ABSTRACT
Compelling evidence supports the hypothesis that stress negatively impacts cancer development and prognosis. Irrespective of its physical, biological or psychological source, stress triggers a physiological response that is mediated by the hypothalamic-pituitary-adrenal axis and the sympathetic adrenal medullary axis. The resulting release of glucocorticoids and catecholamines into the systemic circulation leads to neuroendocrine and metabolic adaptations that can affect immune homeostasis and immunosurveillance, thus impairing the detection and eradication of malignant cells. Moreover, catecholamines directly act on β-adrenoreceptors present on tumor cells, thereby stimulating survival, proliferation, and migration of nascent neoplasms. Numerous preclinical studies have shown that blocking adrenergic receptors slows tumor growth, suggesting potential clinical benefits of using β-blockers in cancer therapy. Much of these positive effects of β-blockade are mediated by improved immunosurveillance. The present trial watch summarizes current knowledge from preclinical and clinical studies investigating the anticancer effects of β-blockers either as standalone agents or in combination with conventional antineoplastic treatments or immunotherapy.
KEYWORDS: Beta-adrenoreceptors, beta-blockers, cancer, catecholamines, stress
Introduction
Stress typically leads to the co-activation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic adrenal medullary (SAM) axis, resulting in the systemic elevation of stress hormones, namely glucocorticoids produced by the adrenal cortex and catecholamines (including epinephrine and norepinephrine) that are released from the adrenal medulla into the circulation.1,2 Catecholamines act on adrenergic receptors (ARs), thus increasing heart rate and blood pressure to prepare the organism for a fight-or-flight response.3 ARs belong to the G-protein-coupled receptor super-family and are categorized into α- and β-ARs based on their location and function. α-ARs are divided into two classes: α1-AR, predominantly found on blood vessels, which increase blood pressure upon activation, and α2-AR, mainly located within solid organs such as the pancreas, where they control insulin synthesis. β-receptors can be further classified into three types: β1-AR (found in the heart, blood vessels, kidney, and ciliary muscle), β2-AR (located in the lungs and ciliary muscle), and β3-AR (present only in smooth muscle tissue). β-ARs play a major role in stimulating cardiovascular functions and promoting the relaxation of the smooth muscles in bronchi and blood vessels. Table 1
Table 1.
Adrenergic receptors and their main agonists and antagonists in clinic use (non-exhaustive list).
| Receptors | Endogenous agonists affinity | Pharmacological agonists |
Agonist properties | Pharmacological antagonists |
|---|---|---|---|---|
| 1 | Epinephrine < norepinephrine | Epinephrine, norepinephrine, phenylephrine | Smooth muscle constriction in viscera, skin, sphincter, mucosa, vessels, iris mydriasis | Alfuzosin, hydroxyzine, tamsulosin |
| 2 | Epinephrine = norepinephrine | Clonidine, dexmedetomidine, epinephrine, norepinephrine |
Smooth muscle constriction, decreased insulin and glucagon production, decreased thyroid hormone production, decrease platelet aggregation | Diverse antipsychotics |
| 1 | Epinephrine | Dobutamine, epinephrine, isoprenaline | Chronotropic, dromotropic, inotropic, bathmotropic effects, increased renin production | Atenolol, bisoprolol, metoprolol, nebivolol, propranolol, timolol |
| 2 | Epinephrine > norepinephrine | Epinephrine, isoprenaline, salbutamol, terbutaline | Smooth muscle relaxation, vessels dilatation, enhanced lipolysis, bronchodilatation | Propranolol, timolol |
| 3 | Epinephrine = norepinephrine | Amibegron, mirabegron, solabegron | Enhanced lipolysis smooth muscle relaxation of bladder and bowel | SR 59230A |
β-blockers are specific antagonists targeting β-ARs. The first synthetic molecules dichloroisoprenaline and pronethalol were derived from epinephrine in the late 1950s, but were withdrawn from clinical use several years later due to severe cardiotoxic side effects.4 However, in 1962, propranolol, a non-cardioselective agent that targets both β1 and β2 receptors, was found to promote negative chronotropic, bathmotropic, inotropic, and dromotropic cardiac effects and a decrease in kidney renin production (resulting in decreased blood pressure), thus providing safe clinical use.5 Subsequently, cardioselective agents including acebutolol, atenolol, bisoprolol, and nebivolol were introduced. These agents specifically target β1-ARs, thus limiting side effects such as vaso- and bronchoconstriction. Currently, β-blockers are broadly used in clinical practice, primarily for regulating dysrhythmia, and arterial hypertension, preventing heart attacks and migraines, as well as for treating glaucoma. However, misuse of these agents can cause severe hypotension, bradycardia, asthenia, asthma or Raynaud’s syndrome, and contraindications, such as cardiac conduction disturbances or chronic obstructive pulmonary disease have to be respected. Table 1
β-blockers can be subdivided into two pharmacological classes: phenylethanolamines and aryloxypropanolamines. Phenylethanolamines are composed of an aromatic group linked to an ethylamine substituted by a hydroxyl on position 1. β -blockers of this class such as sotalol or labetalol are non-cardioselective and act on-target on β1 receptors while often causing off-target β2 receptor-mediated side effects. Compounds from the group of aryloxypropanolamines including carvedilol, nebivolol and propafenone possess an aromatic group linked to a propylamine substituted by a hydroxyl on position 2 and have an additional methyl group on the amine chain, which increases their activity and selectivity. Of note, the size of the aromatic group of a β-blocker determines its ability to activate adrenergic receptors. Thus, a small aromatic group as the one of epinephrine allows activation of adrenergic receptors, whereas a voluminous group such as the one of pronethalol induces an antagonistic effect. Moreover, a β-blocker becomes cardioselective if its activity on β1 receptors is higher than that on β2 receptors. Agonistic activity on β1 receptor allows vasodilatation that decreases blood pressure, while activating β2 promotes side effects such as bronchoconstriction. This selectivity is due to a hydrogen group inducing a preferential interaction with the β1 receptor. Cardioselective β-blockers include acebutolol, atenolol, bisoprolol, and nebivolol, while the most employed non-cardioselective β-blockers are propranolol, sotalol and timolol. (Figure 1)
Figure 1.

Chemical structures of the most employed cardioselective β-blockers (a) and non-selective β-blockers (b). Red = aryloxypropanolamine and green = phenylethanolamine β-blockers.
Surgical removal of the primary tumor plays a crucial role in improving the overall survival of cancer patients. However, the physical excision of the tumor by the surgeon can release circulating tumor cells, which can spread micrometastases to distant organs. Additionally, surgical procedures can cause stress such as pain, nociception, inflammation, and tissue damage, which in turn trigger a cascade of local and systemic signaling pathways, activating corticotropic signaling. This results in the secretion of adrenocorticotropic hormone (ACTH), catecholamines, and cortisol, proportional to the stress caused during surgery, leading to critical neuroendocrine and metabolic changes known as ‘glucocorticoid stress’.6,7 Moreover, stress hormones released into the systemic circulation can negatively impact both humoral and cellular immunity.8,9 Thus, ACTH inhibits the synthesis of immunoglobulins by plasma cells, while catecholamines can act on adrenoceptors expressed on natural killer (NK) cells, peripheral mononuclear cells and CD4+/8+ T lymphocytes,10 triggering the production of intracellular cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA), altogether abrogating chemotaxis, migration and cytotoxic functions.11–13 Moreover, glucocorticoid stress can impact type I interferon (IFN) production by dendritic cells, as well as the release of IFN- γ by cytotoxic T lymphocytes (CTLs), thus broadly compromising adaptive antitumor immune responses.14,15 Studies in a rat mammary adenocarcinoma model also showed that injection of catecholamines inhibited NK cell-mediated tumor lysis and suppressed resistance to NK-sensitive metastasis.16 Furthermore, endogenous stress hormones have been shown to activate β2-ARs and downstream cAMP-PKA signaling pathways that increased the activity of matrix metalloproteinases (MMP), further promoting the dissemination of malignant cells. They were also shown to stimulate STAT3 signaling in cells surrounding the tumor tissue, leading to the release of pro-inflammatory cytokines, including IL-6 and IL-8, and an increase in the secretion of vascular endothelial growth factor (VEGF), thus promoting tumor growth and neovascularization, respectively.17–20 In a murine model of pancreatic cancer, chronic stress increased levels of circulating steroids and adrenal tyrosine resulting in impaired immune responses, with a decreased response of ex vivo splenocytes to lipopolysaccharide, a decrease in cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) expression in CD4+ cells, and an increase in regulatory T cells in the tumor bed, altogether stimulating tumor growth and impacting on overall survival.21 In yet another study using a murine model of MDA-MB-231 breast cancer, stress-induced epinephrine production was found to promote tumor growth in a time- and concentration-dependent manner.22 Additionally, in an observational retrospective trial, Cox regression analysis revealed low serum epinephrine as a predictor of positive prognosis. Thus, breast cancer patients with low serum epinephrine levels had a significantly better overall (OS) and disease-free survival (DFS) compared to patients with high epinephrine levels.22 Surprisingly, colon cancer cells are also able to produce immunoregulatory glucocorticoids to suppress the activation of immune cells. Finally, many data support that both endogenous as well as exogenous corticoids might diminish therapies-induced anti-tumor response. In summary, while oncological interventions are crucial for achieving remission in clinical routine, they paradoxically decrease the immunosurveillance necessary to avoid immune escape thus promoting the development of secondary lesions due to ‘glucocorticoid stress’.14,23,24
Psychosocial stress has been suggested as a putative cause of cancer incidence and mortality for a long time, however, determining a cause–effect relationship has been challenging and was not firmly established.25 The influence of persistent corticotropic signaling on major antitumor immunological effectors, such as NK cells, dendritic cells (DC), and T-lymphocytes, has been explored through various approaches.26 T lymphocytes obtained from the serum of stressed individuals exhibited a shift in phenotype from Th1 to Th2, potentially affecting immune signaling pathways.27 Moreover, in both animal models and human studies, psychosocial stress was found to negatively impact NK cell activity and promote tumor growth. In a murine colon carcinoma model, social isolation stress decreased splenic NK cell activity while increasing angiogenesis leading to the formation of secondary tumors.28,29 Conversely, NK cells from ovarian cancer patients became more efficient at lysing tumor cells after receiving psychosocial support during the perioperative period.30 Furthermore, preclinical data suggest that psychosocial stress activates β-adrenergic signaling and promotes tumor progression. Thus, in a murine hepatocellular carcinoma model, restraint stress promoted tumor growth and increased norepinephrine levels through β-adrenergic signaling.31 In an orthotopic ovarian carcinoma model, mice subjected to restraint stress experienced increased tumor growth and VEGF-mediated vascularization, which correlated with the level of circulating stress hormones. However, premedication with propranolol, a β-blocker with anxiolytic properties, reversed the tumor-promoting effects.11 Similar results were observed in mice exposed to psychosocial stress through crowded or isolated housing conditions where stress-enhanced melanoma and fibrosarcoma growth was decreased by the oral administration of propranolol.32 In a social defeat model in mice; we observed stress-elevated plasma corticosterone levels and an increase in the expression of glucocorticoid-inducible factor Tsc22d3 that blocked type I Interferon (IFN) responses in dendritic cells (DC) and T cells, thus dampening therapeutic responses against carcinogen-induced and transplantable tumors. In this setting, the administration of a glucocorticoid receptor antagonist reversed the negative impact of psychosocial stress on therapeutic outcomes.14 Taken together, these findings suggest that effectively preventing or managing psychological stress by pharmacological strategies could significantly improve oncological prognosis. However, putative effects of psychological and medical stress on cancer induction and progression are currently discussed, and additional clinical data are expected to support this evidence and to further substantiate cause–effect relationships14,33,34
Preclinical investigations
β-ARs can be found on the cell surface of different types of primary and metastatic tumor cells directly linking persistent stress with oncogenesis and disease progression.17,35–40 Thus, epinephrine, norepinephrine, and other AR agonists can directly act on malignant cells, leading to various pro-tumorigenic effects including enhanced proliferation and increased migratory potential.18,35,37,39,41–43 These stress mediators also cause intracellular hypermetabolism, as evidenced by the accumulation of lipid droplets in MCF-7 breast cancer cells treated with AR agonists in vitro.44,45 Several downstream molecular mediators have been identified as responsible for pro-tumorigenic β-AR signaling, including the intracellular second messengers cAMP and PKA, which transactivates epidermal growth factor receptor (EGFR)46 and triggers the initiation of the mitogen-activated protein kinase 1 (MAP2K1 better known as MEK1)/mitogen-activated protein kinase 1(MAPK1) and MAPK3 (better known as ERK1/2) cascade47 thus stimulating cyclin D1, cyclin E2, and cyclin-dependent kinases CDK 4/6 to promote proliferation.17 Moreover, β-AR signaling leads to the activation of cytosolic phospholipase-A2, the release of arachidonic acid (AA), as well as to an increased expression of the AA-metabolizing and tumor-growth-promoting enzymes cyclooxygenase-2 (COX-2) and 5-lipoxygenase in cancer cells.48–50 PKA also upregulates transcription factors including nuclear factor kappa B (NF-kB), activator protein 1 (AP1), and cAMP response element-binding protein (CREB) in the context of lung and pancreatic adenocarcinoma development.51 Additionally, β-AR activation results in enhanced retinoblastoma protein phosphorylation and suppresses Rap1B prenylation, leading to reduced cell–cell adhesion and a migratory phenotype.35,38,42,46,52 Interestingly, the autocrine secretion of epinephrine by certain cancer cells can stimulate β-AR, and this effect is further increased by oncogenic factors such as nicotine.53,54 Altogether these observations support the hypothesis that β-AR antagonists have clinical potential by halting malignant disease and decreasing the incidence of recurrences as suggested by the inverse correlation between the incidence of prostate adenocarcinoma and the use of antihypertensive drugs including β-blockers (n = 2442, HR = 0.7, 95% [0.5–0.9]).55
The exploration of different types of β-AR has been subject to extensive research. However, among cardioselective β-blockers, the nonselective propranolol appears to be particularly effective.56 Propranolol acts on both β1 and β2 receptors and showed the capacity to effectively mitigate oxidative stress as well as pro-tumorigenic inflammatory response such as IL-6 and TNF-α production.57,58 Additionally, propranolol impairs tumorigenesis in a time- and concentration-dependent manner through various molecular and cellular processes.42,59–61 Moreover, AR antagonists, including propranolol, have shown potential to reverse stress-induced tumorigenesis and disease progression.19,35–39,41,52,62,63 Thus, by blocking β-adrenergic signaling and counteracting the metastatic potential of epinephrine and norepinephrine, propranolol inhibits the invasion of malignant cells and reduces their metastatic spread to distant organs.64 Propranolol can intercept voltage-gated sodium channels expressed in malignant cells that are crucial for cell mobility and additionally impair the enzymes MMP2 and MMP9, which dysregulate anti-tumor immunity and facilitate the migration of malignant cells through the extracellular space.65–67 Moreover, AR antagonists can halt cellular proliferation through anti-mitotic effects paralyzing the cell cycle at G0/G1/S phase or G2/M phase, strongly affecting DNA synthesis.68–76 AR antagonists also exhibit antiangiogenic properties by decreasing the expression and activity of vascular endothelial growth factor (VEGF) thus further impacting cancer progression.66,77,78
Of note β-blockers can induce distinct types of cellular stress including autophagy,79,80 endoplasmic reticulum (ER) stress and mitochondrial dysfunction resulting in the production of reactive oxygen species (ROS) and affecting glucose metabolism,81,82 altogether triggering apoptotic or necro(pto)tic cell death83–90 of cancer cells. In addition, these stresses deeply contribute to the anti-cancer response,91 especially by enhancing immune infiltration of the tumor microenvironment.92–94 AR antagonists also exert trans-inhibitory effects on certain signaling pathways and transcription factors involved in carcinogenesis. Epidermal growth factor receptor (EGFR) is frequently overexpressed in epithelial tumors, resulting in elevated levels of intracellular cAMP and PKA, which promotes angiogenesis, invasiveness, and renders cells resistant to apoptosis. Both propranolol and atenolol can prevent the development and progression of tumors by competitively decreasing intracellular cAMP and PKA levels.18,95 Moreover, propranolol can arrest the cell cycle in various malignant cell types by downregulating components of the pro-invasive signaling pathways ERK/COX-2 or EGFR-Akt/ERK1–2, as well as by modifying the phosphorylation level of survival (Akt, p53, GSK3β) and mitogenic regulators (p44/42 MAPK, p38 MAPK, JNK, CREB), ultimately leading to cancer cell apoptosis.35,47,59,68,69,96–99 Finally, β-AR antagonists have been shown to reverse nicotine-induced mitogenic and protooncogenic factors such as COX-2, ERK1/2, PGE2, PKC, and VEGF in colon, gastric, and lung cancer cells in a dose-dependent manner.54,100,101
Carvedilol, yet another β- and β-AR antagonist, exerts cytotoxic effects on many human hematopoietic and solid tumor cell lines.102 Compared with other adrenergic agents, carvedilol exhibits a unique Ca2+ mobilization capacity by exerting control over extracellular Ca2+ influx and the release of Ca2+ from ER stores. The carvedilol-orchestrated increases in cytosolic Ca2+ movement triggers cytotoxicity and inhibits the migratory capacity of human osteosarcoma, hepatoma and oral cancer cells in a concentration-dependent manner.85,87,103 Carvedilol inhibits signaling pathways promoting invasiveness, including the cAMP, PKA/SCR, PKCs/Src pathways.104 Moreover, carvedilol inhibits EGF-mediated malignant skin transformation in a dose-dependent manner by impairing AP-1 activation.105 Conversely, atenolol, another β1-AR specific antagonist, failed to prevent neoplastic transformation after application to mouse epidermal cells JB6 P.+105
Until now β3-AR blockade was less investigated. However, an increased expression of β3-AR was reported for neuroblastoma and melanoma.86 Moreover, β3-ARs favor the recruitment of tumor-associated pro-inflammatory and pro-tumor effectors such as fibroblasts and M2 macrophages. The use of β3-AR antagonists, such as SR59230A and L-748 337, reportedly impacts tumor vasculature and reduces the growth of melanoma.86,106 SR59230A induces a significant reduction of mitochondrial activity, halting ATP synthesis and triggering the generation of reactive oxygen species, resulting in tumor cell death.107 Moreover, the inhibition of β3-AR reduces the proliferation of neuroblastoma by dysregulation of bioactive lipid sphingosine kinase 2/sphingosine 1-phosphate metabolism, which is implicated in various cancers and anticancer therapy resistance.108 Furthermore, β3-AR antagonists were shown to reduce the phosphorylation of the mTOR/p70S6K pathway, thus reducing malignant growth.109
β-AR antagonists have shown a significant potential in mitigating the immunosuppressive effect of chronic stress, thereby improving immunosurveillance. Thus, propranolol was shown to suppress stress-induced lung metastases in a preclinical model of murine breast cancer, while nadolol decreased the incidence of metastases promoted by surgical stress by 50%.61,110,111 In various models of subcutaneous cancers, propranolol prevented a stress-induced ileopathy that led to immunosuppressive dysbiosis.112 Propranolol also suppresses the progression of hematopoietic cancers such as acute lymphoblastic leukemia by impairing α-adrenergic signaling activated during psychological stress.113 Furthermore, α-blockers decrease the number of myeloid-derived suppressor cells (MDSCs). Conversely, MDSCs are induced by α2 adrenergic signaling in response to chronic stress.114–118
β-adrenergic receptors play an important role in shaping the immune orientation of the tumor microenvironment.119,120 Thus, decreasing adrenergic stress by different approaches including physiological manipulation such as placement of mice in a thermoneutral environment, genetic interventions such as the knockout of β-AR or pharmacological β-blockade, increases glycolysis and oxidative phosphorylation in tumor-infiltrating lymphocytes. Reduction of adrenergic stress upregulates the expression of the costimulatory molecule CD28, stimulates cytokine release121 and enhances the ration of cytotoxic over regulatory T cells. It also increases the secretion of granzyme B and IFN-γ contributing to immune-mediated anti-cancer responses in mice.96,99,122 Additionally, SR59230A promotes the differentiation of stromal cells and increases the abundance of lymphoid, myeloid, and NK progenitor cells in the tumor microenvironment. Altogether, these effects may inhibit tumor progression, inflammation and angiogenesis.123
Propranolol has demonstrated a remarkable synergism with current antineoplastic therapies. Specifically, it enhances radiosensitivity, thereby increasing radiotherapy-induced abscopal antitumor effect and exerts T cell-dependent immune response that effectively slows tumor growth. Moreover, propranolol decreases the expression of prometastatic, proinflammatory and proangiogenic genes such as EGFR, COX-2 and VEGF, respectively, thus impairing cell viability and inducing apoptosis.60,124–127
The cytotoxic effects of conventional chemotherapeutic agents such as platinum salts, anthracyclines (such as doxorubicin), 5-fluorouracil, mitotic spindle poisons (such as taxanes or vincristine), topoisomerase inhibitors and gemcitabine were increased in the presence of β-blocking agents including propranolol.128–134 β-blocking agents also boosted the anticancer effect in combination with targeted therapies such as U0126 (a MAPK inhibitor),79 sorafenib (a multikinase inhibitor),80 vemurafenib (a B-Raf gene inhibitor),135 and sunitinib (an inhibitor of tyrosine kinase).136 Importantly, propranolol was found to decrease the expression of programmed death receptor-1 (PD-1) in tumors and to increase CD8+ T cell infiltration within the tumor microenvironment, thus enhancing the efficacy of immune checkpoint blockade.93,122,137–142 If combined with the antitumor vaccine STxBE7, propranolol strongly enhanced the amount of tumor infiltrating CD8+ T cells, although without improving their activity.143
Even agents without any direct antitumor activity have been found to potentiate the cytotoxic effects of β-blockers. Thus, the combination of propranolol with metformin, an oral antidiabetic agent, revealed an unexpected synergistic inhibitory effect on proliferation, invasion, and migration in vitro and reduced tumor growth and metastasis in vivo.144,145 Similarly, when combined with the COX-2 inhibitor etodolac, propranolol increased the cytotoxicity of NK cells, reduced postsurgical local relapse and metastasis and improved overall free-survival.146–148 In conjunction with 2-deoxy-D-glucose (2DG), propranolol significantly reduced glucose metabolism associated with alterations in mitochondrial morphology and the subsequent activation of endoplasmic reticulum stress, autophagy, proliferative arrest ultimately resulting in apoptosis.149 Altogether, this underscores the potential of AR blocking agents alone or in combination with additional medication as novel anticancer (immuno)therapies. Table 2, Figure 2.
