Abstract
Background and Objectives
Haemodilution leads to complications in clinical practice. It is exactly unknown whether this damage is caused by the fluid or by the stretching of the vascular bed. We aimed to compare two different haemodilution techniques at the same anaemic level.
Methods
Normovolemic or hypervolemic haemodilution was performed on twelve adult male Wistar rats. In the normovolemic procedure, blood was withdrawn and instantaneously administered with similar amounts of 6% hydroxyethyl starch (HES 130/0.4). Fluid was administered without withdrawing blood in the hypervolemic procedure. In both models, a 25% haematocrit level was targeted and kept at this level for 90 min to deepen the anaemia effect. Besides haemodynamics measurement, renal function (creatinine, blood urea nitrogen) and injury (tissue norepinephrine, malondialdehyde) were evaluated. Also, systemic hypoxia (lactate), oxidative stress (malondialdehyde, ischaemia-modified albumin), inflammation (tumour necrosis factor-alpha [TNF-α]), osmotic stress, adrenal stress (norepinephrine, epinephrine), and vascular stretching (atrial natriuretic peptide [ANP]) were assessed.
Results
Arterial pressure in the normovolemic group was lower than in the hypervolemic group. Serum creatinine, blood urea nitrogen, and lactate levels were higher in the normovolemic group. Tissue norepinephrine and malondialdehyde levels were higher in the normovolemic group. Serum ANP, malondialdehyde, ischaemia-modified albumin, free haemoglobin, syndecan-1, and TNF-α were higher in both groups compared to respective baseline.
Conclusions
Normovolemic haemodilution may lead to hypoxic kidney injury. The hypervolemic state may be advantageous if fluid is to be administered. Thus, the effect of the fluid itself can be relatively masked.
Key words: haemodilution, kidney injury, anaemia, fluid therapy, resuscitation, glycocalyx
Introduction
Fluid therapy is used to maintain intravascular blood volume in shock, to reduce the effect of blood loss before autologous blood transfusion and to fill the pump circuit in bypass surgery. Currently, two different types of fluids such as colloids and crystalloids are used in the clinics. Regardless of the type of the fluid, the blood becomes diluted after administration, and therefore, anaemia occurs.[1]
During dilutional anaemia, haematocrit is reduced and the oxygen-carrying capacity of the blood is decreased due to reduced perfused vessel density unless cardiac output increases in response to dilutional stress.[2] Haemodilution and subsequently anaemia result in organ damage (heart, brain, kidney) and mortality in patients requiring surgical intervention.[3] In particular, the renal tolerance to anaemia is quite low.[4] Kidney dysfunction related to dilutional anaemic injury involves many processes such as inflammation, oxidative stress and glycocalyx damage.[5,6]
Although quite a few suggestions indicate the need for fluid therapy to reduce blood loss, there are no clear cues to terminate fluid administration.[7,8] Hence, since the fluid status of patients cannot be known exactly, fluid administration may lead the patient to a hypervolemic state. Dilutional anaemia accompanies vascular distension as long as fluid treatment continues. Moreover, apart from the anaemic effect of the fluid, the increment in fluid load causes the heart wall to stretch and atrial natriuretic peptide (ANP) is released. ANP is one of the leading players in the destruction mechanism.[9] Also, in the absence of vascular stretch, free radicals may be a trigger for ANP release per se.[10] ANP cleaves the endothelial glycocalyx via an oxidative mediator.[10] As a result of the oxidative process, ANP contributes to oedema formation by increasing vascular permeability.[10,11] In response to the shedding of glycocalyx fragments into the plasma, secretion of the sympathetic adrenal nerves increases.[12,13] However, ANP released from the cardiac atrium may complicate the relationship between catecholamines and glycocalyx fragments.[14]
Despite the contrary statements for stress caused by fluids stated above, it is unexplained how vascular stretching contributes to the dilutional injury process. In this study, we aimed to investigate whether the source of the adverse effects of haemodilution is anaemia or stretching of the vascular bed. To substantiate this, we constructed two different models of dilutional stresses at the same anaemic level.
Materials and methods
Ethics
All experiments in this study were approved by the Local Ethics Committee on Animal Experiments of Istanbul University, Istanbul, Turkey (Chairperson Prof. A. Akdogan Kaymaz) on 28 September 2017 (Protocol-Number 2017/105706). All animals were obtained from Istanbul University Aziz Sancar Institute of Experimental Medicine. Care and handling of the animals were performed according to ARRIVE guidelines, the U.K. animals Act 1986 and EU directive 2010/63/EU. Individual cages in a temperature-controlled room (23°C ± 1°C) and a light-dark cycle-controlled environment (12 h) with free access to food and water were used for animal housing. Male Wistar albino rats (n = 12) (335 ± 14 g) were used for the experiments.
