ABSTRACT
Herpes zoster (HZ) is caused by the reactivation of latent varicella zoster virus (VZV). Severe immunocompromising conditions, such as solid tumors, have been largely associated with an increased risk for HZ due to waning VZV-specific cellular immunity. With the approval of the adjuvanted glycoprotein E (gE)-based recombinant vaccine (RZV; Shingrix™, GSK) also in immunocompromised subjects, HZ is considered a vaccine-preventable disease changing perspectives in immunocompromised subjects. To date, no clinical trial has evaluated the immunogenicity in the patients with cancer undergoing immunotherapy. In this study, we describe the humoral and cell-mediated immune responses in 38 cancer patients treated with immune checkpoint inhibitors (ICIs) and receiving RZV. We used samples collected at baseline (T0), 3 weeks (T2), and 6 months (T3) after the complete RV vaccination schedule. Our data showed that a significant proportion (40,5%) of RZV recipients mounted a stronger humoral and cell-mediated immune response at 3 weeks (T2) after complete RZV vaccination schedule. Interestingly, both humoral and cell-mediated immune responses were mostly stable over 6 months (T3). Interestingly, the overall IFNγ-producing lymphocytes was mainly associated with CD4 T cell response (p = .0012). In conclusion, data from our pilot study suggest a strong and long-lasting immunogenicity of RZV in ICI-treated patients. Prospective analyses at 1 year after vaccination will be performed in order to evaluate the long-term persistence of humoral and cell-mediated response against RZV.
KEYWORDS: RZV, vaccine, immunotherapy, cancer, herpes zoster, PHN, CD4, CD8
Introduction
Herpes Zoster (HZ) is the infectious reactivation of the Varicella-Zoster Virus (VZV). In immunocompromised subjects such as the patients with cancer, HZ can cause additional morbidity1 with a more prolonged phase of cutaneous eruption and higher risk for cutaneous dissemination and visceral involvements such as pneumonitis, hepatitis, and central nervous system disease.2 The risk of HZ is higher among cancer patients undergoing chemotherapy compared to those without active treatment.3 Relatively few papers have described the occurrence of HZ during immunotherapy.4
Immune Checkpoint Inhibitors (ICIs) may induce HZ in two ways: during the treatment of the immune-related adverse events (irAEs) with high-dose steroids and in the context of the immune reconstitution inflammatory syndrome (IRIS).5
Indeed, HZ may be one of the manifestations of IRIS in the setting of drug-induced hypersensitivity syndrome (DIHS) and drug reaction with eosinophilia and systemic symptoms (DRESS).6
With the approval of the adjuvanted glycoprotein E (gE)-based recombinant vaccine (RZV; Shingrix™, GSK) also in immunocompromised subjects on 23 July, 2021,7 HZ is considered a vaccine-preventable disease (VPD) now. Worldwide, vaccination guidelines were recently updated to prioritize RZV for risk groups such as patients with cancer.8,9
The immunogenicity of RZV has been studied in the patients with cancer during chemotherapy with the evidence of the persistence of humoral and cell-mediated immune responses 12 months after RZV.10
To date, no clinical trial has evaluated the immunogenicity and the safety of RZV specifically in the patients with cancer undergoing immunotherapy.
The aim of this study is to investigate the humoral and cell-mediated immune responses in cancer patients treated with ICIs and receiving RZV.
Patients and methods
Patients and study design
This was a prospective single-center cohort study. Between November and December 2022, 38 patients were enrolled. We included all the types of cancer and all the types of immunotherapy (anti CTLA-4 or anti-PD-1 or anti-PD-L1 or a combination of anti-CTLA-4 and anti-PD1) with or without chemotherapy. Blood samples for humoral and cell-mediated immune response evaluation were obtained at baseline (at the first dose of RZV; T0), 28 days after the second dose (T2) and 6 months after the second dose (T3) (Figure 1).
Figure 1.
The flowchart illustrates the sampling schedule of the patients enrolled in the study. RZV is given as a two-dose series as shown in the figure.