Table 2.
Antitumor effects of β-blockers.
| Cancer type | β-blocker | Cell lines; animal model; patient sample | Mode of action | Ref. |
|---|---|---|---|---|
| Angiosarcoma, hemangio-endothelioma | Propranolol | Murine angiosarcoma SVR and hemangioendothelioma EOMA cells; canine angiosarcoma Emma, Frog and SB cells | Concentration-dependent inhibition of proliferation, migration and tumor growth; apoptosis induction | 59 |
| Bone sarcoma | Propranolol, carvedilol | Canine osteosarcoma OSCA 32, OSCA 40 cells; human osteosarcoma HOS cells and Ewing sarcoma A673 cells | Concentration-dependent inhibition of viability; enhanced radiosensitivity | 60 |
| Breast cancer | Atenolol, L-748,337 (β3 antagonist) |
Human breast cancer MCF-7 cells | Reduced adrenaline and isoprenaline induced lipid droplets cAMP, PKA, EPAC-dependent way | 44 |
| Breast cancer | Propranolol | Murine breast tumor 4T1 cells | Reduction of inducible MDSC in vitro; inhibition of IL-6 expression | 116 |
| Breast cancer, colon cancer, melanoma | Propranolol | Murine CT26.CL25, B16-F10, 4T1 cells, C57BL/6 and BALB/c mice | Potentiation of abscopal antitumor effects and T cell immune responses to radiotherapy; reduction of metastasis | 124 |
| Breast cancer | Propranolol | Murine breast cancer 4T1 and AT-3 cell, C57BL/6 and BALB/c mice | Reduction of MDSCs and impairment of immunosuppressive function | 114 |
| Breast cancer | Propranolol | Human breast cancer MDA-MB-231 cells | Inhibition of voltage-gated sodium channels, inhibition of lateral motility and invasion | 65 |
| Breast cancer | Propranolol, ICI 118,551 (selective β2 blocker) | Human breast cancer MDA-MB-231 cells | Inhibition of viability by downregulation of ERK, COX-2 pathways; cell cycle arrest; apoptosis | 68 |
| Breast cancer | Propranolol | Murine breast cancer 4T1 cells, BALB/c, NSG and C57BL/6 mice | Suppression of stress-induced lung metastasis | 110 |
| Breast cancer | Propranolol | Murine breast cancer 4T1 cells, BALB/c and C57BL/6 mice | Increased CTL/Treg ratio and decrease PD-1 expression in the TME; potentiation of anti-PD-1 checkpoint blockade | 122 |
| Breast cancer | Propranolol | Human breast cancer MDA-MB-231, IBH-6 cells | Inhibition of migration | 61 |
| Breast cancer | Propranolol | Human breast SK-BR-3 cells | Anti-proliferation; decreased p44/42 MAPK, p38 MAPK, JNK, and CREB signaling, increased phosphorylation of survival/apoptosis regulators AKT, p53, and GSK3β). | 97 |
| Breast cancer | Propranolol | Human breast M-406, MCF7, MDA-MB-231 cells; murine breast cancer M-234p, 4T1 cells; BALB/c and CBi mice | Synergistic effects with metformin, inhibition of viability, migration, invasion, mitochondrial function, tumor growth and metastasis | 144 |
| Breast cancer | Propranolol; CGP-20712A (β1 antagonist); ICI-118,551; L-748,337 |
Human breast cancer MDA-MB-231 cells; BALB/c nude mice | Inhibition of invadopodia formation (role in invasion via Src pathway) | 186 |
| Breast cancer | Propranolol | Human breast cancer MDA-MB-231 cells | Inhibition of migration | 42 |
| Breast cancer | Carvedilol | Human breast cancer MDA-MB-231 and MCF-7 cells | Inhibition of migration and invasion via inhibition of Src pathway | 104 |
| Breast cancer | Propranolol | Murine breast cancer 4T1 cells, human breast cancer MDA-MB-231 and MCF-7 cells | Decreased HK-2 expression; inhibition of glucose metabolism | 187 |
| Breast cancer | Propranolol | Human breast cancer MDA-MB-231, MDA-MB-468, MDA-MB-435S cells | Reversed norepinephrine-mediated adhesion promoted by the release of GROα and β1 integrin | 43 |
| Breast cancer | Propranolol | Human breast cancer MCF-7, MDA-MB-231 and SKBR3 cells, glioblastoma U87 cells, lung carcinoma A549 cells, neuroblastoma SK-N-SH cells; NMRI nude mice | Anti-proliferation (dose-dependent); anti-angiogenesis; synergistic effect in vitro and in vivo with 5-fluorouracil and paclitaxel in cell type and dose dependent manner | 128 |
| Breast cancer | Propranolol | Human estrogen responsive breast cancer MCF-7, ZR-75, MDA-MB-361; estrogen non-responsive breast cancer MDA-453, MDA-435, MDA-468 cells | Short exposure of MDA-453 to propranolol increases GIRK1 mRNA (potassium channel) and decrease B2-adrenergic mRNA levels | 188 |
| Breast cancer | Atenolol, propranolol, ICI 118,551 | Human estrogen responsive breast cancer MCF-7, ZR-75, MDA-MB-361; estrogen non-responsive breast cancer MDA-453, MDA-435, MDA-468 cells | Inhibition of DNA synthesis | 76 |
| Cervical cancer | Propranolol | Human cervical carcinoma Siha, HeLa cells | Anti-proliferation by suppressing cGMP/PKG pathway; inhibition of clone formation; apoptosis induction | 83 |
| Colorectal cancer | Propranolol | Human colorectal cancer HCT116, HT29, RKO cells | Apoptosis induction; decreased mitochondria and proteins involved in oxidative phosphorylation; reduced metastatic potential, viability and proliferation | 81 |
| Colorectal cancer | Propranolol | Murine colorectal cancer CT26 cells; BALB/c mice | Decreased tumor growth by activating CD8+ T cells and by inhibiting AKT/MAPK pathway | 96 |
| Colorectal cancer | Atenolol, carvedilol, propranolol, ICI 118,551 | Human colorectal cancer HT-29 cells | Reversion of AR agonists-induced proliferation | 62 |
| Colorectal cancer | Propranolol | Murine colorectal cancer CT26 cells; BALB/c mice | Improved resistance to metastasis in combination with etodolac | 146 |
| Colorectal cancer | Atenolol, propranolol, ICI 118,551 | Human colorectal cancer SW1116, SW480, SW620, DLD1, HCT116, Colo205, HT29 cells, BALB/c mice | Inhibition of viability and tumor growth (G1-phase cell cycle arrest and inhibition of EGFR-Akt/ERK1/2 pathway), apoptosis induction | 69 |
| Colorectal cancer | Atenolol, ICI 118,551 | Human colorectal cancer HT-29 cells | Dose-dependent inhibition of nicotine-induced tumor growth, microvessel densities; expression of COX2, PGE2, VEGF | 100 |
| Colorectal cancer | Atenolol, propranolol | Human colorectal cancer SW 480 cells | Inhibition of norepinephrine-promoted migration | 41 |
| Cutaneous squamous-cell carcinoma | Butoxamine (β2 antagonist), ICI 118,551 | Human skin epidermoid carcinoma A-431 cells | Inhibition of VEGF-A-induced angiogenesis; decreased tumor development | 77 |
| Gastric cancer | Propranolol | Human gastric cancer MKN45, NUGC3 cells | Inhibition of tumor growth, viability (G1 cycle arrest), and migration; apoptosis induction; decreased expression of P-CREB-ATF and MEK-ERK pathways; suppression of MMP2, MMP9 and VEGF expression | 70 |
| Gastric cancer | Propranolol ICI 118,551 |
Human gastric cancer HGC27, MKN45, MGC803, BGC823, SGC7901, AGS cells | G1/S phase cell cycle arrest; apoptosis; inhibition of tumor growth, proliferation, invasion, and metastasis by inhibiting ERK1/2-JNK-MAPK pathway and transcription factors (NFkB, CREB, STAT3) | 71 |
| Gastric cancer | Propranolol | Human gastric cancer SGC7901l cells; nude mice | Potentiation of radiotherapy effects, decreased tumor growth, decreased expression of NFkB and then COX-2, EGFR, VEGF expression) | 125 |
| Gastric cancer | ICI 118,551 | Human gastric cancer NCI-N87, MGC803, HGC27, BCG823 cells | Decreased catecholamine-stimulated MUC4 expression involved in the resistance of trastuzumab in HER2+ gastric cancer | 189 |
| Gastric cancer | Propranolol | Human gastric cancer SGC7901, BCG823 cells | Inhibition of proliferation (G0/G1 arrest and G2/M arrest) in a concentration-dependent manner; apoptosis; decreased expression of NFkB, VEGF, COX-2, MMP2, MMP9 | 72 |
| Gastric cancer | Propranolol | Human gastric cancer SGC7901, BCG823 cells | Potentiation of radiation effects, decreased viability and clonogenic potential, apoptosis through the inhibition of NFkB/VEGF/EGFR/COX-2 pathways | 126 |
| Gastric cancer | Atenolol, ICI 118,551 |
Human gastric cancer AGS cells | Reversion of nicotine-induced expression of PKC, ERK1/2 phosphorylation, and COX-2 with cell proliferation | 100 |
| Glioma | Propranolol | C6 rat glioma | Inhibition of TNFα-induced proliferation | 190 |
| Kidney cancer | Propranolol, ICI 118,551 | Patient derived ccRCC cells; human renal cell carcinoma 786-O cells | Decreased viability; decreased oxidative stress and mRNA expression of proinflammatory cytokines | 58 |
| Kidney cancer | Propranolol, ICI 118,551 | Human renal cell carcinoma 786-O cells; NSG mice | Apoptosis induction; inhibited expression of HIF2α, CAIX, VEGF; impaired nuclear internalization of HIF2α and NFkB/p65, reduced tumor growth | 84 |
| Leukemia | Propranolol | Human leukemia Molt-4, Jurkat, U937 cells | Decreased VEGF and MMP2 activity | 66 |
| Leukemia | SR59230A (β3 antagonist) |
Human myeloid leukemia K562, KCL22, HEL, HL60 cells | Apoptosis induction, doxorubicin-resistance reversion | 123 |
| Leukemia | Propranolol | Human pre-B acute lymphoblastic leukemia Nalm-6 cells; NCID mice | Reversed stress effect of by decreasing tumor burden and dissemination | 113 |
| Liver cancer | Propranolol | Murine hepatocellular carcinoma H22 cells; BALB/c mice | Inhibition of tumor growth induced by chronic stress and inactivation of CXCL5-CXCR2-ERK pathway | 191 |
| Liver cancer | Propranolol | Murine hepatocellular carcinoma H22 cells | Inhibition of tumor growth; prevention of the redistribution of splenic myeloid cells | 31 |
| Liver cancer | Propranolol | Human liver cancer HepG2, HepG2.2.15, HL-7702 cells | Anti-proliferation; apoptosis induction; S-phase cycle arrest | 73 |
| Liver cancer | Carvedilol | Wistar rats with hepatic cirrhosis | Decreased hepatocarcinogenesis by suppression of circulating IL-6, ALT, AST, ALP, Bilm, and hepatic IL-6, STAT-3, MDA levels and hydroxyproline content | 192 |
| Liver cancer | Carvedilol | Human hepatoma HA59T cells | Increased intracellular calcium; apoptosis | 85 |
| Liver cancer | ICI 118,551 | HCC cell lines, C57BL/6 mice | Autophagy; HIF1α destabilization; tumor growth suppression; improved anti-tumor activity of sorafenib | 80 |
| Lung cancer | Atenolol, betaxolol, esmolol, metoprolol, pindolol, propranolol, timolol | Human lung carcinoma A549, H1299 cells | Apoptosis, necrosis induction; inhibition of lung cancer cell colony formation | 90 |
| Lung cancer | ICI 118,551 | Human NSCC lung cancer UMSCC103, Cal33 cells | Inhibition of p38 and NFkB oncogenic pathways; affect ERK and PI3K pathways, Nrf2-Keap1 stability and its nuclear translocation; induction of ROS and oxidative stress; synergistic effect with U0126 (MAPK inhibitor) on viability and induction of autophagy | 79 |
| Lung cancer | Propranolol | Patient-derived head and neck cancer cells | Inhibition of proliferation and viability | 39 |
| Lung cancer | Esmolol, ICI 118,511, nadolol |
Human lung cancer A549, MRC-5 cells | Apoptosis ROS induction |
82 |
| Lung cancer | Propranolol | Rat breast cancer MADB106 cells; F344 rats | Combined with etodolac (COX-2 inhibitor): reduction of tumor retention induced by surgery, increase NK cytotoxicity | 147 |
| Lung cancer | Nadolol | Rat breast cancer MADB106 cells; F344 rats | Decrease surgery-induced metastasis | 111 |
| Melanoma | Propranolol, ICI 118,551 | Murine melanoma B16F10 cells | Inhibition of AR agonist induced proliferation | 176 |
| Melanoma | SR59230A | Murine melanoma B16F10 cells | Decreased tumor growth, proliferation and viability; induced differentiation of stromal cells in the TME, promote hematopoietic differentiation; increased number of NK cells |
129 |
| Melanoma | Propranolol | Human melanoma MEL270, OMM2.5, MP41, MP46, WM115, WM266.4 cells | Anti-proliferation; anti-migration; VEGF reduction; cell cycle arrest; apoptosis | 78 |
| Melanoma | SR59230A | Human melanoma A375 cells | Reversion of reduction of mitochondrial activity mediated by β3-AR/UCP2 axis | 107 |
| Melanoma | Propranolol | Human melanoma A375 cells; patient derived- melanoma cells; NOD/SCID mice | Synergistic effect with sunitinib (anti-proliferation, cell cycle arrest through suppressing ERK/Cyclin D1/Rb/Cyclin E, decrease tumor growth) | 136 |
| Melanoma | Propranolol | MT/ret mice | Decreased angiogenesis, proliferation and survival; decreased infiltration of immunosuppressive myeloid cells in TME | 115 |
| Melanoma | Propranolol | Human melanoma A375 cells, patient derived melanoma cells; BALB/c mice | Cell cycle arrest; apoptosis via AKT/MAPK pathway | 98 |
| Melanoma | L 748,337 | Murine melanoma B16F10 cells | Decreased tumor growth, neoangiogenesis and proliferation; increased tumor cell death | 106 |
| Melanoma | Propranolol | Patient derived melanoma cells | Anti-proliferation; anti-angiogenesis; apoptosis; decreased tumor growth | 56 |
| Melanoma | SR59230A L 748,337 |
Murine melanoma B16F10 cells | Anti-proliferation; apoptosis; decreased tumor growth | 86 |
| Melanoma | Propranolol | Human melanoma A375, Hs29-4T cells | Decrease mobility and released IL6/VEGF induced by catecholamines | 17 |
| Melanoma | Metoprolol, Propranolol, ICI 118 551 |
Murine melanoma B16F10 cells | Potentiation of anti-PD-1 checkpoint blockade | 137 |
| Multiple myeloma | Bisoprolol, Propranolol ICI 118 551 |
Human multiple myeloma LP-1, OPM-2, RPMI-8226, ANBL-6, XG-2 cells | Apoptosis; autophagy; mitochondrial respiratory chain alteration; decreased glycolysis; increased chemosensitivity to melphalan and bortezomib | 193 |
| Neuroblastoma | Propranolol | Human neuroblastoma KELLY, CHLA-20, LAN-5, IMR-32, SK-N-BE1, SK-N-BE2, SK-N-BE2c, SK-N-SH, SK-N-AS, LAN-6, SH-EP, CHLA-15, CHLA-90, SK-N-FI cells | Inhibition of growth and proliferation; apoptosis via activation of p53 and p73; synergistic effect with SN-38 (topoisomerase I inhibitor) | 130 |
| Neuroblastoma | Atenolol, butoxamine, carvedilol, labetalol, metoprolol nebivolol, propranolol | Human neuroblastoma BE2-C, SHEP, SK-N-SH cells | Synergistic effect with vincristine (anti-angiogenic, anti-mitochondrial, anti-mitotic, pro-apoptotic effects, decrease tumor growth) | 131 |
| Neuroblastoma | SR59230A | Human neuroblastoma SK-N-BE2 and BE2C cells; murine neuroblastoma Neuro-2A cells; NCI A/JCr mice | Decreased proliferation; inhibition of tumor growth and progression through blockade of SK2/S1P2 signaling | 108 |
| Neuroblastoma | SR59230A | Neuroblastoma cell lines from patients | Anti-proliferation; Inhibition of tumor growth and colony formation through the inactivation of the mTOR/p70S6K pathway | 109 |
| Osteosarcoma | Carvedilol | Human osteosarcoma MG63 cells | Anti-proliferation Increase in intracellular calcium level, which may lead to cytotoxicity |
103 |
| Osteosarcoma | Propranolol | Human osteosarcoma MG63, U2OS cells | Decreased tumor growth; anti-mitotic; G0/G1 cycle arrest; impaired colony formation, 3D spheroid growth, cell chemotaxis and capillary-like tube formation; alteration of cytoskeleton | 74 |
| OSCC | Propranolol | Human tongue cancer SCC-9, SCC-25, Cal27 cells | Dose- and time-dependent decrease in viability; downregulated p-P65 NFkB and VEGF expression; inhibited cell migration; synergism with CDDP and 5-FU | 132 |
| OSCC | Propranolol | OSCC cells; BALB/c mice | Reversed anti-migratory effect induced by AR agonist | 63 |
| OSCC | Propranolol | 4NQO induced oral carcinogenesis in Wistar rats | Decreased occurrence of tumors; decreased thickness of OSCC; reduced pro-inflammatory cytokines IL-6 and TNF-alpha | 57 |