Surgical protocol and study design
The rats were anaesthetized by using sodium pentothal (70 mg/kg intraperitoneal) and allowed to breathe spontaneously through a tube placed into the trachea. The body temperature of the rats was set at 37°C ± 0.5°C and controlled by a rectal probe during the entire experiment. Carotid artery—to record the systemic arterial pressure— and femoral vein—to administer the fluids—were then cannulated. Artery pressure waves were recorded in real time via a pressure transducer (Biopac MP45). At the end of the surgical preparation, 20 min were allowed for haemodynamic stabilization. Then, the animals were randomly assigned to two groups: Acute normovolemic haemodilution group (ANH) (n = 6): Blood was withdrawn via the femoral artery (1 mL/min) and 6% hydroxyethyl starch (HES) was given via the femoral vein at the same rate. Acute hypervolemic haemodilution group (AHH) (n = 6): 6% HES solution was intravenously administered (1 mL/min) without withdrawing blood. For both groups, the targeted haematocrit as 25% was provided with sporadic haematocrit measurement, and when the target haematocrit was reached 25%, the rats were monitored for 1 h.
Blood sampling and haemodynamic measurements
Blood samples were gathered immediately after haemodynamic stabilization (T0), at the end of reaching the target haematocrit (T1), and 90 min after reaching the target haematocrit (T2). Mean arterial blood pressure (MAP) and heart rate (HR) were recorded at these time points. MAP values were calculated according to the formula as “MAP = 2/3 diastolic arterial pressure + 1/3 systolic arterial pressure”. HR was calculated as the derivation of the arterial pressure signal. In addition, the amount of fluid applied for both methods was recorded. The rats were then euthanized by an intracardiac puncture. Kidney and blood samples were collected.
The taken blood samples were centrifuged at 5000 rpm for 5 min. The tissue samples were weighed and washed with 0.9% NaCl solution. The acquired supernatants and other tissue samples were stored at −80°C until the day of measurement.
Biochemical evaluations
The kidney tissues were weighed and diluted 10% (w/v) in 20 mmol/L ice-cold PBS, pH 7.4, and homogenized with a tissue grinder. The homogenates were centrifuged at 3000 × g for 10 min, and biochemical measurements were performed in the supernatant fraction.
Serum levels of blood urea nitrogen, creatinine, and lactate were measured by using an autoanalyzer. The level of free haemoglobin was determined according to Noe’s method.[15] The measurement of malondialdehyde (MDA) level was based on the principle that the MDA reacts with thiobarbituric acid and gives a pink colour in a hot environment.[16] The measurements of ischaemia-modified albumin (IMA) level are based on the spectrophotometric reading of the resulting coloration of the reaction with albumin cobalt.[17]
Atrial natriuretic peptide, norepinephrine, epinephrine, tumour necrosis factor-alpha and syndecan-1 levels were measured with commercial enzyme-linked immunosorbent assays (ELISA) kits.
Statistical Analysis
Data sets are expressed as means ± SEM. Statistical analysis was performed with a statistical package program (GraphPad Software v5.0, San Diego, CA, USA). All data were compared by performing Student’s t-test, one-way ANOVA analysis was used for comparisons and post hoc analyses were used with Tukey’s multiple comparison test when P < 0.05. For all analyses, P < 0.05 was considered statistically significant.
Results
Haemodynamic variables
The baseline levels of the measured parameters were not significantly different between the groups. Both normovolemic (NH) and hypervolemic (HH) haemodilution caused marked effects on mean arterial pressure (MAP), when compared to baseline (ANH T0: 106.1 ± 2.9 mmHg, T2: 67.1 ± 4.1 mmHg P < 0.01) (AHH T0: 111.4 ± 2.4 mmHg, T2: 84.7 ± 5.8 mmHg P < 0.01). At the T2 time point, MAP values in the AHH group were higher than in the ANH group (P < 0.05). Additionally, heart rate (HR) values in both groups remain unchanged (P > 0.05) (Table 1).
Table 1.
Haemodynamic variables in ANH and AHH
| ANH | AHH | |
|---|---|---|
| MAP (mmHg) | ||
| T0 | 106.1 ± 2.9 | 111.4 ± 2.4 |
| T1 | 83.5 ± 4.6 | 98.2 ± 5.2* |
| T2 | 67.1 ± 4.1**+† | 84.7 ± 5.8** |
| HR (beat/min) | ||
| T0 | 380.0 ± 12.6 | 335.0 ± 16.3 |
| T1 | 375.0 ± 24.2 | 330.0 ± 13.4 |
| T2 | 355.0 ± 22.5 | 330.0 ± 18.9 |
MAP: mean arterial pressure; HR: heart rate; ANH: acute normovolemic haemodilution; AHH: acute hypervolemic haemodilution. *P < 0.05, **P < 0.01, vs. T0, +P < 0.05 vs. T1, †P < 0.05 vs. AHH T2.