The primary end-point was the evaluation of the gE-specific T-cell response 28 days after the second dose (T2).
The secondary end-points were:
Evaluation of the quantification of the VZV-antibodies at the baseline (T0).
Evaluation of the overall IFNγ-producing lymphocytes increase measured by flow cytometric assay.
Evaluation of the incidence of adverse reactions to the RZV, local and systemic, solicited and unsolicited, within the period of 4 weeks after each dose of vaccination.
Data collection
Patients with solid tumors undergoing to ICIs were enrolled. The Inclusion Criteria were as follows: i) patients aged 18 and older; ii) life-expectancy (as estimated by treating physician) ≥ 3 months; iii) confirmed histological diagnosis of solid tumors; iv) treatment with ICIs; v) signing of informed consent; vi) patients with a history of a previous HZ were also enrolled. Patients with psychiatric illness/social situations that would limit compliance with study requirements were excluded from the study.
Patients were examined by type of tumor, TNM, stage disease, type of treatment (anti-PD-1, anti-PD-L1, anti-CTLA-4, or a combination of ICIs), treatment setting (first- or second-line, maintenance after chemo-radiotherapy), the time gap between the start of immunotherapy and vaccine administration, the history of a previous HZ.
Peptide pools and ELISpot assays
Serum was used for antibody titer. Peripheral blood mononuclear cells (PBMCs) were isolated by standard density gradient centrifugation and used for ELISpot assay.
For the ELISpot setup, two pools of lyophilized synthetic peptides (15 mers with 11 amino acids overlap) were used, spanning the glycoprotein E (gE, 153 peptides) and the Immediate-early protein 63 (IE63; 67 peptides) (JPT, innovative peptide solution, Berlin, Germany). A final concentration of 0.25 μg/mL of each peptide was used. Phytoheamagglutinin (PHA; 5 µg/mL) and medium were used as a positive and negative control, respectively. ELISpot assay was performed as previously described.11 Results were given as IFNγ spot forming units (SFU)/106 PBMC, after subtracting the medium alone response. Values higher than 13 net spot/million PBMC for gE and 24 net spot/million PBMC for IE63-specific T-cell response were considered positive.12
Evaluation of antigen-specific T cell response
Cryopreserved cells were thawed by diluting them in 5 mL culture medium and rested overnight at 37°C in a humidified atmosphere at 5% CO2. Subsequently, 4 × 105 cells/well were stimulated for 24 h with gE pool peptides [0.25 μg/mL]. A peptide pool of human actin (15 mers, overlapping by 10 amino acids, Pepscan, Lelystad, The Netherlands) was used as a negative control. Golgi-Plug containing brefeldin A (BD Biosciences, San Diego, CA) and Golgi-Stop containing monensin (BD Biosciences) were added for the last 14 h of incubation. LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit (Thermo Fisher Scientific) was used to determine cell viability. The cells were then membrane-stained with anti-CD3, -CD56, -CD57, -CD16, -CD8 (BD Biosciences) for 30 min at 4°C. After fixation and permeabilization (Fixation/Permeabilization Solution Kit; BD Biosciences), cells were stained with anti-IFN-γ. Analysis was performed with BDFACS Lyric (BD Biosciences) flow cytometer. Data were analyzed using the FlowJo software (TreeStar, Ashland, OR).
Antibody titer
For quantifications of anti-VZV IgG antibody titer in serum, the automated chemiluminescence analyzer technology (CLIA) was used (LIASON XL, Diasorin). Values lower than 135 mUI/ml were considered negative.
Statistical analysis
The patients’ characteristics are described as median and interquartile range (Shapiro–Wilks test excluded the normal distribution hypothesis) if quantitative variables. Qualitative variables are described as count and percentages. Friedman test was used for comparison of repeated measured values at the three time points, followed by the Dunn’s multiple comparison test to detect differences between each time points.