| OSCC | Propranolol | Human tongue cancer SCC-9, SCC-25 cells | Reversion of migratory effect of norepinephrine | 36 |
| OSCC | Carvedilol | Human oral cancer OC2 cells | Apoptosis | 87 |
| OSCC | Propranolol | Human tongue cancer TCa8113 cells; human salivary adenoid cystic carcinoma ACC cells | Reversed migratory and mitogenic effect of norepinephrine | 37 |
| Esophagus cancer | Atenolol, ICI 118,551 |
Human esophageal squamous cell carcinoma HKESC-1 cells |
Reversion of proliferative effect of epinephrine | 18 |
| Esophagus cancer | Atenolol, ICI 118,551 |
Human esophageal squamous cell carcinoma HKESC-1 cells |
Inhibition of proliferative effects mediated by epidermal growth factor | 38 |
| Ovary cancer | Propranolol | Murine ovarian cancer HeyA8, SKOV3ip1, A2780, RMG-II, MB-231 cells | Reversion of AR agonist and daily stress-induced tumor growth and angiogenesis. | 11 |
| Pancreas cancer | ICI 118,551, metoprolol | Human pancreatic adenocarcinoma MIA PaCa-2, BxPC-3 cells | G1/S phase cell cycle arrest; apoptosis; anti-proliferative, anti-invasive effect | 75 |
| Pancreas cancer | Atenolol, propranolol, ICI 118,551 | Pancreatic ductal adenocarcinoma patient cohort | Synergistic effects with gemcitabine on survival | 133 |
| Pancreas cancer | Propranolol | Human pancreatic carcinoma Panc-1 cells | Reversion of pro-tumor effects of norepinephrine | 35 |
| Pancreas cancer | Propranolol | Human pancreatic cancer Colo-357, BxPC-3, MIA PaCa-2, Panc-1, PaTus cells; murine pancreatic cancer 6606PDA, TD1, TD2, Panc02 cells; C57BL/6 mice | Reversion of catecholamine-induced proliferation and migration | 21 |
| Pancreas cancer | ICI 118,551 | Human pancreatic cancer BxPC-3, MIA PaCa-2 cells | Synergistic effect with gemcitabine (anti-proliferative and pro-apoptotic effects); inhibition of NFkB | 134 |
| Pancreas cancer | Metoprolol, propranolol, ICI 118,551 | Human pancreatic cancer BxPC-3, MIA PaCa-2 cells | Anti-proliferative and anti-invasive effect by inhibition of cAMP/PKA and Ras, NFkB, AP-1, CREB, VEGF, MMP2, MMP9, COX-2 | 67 |
| Pancreas cancer | Propranolol | Human pancreatic cancer BxPC-3, MIA PaCa-2 cells | Reversion of the invasive effect of norepinephrine (concentration-dependent) | 19 |
| Pancreas cancer | Propranolol | Nitrosamine-induced pancreas cancer in Syrian golden hamsters | Anti-tumor effect by blocking cAMP-dependent intracellular signaling, cAMP-dependent release of EGF and PKA-dependent release of VEGF | 95 |
| Pancreas cancer | Butoxamine, metoprolol, propranolol | Human pancreatic adenocarcinoma PC-2 cells | Apoptosis | 88 |
| Pancreas cancer | Propranolol | Human pancreatic adenocarcinoma Panc-1 cells | Reversion of the pro-tumor effect (tumor growth) of AR agonist | 52 |
| Pancreas cancer | Propranolol | Human pancreatic adenocarcinoma Panc-1, HPAF-II, Capan-1 cells; BALB/c-Foxn1nu mice | Reversion of proliferative and invasive effects induced by the activation of AR by chronic stress | 23 |
| Prostate cancer | Propranolol | Human pancreatic adenocarcinoma PC-3 cells | Propranolol + 2DG (glycolysis inhibitor) induced autophagy, anti-proliferative effects, apoptosis, mitochondrial morphology alteration, endoplasmic reticulum stress, and suppression of tumor growth | 149 |
| Prostate cancer | Propranolol | Human pancreatic adenocarcinoma PC-3 cells; BALB/c mice | Reversion of norepinephrine-induced lumbar lymph metastasis | 64 |
| Prostate cancer | ICI 118,551 | Human prostate cancer C42 cells | Prevention of stress-induced effects | 194 |
| Bladder cancer, prostate cancer | Propranolol | Human prostate cancer C4, human prostate cancer, human urinary bladder carcinoma T24 cells | Apoptosis; inhibition of MAPK pathway | 89 |
| Thyroid cancer | Propranolol | Human thyroid cancer K1, BCPAP, ATC, BHP27 cells | Inhibition of tumor growth; apoptosis; cell cycle arrest; decreased expression HK2 and GLUT1; Synergism with vemurafenib | 135 |
| Uveal melanoma | Carvedilol | Human uveal melanoma Mel270, 92–1, UPMD2, UPMM3 cells | Inhibition of tumor growth; prevention of long-term survival; additive cytotoxic effect with radiation | 127 |
| Colorectal cancer, melanoma | Propranolol | Murine melanoma B16-OVA cells, colorectal cancer CT26.CL25 cells; C57BL/6NCr and BALB/c mice | Decreased tumor growth in stressed mice; reduced checkpoint receptors; increased glycolysis/mitochondrial dysfunction, oxidative phosphorylation and CD28 expression in TILs; increased expression of anti-tumor cytokines | 121 |
| Lung cancer, skin cancer | Carvedilol | Murine epidermal cancer JB6 P+ cells; human lung cancer A549 cells | Inhibition of EGF-induced malignant transformation; inhibition of EGF-mediated activator protein-1 (AP-1) activation | 105 |
| Breast cancer, Colorectal cancer, liver cancer |
Atenolol, propranolol, ICI 118,551 | Human breast cancer MCF7, colorectal cancer HT29, liver cancer HepG2 cells | Anti-proliferative, anti-migratory and anti-invasive effect | 40 |
| Lung cancer, melanoma | Propranolol | Murine melanoma B16F10.9, Lewis lung carcinoma 3LL cells; C57BL/6J mice |
Combined with COX-2 inhibitor etodolac: improved overall survival and recurrence free-survival; reversed surgical glucocorticoid stress: increase NK cytotoxicity and expression of Fas ligand and CD11a; decreased corticosterone level | 148 |
| Breast cancer, cervix adenocarcinoma, melanoma, leukemia | Carvedilol | Human breast cancer MDA-MB-231; melanoma Fem-x; cervix adenocarcinoma HeLa, leukemia K562 cells | Anti-proliferative effect | 102 |
| Fibrosarcoma, melanoma | Propranolol | Murine melanoma B16, fibrosarcoma Meth A cells; C57BL/6 and BALB/c mice | Inhibit psychological stress-enhanced tumor growth | 32 |
| / | Atenolol, propranolol, ICI 118,551 | Preclinical in vivo model (xenografts) | Inhibition of cell viability and xenograft growth; decreased phosphorylation of AKT/MEK/ERK; activation of CD8+ T cells in TME | 99 |
| / | Propranolol | Mouse model of vaccine-based immunotherapy (C57BL/6J and OT-IxSJL) + TC1 expressing HPV16-E6/E7 | Improved efficacy of antitumor STxBE7 vaccine by enhanced the CD8+ T cell infiltration | 143 |
Figure 2.
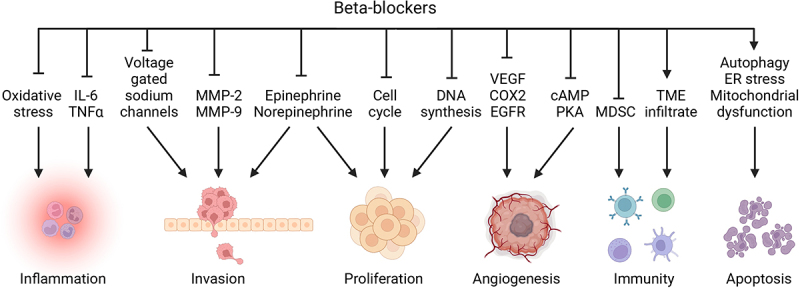
Scheme of the biological effects of β-blockers.
cAMP, cyclic adenosine monophosphate; COX-2, cyclooxygenase 2; EGFR, epithelial growth factor receptor; ER, endoplasmic reticulum; IL, interleukin; MDSCs, myeloid-derived suppressor cells; MMP, matrix metalloproteinase; NK, natural killer; PKA, protein kinase A; TME, tumor microenvironment; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor. Created with BioRender.com.
Published clinical trials
The aforementioned preclinical findings spurred the initiation of numerous clinical trials most of which are observational (12 retrospective trials, 1 prospective trial and 6 meta-analyses). Retrospective studies come from cohorts of oncological patients treated with β-blockers for a history of cardiovascular disease or arterial hypertension or from data extracted of prospective trials with incidental use of β-blockers. Most of the results concluded to a positive impact of β-blockers on oncological outcomes. β-blockers significantly decreased tumor growth and the risk of metastasis into distant organs. As a result, most analyses reported a strong positive correlation between the use of β-blockers and overall and progression free-survival in various treated solid tumors.150–161 These positive and encouraging results need to be confirmed in prospective randomized controlled trials. Indeed, these studies involved many sources of bias such as combination of antihypertensive drugs (β-blockers plus angiotensin-II-receptor inhibitor or angiotensin-converting enzyme inhibitors) and positive effects of β-blockers on cardiovascular mortality. Only three trials failed to report prognostic effects of β-blockers162–164 except a better response to pembrolizumab in the treatment of stage III melanoma.162 Only one study reported negative effects of β-blockers in a cohort of HER2+ breast cancer patients treated with trastuzumab, where β-blocker appeared to decrease survival (PFS adjusted HR = 2.21, 95%[1.56–3.12]; p < 0.001 and OS adjusted HR = 2.46, 95%[1.69–3.57]; p < 0.001)165. However, the higher rate of mortality observed in this trial might involve cardiovascular mortality or immune toxicity. Table 3
Table 3.
Published observational studies (prospective, retrospective, and meta-analyses) investigating the inhibition of adrenergic signaling pathway in cancer patients.
| Cancer | Beta-blockers | Conventional anticancer agents | Results | Study | Ref. |
|---|---|---|---|---|---|
| Brain cancer | β-blockers | NA | 225 patients included. Control of tumor growth (p=0.001), tumor progression (p=0.0001) and higher survival (p=0.015). Strong correlation between β-blockers and survival (p=0.049) | Retrospective | 150 |
| Breast cancer (HER2+) | Atenolol, bisoprolol, carvedilol, propranolol |
Trastuzumab | PFS adjusted HR=2.21, 95%CI[1.56–3.12]; p<0.001 OS adjusted HR=2.46, 95% CI[1.69–3.57]; p<0.001 |
Retrospective | 165 |
| Breast cancer (HER2-) | Atenolol, bisoprolol, metoprolol, propranolol |
Docetaxel and/or ramucirumab | Improved PFS (15.5 vs 8.3 months); p=0.038 No significant difference in OS |
Retrospective | 151 |
| Breast cancer | Atenolol, bisoprolol, propranolol, timolol |
NA | Reduction in metastasis (p=0.026), tumor recurrence (p=0.001) and longer disease-free interval (p=0.01). A 57% reduction in risk of metastasis (HR=0.430; 95%CI[0.200–0.926], p=0.031). A 71% reduction in mortality after 10 years (HR=0.291; 95%CI[0.119–0.715]; p=0.007). No significant difference in vascular invasion. | Retrospective | 152 |
| Breast cancer | β-blockers | NA | 46 245 patients included. Survival HR=0.44; 95%CI[0.26–0.73] with I2=78%. DFS HR=0.71; 95%CI[0.19–1.03] | Meta-analysis | 195 |
| Colo-rectal cancer | β-blockers | Chemotherapy (2628 patients) Radiotherapy (1427 patients) |
4794 patients included. β-blockers decreased mortality (adjusted OR=0.88; 95%CI[0.77–1.00]; p=0.04) | Retrospective | 153 |
| Hepato-cellular carcinoma | Carvedilol, nadolol, propranolol, timolol |
NA | 47 studies (28 RCT + 19 cohorts) included. No significant association between propranolol (OR=0.94;95%CI [0.62–1.44]) or timolol (OR=1.32; 95%CI [0.44–3.95]) and HCC incidence. Risk of HCC decreased by 26% and 38% with nadolol (OR=0.74; 95%CI[0.64–0.86]; p=0.796) and carvedilol (OR=0.62; 95%CI[0.52–0.74]; p=0.776). | Meta-analysis | 154 |
| Hepato-cellular carcinoma | Carvedilol, propranolol, nadolol, timolol |
NA | 23 studies were included (totaling 2618 patients). β-blockers do not reduce mortality. | Meta-analysis | 196 |
| Lung cancer | Landiolol | Adjuvant chemotherapy (8 patients) | 28 patients included in the landiolol group and 29 in the control group. HR for RFS in the landiolol group was 0.41; 95%CI[0.13–1.34]; p = 0.1294. HR for RFS in the landiolol group without adjuvant chemotherapy was 0.50; 95%CI[0.15–1.62]; p = 0.2363. |
Retrospective | 197 |
| Lung cancer | Selective agents: atenolol, bisoprolol, metoprolol Non selective agents: carvedilol, labetolol, nadolol, propranolol, sotalol |
Radiotherapy (100% patients) Chemotherapy (90% patients) |
722 patients (155 patients received β-blockers). Univariate analysis: better Distant Metastasis Free Survival (p<0.01), DFS (p<0.01), and OS (p=0.01) compared with no β-blockers. Multivariate analysis: better DMFS (HR=0.67; p=0.01), DFS (HR=0.74; p=0.02), and OS (HR=0.78; p=0.02) | Retrospective | 155 |
| Melanoma | β-blockers | NA | 121 patients (30 patients treated with β-blockers). A 36% risk reduction of progression each year in the treated group (95%CI[11%-54%]; p=0.002) | Retrospective | 156 |
| Melanoma | Selective agents: acebutolol, atenolol, betaxolol, bisoprolol, celiprolol, esmolol, metoprolol, nebivolol Non-selective agents: carteolol, carvedilol, labetatol, levobunolol, metipranolol, nadolol, oxprenolol, penbutolol, pindolol, practolol, propranolol, sotalol, timolol |
± pembrolizumab | No prognostic effect of β-blockers on RFS (HR=0.67; 95%CI [0.38–1.19] in the pembrolizumab group and HR=1.15; 95%CI [0.80–1.66] in the placebo group). | Retrospective | 164 |
| Ovary cancer | Atenolol, bisoprolol, metoprolol, oxprenolol, pindolol, propranolol | NA | Extension of at least 8 years post-surgery if use non selective β-blockers | Retrospective | 157 |
| Ovary cancer | Metoprolol | No chemotherapy (9 patients) IV platinum (146 patients) IV-IP platinum (30 patients) |
Metoprolol given before and during cytoreduction for 70 patients vs 115 patients (control group). OS was significantly higher in β-blocker group (44.2 vs 39.3 months; p=0.01). In multivariate analysis, β-blocker was associated with significant improvement in OS (HR 0.68; 95%CI[0.46–0.99]; p=0.046). | Retrospective | 158 |
| Ovary cancer | Acebutolol, atenolol, betaxolol, bisoprolol, metoprolol, nebivolol, penbutolol, propranolol, talinolol, soltalol | carboplatin, gemcitabine | No difference in PFS (7.79 vs 7.62 months; p=0.95) and OS (21.2 vs 17.3 months; p=0.18) between β-blockers group and control group. | Retrospective | 163 |
| Prostate cancer | β-blockers | No hormono- radio- or chemotherapy prior surgery (exclusion criteria) | 11 117 men were included. β-blockers at time of surgery were significantly associated with a lower risk of treatment for cancer recurrence (adjusted HR=0.64; 95% CI[0.42–0.96]; p=0.03) | Prospective | 159 |
| Solid tumors | Atenolol, bisoprolol, carvedilol, labetalol, metoprolol, nebivolol, solatol | Immunotherapy (PD-1, PD-L1, CTLA-4 inhibitors) ± chemotherapy |
11 studies included (=10 156 patients). No association between β-blockers and OS (HR=0.97; 95%CI[0.85–1.11]) or PFS (HR=0.98; 95%CI[0.90–1.06]). Significant better response to immunotherapy in the cohort (OR=0.42; 95%CI[0.19–0.94]; p=0.036) and lung cancer subgroup (OR= 0.25; 95%CI [0.08–0.83]); p=0.024) | Meta-analysis | 162 |
| Solid tumors | Acebutolol, atenolol, bisoprolol, carvedilol, celiprolol, labetolol, metoprolol, nadolol, nebivolol, oxprenolol, pindolol, propranolol, timolol | NA | Reduction in risk of cancer (HR=0.33 95%CI[0.13; 0.83]; p=0.019). In the meta-analysis sub-analysis: lower risk of cancer (MH-OR=0.93 95%CI[0.86; 1.01]; p=0.070). | Observational and meta-analysis | 160 |
| Solid tumors | β-blockers | Chemotherapy and radiotherapy in some studies | 20 cohorts and 4 case controls (76 538 patients). β-blockers were given after the diagnosis of cancer. HR all causes mortality=0.89 (95%CI [0.81–0.98]). HR cancer mortality=0.89 (95%CI [0.79–0.99]). | Meta-analysis | 161 |
| Solid tumors | β-blockers | Immunotherapy (PD-1, PD-L1, CTLA-4 inhibitors) | 13 studies included (=3 331 patients). Concomitant use of NSAIDs, β-blockers and metformin is not associated with improved OS or PFS. | Meta-analysis | 198 |
Abbreviations: CI, confidence interval; DMFS, distant metastasis free survival; HR, hazard ratio; IV, intravenous; IP, intraperitoneal; NSAIDs, non-steroidal anti-inflammatory drugs; OS, overall survival; PFS, progression free-survival; RFS, recurrence free-survival.
