observations made using an inverted fluorescence microscope (IX71, Olympus, Japan) showed that PTCM@PMet at an effective drug concentration of 50 μM had no detectable apoptotic response when exposed to 50 μM H2O2 stimulation (fig. S10B). This result indicated that PTCM@PMet at an effective drug concentration of 50 μM remained safe even in the presence of 50 μM H2O2. In addition, other components of the conduit, including the mPEG-PA-PP hydrogel precursor and P(MMD-CL) membrane, exhibited excellent biocompatibility (fig. S11).
Anti-inflammatory effects of H2S
To explore the biological effect of H2S in neuroregeneration, RAW264.7 cells, capable of exerting both pro- or anti-inflammatory effects, were incubated with PTCM@PMet NPs to investigate the influence of H2S on cellular immune responses. Differential proteomic analysis was performed on RAW264.7 cells treated with PCM@PMet and PTCM@PMet NPs. In each comparison, the PCM@PMet NPs (CO2) group served as the control of H2S group. This choice was made to elucidate the biological effects of H2S and mitigate potential interference from the by-products of H2S donor activation. Principal components analysis (PCA) revealed a distinct differentiation between the H2S and CO2 groups, primarily along PC1 (Fig. 3A). Volcano plots of differentially expressed proteins (DEPs) showed that 159 proteins were statistically up-regulated, while 192 were down-regulated in the H2S group (Fig. 3B). Radar charts were used to display the relative expression levels of the top 30 differential proteins (Fig. 3C). To discern whether the DEPs exhibited notable enrichment in specific functional categories, we conducted Gene Ontology (GO) categorization of DEPs from each comparison group (fig. S12A). Enrichment chord diagrams indicated that in the cellular component category, DEPs predominantly localized in the extracellular space, extracellular region, and cell surface. Molecular function categorization revealed that these DEPs involved in signaling receptor and molecular transducer activity. Analysis of biological processes suggested that DEPs modulated by H2S are implicated in regulating immune response and the endoplasmic reticulum–nucleus signaling pathway. To compare functional distinctions among proteins with varying differential expression magnitudes, we divided the proteins into four quartiles designated as Q1 through Q4 (Fig. 3D). We performed GO enrichment for each quartile, followed by functional clustering analysis. Using a hierarchical clustering approach based on Fisher’s exact test P values obtained from enrichment analysis, related functions across different quartiles were clustered together. Heatmaps revealed that greatly down-regulated DEPs in the H2S group (Q1) are primarily enriched in processes such as regulation of inflammatory response and neutral amino acid transport. On the basis of these bioinformatics insights, H2S may hold potential therapeutic value in inhibiting macrophage inflammatory processes (fig. S12B).
To elucidate the impact of H2S on macrophages, we incubated the cells with PTCM@PMet or PTCM@PMet NP for 3 days and subsequently examined their morphology. Macrophage phenotype changes reflect macrophage function alterations (Fig. 3G) (11). Immunofluorescence staining was performed for the M2 macrophage surface marker CD206 (Fig. 3F). The CON group and CO2 group showed round macrophages. In contrast, the H2S group showed more slender macrophages with cell elongation 1.24 times greater than the CO2 group (P < 0.05) (Fig. 3, I and J). The cell elongation is closely related to the polarization of macrophages toward the M2 phenotype (12). The fluorescence intensity of CD206 on macrophages treated with PTCM@PMet NPs was significantly increased, further confirming that H2S can induce polarization toward the M2 phenotype, which was also consistent with the Western blot results (Fig. 3H). In addition, enzyme-linked immunosorbent assay (ELISA) results showed that macrophages treated with PTCM@PMet NPs significantly down-regulated the proinflammatory factor tumor necrosis factor–α (TNF-α) and promoted the expression of the anti-inflammatory factor interleukin-10 (IL-10) (Fig. 3K). This indicated that H2S could affect the morphology and polarization of macrophages and alter their protein expression and factor secretion. These studies collectively suggest that H2S has the potential to modulate immune function by influencing macrophage phenotypes.
The inflammatory response is the first stage of nerve regeneration. After nerve injury, damage-associated molecular patterns (DAMPs) and external infections trigger an early acute inflammatory response, during which M1 macrophages dominate and play a role in phagocytosis of tissue debris and pathogens (Fig. 4E). After the acute inflammatory phase, the inflammatory response gradually subsides, and the immune homeostasis is restored, and M2 macrophages gradually dominate and play an anti-inflammatory and pro-repair role (Fig. 4E). To further evaluate the role of H2S in regulating the M2 polarization process of macrophages in vivo, immunofluorescence staining for CD68, CD206, CCR7, IL-10, and TNF-α was used to investigate early macrophage polarization, following our in vitro results that showed H2S promoted the polarization of macrophages toward the M2 phenotype. The first and second weeks were chosen as the assessment time points to detect changes in the immune response. At day 7, CCR7-positive expression was higher in the CO2 group than in the H2S group, whereas CD206-positive expression was lower than in the H2S group, and M1 macrophages dominated (Fig. 4A). Five regions in the immunofluorescence images were randomly selected, and the fluorescence intensity was counted for CCR7/CD68 and CD206/CD68. It was found that the proportion of M1 macrophages was higher in the CO2 group than in the H2S group (P < 0.05), while the proportion of M2 macrophages was lower than in the H2S group (P < 0.001) (Fig. 4, F and H). The same method was used to count TNF-α and IL-10. The results showed that the expression of inflammatory factors was lower in the H2S group than in the CO2 group (P < 0.05), while the expression of anti-inflammatory factors was higher than in the CO2 group (P < 0.05), as further verified by the Western blot results (Fig. 4, G, I, and N). The results in Fig. 4 (C and D) showed that the macrophage ratio had shifted at day 14, with M2 macrophages dominating. Notably, the proportion of M2 macrophages (P < 0.001) and the expression of anti-inflammatory factor (IL-10) (P < 0.05) were higher in the H2S group than in the CO2 group. In comparison, the proportion of M1 macrophages (P < 0.01) and the expression of inflammatory factor (TNF-α) (P < 0.01) were lower than in the CO2 group (Fig. 4, J to M). The above results suggested that H2S could induce the polarization of macrophages in vivo to the M2 phenotype, suppress the inflammatory response, and accelerate the restoration of immune homeostasis.
Fig. 4. H2S induces macrophage polarization in vivo.
(A and B) DAPI/CCR7/CD68/TNF-α and DAPI/CD206/CD68/IL-10 immunofluorescent staining of proximal nerve stumps 1 week postsurgery. Scale bars, 20 μm. (C and D) DAPI/CCR7/CD68/TNF-α and DAPI/CD206/CD68/IL-10 immunofluorescent staining of regenerated nerves 2 weeks postsurgery. Scale bars, 20 μm. (E) Schematic diagram of nerve injury leading to inflammatory response and the effect of H2S treatment on immune microenvironment balance. Statistical results of (F) CCR7/CD68, (G) TNF-α, (H) CD206/CD68, and (I) IL-10 immunofluorescence 1 week post-operation. Statistical results of (J) CCR7/CD68, (K) TNF-α, (L) CD206/CD68, and (M) IL-10 immunofluorescence 2 weeks post-operation. (N) Western blot results of TNF-α and IL-10 in proximal nerves 1 week post-operation. *P < 0.05, **P < 0.01, ***P < 0.001. iNOS, inducible nitric oxide synthase; TGF-β, transforming growth factor–β.
To observe the early changes in inflammatory, fibrotic, and regenerative signaling pathways following H2S treatment, we conducted RNA sequencing (RNA-seq) on the proximal nerve stumps in rats. Compared with the CO2 group, a total of 2802 DEGs (differentially expressed genes) were identified in the H2S group, with 1399 up-regulated and 1403 down-regulated [|log2(fold change)| > 2, P < 0.05] (Fig. 5A). GO analysis suggested strong associations of the DEGs with inflammation, oxidative stress, cell adhesion, and axonal growth (Fig. 5B). The KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment chart showed functional annotations related to anti-inflammation and antioxidation, including nuclear factor κB (NF-κB), TNF, and IL-17 signaling pathways (Fig. 5C). Key downstream expression factors in these pathways, such as cytokines and chemokines like TNF-α and IL-17, also play pivotal roles in fibrosis. Suppression of these inflammatory pathways may help establish an immune microenvironment conducive to nerve regeneration and reduce neurotissue fibrosis. Using the GSEA (gene set enrichment analysis) method on the gene expression data for GO and KEGG enrichment revealed that H2S treatment attenuated the inflammatory response process and inhibited the activation of NF-κB, TNF, and IL-17 signaling pathways, resulting in decreased expression of inflammatory factors (Fig. 5, D and E, and fig. S13, A and B). The DEGs were also enriched in cellular adhesion molecule signaling pathways, PI3 kinase (PI3K)–Akt signaling pathway, and the JAK-STAT signaling pathway, pathways crucial in various biological processes such as muscle contraction, neurotransmission, enzymatic activity, cell growth, and differentiation (Fig. 5C). Regarding the modulation of nerve growth, the DEGs were enriched in the calcium signaling pathway, and cyclic adenosine 3′,5′-monophosphate (cAMP) signaling pathway, both of which are crucial in synapse formation, axonal guidance, axonal growth, and nerve regeneration (Fig. 5C). The GSEA method on the gene expression data for GO and KEGG enrichment indicated that H2S treatment promoted the biological process of calcium ion binding, activating the calcium signaling pathway. For further analysis, the normalized heatmap also revealed an up-regulation of M2 macrophage polarization marker genes and a significant down-regulation in the expression of genes associated with inflammation, fibrosis, and chemotactic factors following H2S treatment (Fig. 5F). In conclusion, our findings suggest that H2S can enhance the nerve regenerative microenvironment, thereby promoting peripheral nerve regeneration.
Fig. 5. Regulation of inflammation, fibrosis, and regeneration by H2S.