Ethical considerations
This study was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement for reporting observational studies13 and was approved by the local Ethics Committee (Comitato Etico Area Pavia) and Institutional Review Board (P-0059768/22). All the subjects signed the informed written consent.
Results
Patients’ characteristics
Thirty-eight patients (10 females/28 males; median age 71 years) were enrolled between 9th November and 21st December 2022. Twenty-one patients (55.3%) had lung cancer. The most common ICI was pembrolizumab (15 patients, 39.5%). Twelve patients (30.8%) had no comorbidities; in those with comorbidities, the most common were cardiovascular diseases (Table 1).
Table 1.
Demographic and clinical characteristics of the enrolled patients who received RZV.
| Sex | Num patients | % |
|---|---|---|
| Female/Male | 10/28 | 27/73 |
| Comorbidities | ||
| Cardiovascular | 20 | 52.6 |
| COPD/Asthma | 6 | 15.8 |
| Coronaric heart disease | 4 | 10.5 |
| Diabetes mellitus | 6 | 15.8 |
| Autoimmune disorders | 1 | 2.6 |
| HCV | 5 | 13.2 |
| HBV | 2 | 5.3 |
| HIV | 0 | 0 |
| No comorbitidies | 11 | 28.9 |
| ≥1 comorbidity | 27 | 71.1 |
| Previous HZ | ||
| Yes/No | 2/36 | 5.3/94.7 |
| Type of tumor | ||
| Lung Cancer | 21 | 55.3 |
| Kidney cancer | 7 | 18.4 |
| Melanoma | 5 | 13.2 |
| HCC | 3 | 7.9 |
| Head&Neck cancer | 2 | 5.2 |
| Type of ICIs | ||
| Pembrolizumab | 15 | 39.5 |
| Nivolumab | 12 | 31.6 |
| Nivolumab + Ipilimumab | 6 | 15.7 |
| Cemiplimab | 2 | 5.3 |
| Tislelizumab | 2 | 5.3 |
| Atezolizumab | 1 | 2.6 |
Legend: COPD: chronic obstructive pulmonary disease; HBV: Hepatitis B virus; HCV: Hepatitis C virus; HIV: Human immunodeficiency virus; HCC: hepatocellular carcinoma; ICIs: immune-checkpoints inhibitors.
Humoral and cell-mediated response
The quantification of the VZV antibodies revealed that all the patients except one were seropositive before vaccination. VZV-IgG was significantly increased at all timepoints when compared with T0, We did not observe a decrease of the IgG level between T2 and T3. In particular, among vaccine recipients found an increase of VZV-IgG level of at least twofold at all follow-up time points in 25/38 (65.7%) subjects (Figure 3a).
Figure 3.
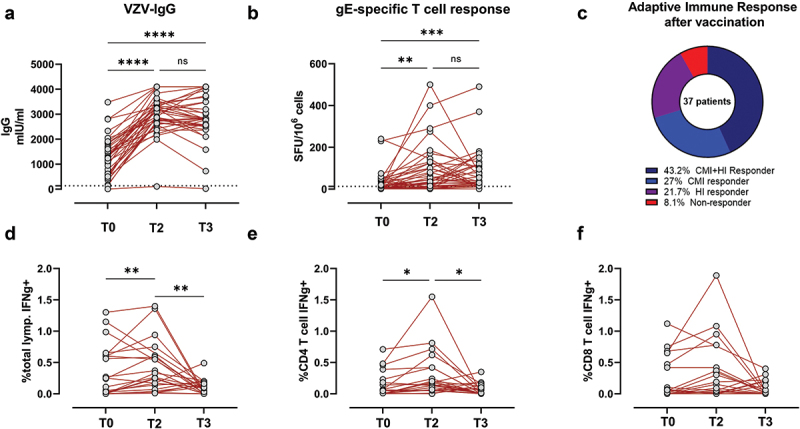
Humoral and cell-mediated response after the two doses of the adjuvanted glycoprotein E (gE)-based recombinant vaccine against Herpes zoster (RZV).