Nine prospective interventional or randomized controlled trials have been published. One study enrolled 25 participants with multiple myeloma to receive either propranolol in titrated doses or placebo for 5 weeks. This trial concluded on the tolerability and the efficacy of propranolol to minimize β-adrenergic stress during hematopoietic cell transplantation. It also showed successful engraftment and effective response against myeloma when propranolol was administered.166 Safety and efficacy of β-blockers on adrenergic stress during the treatment of 26 ovary cancers were also confirmed.167 Used as a standalone agent during breast cancer and melanoma care, propranolol increases immune cell infiltration into the tumor bed and significantly reduces the risk of recurrence.168,169 When associated with conventional antineoplastic treatments such as taxanes or anthracyclines, β-blockers are also perfectly tolerable and safe.170 Moreover, propranolol potentiates the immune effects of pembrolizumab against melanoma.171 Combined with anti-COX-2 during the peri-operative period of breast and colorectal cancer surgery in three randomized controlled trials, propranolol minimizes surgical stress, improves tumor molecular markers, reinforces the anti-tumor immune response, impairs pro-inflammatory and metastatic transcription factors and finally decreases the risk of relapse.172–175
Finally, a few prospective studies all reported promising oncological outcomes. Thus, the study of Ramondetta et al. showed that patients with ovary cancer treated with propranolol had 55.5% complete response, 33.3% partial response, 5.6% stable response, and only 5.6% progressive disease165. In the randomized controlled trial by De Giorgi et al. the administration of β-blocker to patients with melanoma decreased the risk of recurrence (80%, HR = 0.18, 95% CI [0.04–0.89], p = 0.03) with a DFS 89% vs 64% (p = 0.04). Treatment with β-blocker also decreased the rate of progressive disease (15.8% vs 41.2%) and death (10.5% vs 17.7%).167 Associated with pembrolizumab, β-blockers allowed to achieve 7 partial responses and 1 stable disease in a cohort of 9 metastatic melanoma patients.169 Of note, four studies notified cardiovascular events such as hypotension and bradycardia due to the consumption of β-blockers without major consequences.164,166,168,169 Table 4
Table 4.
Published interventional and randomized controlled trials investigating the inhibition of adrenergic signaling pathway in cancer patients.
| Cancer | Beta-blockers | Conventional anticancer treatment |
Results | Cardiovascular effects | Study | Phase | Ref. |
|---|---|---|---|---|---|---|---|
| Breast cancer (HER2+) | Propranolol | Neoadjuvant chemotherapy (taxanes/ anthracyclines) | 10 patients included. Propranolol started at 20mg and then increased to 80mg daily. Feasibility of combining propranolol with chemotherapy. | Bradycardia in 3 patients | Interventional | II | 170 |
| Breast cancer | Propranolol | No neoadjuvant chemotherapy (exclusion criteria) | 60 patients randomized to receive placebo or propranolol (escalating doses 80-160mg daily for 7 days before surgery). Reduction in intratumoral mesenchymal polarization and increase in immune cell infiltration. | Minimal reduction in blood pressure (<20 mmHg) and heart rate (<10 mmHg) Bradycardia in 1 patient Hypotension in 3 patients |
RCT | II | 168 |
| Breast cancer | Propranolol + etodolac | NA | 38 patients randomized to receive propranolol + etodolac 5 days before and 6 days after surgery or placebo. Significant decrease in epithelial to mesenchymal transition, prometastatic/proinflammatory transcription factors and tumor-infiltrating monocytes. Increase in tumor-infiltrating B cells. Abrogation of serum IL-6 and C-reactive protein levels, and IL-12/IFNγ production. | NA | RCT | II | 172 |
| Breast cancer | Propranolol | With or without parecoxib No neoadjuvant chemo- or radiotherapy (exclusion criteria) |
101 women were randomized to receive propranolol ± parecoxib or placebo before and after mastectomy. β-blockers reduced surgical stress-induced Treg cells. No additive or synergic effect with parecoxib. | Propranolol group: significant decrease in heart rate during the per-operative period | RCT | NA | 173 |
| Colo-rectal cancer | Propranolol + etodolac | NA | 34 patients included to receive propranolol + etodolac for 5 days before and 15 days after surgery or placebo. Significant improvement (p<0.05) of tumor molecular markers. In compliant patients group, recurrence rates were 0% in the treatment group and 29.4% in the placebo group (p=0.054). | NA | RCT | II | 174 |
| Melanoma | Propranolol | Pembrolizumab | 9 patients included in three groups (10 mg, 20 mg or 30 mg propranolol twice a day). Association with immunotherapy was safe, tolerable, increased IFN-γ and decreased IL-6. 10 mg group: 2 partial responses and 1 stable disease 20 mg group: 2 partial responses and 1 progressive disease 30 mg group: 3 partial responses |
Hypotension in 1 patient (30 mg) | Interventional | I | 171 |
| Melanoma | Propranolol | NA | 53 patients included (19 received propranolol). β-blockers are associated with a 80% risk reduction in recurrence (HR=0.18; 95%CI [0.04–0.89]; p=0.03). Propranolol group 15.8% disease progression and 10.5% death vs 41.2% and 17.7% in the control group. DFS 89% propranolol group vs 64% at 3 years, p=0.04). Cox model : HR DFS 0.18, 95%CI [0.04–0.89], p=0.03; HR OS 0.64, 95%CI [0.1–3.96], p=0.63 |
NA | RCT | NA | 169 |
| Multiple myeloma | Propanolol | Melphalan | 25 patients included. Feasibility to recruit and treat multiple myeloma during hematopoietic cell transplantation. | Hypotension in 1 patient | RCT | II | 166 |
| Ovary cancer | Propranolol | Carboplatin and paclitaxel | 26 patients included. Feasibility of propranolol before chemotherapy or surgery. Decrease in adrenergic stress markers. 55.5% complete response/33.3% partial response/5.6% stable response/5.6% progressive response |
NA | Interventional | I | 167 |
Abbreviations: DFS, disease free-survival; HR, hazard ratio; IFN, interferon; IL, interleukin; RCT, randomized controlled trial.
Completed clinical trials
The primary focus of these trials was to study the effect of β-blockers as standalone agents. Among these trials, NCT01544959 evaluated the possibility of substituting fentanyl with esmolol for anesthesia induction, followed by metoprolol during mastectomy, with the aim to manage hemodynamic, perioperative pain and postoperative nausea and vomiting. NCT02596867, a non-randomized phase II trial, studied the effect of β-adrenergic blockade with 0.75 mg/kg propranolol, administered twice daily for 3 weeks, prior to surgical resection of breast cancer. The trial assessed the tumor proliferative index using Ki-67 before and 3 weeks after propranolol administration and assessed the capacity of propranolol to decrease tumor proliferation in breast cancer. Similarly, the early phase I study NCT02013492 evaluated the potential of propranolol administered for 4 weeks in patients with locally-recurrent or metastatic solid tumors to decrease tumor growth by inhibiting the effects of adrenergic hormones on the tumor cells. Furthermore, the study NCT03861598 aimed at establishing a correlation between circulating tumor cells and favorable magnetic resonance imaging (MRI) results in patients with grade IV glioblastoma receiving chemotherapy with a combination of escalated doses of carvedilol. Further trials investigated whether the synergistic effects observed between β-blockers and conventional chemotherapies observed in preclinical studies were assessable in clinical practice. Both NCT01308944 and NCT01504126 aimed at confirming feasibility of administering β-blockers before, during and after surgical ovarian cancer debulking until completion of chemotherapy to mitigate depression and anxiety and to evaluate an impact on immune response and survival. The randomized multicenter phase 2 study NCT01857817 was designed to evaluate clinical benefits and changes in circulating prostate-specific antigen (PSA) of the VT-122 protocol consisting of a co-administration of 22 mg propranolol plus etodolac 340 mg in clinically progressive prostate cancer. Table 5
Table 5.
Completed and terminated clinical trials investigating the inhibition of adrenergic signaling pathway in cancer patients (not yet published).
| Cancer | Drug | Assessed outcomes | Status | Phase | Co-therapy | Study | NCT |
|---|---|---|---|---|---|---|---|
| Breast cancer | Esmolol Metoprolol | –Postoperative consumption of narcotic –Impact of postoperative acute and chronic pain –Nausea, vomiting –Recurrence |
Completed | NA | As a single agent | RCT | NCT01544959 |
| Breast cancer | Propanolol | –Decrease in tumor proliferative index (Ki-67) | Terminated | II | As a single agent | Interventional | NCT02596867 |
| Breast cancer | Propranolol | –Number of patients with pathologic complete response at 6 months | Completed | II | Combined with neoadjuvant chemotherapy | Interventional | NCT01847001 |
| Gliobla-stoma | Carvedilol | –Correlation between RT-qPCR assay for circulating tumor cells and the change in response to MRI results –Evaluate response |
Terminated | Early phase I | Combined with standard chemotherapy | Interventional | NCT03861598 |
| Ovary cancer | Propanolol | –Feasibility of concurrent β–blocker administration with chemotherapy –DFS –Blood markers –Immunohistochemistry of angiogenic markers on tumor samples |
Completed | I | Concurrent chemotherapy | Interventional | NCT01308944 |
| Ovary cancer | Propanolol | –Complete 6 cycles of chemotherapy –Change in mood state –DFS and OS –Change in immune response (IL-6, IL-8, VEGF) |
Completed | Early phase I | Combined with chemotherapy | Interventional | NCT01504126 |
| Prostate cancer | Propanolol | –Change in PSA –Pain –Time to symptom progression –Change in correlative biomarkers |
Terminated | II | Combined with etodolac | RCT | NCT01857817 |
| Solid tumors | Propanolol | –Toxicity –Change in VEGF –Effect of β-adrenergic blockade on the TME and on the host immune system –DFS and OS |
Completed | Early Phase I | As a single agent | Interventional | NCT02013492 |
Abbreviations: CRS, cytokine release syndrome; DFS, disease-free-survival; IL, Interleukin; MRI, magnetic resonance imaging; ORR, overall response rate; OS, overall survival; PSA, prostate specific antigen; RCT, randomized controlled trial; TME, tumor microenvironment; VEGF, Vascular Endothelial Growth Factor.
Ongoing clinical trials
Most registered currently ongoing studies aim at evaluating the clinical impact, in particular overall survival and disease-free survival, after administration of β-blockers during the management of various solid tumors and hematological malignancies. Some of these trials also investigate potential advantages arising from the combination of β-blockers with additional anticancer or non-anticancer agents.
β-blockers as single agents
The randomized placebo-controlled clinical trial MELABLOCK (NCT02962947) is designed to evaluate the efficacy and safety of a daily dose of 80 mg propranolol in patients with stage II/IIIA melanoma. NCT04518124 consists of a single arm propranolol administered in a dose of 40–80 mg 2–3 times per day immediately after diagnosis of cutaneous angiosarcoma extending over a period of 3–6 weeks. Clinical and histological responses defined as a decrease in Ki-67 index will be measured until study completion. The early phase I interventional trial NCT03245554 intends to enroll in total 80 patients with gastric adenocarcinoma or non-metastasized colon cancer without any prior treatment. Propranolol will be administered for 1 week as a neoadjuvant treatment during the preoperative period and CT-scan and Ki-67 index will be used to evaluate tumor growth. NCT02944201 aims to investigate whether β-blockers administered to prostate cancer patients from the point of diagnosis until prostatectomy can decrease tumor growth measured by Ki-67 index and TUNEL assay performed on prostatectomy tissues. Notably, the phase II study NCT05679193 presents as a large randomized controlled trial that aims to assess the potential of a 3 weeks propranolol regimen in reducing recurrences after robot-assisted laparoscopic prostatectomy, a surgical procedure significantly less inflammatory and stressful as compared to conventional laparotomy. The study will assess changes in catecholamines and PSA levels during the perioperative period, the bioavailability of propranolol as well as the impact on anesthesiological and surgical strategies including the use of vasopressors, and the occurrence of complications. Finally, NCT05312255 focuses on the evaluation of immune effects, stress reduction and quality of life in non-chemotherapeutic interventions such as physical activity, specific nutritional regimen and propranolol treatment in patients with multiple myeloma.
β-blockers combined with conventional anticancer therapies
Four interventional clinical trials are planned to examine safety and efficacy of propranolol administered in combination with conventional chemotherapy to potentiate antitumor responses. NCT04005365 aims at incorporating propranolol into neoadjuvant chemotherapy in gastric cancer patients with the aim to improve overall response. NCT02641314 aims at administering a combination of propranolol with NSAID (celecoxib) anti-neuroblastic drugs (cyclophosphamide, etoposide and vinblastine) in children and adolescents with recurrent or progressive neuroblastoma. The primary objective is to demonstrate the non-inferiority in survival compared with controls, while as secondary outcomes safety, tolerance, and disease response rate will be assessed. NCT03108300 aims at assessing overall and progression-free survival in patients with metastatic soft tissue sarcoma co-administered propranolol 40 mg twice daily and doxorubicin. NCT02897986 is a dose escalation trial to determine the maximal tolerated dose of vinorelbine administered in combination with daily oral propranolol in children and teenagers with refractory solid tumors. NCT04682158 aims to determine the safety and efficacy of propranolol combined with standard neoadjuvant chemoradiation, as well as an impact on overall survival and pathologic response rate in patients undergoing esophageal cancer resection. The randomized phase II clinical trial NCT04493489 focuses on safety and efficacy of propranolol in adjuvant BCG therapy after the transurethral resection of bladder cancer and evaluates the capacity of the combination treatment to decrease relapses over a 2-year period. Table 6
Table 6.
Ongoing clinical trials investigating the inhibition of adrenergic signaling pathway in cancer patients.