(A) Differential gene expression volcano plot. (B) GO enrichment bar graphs (cell adhesion, inflammation, and nerve growth). BP, biological process. CC, cell component. MF, molecular function. MHC, major histocompatibility complex. (C) Scatterplot of KEGG enrichment (cell adhesion, signaling, inflammation, nerve regeneration, and growth). TH17, T helper cell 17; PPAR, peroxisome proliferator–activated receptor; MAPK, mitogen-activated protein kinase; ECM, extracellular matrix. (D) GO term GSEA. (E) KEGG pathway GSEA. (F) Heatmap showing relative changes in expression levels of selected inflammation-related genes compared to the blank CON group. (G) Representative images of NF-κB immunofluorescence staining of proximal nerves 1 week after surgery. Scale bar, 50 μm. (H) Western blot results of protein expression of NF-κB,caspase-1, and IL-1β in proximal nerve stumps 1 week after the operation. (I) The expression of NF-κB and TNF-α genes in proximal nerve stumps 1 week after the operation. (J) Schematic diagram of H2S inhibiting NF-κB signaling pathways. *P < 0.05, **P < 0.01.
To further investigate the anti-inflammatory effects of H2S in the early stages of postnerve injury, we assessed the nerve stumps 1 week after surgery. The inflammasomes are composed of sensor molecules, adaptor proteins, and the effectorcaspase-1 and are activated by the initiating signals mediated by proinflammatory pathways. For instance, the Toll-like receptor (TLR)–mediated DAMPs activate NF-κB, which in turn further activatescaspase-1, promoting the maturation and secretion of the cytokine IL-1β (Fig. 5J) (13). NF-κB regulates the transcription of proinflammatory mediators, such as TNF-α, IL-6, IL-1β, and inducible nitric oxide synthase (14). A key step in NF-κB activation and its subsequent translocation to the nucleus is the degradation of IκBα, a protein that sequesters NF-κB in the cytoplasm (15). Upstream TLR activation would lead to phosphorylation and degradation of IκBα, thereby releasing NF-κB and translocating it into the nucleus (Fig. 5J). To monitor the activation of inflammatory pathways and expression of inflammatory factors in regenerating nerve terminals 1 week after surgery in the H2S conduit treatment group, NF-κB, Capase-1, TNF-α, and IL-1β expression were measured by immunofluorescence, reverse transcription quantitative polymerase chain reaction (RT-qPCR), and Western blot. The fluorescence intensity of NF-κB, NF-κB gene expression (P < 0.01), and P65 protein expression were lower in the H2S group compared to the CO2 group, suggesting that H2S could inhibit NF-κB activation and thus reduce the secretion of inflammatory factors (Fig. 5, H and I). The blockade of NF-κB nuclear translocation by H2S also implies a reduction in thecaspase-1 cascade. In conclusion, exogenous H2S appeared to inhibit the secretion of proinflammatory mediators, an effect that was likely due to the inhibition of NF-κB pathways.
H2S-induced cell proliferation and angiogenesis
To test the ability of H2S to promote angiogenesis in nerve repair, HUVECs, a commonly used cell model for vascular endothelial cell experiments (16), were incubated to study the effect of H2S on endothelial cell migration and tube formation during nerve regeneration. Scratch closure was observed at the 12 and 24-hour time points. Figure 6A showed representative images of wound healing in all groups. The statistical results indicated a significantly higher rate of wound healing in the PTCM@PMet NP–treated group after 12 hours of incubation compared to the PCM@PMet NP–treated group (P < 0.05) (Fig. 6J). As the incubation time was extended to 24 hours, the scratches in the H2S group were almost completely closed due to the promotion of cell migration by H2S. Further, the effect of H2S release in the medium on the migration of HUVECs was investigated using the Transwell’s method, as shown in Fig. 6B. The blank group and CO2 group were used as controls. Cell migration statistics after crystalline violet staining showed that the migration of HUVECs was enhanced in the H2S group compared to the other groups (Fig. 6K). In addition to endothelial cell migration, tube formation is an important step in angiogenesis. Figure 6C showed representative images of HUVECs tube formation in vitro for all groups at 6 hours. The number of tubes formed was calculated to quantify the angiogenesis rate. After 6 hours of incubation, the H2S group showed many tube branches and a distinct network of tubes, nearly twice as many as the CO2 group (Fig. 6L). The above results suggested that H2S produced by PTCM@PMet NPs stimulated by H2O2 has the potential to promote angiogenesis rapidly and holds promise for solving the problem of slow nerve regeneration due to difficulty in vivo angiogenesis.
Fig. 6. The angiogenic, cytoprotective, and proliferative cell effects of H2S.
(A) Scratch wound healing tests assessed the effect of H2S on HUVEC migration from 0 to 24 hours. Scale bar, 500 μm. (B) The effect of H2S on HUVEC migration was assessed by a 24-hour Transwell method. Scale bar, 100 μm. (C) A 6-hour tube formation assay assessed the ability of H2S to promote angiogenesis. Scale bar, 100 μm. (D) Differential gene expression volcano plot. (E) Functional annotation results of the differential genes in the KEGG database. (F) Gene expression of HUVECs after 3 days of treatment with PTCM@PMet NPs at an effective H2S concentration of 50 μM. (G) Immunofluorescent staining of VEGF/CD31 and (H) Ki67 in the proximal nerve stump 1 week postoperatively. Scale bar, 50 μm. (I) Western blotting assay for PI3K and Akt protein expression. (J) Summarized data on cell migration by Transwell assays. (K) Summarized data showing the difference in the number of meshes in HUVECs. (L) Summarized data showing the difference in wound healing rate after making HUVECs scratched for 12 and 24 hours. (M) Gene expression of eNOS, VEGF, and CD31 in the proximal nerve stump 1 week postoperatively. (N) Gene expression of mTOR and Ki67 gene expression in the proximal nerve stump 1 week postoperatively. (O) Schematic diagram of the mechanism of H2S promoting angiogenesis and cell proliferation. *P < 0.05, **P < 0.01, ***P < 0.001.
To gain insights into the regulatory mechanisms of H2S in angiogenesis, we conducted a transcriptomic analysis after culturing HUVECs with PTCM@PMet or PCM@PMet NPs. We set thresholds of |log2fold change| > 2 and P < 0.05 to filter significantly DEGs. Our results (Fig. 6D) showed that after 72 hours of coculturing with PTCM@PMet and PCM@PMet NPs, there were 478 DEGs compared to the CON group, with 376 genes up-regulated and 102 genes down-regulated. This significant alteration in gene expression suggests a profound influence of H2S on the behavior of HUVECs. GO analysis between the two groups indicated a strong association of DEGs with cellular adhesion and cellular bioprocess formation (Fig. 6E). The KEGG enrichment chart illustrated the enrichment of DEGs in various pathways, including the Ras signaling pathway, Rap1 signaling pathway, and the PI3K/Akt signaling pathway, which regulate cellular proliferation, differentiation, migration, survival, and angiogenesis (fig. S14). We then specifically evaluated the expression of key genes associated with the PI3K/Akt signaling pathway, including PI3K, Akt, endothelial nitric oxide synthase (eNOS), mammalian target of rapamycin (mTOR), vascular endothelial growth factor (VEGF), and von willebrand factor (vWF). Compared to the CO2 group, H2S demonstrated a pronounced up-regulatory trend in the expression of angiogenesis-related genes in the PI3K/Akt pathway (Fig. 6F).
eNOS is a calcium-dependent enzyme expressed in vascular endothelial cells. eNOS activity is regulated by phosphorylation/dephosphorylation of several amino acid residues in addition to calcium ions (17). The most effective way to increase the specific activity of eNOS is to phosphorylate the amino acid residue Ser1177, which is caused by the activation of protein kinase B (Akt) in endothelial cells (18). The activation (phosphorylation) of Akt is controlled by the PI3K system (19). It has been shown that exogenous H2S can promote eNOS expression by activating the PI3K/Akt pathway (Fig. 6O) (16). We detected the expression of PI3K/Akt vascularization-related genes and proteins in vivo and verified whether H2S accelerated angiogenesis in regenerated nerves. Western blotting experiments first examined the phosphorylation levels of PI3K and Akt 1 week after injury to the proximal nerve. The phosphorylation levels of PI3K/Akt were greatly higher in the H2S group compared to the CO2 group after 1 week of treatment (Fig. 6I). RT-qPCR results showed that the eNOS mRNA expression levels were 1.3-fold higher in the H2S group compared to the CO2 group (P < 0.01) (Fig. 6M). We further examined angiogenesis and representative images of VEGF/CD31 immunofluorescence staining were shown in Fig. 6G, indicating neovascularization in the conduits 1 week postsurgery and compared to the CO2 group, the H2S, and autologous groups exhibited more significant positive expression of VEGF and CD31, with a larger and more well-defined vascular network. Nerve regeneration is closely related to cell proliferation, growth, motility, and metabolism, and the mTOR is the main transduction pathway that regulates cell activity. RT-qPCR results showed that H2S up-regulated the expression of mTOR (P < 0.001) and Ki67 (P < 0.05) genes (Fig. 6N). In addition, immunofluorescence detection on proximal nerve stumps further indicated that H2S promoted cell proliferation in nerve tissue (Fig. 6H). Our results suggest that H2S exerts certain effects on the PI3K/Akt signaling pathway, promoting cell proliferation and migration and accelerating angiogenesis.
H2S-induced oxidative stress reduction and mitochondrial function recovery
The ischemic and inflammatory response to PNI in the lesion and distal stump leads to the production of large amounts of ROS in the mitochondria, which are the signals that initiate the phenotypic transformation of neurons after nerve injury and have a role in maintaining neuronal cell function (20). In normal neural tissue, ROS are readily cleared by endogenous antioxidant enzymes, whereas when damaged, endogenous antioxidant enzymes have difficulty resisting the large accumulation of ROS, and sustained oxidative stress can easily cause oxidative DNA damage, leading to mitochondrial dysfunction and neuronal apoptosis (21). Therefore, regulating the balance between oxidation and antioxidation is essential to prevent further oxidative damage to neuronal cells and create a microenvironment conducive to nerve regeneration. We treated PTCM@PMet NP pretreated RSCs with H2O2 to investigate whether H2S could protect RSCs’ mitochondrial function from H2O2 damage. Intracellular levels of ROS were examined in material and H2O2-treated RSCs using 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA). After H2O2 incubation, intracellular ROS concentrations in RSCs increased greatly, while H2S reduced ROS production (fig. S15, A and B). We then examined the apoptosis of RSCs after H2O2 oxidative damage. Comparing the CON and H2O2 groups found that H2O2 induced apoptosis in RSCs, and the percentage of apoptotic cells in the group pretreated with PTCM@PMet NPs decreased from 55.5 and 26.58 to 19.59% compared to the H2O2 and PCM@PMet NP pretreatment groups (fig. S16). Apoptosis of RSCs was evident after H2O2 incubation, while H2S reduced the occurrence of apoptosis in RSCs. The gene expression analysis of antioxidant proteins Mn-superoxide dismutase (MnSOD), catalase (CAT), and glutathione peroxidase (GSH-Px) revealed a notable up-regulation in the H2S group (fig. S17). These data suggest that H2S can enhance the expression of antioxidant protein genes and decrease ROS levels.
