ABSTRACT
The aim of this study was to evaluate the therapeutic effect of idebenone in patients with OPA1-dominant optic atrophy (DOA). Sixteen patients with genetically confirmed OPA1-DOA were treated with 900 mg idebenone daily for 12 months. The primary endpoint was the best recovery/least deterioration of visual acuity. Secondary endpoints were the changes of visual acuity, colour vision, contrast sensitivity, visual field, peripapillary retinal nerve fibre layer thickness (pRNFLT), and visual-related quality of life. For the primary endpoint, a significant increase was observed for the right eye (p = .0027), for the left eye (p = .0111) and for the better-seeing eye (p = .0152). For visual fields, a significant improvement was observed for the left eye between baseline and 9 months (p = .0038). Regarding pRNFLT, a significant decrease was found for the left eye between baseline and 3 months (p = .0413) and between baseline and 6 months (p = .0448). In the visual function questionnaire, a significant improvement was observed in the subscale general vision (p = .0156) and in the composite score (p = .0256). In conclusion, best recovery of visual acuity improved, even though the amount of improvement was small. Furthermore, a maintenance of visual function after 12 months of idebenone intake could be observed as well as a significant improvement in vision-related quality of life.Whether this effect is due to idebenone treatment, the placebo effect, or is explainable by the natural progression of DOA, remains unclear.
Trial registration: EU Clinical Trials Register, EudraCT Number: 2019-001493-28
KEYWORDS: Dominant optic atrophy, OPA1, idebenone, optic neuropathy, visual function
Introduction
Dominant optic atrophy (DOA) is a disease of the retinal ganglion cells1–4 with a prevalence between 1 in 10 000 and 1 in 50 000.3,5–7 Currently, there is no approved therapy for DOA.8 The hallmark of this disease is an insidious onset of bilateral vision loss during childhood, but visual impairment may remain subclinical until adolescence. This is followed by a slow progression in some families, while in others, vision may remain stable. Rapid deterioration of visual acuity is rare.5–7,9–14 Other clinical features are bitemporal optic atrophy in the early stages followed by total optic atrophy later, reduced colour vision as well as caecocentral, central, or paracentral scotomas in the visual field. Also, pseudo-cupping or excavation of the optic discs can be observed. Phenotypic expression is characterised by large intra- and interfamilial varieties ranging from asymptomatic carriers to patients classified as legally blind.3–7,9–12,14,15
In most cases, DOA is caused by a mutation in the OPA1 gene (MIM 165500).1–4 OPA1 regulates different mitochondrial functions including mitochondrial fusion, cristae derangement, and regulation of apoptosis controlled by cytochrome c. Dysregulation of these processes has been postulated to cause defective oxidative phosphorylation and reduced adenosine triphosphate (ATP) synthesis by complex I.16,17 Furthermore, an augmented production of reactive oxygen species has been observed in DOA.17 It is assumed that the retinal ganglion cells are affected by reduced ATP synthesis and increased oxidative stress.18
Ubiquinone, also called coenzyme Q10, is a long-chain quinone that plays an important role in the transport of electrons in the respiratory chain as a lipophilic electron carrier. It is able to bypass mitochondrial complex I and can transfer electrons directly to complex III.4,18,19 In contrast to coenzyme Q10, idebenone is less lipophilic and is analogous to the short chain of coenzyme Q10. Due to this biochemical characteristic, idebenone can pass the mitochondrial membrane better than coenzyme Q10.18,19 Because of its high affinity to absorb electrons, idebenone also functions as an antioxidant, scavenging reactive oxygen species.17,19 Those two functions tackle the two biochemical problems that OPA1 defects create. Currently, idebenone is approved as Raxone® for the treatment of Leber’s hereditary optic neuropathy (LHON) by the European Medicines Agency.20 LHON and DOA share some similarities such as the clinical presentation and the dysfunction in complex I of the respiratory chain.1,21 Because of the common features between LHON and DOA, the mitochondrial dysfunctions caused by OPA1 mutations, and the beneficial biochemical functions of idebenone, it is assumed that idebenone has a therapeutic effect in DOA patients.16
To this day, two studies have described the use of idebenone in patients with OPA1-DOA. The first publication was a prospective study by Barboni et al. on seven patients.1 The second one was a retrospective analysis by Romagnoli et al. on 87 patients, 50 of whom were treated with idebenone.17 Romagnoli et al. reported the effect of idebenone on visual acuity only.17 In both studies, varying doses of idebenone were used (Barboni et al. used 270–1000 mg/day,1 Romagnoli et al. used 135-675mg/day17). Both described a favourable effect of idebenone.
However, no structured study about the long-term effects of idebenone as treatment for DOA patients with OPA1 mutations has been carried out as of yet. The aim of this study was to observe the therapeutic effect of 900 mg idebenone daily over 12 months in patients with OPA1-DOA.
Materials and methods
Study design and patients
In this prospective, monocentric clinical trial of a registered pharmaceutical product not according to its label (phase 2), patients with OPA1-DOA were treated with 900 mg idebenone (Raxone® 150 mg, Santhera Pharmaceuticals, 3 × 2 film-coated tablets taken with food), the approved dosage for LHON,20 for 12 months according to recommendations in LHON to assess treatment response.22
Subjects with OPA1-DOA were informed about the trial during routine clinical visits at the Department of Ophthalmology, Medical University of Graz, Austria. Trial participation was offered to all subjects. The first 16 subjects that were interested in study participation and fitted the criteria were enrolled. Inclusion and exclusion criteria are depicted in Table 1.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | |
|
|
|
|
|
|
|
|
|
|
| Exclusion criteria | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The study was designed according to the Declaration of Helsinki, approved by the Ethics Committee of the Medical University of Graz (EC number: 32-250 ex 19/20) and the Federal Agency for Safety and Health Care in Austria (reference number: 13371525). Furthermore, 9 September 2020, the trial was registered at the European Union Clinical Trials Register (EudraCT number: 2019-001493-28). Informed consent was given by all participants.
Endpoints and examinations
The primary endpoint of this study was the best recovery of visual acuity from baseline to 12 months. In patients without recovery, stabilisation or least deterioration of visual acuity was evaluated as recovery. Secondary endpoints were the changes of visual acuity, visual field, colour vision, contrast sensitivity, peripapillary retinal nerve fibre layer thickness (pRNFLT) on optical coherence tomography (OCT), and visual performance-related quality of life within a 12-month period. All visual function endpoints were determined for the right eye and for the left eye. Furthermore, we evaluated all parameters for the better-seeing eye assuming that it has a higher probability to regenerate visual function under idebenone treatment.