| Cancer | Drug | Outcomes | Status | Phase | Co-therapy | Study | NCT |
|---|---|---|---|---|---|---|---|
| Angio-sarcoma | Propanolol | –Clinical and histological response (decrease of >30% of Ki-67 index) | Recruiting | II | As a single agent | Interventional | NCT04518124 |
| Bladder | Propanolol | –Two-year recurrence-free survival | Not yet recruiting | II | Combined with BCG vaccine | RCT | NCT04493489 |
| Breast | Propanolol | –Cytotoxic activity of NK cells, levels of NKT cells, lymphocytes, monocytes and granulocytes; cytokine levels; In vitro cytokine secretion; levels of cortisol and VEGF. −5-year recurrence |
Unknown | NA | Combined with etodolac during perioperative period | RCT | NCT00502684 |
| Colo-rectal | Propanolol | −5-year DFS –Tumor-infiltrating leucocytes. Pro-tumor and inflammatory cytokines –Adverse effects (Clavien-Dindo classification, depression, anxiety, global distress, fatigue) |
Recruiting | II | Combined with etodolac during perioperative period | RCT | NCT03919461 |
| Colo-rectal | Propanolol | –Recurrence –Magnitude and duration of surgically induced immune depression –Morbidity. Mortality |
Unknown | III | Combined with etodolac during perioperative period | RCT | NCT00888797 |
| Esophagus | Propanolol | −5-year DFS and OS. | Recruiting | II | Combined with chemoradiation | RCT | NCT04682158 |
| Gastro-esophagus | Propanolol | –Toxicities and adverse events –DFS. ORR. OS. |
Not yet recruiting | II | Combined with pembrolizumab, fluorouracil, oxaliplatin and leucovorin | Interventional | NCT05651594 |
| Gastric | Propanolol | –ORR | Unknown | II | Combined with neochemo-therapy | Interventional | NCT04005365 |
| Gastro-intestinal | Propanolol | –Tumor size (Computed tomography and Ki-67) | Unknown | Early phase I | As a single agent | Interventional | NCT03245554 |
| Liver | Propanolol | –Failure free survival –Clinical benefit response |
Unknown | II | Combined with etodolac and sorafenib | RCT | NCT01265576 |
| Liver, pancreas, gallbladder | Propanolol | –Efficacy in boosting the effects of immunotherapy –Feasibility. Safety –Tolerability –DFS. OS |
Not yet recruiting | II | Combined with durvalumab, gemcitabine, paclitaxel, tremelimumab, cisplatin | Interventional | NCT05451043 |
| Lung | Landiolol | −2-year relapse-free survival and OS after surgery –additional treatment after recurrence. Safety events. Postoperative complications |
Unknown | III | As a single agent | RCT | No NCT PMID31829248 |
| Melanoma | Propanolol | –Dose limiting toxicities –DFS. ORR. OS. |
Recruiting | I/II | Combined with pembrolizumab | Interventional | NCT03384836 |
| Melanoma | Propanolol | –DFS. OS. Mortality –Long-term safety |
Unknown | II/III | As a single agent | RCT | NCT02962947 |
| Melanoma | Propranolol | –PFS. ORR. OS | Not yet recruiting | I | Combined with ipilimumab and nivolumab | Interventional | NCT05968690 |
| Multiple myeloma | Propanolol | –Changes in immune cell subsets –Changes in the gut microbiome –Comparison in bone markers –Changes in body composition –Changes in stress, anxiety, fatigue, functional status, nutritional behavior before and after intermittent fasting and stress-related biomarkers |
Recruiting | NA | As a single agent | Interventional | NCT05312255 |
| Neuro-blastoma | Propanolol | –Event free survival –Disease control rate at 6 and 12 months –OS –Hospitalization days. –Number of transfusion days –Drop-out rate |
Recruiting | II | Combined with celecoxib, cyclo-phosphamide, vinblastine, etoposide | Interventional | NCT02641314 |
| Pancreas | β-blockers | –DFS. OS. | Recruiting | NA | As a single agent or combined with aspirin, metformin, ACE-inhibitors, statins | Observational Prospective |
NCT04245644 |
| Pancreas | Propanolol | –Recurrence –Tumor-infiltrating leukocytes. Protumor and inflammatory cytokines –Adverse effects (Clavien-Dindo classification, depression, anxiety, global distress, fatigue) |
Recruiting | II | Combined with etodolac | RCT | NCT03838029 |
| Prostate | Carvedilol | –Change in biomarkers (Ki-67, TUNEL assay) in prostate biopsy –Change in serum PSA |
Not yet recruiting | II | As a single agent prior to surgery | Interventional | NCT02944201 |
| Prostate | Propanolol | –Feasibility –Safety and tolerability –Bioavailability –Changes in catecholamines levels in perioperative period. Serum level of PSA –Change in distress –Surgical complications Proportion of patients requiring vasopressors. Procedure time. Blood loss. –Tumor-infiltrating leucocytes. –Prognostic and predictive markers |
Recruiting | II | As a single agent | RCT | NCT05679193 |
| Sarcoma | Propanolol | –DFS. OS | Unknown | II | Combined with doxorubicin | Interventional | NCT03108300 |
| Solid tumors Hematolo-gical malignancy |
Metoprolol | –Safety and tolerability –Efficacy for CRS control and precaution |
Recruiting | I/II | Combined with CAR-T cells therapy ± infliximab, etanercept, tocilizumab and/or other agents | Interventional | NCT04082910 |
| Solid tumors | Propanolol | –Decrease in toxicity of chemotherapy | Unknown | I | Combined with vinorelbine | Interventional | NCT02897986 |
| Urothelial | Propanolol | –DFS. ORR. OS. –Adverse events |
Recruiting | II | Combined with pembrolizumab | Interventional | NCT04848519 |
Abbreviations: ACE, angiotensin-converting enzyme; BCG, Bacillus Calmette-Guérin; CAR-T cells, chimeric antigenic receptor-T cells; CRS, cytokine release syndrome; DFS, disease-free survival; NK, natural killer; ORR, overall response rate; OS, overall survival; PFS, progression free survival; PSA, prostate specific antigen; RCT, randomized controlled trial; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
β-blockers combined with immunotherapy
Five interventional trials (NCT05651594, NCT05451043, NCT03384836, NCT04848519, NCT01265576, NCT05968690) aim at exploring the addition of β-AR antagonists to immunotherapy (with pembrolizumab, durvalumab, tremelimumab, ipilimumab or nivolumab) alone or together with standard chemotherapy in the treatment of unresectable advanced and/or metastatic digestive adenocarcinoma, stage III/IV melanoma and urothelial cancers. The primary objective is to evaluate the efficacy of β-blockade in boosting immunotherapy as measured by response evaluation criteria in solid tumors (RECIST). Secondary explorative objectives encompass correlations of biomarkers (such as immune effectors, circulating cytokines and stress) with efficacy, progression-free survival and overall survival.
NCT04082910 will test the β1 adrenergic receptor blocker metropolol for its ability to control and prevent of cytokine release syndrome (CRS) in patients with lymphoma and leukemia enrolled for chimeric antigen receptor (CAR)-T cell infusion. Safety and tolerability of metropolol will be confirmed by evaluating heart rate and blood pressure, while the efficacy in the control of CRS will be assessed by monitoring body temperature and serum IL-6 levels.
β-blockers combined with other agents
Four prospective trials (NCT00502684, NCT03919461, NCT00888797, NCT03838029) aim at combining β-blockers with COX-2 inhibitors before, during, and after breast or colorectal cancer surgery to evaluate the impact on immune effector activity and tumor-infiltrating leucocytes. Moreover, cytokine secretion and serum levels of cortisol and VEGF will be assessed alongside psychological examinations. Oncological outcomes such as morbidity and mortality will be assessed in a 5-year follow up. Lastly, the monocentric observational prospective study NCT04245644 will evaluate if the combination of β-blockers with four chemopreventive agents (angiotensin converting enzyme inhibitors, aspirin, metformin, and statin) can improve the overall and disease-free survival in 800 patients with pancreatic ductal adenocarcinoma.
Discussion
Stress-related hormones and signaling via β-adrenergic receptors impact oncogenesis. Preclinical researches and pilot clinical trials support the hypothesis that β-blockers administered at clinically relevant concentrations during anticancer therapy extend the lifespan of individuals with malignant disease. Whether this effect is due to the direct blockage of β-AR and/or due to an indirect systemic effect via the reduction of stress and corticotropic activity is still under discussion. Indeed, upon physical, biological, and psychological stress, the corticotropic HPA axis is activated releasing substantial amounts of immunosuppressive catecholamines and cortisol. Catecholamines are ligands of β-AR located at the plasma membrane of cytolytic immune effectors, negatively impacting mobility and activity, but also on β-AR present on cancer cells where they can stimulate proliferation, invasion, and migration. Of note, different β-AR subtypes are present with a strong predominance of the β2 sub-group.18 ICI 118 551, the most selective β2-AR antagonist, demonstrated consistent antitumor activity while no or partial effects were observed with the β1-specific blocker atenolol.41,105 The implication of β1-receptors is still uncertain, and further investigation is required to understand the contribution of each type of receptor in tumor development. Selective β3-AR antagonists have been even less studied, however, preclinical data noticed antiproliferative and apoptotic effects, suggesting β3-AR could be a target for anticancer therapy.
At present, in the field of oncology, preference is giving to nonspecific β-blockers such as propranolol, which targets both β1/2 ARs. However, it is important to consider potential side effects arising from dual β1/2 blocking. Due to the expression of β-ARs at the tumor cell surface, some research has explored the use of carvedilol, a α-blocker, with alpha-blocking property and has demonstrated an anti-tumor effect. Thus, reduced tumor cell proliferation was observed following the use of α2-AR agonists such as clonidine, a common anti-hypertensive agent.176 This might indicate a crosstalk between α2- and β2-ARs in stimulating cancer cell growth. Further research is needed to elucidate the pro- or anti-tumor profiles of α-ARs.
An interesting aspect was advanced with clinical trial NCT01544959 in which authors hypothesized that fentanyl, an opioid used to control acute pain during induction and maintenance of anesthesia, could be replaced by β-blockers. Opioids have been ascribed with pro-tumorigenic effects, such as stimulating tumor cell migration by the activation of MMPs, activation of the oncogene c-MYC and inhibition of anti-angiogenic TSP-1.177–179 Alternative techniques such as “opioid-free-anesthesia”, a specific protocol mixing local anesthetics, ketamine, magnesium and dexmedetomidine, are emerging for the management of acute pain during the per-operative period. In this setting, due to their anti-tumor effect and immunostimulatory properties, β-blockers appear as an appealing option for opioid replacement. Reportedly, β-adrenergic stimulation inhibits the primary phase of CD8+ T lymphocytes activation, thus providing a rationale for the administration of β-blockers together with T cell targeted immunotherapies.122
Surgical glucocorticoid stress is significantly activated by local and systemic inflammatory pain that can be caused by surgical tissue damage and is mediated by pro-tumorigenic COX-2. Several randomized controlled trials advanced the possibility to potentiate antitumor effects by blocking sympathetic signaling and managing pain with a combination of β-AR antagonists and COX-2 inhibitors (NCT00502584; NCT03919461; NCT00888797; NCT03838029). COX-2 inhibitors belong to the family of non-steroidal anti-inflammatory drugs (NSAIDs) and previous observational clinical trials supported the notion that NSAIDs might improve oncological outcomes. Thus, Desmedt et al. observed that intraoperative administration of ketorolac, a COX-1 and COX-2 inhibitor, significantly reduced the incidence of distant recurrences after breast cancer surgery.180 In a study involving 327 women undergoing mastectomy, Forget et al. reported a significantly lower recurrence rate (p = 0.019) after administration of ketorolac prior to surgery, while other analgesics, such as sufentanil, ketamine and clonidine, showed no effect.181 A retrospective analysis in a cohort of 720 breast cancer patients revealed that injection of the NSAIDs ketorolac and diclofenac during conservative breast cancer surgery correlated with improved disease-free (HR = 0.57, p = 0.01) and overall survival (HR = 0.35, p = 0.03).182 Finally, in a propensity score matching study involving 2502 patients with non-small cell lung cancer, immunotherapy combined with the administration of NSAIDs was associated with a better overall survival (HR = 0.85, p < 0.001).183 In vitro, ketorolac was found to decrease proliferation, migration and angiogenesis, and to enhance the sensitivity of renal cancer to apoptosis. In mice, ketorolac caused tumor growth inhibition when used as a standalone agent or combined with sunitinib.184 In a model of head and neck squamous cell carcinoma, COX-2 inhibition showed additive and synergistic effects with EGFR inhibitors enhancing tumor cell death both in vitro and in vivo, especially in PIK3CA-mutated cancers.99 Altogether, the combination of COX-2 inhibitors with β-blockers seems to be a promising option to potentiate antitumor responses.
In addition, activation of β-ARs in response to surgical stress promotes the survival of circulating tumor cells and increases the incidence of metastases. Surgical glucocorticoid stress can be optimally managed by spinal anesthesia or epidural anesthesia during the perioperative period.185 By controlling stress and inflammatory pain, medullar anesthesia also positively impacted oncological outcome by decreasing the incidence of recurrence. However, these analgesic procedures are contraindicated in bleeding disorders, infection, backbone osteosynthesis or upon patient refusal, necessitating alternative analgesia techniques. In this case, anxiolytic premedication (the day before and 2 h before surgery), for instance with benzodiazepines, can control surgical stress. Premedication has four main objectives: i) decreasing the level of anxiety and its physical consequences such as tachycardia, perspiration and polypnea; ii) minimizing the intensity of biochemical reactions and metabolic activity; iii) enhancing the analgesic potency of anesthetics; and iv) blocking parasympathetic effects induced by anesthesia and surgery such as salivary hypersecretion, nausea, vomiting, laryngeal spasms, and dysrhythmia. Here, β-blockers offer an appealing premedication option because they address all the aforementioned objectives without the known side effects of benzodiazepines such as sleepiness, confusion, and balance disorder. In contrast to other cardiologic agents targeting the angiotensin system, β-blockers are not contraindicated prior to anesthetic procedures. To reverse or inhibit the surgical glucocorticoid stress-induced immune system suppression, β-blockers used at a dose not triggering hypotension, could be considered for cancer patients, especially in the case of contraindication for medullar anesthesia. So far, specific guidelines for the use of β-blockers during the preoperative period are still lacking, and the efficacy of such premedications still requires further demonstration.
Conclusion
All forms of stress, be they physical, biological, or of psychological origin, can activate sympathetic signaling pathways promoting the release of catecholamines and other stress mediators into the systemic circulation. Catecholamines promote the proliferation, migration, and invasion of tumor cells, while suppressing the cytolytic function of immune effectors, through direct interaction with β-AR located on their plasma membrane. β-blockers can counteract such deleterious effects by competitively interfering with the binding of catecholamines to AR. Promising results from preclinical and clinical pilot studies suggesting that β-blockade can improve the outcome of cancer treatments now await confirmation in ongoing prospective randomized controlled trials. The facts that β-blockers are already approved and widely used for cardiovascular indications may accelerate their deployment in oncological practice.
Abbreviations
- AA
arachidonic acid
- ACE
angiotensin-converting enzyme
- ACTH
adrenocorticotropic hormone
- AP-1
activator protein 1
- AR
adrenergic receptor
- BCG
Bacillus Calmette-Guérin
- cAMP
cyclic adenosine monophosphate
- CAR-T cells
chimeric antigenic receptor-T cells
- ccRCC
clear cell Renal Cell Carcinoma
- CDDP
cisplatin
- CI
confidence interval
- COX-2
cyclooxygenase 2
- CREB
cAMP response element-binding protein
- CRS
cytokine release syndrome
- CTLA4
cytotoxic T-lymphocyte-associated protein 4
- CTLs
cytotoxic T lymphocytes
- DC
dendritic cell
- DFS
disease-free survival
- EGF
epidermal growth factor
- EGFR
epithelial growth factor receptor
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated kinase
- 5-FU
5-fluorouracil
- HPA
hypothalamic-pituitary-adrenal
- HR
Hazard ratio
- HPA
hypothalamic-pituitary-adrenal
- IFN
interferon
- IL
interleukin
- MAPK
mitogen activated protein kinase
- MDSCs
myeloid-derived suppressor cells
- MMP
matrix metalloproteinase
- NFkB
nuclear factor kappa B
- NK
natural killer
- NSAIDs
non-steroidal anti-inflammatory drugs
- ORR
overall response rate
- OS
overall survival
- OSCC
oral squamous cell carcinoma
- PD-1
programmed death receptor 1
- PFS
progression-free survival
- PGE2
prostaglandin E2
- PKA/C
protein kinase A/C
- PSA
prostate-specific antigen
- RCT
randomized controlled trial
- RFS
recurrence-free survival
- ROS
reactive oxygen species
- TME
tumor microenvironment
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- VEGF
vascular endothelial growth factor
- VHL
Von Hippel Lindau
Acknowledgments
KCLP receives funding from the Agence Régionale de Santé (ARS) Ile-de-France (Année-recherche Pharmacie). OK receives funding from Institut National du Cancer (INCa) and Agence Nationale de la Recherche (ANR); GK is supported by the Ligue contre le Cancer (équipe labellisée); ANR – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); European Research Council (ICD-Cancer), European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; INCa; Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Immunolife; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosures statement
OK and GK have been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Tollys, and Vascage. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. OK is a scientific co-founder of Samsara Therapeutics. GK is in the scientific advisory boards of Hevolution, Institut Servier and Longevity Vision Funds. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders. GK’s brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. GK’wife, Laurence Zitvogel, has held research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9 m, Tusk and Roche, was on the on the Board of Directors of Transgene, is a cofounder of everImmune, and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. The funders had no role in the design of the study; in the writing of the manuscript, or in the decision to publish the results. The other authors declare no conflicts of interest.