On the basis of the antioxidant results of H2S, we further investigated whether H2S could improve cellular mitochondrial function. RSCs were pretreated with PTCM@PMet NPs and then incubated with H2O2. ATP production by RSCs was analyzed using an ATP assay kit, which showed that H2O2 treatment impaired ATP synthesis in RSCs; this effect was alleviated by pretreatment with PTCM@PMet NPs (fig. S18A). To investigate how PTCM@PMet NPs mitigate H2O2-induced mitochondrial damage, MMP was examined using JC-1 (fig. S19). Normal MMP is a prerequisite for maintaining mitochondrial oxidative phosphorylation and ATP production, and its stability contributes to maintaining normal cellular physiological functions (22). H2O2 treatment reduced the MMP of RSCs, PCM@PMet NP incubation provided no protection, and PTCM@PMet NP incubation improved the downward trend in MMP. Electron transfer in the respiratory chain is the main cause of potential membrane changes, and respiratory chain complex I is the electron carrier responsible for respiratory chain oxidation and electron transfer. On the basis of changes in ATP synthesis and MMP, we examined the improvement in the activity of mitochondrial respiratory chain complexes I and V. The activity of both enzymes was greatly reduced after disruption by H2O2, and this change was attenuated by pretreatment with PTCM@PMet NPs (fig. S18, B and C). In addition, the expression of mitochondrial DNA repair genes PLOG and OGG-1 was greatly increased (fig. S18D). In summary, H2S enhanced the expression of mitochondrial DNA repair genes and improved mitochondrial respiratory chain enzyme activity, essential for increasing MMP and ATP synthesis (fig. S18E).
The effect of H2S on promoting nerve regeneration
To verify the ability of the adaptive H2S release system to promote nerve regeneration in vivo, SD rats with sciatic nerve defects were used as a model. Rats with a 10-mm nerve defect were randomly selected for autologous nerves and artificial conduit treatment. Figure S22 showed the NF200/S100 immunofluorescence staining images of the H2S conduit group after 1, 2, 3, and 4 weeks of treatment. In the first week, only some cells migrated and grew from the proximal to the distal end of the nerve. Two weeks after the operation, the cells in the H2S conduit group were able to fill the gap between the proximal and distal nerves. This may benefit from the hydrogel’s larger surface area for the adhesion and migration of nerve cells and the regulation of the H2S microenvironment. The H2S conduit group regenerated nerve axons (NF200) faster, growing about 3 mm in length in the second week, thanks to the ability of H2S to direct rapid angiogenesis and promote mitochondrial energy metabolism, thus providing a pathway for SC migration, as well as energy for the rapid growth of regenerated axons. In the third week, the regenerated axons in the H2S conduit group approached the distal nerve. The number of mature SCs gradually increased (S100), more than that in the CO2 conduit group (fig. S23). Four weeks after the operation, the regenerated axons in the H2S conduit group had completely docked with the distal nerves, and the positive expression area of mature SCs increased further. Although the CO2 conduit group also completed docking, the number of regenerated nerve fibers and mature SCs was greatly less than the H2S conduit group.
Muscle reinnervation-promoting properties of H2S
The neuromuscular junction (NMJ) is a peripheral synapse responsible for efficiently transmitting information from the motor neuron to the muscle fibers, thereby facilitating the stimulation of muscle contraction. It consists of three primary components: the nerve terminal, synaptic cleft, and postsynaptic motor endplate or acetylcholine receptors (23). The successful restoration of motor function following PNI ultimately depends on the effective reinnervation of the target muscle by the NMJ (24). We examined the reinnervation of the tibialis anterior (TA) and gastrocnemius muscles in SD rats 12 weeks postsurgery to assess the state of nerve-muscle connectivity. We evaluated the morphology of α-bungarotoxin (α-BTX)–positive clusters, which were primarily categorized into three types: “pretzel” (mature, with a complex, perforated, net-like pattern), “plaque” (immature and smaller in size, lacking perforations), and “intermediate” (a morphology between plaque and pretzel forms). Figure S24 shows all groups’ histological evaluation results for the TA and gastrocnemius muscles. At 12 weeks postsurgery, the average number of α-BTX–positive clusters per square millimeter in the TA was highest in the autograft group, followed by the H2S group, and lowest in the CO2 group. The proportion of mature NMJs followed a similar trend (15.00, 40.83, and 67.94%). The findings in the gastrocnemius muscle were consistent with those of the TA, and the trend among experimental groups indicated that the reinnervation was most effective in the autograft group, followed by H2S, and least in CO2. Staining with α-BTX is a robust indicator of reinnervation, but additional functional and structural evaluations, such as electrophysiology and gait analysis, are needed to understand the recovery of nerve-muscle connectivity comprehensively.
Assessment of nerve function recovery in H2S-involved treatment
The walking trajectory analysis of each group of rats was performed 12 weeks after surgery to evaluate the beneficial effect of the H2S scaffold on the recovery of motor function (Fig. 7A). Figure 7D shows each group’s sciatic nerve function index (SFI) values. The SFI value of the H2S group was higher than that of the CO2 group (P < 0.05) but lower than that of the autograft group (P < 0.001) (Fig. 7D). There were differences between the experimental groups in the gastrocnemius muscle wet weight ratio (injured site versus contralateral normal site), with the H2S group’s ratio being higher than that in the CO2 group and lower than that in the autograft (P < 0.05) (Fig. 7E). Masson’s trichrome staining was used to further evaluate the muscle fiber morphology and collagen deposition in gastrocnemius muscle tissue (the red areas are muscle fibers, and the blue areas are collagen). As shown in the representative images, the CO2 group had some myofiber (red) disorganization and collagen deposition (blue) (Fig. 7B). In contrast, the H2S and autologous groups had good myofiber morphology and no noticeable collagen deposition. The collagen-positive area in Masson-stained images was counted using ImageJ. Figure 7F showed that the collagen-positive area in the CO2 group was significantly higher than that in the other two groups (P < 0.05), whereas the H2S group was lower than that of the autologous group (P < 0.05). These results supported the positive role of ROS-triggered H2S release in PNI repair to improve motor capacity and function in PNI rats.
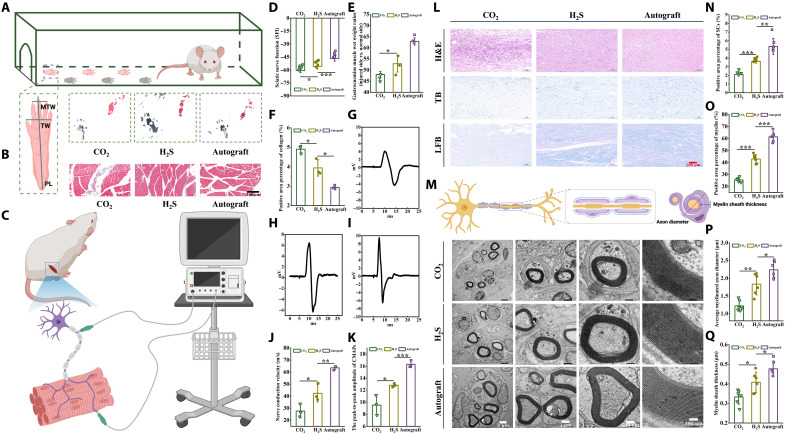
Fig. 7. Nerve regeneration and functional recovery evaluation 12 weeks after the operation.
(A) Typical footprint photographs. (B) Masson-stained images of a cross section of the muscle in an injured limb. Scale bar, 200 μm. (C) Schematic diagram of electrophysiological examination of the injured sciatic nerve 12 weeks after surgery. (D) Statistical results of SFI. (E) Gastrocnemius wet weight ratio (injured side versus normal side) statistics. (F) Summarized data of positive collagen area percentage. (G to I) Electrophysiological assessment of regenerating nerves. (J) Summarized data of regenerating nerve conduction velocity. (K) The peak-to-peak amplitude of CMAPs. (L) Hematoxylin and eosin (H&E) staining, TB staining, and LFB staining. Scale bar, 100 μm. (M) TEM images of transverse sections of regenerated nerves. Scale bars, 2 μm, 1 μm, 500 nm, and 100 nm. (N) Percentage of SC-positive areas obtained by statistics of TB-stained images. (O) Myelin sheath area percentage obtained by statistics TEM images. (P) Summarized data of mean axon diameter. (Q) Summarized data of mean myelin thickness. *P < 0.05, **P < 0.01, ***P < 0.001.
Next, electromyography was further examined (Fig. 7, C, G, H, and I). The peak-to-peak compound motor action potential (CMAP) amplitude of the H2S group (12.81 mV) was closer to that of the autograft group (16.29 mV), while the CO2 group had a lower amplitude (9.52 mV) (Fig. 7K). Likewise, the nerve conduction velocity was higher in the H2S group (88.16 ms−1) than in the CO2 group (72.05 ms−1) and lower than that in the autograft (110.01 ms−1) at 12 weeks postoperatively (Fig. 7J). These findings suggested that ROS-triggered H2S release improved the electrophysiological properties of the PNI.
Assessment of nerve morphological recovery in H2S-involved treatment
The regenerated nerves were dissected for histological analysis following behavioral and electrophysiological experiments. Hematoxylin and eosin, toluidine blue (TB), Lasker’s fast blue (LFB) staining, and TEM observations were performed on sciatic nerve sections to assess nerve tissue regeneration and myelination. As shown in Fig. 7L, the nerve tissue in each group regenerated well without obvious inflammation or scarring. Many mature SCs were in the regenerated nerve tissue, and most of the regenerated nerves had been myelinated. To quantify the number of SCs and the degree of myelination per unit area of regenerating neurons, we performed a quantitative analysis of TB- and LFB-stained images. The results showed that the density of SCs and myelin was highest in the autologous transplantation group, followed by the H2S group, and lowest in the CO2 group (P < 0.001) (Fig. 7, N and O). The TEM results in Fig. 7M showed the morphology of the regenerated axons, where the diameter (P < 0.01) and myelin sheath thickness (P < 0.05) of the regenerated axons in the H2S group were larger than those in the CO2 group. These results confirmed the positive effect of H2S on PNI repair (Fig. 7, P and Q).