Before inclusion, participants underwent all examinations at least once. A full medical history was taken including pre-existing conditions, medications, and the age of onset of vision loss. Other reasons for optic atrophy, for example intracerebral or intraorbital expanding lesions, were ruled out by magnetic resonance imaging of the brain and orbits.
Examinations were performed at baseline and then at 3-monthly intervals (±10 days) according to current recommendations for LHON treatment.22 At each visit, all primary and secondary endpoints were evaluated. Furthermore, at each follow-up visit the medical history, adverse events, and reactions were evaluated. Compliance of medication intake was checked by counting the remaining tablets at each follow-up visit.
Best-corrected visual acuity (BCVA) was measured using illuminated Early Treatment Diabetic Retinopathy Study (ETDRS) charts according to guidelines.23,24 During each visit, refraction was checked and, if necessary, updated.24 The smallest line with one or no errors was converted to the logarithm of the minimum angle of resolution (logMAR). Colour vision was tested with the Ishihara plates at a distance of 75 cm with 38 plates presented in a well-illuminated location; 21 of them counted for the test, and the remaining 17 were placebo plates. The participant had to give the answer in 3 seconds for every plate (forced-choice). The sum of the correct answers was documented as fraction (n/21). Contrast sensitivity was evaluated using Pelli Robson charts at a distance of 1 m in a well-illuminated location according to the examination guidelines.25 The triplet with the poorest contrast, in which at least two out of three optotypes were recognised, was documented as a logarithm. The change of visual field was assessed through mean deviation (MD) of the 30-2 programme of Octopus 900 (Haag-Streit Switzerland).
A peripapillary 12° scan centred at the optic nerve head with 100 ART (automatic real time) was performed using spectral-domain OCT (Heidelberg Engineering GmbH, Germany, Spectralis Family Acquisition Module Software Version 6.16.8.0) to measure the pRNFLT.26 Baseline scans were used as reference location for follow-up scans. The retinal nerve fibre layer was segmented by the built-in segmentation software and, if necessary, corrected by a single reviewer. For the comparison of follow-up visits, global pRNFLT was analysed.
To assess the visual performance-related quality of life, the German version of the National Eye Institute 25-Item Visual Function Questionnaire (Version 2000) was used.27,28 The questionnaire was completed within an interview at baseline and at the 12-month visit. It was evaluated according to the description.29
Every 3 months peripheral venous blood was analysed to observe the effect of idebenone on full blood count, liver, and kidney parameters, as well as electrolytes. Furthermore, childbearing women underwent pregnancy tests monthly according to the Medicinal Products Act BGBl. I Nr. 35/2004 of the republic of Austria.30
Statistical analysis
The sample size was limited due to the orphan disease status of OPA1-DOA. The number of participants was determined by the expected number of patients presented at the Department of Ophthalmology of Graz. The planned number of participants was 16, and the expected drop-out-rate was about 10%.
For statistical analysis, the better-seeing eye was defined as the eye with the lower logMAR BCVA at baseline. If both eyes had equal BCVA, then the eye with the lower MD in visual field was determined as the better-seeing eye.
Categorical data were presented with quantity and percent, continuous data with mean, standard deviation (SD) or median, minimum, and maximum.
Changes over time for the primary endpoint were estimated via a mixed model accounting for repeated measures (baseline, follow-up) and included time, eye, time–eye interaction as fixed effects, and a random intercept for subject. P values and the corresponding 95% confidence intervals (CIs) for the differences in means (follow-up – baseline value), overall, and within eye were estimated by least squares means (LSM). The secondary endpoints were analysed via a mixed model with repeated measures (baseline, 3-, 6-, 9-, and 12 months) and included time, eye, time–eye interaction as fixed effects, and a random intercept for subject and eye. For repeated measures, a first-order autoregressive [AR(1)] covariance structure was modelled.
Additionally, analyses were repeated by including only one eye (the better-seeing eye at baseline). Mixed models including time as a fixed effect, a random intercept for subject and an AR(1) covariance structure for the time points were used.
The p values for the comparison of the questionnaire results from baseline to 12 months were obtained by Wilcoxon signed-rank test. P values below .05 were defined as statistically significant. No imputation of missing data was applied. SAS version 9.4 (Cary, NC, USA) was used for statistical analysis.
Results
Eleven male (69%) and five female (31%) white Caucasian participants were included between 1 October 2020 and 4 May 2021. The follow-up period ended on 26 April 2022. Characteristics of our study population showed variability in age and baseline BCVA corresponding to descriptions of other studies (Table 2).
Table 2.
Demographic, genetic, and baseline clinical data.
| Patient | Gender | Age at baseline (years) |
Age at onset of vision loss (years) |
BCVA at baseline (logMAR) |
Mutation | ||
|---|---|---|---|---|---|---|---|
| OD | OS | ||||||
| 1 | male | 37 | 11 | 0.7 | 0.6 | c.1879A>T | p.Arg627Ter |
| 2 | male | 19 | 6 | 0.2 | 0.2 | c.1780C>T | p.Arg594* |
| 3 | female | 43 | 20 | 0.3 | 0.1 | c.2232dupT | p.lle745Tyrfs*16 |
| 4 | female | 57 | NVL | 0.1 | 0.3 | c.2708_2711delTTAG | p.Val903Glyfs*3 |
| 5 | male | 31 | 13 | 0.6 | 0.5 | c.2131C>T | p.Arg711Ter |
| 6 | male | 62 | 22 | 0.2 | 0.5 | c.2708_2711delTTAG | p.Val903Glyfs*3 |
| 7 | male | 28 | 10 | 0.1 | 0.2 | c.116_119del | p.Ser39llefs*9 |
| 8 | female | 22 | 14 | 0.3 | 0.3 | c.2708_2711delTTAG | p.Val903Glyfs*3 |
| 9 | male | 54 | 16 | 0.7 | 0.4 | c.687T>A | p.Tyr229Ter |
| 10 | male | 19 | 6 | 0.7 | 0.6 | c.687T>A | p.Tyr229Ter |
| 11 | female | 37 | 4 | 0.9 | 1.2 | c.1313A>G | p.Asp438Gly |
| 12 | female | 14 | 2 | 1 | 1.3 | c.1313A>G | p.Asp438Gly |
| 13 | male | 62 | 18 | 0.3 | 0.3 | c.2708_2711delTTAG | p.Val903Glyfs*3 |
| 14 | male | 53 | 18 | 0.7 | 0.6 | c.2708_2711delTTAG | p.Arg904Aspfs*2 |
| 15 | male | 55 | 43 | 1.1 | 1.1 | c.(32 + 1_33–1)_(678 + 1_679–1)del, c.(32 + 1_33–578)_(678 + 1_679–1)del | |
| 16 | male | 16 | 9 | 0.4 | 0.4 | c.(32 + 1_33–578)_(678 + 1_679–1)del | |
BCVA = best-corrected visual acuity; NR = NVL = perceived no vision loss; OD = right eye; OS = left eye.