An increase in T cell response of at least twofold was considered for definition of “responder patients” and observed in 25/37 patients (67.5%) at all follow-up time points. On the other hand, no increase was observed against IE63 peptide points. On the other hand, no increase was observed against IE63 peptide pool. **p < .01, ****p < .0001.
In parallel, T-cell-mediated response was evaluated in 37 enrolled patients at the same time points. At baseline, 45.7% subjects were positive for gE-specific T-cell response and levels of response significantly increased at 28 days after the complete vaccination schedule. The median level of response increased from 10 [Interquartile range (IQR) 1–40] net spots/million PBMC to 45 [IQR 15–125] net spots/million PBMC (p= .0018). At T3, gE-specific T cells response was higher than that observed at baseline but had not increased further compared T2 (p .99). The median level of response increased from 10 [IQR 1–40] net spots/million PBMC to 40 [IQR 20–120] net spots/million PBMC (p = .0005).
An increase in T cell response of at least twofold was considered for definition of “responder patients” and observed in 26/37 patients at all follow-up time points. On the other hand, no increase was observed against IE63 peptide pool (Figure 2).
Figure 2.
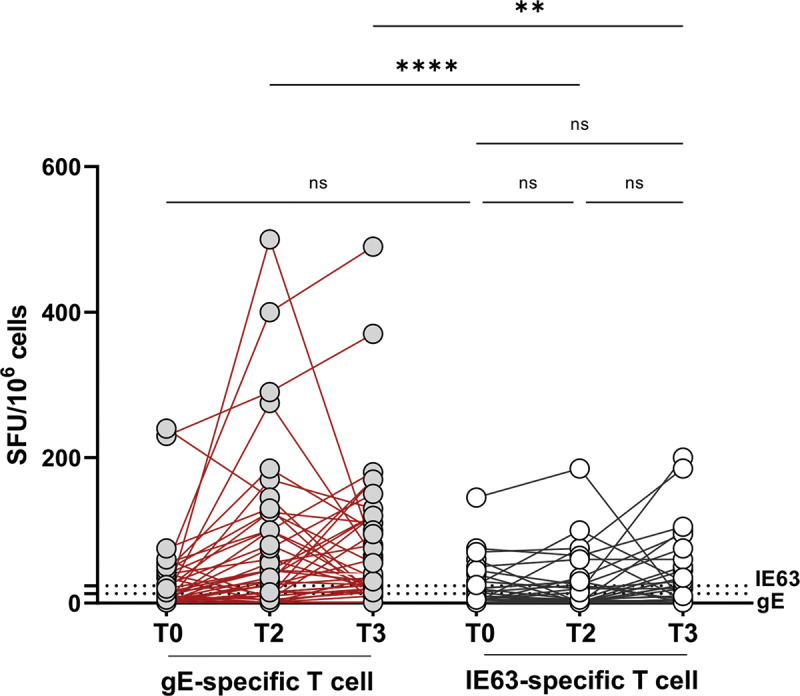
Evaluation of T cell response.
(A) VZV-specific IgG antibodies were quantified at each time point in plasma sample. A cutoff of 135 mUI/mL was considered for the definition of positive samples (Dotted lines, n = 38). (B) A standard IFN-γ ELISPOT assay using 0,25 mcg/ml lyophilized synthetic peptides (15-mer peptides with an overlap of 11aa) spanning gE (153 peptides, gray dots) or IE63 (67 peptides, white dots) was set up using isolated PBMCs at T0, T2, and T3 (n = 37). The graph showed ELISpot results in spot-forming colonies (SFC)/106 PBMCs. (C) Pie chart distinguished responder patients who showed an increase in humoral (HI) and/or cell-mediated immune (CMI) response at T3 compared to baseline from non-responder patients. An increase in response of at least twofold was considered for definition of “responder patients.” (D-G) Cytokine flow cytometry assay for the enumeration of antigen-specific cells was performed using PBMC stimulated in vitro with 0,25 mcg/ml gE pool peptides for 24 h (n = 25). A peptide pool of human actin (15 mers, overlapping by 10 amino acids) was used as a negative control. The frequency of IFN-γ -secreting cells were detected in total lymphocytes (D), CD4 T cells (E), CD8 T cells (F), and NK cells (G) at T0, T2, and T3. Paired data were analyzed using the Wilcoxon signed rank test. *p < .05, **p < .01, ***p < .001, ****p < .0001.