Administered fluid volumes and haematocrit values
Administered fluid volumes in the AHH group (25.3 ± 3.7 mL) were higher than in the ANH group (8.4 ± 0.9 mL, P < 0.01). The baseline levels of haematocrit were not significantly different between the groups (P > 0.05). In the ANH group, haematocrit levels were found as 47.5 ± 1.7, 24.8 ± 0.5, and 25.0 ± 1.0, and as 50.2 ± 1.8, 25.1 ± 0.5, and 25.9 ± 1.8, respectively, in the AHH group. In both groups, haematocrit values were significantly changed during the haemodilution (T0 vs. T1 and T2 P < 0.001) (Figure 1).
Figure 1.
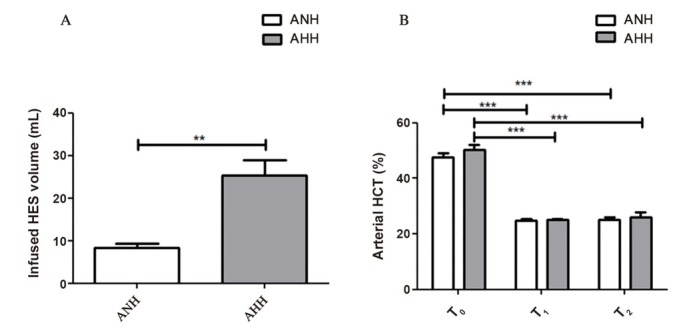
Haemodilution protocol. Infused HES volume at the procedure (A), arterial HCT (%) levels after hemodynamic stabilization (T0), at the end of fluid treatment (target haematocrit of 25%) (T1) and the end of experimentation (T2) (B). HES: hydroxyethyl starch; ANH: acute normovolemic haemodilution; AHH: acute hypervolemic haemodilution. **P < 0.01, ***P < 0.001.
Renal function markers and lactate levels
Baseline creatinine, blood urea nitrogen (BUN) and lactate levels in serum showed no difference between the ANH and AHH groups (P > 0.05). Serum creatinine levels were significantly found to be higher in the ANH group (T2: 0.4 ± 0.02 mg/dL) compared to the respective baseline (0.2 ± 0.02 mg/dL, P < 0.01) and to the AHH (0.2 ± 0.04 mg/dL) group at the T2 time point. In terms of BUN levels were significantly found to be higher in the ANH group (T2: 30.7 ± 3.2 mg/dL) compared to the respective baseline (12.5 ± 2.1 mg/dL, P < 0.01) and to the AHH (15.8 ± 3.5 mg/dL, P < 0.05) group at the T2 time point. Serum lactate levels were significantly higher in the ANH group (T2: 6.2 ± 0.9 mmol/L) compared to the respective baseline (1.4 ± 0.2 mmol/L, P < 0.01) and to the AHH (3.8 ± 1.3 mmol/L, P < 0.01) group at the T2 time point (Table 2).
Table 2.
Levels of serum biochemistry in ANH and AHH
| ANH | AHH | |
|---|---|---|
| Creatinine (mg/dL) | ||
| T0 | 0.2 ± 0.00 | 0.2 ± 0.01 |
| T2 | 0.4 ± 0.02**† | 0.2 ± 0.04 |
| BUN (mg/dL) | ||
| T0 | 12.5 ± 2.1 | 15.8 ± 3.5 |
| T2 | 30.7 ± 3.2**† | 15.1 ± 1.2 |
| Lactate (mmol/L) | ||
| T0 | 1.4 ± 0.2 | 1.9 ± 0.4 |
| T2 | 6.2 ± 0.9**† | 3.8 ± 1.3 |
| ANP (pg/mL) | ||
| T0 | 25.1 ± 1.7 | 27.1 ± 5.1 |
| T2 | 66.2 ± 11.6* | 55.4 ± 5.2*** |
| MDA (mmol/L) | ||
| T0 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| T2 | 1.6 ± 0.2* | 1.5 ± 0.4* |
| IMA (ABSU) | ||
| T0 | 0.8 ± 0.03 | 0.8 ± 0.01 |
| T2 | 1.1 ± 0.10* | 0.9 ± 0.03 |
| Free haemoglobin (g/L) | ||
| T0 | 0.005 ± 0.002 | 0.007 ± 0.003 |
| T2 | 0.042 ± 0.017* | 0.051 ± 0.011* |
| Syndecan-1 (ng/mL) | ||
| T0 | 4.2 ± 1.8 | 6.7 ± 2.5 |
| T2 | 8.9 ± 2.7* | 11.7 ± 3.7* |
| TNF-α (pg/mL) | ||
| T0 | 31.4 ± 11.0 | 22.2 ± 8.3 |
| T2 | 177.1 ± 25.7** | 147.0 ± 25.2** |
ANH: acute normovolemic haemodilution; AHH: acute hypervolemic haemodilution; ANP: atrial natriuretic peptide; MDA: malondialdehyde; IMA: ischaemia modified albumin; TNF-α: tumour necrosis factor-alpha. *P < 0.05, **P < 0.01, ***P < 0.001 vs. T0, †P < 0.05 vs. AHH.