DISCUSSION
In the last two decades, artificial nerve grafts have developed rapidly. Many synthetic polymers or natural compounds and their derivatives have been developed and used to prepare nerve conduits. However, they still need to meet the requirements of tissue adaptation, degradation adaptation, and microenvironment adaptation for nerve regeneration. Among these researches, PCL is one of the most favored nerve repair materials due to its excellent mechanical properties and low immunogenicity. However, its strong hydrophobicity, poor cell affinity, and slow degradation reduce its superiority. Therefore, PCL is often used to prepare nerve conduits through physical blending or copolymerization, such as Neurolac, a US Food and Drug Administration–approved nerve conduit that combines polylactic acid and PCL. The copolymerization of ɛ-caprolactone (CL) with glycolide or lactide is also an effective way to improve the degradation performance of PCL. Although these approaches increase the degradation rate of PCL, more ester linkages are introduced, increasing the risk of aseptic inflammation. Morpholine-2,5-dione derivatives are six-membered cyclic lactone amides composed of α-hydroxy and α-amino acid. As cyclic monomers, morpholine-2,5-dione derivatives can prepare biodegradable materials with high medical value by self-polymerization or random copolymerization. In this study, the copolymer of CL with MMD not only maintained good mechanical properties but also improved the hydrophilicity and degradation rate. In addition, the degradation products of MMD units in the copolymer are glycolic acid and alanine, which avoid adverse factors in the microenvironment. PCL is a slowly degrading material that may take over 2 years to completely degrade in vivo (25). The introduction of MMD in the copolymer P(MMD-CL) enhanced the hydrophilicity of PCL and reduced its crystallinity. According to the degradation trend observed in vitro, it is estimated that P(MMD-CL) (MMD:CL = 10:1) will take approximately 1 year to degrade in vivo fully. The maturity of a 10-mm gap in the rat sciatic nerve typically occurs within 16 weeks (26). However, in humans, the time required for complete nerve regeneration and maturation can vary depending on the size and severity of the injury and the type of nerve injury, ranging from 1 year to several years (27). Considering the timeline of nerve regeneration, adjusting the degradation characteristics of the polymer by changing the ratio between different components to align with the pace of nerve regeneration could be crucial.
The topology of the graft can greatly influence cell adhesion, proliferation, and migration (28–30), and many studies have been devoted to optimizing the physical structure of the nerve conduit to affect nerve regeneration directly (31–35). Among them, intraluminal hydrogel filler conduit has certain advantages in biomimetic structure design. It can provide a larger specific surface area for cell growth and guidance and support axon regeneration. Hydrogels are very suitable for peripheral nerve repair due to their soft 3D structure, similarity to nerve tissue, and ability to simulate the extracellular matrix environment. In particular, injectable thermosensitive hydrogels have unique advantages in functionalizing nerve conduits due to their high integration, hierarchical mimicry of natural tissue, and in situ gelation. Here, we synthesized injectable mPEG-PA-PP thermosensitive hydrogel as intraluminal filler for P(MMD-CL) electrospinning conduit, providing space for nerve cell adhesion and growth while providing sites for H2S donor loading.
After peripheral nerve damage, the lesions and distal stumps are accompanied by ischemia, inflammation, and oxidative stress (Fig. 8). Inflammation and oxidative stress interact, complement each other, and are necessary links in peripheral nerve regeneration (2). Early inflammation and oxidative stress are conducive to bactericidal degradation of damaged tissue, remodeling the regenerative microenvironment and initiating a neuronal phenotypic transformation. However, the persistent and excessive immune response will continuously recruit monocytes and cause high oxidant insult, resulting in collagen deposition, tissue fibrosis, and mitochondrial dysfunction, preventing vascularization and tissue regeneration (21, 36). Therefore, regulating the immune microenvironment balance is crucial to creating a microenvironment conducive to nerve regeneration. Macrophages are primary effector cells involved in the immune response to injured peripheral nerves and have multiple functions, including pathogen, apoptotic cell clearance, inflammatory activation, resolution, and tissue healing (37, 38). Our in vitro proteomic results showed that H2S was closely related to macrophage immune response, and the specific reasons for this result were further explored. Macrophages’ phenotypic and functional plasticity plays an important regulatory role in PNI repair (39). Inducing the polarization of macrophages to an anti-inflammatory phenotype (M2 type) is an effective way to promote inflammation resolution and nerve regeneration (12, 40–43). Unlike physical structures that induce macrophage polarization only upon direct cell contact, H2S can freely permeate cells and tissues, inducing macrophage polarization within a 3D space. This promotes the expression of anti-inflammatory and pro-reparative factors, which are crucial for enhancing the regenerative immune microenvironment. This might represent a pathway through which H2S modulates macrophage immune responses. RNA-seq of the proximal nerve stump in rats revealed that H2S is closely associated with inflammation, oxidative stress, cell adhesion, and axonal growth. H2S inhibited the activation of the NF-κB, TNF, and IL-17 signaling pathways, thereby reducing inflammatory responses and neural fibrosis. In addition, H2S activated the calcium and cAMP signaling pathways, enhancing synapse formation, axonal guidance, axonal growth, and neural regeneration. Western blot and immunofluorescence results confirmed that H2S indeed attenuates inflammation by blocking the activation of the NF-κB signaling pathway and inhibiting thecaspase-1 cascade reaction, subsequently reducing the maturation and secretion of inflammatory cytokines (Fig. 8). Regarding antioxidants, H2S elevated the activities of SOD, GSH-Px, and CAT, thereby scavenging excessive ROS and minimizing oxidative damage to tissues caused by free radicals.
Fig. 8. Microenvironmental changes after PNI and H2S regulating microenvironmental nerve regeneration.
After PNI, the surrounding microenvironment faces adverse conditions characterized by inflammation, oxidative stress, ischemia, and insufficient energy supply, all of which hinder the process of nerve regeneration. The implantation of an adaptable H2S delivery system mitigates these challenges by improving the immune microenvironment, facilitating pathways for cellular migration, enhancing vascularization, and boosting energy supply. These improvements collectively accelerate the process of nerve regeneration.
Revascularization is a prerequisite for tissue survival within the graft and the basis for reconstructing physiological functions (44). After graft bridging, the initial supply of O2 and nutrients to intraductal cells mainly depends on the damaged nerve trunk and the fluid in the surrounding tissue [the maximum diffusion distance of O2 in the tissue is 200 μm (45, 46)]. If the vascular network cannot be rebuilt in time, it will affect the nutrient supply to the cells in the graft, which is not conducive to the survival and regeneration of tissue or axon growth. In addition, neovascularization is the “track” through which SCs drive axonal growth, and rapid vascularization facilitates the process of axonal regeneration. In vitro cell experiments confirmed that H2S can promote the migration and tube formation of HUVECs. However, the mechanism requires further investigation. VEGF is the strongest mitogen of endothelial cells and one of the most important growth factors in promoting angiogenesis, which plays an important role in angiogenesis initiation, vascular endothelial cell extension, lumen formation, vascular stability, and maturation (47). Studies have shown that H2S can promote VEGF’s expression and mediate the key signaling molecules involved in VEGF-induced angiogenesis (16). Transcriptomic analysis has confirmed the impact of H2S on HUVECs. DEGs were closely related to biological processes such as cell adhesion and migration. Enrichment analysis revealed that many of these DEGs are associated with critical signaling pathways, including the Ras, Rap1, and PI3K/Akt pathways, which regulate cellular proliferation, differentiation, migration, survival, and angiogenesis. In vivo, animal experiments further substantiated the role of H2S in up-regulating key genes related to vascularization in the PI3K/Akt signaling pathway, such as eNOS and VEGF. This may account for the accelerated cell migration and proliferation facilitated by H2S, thereby promoting rapid vascular formation. In conclusion, the targeted delivery of H2S endows grafts with pro-angiogenic properties, which not only improves the survival rate of cellular tissues but also expedites the process of nerve regeneration, thereby enhancing the likelihood of successful nerve repair.
Mitochondria are the structures that produce energy in cells and are central to aerobic respiration. In addition to synthesizing ATP to provide energy for cells, mitochondria are also the main place for producing ROS (48). The concentration of ROS increases greatly within 2 hours of PNI, and excessive oxidative stress can cause oxidative damage to mitochondria, disrupting the normal cell cycle and interrupting the energy supply (20, 49). This eventually leads to a deadly energetic collapse of the axon, followed by structural disintegration (50, 51). SCs are glia that form a symbiotic relationship with axons, providing drive and guidance for regenerating axons to cross the gap successfully (52, 53). Recent evidence suggests that glial cells, including SCs, are metabolically coupled to axons and may provide energy-rich substrates to modulate axonal bioenergetics and integrity (54–56). Furthermore, axon regeneration is an energy-intensive process dependent on proper mitochondrial function (57, 58). Local mitochondrial density is closely related to axon regeneration, and inhibition of distal mitochondrial transport greatly reduces the length of regenerated axons (59). Therefore, increasing the ATP concentration in injured axons and the energy metabolism of glial cells may be an effective intervention to protect and promote axon regeneration. We assessed the effect of H2S on mitochondrial repair in an SC model of oxidative damage. The results showed that H2S promoted mitochondrial function and ATP synthesis by reducing the concentration of ROS in RSCs, up-regulating the expression of mitochondrial DNA repair genes POLG and OGG-1 and stimulating the activity of mitochondrial respiratory chain complexes I and V. This result indicated that H2S had potential application value in the protection of injured axons and induction of regenerated axons.