References
- 1.Russell G, Lightman S.. The human stress response. Nat Rev Endocrinol. 2019;15(9):525–28. doi: 10.1038/s41574-019-0228-0. [DOI] [PubMed] [Google Scholar]
- 2.Wick G, Hu Y, Schwarz S, Kroemer G.. Immunoendocrine communication via the hypothalamo-pituitary-adrenal axis in autoimmune diseases. Endocr Rev. 1993;14(5):539–563. doi: 10.1210/edrv-14-5-539. [DOI] [PubMed] [Google Scholar]
- 3.Gatica S, Aravena D, Echeverria C, Santibanez JF, Riedel CA, Simon F. Effects of adrenergic receptor stimulation on Human hemostasis: a systematic review. Adv Exp Med Biol. 2023;1408:49–63. doi: 10.1007/978-3-031-26163-3_3. [DOI] [PubMed] [Google Scholar]
- 4.Glover WE, Greenfield AD, Shanks RG. Effect of dichloroisoprenaline on the peripheral vascular responses to adrenaline in man. Br J Pharmacol Chemother. 1962;19(2):235–244. doi: 10.1111/j.1476-5381.1962.tb01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prichard BN, Gillam PM. Use of Propranolol (inderal) in treatment of hypertension. British Med J. 1964;2(5411):725–727. doi: 10.1136/bmj.2.5411.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Udelsman R, Holbrook NJ. Endocrine and molecular responses to surgical stress. Curr Probl Surg. 1994;31(8):658–720. doi: 10.1016/0011-3840(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 7.Udelsman R, Chrousos GP. Hormonal responses to surgical stress. Adv Exp Med Biol. 1988;245:265–272. doi: 10.1007/978-1-4899-2064-5_21. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Otin C, Kroemer G. Hallmarks of health. Cell. 2021;184(1):33–63. doi: 10.1016/j.cell.2020.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G, McQuade JL, Merad M, Andre F, Zitvogel L. Bodywide ecological interventions on cancer. Nat Med. 2023;29(1):59–74. doi: 10.1038/s41591-022-02193-4. [DOI] [PubMed] [Google Scholar]
- 10.Jetschmann JU, Benschop RJ, Jacobs R, Kemper A, Oberbeck R, Schmidt RE, Schedlowski M. Expression and in-vivo modulation of α- and β-adrenoceptors on human natural killer (CD16+) cells. J Neuroimmunol. 1997;74(1–2):159–164. doi: 10.1016/s0165-5728(96)00221-4. [DOI] [PubMed] [Google Scholar]
- 11.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 12.Thivierge M, Parent JL, Stankova J, Rola-Pleszczynski M. Modulation of formyl peptide receptor expression by IL-10 in human monocytes and neutrophils. J Immunol. 1999;162(6):3590–3595. doi: 10.4049/jimmunol.162.6.3590. [DOI] [PubMed] [Google Scholar]
- 13.Whalen MM, Bankhurst AD. Effects of beta-adrenergic receptor activation, cholera toxin and forskolin on human natural killer cell function. Biochem J. 1990;272(2):327–331. doi: 10.1042/bj2720327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Xia L, Chen J, Zhang S, Martin V, Li Q, Lin S, Chen J, Calmette J, Lu M, et al. Stress–glucocorticoid–TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med. 2019;25(9):1428–1441. doi: 10.1038/s41591-019-0566-4. [DOI] [PubMed] [Google Scholar]
- 15.Hsiehchen D, Naqash AR, Espinoza M, Von Itzstein MS, Cortellini A, Ricciuti B, Owen DH, Laharwal M, Toi Y, Burke M, et al. Association between immune-related adverse event timing and treatment outcomes. Oncoimmunology. 2022;11(1):2017162. doi: 10.1080/2162402X.2021.2017162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and β-adrenoceptors. Neuroimmunomodulation. 2000;8(3):154–164. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- 17.Moretti S, Massi D, Farini V, Baroni G, Parri M, Innocenti S, Cecchi R, Chiarugi P. β-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Invest. 2013;93(3):279–290. doi: 10.1038/labinvest.2012.175. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Wu WK, Yu L, Li ZJ, Sung JJ, Zhang ST, Cho CH. Epidermal growth factor-induced esophageal cancer cell proliferation requires transactivation of β-adrenoceptors. J Pharmacol Exp Ther. 2008;326(1):69–75. doi: 10.1124/jpet.107.134528. [DOI] [PubMed] [Google Scholar]
- 19.Guo K, Ma Q, Wang L, Hu H, Li J, Zhang D. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol Rep. 2009;22(4):825–830. doi: 10.3892/or_00000505. [DOI] [PubMed] [Google Scholar]
- 20.Louault K, Porras T, Lee MH, Muthugounder S, Kennedy RJ, Blavier L, Sarte E, Fernandez GE, Yang F, Pawel BR, et al. Fibroblasts and macrophages cooperate to create a pro-tumorigenic and immune resistant environment via activation of TGF-β/IL-6 pathway in neuroblastoma. Oncoimmunology. 2022;11(1):2146860. doi: 10.1080/2162402X.2022.2146860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Partecke LI, Speerforck S, Kading A, Seubert F, Kuhn S, Lorenz E, Schwandke S, Sendler M, Keßler W, Trung DN, et al. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Int J Pancreatol. 2016;16(3):423–433. doi: 10.1016/j.pan.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Cui B, Luo Y, Tian P, Peng F, Lu J, Yang Y, Su Q, Liu B, Yu J, Luo X, et al. Stress-induced epinephrine enhances lactate dehydrogenase a and promotes breast cancer stem-like cells. J Clin Invest. 2019;129(3):1030–1046. doi: 10.1172/JCI121685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim-Fuchs C, Le CP, Pimentel MA, Shackleford D, Ferrari D, Angst E, Hollande F, Sloan EK. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun. 2014;40:40–47. doi: 10.1016/j.bbi.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angelova M, Mascaux C, Galon J. Evasion before invasion: Pre-cancer immunosurveillance. Oncoimmunology. 2021;10(1):1912250. doi: 10.1080/2162402X.2021.1912250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans E. A psychological study of cancer. Oxford, England: Dodd Mead; 1926. [Google Scholar]
- 26.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 27.Glaser R, MacCallum RC, Laskowski BF, Malarkey WB, Sheridan JF, Kiecolt-Glaser JK. Evidence for a shift in the th-1 to th-2 cytokine response associated with chronic stress and aging. J Gerontol A Biol Sci Med Sci. 2001;56(8):M477–82. doi: 10.1093/gerona/56.8.m477. [DOI] [PubMed] [Google Scholar]
- 28.Wu W, Yamaura T, Murakami K, Murata J, Matsumoto K, Watanabe H, Saiki I. Social isolation stress enhanced liver metastasis of murine colon 26-L5 carcinoma cells by suppressing immune responses in mice. Life Sci. 2000;66(19):1827–1838. doi: 10.1016/s0024-3205(00)00506-3. [DOI] [PubMed] [Google Scholar]
- 29.Wu W, Murata J, Murakami K, Yamaura T, Hayashi K, Saiki I. Social isolation stress augments angiogenesis induced by colon 26-L5 carcinoma cells in mice. Clin Exp Metastasis. 2000;18(1):1–10. doi: 10.1023/a:1026548715669. [DOI] [PubMed] [Google Scholar]
- 30.Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, Sorosky JI, De Geest K, Ritchie J, Lubaroff DM, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23(28):7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, Li Y, Li ZZ, Sun J, Li JW, Wei W, Li L, Zhang C, Huang C, Yang S-Y, et al. Chronic restraint stress promotes hepatocellular carcinoma growth by mobilizing splenic myeloid cells through activating β-adrenergic signaling. Brain Behav Immun. 2019;80:825–838. doi: 10.1016/j.bbi.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa H, Saiki I. Psychosocial stress augments tumor development through β-adrenergic activation in mice. Jpn J Cancer Res. 2002;93(7):729–735. doi: 10.1111/j.1349-7006.2002.tb01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma Y, Yang H, Kroemer G. Endogenous and exogenous glucocorticoids abolish the efficacy of immune-dependent cancer therapies. Oncoimmunology. 2020;9(1):1673635. doi: 10.1080/2162402X.2019.1673635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Y, Kroemer G. The cancer-immune dialogue in the context of stress. Nat Rev Immunol. 2023. doi: 10.1038/s41577-023-00949-8. [DOI] [PubMed] [Google Scholar]
- 35.Huang XY, Wang HC, Yuan Z, Huang J, Zheng Q. Norepinephrine stimulates pancreatic cancer cell proliferation, migration and invasion via ß-adrenergic receptor-dependent activation of P38/MAPK pathway. HGE. 2011;59(115–116):889–893. doi: 10.5754/hge11476. [DOI] [PubMed] [Google Scholar]
- 36.Bravo-Calderon DM, Assao A, Garcia NG, Coutinho-Camillo CM, Roffe M, Germano JN, Oliveira DT. Beta adrenergic receptor activation inhibits oral cancer migration and invasiveness. Arch Oral Biol. 2020;118:104865. doi: 10.1016/j.archoralbio.2020.104865. [DOI] [PubMed] [Google Scholar]
- 37.Shang ZJ, Liu K, Liang DF. Expression of β 2 -adrenergic receptor in oral squamous cell carcinoma. J Oral Pathol Med. 2009;38(4):371–376. doi: 10.1111/j.1600-0714.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Wu WK, Yu L, Sung JJ, Srivastava G, Zhang ST, Cho CH. Epinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via β-adrenoceptor-dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathway. J Cell Biochem. 2008;105(1):53–60. doi: 10.1002/jcb.21802. [DOI] [PubMed] [Google Scholar]
- 39.Kwon SY, Chun KJ, Kil HK, Jung N, Shin HA, Jang JY, Choi H, Oh K-H, Kim M-S. β2‑adrenergic receptor expression and the effects of norepinephrine and propranolol on various head and neck cancer subtypes. Oncol Lett. 2021;22(5):804. doi: 10.3892/ol.2021.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iseri ÖD, Sahin FI, Terzi YK, Yurtcu E, Erdem SR, Sarialioglu F. Beta-adrenoreceptor antagonists reduce cancer cell proliferation, invasion, and migration. Pharm Biol. 2014;52(11):1374–1381. doi: 10.3109/13880209.2014.892513. [DOI] [PubMed] [Google Scholar]
- 41.Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866–2869. [PubMed] [Google Scholar]
- 42.Wilson JM, Lorimer E, Tyburski MD, Williams CL. β-adrenergic receptors suppress Rap1B prenylation and promote the metastatic phenotype in breast cancer cells. Cancer Biol Ther. 2015;16(9):1364–1374. doi: 10.1080/15384047.2015.1070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strell C, Niggemann B, Voss MJ, Powe DG, Zanker KS, Entschladen F. Norepinephrine promotes the β1-Integrin–Mediated adhesion of MDA-MB-231 cells to vascular endothelium by the induction of a GROα release. Mol Cancer Res. 2012;10(2):197–207. doi: 10.1158/1541-7786.MCR-11-0130. [DOI] [PubMed] [Google Scholar]
- 44.Silva D, Kacprzak K, Quintas C, Goncalves J, Fresco P. Activation of β-adrenoceptors promotes lipid droplet accumulation in MCF-7 breast cancer cells via cAMP/PKA/EPAC pathways. IJMS. 2023;24(1):767. doi: 10.3390/ijms24010767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z, Gao Z, Li B, Li J, Ou Y, Yu X, Zhang Z, Liu S, Fu X, Jin H, et al. Lipid-associated macrophages in the tumor-adipose microenvironment facilitate breast cancer progression. Oncoimmunology. 2022;11(1):2085432. doi: 10.1080/2162402X.2022.2085432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Askari MD, Tsao MS, Schuller HM. The tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through β-adrenergic transactivation of EGF receptors. J Cancer Res Clin Oncol. 2005;131(10):639–648. doi: 10.1007/s00432-005-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alaskar A, Abdulraqeb Ali A, Hassan S, Shinwari Z, Alaiya A, von Holzen U, Miller L, Kulik G. Inhibition of signaling downstream of beta-2 adrenoceptor by propranolol in prostate cancer cells. The Prostate. 2023;83(3):237–245. doi: 10.1002/pros.24455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T, et al. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58(17):3761–3764. [PubMed] [Google Scholar]
- 49.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 50.Weddle DL, Tithoff P, Williams M, Schuller HM. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22(3):473–479. doi: 10.1093/carcin/22.3.473. [DOI] [PubMed] [Google Scholar]
- 51.Schuller HM. Mechanisms of smoking-related lung and pancreatic adenocarcinoma development. Nat Rev Cancer. 2002;2(6):455–463. doi: 10.1038/nrc824. [DOI] [PubMed] [Google Scholar]
- 52.Lin X, Luo K, Lv Z, Huang J. Beta-adrenoceptor action on pancreatic cancer cell proliferation and tumor growth in mice. Hepatogastroenterology. 2012;59(114):584–588. doi: 10.5754/hge11271. [DOI] [PubMed] [Google Scholar]
- 53.Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH. Nicotine promotes colon tumor growth and angiogenesis through -adrenergic activation. Toxicol Sci. 2007;97(2):279–287. doi: 10.1093/toxsci/kfm060. [DOI] [PubMed] [Google Scholar]
- 54.Wong HP, Yu L, Lam EK, Tai EK, Wu WK, Cho CH. Nicotine promotes cell proliferation via α7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol Appl Pharm. 2007;221(3):261–267. doi: 10.1016/j.taap.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Fitzpatrick AL, Daling JR, Furberg CD, Kronmal RA, Weissfeld JL. Hypertension, heart rate, use of antihypertensives, and incident prostate cancer. Ann Epidemiol. 2001;11(8):534–542. doi: 10.1016/s1047-2797(01)00246-0. [DOI] [PubMed] [Google Scholar]
- 56.Wrobel LJ, Le Gal FA. Inhibition of Human melanoma growth by a non-cardioselective β-blocker. J Invest Dermatol. 2015;135(2):525–531. doi: 10.1038/jid.2014.373. [DOI] [PubMed] [Google Scholar]
- 57.Cecilio HP, Valente VB, Pereira KM, Kayahara GM, Furuse C, Biasoli ÉR, Miyahara GI, Oliveira SHP, Bernabé DG. Beta-adrenergic blocker inhibits oral carcinogenesis and reduces tumor invasion. Cancer Chemother Pharmacol. 2020;86(5):681–686. doi: 10.1007/s00280-020-04149-2. [DOI] [PubMed] [Google Scholar]
- 58.Albinana V, Recio-Poveda L, Gonzalez-Peramato P, Martinez-Pineiro L, Botella LM, Cuesta AM. Blockade of β2-adrenergic receptor reduces inflammation and oxidative stress in clear cell renal cell carcinoma. IJMS. 2022;23(3):1325. doi: 10.3390/ijms23031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stiles JM, Amaya C, Rains S, Diaz D, Pham R, Battiste J, Modiano JF, Kokta V, Boucheron LE, Mitchell DC, et al. Targeting of beta adrenergic receptors results in therapeutic efficacy against models of hemangioendothelioma and angiosarcoma. PLoS ONE. 2013;8(3):e60021. doi: 10.1371/journal.pone.0060021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duckett MM, Phung SK, Nguyen L, Khammanivong A, Dickerson E, Dusenbery K, Lawrence J. The adrenergic receptor antagonists propranolol and carvedilol decrease bone sarcoma cell viability and sustained carvedilol reduces clonogenic survival and increases radiosensitivity in canine osteosarcoma cells. Vet Comp Oncol. 2020;18(1):128–140. doi: 10.1111/vco.12560. [DOI] [PubMed] [Google Scholar]
- 61.Rivero EM, Pinero CP, Gargiulo L, Entschladen F, Zanker K, Bruzzone A, Lüthy IA. The β2-adrenergic agonist salbutamol inhibits migration, invasion and metastasis of the Human breast cancer MDA-MB- 231 cell line. Curr Cancer Drug Targets. 2017;17(8):756–766. doi: 10.2174/1568009617666170330151415. [DOI] [PubMed] [Google Scholar]
- 62.Coelho M, Moz M, Correia G, Teixeira A, Medeiros R, Ribeiro L. Antiproliferative effects of β-blockers on human colorectal cancer cells. Oncol Rep. 2015;33(5):2513–2520. doi: 10.3892/or.2015.3874. [DOI] [PubMed] [Google Scholar]
- 63.Sakakitani S, Podyma-Inoue KA, Takayama R, Takahashi K, Ishigami-Yuasa M, Kagechika H, Harada H, Watabe T. Activation of β2-adrenergic receptor signals suppresses mesenchymal phenotypes of oral squamous cell carcinoma cells. Cancer Sci. 2021;112(1):155–167. doi: 10.1111/cas.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palm D, Lang K, Niggemann B, Drell TL, Masur K, Zaenker KS, Entschladen F. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by β-blockers. Int J Cancer. 2006;118(11):2744–2749. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 65.Lee A, Fraser SP, Djamgoz MBA. Propranolol inhibits neonatal Nav1.5 activity and invasiveness of MDA-MB-231 breast cancer cells: effects of combination with ranolazine. J Cell Physiol. 2019;234(12):23066–23081. doi: 10.1002/jcp.28868. [DOI] [PubMed] [Google Scholar]
- 66.Hajighasemi F, Hajighasemi S. Effect of propranolol on angiogenic factors in human hematopoietic cell lines in vitro. Iran Biomed J. 2009;13:223–228. [PubMed] [Google Scholar]
- 67.Zhang D, Ma QY, Hu HT, Zhang M. β 2 -adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NF-κB and AP-1. Cancer Biol Ther. 2010;10(1):19–29. doi: 10.4161/cbt.10.1.11944. [DOI] [PubMed] [Google Scholar]
- 68.Xie WY, He RH, Zhang J, He YJ, Wan Z, Zhou CF, Tang Y, Li Z, Mcleod H, Liu J, et al. β‑blockers inhibit the viability of breast cancer cells by regulating the ERK/COX‑2 signaling pathway and the drug response is affected by ADRB2 single‑nucleotide polymorphisms. Oncol Rep. 2018;41(1):341–350. doi: 10.3892/or.2018.6830. [DOI] [PubMed] [Google Scholar]
- 69.Chin CC, Li JM, Lee KF, Huang YC, Wang KC, Lai HC, Cheng C-C, Kuo Y-H, Shi C-S. Selective β2-AR blockage suppresses colorectal cancer growth through regulation of EGFR-Akt/ERK1/2 signaling, G1-phase arrest, and apoptosis. J Cell Physiol. 2016;231(2):459–472. doi: 10.1002/jcp.25092. [DOI] [PubMed] [Google Scholar]
- 70.Koh M, Takahashi T, Kurokawa Y, Kobayashi T, Saito T, Ishida T, Serada S, Fujimoto M, Naka T, Wada N, et al. Propranolol suppresses gastric cancer cell growth by regulating proliferation and apoptosis. Gastric Cancer. 2021;24(5):1037–1049. doi: 10.1007/s10120-021-01184-7. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X, Zhang Y, He Z, Yin K, Li B, Zhang L, Xu Z. Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Disease. 2019;10(11):788. doi: 10.1038/s41419-019-2030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liao C. The β-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor κB signaling. Oncol Rep. 2010;24(6):1669–1676. doi: 10.3892/or_00001032. [DOI] [PubMed] [Google Scholar]
- 73.Wang F, Liu H, Wang F, Xu R, Wang P, Tang F, Zhang X, Zhu Z, Lv H, Han T, et al. Propranolol suppresses the proliferation and induces the apoptosis of liver cancer cells. Mol Med Rep. 2018;17(4):5213–5221. doi: 10.3892/mmr.2018.8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Solerno LM, Sobol NT, Gottardo MF, Capobianco CS, Ferrero MR, Vasquez L, Alonso DF, Garona J. Propranolol blocks osteosarcoma cell cycle progression, inhibits angiogenesis and slows xenograft growth in combination with cisplatin-based chemotherapy. Sci Rep. 2022;12(1):15058. doi: 10.1038/s41598-022-18324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang D, Ma Q, Wang Z, Zhang M, Guo K, Wang F, Wu E. β2-adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFκB pathway. Mol Cancer. 2011;10(1):146. doi: 10.1186/1476-4598-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cakir Y, Plummer HK 3rd, Tithof PK, Schuller HM. Beta-adrenergic and arachidonic acid-mediated growth regulation of human breast cancer cell lines. Int J Oncol. 2002;21(1):153–157. doi: 10.3892/ijo.21.1.153. [DOI] [PubMed] [Google Scholar]
