Abstract
Background
Atopic dermatitis (AD) is a common skin disease which, depending on its severity, can have a significant impact on the quality of life of affected individuals. In cases of severe AD, systemic immunomodulatory agents can be considered for treatment. However, the available treatment options for moderate AD are limited. According to previous reports, however, 308-nm excimer light is a potential treatment for localized, moderate AD.
Objective
This study aimed to assess the clinical efficacy and safety of 308-nm excimer light in Korean adults with AD.
Methods
This study included Korean patients aged over 19 years, who were diagnosed with AD by a dermatologist, with bilateral, symmetric, and eczematous lesions. The symmetrical lesions in each patient were treated as control-test pairs. Treatment with 308-nm excimer light was applied to the test lesion twice a week for 4 weeks. The severity of the eczema, trans-epidermal water loss, and epidermal capacitance were measured.
Results
A total of 25 participants were enrolled in the study. After the first visit, two participants withdrew, whereas the remaining 23 completed the study. There was a statistically significant improvement in AD severity in the test group than in the control group (p<0.001). Skin barrier function also improved in the test than in the control group (p<0.01).
Conclusion
This study provides preliminary evidence for the use of 308-nm excimer light as a treatment option to improve symptoms and skin barrier function in moderately localized AD.
Keywords: Dermatitis, atopic, Lasers, excimer, Phototherapy, Ultraviolet therapy
INTRODUCTION
Atopic dermatitis (AD) is a common inflammatory skin disease that, depending on its severity, has a significant impact on the quality of life of affected individuals. It has a prevalence of 10% to 20% in children and 1% to 3% in adults; moreover, its prevalence is increasing worldwide1. AD is characterized by abnormal skin barrier function, hypersensitivity to allergens, recurrent skin infections, and a chronic course. Although the pathogenesis of AD is not precisely known, various genetic and environmental factors are thought to cause skin barrier dysfunction and subsequent inflammation2.
The treatment of AD depends on its severity. For severe AD, systemic immune-modulating agents are effective options3. Recently, a systemic biological immunomodulator has been developed and widely adapted owing to its profile of favorable side effects4. However, there are limited treatment options available for moderate AD, which is not severe enough to require systemic immune-modulating treatment and does not respond well to emollients, topical corticosteroids, and lifestyle modifications. In particular, high-severity but small-area lesions present a tricky problem in treatment selection since the range of options is limited.
Monochromatic 308-nm excimer laser and light are super-narrow-band high-intensity ultraviolet B (UVB). This portion of the electromagnetic spectrum has been shown to be the most effective for targeted phototherapy against psoriasis and AD5. According to previous studies, 308-nm excimer light is effective in the treatment of localized lesions of AD in adults and children5,6,7,8,9,10. In this study, we aimed to determine the clinical effect and safety profile of 308-nm excimer light in Korean adult patients with AD.
MATERIALS AND METHODS
Study population
This study included patients diagnosed with AD by a dermatologist at the Korea University Ansan Hospital between May 2020 and April 2021. The diagnosis was based on the diagnostic criteria of Hanifin and Rajka11. Inclusion criteria were patients aged over 19 years with symmetrical AD lesions relative to the midline of the body. All patients reported a poor response to oral antihistamines and topical treatment with corticosteroids and calcineurin inhibitors. For patients who had been treated with topical corticosteroids or calcineurin inhibitors for existing skin lesions, a wash-out period of 2 weeks was set before beginning the test treatment. Exclusion criteria included the following: diagnosis of active infectious skin disease, history of keloids or photosensitivity, pregnancy or current breastfeeding status, and history of having received narrowband UVB (NB-UVB) or 308-nm excimer light treatment within the last month.
Treatment with 308-nm UV light
The AD lesions of participants were treated using a 308-nm excimer light device (CAREVEAM [EX-3000]; LAMEDITECH Inc.). The wavelength of light from this instrument was 308 nm, with a pulse duration of 10~180 seconds and a beam diameter of 2~5 cm. The excimer light can deliver a fixed fluence starting at 100 mJ/cm2, increasing in increments of 50 mJ/cm2 up to 2, 100 mJ/cm2. Before the treatment, all patients underwent a photosensitivity test by exposing an area of their skin to a dose range of 100~300 mJ/cm2 to determine the minimum erythema dose (MED) of the excimer light. The initial treatment dose was set at 50% to 100% of the MED, and the dosage was increased by 50 mJ/cm2 during each treatment session. Among the subjects who completed the study, symmetrical lesion distribution was observed as follows: 12 subjects had lesions on the upper extremities, 6 subjects had lesions on the lower extremities, 2 subjects had trunk lesions, and 3 subjects had lesions on the face and neck.
