Dear Editor:
The prevalence of skin cancer, including melanoma, is increasing worldwide. Although it is known that melanoma is the fourth most common cancer in the West, the incidence of melanoma is steadily increasing in Korea as well. Because of its rapid proliferation and metastasis, melanoma has a high mortality rate among various types of skin cancers.
Aldo-keto reductases (AKR) subfamily plays an important biochemically role in steroid metabolism and exist as several isoforms. It has been reported that AKR family 1 member C3 (AKR1C3), one of AKR subfamily including AKR1C1-4, could govern indirectly ligand binding to various receptors, such as androgen receptor, estrogen receptor, progesterone receptor, and peroxisome proliferator activated receptor, and regulate trans-activation activities of these nuclear receptors1. Dysregulated expression of AKR1C3 has been established in various hormone-dependent and hormone-independent cancers (Table 1)1,2,3,4,5. However, there are no reports to date on the expression of AKR1C3 in melanoma, known as hormone-independent cancer.
Table 1. The expression of AKR1C3 in hormone-dependent or independent cancers.
| Hormone dependency | Malignancy type | The expression of AKR1C3 | Reference |
|---|---|---|---|
| Dependent | Breast cancer | High expression | 1 |
| Prostate cancer | High expression | 1 | |
| Endometrial cancer | Low expression | 2 | |
| Lung small cell carcinoma | Low expression | 3 | |
| Lung adenocarcinoma | High expression | 3 | |
| Lung squamous cell carcinoma | High expression | 3 | |
| Gastric cancer | Low expression | 4 | |
| Independent | Papillary urothelial carcinoma | High expression | 5 |
| Renal cell carcinoma | High expression | 5 |
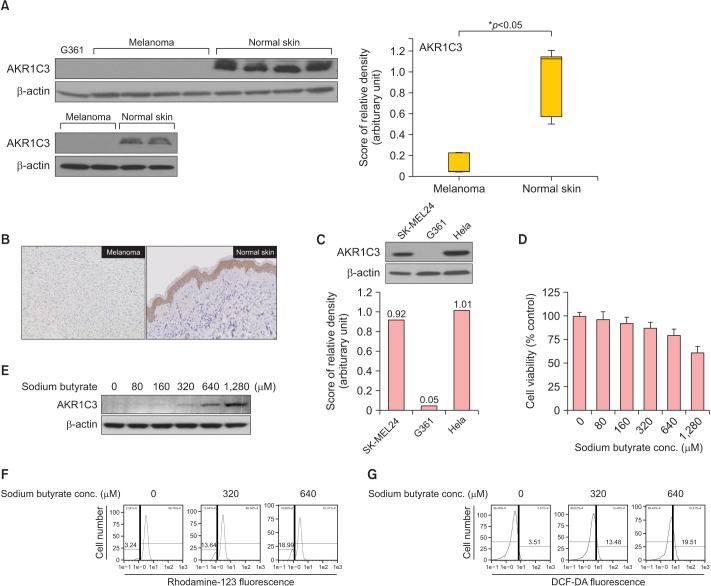
Therefore, we investigated the levels of AKR1C3 expression in six human cutaneous melanoma tissues and six normal skin tissues. Details about the materials and methods are provided in the Supplementary Materials. Melanoma samples were obtained from Korean patients with 4 nodular melanoma and 2 acral lentiginous melanoma. The Institutional Review Board of Soonchunhyang University Seoul Hospital reviewed and approved this research protocol, which involved the use of tissue samples (IRB File no. 2018-04-009). To determine the expression levels of AKR1C3 proteins in melanoma tissues and normal skin tissues, Western blot analysis was carried out. AKR1C3 was significantly decreased in melanoma tissues compared to normal skin tissues (Fig. 1A). The relative protein expressions were shown to be statistically significant (p<0.05) (Fig. 1A). Data revealed a median (interquartile range) of 0.0443 (0.04~0.23) and 1.1253 (0.50~1.21) for melanoma tissues and normal skin tissues, respectively. Representative immunohistochemical (IHC) staining results for AKR1C3 in melanoma tissues and normal skin tissues are shown in Fig. 1B. The staining intensities of AKR1C3 in melanoma tissues were significantly lower than those in normal skin tissue. We also investigated the expression of AKR1C3 in human melanoma cell lines SK-MEL24, G361 and human cervical cancer cell line Hela (Fig. 1C). Interestingly, the results showed significant overexpression of AKR1C3 in SK-MEL24 and Hela except G361.