- 77.Lu K, Bhat M, Peters S, Mitra R, Oberyszyn T, Basu S. Suppression of beta 2 adrenergic receptor actions prevent UVB mediated cutaneous squamous cell tumorigenesis through inhibition of VEGF-A induced angiogenesis. Mol Carcinog. 2021;60(3):172–178. doi: 10.1002/mc.23281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bustamante P, Miyamoto D, Goyeneche A, de Alba Graue PG, Jin E, Tsering T, Dias AB, Burnier MN, Burnier JV. Beta-blockers exert potent anti-tumor effects in cutaneous and uveal melanoma. Cancer Med. 2019;8(17):7265–7277. doi: 10.1002/cam4.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mele L, Del Vecchio V, Marampon F, Regad T, Wagner S, Mosca L, Bimonte S, Giudice A, Liccardo D, Prisco C, et al. β2-AR blockade potentiates MEK1/2 inhibitor effect on HNSCC by regulating the Nrf2-mediated defense mechanism. Cell Death Disease. 2020;11(10):850. doi: 10.1038/s41419-020-03056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu FQ, Fang T, Yu LX, Lv GS, Lv HW, Liang D, Li T, Wang C-Z, Tan Y-X, Ding J, et al. ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1α. J Hepatol. 2016;65(2):314–324. doi: 10.1016/j.jhep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 81.Barathova M, Grossmannova K, Belvoncikova P, Kubasova V, Simko V, Skubla R, Csaderova L, Pastorek J. Impairment of hypoxia-induced CA IX by beta-blocker Propranolol—impact on progression and metastatic potential of colorectal cancer cells. IJMS. 2020;21(22):8760. doi: 10.3390/ijms21228760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kavakcioglu Yardimci B, Geyikoglu F, Aysin F, Koc K, Simsek Ozek N, Kucukatay V. The cytotoxic and apoptotic effects of beta-blockers with different selectivity on cancerous and healthy lung cell lines. Mol Biol Rep. 2021;48(5):4009–4019. doi: 10.1007/s11033-021-06409-7. [DOI] [PubMed] [Google Scholar]
- 83.Gong L, Lei Y, Tan X, Dong Y, Luo Z, Zhang D, Han S. Propranolol selectively inhibits cervical cancer cell growth by suppressing the cGMP/PKG pathway. Biomed Pharmacother. 2019;111:1243–1248. doi: 10.1016/j.biopha.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 84.Albinana V, Gallardo-Vara E, de Rojas PI, Recio-Poveda L, Aguado T, Canto-Cano A, Aguirre DT, Serra MM, González-Peramato P, Martínez-Piñeiro L, et al. Targeting β2-adrenergic receptors shows therapeutical benefits in clear cell renal cell carcinoma from Von Hippel–Lindau disease. JCM. 2020;9(9):2740. doi: 10.3390/jcm9092740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng JS, Huang CC, Chou CT, Jan CR. Mechanisms of carvedilol-induced [Ca2+] i rises and death in human hepatoma cells. Naunyn-Schmiedeberg’s Arch Pharmacol. 2007;376(3):185–194. doi: 10.1007/s00210-007-0191-5. [DOI] [PubMed] [Google Scholar]
- 86.Dal Monte M, Casini G, Filippi L, Nicchia GP, Svelto M, Bagnoli P. Functional involvement of β3-adrenergic receptors in melanoma growth and vascularization. J Mol Med. 2013;91(12):1407–1419. doi: 10.1007/s00109-013-1073-6. [DOI] [PubMed] [Google Scholar]
- 87.Hsieh YD, Chi CC, Chou CT, Cheng JS, Kuo CC, Liang WZ, Lin K-L, Tseng L-L, Jan C-R. Investigation of carvedilol-evoked Ca 2+ movement and death in human oral cancer cells. J Recept Signal Transduct Res. 2011;31(3):220–228. doi: 10.3109/10799893.2011.577785. [DOI] [PubMed] [Google Scholar]
- 88.Zhang D, Ma Q, Shen S, Hu H. Inhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction: the study of beta-adrenoceptor antagonist’s anticancer effect in pancreatic cancer cell. Pancreas. 2009;38(1):94–100. doi: 10.1097/MPA.0b013e318184f50c. [DOI] [PubMed] [Google Scholar]
- 89.Ozler S, Pazarci P. Anti-tumoral effect of beta-blockers on prostate and bladder cancer cells via mitogen-activated protein kinase pathways. Anticancer Drugs. 2022;33(4):384–388. doi: 10.1097/CAD.0000000000001271. [DOI] [PubMed] [Google Scholar]
- 90.Sidorova M, Petrikaite V. The effect of beta adrenoreceptor blockers on viability and cell colony formation of non-small cell lung cancer cell lines A549 and H1299. Molecules. 2022;27(6):1938. doi: 10.3390/molecules27061938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bezu L, Sauvat A, Humeau J, Leduc M, Kepp O, Kroemer G. eIf2α phosphorylation: a hallmark of immunogenic cell death. Oncoimmunology. 2018;7(6):e1431089. doi: 10.1080/2162402X.2018.1431089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salvagno C, Mandula JK, Rodriguez PC, Cubillos-Ruiz JR. Decoding endoplasmic reticulum stress signals in cancer cells and antitumor immunity. Trends Cancer. 2022;8(11):930–943. doi: 10.1016/j.trecan.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kamal Y, Dwan D, Hoehn HJ, Sanz-Pamplona R, Alonso MH, Moreno V, Cheng C, Schell MJ, Kim Y, Felder SI, et al. Tumor immune infiltration estimated from gene expression profiles predicts colorectal cancer relapse. Oncoimmunology. 2021;10(1):1862529. doi: 10.1080/2162402X.2020.1862529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bouaoud J, Foy JP, Tortereau A, Michon L, Lavergne V, Gadot N, Boyault S, Valantin J, De Souza G, Zrounba P, et al. Early changes in the immune microenvironment of oral potentially malignant disorders reveal an unexpected association of M2 macrophages with oral cancer free survival. Oncoimmunology. 2021;10(1):1944554. doi: 10.1080/2162402X.2021.1944554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Wadei HA, Al-Wadei MH, Schuller HM. Prevention of pancreatic cancer by the beta-blocker propranolol. Anticancer Drugs. 2009;20(6):477–482. doi: 10.1097/CAD.0b013e32832bd1e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liao P, Song K, Zhu Z, Liu Z, Zhang W, Li W, Hu J, Hu Q, Chen C, Chen B, et al. Propranolol suppresses the growth of colorectal cancer through simultaneously activating autologous CD8 + T cells and inhibiting tumor AKT/MAPK pathway. Clin Pharmacol Ther. 2020;108(3):606–615. doi: 10.1002/cpt.1894. [DOI] [PubMed] [Google Scholar]
- 97.Montoya A, Amaya CN, Belmont A, Diab N, Trevino R, Villanueva G, Rains S, Sanchez LA, Badri N, Otoukesh S, et al. Use of non-selective β-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget. 2017;8(4):6446–6460. doi: 10.18632/oncotarget.14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou C, Chen X, Zeng W, Peng C, Huang G, Li X, Ouyang Z, Luo Y, Xu X, Xu B, et al. Propranolol induced G0/G1/S phase arrest and apoptosis in melanoma cells via AKT/MAPK pathway. Oncotarget. 2016;7(42):68314–68327. doi: 10.18632/oncotarget.11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li H, Peyser ND, Zeng Y, Ha PK, Johnson DE, Grandis JR. Nsaids overcome PIK3CA mutation-mediated resistance to EGFR inhibition in head and neck cancer preclinical models. Cancers Basel. 2022;14(3):506. doi: 10.3390/cancers14030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shin VY, Wu WK, Chu KM, Koo MW, Wong HP, Lam EK, Tai EKK, Cho CH. Functional role of -adrenergic receptors in the mitogenic action of nicotine on gastric cancer cells. Toxicol Sci. 2006;96(1):21–29. doi: 10.1093/toxsci/kfl118. [DOI] [PubMed] [Google Scholar]
- 101.Jin Z, Gao F, Flagg T, Deng X. Nicotine induces multi-site phosphorylation of bad in association with suppression of apoptosis. J Biol Chem. 2004;279(22):23837–23844. doi: 10.1074/jbc.M402566200. [DOI] [PubMed] [Google Scholar]
- 102.Stanojkovic TP, Zizak Z, Mihailovic-Stanojevic N, Petrovic T, Juranic Z. Inhibition of proliferation on some neoplastic cell lines-act of carvedilol and captopril. J Exp Clin Cancer Res. 2005;24:387–395. [PubMed] [Google Scholar]
- 103.Liu CP, Jan CR. Effect of carvedilol on Ca2+ movement and cytotoxicity in human MG63 osteosarcoma cells. Basic Clin Pharmacol Toxicol. 2004;95(2):59–65. doi: 10.1111/j.1742-7843.2004.950203.x. [DOI] [PubMed] [Google Scholar]
- 104.Dezong G, Zhongbing M, Qinye F, Zhigang Y. Carvedilol suppresses migration and invasion of malignant breast cells by inactivating src involving cAMP/PKA and PKCδ signaling pathway. J Cancer Res Ther. 2014;10(4):991–1003. doi: 10.4103/0973-1482.137664. [DOI] [PubMed] [Google Scholar]
- 105.Chang A, Yeung S, Thakkar A, Huang KM, Liu MM, Kanassatega RS, Parsa C, Orlando R, Jackson EK, Andresen BT, et al. Prevention of Skin Carcinogenesis by the β-Blocker Carvedilol. Cancer Prev Res. 2015;8(1):27–36. doi: 10.1158/1940-6207.CAPR-14-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sereni F, Dal Monte M, Filippi L, Bagnoli P. Role of host β1- and β2-adrenergic receptors in a murine model of B16 melanoma: functional involvement of β3-adrenergic receptors. Naunyn-Schmiedeberg’s Arch Pharmacol. 2015;388(12):1317–1331. doi: 10.1007/s00210-015-1165-7. [DOI] [PubMed] [Google Scholar]
- 107.Calvani M, Cavallini L, Tondo A, Spinelli V, Ricci L, Pasha A, Bruno G, Buonvicino D, Bigagli E, Vignoli M, et al. β 3-adrenoreceptors control mitochondrial dormancy in melanoma and embryonic stem cells. Oxid Med Cell Longev. 2018;2018:1–10. doi: 10.1155/2018/6816508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bruno G, Cencetti F, Pini A, Tondo A, Cuzzubbo D, Fontani F, Strinna V, Buccoliero AM, Casazza G, Donati C, et al. β3-adrenoreceptor blockade reduces tumor growth and increases neuronal differentiation in neuroblastoma via SK2/S1P2 modulation. Oncogene. 2020;39(2):368–384. doi: 10.1038/s41388-019-0993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deng J, Jiang P, Yang T, Huang M, Qi W, Zhou T, Yang Z, Zou Y, Gao G, Yang X, et al. Targeting β3-adrenergic receptor signaling inhibits neuroblastoma cell growth via suppressing the mTOR pathway. Biochem Bioph Res Co. 2019;514(1):295–300. doi: 10.1016/j.bbrc.2019.04.099. [DOI] [PubMed] [Google Scholar]
- 110.Chen H, Liu D, Guo L, Cheng X, Guo N, Shi M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating β-adrenergic signaling. J Pathol. 2018;244(1):49–60. doi: 10.1002/path.4988. [DOI] [PubMed] [Google Scholar]
- 111.Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a β-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19(2):114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 112.Yonekura S, Terrisse S, Alves Costa Silva C, Lafarge A, Iebba V, Ferrere G, Goubet A-G, Fahrner J-E, Lahmar I, Ueda K, et al. Cancer induces a stress ileopathy depending on β-adrenergic receptors and promoting dysbiosis that contributes to carcinogenesis. Cancer Discov. 2022;12(4):1128–1151. doi: 10.1158/2159-8290.CD-21-0999. [DOI] [PubMed] [Google Scholar]
- 113.Lamkin DM, Sloan EK, Patel AJ, Chiang BS, Pimentel MA, Ma JC, Arevalo JM, Morizono K, Cole SW. Chronic stress enhances progression of acute lymphoblastic leukemia via β-adrenergic signaling. Brain Behav Immun. 2012;26(4):635–641. doi: 10.1016/j.bbi.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mohammadpour H, MacDonald CR, Qiao G, Chen M, Dong B, Hylander BL, McCarthy PL, Abrams SI, Repasky EA. β2 adrenergic receptor–mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest. 2019;129(12):5537–5552. doi: 10.1172/JCI129502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jean Wrobel L, Bod L, Lengagne R, Kato M, Prevost-Blondel A, Le Gal FA. Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget. 2016;7(47):77825–77837. doi: 10.18632/oncotarget.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.An J, Feng L, Ren J, Li Y, Li G, Liu C, Yao Y, Yao Y, Jiang Z, Gao Y, et al. Chronic stress promotes breast carcinoma metastasis by accumulating myeloid-derived suppressor cells through activating β-adrenergic signaling. Oncoimmunology. 2021;10(1):2004659. doi: 10.1080/2162402X.2021.2004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kato T, Fukushima H, Furusawa A, Okada R, Wakiyama H, Furumoto H, Okuyama S, Takao S, Choyke PL, Kobayashi H, et al. Selective depletion of polymorphonuclear myeloid derived suppressor cells in tumor beds with near infrared photoimmunotherapy enhances host immune response. Oncoimmunology. 2022;11(1):2152248. doi: 10.1080/2162402X.2022.2152248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tang Y, Zhou C, Li Q, Cheng X, Huang T, Li F, He L, Zhang B, Tu S. Targeting depletion of myeloid-derived suppressor cells potentiates PD-L1 blockade efficacy in gastric and colon cancers. Oncoimmunology. 2022;11(1):2131084. doi: 10.1080/2162402X.2022.2131084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cho H, Kim JE, Hong YS, Kim SY, Kim J, Ryu YM, Kim S-Y, Kim TW. Comprehensive evaluation of the tumor immune microenvironment and its dynamic changes in patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy: from the phase II ADORE study. Oncoimmunology. 2022;11(1):2148374. doi: 10.1080/2162402X.2022.2148374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barnestein R, Galland L, Kalfeist L, Ghiringhelli F, Ladoire S, Limagne E. Immunosuppressive tumor microenvironment modulation by chemotherapies and targeted therapies to enhance immunotherapy effectiveness. Oncoimmunology. 2022;11(1):2120676. doi: 10.1080/2162402X.2022.2120676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qiao G, Chen M, Mohammadpour H, MacDonald CR, Bucsek MJ, Hylander BL, Barbi JJ, Repasky EA. Chronic adrenergic stress contributes to metabolic dysfunction and an Exhausted phenotype in T cells in the tumor microenvironment. Cancer Immunol Res. 2021;9(6):651–664. doi: 10.1158/2326-6066.CIR-20-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B, Liu H, Kokolus KM, Eng JWL, Messmer MN, et al. β-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8+ T cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017;77(20):5639–5651. doi: 10.1158/0008-5472.CAN-17-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Calvani M, Bruno G, Dabraio A, Subbiani A, Bianchini F, Fontani F, Casazza G, Vignoli M, De Logu F, Frenos S, et al. β3-adrenoreceptor blockade induces stem cells differentiation in melanoma microenvironment. IJMS. 2020;21(4):1420. doi: 10.3390/ijms21041420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen M, Qiao G, Hylander BL, Mohammadpour H, Wang XY, Subjeck JR, Singh AK, Repasky EA. Adrenergic stress constrains the development of anti-tumor immunity and abscopal responses following local radiation. Nat Commun. 2020;11(1):1821. doi: 10.1038/s41467-020-15676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liao X, Chaudhary P, Qiu G, Che X, Fan L. The role of propranolol as a radiosensitizer in gastric cancer treatment. Drug Des Devel Ther. 2018;12:639–645. doi: 10.2147/DDDT.S160865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liao X, Che X, Zhao W, Zhang D, Long H, Chaudhary P, LI H. Effects of propranolol in combination with radiation on apoptosis and survival of gastric cancer cells in vitro. Radiat Oncol. 2010;5(1):98. doi: 10.1186/1748-717X-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Farhoumand LS, Fiorentzis M, Kraemer MM, Sak A, Stuschke M, Rassaf T, Hendgen-Cotta U, Bechrakis NE, Berchner-Pfannschmidt U. The adrenergic receptor antagonist carvedilol elicits anti-tumor responses in uveal melanoma 3D tumor spheroids and May serve as co-adjuvant therapy with radiation. Cancers Basel. 2022;14(13):3097. doi: 10.3390/cancers14133097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pasquier E, Ciccolini J, Carre M, Giacometti S, Fanciullino R, Pouchy C, Montero M-P, Serdjebi C, Kavallaris M, André N, et al. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget. 2011;2(10):797–809. doi: 10.18632/oncotarget.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Calvani M, Dabraio A, Bruno G, De Gregorio V, Coronnello M, Bogani C, Ciullini S, Marca GL, Vignoli M, Chiarugi P, et al. β3-adrenoreceptor blockade reduces hypoxic myeloid leukemic cells survival and Chemoresistance. IJMS. 2020;21(12):4210. doi: 10.3390/ijms21124210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wolter JK, Wolter NE, Blanch A, Partridge T, Cheng L, Morgenstern DA, Podkowa M, Kaplan DR, Irwin MS. Anti-tumor activity of the beta-adrenergic receptor antagonist propranolol in neuroblastoma. Oncotarget. 2014;5(1):161–172. doi: 10.18632/oncotarget.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pasquier E, Street J, Pouchy C, Carre M, Gifford AJ, Murray J, Norris MD, Trahair T, Andre N, Kavallaris M, et al. β-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br J Cancer. 2013;108(12):2485–2494. doi: 10.1038/bjc.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shibuya CM, Tjioe KC, Oliveira SHP, Bernabe DG. Propranolol inhibits cell viability and expression of the pro-tumorigenic proteins Akt, NF-ĸB, and VEGF in oral squamous cell carcinoma. Arch Oral Biol. 2022;136:105383. doi: 10.1016/j.archoralbio.2022.105383. [DOI] [PubMed] [Google Scholar]
- 133.Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, Maurer HC, Chen X, Jiang Z, Westphalen CB, et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;34(5):863–867. doi: 10.1016/j.ccell.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shan T, Ma Q, Zhang D, Guo K, Liu H, Wang F, Wu E. β2-adrenoceptor blocker synergizes with gemcitabine to inhibit the proliferation of pancreatic cancer cells via apoptosis induction. Eur J Pharmacol. 2011;665(1–3):1–7. doi: 10.1016/j.ejphar.2011.04.055. [DOI] [PubMed] [Google Scholar]
- 135.Wei WJ, Shen CT, Song HJ, Qiu ZL, Luo QY. Propranolol sensitizes thyroid cancer cells to cytotoxic effect of vemurafenib. Oncol Rep. 2016;36(3):1576–1584. doi: 10.3892/or.2016.4918. [DOI] [PubMed] [Google Scholar]