Study protocol
Pairs of symmetrical AD lesions in each patient were used as control-test pairs. The treatment arm was allocated through randomization by blinded investigator. The 308-nm excimer light treatment was applied on the test-side lesion twice a week for 4 weeks at 2 to 3 day intervals. Nine excimer light treatment sessions were performed for all patients. UV protection glasses were used to protect the eyes of the patients. During the study period, no other systemic or localized treatment for AD was permitted, with the exception of emollients. The patients were instructed to apply topical emollients to both the test and control surfaces. Clinical photographs were taken under the same conditions and at the same locations before the procedure and at each evaluation.
Evaluation of treatment efficacy
Treatment response was evaluated three times (first, fifth visit, and last visits), and a total of nine visits were made during the treatment. To evaluate the degree of improvement of the AD lesions, two independent investigators, who were blinded to the treatment allocation of each lesion, measured the eczema severity evaluation index without the area score for all the lesions during each evaluation. The eczema severity evaluation index without the area score is the sum of the scores for the four clinical symptoms (erythema, edema, excoriation, and lichenification), each on a scale of 0 to 3 (0: absent, 1: mild, 2: moderate, and 3: severe)8. In addition, transdermal water loss (TEWL) and epidermal capacitance were measured during each evaluation to determine epidermal barrier function and skin hydration. TEWL was measured using a Tewameter® TM300 (Courage+Khazaka Electronic GmbH), and epidermal capacitance was measured using a Corneometer® CM825 (Courage+Khazaka Electronic GmbH). The subjective degree of improvement was evaluated at the final visit using a questionnaire that required each participant to rate each lesion on a 5-point scale ranging from –1 to 3 (–1: aggravated, 0: no change, 1: slight improvement, 2: moderate improvement, and 3: much improvement).
Statistical analyses
Patient characteristics, including demographic data and observed outcomes, are summarized in the Table 1. Repeated measures analysis of variance and paired t-tests were conducted to compare the test and control group lesions. p-values <0.05 were regarded as statistically significant. All analyses were performed using SPSS version 23 (IBM Corp.).
Table 1. Demographic data of the patients.
| Value (n=23) | |||
|---|---|---|---|
| Sex | |||
| Male | 11 (47.83) | ||
| Female | 12 (52.17) | ||
| Age (yr) | 33.26±8.98 | ||
| MED | |||
| 150 mJ | 12 (52.17) | ||
| 200 mJ | 11 (47.83) | ||
| Sites of presentation | |||
| Upper extremity | |||
| Hand | 3 (13.04) | ||
| Forearm | 9 (39.13) | ||
| Lower extremity | |||
| Upper leg | 2 (8.70) | ||
| Lower leg | 4 (17.39) | ||
| Trunk | 2 (8.70) | ||
| Face or neck | 3 (13.04) | ||
Values are presented as number (%) or mean±standard deviation. MED: minimal erythema dose. A total of 23 patients with symmetrical atopic dermatitis completed the study. The results show a similar distribution of demographic characteristics. The MED is set at 150 or 200 mJ depending on the patient.
Ethical approval
Informed consent for both treatment and photography of the results were obtained from all the patients. This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization and Good Clinical Practice Guidelines. The study was approved by the Institutional Review Board of Korea University Ansan Hospital (IRB no. 2020AS0026).
RESULTS
Patient characteristics
A total of 25 patients were enrolled in this study. After the first screening/baseline visit, 2 patients withdrew from the study due to loss of follow-up; however, the remaining 23 completed the study. Among them, 12 (52.17%) were females and 11 (47.83%) were males. Their ages ranged between 20 and 51 years (mean±standard deviation [SD], 33.26±8.98 years). All lesions that were symmetric with respect to the sagittal midline of each patient were paired as control-test pairs (Table 1).