Although the “multi-effect messenger” system has demonstrated some regenerative capabilities, there remains a notable gap compared to autologous nerve transplantation. This is because peripheral nerve regeneration is a complex pathophysiological process involving changes at multiple levels, from molecules and cells to the whole organism, and is influenced by various factors (1). Autologous nerves, derived from the patients themselves, eliminate immune rejection concerns. Moreover, these transplanted nerves have a complete neural sheath and blood supply, providing an optimal environment for axonal regeneration. Furthermore, they integrate well with the surrounding environment, effectively transmitting nerve signals (60). Autologous nerve transplantation has long been considered the “gold standard” for treating nerve defects following peripheral nerve injuries, and to date, it has achieved the best regenerative results. Donor’s nerves typically originate from less functionally critical nerves such as the sural nerve, medial and lateral antebrachial cutaneous nerves, dorsal branch of the ulnar nerve, and superficial and deep peroneal nerves (61). In our study, we used an in situ bridging technique using the rat sciatic nerve, circumventing the limitations of conventional autologous nerve grafts, such as the use of sensory nerves to replace motor nerves, which can lead to decreased nerve function Although the multi-effect messenger system has shown therapeutic potential in many aspects, it still lags in situ autologous transplantation regarding biocompatibility, bioactivity, nourishment (blood supply and growth factors), and the establishment of axonal regeneration pathways. By equipping nerve conduits with the ability to promote cell adhesion [hydrogel grafting with RGD (Arg-Gly-Asp sequence polypeptide composed of three amino acids)], constructing porous hydrogel structures, and providing electrical stimulation (which enhances the secretion of nerve growth factors), we can further optimize nerve conduits in terms of constructing axonal growth pathways and the regeneration microenvironment, promoting axonal budding and extension, and potentially achieving regenerative results closer to those of autologous transplantation.
Our current study demonstrates that pleiotropic messengers are important in regulating, protecting, and inducting nerve regeneration. The biggest advantage of the intelligent bionic scaffold we developed is that it integrates gas transmitters and bionic structures and combines the temporal and spatial changes of nerve regeneration to precisely adjust the regenerative microenvironment, simulate the microenvironment of each stage of regeneration to the greatest extent, and accelerate the process of nerve regeneration (Fig. 8). This study provides an important strategy for constructing gas transmitter biomimetic nerve grafts. However, it should be mentioned that despite the multiple biological effects of gaseous transmitters, the deeper reasons for this multitarget, multipath therapeutic capability should be further elucidated. Research in the future should explore the deep-level mechanisms of gas transmitters regulating various physiological processes such as immunity, vascularization, and mitochondrial function from a cellular metabolism perspective.
In conclusion, given the difficulty of repairing the damaged nerve regeneration microenvironment and the slow regeneration speed, this study successfully fabricated an intelligent pleiotropic messenger bionic nerve scaffold using electrospinning and injection molding, integrating with polyester amide, thermosensitive hydrogel, and H2S to synergistically promote peripheral nerve regeneration. We have constructed a responsive drug-loaded NP system, and ROS, one of the markers of pathological environment, was used as the trigger source. This system allowed for the “adaptive” release of H2S in the damaged area, avoiding the blood-nerve barrier, which may block effective drug uptake. This strategy efficiently and dynamically regulated the inflammation, oxidative stress, ischemia, and insufficient energy supply of injured peripheral nerves, thereby protecting the damaged nerves and accelerating the remodeling of the regenerative microenvironment in the graft. In addition, the highly mimetic 3D structure and chemical signaling cues facilitated the adhesion, proliferation, and migration of neuronal cells and the formation of “cell bridges,” after which chemical signals promoted the polarization of the vascular system on the bridge, thus providing a pathway for the migration of SCs that drive axonal growth. The graft guided the regenerated axons by combining the spatiotemporal changes of nerve regeneration and accelerated nerve regeneration and functional recovery. The research results help to explore the feasibility of polyester amide materials and injectable gas delivery systems for peripheral nerve repair, providing technical support and a theoretical basis for solving the common problems of delayed regeneration and slow regeneration due to difficult reconstruction of the nerve injury microenvironment.
MATERIALS AND METHODS
Characterization
1H NMR spectra of the products of each step of the synthesis reaction were obtained using an Avance III HD 500 MHz NMR spectrometer (Bruker, Germany). The peroxyTCM and peroxyCM molecular weight information was obtained via atmospheric pressure chemical ionization using solanX 70 FT-MS HRMS (Bruker, Germany). The molecular weight of P(MMD-CL) was determined using an Agilent infinity 1260II gel permeation chromatography (American) detection system. CHCl3 was the mobile phase. The hydrogel and electrospun membrane surface morphologies were tested using JSM-IT200 SEM (JEOL, Japan). The mechanical properties of fiber membranes were obtained on an Instron 5967 electronic universal material testing machine (Instron, UK) according to ISO 1040-2006 at 100 mm/min speed. The wettability and hydrophobicity of the P(MMD-CL) and PCL fiber membranes were monitored using the FACE CA-XP150 contact angle meter (Shanghai Powereach Company, China).
To evaluate the ROS responsiveness of the donor molecules and the release of H2S, we added peroxyTCM to CD3CN/D2O (1:1) containing H2O2 [5 equivalent (equiv.)]. The chemical structure changes of peroxyTCM were subsequently monitored at corresponding times by 1H NMR to reveal the release of H2S indirectly.
PTCM@PMet NPs’ topography images were acquired on a JEM-1400Plus TEM (JEOL, Japan). The absorbance of NPs (0.2 mg/ml) dissolved in a mixed solution (dimethyl sulfoxide:H2O = 9:1) at 275 nm was measured using a ultraviolet-visible spectroscopy (UV-Vis, UV-1900i, SHIMADZU, Japan). The content of peroxyTCM in a unit mass of NPs was calculated according to the absorbance-concentration standard curve. The formula calculated the loading efficiency of peroxyTCM in mPEG-PMet NPs: loading efficiency of peroxyTCM in mPEG-PMet NPs = (mass of peroxyTCM/total weight of NPs) × 100%. The mean hydrodynamic diameter and size distribution of the NPs were determined using DLS (Zetasizer Nano ZS, Malvern Instruments, UK). The NPs were formulated into an aqueous solution with a concentration of 0.5 mg/ml and filtered with a disposable 0.45-μm filter for DLS measurement. The drug release of PTCM@PMet NPs was determined by a dialysis method. Briefly, 40 ml of PBS buffer solution with pH 7.4 was placed in a 50-ml centrifuge tube, and 0.2 ml of Tween 80 was added to the centrifuge tube. The above centrifuge tubes were placed in a constant temperature shaking incubator at 37°C and 100 rpm. After the buffer temperature stabilized, the dialysis bag [molecular weight cut-off (MwCO), 1000 Da] filled with 2 ml and PTCM@PMet NPs (1 mg/ml) was placed into a 50-ml centrifuge tube. At 2-, 8-, 24-, and 48-hour time points, 3 ml of release medium was taken, and an equal volume of fresh buffer was added. The experiment was divided into four groups: PBS, 50 μM H2O2, 200 μM H2O2, and 500 μM H2O2, with three samples in each group, and the release medium was refreshed daily. After sampling, high-performance liquid chromatography was used to detect the absorption peak of p-hydroxy benzyl alcohol at 275 nm (the product of the decomposition of peroxyTCM) to determine the drug release concentration.
The phase transition temperature and rheological properties of the mPEG-PA-PP hydrogels were tested using the HAAKE RheoStress 6000 rotational rheometer (Thermo Fisher Scientific, USA). A parallel plate rotor with a diameter of 25 mm was chosen, and the distance from the platform was set to 1 mm. The mPEG-PA-PP precursor solution was injected into the gap between the parallel plate rotor and the platform, and the rotational rheological (10 rad/s; 1% strain) to obtain real-time changes in the storage modulus (G′) and loss modulus (G″) of the hydrogel. The platform temperature was lowered to 37°C and frequency sweep (0.01 to 100 rad/s; 1% strain) and strain sweep (0.01 to 10,000% strain; 10 rad/s) were performed to observe the hydrogel rheological properties.
Determination of textural properties of mPEG-PA-PP hydrogels
Briefly, 0.5 ml of 6.0 wt % mPEG-PA-PP was injected into a round tetrafluoroethylene mold (inner diameter of 10 mm × height of 5 mm) to form a gel at 37°C. The texture profile analysis (TPA) was chosen, and a probe P/0.5 (12.7 mm in diameter) was used, with a trigger force of 0.05 N. The probe was lowered by 4 mm at a test speed of 1 mm/s, and the stress-strain data were obtained by automatic computer processing.
The sol-gel transition behavior of mPEG-PA-PP was studied by the test tube inversion method. Briefly, the samples were dissolved in PBS at 4°C to configure solutions of different concentrations (4.0, 6.0, 8.0, and 10.0 wt %). The solution was poured into an 8-mm-diameter vial and placed in a water bath, the temperature of which was increased by 1°C every 10 min. The vials were turned over once for every 1°C increase, and the phase transition was judged to be complete if no liquid flow was observed within 0.5 min.
P(MMD-CL) and PCL degradation properties were performed in 0.1 M PBS. Briefly, 0.1 g of electrospinning conduit and 10 ml of 0.01 M PBS buffer [pH 7.4, containing 0.03% (w/v) sodium azide] were added to a 15-ml centrifuge tube and placed on a shaker (37°C, 100 rpm) for degradation experiments. Conduit weight and buffer pH were monitored at 1, 2, 3, 4, 5, 6, 7, and 8 weeks, and 100 volumes of fresh PBS were readded on the basis of the degraded conduit weight.
Cell imaging of H2O2-induced H2S release from PeroxyTCM
RAW 264.7 and RSCs were seeded in six-well plates containing 2 ml of Dulbecco’s modified Eagle’s medium (DMEM) medium and cultured to 80% confluency. The medium was discarded, and confluent cells were washed twice with PBS, following incubation with fetal bovine serum (FBS)–free DMEM medium containing peroxyTCM (50 μM) and AzMC (5 μM, Sigma-Aldrich) for 30 min. Extracellular peroxyTCM and AzMC were removed by washing with PBS and incubated with different concentrations of H2O2 (0, 25, and 50 μM) in an FBS-free DMEM medium for 30 min. After washing with PBS, cells were imaged using an IX71 inverted fluorescence microscope (Olympus, Japan) under excitation with a 365-nm laser.
H2S treatment on RAW 264.7
RAW 264.7 were seeded in plates (1 × 106 cells) and cultured in the incubator for 24 hours. The confluent cells were washed twice with PBS, following incubation with a DMEM medium containing H2O2 (50 μM) and PCM@PMet NPs (50 μM) or PTCM@PMet NPs (50 μM), respectively. After 48 hours of culture, extracellular NPs were removed by washing with PBS.