Cellular injury markers
The levels of measured parameters of both groups were similar at baseline (P > 0.05) and also were similar at the T2 time point (P > 0.05). At the T2 time point, serum atrial natriuretic peptide (ANP) levels significantly increased in both groups (ANH 66.2 ± 11.6 pg/mL, P < 0.05 and AHH 55.4 ± 5.2 pg/mL, P < 0.001) compared to the respective baseline (25.1 ± 1.7 pg/mL and 27.1 ± 5.1 pg/mL). The level s of m a l on d i a l d ehyd e ( MDA ) in serum were significantly increased in both (ANH 1.6 ± 0.2 mmol/L, P < 0.05 and AHH 1.5 ± 0.4 mmol/L, P < 0.05) groups compared to the respective baseline (0.4 ± 0.1 mmol/L and 0.4 ± 0.1 mmol/L). Ischaemia modified albumin(IMA) levels were significantly higher at the T2 time point of the ANH group (P < 0.05). Free haemoglobin levels were significantly increased in both groups (ANH 0.042 ± 0.017 g/L, P < 0.05 and AHH 0.051 ± 0.011 g/L, P < 0.05) compared to respective baseline levels in both groups (ANH 0.005 ± 0.002 g/L and AHH 0.007 ± 0.003 g/L). At the T2 time point, syndecan-1 levels in serum were significantly increased in both (ANH 8.9 ± 2.7 ng/mL, P < 0.05 and AHH 11.7 ± 3.7 ng/mL, P < 0.05) groups compared to the respective baseline (4.2 ± 1.8 ng/mL and 6.7 ± 2.5 ng/mL). As last, serum tumour necrosis factor-alpha (TNF-α) levels were significantly increased at the T2 time point in both (ANH 177.1 ± 25.7 pg/mL, P < 0.01 and AHH 147.0 ± 25.2 pg/mL, P < 0.01) groups compared to baseline (31.4 ± 11.0 pg/mL and 22.2 ± 8.3 pg/mL, respectively) (Table 2).
Renal tissue MDA and norepinephrine levels
Norepinephrine (NE) levels in renal tissue were higher in the ANH group (0.26 ± 0.03 ng/mL) compared to the AHH (0.08 ± 0.04 ng/mL) group (P < 0.01). Besides, kidney tissue levels of MDA were slightly increased in the ANH group (1.3 ± 0.4 mmol/L) compared to the AHH (0.9 ± 0.3 mmol/L) (Figure 2).
Figure 2.
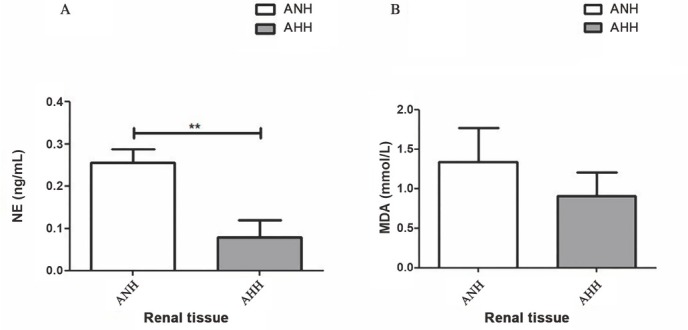
Renal injury. Renal tissue NE (A) and MDA levels (B). ANH: acute normovolemic haemodilution; AHH: acute hypervolemic haemodilution; NE: norepinephrine; MDA: malondialdehyde. **P < 0.01.
Serum catecholamine levels
The baseline NE levels in serum showed no difference between the ANH and AHH groups (P > 0.05). At the T2 time points, NE levels in serum were significantly increased in both ANH (0.3 ± 0.02 ng/mL vs. 1.2 ± 0.1 ng/mL, P < 0.01) and AHH (0.4 ± 0.1 ng/mL vs. 1.2 ± 0.3 ng/mL, P < 0.05) groups compared to the respective baseline. However, both groups were similar at the T2 (P > 0.05). Likewise, baseline epinephrine (EPI) levels in serum showed no difference between the ANH and AHH groups (P > 0.05). At the T2 time point, EPI levels in serum were significantly increased in both ANH (136.4 ± 23.4 pg/mL vs. 210.8 ± 20.9 pg/mL, P < 0.001) and AHH (95.1 ± 19.4 pg/mL vs. 180.5 ± 36.0 pg/mL, P < 0.05) groups compared to the respective baseline. However, both groups were similar at the T2 (P > 0.05) (Figure 3).