Effect of excimer light on eczema severity
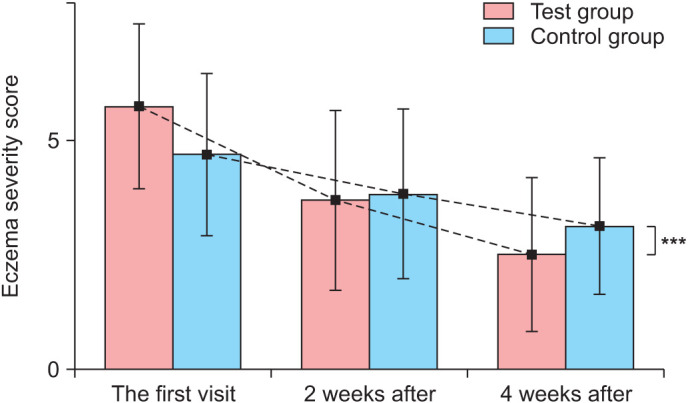
At the start of treatment, the mean severity index, as measured by the eczema severity evaluation index without the area score, was 5.74±1.79 in the test group and 4.70±1.77 in the control group. Looking at each criterion separately, the initial erythema, edema, excoriation, and lichenification indices were 1.52±0.73, 1.13±0.81, 1.30±0.47, and 1.96±0.77, respectively, for the test group lesions and 1.17±0.65, 0.74±0.75, 1.26±0.62, and 1.52±0.66, respectively, for the control group lesions. In the test group, the severity of eczema was 3.7±1.96 after 2 weeks and 2.52±1.68 after 4 weeks. In the control group, the severity index was 3.83±1.85 after 2 weeks and 3.13±1.49 after 4 weeks.
Over time, the severity index of the AD decreased in both test and control groups (Fig. 1). However, there was a statistically significant difference between the average severity indices of the test group and the control group throughout the test period (p<0.001). A significant improvement in the mean severity score was achieved after 2 weeks (p<0.001) and was maintained until the final assessment after 4 weeks (p<0.001; Fig. 2).
Fig. 1. Clinical photographs of patients. Treatment with 308-nm ultraviolet (UV) excimer light is applied to the intervention side lesion twice a week for 4 weeks. (A, B) Clinical photographs of intervention side before 308-nm UV excimer light treatment. (C, D) Clinical photographs of intervention side after 4 weeks 308-nm UV excimer light treatment.
Fig. 2. Effect of the 308-nm excimer light on eczema severity scores of atopic dermatitis lesions. Treatment with 308-nm excimer light significantly improves eczema severity scores at week 4. Eczema severity scores are calculated as the sum of the individual scores, as described in the Materials and Methods section. ***p<0.001 compared with the control group (n=23).
Treatment efficacy on skin barrier function
To estimate skin barrier function, TEWL was measured, and changes over time were evaluated. The mean TEWL (±SD) at the first visit was 31.79±15.29 g/h/m2 in the test group and 29.54±15.21 g/h/m2 in the control group. In the test group, the TEWL gradually decreased with time to 21.33±11.34 g/h/m2 at 2 weeks after excimer light treatment and to 17.03±7.92 g/h/m2 at 4 weeks. In the control group, TEWL was 27.01±17.03 g/h/m2 at 2 weeks and 27.59±14.70 g/h/m2 at 4 weeks; this does not suggest a clear pattern of improvement. The differences in TEWL in the test group between the first visit and 2 weeks, 2 and 4 weeks, and between the first visit and 4 weeks were all statistically significant (p<0.001, p=0.021, p<0.001, respectively). However, there were no significant differences in TEWL over time in the control group (p=0.389, p=0.826, and p =0.483, respectively). In addition, the difference in the level of change in TEWL between the test and control groups was statistically significant (p<0.01; Fig. 3).
Fig. 3. Effect of the 308-nm excimer light on the trans-epidermal water loss (TEWL) scores of atopic dermatitis lesions. Treatment with 308-nm excimer light significantly decreases TEWL at week 4. Reduced TEWL indicates an improvement in skin barrier function. **p<0.01 compared with the control group (n=23).

Treatment efficacy on skin moisturization
To evaluate the degree of skin moisturization, epidermal capacitance was measured in the test and control group lesions, and changes in epidermal capacitance over time were evaluated. At the first visit, the mean epidermal capacitance±SD was 41.24±8.92 A.U. in the test group and 46.80±8.57 A.U. in the control group. In the test group, the epidermal capacitance gradually increased with time to 47.06±8.40 A.U. at 2 weeks after excimer light treatment and 50.08±8.05 A.U. at 4 weeks. In the control group, the epidermal capacitance was 40.52±8.44 A.U. at 2 weeks and 45.18±7.92 A.U. at 4 weeks; thus, there was no clear pattern of improvement. The difference in the average change in epidermal capacitance between the test and control groups was significant throughout the test period (p<0.001; Fig. 4).