It has been shown synergy between humoral (HI) and cell-mediated immunity (CMI) appears to be critical to prevent infection. To examine the magnitude of these relationships after vaccination, we stratified RZV recipients based on the breadth of the antigen-specific response. In particular, patients were stratified as responder or nonresponder based on at least a twofold increase in T cell-response (CMI) or IgG titer (HI) at T3 point of the study. We counted: 16/37 (43,2%) responder patients for both humoral and cell-mediate immunity, 10/37 (27%) responder patients for cell-mediate immunity, 8/37 (21,7%) responder patients for humoral immunity but only 3/37 (8,1%) did not develop a sustained immune response after complete vaccination schedule (Figure 3c).
When measured using a flow cytometric assay, the overall IFNγ-producing lymphocytes significantly increased at T2 compared with baseline (Figure 3d). The data showed the production of IFN-γ was mainly associated with CD4 T cell response (p = .0012) (Figure 3e–f).
Safety
The most common side effect was pain at the injection site (21.1% after the first dose and 44.7% after the second dose). Nobody had grade 3–4 side effects (Table 2).
Table 2.
Side effects after the two doses of the adjuvanted glycoprotein E (gE)-based recombinant vaccine against herpes zoster (RZV).
| Side effects after the first dose | Num patients/percentage |
|---|---|
| Pain at the injection site | 8 (21.1) |
| Fever | 0 (0) |
| Arthralgia | 0 (0) |
| Myalgia | 0 (0) |
| Side effects after the second dose | Num patients/percentage |
| Pain at the injection site | 17 (44.7) |
| Fever | 0 (0) |
| Arthralgia | 0 (0) |
| Myalgia | 1 (2.6) |
Discussion
HZ and its complications have an unfavorable impact in the patients with cancer. A recent paper has evaluated the incidence of HZ in lung cancer patients during ICIs or chemotherapies. The authors have demonstrated no significant difference in the rate of HZ between the two treatments (5% versus 4.5%, respectively, p = .782), while the younger patients (<65 years old) on ICIs have presented a higher risk of HZ than those treated with chemotherapy alone (12.5% versus 1.2%, respectively, p = .0365).14
All the peripheral blood mononuclear cells (PBMCs) subsets (monocytes, B cells, and Natural Killer (NK) T cells) can be infected with VZV. T-lineage cells and NK cells are able to withstand the viral infection over a long period due to their slight increases in surface VZV-gE expression. The immune evasion pathway triggered by VZV infection may be due to the ability of infected immune cells to transmit virus together with the induction of PD-L1 expression in PBMCs.15 Thus, the role of VZV-specific T-cell response in high-risk subjects should be considered.
We have data about the immunogenicity and safety only in the patients during chemotherapy, but we have no data during other oncological treatments such as immunotherapy. In the absence of available data on the immunogenicity of the vaccine for different types of cancer and therapy, the recommendations for HZ vaccination are given basing on the patient’s general condition and life expectancy.
This study presents the preliminary results about the humoral and cell-mediated response elicited by RZV in a small cohort of the cancer patients during immunotherapy. Our data showed, both humoral and cell-mediated immune responses were increased in RZV recipients at T2 compared with baseline and were mostly stable over 6 months after the complete vaccination schedule. Even if no correlation was observed at the different time points, a weak positive association was observed at 6 months after vaccination (p = .042: r = 0.333, data not shown). As known, VZV antibody is an important factor in varicella prevention, but it is insufficient for preventing herpes zoster. On the other hand, VZV-specific T cells are essential for preventing reactivation by controlling latency and the reduction in the number of these immune cells facilitates the development of herpes zoster.16 Interestingly, in this pilot study 43.2% of RZV recipients showed coordinated responses by all branches of adaptive immunity and more than 70% of individuals showed a sustained VZV-specific T cell response.