For the primary endpoint (best recovery/least deterioration), a significant change of the least square mean difference (LSMD) of −0.08 logMAR (95% CI: −0.12, −0.03; p = .0027) was observed for the right eye (Table 3). Further significant improvement could be shown for the left eye (Table 4) as well as for the better-seeing eye (Table 5) with a LSMD of −0.06 logMAR (95% CI: −0.11, −0.01; p = .0111) and −0.05 logMAR (95% CI: −0.09, −0.01; p = .0152), respectively (Figure 1a).
Table 3.
Visual function data for the right eye.
| Time | n | Median (Min, Max) | Mean ± SD | LSMD (95% CI) | p value |
|---|---|---|---|---|---|
| Visual acuity: best recovery/least deterioration (logMAR) | |||||
| Baseline | 16 | 0.50 (0.10, 1.10) | 0.52 ± 0.32 | - | |
| Follow-up | 16 | 0.40 (0.00, 1.00) | 0.44 ± 0.32 | −0.08 (−0.12, −0.03) | .0027* |
| Visual acuity within a 12-month period (logMAR) | |||||
| Baseline | 16 | 0.50 (0.10, 1.10) | 0.52 ± 0.32 | - | |
| 3 months | 16 | 0.50 (0.10, 1.30) | 0.53 ± 0.34 | 0.01 (−0.04, 0.06) | .8011 |
| 6 months | 15 | 0.50 (0.10, 1.10) | 0.55 ± 0.30 | 0.00 (−0.05, 0.06) | .8876 |
| 9 months | 15 | 0.50 (0.10, 1.00) | 0.53 ± 0.29 | −0.01 (−0.06, 0.04) | .7164 |
| 12 months | 15 | 0.60 (0.00, 1.10) | 0.52 ± 0.35 | −0.02 (−0.07, 0.03) | .3825 |
| Visual field (dB) | |||||
| Baseline | 15 | 3.40 (0.20, 12.80) | 4.41 ± 3.27 | - | |
| 3 months | 15 | 4.00 (0.00, 15.30) | 4.73 ± 3.87 | 0.33 (−0.72, 1.37) | .5370 |
| 6 months | 14 | 3.05 (0.60, 12.90) | 4.36 ± 3.35 | −0.19 (−1.26, 0.88) | .7263 |
| 9 months | 13 | 2.20 (0.40, 9.90) | 3.09 ± 2.97 | −0.83 (−1.93, 0.26) | .1351 |
| 12 months | 14 | 3.20 (−0.60, 13.80) | 4.31 ± 3.93 | −0.24 (−1.31, 0.83) | .6582 |
| Colour vision (n/21) | |||||
| Baseline | 16 | 1.50 (0.00, 13.00) | 3.81 ± 4.42 | - | |
| 3 months | 16 | 1.00 (0.00, 18.00) | 3.56 ± 4.75 | −0.25 (−1.13, 0.63) | .5747 |
| 6 months | 15 | 2.00 (0.00, 20.00) | 3.80 ± 5.47 | −0.16 (−1.13, 0.81) | .7405 |
| 9 months | 15 | 2.00 (0.00, 20.00) | 4.07 ± 5.28 | 0.11 (−0.87, 1.09) | .8279 |
| 12 months | 15 | 2.00 (0.00, 19.00) | 4.25 ± 5.08 | 0.29 (−0.69, 1.27) | .5575 |
| Contrast sensitivity (log) | |||||
| Baseline | 16 | 1.20 (0.45, 1.50) | 1.11 ± 0.31 | - | |
| 3 months | 16 | 1.13 (0.60, 1.35) | 1.13 ± 0.23 | 0.02 (−0.1, 0.13) | .7462 |
| 6 months | 15 | 1.20 (0.30, 1.65) | 1.16 ± 0.34 | 0.07 (−0.06, 0.20) | .3189 |
| 9 months | 15 | 1.35 (0.45, 1.50) | 1.19 ± 0.28 | 0.10 (−0.04, 0.23) | .1624 |
| 12 months | 15 | 1.05 (0.45, 2.00) | 1.12 ± 0.40 | 0.03 (−0.11, 0.16) | .6762 |
| Peripapillary retinal nerve fibre layer thickness (µm) | |||||
| Baseline | 15 | 60.00 (45.00, 70.00) | 58.47 ± 8.02 | - | |
| 3 months | 15 | 60.00 (46.00, 69.00) | 58.40 ± 8.14 | −0.07 (−0.71, 0.57) | .8367 |
| 6 months | 13 | 59.00 (48.00, 68.00) | 59.23 ± 7.36 | 0.25 (−0.57, 1.07) | .5487 |
| 9 months | 14 | 58.50 (43.00, 69.00) | 57.57 ± 8.46 | −0.38 (−1.26, 0.50) | .3984 |
| 12 months | 14 | 58.50 (44.00, 68.00) | 57.36 ± 8.24 | −0.58 (−1.5, 0.34) | .2109 |
*p value < .05.
CI = confidence interval; logMAR = logarithm of the minimum angle of resolution; LSMD = least square mean difference; Max = maximum; Min = minimum; SD = standard deviation.
Table 4.
Visual function data for the left eye.