- 136.Kuang X, Qi M, Peng C, Zhou C, Su J, Zeng W, Liu H, Zhang J, Chen M, Shen M, et al. Propranolol enhanced the anti-tumor effect of sunitinib by inhibiting proliferation and inducing G0/G1/S phase arrest in malignant melanoma. Oncotarget. 2018;9(1):802–811. doi: 10.18632/oncotarget.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kokolus KM, Zhang Y, Sivik JM, Schmeck C, Zhu J, Repasky EA, Drabick JJ, Schell TD. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology. 2018;7(3):e1405205. doi: 10.1080/2162402X.2017.1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shirinbak S, Chan RY, Shahani S, Muthugounder S, Kennedy R, Hung LT, Fernandez GE, Hadjidaniel MD, Moghimi B, Sheard MA, et al. Combined immune checkpoint blockade increases CD8+CD28+PD-1+ effector T cells and provides a therapeutic strategy for patients with neuroblastoma. Oncoimmunology. 2021;10(1):1838140. doi: 10.1080/2162402X.2020.1838140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carretero-Gonzalez A, Otero I, Lora D, Carril-Ajuria L, Castellano D, de Velasco G. Efficacy and safety of anti-PD-1/PD-L1 combinations versus standard of care in cancer: a systematic review and meta-analysis. Oncoimmunology. 2021;10(1):1878599. doi: 10.1080/2162402X.2021.1878599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Smolle MA, Herbsthofer L, Goda M, Granegger B, Brcic I, Bergovec M, Scheipl S, Prietl B, El-Heliebi A, Pichler M, et al. Influence of tumor-infiltrating immune cells on local control rate, distant metastasis, and survival in patients with soft tissue sarcoma. Oncoimmunology. 2021;10(1):1896658. doi: 10.1080/2162402X.2021.1896658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boesch M, Baty F, Albrich WC, Flatz L, Rodriguez R, Rothschild SI, Joerger M, Früh M, Brutsche MH. Local tumor microbial signatures and response to checkpoint blockade in non-small cell lung cancer. Oncoimmunology. 2021;10(1):1988403. doi: 10.1080/2162402X.2021.1988403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goedegebuure RSA, Harrasser M, de Klerk LK, van Schooten TS, van Grieken NCT, Eken M, Grifhorst MS, Pocorni N, Jordanova ES, van Berge Henegouwen MI, et al. Pre-treatment tumor-infiltrating T cells influence response to neoadjuvant chemoradiotherapy in esophageal adenocarcinoma. Oncoimmunology. 2021;10(1):1954807. doi: 10.1080/2162402X.2021.1954807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Daher C, Vimeux L, Stoeva R, Peranzoni E, Bismuth G, Wieduwild E, Lucas B, Donnadieu E, Bercovici N, Trautmann A, et al. Blockade of β-adrenergic receptors improves CD8+ T-cell priming and cancer vaccine efficacy. Cancer Immunol Res. 2019;7(11):1849–1863. doi: 10.1158/2326-6066.CIR-18-0833. [DOI] [PubMed] [Google Scholar]
- 144.Rico M, Baglioni M, Bondarenko M, Laluce NC, Rozados V, Andre N, Carré M, Scharovsky OG, Márquez MM. Metformin and propranolol combination prevents cancer progression and metastasis in different breast cancer models. Oncotarget. 2017;8(2):2874–2889. doi: 10.18632/oncotarget.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xia W, Qi X, Li M, Wu Y, Sun L, Fan X, Yuan Y, Li J. Metformin promotes anticancer activity of NK cells in a p38 MAPK dependent manner. Oncoimmunology. 2021;10(1):1995999. doi: 10.1080/2162402X.2021.1995999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sorski L, Melamed R, Matzner P, Lavon H, Shaashua L, Rosenne E, Ben-Eliyahu S. Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through β-adrenoceptors blockade and COX2 inhibition. Brain Behav Immun. 2016;58:91–98. doi: 10.1016/j.bbi.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, Ben-Eliyahu S. Perioperative use of β-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15(7):2042–2052. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, Meiboom H, Ben-Eliyahu S. Improving survival rates in two models of spontaneous Postoperative metastasis in mice by Combined administration of a β-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184(5):2449–2457. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- 149.Brohee L, Peulen O, Nusgens B, Castronovo V, Thiry M, Colige AC, Deroanne CF. Propranolol sensitizes prostate cancer cells to glucose metabolism inhibition and prevents cancer progression. Sci Rep. 2018;8(1):7050. doi: 10.1038/s41598-018-25340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nanda A, Ahmed O, Bir S, Bollam P, Kalakoti P. Elucidating the role of incidental use of beta-blockers in patients with metastatic brain tumors in controlling tumor progression and survivability. Neurol India. 2015;63(1):19–23. doi: 10.4103/0028-3886.152625. [DOI] [PubMed] [Google Scholar]
- 151.Spera G, Fresco R, Fung H, Dyck JRB, Pituskin E, Paterson I, Mackey JR. Beta blockers and improved progression-free survival in patients with advanced HER2 negative breast cancer: a retrospective analysis of the ROSE/TRIO-012 study. Ann Oncol : Off J Eur Soc Medi Oncol. 2017;28(8):1836–1841. doi: 10.1093/annonc/mdx264. [DOI] [PubMed] [Google Scholar]
- 152.Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1(7):628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hicks BM, Murray LJ, Powe DG, Hughes CM, Cardwell CR. β-blocker usage and colorectal cancer mortality: a nested case–control study in the UK clinical practice research Datalink cohort. Ann Oncol : Off J Eur Soc Medi Oncol. 2013;24(12):3100–3106. doi: 10.1093/annonc/mdt381. [DOI] [PubMed] [Google Scholar]
- 154.He X, Zhao Z, Jiang X, Sun Y. Non-selective beta-blockers and the incidence of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Front Pharmacol. 2023;14:1216059. doi: 10.3389/fphar.2023.1216059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang HM, Liao ZX, Komaki R, Welsh JW, O’Reilly MS, Chang JY, Zhuang Y, Levy LB, Lu C, Gomez DR, et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol : Off J Eur Soc Medi Oncol. 2013;24(5):1312–1319. doi: 10.1093/annonc/mds616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.De Giorgi V, Grazzini M, Gandini S, Benemei S, Lotti T, Marchionni N, Geppetti P. Treatment with β-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med. 2011;171(8):779–781. doi: 10.1001/archinternmed.2011.131. [DOI] [PubMed] [Google Scholar]
- 157.Spilsbury K, Tuesley KM, Pearson SA, Coory MD, Donovan P, Steer CB, Stewart LM, Pandeya N, Jordan SJ. Perioperative beta-blocker supply and survival in women with epithelial ovarian cancer and a history of cardiovascular Conditions. J Clin Oncol. 2023;41(2):266–275. doi: 10.1200/JCO.22.00097. [DOI] [PubMed] [Google Scholar]
- 158.Al-Niaimi A, Dickson EL, Albertin C, Karnowski J, Niemi C, Spencer R, Shahzad MMK, Uppal S, Saha S, Rice L, et al. The impact of perioperative β blocker use on patient outcomes after primary cytoreductive surgery in high-grade epithelial ovarian carcinoma. Gynecol Oncol. 2016;143(3):521–525. doi: 10.1016/j.ygyno.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 159.Sivanesan S, Tasken KA, Grytli HH. Association of β-blocker use at time of Radical prostatectomy with rate of treatment for prostate cancer recurrence. JAMA Netw Open. 2022;5(1):e2145230. doi: 10.1001/jamanetworkopen.2021.45230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Monami M, Filippi L, Ungar A, Sgrilli F, Antenore A, Dicembrini I, Bagnoli P, Marchionni N, Rotella CM, Mannucci E, et al. Further data on beta-blockers and cancer risk: observational study and meta-analysis of randomized clinical trials. Curr Med Res Opin. 2013;29(4):369–378. doi: 10.1185/03007995.2013.772505. [DOI] [PubMed] [Google Scholar]
- 161.Zhong S, Yu D, Zhang X, Chen X, Yang S, Tang J, Zhao J, Wang S. β-blocker use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Eur J Cance Prev. 2016;25(5):440–448. doi: 10.1097/CEJ.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 162.Yan X, Liu P, Li D, Hu R, Tao M, Zhu S, Wu W, Yang M, Qu X. Novel evidence for the prognostic impact of β-blockers in solid cancer patients receiving immune checkpoint inhibitors. Int Immunopharmacol. 2022;113(Pt A):109383. doi: 10.1016/j.intimp.2022.109383. [DOI] [PubMed] [Google Scholar]
- 163.Heitz F, du Bois A, Harter P, Lubbe D, Kurzeder C, Vergote I, Plante M, Pfisterer J. Impact of beta blocker medication in patients with platinum sensitive recurrent ovarian cancer—a combined analysis of 2 prospective multicenter trials by the AGO study group, NCIC-CTG and EORTC-GCG. Gynecol Oncol. 2013;129(3):463–466. doi: 10.1016/j.ygyno.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 164.Kennedy OJ, Kicinski M, Valpione S, Gandini S, Suciu S, Blank CU, Long GV, Atkinson VG, Dalle S, Haydon AM, et al. Prognostic and predictive value of β-blockers in the EORTC 1325/KEYNOTE-054 phase III trial of pembrolizumab versus placebo in resected high-risk stage III melanoma. Eur J Cancer. 2022;165:97–112. doi: 10.1016/j.ejca.2022.01.017. [DOI] [PubMed] [Google Scholar]
- 165.Hsieh HH, Wu TY, Chen CH, Kuo YH, Hour MJ. Survival outcomes of beta-blocker usage in HER2-positive advanced breast cancer patients: a retrospective cohort study. Ther Adv Drug Saf. 2023;14:20420986231181338. doi: 10.1177/20420986231181338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Knight JM, Kerswill SA, Hari P, Cole SW, Logan BR, D’Souza A, Shah NN, Horowitz MM, Stolley MR, Sloan EK, et al. Repurposing existing medications as cancer therapy: design and feasibility of a randomized pilot investigating propranolol administration in patients receiving hematopoietic cell transplantation. BMC Cancer. 2018;18(1):593. doi: 10.1186/s12885-018-4509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ramondetta LM, Hu W, Thaker PH, Urbauer DL, Chisholm GB, Westin SN, Sun Y, Ramirez PT, Fleming N, Sahai SK, et al. Prospective pilot trial with combination of propranolol with chemotherapy in patients with epithelial ovarian cancer and evaluation on circulating immune cell gene expression. Gynecol Oncol. 2019;154(3):524–530. doi: 10.1016/j.ygyno.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hiller JG, Cole SW, Crone EM, Byrne DJ, Shackleford DM, Pang JMB, Henderson MA, Nightingale SS, Ho KM, Myles PS, et al. Preoperative β-blockade with Propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin Cancer Res. 2020;26(8):1803–1811. doi: 10.1158/1078-0432.CCR-19-2641. [DOI] [PubMed] [Google Scholar]
- 169.De Giorgi V, Grazzini M, Benemei S, Marchionni N, Botteri E, Pennacchioli E, Geppetti P, Gandini S. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 2018;4(2):e172908. doi: 10.1001/jamaoncol.2017.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hopson MB, Lee S, Accordino M, Trivedi M, Maurer M, Crew KD, Hershman DL, Kalinsky K. Phase II study of propranolol feasibility with neoadjuvant chemotherapy in patients with newly diagnosed breast cancer. Breast Cancer Res Treat. 2021;188(2):427–432. doi: 10.1007/s10549-021-06210-x. [DOI] [PubMed] [Google Scholar]
- 171.Gandhi S, Pandey MR, Attwood K, Ji W, Witkiewicz AK, Knudsen ES, Allen C, Tario JD, Wallace PK, Cedeno CD, et al. Phase I clinical trial of combination Propranolol and pembrolizumab in locally advanced and metastatic melanoma: safety, tolerability, and preliminary evidence of antitumor activity. Clin Cancer Res. 2021;27(1):87–95. doi: 10.1158/1078-0432.CCR-20-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shaashua L, Shabat-Simon M, Haldar R, Matzner P, Zmora O, Shabtai M, Sharon E, Allweis T, Barshack I, Hayman L, et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res. 2017;23(16):4651–4661. doi: 10.1158/1078-0432.CCR-17-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou L, Li Y, Li X, Chen G, Liang H, Wu Y, Tong J, Ouyang W. Propranolol attenuates surgical stress–induced Elevation of the regulatory T cell response in patients undergoing radical mastectomy. J Immunol. 2016;196(8):3460–3469. doi: 10.4049/jimmunol.1501677. [DOI] [PubMed] [Google Scholar]
- 174.Haldar R, Ricon-Becker I, Radin A, Gutman M, Cole SW, Zmora O, Ben‐Eliyahu S. Perioperative COX2 and β-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: a randomized controlled trial. Cancer. 2020;126(17):3991–4001. doi: 10.1002/cncr.32950. [DOI] [PubMed] [Google Scholar]
- 175.Bertucci F, Boudin L, Finetti P, Van Berckelaer C, Van Dam P, Dirix L, Viens P, Gonçalves A, Ueno NT, Van Laere S, et al. Immune landscape of inflammatory breast cancer suggests vulnerability to immune checkpoint inhibitors. Oncoimmunology. 2021;10(1):1929724. doi: 10.1080/2162402X.2021.1929724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Matarrese P, Maccari S, Ascione B, Vona R, Vezzi V, Stati T, Grò MC, Marano G, Ambrosio C, Molinari P. Crosstalk between β2- and α2-adrenergic receptors in the regulation of B16F10 melanoma cell proliferation. IJMS. 2022;23(9):4634. doi: 10.3390/ijms23094634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Khabbazi S, Hassanshahi M, Hassanshahi A, Peymanfar Y, Su YW, Xian CJ. Opioids and matrix metalloproteinases: the influence of morphine on MMP-9 production and cancer progression. Naunyn-Schmiedeberg’s Arch Pharmacol. 2019;392(2):123–133. doi: 10.1007/s00210-019-01613-6. [DOI] [PubMed] [Google Scholar]
- 178.Liu Z, Cheng S, Fu G, Ji F, Wang C, Cao M. Postoperative administration of ketorolac averts morphine-induced angiogenesis and metastasis in triple-negative breast cancer. Life Sci. 2020;251:117604. doi: 10.1016/j.lfs.2020.117604. [DOI] [PubMed] [Google Scholar]
- 179.Liu S, Qin Z, Mao Y, Zhang W, Wang Y, Jia L, Peng X. Therapeutic Targeting of MYC in head and neck squamous cell carcinoma. Oncoimmunology. 2022;11(1):2130583. doi: 10.1080/2162402X.2022.2130583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Desmedt C, Demicheli R, Fornili M, Bachir I, Duca M, Viglietti G, Berlière M, Piccart M, Sotiriou C, Sosnowski M, et al. Potential Benefit of Intra-operative administration of ketorolac on breast cancer recurrence according to the patient’s body Mass index. JNCI J Nat Cancer Inst. 2018;110(10):1115–1122. doi: 10.1093/jnci/djy042. [DOI] [PubMed] [Google Scholar]
- 181.Forget P, Vandenhende J, Berliere M, Machiels JP, Nussbaum B, Legrand C, De Kock M. Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg. 2010;110(6):1630–1635. doi: 10.1213/ANE.0b013e3181d2ad07. [DOI] [PubMed] [Google Scholar]
- 182.Forget P, Bentin C, Machiels JP, Berliere M, Coulie PG, De Kock M. Intraoperative use of ketorolac or diclofenac is associated with improved disease-free survival and overall survival in conservative breast cancer surgery. British Journal Of Anaesthesia. 2014;113(Suppl 1):i82–7. doi: 10.1093/bja/aet464. [DOI] [PubMed] [Google Scholar]
- 183.Sebastian NT, Stokes WA, Behera M, Jiang R, Gutman DA, Huang Z, Burns A, Sukhatme V, Lowe MC, Ramalingam SS, et al. The Association of improved overall survival with NSAIDs in non–small cell lung cancer patients receiving immune checkpoint inhibitors. Clin Lung Cancer. 2023;24(3):287–294. doi: 10.1016/j.cllc.2022.12.013. [DOI] [PubMed] [Google Scholar]
- 184.Sonawane V, Ghosalkar J, Achrekar S, Joshi K. Ketorolac modulates Rac-1/HIF-1alpha/DDX3/beta-catenin signalling via a tumor suppressor prostate apoptosis response-4 (Par-4) in renal cell carcinoma. Sci Rep. 2023;13(1):5659. doi: 10.1038/s41598-023-32627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Carli F, Webster J, Pearson M, Pearson J, Bartlett S, Bannister P, HALLIDAY D. Protein metabolism after abdominal surgery: effect of 24-h extradural block with local anaesthetic. Br J Anaesth. 1991;67(6):729–734. doi: 10.1093/bja/67.6.729. [DOI] [PubMed] [Google Scholar]
- 186.Creed SJ, Le CP, Hassan M, Pon CK, Albold S, Chan KT, Berginski ME, Huang Z, Bear JE, Lane JR, et al. β2-adrenoceptor signaling regulates invadopodia formation to enhance tumor cell invasion. Breast Cancer Res. 2015;17(1):145. doi: 10.1186/s13058-015-0655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kang F, Ma W, Ma X, Shao Y, Yang W, Chen X, Li L, Wang J. Propranolol inhibits glucose metabolism and 18F-FDG uptake of breast cancer through posttranscriptional downregulation of hexokinase-2. J Nucl Med. 2014;55(3):439–445. doi: 10.2967/jnumed.113.121327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Plummer HK 3rd, Yu Q, Cakir Y, Schuller HM. Expression of inwardly rectifying potassium channels (GIRKs) and beta-adrenergic regulation of breast cancer cell lines. BMC Cancer. 2004;4(1):93. doi: 10.1186/1471-2407-4-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shi M, Yang Z, Hu M, Liu D, Hu Y, Qian L, Zhang W, Chen H, Guo L, Yu M, et al. Catecholamine-induced β2-adrenergic receptor activation mediates desensitization of gastric cancer cells to trastuzumab by upregulating MUC4 expression. J Immunol. 2013;190(11):5600–5608. doi: 10.4049/jimmunol.1202364. [DOI] [PubMed] [Google Scholar]
- 190.Lung HL, Shan SW, Tsang D, Leung KN. Tumor necrosis factor-α mediates the proliferation of rat C6 glioma cells via β-adrenergic receptors. J Neuroimmunol. 2005;166(1–2):102–112. doi: 10.1016/j.jneuroim.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 191.Cao M, Huang W, Chen Y, Li G, Liu N, Wu Y, Wang G, Li Q, Kong D, Xue T, et al. Chronic restraint stress promotes the mobilization and recruitment of myeloid-derived suppressor cells through β-adrenergic-activated CXCL5-CXCR2-erk signaling cascades. Intl Journal Of Cancer. 2021;149(2):460–472. doi: 10.1002/ijc.33552. [DOI] [PubMed] [Google Scholar]
- 192.Balaha M, Kandeel S, Barakat W. Carvedilol suppresses circulating and hepatic IL-6 responsible for hepatocarcinogenesis of chronically damaged liver in rats. Toxicol Appl Pharm. 2016;311:1–11. doi: 10.1016/j.taap.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 193.Satilmis H, Verheye E, Vlummens P, Oudaert I, Vandewalle N, Fan R, Knight JM, De Beule N, Ates G, Massie A, et al. Targeting the β 2 -adrenergic receptor increases chemosensitivity in multiple myeloma by induction of apoptosis and modulating cancer cell metabolism. J Pathol. 2023;259(1):69–80. doi: 10.1002/path.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, Register T, Cline JM, D’Agostino R, Danial N, et al. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest. 2013;123(2):874–886. doi: 10.1172/JCI63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Raimondi S, Botteri E, Munzone E, Cipolla C, Rotmensz N, DeCensi A, Gandini S. Use of beta-blockers, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and breast cancer survival: systematic review and meta-analysis. Intl Journal Of Cancer. 2016;139(1):212–219. doi: 10.1002/ijc.30062. [DOI] [PubMed] [Google Scholar]
- 196.Thiele M, Albillos A, Abazi R, Wiest R, Gluud LL, Krag A. Non-selective beta-blockers may reduce risk of hepatocellular carcinoma: a meta-analysis of randomized trials. Liver Int. 2015;35(8):2009–2016. doi: 10.1111/liv.12782. [DOI] [PubMed] [Google Scholar]
- 197.Sakamoto A, Yagi K, Okamura T, Harada T, Usuda J. Perioperative administration of an intravenous beta-blocker landiolol hydrochloride in patients with lung cancer: a Japanese Retrospective exploratory clinical study. Sci Rep. 2019;9(1):5217. doi: 10.1038/s41598-019-41520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang Y, Chen H, Chen S, Li Z, Chen J, Li W. The effect of concomitant use of statins, NSAIDs, low-dose aspirin, metformin and beta-blockers on outcomes in patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Oncoimmunology. 2021;10(1):1957605. doi: 10.1080/2162402X.2021.1957605. [DOI] [PMC free article] [PubMed] [Google Scholar]