The strength of our data is the simultaneous detection of antibody titers and cell-mediated response, which allows us to comprehensively track the immune response. At the same time, we collected data after both doses in order to assess any changes after each dose. Our work has also various limitations. Firstly, the lack of a control group and secondly the small sample size of the patients tested that limited the probability of detecting rare adverse effects of vaccination.
The “real-life” data on RZV administration in a cohort of solid tumor patients revealed a high immunogenicity of the vaccine with more than 70% of responder patients. Moreover, RZV is well-tolerated in the patients with cancer undergoing ICIs, with a low rate of side-effects. The safety results of RZV have reassured clinicians to use it in patients undergoing immunotherapy. No immune-mediated adverse events have been reported, unlike other types of vaccines.17,18 However, it is important to note that the number of patients involved in this study was too small to make any conclusive statements. During the counseling, it is crucial to reassure the patients based on about the safety of the vaccine. In a recent survey promoted by AIOM on the attitudes of oncologists toward vaccination, it emerged that over 60% of them are concerned about possible adverse events/drug–drug interactions (DDI).19 Therefore, it is important to conduct both randomized clinical trials and observational real-life studies to strengthen the concept of vaccine safety in the oncology population. Larger future studies will be needed to confirm our results.
Funding Statement
This work was supported by Ricerca Corrente grant no 08067620, Fondazione IRCCS Policlinico San Matteo.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability Statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.
Author contributions
All the authors have contributed to writing this manuscript, read, and approved the final manuscript.
Ethics Statement
The study was approved by the local Ethics Committee (Comitato Etico Area Pavia) and Institutional Review Board (P-0059768/22). All the subjects signed the informed written consent.
References
- 1.Kawai K, Yawn B.. Risk of herpes zoster in cancer patients and the promise of new vaccines. J Infect Dis. 2019;220(1):1–6. doi: 10.1093/infdis/jiy626. [DOI] [PubMed] [Google Scholar]
- 2.Tayyar R, Ho D. Herpes simplex virus and varicella zoster virus infections in cancer patients. Viruses. 2023;15(2):439. doi: 10.3390/v15020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qian J, Heywood AE, Karki S, Banks E, Macartney K, Chantrill L, Liu B. Risk of herpes zoster prior to and following cancer diagnosis and treatment: a population-based prospective cohort study. J Infect Dis. 2019;220(1):3–11. doi: 10.1093/infdis/jiy625. [DOI] [PubMed] [Google Scholar]
- 4.Serra F, Cassaniti I, Lilleri D, Pedrazzoli P, Baldanti F, Lasagna A. Herpes zoster in patients with solid tumors treated with immune checkpoint inhibitors. Immunotherapy. 2022;14(6):389–93. doi: 10.2217/imt-2021-0333. [DOI] [PubMed] [Google Scholar]
- 5.Lasagna A, Cassaniti I, Sacchi P, Baldanti F, Bruno R, Pedrazzoli P. Infectious complications and immunotherapy: old pitfalls and new horizons. Future Oncol. 2022;18(22):2377–81. doi: 10.2217/fon-2022-0277. [DOI] [PubMed] [Google Scholar]
- 6.Kano Y, Tohyama M, Aihara M, Matsukura S, Watanabe H, Sueki H, Iijima M, Morita E, Niihara H, Asada H, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42(3):276–82. doi: 10.1111/1346-8138.12770. [DOI] [PubMed] [Google Scholar]
- 7.SHINGRIX: product information. [accessed 2023 July 28]. https://www.fda.gov/vaccines-blood-biologics/vaccines/shingrix.