| Time | n | Median (Min, Max) | Mean ± SD | LSMD (95% CI) | p value |
|---|---|---|---|---|---|
| Visual acuity: best recovery/least deterioration (logMAR) | |||||
| Baseline | 16 | 0.45 (0.10, 1.30) | 0.54 ± 0.36 | - | |
| Follow-up | 16 | 0.40 (0.10, 1.30) | 0.48 ± 0.38 | −0.06 (−0.11, −0.01) | .0111* |
| Visual acuity within a 12-month period (logMAR) | |||||
| Baseline | 16 | 0.45 (0.10, 1.30) | 0.54 ± 0.36 | - | |
| 3 months | 16 | 0.40 (0.20, 1.30) | 0.55 ± 0.38 | 0.01 (−0.04, 0.06) | .6145 |
| 6 months | 15 | 0.40 (0.10, 1.30) | 0.52 ± 0.41 | −0.04 (−0.09, 0.01) | .1546 |
| 9 months | 15 | 0.40 (0.10, 1.30) | 0.54 ± 0.40 | −0.02 (−0.07, 0.03) | .5064 |
| 12 months | 15 | 0.40 (0.10, 1.30) | 0.52 ± 0.38 | −0.04 (−0.09, 0.01) | .1537 |
| Visual field (dB) | |||||
| Baseline | 14 | 5.10 (0.80, 11.00) | 4.81 ± 2.48 | - | |
| 3 months | 14 | 3.40 (1.30, 8.30) | 4.08 ± 2.10 | −0.74 (−1.82, 0.35) | .1807 |
| 6 months | 13 | 3.10 (−1.00, 10.80) | 4.08 ± 3.28 | −0.83 (−1.94, 0.27) | .1383 |
| 9 months | 13 | 2.60 (−0.60, 9.40) | 3.26 ± 2.81 | −1.66 (−2.77, −0.55) | .0038* |
| 12 months | 13 | 3.90 (−0.40, 10.80) | 3.90 ± 3.01 | −1.02 (−2.13, 0.09) | .0711 |
| Colour vision (n/21) | |||||
| Baseline | 16 | 1.00 (0.00, 15.00) | 3.50 ± 4.63 | - | |
| 3 months | 16 | 2.00 (0.00, 16.00) | 4.19 ± 5.31 | 0.69 (−0.19, 1.57) | .1245 |
| 6 months | 15 | 1.00 (0.00, 21.00) | 3.87 ± 5.95 | 0.22 (−0.75, 1.19) | .6565 |
| 9 months | 15 | 1.00 (0.00, 15.00) | 3.67 ± 4.69 | 0.02 (−0.96, 1.00) | .9718 |
| 12 months | 15 | 3.00 (0.00, 15.00) | 4.22 ± 4.97 | 0.57 (−0.41, 1.56) | .2494 |
| Contrast sensitivity (log) | |||||
| Baseline | 16 | 1.35 (0.45, 1.50) | 1.20 ± 0.28 | - | |
| 3 months | 16 | 1.28 (0.30, 1.50) | 1.18 ± 0.33 | −0.02 (−0.13, 0.10) | .7462 |
| 6 months | 15 | 1.35 (0.60, 1.65) | 1.26 ± 0.31 | 0.07 (−0.06, 0.20) | .2819 |
| 9 months | 15 | 1.35 (0.15, 1.65) | 1.17 ± 0.41 | −0.02 (−0.15, 0.12) | .7852 |
| 12 months | 15 | 1.35 (0.30, 2.00) | 1.19 ± 0.45 | 0.00 (−0.13, 0.14) | .9441 |
| Peripapillary retinal nerve fibre layer thickness (µm) | |||||
| Baseline | 15 | 62.00 (46.00, 83.00) | 60.73 ± 10.15 | - | |
| 3 months | 15 | 60.00 (45.00, 83.00) | 60.07 ± 10.05 | −0.67 (−1.31, −0.03) | .0413* |
| 6 months | 14 | 58.50 (44.00, 83.00) | 59.43 ± 10.31 | −0.83 (−1.63, −0.02) | .0448* |
| 9 months | 14 | 60.00 (45.00, 81.00) | 59.36 ± 10.06 | −0.87 (−1.75, 0.01) | .0532 |
| 12 months | 14 | 60.50 (45.00, 81.00) | 59.43 ± 10.20 | −0.78 (−1.70, 0.13) | .0937 |
*p value < .05.
CI = confidence interval; logMAR = logarithm of the minimum angle of resolution; LSMD = least square mean difference; Max = maximum; Min = minimum; SD = standard deviation.
Table 5.
Visual function data for the better-seeing eye.
| Time | n | Median (Min, Max) | Mean ± SD | LSMD (95% CI) | p value |
|---|---|---|---|---|---|
| Visual acuity: best recovery/least deterioration (logMAR) | |||||
| Baseline | 16 | 0.40 (0.10, 1.10) | 0.46 ± 0.32 | - | |
| Follow-up | 16 | 0.40 (0.00, 1.20) | 0.41 ± 0.35 | −0.05 (−0.09, −0.01) | .0152* |
| Visual acuity within a 12-month period (logMAR) | |||||
| Baseline | 16 | 0.40 (0.10, 1.10) | 0.46 ± 0.32 | - | |
| 3 months | 16 | 0.40 (0.10, 1.30) | 0.49 ± 0.36 | 0.03 (−0.02, 0.08) | .2342 |
| 6 months | 15 | 0.40 (0.10, 1.20) | 0.50 ± 0.33 | 0.02 (−0.04, 0.07) | .5560 |
| 9 months | 15 | 0.40 (0.10, 1.20) | 0.50 ± 0.33 | 0.02 (−0.04, 0.07) | .5527 |
| 12 months | 15 | 0.40 (0.00, 1.20) | 0.46 ± 0.37 | −0.02 (−0.08, 0.03) | .3823 |
| Visual field (dB) | |||||
| Baseline | 15 | 4.50 (0.20, 11.00) | 4.73 ± 2.90 | - | |
| 3 months | 15 | 4.00 (0.00, 15.30) | 4.74 ± 3.71 | 0.01 (−1.03, 1.04) | .9898 |
| 6 months | 14 | 3.40 (0.70, 12.90) | 4.66 ± 3.66 | −0.21 (−1.33, 0.91) | .7085 |
| 9 months | 13 | 2.70 (0.40, 9.40) | 3.22 ± 2.82 | −1.03 (−2.19, 0.12) | .0787 |
| 12 months | 14 | 3.75 (−0.60, 13.80) | 4.49 ± 3.90 | −0.38 (−1.51, 0.75) | .5071 |
| Colour vision (n/21) | |||||
| Baseline | 16 | 2.00 (0.00, 13.00) | 3.81 ± 4.32 | - | |
| 3 months | 16 | 2.00 (0.00, 18.00) | 3.81 ± 4.72 | 0.00 (−0.96, 0.96) | 1.0000 |
| 6 months | 15 | 1.00 (0.00, 20.00) | 3.73 ± 5.57 | −0.23 (−1.26, 0.80) | .6571 |
| 9 months | 15 | 2.00 (0.00, 20.00) | 4.07 ± 5.38 | 0.11 (−0.93, 1.14) | .8369 |
| 12 months | 15 | 4.00 (0.00, 19.00) | 4.42 ± 5.01 | 0.46 (−0.57, 1.50) | .3738 |
| Contrast sensitivity (log) | |||||
| Baseline | 16 | 1.35 (0.45, 1.50) | 1.15 ± 0.31 | - | |
| 3 months | 16 | 1.20 (0.60, 1.50) | 1.15 ± 0.27 | 0.00 (−0.12, 0.12) | 1.0000 |
| 6 months | 15 | 1.20 (0.30, 1.65) | 1.20 ± 0.34 | 0.08 (−0.07, 0.22) | .2814 |
| 9 months | 15 | 1.35 (0.45, 1.50) | 1.20 ± 0.29 | 0.02 (−0.13, 0.17) | .8246 |
| 12 months | 15 | 1.05 (0.45, 2.00) | 1.15 ± 0.41 | 0.02 (−0.13, 0.17) | .7979 |
| Peripapillary retinal nerve fibre layer thickness (µm) | |||||
| Baseline | 15 | 60.00 (46.00, 83.00) | 60.00 ± 10.09 | - | |
| 3 months | 15 | 59.00 (45.00, 83.00) | 59.60 ± 10.45 | −0.40 (−1.03, 0.23) | .2086 |
| 6 months | 14 | 57.00 (44.00, 83.00) | 59.21 ± 10.79 | −0.36 (−1.10, 0.38) | .3290 |
| 9 months | 14 | 58.00 (45.00, 81.00) | 59.00 ± 10.21 | −0.57 (−1.33, 0.20) | .1456 |
| 12 months | 14 | 58.50 (45.00, 81.00) | 58.79 ± 10.15 | −0.78 (−1.55, 0.00) | .0501 |
*p value < .05.