Fig. 4. Effect of the 308-nm excimer light on the epidermal capacitance of atopic dermatitis lesions. Treatment with 308-nm excimer light significantly increases epidermal capacitance at week 4. Increased epidermal capacitance indicates an improvement in skin moisturization. ***p<0.001 compared with the control group (n=23).

Patient questionnaire
After 2 weeks of excimer light treatment, the average degree of subjective improvement was 0.91 points in the test group (no change to slight improvement) and 0.31 points in the control group (from no change to slight improvement). After 4 weeks of treatment, the average score for the test group was 1.35 points (from slight improvement to moderate improvement) and 0.28 for the control group (from no change to slight improvement).
Evaluation of safety profile
Follow-up was performed for up to 4 weeks after treatment. No adverse reactions were reported in any of the 23 patients who completed the study or in the 2 patients who withdrew earlier.
DISCUSSION
The management of AD requires a complex approach depending on the severity and distribution of lesions, as well as the demographic characteristics of the patient4. For patients with mild AD, lifestyle modifications, emollients, and topical steroids are considered as first-line treatments. However, severe AD often has a chronic course that does not respond to these treatments. For severe AD, systemic immunomodulatory drugs, such as cyclosporin A, may be used for a set period. Novel medications, such as biologics and Janus kinase inhibitor, are emerging as important treatment options for patients with severe AD and are proving to be effective12.
However, these drugs are often inaccessible owing to their high costs and limited insurance coverage; they are currently used only for patients with widespread and severe lesions that do not respond to systemic immunomodulatory drugs. This leaves the group of patients with localized but large and severe lesions with oozing and lichenification (i.e., moderately localized AD) with limited treatment options, as the only available therapies for them are topical agents or systemic immunomodulators such as cyclosporin and methotrexate. The severity of AD is currently measured using the Eczema Area and Severity Index (EASI) score, which determines the severity by multiplying the area and severity of each lesion. This means that patients with lesions that are relatively small in area have lower EASI scores and are deemed ineligible for biological therapy under insurance coverage. This study aimed to assess the effects of 308-nm excimer light on patients with moderate, localized AD and to investigate the feasibility of using 308-nm excimer light as an option for them.
The most widely used phototherapies are NB-UVB and psoralen plus UVA. They are used to treat patients with moderate-to-severe AD. The 308-nm excimer laser and light are a new form of phototherapy, and although their wavelength is similar to that of NB-UVB (311 nm), many studies have shown them to be more effective in treating skin lesions13. In particular, previous studies have revealed that the 308-nm excimer light is more effective than NB-UVB in inducing T cell apoptosis13,14. In addition, while conventional phototherapy or photochemotherapy exposes the whole body to UV light, the 308-nm excimer light has the advantage that the treatment area can be limited to the relevant area. Excimer light was initially introduced for the treatment of psoriasis. While excimer laser and light already constitute the standard of care for psoriasis and vitiligo, its use in the treatment of AD is still not widely accepted. A larger body of evidence is warranted in this regard15. So far, studies have shown that excimer light can improve skin barrier function in patients with AD, mostly based on animal studies10. Investigations in humans have been limited and a few studies from Europe have demonstrated the effectiveness of reducing the severity of AD7,15. In Korea, there have only been animal studies and studies measuring changes in the skin microbiome10,16. Based on our understanding, there have not been many studies investigating the effect of 308-nm excimer light on skin barrier function in patients with AD.
Evaluation of skin barrier function using TEWL confirmed that the skin barrier function improved significantly after 4 weeks of treatment than before treatment or than that in the control group. Similarly, skin moisturization measured by the Corneometer showed a statistically significant improvement in the test group than in the control group. The differences between the test and control groups were statistically significant throughout the experimental period. Patients’ subjective evaluations also indicated a greater improvement in lesions within the test group.
No adverse effects, such as burning or pruritus in the treatment area, were described by the patients. Our results suggest that 308-nm excimer light is effective and well-tolerated for moderate, localized AD.
Limitation of this study is that the effects were compared over a relatively short period of time, which results in limitations in evaluating long-term effects and safety profiles. Further research is needed to investigate whether the effects of improving skin barrier function and reducing the severity of eczema in AD patients persist with long-term treatment. Additionally, it is necessary to conduct comparative studies with other treatment modalities targeting a larger number of patients. Evaluating the effectiveness of combination therapy with topical corticosteroid or topical calcineurin inhibitor is also needed. Although long-term results have not yet been analyzed, this treatment appears to be a promising treatment option for patients with AD.