Figure 3.
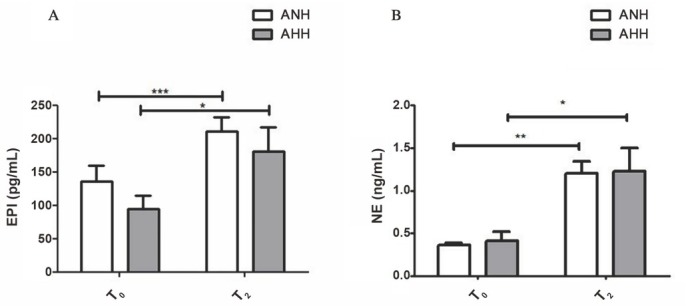
Sympathoadrenal activation. Serum EPI (A) and NE (B) levels. ANH: acute normovolemic haemodilution; AHH: acute hypervolemic haemodilution; EPI: epinephrine; NE: norepinephrine. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In this study, we compared two different haemodilution models (normovolemic vs. hypervolemic) with the same anaemic levels for their effects on cellular injury and renal function in rat models. The main points were that (1) both haemodilution approaches caused haemolysis (free haemoglobin), systemic oxidative stress (MDA), inflammation (TNF-α), glycocalyx disruption (syndecan-1), and led to adrenergic stress (epinephrine, norepinephrine); (2) moreover, NH decreased blood pressure, led to systemic hypoxia resulting in renal function loss; (3) more volume fluid was needed to achieve target haematocrit in AHH.
Haemodilution is an inevitable result in some cases such as cardiopulmonary bypass surgery[18,19] and shock treatment.[20] Despite the widespread use of fluids, the side effects of fluids are much higher than expected. Several studies have showed that fluids cannot improve the oxygen-carrying capacity of the blood resulting in disrupting oxygen delivery to the tissues.[20,21] At the systemic level, haemodilution causes to decrease in peripheral resistance and an increase in venous return and cardiac output. Although increments in macrohaemodynamics try to improve the aerobic metabolism via oxygen delivery, the tissue oxygenation is already impaired.[22,23]
In our study, two different haemodilution models with the same anaemic level were performed on rats. In the NH procedure, blood was withdrawn and instantaneously administered with similar amounts of 6% HES. However, fluid was administered without withdrawing blood in the hypervolemic procedure. In both models, a 25% haematocrit level was targeted and kept at this level for 90 min to deepen the anaemia effect. At the end of the experiment, the dilution levels were similar in both groups, even if more fluid is given in the hypervolemic model. The same anaemic level in both models facilitated the comparison of different fluid regimens in terms of vascular distension.
Dilution of the blood first reduces the oxygen-carrying capacity of the blood, leading to hypoxic tissue injury.[24] Another major factor that can lead to organ damage is the change in the osmotic balance of the blood. As a result of excessive fluid administration, erythrocytes can lose their membrane integrity, and the level of free haemoglobin in the plasma may increase.[25] In our study, the disruption of membrane integrity was demonstrated by the increase in free haemoglobin levels. Indeed, both protocols created similar osmotic stress, reflecting the related increase in free haemoglobin levels. Many diseases have been associated with free haemoglobin. The basis of all diseases is haemoglobin is an extremely reactive and oxidative molecule. Initially, haemoglobin passes to extravascular areas in sensitive tissues such as the kidney and lung. After and during this transition, the pro-oxidant and nitric oxide scavenger properties of haemoglobin create toxicity where it is present. The main product of this toxicity is Fe3+ carrying haemoglobin, which is separated from hemin. After that, hemin continues to damage as a signal initiator on its own.[26] Considering all these processes, free haemoglobin, which was increased in our study, may have created a domino effect. In this way, enhanced oxidant mediators cause a boost in other oxidant mediators and initiate inflammation. In our study, both models showed an increment in plasma parameters associated with oxidative stress and inflammation. The primary target of inflammation in the vascular environment is glycocalyx.[6] Glycocalyx forms a vascular barrier with intercellular connections and mediates the association of various hormones and endothelial-leukocyte cells. It allows some vascular factors to work but also regulates receptor sensitivity.[27] We think that the escalated levels of inflammation parameters in both protocols are associated with increased glycocalyx damage.