CI = confidence interval; logMAR = logarithm of the minimum angle of resolution; LSMD = least square mean difference; Max = maximum; Min = minimum; SD = standard deviation.
Figure 1.
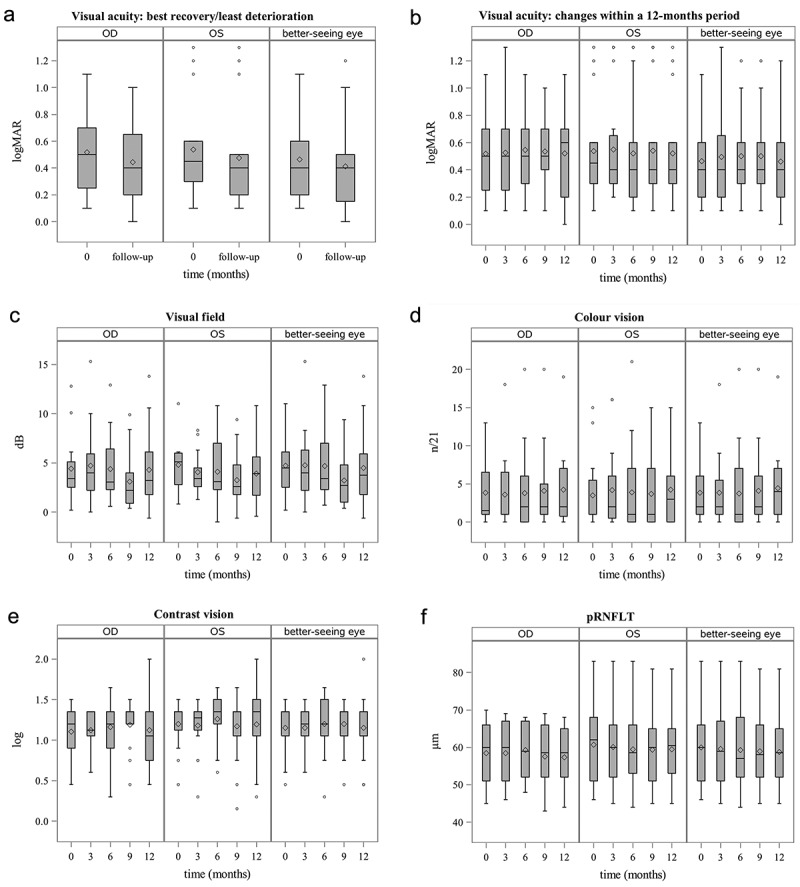
Box plots showing change of visual function within a 12-month period of idebenone treatment. (a) Best recovery or least deterioration of visual acuity. (b) Change of visual acuity. (c) Change of visual field. (d) Change of colour vision. (e) Change of contrast sensitivity. (f) Change of peripapillary retinal nerve fibre layer thickness. Solid lines represent median values and the symbols ‘o’, ‘+’ and ’ ×’ within the boxes represent mean values for OD (right eye), OS (left eye) and the better-seeing eye and ‘o’, ‘+’ and ’ ×’ outside depict outliers. logMAR = logarithm of the minimum angle of resolution; pRNFLT = peripapillary retinal nerve fibre layer thickness.
For the secondary endpoint of change of BCVA within a 12-month period (Figure 1b), no significant change was observed for the right eye (Table 3), the left eye (Table 4) or for the better-seeing eye (Table 5).
For the visual field of the left eye (Table 4), between baseline and the 9-month visit a significant improvement with a LSMD of −1.66 dB (95% CI: −2.77, −0.55; p = .0038) was observed. Between baseline and the other follow-up visits, the change in visual field was not significant (Figure 1c).
For colour vision and contrast sensitivity, no significant change was found (Tables 3–5, Figure 1d,e).
Analysing pRNFLT (Figure 1f), we found a significant decrease in the left eye between baseline and the 3-month visit with a LSMD of −0.67 µm (95% CI: −1.31, −0.03; p = .0413) as well as between baseline and the 6-month visit with a LSMD of −0.83 µm (95% CI: −1.63, −0.02; p = .0448). This significant decrease disappeared between baseline and the other follow-up visits (Table 4).
Regarding the visual performance-related quality of life, the highest deficits of our participants were documented in subscale general vision, role difficulties, and driving at baseline. After 12 months of idebenone therapy, the participants perceived an improvement in all areas. A significant increase was noticed only for the subscale general vision from a median 60.0 (Minimum [Min] 20.0, Maximum [Max] 80.0) to a median 80.0 (Min 40.0, Max 80.0; p = .0156). Improvement of all subscales led to a significant increase in the composite score after 12 months of idebenone treatment from 83.6 (Min 45.9, Max 95.5) to 92.5 (Min 57.8, Max 97.6; p = .0256) (Table 6).
Table 6.