- 8.Pedrazzoli P, Lasagna A, Cassaniti I, Ferrari A, Bergami F, Silvestris N, Sapuppo E, Di Maio M, Cinieri S, Baldanti F. Vaccination for herpes zoster in patients with solid tumors: a position paper on the behalf of the Associazione Italiana di Oncologia Medica (AIOM). ESMO Open. 2022;7(4):100548. doi: 10.1016/j.esmoop.2022.100548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Working Group on Vaccination against Herpes Zoster . Public health commission of the interterritorial council of the national health system. Recomendaciones de vacunación frente a herpes zóster [Vaccination recommendations against shingles]. Madrid: Ministry of Health; 2021. Mar 25 [accessed 2023 July 28]. Spanish. https://www.mscbs.gob.es/fr/profesionales/saludPublica/prevPromocion/vacunaciones/programasDeVacunacion/docs/HerpesZoster_RecomendacionesVacunacion.pdf. [Google Scholar]
- 10.Vink P, Delgado Mingorance I, Maximiano Alonso C, Rubio-Viqueira B, Jung KH, Rodriguez Moreno JF, Grande E, Marrupe Gonzalez D, Lowndes S, Puente J, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer. 2019;125(8):1301–12. doi: 10.1002/cncr.31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasagna A, Agustoni F, Percivalle E, Borgetto S, Paulet A, Comolli G, Sarasini A, Bergami F, Sammartino JC, Ferrari A, et al. A snapshot of the immunogenicity, efficacy and safety of a full course of BNT162b2 anti-SARS-CoV-2 vaccine in cancer patients treated with PD-1/PD-L1 inhibitors: a longitudinal cohort study. ESMO Open. 2021;6(5):100272. doi: 10.1016/j.esmoop.2021.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassaniti I, Ferrari A, Comolli G, Sarasini A, Gregorini M, Rampino T, Lilleri D, Baldanti F. Characterization of varicella-zoster (VZV) specific T cell response in healthy subjects and transplanted patients by using enzyme linked immunospot (ELISpot) assays. Vaccines (Basel). 2021;9(8):875. doi: 10.3390/vaccines9080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31–S4. doi: 10.4103/sja.SJA_543_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taoka M, Ochi N, Yamane H, Yamamoto T, Kawahara T, Uji E, Kosaka Y, Takeda K, Nagasaki Y, Nakanishi H, et al. Herpes zoster in lung cancer patients treated with PD-1/PD-L1 inhibitors. Transl Cancer Res. 2022;11(3):456–62. doi: 10.21037/tcr-21-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones D, Como CN, Jing L, Blackmon A, Neff CP, Krueger O, Bubak AN, Palmer BE, Koelle DM, Nagel MA, et al. Varicella zoster virus productively infects human peripheral blood mononuclear cells to modulate expression of immunoinhibitory proteins and blocking PD-L1 enhances virus-specific CD8+ T cell effector function. PLoS Pathog. 2019;15(3):e1007650. doi: 10.1371/journal.ppat.1007650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laing KJ, Ouwendijk WJD, Koelle DM, Verjans GM. Immunobiology of varicella-zoster virus infection. J Infect Dis. 2018;218(suppl_2):S68–74. doi: 10.1093/infdis/jiy403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasagna A, Pedrazzoli P, Bruno R, Sacchi P. Postvaccination immune-mediated hepatitis: what do we really know? Immunotherapy. 2023;15(9):627–30. doi: 10.2217/imt-2023-0038. [DOI] [PubMed] [Google Scholar]
- 18.Lasagna A, Lenti MV, Cassaniti I, Sacchi P. Development of hepatitis triggered by SARS-CoV-2 vaccination in patient with cancer during immunotherapy: a case report. Immunotherapy. 2022;14(12):915–25. doi: 10.2217/imt-2021-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasagna A, Brunello A, Silvestris N, Pedrazzoli P, Di Maio M, Cinieri S. Italian oncologists and vaccinations against infectious diseases: results of a survey of the Italian Association of Medical Oncology. Tumori. 2023. Aug;10:3008916231191547. doi: 10.1177/03008916231191547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.