In our study, we measured catecholamine concentrations also to understand the stress created by fluid administrations, and the levels of related substances were higher in both groups at the end of the experiment. Molecules reflecting adrenergic stress also have a relationship with other molecules. Even in previous studies, the parallel increase of catecholamine and glycocalyx components was investigated in acute myocardial infarction and trauma models, and this mutual increase was thought to trigger each other.[13] As seen in our study, the build-up in catecholamines and glycocalyx components levels suggests that there is a relationship between them.
In our study, the ANP levels were higher in both protocols. ANP is known to increase due to the stretching of the vascular system under normal physiological conditions. The enhanced level of ANP in the normovolemic condition could be a direct effect of the fluid itself. It is known that ANP itself is a radical generator. Thus, the independent destructive effect of fluids may have occurred via ANP. In fluid administration, apart from free haemoglobin, ANP may be working as another initiator by a different route. As the later injury process, in both models, glycocalyx degradation may also be a direct result of ANP release.[28]
Interestingly, both positive and negative correlation between the release of ANP and catecholamines have been shown in many studies.[29,30] The common axis of this relationship is glycocalyx damage.[13] Moreover, the levels of catecholamines, such as noradrenaline, together with ANP have been previously showed to increase in cases where vascular stress surges.[31] In our study, we showed that both ANP and catecholamine levels get higher in both protocols. Catecholamines (adrenaline, noradrenaline), which increase in circulation and are released from sympathetic nerve endings, also increase blood pressure and cause contractions in the vessels. Catecholamines also play a role in the cleavage of endothelial glycocalyx because they increase blood pressure. Therefore, they may lead to pulmonary oedema.[32] As a result of this strain, the glycocalyx compartments may cause localized inflammation in the environment, allowing the passage of cells and proteins into the tissue, thereby increasing the filtration rate, leading to the formation of oedema or damage to the distal organs by joining the bloodstream.[13] Taken together, ANP, catecholamines, free haemoglobin, and glycocalyx fragments can be independent initiators of cellular damage, or they can act as triggers for each other.
Regardless of the protocol, although we clearly showed the destructive effect of the fluid itself, we can also see that the two approaches ultimately result in a different outcome in terms of the kidney. As previously pointed out, systemic haemodynamic parameters were measured in our study. The most significant systemic effect of NH was lower blood pressure compared to the hypervolemic model. This hypotensive effect can initiate a process that continues in a way independent of the direct action of the fluid itself. Indeed, our findings point to a process in the normovolemic procedure that begins with hypotension, continues with hypoxia, and eventually ends with the loss of kidney function. In detail, serum lactate, creatinine and BUN levels increased in normovolemic protocol compared to hypervolemic protocol. Even in kidney tissue, norepinephrine and MDA levels which are stress markers were higher normovolemia compared to hypervolemia. With these findings, we think that ANH has a more detrimental effect on kidney injury, and the adverse effects of ANH could be related to hypotension.[33] Similarly, there have been many studies showing tissue and organ damage due to haemodilution.[34,35] Previously, the results of ANH in patients with spinal cord ischaemia have been to cause neurological damage.[36]
It has been previously showed that the tolerance of the kidney to anaemia and hypoxia is reasonably low. Therefore, it is certain that fluid administration, whether normovolemic or hypervolemic, will have a wearing effect on the kidney. For instance, the effects of AHH on kidney and blood were also investigated in other posttraumatic patients. It has been predicted that the decrease in respiratory failure, hypertension, increased cardiac output, and renal perfusion may be due to hypervolemia.[37, 38, 39]
Conclusion
Haemodilution could lead to a detrimental injury no matter it is in the case of hypervolemia or normovolemia. However, the normovolemic state may place an additional burden on the body system compared to hypervolemia. Particularly, hypoxic kidney damage could be the result of normovolemic haemodilution. In this respect, the vascular stretching induced by the hypervolemia may be advantageous if fluid administration is a necessity. Thus, the adverse effect of the fluid itself can be relatively masked. Hence, comprehensive and molecular biological studies are needed on the long-term effects of fluid applications.
Acknowledgements
The authors would like to thank Gulsum Karduz, MSc., and Kubra Vardar, MSc., for their assistance.
Funding Statement
This study was funded by Scientific Research Projects Coordination Unit of Istanbul University (FYL-2018-27510).
Footnotes
Author Contributions
Performing experimentation: Aksu U, Cakir MU; Conceptualization: Aksu U; Draft Writing: Cakir MU, Aksu U, Yavuz-Aksu B; Final Writing: Cakir MU, Aksu U, Yavuz-Aksu B; Supervision: Aksu U; Statistical Analyses: Cakir MU.