National Eye Institute Visual Function Questionnaire.
| Scale Name | Baseline Median (Min, Max) |
12-months Median (Min, Max) |
Difference Median (Min, Max) |
p value* |
|---|---|---|---|---|
| General Health | 75.0 (50.0, 100.0) | 75.0 (50.0, 100.0) | 0.0 (−50.0, 0.0) | .2500 |
| General Vision | 60.0 (20.0, 80.0) | 80.0 (40.0, 80.0) | 0.0 (0.0, 20.0) | .0156* |
| Ocular Pain | 100.0 (37.5, 100.0) | 100.0 (87.5, 100.0) | 0.0 (0.0, 50.0) | .0625 |
| Near Activities | 91.7 (33.3, 100.0) | 91.7 (25.0, 100.0) | 0.0 (−16.7, 16.7) | .8105 |
| Distance Activities | 79.2 (37.5, 100.0) | 87.5 (45.8, 100.0) | 4.2 (−20.8, 33.3) | .0898 |
| Vision Specific: | ||||
| Social Functioning | 100.0 (37.5, 100.0) | 100.0 (37.5, 100.0) | 0.0 (−12.5, 50.0) | 1.0000 |
| Mental Health | 87.5 (75.0, 93.8) | 87.5 (57.3, 100.0) | 0.0 (−25.0, 25.0) | .7871 |
| Role Difficulties | 68.8 (25.0, 100.0) | 100.0 (25.0, 100.0) | 0.0 (−50.0, 50.0) | .6328 |
| Dependency | 100.0 (58.3, 100.0) | 100.0 (58.3, 100.0) | 0.0 (−33.3, 41.7) | .3750 |
| Driving | 62.5 (0.0, 91.7) | 79.2 (0.0, 100.0) | 4.2 (−8.3, 91.7) | .0625 |
| Colour Vision | 100.0 (50.0, 100.0) | 100.0 (75.0, 100.0) | 0.0 (−25.0, 25.0) | 1.0000 |
| Peripheral Vision | 100.0 (25.0, 100.0) | 100.0 (2.0, 100.0) | 0.0 (−50.0, 25.0) | .8125 |
| Composite Score | 83.6 (45.9, 95.5) | 92.5 (57.8, 97.6) | 3.7 (−6.5, 13.8) | .0256* |
*p value < .05, calculated with Wilcoxon signed-rank test.
Max = maximum; Min = minimum.
Compliance regarding study medication intake was high. The median pill intake was >95% from baseline to the 12-month visit. Observed adverse events were headache (two participants), anorexia (one participant), anaemia (two female participants), elevation of liver parameters (two participants), sore throat (one participant), and heartburn (one participant). Adverse reactions and serious adverse events were not observed.
One participant (6.25%) was lost to follow-up after the first 3 months. The remaining 15 participants completed the study. Octopus perimetry was unobtainable in two right eyes and one left eye due to insufficient visual function. At the 9-month visit, another participant was unable to perform Octopus perimetry with his right eye. Regarding pRNFLT, both eyes of one participant were excluded because of vitreous detachment with traction to the optic nerve head. Furthermore, at the 6-month visit, another right eye was excluded because of insufficient OCT quality.
Discussion
This is the first study describing the therapeutic effect of a standardised dose of idebenone in OPA1-DOA participants with defined follow-up visits. Limited awareness about the disease among ophthalmologists and the absence of a collective subject registry in Austria and Europe renders sample size calculation and recruitment difficult. The positive results about the effect of idebenone on visual function in OPA1-DOA subjects from Barboni et al.1 and Romagnoli et al.17 encouraged us to make idebenone accessible to all participants and to design our study without a control group.
In the last decades, several studies describing the natural history of OPA1-DOA have been conducted. The rate of visual acuity deterioration varies between 24% and 100%.2,3,7,12,15,17 Yu-Wai-Man et al. observed a mean rate of vision loss of 0.032 logMAR/year3 and 0.070 logMAR/year.2
To our knowledge, a spontaneous improvement of visual acuity has only been described in the two retrospective studies by Cohn et al.15 and Romagnoli et al.17 and in a single-case presentation of a subject with an LHON-like phenotype harbouring the c.740 G>A mutation.31 A closer look to the retrospective studies reveals some limitations in data collection. They used different observation periods, the method of visual acuity testing was not described,17 or differing visual acuity charts were used.15 In the study by Cohn et al., data from previous ophthalmological records were partially included. Additionally, Cohn et al. were uncertain about the validity of their results and could not rule out a learning effect in children.15
In our study, all participants were familiar with the study examinations and had experienced them at least once before. Therefore, a learning effect seems improbable.
Summarising natural history data, most studies describe a stabilisation or slow progression of visual impairment over many years. In LHON, an improvement of 0.2 logMAR on ETDRS charts is accepted as a clinically relevant recovery.22,32
For the primary endpoint of our study, a small increase of visual acuity was found (LSMD: right eye −0.08 logMAR [Table 3], left eye −0.06 logMAR [Table 4] and better-seeing eye −0.05 logMAR [Table 5]). However, the aforementioned threshold for clinically relevant recovery was not reached. Within the 12-month period (secondary endpoint), no significant improvement in visual acuity could be observed. Nevertheless, from baseline to the 12-month visit mean visual acuity was stable without deterioration (LSMD: right eye −0.02 logMAR, left eye −0.04 logMAR, better-seeing eye −0.02 logMAR). In comparison with Yu-Wai-Man et al.’s reported rates of visual decrease of 0.0323 and 0.0702 logMAR/year, maintenance of visual acuity may be a success. Romagnoli et al. observed a change of the median visual acuity in the best-seeing eyes from 0.52 to 0.51 logMAR in the idebenone treated group (observation time of 4.2 ± 2.3 years). In the untreated group, the median visual acuity was unchanged at 0.52 logMAR after an observation period of 3.4 ± 2.5 years.17 In our results, the median visual acuity in the better-seeing eye was 0.40 logMAR at baseline and at the 12-month follow-up (Table 5). Although our study participants were observed for only 12 months, our median visual acuity results are comparable with the data of the untreated and treated groups of Romagnoli et al.17 Barboni et al. were able to show an improvement of mean visual acuity in the right eye from 0.7 logMAR to 0.5 logMAR after 16.4 months of idebenone treatment.1 In our study, the mean visual acuity in the right eye remained stable after 12 months of treatment (0.52 logMAR at baseline and at the 12-month visit [Table 3]). In the left eye, the mean visual acuity in the Barboni et al. study was unchanged at 0.6 logMAR after 16.4 months,1 while we had similar stable results, only changing from 0.54 logMAR to 0.52 logMAR after the 12-month follow-up (Table 4).
A significant improvement of the visual field of the left eye was found between baseline and the 9-month visit. Also, between baseline and the other follow-up visits, the visual field improved, but without a significant change. A trend towards improvement with increasing treatment duration was detectable. In view of the slow disease progression, prolonged treatment duration may be necessary to confirm this positive trend.