Immunofluorescence
RAW 264.7 were seeded in six-well plates at 1 × 106 per well. After being treated for 48 hours, cells were fixed with 4% paraformaldehyde (Beyotime, P0099) for 15 min at room temperature (r.t.) and permeabilized in 0.5% (v/v) Triton X-100/PBS (Beyotime, ST677) for 5 min. After blocking with 1% (w/v) bovine serum albumin (BSA/PBS, Beyotime, ST025) for 30 min, cells were incubated with CD206 (1:1000, rabbit polyclonal antibody, ab64693, Abcam) for 30 min at 4°C. The cells were washed with PBS, following incubation with goat anti-rabbit immunoglobulin G (IgG) (H + L) CoraLite 594 (1:100, SA00013-4, Proteintech) secondary antibodies at 37°C for 1 hour in the dark. Last, cells were incubated with a 4′,6-diamidino-2-phenylindole (DAPI) working solution (10 μg/ml, C0065, Solarbio) at r.t. for 10 min in the dark. Cell immunostaining images were observed and taken by Nikon-Eclipse-Ti laser scanning confocal microscope (LSCM, Nikon, Japan).
Western blot
RAW 264.7 was seeded in six-well plates at 1 × 106 per well and treated for 48 hours. Cells were collected by centrifugation, and 200 μl of radioimmunoprecipitation assay (RIPA) lysate containing 1 mM phenylmethylsulfonyl fluoride was added, and homogenize for 1 hour at 4°C until cells are fully lysed. Solubilized protein was collected by centrifugation at 12,000g, and the supernatant was quantified for protein concentration using bicinchoninic acid (BCA) reagents. Polyacrylamide gels were separated protein samples and were transferred to polyvinylidene fluoride membranes (Sigma-Aldrich, USA). Nonspecific proteins on membranes were blocked with 5% nonfat dry milk at r.t. for 2 hours and then incubated with CD206 (1:1000, rabbit polyclonal antibody, ab64693, Abcam) overnight at 4°C. Membranes were probed with the indicated secondary antibodies and scanned with an Odyssey instrument (LI-COR).
Enzyme-linked immunosorbent assay
To analyze the effect of H2S on RAW 264.7 polarization, the concentrations of TNF-α (KE20001, Proteintech) and IL-10 (KE20003, Proteintech) were quantified using ELISA kits. Briefly, RAW 264.7 was seeded in 96-well plates at 20,000 per well, and after different treatments, culture supernatants were collected by centrifugation and used in ELISA according to the manufacturer’s instructions.
Angiogenesis assays in vitro
Migration assay
HUVECs were seeded at 1 × 106 cells per well in six-well plates. A straight scratch on the monolayer cell was created using a 10-μl sterile pipette tip after the HUVECs reached 80 to 90% confluency, and the scraped cells were gently washed with PBS. Then, PCM@PMet NPs (50 μM) + H2O2 (50 μM) and PTCM@PMet NPs (50 μM) + H2O2 (50 μM) were added and incubated with the cells to observe the effect of H2S on wound healing. Repopulating/migrated HUVECs were visualized on scratches at 0-, 12-, and 24-hour time points and imaged using an IX71 inverted fluorescence microscope. The healing ratio of the scratch area was quantified by ImageJ v1.8.0 software.
Transwell
To characterize the effect of H2S on HUVEC migration, PTCM@PMet or PCM@PMet NPs (50 μM), 50 μM H2O2, and 600 μl of complete medium were added to the lower section of 6.5-mm Transwell chambers with 8-μm pores (3422, Corning). HUVECs in the logarithmic growth stage after 12 hours of serum-free culture were seeded at 2 × 105 cells per well (200 μl containing 1% FBS medium) into the upper section of the cell compartment. The cells were incubated for 24 hours at 37°C and 5% CO2. The culture medium of the upper section of cell chambers was discarded, and the cells were carefully wiped off with a cotton swab. After washing twice with precooled PBS, 4% paraformaldehyde was added and fixed for 30 min. After moderate air drying, the cells were stained with 0.1% crystal violet (G1063, Solarbio) for 20 min and washed with precooled PBS three times. The number of attached cells (migratory cells) on the lower surface of the chamber was observed under an IX71 inverted fluorescence microscope.
Tube formation
Matrigel (356234, Corning) was injected into prechilled 24-well plates (100 μl per well) and kept in a 37°C incubator for 30 min. The suspended HUVECs were seeded into Matrigel-coated 24-well plates at 20,000 cells per well. Following a 6-hour incubation in the incubator, an IX71 inverted fluorescence microscope analyzed the resulting tube network. The number of tube networks formed was quantified by ImageJ v1.8.0 software.
H2S treatment on RSCs
RSCs were seeded in six-well plates containing 2 mL of DMEM medium and cultured for 24 h under a humidified atmosphere, followed by the replacement of medium by fresh DMEM medium containing H2O2 (50 μM) and PCM@PMet NPs (50 μM) or PTCM@PMet NPs (50 μM), respectively. After 24 hours of culture, extracellular NPs were removed by washing with PBS; cells were incubated with H2O2 (400 μM) in DMEM medium for 12 hours.
Detection of intracellular ROS generation
Intracellular ROS levels were detected using the DCF-DA (CA1410, Solarbio) staining method. After RSCs were treated with H2O2, they were incubated with DCF-DA (10 μM) in an FBS-free DMEM medium for 45 min (37°C, in the dark). Intracellular ROS levels will be visualized by imaging cells under 488-nm laser excitation using an IX71 inverted fluorescence microscope.
Detection of Mitochondrial respiratory chain complex I and V activity
A CheKine mitochondrial respiratory chain complex I activity detection kit and a complex V activity detection kit were used to detect mitochondrial respiratory chain complex I and V activities. The mitochondrial respiratory chain complex I (complex I for short) can catalyze the dehydrogenation of NADH [reduced form of nicotinamide adenine dinucleotide (NAD+)] to form NAD+, and this enzyme’s activity was calculated by measuring NADH’s oxidation rate at 340 nm. Complex V is the key enzyme for mitochondrial oxidative phosphorylation to synthesize ATP. Complex V hydrolyzes ATP to generate ADP and Pi, and complex V activity is measured by measuring the rate of Pi increase. All experimental procedures were performed according to the manufacturer’s protocol.
Detection of MMP
An MMP detection kit (JC-1, M8650, Solarbio) was used to detect changes in MMP. After different treatments, RSCs were incubated with JC-1 staining working solution for 20 min in a 37°C incubator. The supernatant was removed by aspiration and washed twice with JC-1 staining buffer (1×). Two milliliters of DMEM medium was added to the six-well plate, and cells were imaged using an IX71 inverted fluorescence microscope.
Detection of ATP Levels
ATP levels in RSCs were tested using an ATP content assay kit (microassay, BC0305, Solarbio). The energy charge is the main parameter to describe the state of energy metabolism of cells. Detecting ATP content and calculating energy charges can reflect the state of energy metabolism. All testing procedures were performed according to the kit instructions.
Animal experiments
All animal experiments were approved by the Laboratory Animal Management and Ethics Committee of the Zhong Nan Hospital of Wuhan University (approval no. ZN2021027) and conformed to the guidelines of the Animal Care and Use Committee. For additional information on experimental procedures, please refer to the Supplementary Materials.
Transcriptomic study of the effects of H2S on peripheral nerve inflammation and regeneration
For sciatic nerve RNA-seq analysis, rats were euthanized 1 week after treatment to harvest proximal nerve stump tissue, which was then cryopreserved in liquid nitrogen. Total RNA was extracted from nerve tissue using TRIzol (Thermo Fisher Scientific, USA) reagent according to the manufacturer’s instructions. The sequencing service was provided by Bioyi Biotechnology Co., Ltd. (Wuhan). Raw data were filtered using the fastp software, and quality control of the clean data was conducted using FastQC software. If the quality control was acceptable, subsequent analyses were performed. For experiments with biological replicates (DEGs in biological replicates were defined as |fold change| ≥ 2 and P adjusted ≤ 0.05), we used DESeq2 software for differential expression analysis. GO functional significance enrichment and KEGG pathway significance enrichment analyses were conducted based on DEGs.
Behavioral assessment
The functional recovery of the injured sciatic nerve in SD rats 12 weeks after surgery was evaluated by walking track analysis. Briefly, non-operated (non) and experimental (exp) hindlimbs of rats were stained with dye, allowed to crawl forward in the tunnel, and podogram length (PL), toe width (TW), and mid-toe width (MTW) were recorded and measured. The SFI value is calculated based on the following formula
Electrophysiology
The recovery of injured nerve function in SD rats was detected by electrophysiology 12 weeks after the operation. After anesthetized rats, the limbs were immobilized on the surgical board, and the surgical site was exposed. The ground electrode was fixed to the rat’s tail, the receiving electrode was inserted into the gastrocnemius muscle, 10 mV of electrical stimulation was applied to the regenerated nerve’s proximal nerve trunk, and the CMAPs were recorded (Fig. 7C).
Immunofluorescence
As previously described, the sciatic nerve sections of rats in the CO2, H2S, and autologous groups were obtained. The sections were placed in a high-temperature resistant container, a citric acid solution was added, and the container was placed in a microwave for 10 min for antigen retrieval. Sections were blocked with 1% BSA for 30 min at r.t. and then washed thrice with tris-buffered saline. Then, the sections were incubated overnight at 4°C with primary antibody solution (antibody dilution: 1 × PBS/5% normal serum/0.3% Triton X-100). The obtained slides were stained as follows: Ki67 (1:50, rabbit polyclonal antibody, A2094, ABclonal), S100 (1:150, rabbit polyclonal antibody, 16027-1-AP, Proteintech), neurofilament (NF200, 1:150, mouse monoclonal antibody, 60331-1-Ig, Proteintech), VEGF (1:150, rabbit polyclonal antibody, A12303, ABclonal), CD31 (1:100, rabbit polyclonal antibody, 11265-1-AP, Proteintech), CD68 (1:100, rabbit polyclonal antibody, A13286, ABclonal), CCR7 (1:100, rabbit monoclonal antibody, ab32527, Abcam), CD206 (1:500, rabbit polyclonal antibody, ab64693, Abcam), TNF-α (1:100, rabbit polyclonal antibody, A11534, ABclonal), IL-10 (1:100, mouse monoclonal antibody, A12255, ABclonal), and NF-κB (1:100, rabbit monoclonal antibody, A19653, ABclonal).