Ethics Approval
All experiments in this study were approved by the Local Ethics Committee on Animal Experiments of Istanbul University, Istanbul, Turkey (Chairperson Prof. A. Akdogan Kaymaz) on 28 September 2017 (Protocol-Number 2017/105706).
Conflicts of Interest
None declared.
References
- 1.Monnet X, Teboul JL. My patient has received fluid. How to assess its efficacy and side effects? Ann Intensive Care. 2018;8:1–7. doi: 10.1186/s13613-018-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamnicki M, Kocian R, van der Linden P, Zaugg M, Spahn DR. Acute normovolemic hemodilution: physiology, limitations, and clinical use. J Cardiothorac Vasc Anesth. 2003;17:747–54. doi: 10.1053/j.jvca.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Hare GM. Tolerance of anemia: understanding the adaptive physiological mechanisms which promote survival. Transfus Apher Sci. 2014;50:10–2. doi: 10.1016/j.transci.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 4.van Bommel J, Siegemund M, Henny ChP, Ince C. Heart, kidney, and intestine have different tolerances for anemia. Transl Res. 2008;151:110–7. doi: 10.1016/j.trsl.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Konrad FM, Mik EG, Bodmer SI, Ates NB, Willems HF, Klingel K. Acute normovolemic hemodilution in the pig is associated with renal tissue edema, impaired renal microvascular oxygenation, and functional loss. Anesthesiology. 2013;119:256–69. doi: 10.1097/ALN.0b013e31829bd9bc. et al. [DOI] [PubMed] [Google Scholar]
- 6.Ergin B, Guerci P, Uz Z, Westphal M, Ince Y, Hilty M. Hemodilution causes glycocalyx shedding without affecting vascular endothelial barrier permeability in rats. J Clin Transl Res. 2020;5:243. et al. [PMC free article] [PubMed] [Google Scholar]
- 7.Veenstra G, Ince C, Boerma EC. Direct markers of organ perfusion to guide fluid therapy: when to start, when to stop. Best Pract Res Clin Anaesthesiol. 2014;28:217–26. doi: 10.1016/j.bpa.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Ince C. The rationale for microcirculatory guided fluid therapy. Curr Opin Crit Care. 2014;20:301–8. doi: 10.1097/MCC.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 9.Chappell D, Bruegger D, Potzel J, Jacob M, Brettner F, Vogeser M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care. 2014;18:1–8. doi: 10.1186/s13054-014-0538-5. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vito P, Incerpi S, Pedersen JZ, Luly P. Atrial natriuretic peptide and oxidative stress. Peptides. 2010;31:1412–9. doi: 10.1016/j.peptides.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Bruegger D, Schwartz L, Chappell D, Jacob M, Rehm M, Vogeser M. Release of atrial natriuretic peptide precedes shedding of the endothelial glycocalyx equally in patients undergoing on- and off-pump coronary artery bypass surgery. Basic Res Cardiol. 2011;106:1111–21. doi: 10.1007/s00395-011-0203-y. et al. [DOI] [PubMed] [Google Scholar]
- 12.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254:194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 13.Ostrowski SR, Pedersen SH, Jensen JS, Mogelvang R, Johansson PI. Acute myocardial infarction is associated with endothelial glycocalyx and cell damage and a parallel increase in circulating catecholamines. Crit Care. 2013;17:1–2. doi: 10.1186/cc12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. 2019;23:1–2. doi: 10.1186/s13054-018-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noe DA, Weedn V, Bell WR. Direct spectrophotometry of serum hemoglobin: an Allen correction compared with a three-wavelength polychromatic analysis. Clin Chem. 1984;30:627–30. [PubMed] [Google Scholar]
- 16.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 17.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J Emerg Med. 2000;19:311–5. doi: 10.1016/s0736-4679(00)00255-9. [DOI] [PubMed] [Google Scholar]
- 18.Arıtürk C, Ozgen ZS, Kilercik M, Ulugöl H, Ökten EM, Aksu U. Comparative effects of hemodilutional anemia and transfusion during cardiopulmonary bypass on acute kidney injury: a prospective randomized study. Heart Surg Forum. 2015;18:E154–60. doi: 10.1532/hsf.1387. et al. [DOI] [PubMed] [Google Scholar]