Colour vision deficits of our study population are consistent with colour vision abnormalities described in natural history studies.3,5–7,9–13,15,33,34 An improvement of colour vision after idebenone treatment could not be observed in our study. However, Barboni et al. showed an improvement of more than 5/15 Ishihara plates in three out of seven OPA1-DOA participants taking idebenone.1
Recently, significantly reduced contrast sensitivity in OPA1-DOA subjects (mean 1.21 log) compared with healthy controls has been documented.35 These deficits are consistent with our baseline characteristics (right eye: mean 1.11 log, left eye: mean 1.20 log, better-seeing eye: 1.15 log). Idebenone therapy had no influence on the change of contrast sensitivity in our data.
Regarding pRNFLT, we found a significant decrease in the left eye between baseline and 3 and 6 months. However, this decrease in the mean global pRNFLT was smaller than 1 µm and did not persist in the later follow-up.
Similar to other hereditary eye diseases, a decreased quality of life has been detected in OPA1-DOA subjects.35 Eckmann-Hansen et al. found a mean composite National Eye Institute Visual Function Questionnaire −39 score of 69.7,35 which is comparable to our baseline median composite score. After 12 months of idebenone treatment, the quality of life ameliorated in our study, particularly general vision improved significantly. In an open-label trial such as our study, it is not possible to distinguish if the increase in quality of life after 12 months is caused by the treatment with idebenone or due to a placebo effect. Although we found pathological colour vision and visual field defects in our data, participants were not impaired in their daily lives. An explanation could be that they had developed coping mechanisms to compensate for their impairments in everyday life. Nevertheless, the prospect of a pharmaceutical treatment is important for subjects’ mental well-being, particularly in such a rare, hereditary disease.
A dose of 900 mg idebenone was well tolerated, and there was no need to interrupt treatment. Observed adverse events were mild, and almost all of them were documented previously in the product information.
Our study has some limitations. We included a small group of subjects. Nevertheless, our sample size is the second largest reported to date of OPA1-DOA subjects taking idebenone. Examiners were not blinded, and some participants were not able to perform all examinations at every follow-up. We planned our study without a control group and compared the results with baseline data. The development of a collective patient registry in Austria and Europe would facilitate recruitment and planning of an adequately powered, placebo-controlled trial. Another problem for power calculation is the absence of clinically standardised endpoints. A detailed natural history study could lead to the definition of meaningful endpoints as a basis for evaluation of therapeutic effects of future treatments. Furthermore, the National Eye Institute 25-Item Visual Function Questionnaire was not designed for the use in patients with inherited optic neuropathies. Some questions are irrelevant for patients with OPA1-DOA (e.g. ocular pain). Potential limitations may arise from the scoring system by converting an ordinal into a continuous scale.
In conclusion, we observed an improvement in best recovery of visual acuity with idebenone treatment, but this improvement did not reach the level of clinically relevant recovery set for LHON. Nevertheless, visual function of idebenone treatment was maintained and vision-related quality of life improved significantly. Whether this effect is due to idebenone treatment, placebo-effect or explainable by the natural progress of OPA1-DOA requires further research. A randomised, placebo-controlled, and double-blind study over at least 2 years could solve this issue.
Acknowledgements
The authors thank all patients for their participation in this study.
Funding Statement
This work was supported by Chiesi Pharmaceuticals GmbH by a project-related grant for monitoring, pregnancy tests, registration fees, and patients’ insurance, as well as provision of study medication free of charge.
Disclosure statement
K. Valentin received travel reimbursements from Chiesi Pharmaceuticals GmbH, H. Aminfar received travel reimbursements from Santhera Pharmaceuticals and Chiesi Pharmaceuticals GmbH. T. Klopstock received travel reimbursements and speaker honoraria from Santhera Pharmaceuticals and Chiesi Pharmaceuticals GmbH and M. Schneider received speaker honoraria from Santhera Pharmaceuticals. T. Georgi, R. Riedl, C. Singer, and A. Wedrich report no competing interests.
References
- 1.Barboni P, Valentino ML, La Morgia C, et al. Idebenone treatment in patients with OPA1-mutant dominant optic atrophy. Brain. 2013;136(2):e231–e231. doi: 10.1093/brain/aws280. [DOI] [PubMed] [Google Scholar]
- 2.Yu-Wai-Man P, Shankar SP, Biousse V, et al. Genetic screening for OPA1 and OPA3 mutations in patients with suspected inherited optic neuropathies. Ophthalmology. 2011;118(3):558–563. doi: 10.1016/j.ophtha.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu-Wai-Man P, Griffiths PG, Burke A, et al. The prevalence and natural history of dominant optic atrophy due to OPA1 mutations. Ophthalmology. 2010;117(8):1538. doi: 10.1016/j.ophtha.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.La Morgia C, Carbonelli M, Barboni P, Sadun AA, Carelli V.. Medical management of hereditary optic neuropathies. Front Neurol. 2014;5(July):1–7. doi: 10.3389/fneur.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohn AC, Toomes C, Potter C, et al. Autosomal dominant optic atrophy: penetrance and expressivity in patients with OPA1 mutations. Am J Ophthalmol. 2007;143(4):656–662.e1. doi: 10.1016/J.AJO.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 6.Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23(1):53–89. doi: 10.1016/J.PRETEYERES.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Puomila A, Huoponen K, Mäntyjärvi M, et al. Dominant optic atrophy: correlation between clinical and molecular genetic studies. Acta Ophthalmol Scand. 2005;83(3):337–346. doi: 10.1111/J.1600-0420.2005.00448.X. [DOI] [PubMed] [Google Scholar]