The sections were washed with PBS, following incubation with goat anti-rabbit IgG (H + L) CoraLite 594 (1:100, SA00013-4, Proteintech), goat anti-mouse IgG (H + L) CoraLite 594 (1:400, SA00013-3, Proteintech), or goat anti-rabbit IgG (H + L) CoraLite 488 (1:100, SA00013-6, Proteintech) secondary antibodies at 37°C for 1 hour in the dark. Last, sections were incubated in the dark with a DAPI working solution (10 μg/ml; C0065, Solarbio) at r.t. for 10 min. Immunostaining images were observed and taken by Nikon-Eclipse-Ti LSCM (Nikon, Japan).
At 12 weeks postsurgery, rats were anesthetized using 5% chloral hydrate at 400 mg/kg. The rat tails were cleaned and then warmed in 50°C water to facilitate the visualization of the tail vein. α-Bungarotoxin (Alexa Fluor 647 conjugate, B35450, Thermo Fisher Scientific) was dissolved in PBS and administered intravenously at 200 μg/kg concentration via the tail vein. After 1.2 hours, rats were perfused with paraformaldehyde, and the TA and gastrocnemius muscles were carefully dissected following complete blood evacuation. After gradient dehydration, samples were embedded in optimal cutting temperature compound (OCT) and cut into 20-μm-thick longitudinal sections. Immunostained images were captured using a Nikon-Eclipse-Ti LSCM (Nikon, Japan).
Reverse transcription quantitative polymerase chain reaction
ON groups were collected 2 weeks after the operation. Total RNA was extracted from the samples using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s protocol. The content of RNA was determined by spectrophotometry. After reverse transcription and amplification, target gene concentrations were quantified using CFX Connect real-time PCR (Bio-Rad, USA). Gene expression levels were calculated using the 2-ΔΔCT method.
Western blot
Nerve-regenerated segments were collected 2 weeks after surgery, and the samples were cut into fine tissue fragments. RIPA lysate was added at 200 μl of lysate per 20 mg of tissue. The lysed samples were centrifuged at 12,000g for 5 min to obtain a supernatant containing total protein, followed by a Western blot operation as previously described.
Data analysis
Statistical analysis was performed using GraphPad Prism 9 (GraphPad Software LLC, USA). Unpaired Student’s t test (two-tailed) was used to compare the mean values of two independent samples. Statistics for multiple comparisons were performed using one-way analysis of variance (ANOVA). The data layout was presented using OriginPro 2021 (OriginLab, USA) and GraphPad Prism 9. The data shown are mean ± SD.
Acknowledgments
We thank BioRender.com for providing the drawing platform.
Funding: This work was supported by grants from the National Natural Science Foundation of China (nos. 52372272, 32201109, and 52003211), the National Key Research and Development Program of China (2022YFB4601402), the Health Commission of Hubei Province Medical Leading Talent Project (no. LJ20200405), the Natural Science Foundation of Hubei Province (no. 2020CFB418), the Guangdong Basic and Applied Basic Research Foundation (nos. 2022B1515120052 and 2021A1515110557), the Key Basic Research Program of Shenzhen (no. JCYJ20200109150218836), and the Chaozhou Branch of Chemistry and Chemical Engineering Guangdong Laboratory (no. HJL202202A002).
Author contributions: X.D. and H.D. conceived this strategy and designed the experiments. X.D. synthesized and characterized the materials. H.Z. performed in vitro and in vivo experiments, and X.D., P.D., K.L., Y.Y., W.We., W.Wa., and Y.L. assisted in completing this part. X.D. wrote the manuscript, and L.Y., W.We., and X.L. revised it. Q.C., X.L., and Y.H. contributed to the blinding method. X.D., H.D., L.Y., A.Y., and H.Z. supervised and managed this work. A.Y., L.Y., and H.D. provided financial support.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Raw data are available on Dryad (https://doi.org/10.5061/dryad.wpzgmsbth).
Supplementary Materials
This PDF file includes:
Supplementary Materials and Methods
Figs. S1 to S24
Table S1
REFERENCES AND NOTES
- 1.A.-L. Cattin, J. J. Burden, L. van Emmenis, F. E. Mackenzie, J. J. A. Hoving, N. Garcia Calavia, Y. Guo, M. McLaughlin, L. H. Rosenberg, V. Quereda, D. Jamecna, I. Napoli, S. Parrinello, T. Enver, C. Ruhrberg, A. C. Lloyd, Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 162, 1127–1139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Y. Qian, H. Lin, Z. Yan, J. Shi, C. Fan, Functional nanomaterials in peripheral nerve regeneration: Scaffold design, chemical principles and microenvironmental remodeling. Mater. Today 51, 165–187 (2021). [Google Scholar]
- 3.O. S. Manoukian, S. Rudraiah, M. R. Arul, J. M. Bartley, J. T. Baker, X. Yu, S. G. Kumbar, Biopolymer-nanotube nerve guidance conduit drug delivery for peripheral nerve regeneration: In vivo structural and functional assessment. Bioact. Mater. 6, 2881–2893 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Y. Qian, Q. Han, X. Zhao, J. Song, Y. Cheng, Z. Fang, Y. Ouyang, W. E. Yuan, C. Fan, 3D melatonin nerve scaffold reduces oxidative stress and inflammation and increases autophagy in peripheral nerve regeneration. J. Pineal Res. 65, e12516 (2018). [DOI] [PubMed] [Google Scholar]
- 5.R. Wang, Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 92, 791–896 (2012). [DOI] [PubMed] [Google Scholar]
- 6.K. Abe, H. Kimura, The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 16, 1066–1071 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Y. Zhao, T. D. Biggs, M. Xian, Hydrogen sulfide (H2S) releasing agents: Chemistry and biological applications. Chem. Commun. (Camb.) 50, 11788–11805 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.M. Whiteman, L. Li, P. Rose, C. H. Tan, D. B. Parkinson, P. K. Moore, The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 12, 1147–1154 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Y. Zhao, M. D. Pluth, Hydrogen sulfide donors activated by reactive oxygen species. Angew. Chem. Int. Ed. Engl. 55, 14638–14642 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.X. Dong, P. Wu, L. Yan, K. Liu, W. Wei, Q. Cheng, X. Liang, Y. Chen, H. Dai, Oriented nanofibrous P(MMD-co-LA)/Deferoxamine nerve scaffold facilitates peripheral nerve regeneration by regulating macrophage phenotype and revascularization. Biomaterials 280, 121288 (2022). [DOI] [PubMed] [Google Scholar]
- 11.K. Kazankov, S. M. D. Jørgensen, K. L. Thomsen, H. J. Møller, H. Vilstrup, J. George, D. Schuppan, H. Grønbæk, The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 16, 145–159 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Y. Jia, W. Yang, K. Zhang, S. Qiu, J. Xu, C. Wang, Y. Chai, Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes. Acta Biomater. 83, 291–301 (2019). [DOI] [PubMed] [Google Scholar]
- 13.B. S. Thawkar, G. Kaur, Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer's disease. J. Neuroimmunol. 326, 62–74 (2019). [DOI] [PubMed] [Google Scholar]
- 14.C. W. H. Huang, W. Feng, M. T. Peh, K. Peh, B. W. Dymock, P. K. Moore, A novel slow-releasing hydrogen sulfide donor, FW1256, exerts anti-inflammatory effects in mouse macrophages and in vivo. Pharmacol. Res. 113, 533–546 (2016). [DOI] [PubMed] [Google Scholar]
- 15.K. M. Dennehy, G. Ferwerda, I. Faro-Trindade, E. Pyż, J. A. Willment, P. R. Taylor, A. Kerrigan, S. V. Tsoni, S. Gordon, F. Meyer-Wentrup, G. J. Adema, B. J. Kullberg, E. Schweighoffer, V. Tybulewicz, H. M. Mora-Montes, N. A. R. Gow, D. L. Williams, M. G. Netea, G. D. Brown, Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur. J. Immunol. 38, 500–506 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.A. Papapetropoulos, A. Pyriochou, Z. Altaany, G. Yang, A. Marazioti, Z. Zhou, M. G. Jeschke, L. K. Branski, D. N. Herndon, R. Wang, C. Szabó, Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 106, 21972–21977 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.C. Szabo, Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol. Cell Physiol. 312, C3–C15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.M. M. Cortese-Krott, G. G. Kuhnle, A. Dyson, B. O. Fernandez, M. Grman, J. DuMond, M. P. Barrow, G. McLeod, H. Nakagawa, K. Ondrias, P. Nagy, S. B. King, J. E. Saavedra, L. K. Keefer, M. Singer, M. Kelm, A. R. Butler, M. Feelisch, Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc. Natl. Acad. Sci. U.S.A. 112, E4651–E4660 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.P. Nagy, Z. Pálinkás, A. Nagy, B. Budai, I. Tóth, A. Vasas, Chemical aspects of hydrogen sulfide measurements in physiological samples. BBA-Gen. Subjects 1840, 876–891 (2014). [DOI] [PubMed] [Google Scholar]
- 20.A. Hervera, F. de Virgiliis, I. Palmisano, L. Zhou, E. Tantardini, G. Kong, T. Hutson, M. C. Danzi, R. B. T. Perry, C. X. C. Santos, A. N. Kapustin, R. A. Fleck, J. A. del Río, T. Carroll, V. Lemmon, J. L. Bixby, A. M. Shah, M. Fainzilber, S. di Giovanni, Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 20, 307–319 (2018). [DOI] [PubMed] [Google Scholar]
- 21.S.-M. Wang, H. P. Tsai, J. J. Huang, H. C. Huang, J. L. Lin, P. H. Liu, Inhibition of nitric oxide synthase promotes facial axonal regeneration following neurorrhaphy. Exp. Neurol. 216, 499–510 (2009). [DOI] [PubMed] [Google Scholar]