ACKNOWLEDGMENT
We would like to thank the patients who allowed us to report this article using their clinical photographs.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: This work was supported by LAMEDITECH. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Lee JH, Han KD, Kim KM, Park YG, Lee JY, Park YM. Prevalence of atopic dermatitis in Korean children based on data from the 2008-2011 Korean National Health and Nutrition Examination Survey. Allergy Asthma Immunol Res. 2016;8:79–83. doi: 10.4168/aair.2016.8.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Yun SJ, Lee JB, Lee SC. Therapeutic efficacy and safety of methotrexate in moderate-to-severe atopic dermatitis: a retrospective study of Korean patients at tertiary referral hospital. Ann Dermatol. 2020;32:402–408. doi: 10.5021/ad.2020.32.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, Kim JE, Park GH, Bae JM, Byun JY, Shin MK, et al. Consensus update for systemic treatment of atopic dermatitis. Ann Dermatol. 2021;33:497–514. doi: 10.5021/ad.2021.33.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemény L, Varga E, Novak Z. Advances in phototherapy for psoriasis and atopic dermatitis. Expert Rev Clin Immunol. 2019;15:1205–1214. doi: 10.1080/1744666X.2020.1672537. [DOI] [PubMed] [Google Scholar]
- 6.Aubin F, Vigan M, Puzenat E, Blanc D, Drobacheff C, Deprez P, et al. Evaluation of a novel 308-nm monochromatic excimer light delivery system in dermatology: a pilot study in different chronic localized dermatoses. Br J Dermatol. 2005;152:99–103. doi: 10.1111/j.1365-2133.2005.06320.x. [DOI] [PubMed] [Google Scholar]
- 7.Baltás E, Csoma Z, Bodai L, Ignácz F, Dobozy A, Kemény L. Treatment of atopic dermatitis with the xenon chloride excimer laser. J Eur Acad Dermatol Venereol. 2006;20:657–660. doi: 10.1111/j.1468-3083.2006.01495.x. [DOI] [PubMed] [Google Scholar]
- 8.Kurosaki Y, Tsurumachi M, Kamata Y, Tominaga M, Suga Y, Takamori K. Effects of 308 nm excimer light treatment on the skin microbiome of atopic dermatitis patients. Photodermatol Photoimmunol Photomed. 2020;36:185–191. doi: 10.1111/phpp.12531. [DOI] [PubMed] [Google Scholar]
- 9.Nisticò SP, Saraceno R, Capriotti E, Felice CD, Chimenti S. Efficacy of monochromatic excimer light (308 nm) in the treatment of atopic dermatitis in adults and children. Photomed Laser Surg. 2008;26:14–18. doi: 10.1089/pho.2007.2116. [DOI] [PubMed] [Google Scholar]
- 10.Oh CT, Kwon TR, Seok J, Choi EJ, Kim SR, Jang YJ, et al. Effect of a 308-nm excimer laser on atopic dermatitis-like skin lesions in NC/Nga mice. Lasers Surg Med. 2016;48:629–637. doi: 10.1002/lsm.22524. [DOI] [PubMed] [Google Scholar]
- 11.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1980;92:44–47. [Google Scholar]
- 12.Ahn J, Choi Y, Simpson EL. Therapeutic new era for atopic dermatitis: part 2. Small molecules. Ann Dermatol. 2021;33:101–107. doi: 10.5021/ad.2021.33.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bónis B, Kemény L, Dobozy A, Bor Z, Szabó G, Ignácz F. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522. doi: 10.1016/S0140-6736(05)63945-1. [DOI] [PubMed] [Google Scholar]
- 14.Novák Z, Bónis B, Baltás E, Ocsovszki I, Ignácz F, Dobozy A, et al. Xenon chloride ultraviolet B laser is more effective in treating psoriasis and in inducing T cell apoptosis than narrow-band ultraviolet B. J Photochem Photobiol B. 2002;67:32–38. doi: 10.1016/s1011-1344(02)00280-4. [DOI] [PubMed] [Google Scholar]
- 15.Wolkerstorfer A, Brenninkmeijer EEA. Excimer laser: a treatment option for the prurigo form of atopic dermatitis. Expert Rev Dermatol. 2011;6:1–3. [Google Scholar]
- 16.Park JY, Kim SM, Kim JH. Efficacy of phototherapy with 308-nm excimer light for skin microbiome dysbiosis and skin barrier dysfunction in canine atopic dermatitis. Front Vet Sci. 2021;8:762961. doi: 10.3389/fvets.2021.762961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.