- 8.Del Dotto V, Fogazza M, Lenaers G, Rugolo M, Carelli V, Zanna C. OPA1: how much do we know to approach therapy? Pharmacol Res. 2018;131:199–210. doi: 10.1016/J.PHRS.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies – disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30(2–2):81. doi: 10.1016/J.PRETEYERES.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Votruba M, Moore AT, Bhattacharya SS, Votruba S, Bhattacharya MS, Votruba M. Clinical features, molecular genetics, and pathophysiology of dominant optic atrophy. J Med Genet. 1998;35(10):793–800. doi: 10.1136/JMG.35.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Votruba M, Fitzke V, Holder GE, Carter A, Bhattacharya SS, Moore AT. Clinical features in affected individuals from 21 pedigrees with dominant optic atrophy. Arch Ophthalmol. 1998;116(3):351–358. doi: 10.1001/ARCHOPHT.116.3.351. [DOI] [PubMed] [Google Scholar]
- 12.Johnston RL, Seller MJ, Behnam JT, Burdon MA, Spalton DJ. Dominant optic atrophy: refining the clinical diagnostic criteria in light of genetic linkage studies1. Ophthalmology. 1999;106(1):123–128. doi: 10.1016/S0161-6420(99)90013-1. [DOI] [PubMed] [Google Scholar]
- 13.Eliott D, Traboulsi EI, Maumenee IH. Visual prognosis in autosomal dominant optic atrophy (Kjer type). Am J Ophthalmol. 1993;115(3):360–367. doi: 10.1016/S0002-9394(14)73589-5. [DOI] [PubMed] [Google Scholar]
- 14.Kjer B, Eiberg H, Kjer P, Rosenberg T. Dominant optic atrophy mapped to chromosome 3q region. Acta Ophthalmol Scand. 1996;74(1):3–7. doi: 10.1111/J.1600-0420.1996.TB00672.X. [DOI] [PubMed] [Google Scholar]
- 15.Cohn AC, Toomes C, Hewitt AW, et al. The natural history of OPA1-related autosomal dominant optic atrophy. Br J Ophthalmol. 2008;92(10):1333–1336. doi: 10.1136/bjo.2007.134726. [DOI] [PubMed] [Google Scholar]
- 16.Zanna C, Ghelli A, Porcelli AM, et al. OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain. 2008;131(2):352–367. doi: 10.1093/brain/awm335. [DOI] [PubMed] [Google Scholar]
- 17.Romagnoli M, La Morgia C, Carbonelli M, et al. Idebenone increases chance of stabilization/recovery of visual acuity in OPA1-dominant optic atrophy. Ann Clin Transl Neurol. 2020;7(4):590–594. doi: 10.1002/acn3.51026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giorgio V, Petronilli V, Ghelli A, et al. The effects of idebenone on mitochondrial bioenergetics. Biochim Biophys Acta. 2012;1817(2):363. doi: 10.1016/J.BBABIO.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaber S, Polster BM. Idebenone and neuroprotection: antioxidant, pro-oxidant, or electron carrier? J Bioenerg Biomembr. 2015;47:111. doi: 10.1007/S10863-014-9571-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raxone | European Medicines Agency . https://www.ema.europa.eu/en/medicines/human/EPAR/raxone#authorisation-details-section. Accessed March 5, 2022.
- 21.Klopstock T, Yu-Wai-Man P, Dimitriadis K, et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011;134(9):2677–2686. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carelli V, Carbonelli M, De Coo IF, et al. International consensus statement on the clinical and therapeutic management of Leber hereditary optic neuropathy. J Neuro-Ophthalmol. 2017;37(4):371–381. doi: 10.1097/WNO.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 23.Told R, Baratsits M, Garhöfer G, Schmetterer L. ETDRS (early treatment diabetic retinopathy study)-visus. Der Ophthalmol. 2013;110(10):960–965. doi: 10.1007/S00347-013-2813-2. [DOI] [PubMed] [Google Scholar]
- 24.Ferris FL, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. doi: 10.1016/0002-9394(82)90197-0. [DOI] [PubMed] [Google Scholar]
- 25.Pelli DG, Robson JG, Wilkins AJ. The DESIGN of a NEW LETTER CHART for MEASURING CONTRAST SENSITIVITY. Clin Vision Sci. 1988;2:187–199. [Google Scholar]
- 26.Subei AM, Eggenberger ER. Optical coherence tomography: another useful tool in a neuro- ophthalmologist’s armamentarium. Curr Opin Ophthalmol. 2009;20(6):462–466. doi: 10.1097/ICU.0B013E3283313D4E. [DOI] [PubMed] [Google Scholar]
- 27.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-list-item national eye institute visual function questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. doi: 10.1001/ARCHOPHT.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 28.Franke GH, Esser J, Voigtländer A, Mähner N. Der National Eye Institute Visual Function Questionnaire (NEI-VFQ)–Erste Ergebnisse zur psychometrischen Überprüfung eines Verfahrens zur Erfassung der Lebensqualität bei Sehbeeinträchtigten. Zeitschrift für Medizinische Psychol. Published online 1998:178–184. https://www.researchgate.net/publication/310595360_Der_National_Eye_Institute_Visual_Function_Questionnaire_NEI-VFQ_-_Erste_Ergebnisse_zur_psychometrischen_Uberprufung_eines_Verfahrens_zur_Erfassung_der_Lebensqualitat_bei_Sehbeeintrachtigten. Accessed June 3, 2022.
- 29.Mangione C. The national eye institute 25-item visual function questionnaire (VFQ-25). https://www.nei.nih.gov/learn-about-eye-health/outreach-campaigns-and-resources/outreach-materials/visual-function-questionnaire-25. Published 2000. Accessed June 3, 2022.
- 30.RIS - Arzneimittelgesetz - Bundesrecht konsolidiert, Fassung vom 30.04.2022 . https://www.ris.bka.gv.at/GeltendeFassung.wxe?Abfrage=Bundesnormen&Gesetzesnummer=10010441. Accessed April 30, 2022.
- 31.Cornille K, Milea D, Amati-Bonneau P, et al. Reversible optic neuropathy with OPA1 exon 5b mutation. Ann Neurol. 2008;63(5):667–671. doi: 10.1002/ANA.21376. [DOI] [PubMed] [Google Scholar]
- 32.Pemp B, Mitsch C, Kircher K, Reitner A. Changes in visual function and correlations with inner retinal structure in acute and chronic Leber’s hereditary optic neuropathy patients after treatment with idebenone. J Clin Med. 2021;10(1):1–13. doi: 10.3390/JCM10010151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eiberg H, Kjer B, Kjer P, Rosenberg T. Dominant optic atrophy (OPA1) mapped to chromosome 3q region. I. Linkage analysis. Hum Mol Genet. 1994;3(6):977–980. doi: 10.1093/HMG/3.6.977. [DOI] [PubMed] [Google Scholar]
- 34.Brown J. Clinical and genetic analysis of a family affected with dominant optic atrophy (OPA1). Arch Ophthalmol. 1997;115(1):95–99. doi: 10.1001/ARCHOPHT.1997.01100150097016. [DOI] [PubMed] [Google Scholar]
- 35.Eckmann-Hansen C, Bek T, Sander B, Larsen M. Vision-related quality of life and visual ability in patients with autosomal dominant optic atrophy. Acta Ophthalmol. 2022;100(7):797–804. Published online 2022. doi: 10.1111/AOS.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]