- 22.D. Gero, R. Torregrossa, A. Perry, A. Waters, S. Le-Trionnaire, J. L. Whatmore, M. Wood, M. Whiteman, The novel mitochondria-targeted hydrogen sulfide (H2S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol. Res. 113, 186–198 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A. K. Snyder-Warwick, A. Satoh, K. B. Santosa, S.-I. Imai, A. Jablonka-Shariff, Hypothalamic Sirt1 protects terminal Schwann cells and neuromuscular junctions from age-related morphological changes. Aging Cell 17, e12776 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.L. Li, H. Yokoyama, H. Kaburagi, T. Hirai, K. Tsuji, M. Enomoto, Y. Wakabayashi, A. Okawa, Remnant neuromuscular junctions in denervated muscles contribute to functional recovery in delayed peripheral nerve repair. Neural Regen. Res. 15, 731 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.J. C. Middleton, A. Tipton, Synthetic biodegradable polymers as medical devices. Med. Plast. Biomater. 5, 30–39 (1998). [Google Scholar]
- 26.P. H. Robinson, B. van der Lei, H. J. Hoppen, J. W. Leenslag, A. J. Pennings, P. Nieuwenhuis, Nerve regeneration through a two-ply biodegradable nerve guide in the rat and the influence of ACTH4-9 nerve growth factor. Microsurgery 12, 412–419 (1991). [DOI] [PubMed] [Google Scholar]
- 27.M. Sun, P. J. Kingham, A. J. Reid, S. J. Armstrong, G. Terenghi, S. Downes, In vitro and in vivo testing of novel ultrathin PCL and PCL/PLA blend films as peripheral nerve conduit. J. Biomed. Mater. Res A. 93, 1470–1481 (2010). [DOI] [PubMed] [Google Scholar]
- 28.S. J. Hollister, Scaffold design and manufacturing: From concept to clinic. Adv. Mater. 21, 3330–3342 (2009). [DOI] [PubMed] [Google Scholar]
- 29.J. Xie, M. R. MacEwan, W. Liu, N. Jesuraj, X. Li, D. Hunter, Y. Xia, Nerve guidance conduits based on double-layered scaffolds of electrospun nanofibers for repairing the peripheral nervous system. ACS Appl. Mater. Interfaces 6, 9472–9480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Y. Peng, K. Y. Li, Y. F. Chen, X. J. Li, S. Zhu, Z. Y. Zhang, X. Wang, L. N. Duan, Z. J. Luo, J. J. du, J. C. Wang, Beagle sciatic nerve regeneration across a 30 mm defect bridged by chitosan/PGA artificial nerve grafts. Injury 49, 1477–1484 (2018). [DOI] [PubMed] [Google Scholar]
- 31.G. Abagnale, M. Steger, V. H. Nguyen, N. Hersch, A. Sechi, S. Joussen, B. Denecke, R. Merkel, B. Hoffmann, A. Dreser, U. Schnakenberg, A. Gillner, W. Wagner, Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials 61, 316–326 (2015). [DOI] [PubMed] [Google Scholar]
- 32.C. Simitzi, A. Ranella, E. Stratakis, Controlling the morphology and outgrowth of nerve and neuroglial cells: The effect of surface topography. Acta Biomater. 51, 21–52 (2017). [DOI] [PubMed] [Google Scholar]
- 33.J. He, C. Sun, Z. Gu, Y. Yang, M. Gu, C. Xue, Z. Xie, H. Ren, Y. Wang, Y. Liu, M. Liu, F. Ding, K. W. Leong, X. Gu, Morphology, migration, and transcriptome analysis of Schwann cell culture on butterfly wings with different surface architectures. ACS Nano 12, 9660–9668 (2018). [DOI] [PubMed] [Google Scholar]
- 34.F. Wu, J. Zheng, Z. Li, M. Liu, Halloysite nanotubes coated 3D printed PLA pattern for guiding human mesenchymal stem cells (hMSCs) orientation. Chem. Eng. J. 359, 672–683 (2019). [Google Scholar]
- 35.L. Fan, J. L. Li, Z. Cai, X. Wang, Creating biomimetic anisotropic architectures with co-aligned nanofibers and macrochannels by manipulating ice crystallization. ACS Nano 12, 5780–5790 (2018). [DOI] [PubMed] [Google Scholar]
- 36.B. O. O. Boni, L. Lamboni, T. Souho, M. Gauthier, G. Yang, Immunomodulation and cellular response to biomaterials: The overriding role of neutrophils in healing. Mater. Horiz. 6, 1122–1137 (2019). [Google Scholar]
- 37.P. Liu, J. Peng, G. H. Han, X. Ding, S. Wei, G. Gao, K. Huang, F. Chang, Y. Wang, Role of macrophages in peripheral nerve injury and repair. Neural Regen. Res. 14, 1335–1342 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.T. J. Koh, L. A. DiPietro, Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 13, e23 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.T. D. Smith, R. R. Nagalla, E. Y. Chen, W. F. Liu, Harnessing macrophage plasticity for tissue regeneration. Adv. Drug Deliv. Rev. 114, 193–205 (2017). [DOI] [PubMed] [Google Scholar]
- 40.K. A. Sarhane, Z. Ibrahim, R. Martin, K. Krick, C. R. Cashman, S. H. Tuffaha, J. M. Broyles, N. Prasad, Z. C. Yao, D. S. Cooney, R. Mi, W. P. A. Lee, A. Hoke, H. Q. Mao, G. Brandacher, Macroporous nanofiber wraps promote axonal regeneration and functional recovery in nerve repair by limiting fibrosis. Acta Biomater. 88, 332–345 (2019). [DOI] [PubMed] [Google Scholar]
- 41.D. Zhang, Y. Yao, Y. Duan, X. Yu, H. Shi, J. R. Nakkala, X. Zuo, L. Hong, Z. Mao, C. Gao, Surface-anchored graphene oxide nanosheets on cell-scale micropatterned poly(d,l-lactide-co-caprolactone) conduits promote peripheral nerve Regeneration. ACS Appl. Mater. Interfaces 12, 7915–7930 (2020). [DOI] [PubMed] [Google Scholar]
- 42.A. Shapouri-Moghaddam, S. Mohammadian, H. Vazini, M. Taghadosi, S. A. Esmaeili, F. Mardani, B. Seifi, A. Mohammadi, J. T. Afshari, A. Sahebkar, Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 233, 6425–6440 (2018). [DOI] [PubMed] [Google Scholar]
- 43.X. Dong, S. Liu, Y. Yang, S. Gao, W. Li, J. Cao, Y. Wan, Z. Huang, G. Fan, Q. Chen, H. Wang, M. Zhu, D. Kong, Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials 272, 120767–120767 (2021). [DOI] [PubMed] [Google Scholar]
- 44.J. H. Lee, P. Parthiban, G. Z. Jin, J. C. Knowles, H. W. Kim, Materials roles for promoting angiogenesis in tissue regeneration. Prog. Mater. Sci. 117, 100732 (2021). [Google Scholar]
- 45.P. Carmeliet, R. K. Jain, Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000). [DOI] [PubMed] [Google Scholar]
- 46.R. Y. Kannan, H. J. Salacinski, K. Sales, P. Butler, A. M. Seifalian, The roles of tissue engineering and vascularisation in the development of micro-vascular networks: A review. Biomaterials 26, 1857–1875 (2005). [DOI] [PubMed] [Google Scholar]
- 47.S. Oladipupo, S. Hu, J. Kovalski, J. Yao, A. Santeford, R. E. Sohn, R. Shohet, K. Maslov, L. V. Wang, J. M. Arbeit, VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proc. Natl. Acad. Sci. U.S.A. 108, 13264–13269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.R. Scherz-Shouval, Z. Elazar, ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 17, 422–427 (2007). [DOI] [PubMed] [Google Scholar]
- 49.M. Mittal, M. R. Siddiqui, K. Tran, S. P. Reddy, A. B. Malik, Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20, 1126–1167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.J. Gerdts, E. J. Brace, Y. Sasaki, A. DiAntonio, J. Milbrandt, SARM1 activation triggers axon degeneration locally via NAD(+) destruction. Science 348, 453–457 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.K. Essuman, D. W. Summers, Y. Sasaki, X. Mao, A. DiAntonio, J. Milbrandt, The SARM1 Toll/interleukin-1 receptor domain possesses intrinsic NAD(+) cleavage activity that promotes pathological axonal degeneration. Neuron 93, 1334–1343.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.P. J. Arthur-Farraj, M. Latouche, D. K. Wilton, S. Quintes, E. Chabrol, A. Banerjee, A. Woodhoo, B. Jenkins, M. Rahman, M. Turmaine, G. K. Wicher, R. Mitter, L. Greensmith, A. Behrens, G. Raivich, R. Mirsky, K. R. Jessen, c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75, 633–647 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.S. Parrinello, I. Napoli, S. Ribeiro, P. W. Digby, M. Fedorova, D. B. Parkinson, R. D. S. Doddrell, M. Nakayama, R. H. Adams, A. C. Lloyd, EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 143, 145–155 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.B. Beirowski, E. Babetto, J. P. Golden, Y. J. Chen, K. Yang, R. W. Gross, G. J. Patti, J. Milbrandt, Metabolic regulator LKB1 is crucial for Schwann cell-mediated axon maintenance. Nat. Neurosci. 17, 1351–1361 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.A. S. Saab, I. D. Tzvetavona, A. Trevisiol, S. Baltan, P. Dibaj, K. Kusch, W. Möbius, B. Goetze, H. M. Jahn, W. Huang, H. Steffens, E. D. Schomburg, A. Pérez-Samartín, F. Pérez-Cerdá, D. Bakhtiari, C. Matute, S. Löwel, C. Griesinger, J. Hirrlinger, F. Kirchhoff, K. A. Nave, Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.M. K. Jha, Y. Lee, K. A. Russell, F. Yang, R. M. Dastgheyb, P. Deme, X. H. Ament, W. Chen, Y. Liu, Y. Guan, M. J. Polydefkis, A. Hoke, N. J. Haughey, J. D. Rothstein, B. M. Morrison, Monocarboxylate transporter 1 in Schwann cells contributes to maintenance of sensory nerve myelination during aging. Glia 68, 161–177 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.L. A. Patron, K. E. Zinsmaier, Mitochondria on the road to power axonal regeneration. Neuron 92, 1152–1154 (2016). [DOI] [PubMed] [Google Scholar]
- 58.S. Kiryu-Seo, H. Kiyama, Mitochondrial behavior during axon regeneration/degeneration in vivo. Neurosci. Res. 139, 42–47 (2019). [DOI] [PubMed] [Google Scholar]
- 59.S. M. Han, H. S. Baig, M. Hammarlund, Mitochondria localize to injured axons to support regeneration. Neuron 92, 1308–1323 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.W. Z. Ray, S. E. Mackinnon, Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 223, 77–85 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D. Grinsell, C. P. Keating, Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed. Res. Int.. 2014, 698256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.T. Norkus, M. Norkus, T. Ramanauskas, Donor, recipient and nerve grafts in brachial plexus reconstruction: Anatomical and technical features for facilitating the exposure. Surg. Radiol. Anat. 27, 524–530 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials and Methods
Figs. S1 to S24
Table S1