- 19.Saito J, Hirota K. The volume of acute normovolemic hemodilution. Gynecol Oncol Rep. 2019;29:132. doi: 10.1016/j.gore.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aksu U, Bezemer R, Yavuz B, Kandil A, Demirci C, Ince C. Balanced vs unbalanced crystalloid resuscitation in a near-fatal model of hemorrhagic shock and the effects on renal oxygenation, oxidative stress, and inflammation. Resuscitation. 2012;83:767–73. doi: 10.1016/j.resuscitation.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Legrand M, Mik EG, Balestra GM, Lutter R, Pirracchio R, Payen D. Fluid resuscitation does not improve renal oxygenation during hemorrhagic shock in rats. Anesthesiology. 2010;112:119–27. doi: 10.1097/ALN.0b013e3181c4a5e2. et al. [DOI] [PubMed] [Google Scholar]
- 22.van Bommel J, Trouwborst A, Schwarte L, Siegemund M, Ince C, Henny ChP. Intestinal and cerebral oxygenation during severe isovolemic hemodilution and subsequent hyperoxic ventilation in a pig model. Anesthesiology. 2002;97:660–70. doi: 10.1097/00000542-200209000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Van Woerkens EC, Trouwborst A, Duncker DJ, Koning MM, Boomsma F, Verdouw PD. Catecholamines and regional hemodynamics during isovolemic hemodilution in anesthetized pigs. J Appl Physiol. 1992;72:760–9. doi: 10.1152/jappl.1992.72.2.760. [DOI] [PubMed] [Google Scholar]
- 24.Johannes T, Mik EG, Nohé B, Unertl KE, Ince C. Acute decrease in renal microvascular PO2 during acute normovolemic hemodilution. Am J Physiol Renal Physiol. 2007;292:F796–803. doi: 10.1152/ajprenal.00206.2006. [DOI] [PubMed] [Google Scholar]
- 25.Ulugol H, Aksu U, Kocyigit M, Kilercik M, Karduz G, Okten M. Comparative Effects of Blood and Crystalloid Cardioplegia on Cellular Injury and Oxidative Stress in Cardiovascular Surgery. Ann Thorac Cardiovasc Surg. 2019;25:10–7. doi: 10.5761/atcs.oa.18-00113. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–84. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radeva MY, Waschke J. Mind the gap: mechanisms regulating the endothelial barrier. Acta Physiol (Oxf) 2018. p. 222. [DOI] [PubMed]
- 28.Choi MR, Fernández BE. Protective Renal Effects of Atrial Natriuretic Peptide: Where Are We Now? Front Physiol. 2021;12:680213. doi: 10.3389/fphys.2021.680213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanwal S, Elmquist BJ, Trachte GJ. Atrial natriuretic peptide inhibits evoked catecholamine release by altering sensitivity to calcium. J Pharmacol Exp Ther. 1997;283:426–33. [PubMed] [Google Scholar]
- 30.Quan HX, Jin JY, Wen JF, Cho KW. Beta1-adrenergic receptor activation decreases ANP release via cAMP-Ca2+ signaling in perfused beating rabbit atria. Life Sci. 2010;87:246–53. doi: 10.1016/j.lfs.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Sanderson JE. Pathogenesis of oedema in chronic severe anaemia: studies of body water and sodium, renal function, haemodynamic variables and plasma hormones. Br Heart J. 1994;71:490. doi: 10.1136/hrt.71.5.490-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dünser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. 2009;24:293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 33.Corrêa TD, Rocha LL, Pessoa CM, Silva E, Assuncao MS. Fluid therapy for septic shock resuscitation: which fluid should be used? Einstein (Sao Paulo) 2015;13:462–8. doi: 10.1590/S1679-45082015RW3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikami N, Saito J, Ohyama T, Kubota M, Noguchi S, Kitayama M. Acute normovolemic hemodilution and acute kidney injury after open abdominal cancer surgery. J Clin Anesth. 2020;61:109657. doi: 10.1016/j.jclinane.2019.109657. et al. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Pei F, Wu J, Ouyang B, Guan X. Kidney Injury in a Hemodilution Model of Hemorrhagic Shock and Fluid Resuscitation. Am J Med Sci. 2021;362:506–11. doi: 10.1016/j.amjms.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Kim J, Hwang J, Huh J, Nahm SF, Lim C, Park S. Acute normovolemic hemodilution can aggravate neurological injury after spinal cord ischemia in rats . Anesth Analg. 2012;114:1285–91. doi: 10.1213/ANE.0b013e31824d2723. et al. [DOI] [PubMed] [Google Scholar]
- 37.Lucas CE, Ledgerwood AM, Shier MR, Bradley VE. The renal factor in the post-traumatic “fluid overload” syndrome. J Trauma. 1977;17:667–76. [PubMed] [Google Scholar]
- 38.Hansen B. Fluid Overload. Front Vet Sci. 2021;8:668688. doi: 10.3389/fvets.2021.668688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patil VP, Salunke BG. Fluid Overload and Acute Kidney Injury. Indian J Crit Care Med. 2020;24:S94–7. doi: 10.5005/jp-journals-10071-23401. [DOI] [PMC free article] [PubMed] [Google Scholar]