Fig. 1. AKR1C3 was downregulated in melanoma tissue, and upregulated following sodium butyrate. (A) The expression of AKR1C3 was almost decreased in melanoma but was significantly increased in normal human skin tissues. The relative protein expression of AKR1C3 according to the Mann–Whitney U-test was analyzed. (B) Representative immunohistochemistry (IHC) staining for AKR1C3 protein expression in paraffin-embedded melanoma and normal skin tissue. Weakly positive staining of AKR1C3 in melanoma (IHC stain, ×200). Strongly positive staining of AKR1C3 in normal skin (IHC stain, ×200). (C) AKR1C3 was expressed in SK-MEL24 and Hela, but almost decreased in G361. (D) The percentage of viable G361 cells was measured by MTT assay. (E) AKR1C3 expression in G361 cells was upregulaged following sodium butyrate treatment (0, 80, 160, 320, 640, and 1,280 µM). (F) ΔΨm was measured by staining the cells with 30 nM of Rhodamine-123. (G) Cellular ROS levels were measured by staining the cells with DCF-DA (10 µM). DCF-DA: 2′,7′-dichlorodihydrofuorescein diacetate.
In this study, we treated G361 cells with sodium butyrate (Nabu) to examine the effect on cell viability and association with the expression of AKR1C3. MTT assay showed the viability of G361 cells decreased dose dependently in response to different concentrations (0, 80, 160, 320, 640, and 1280 µM) of Nabu (Fig. 1D). Furthermore, it was observed that the expression of AKR1C3 in G361 cells was increased following the treatment of Nabu, more distinctively at higher concentration (Fig. 1E). To determine whether the cytotoxicity on G361 cells of Nabu treatment was associated with oxidative stress, mitochondrial membrane potential (ΔΨm) and intracellular reactive oxygen species (ROS) levels were measured by flow cytometry using fluorescent dye rhodamine-123 and DCF-DA, respectively. The results showed that treatment of Nabu caused mitochondrial dysfunction and increased ROS production in G361 cells. The percentage of cells with ΔΨm loss increased to 3.24%, 13.64%, and 18.99% after treatment with 0, 320, and 640 µM of Nabu, respectively (Fig. 1F). Also, the production of intracellular ROS levels was increased to 3.51%, 13.48%, and 19.51% when Nabu concentration was 0, 320, and 640 µM of Nabu, respectively (Fig. 1G). These findings suggest that the increased level of ROS induced by Nabu impaired mitochondrial function.
According to previous reports, the functions of AKR1C3 in each cancer have been controversial. These reports suggest that the roles of AKR1C3 may be more complex in human cancers. In this study, we identified downregulation of AKR1C3 in melanoma ex vivo , using IHC staining and Western blotting. It has been reported that oxidative stress induced by increased ROS may contribute to development of melanoma, and one of the reasons about increased ROS levels in melanoma cells may be decreased antioxidant capability resulting from reduced catalase, glutathione-S-transferase and manganese superoxide dismutase enzymatic activity and low levels of glutathione6. In addition, AKR1C3 is also involved in removal of cellular ROS, as an antioxidant7. Therefore, suppressed expression of AKR1C3 in melanoma may be associated with carcinogenesis.
Histone modification, one of the epigenetic modifications, is suggested to be an important regulatory mechanism in various genetic processes such as transcription, replication and repair8. Several studies suggest that histone acetylation and deacetylation are associated with the expression of genes related to cancer progression. Also, it has been reported that high expression of histone deacetylase (HDAC) is associated with poor prognosis in cancer patients. HDACs are often dysregulated in melanomas, which regulate mitogen activated protein kinase pathway, immune checkpoint molecules, such as programmed death-1/programmed death ligand-1, related immune evasion, and response to chemotherapies9. Thus, HDAC inhibitors might be employed as an anti-melanoma drug, or as an adjuvant with current melanoma therapy.
Nabu, comprised of short chain fatty acid, has many effects on cellular activities including cell proliferation and differentiation by regulating other gene expression, by serving as a HDAC inhibitor10. In line with our results about AKR1C3 upregulation following Nabu treatment, there is a report disclosing that Nabu increased AKR1C3 expression levels in gastric cancer cell lines, and decreased cancer cell viability4. Furthermore, Nabu can stimulate the production of intracellular ROS, arrest cell cycle, and promote apoptosis10. Although we observed decreased cell viability and increased ROS level following Nabu treatment, we couldn’t make a conclusion that it was due to apoptosis because we haven’t conducted additional investigations.
Collectively, our study showed that downregulation of AKR1C3 expression may be associated with melanoma development. Also, we showed that inhibition of HDAC following Nabu treatment can increase AKR1C3 expression in melanoma cells. However, our study is limited by relatively small patient group and yet to clarify the effect of AKR1C3 on various melanoma cell lines including G361. Thus, further studies are needed to explain what the exact roles of AKR1C3 expression are in melanoma and difference of AKR1C3 expression between SK-MEL24 and G361 cells.
ACKNOWLEDGMENT
This work was supported by the Soonchunhyang University Research Fund.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
FUNDING SOURCE: None.
SUPPLEMENTARY MATERIALS
Supplementary data can be found via http://anndermatol.org/src/sm/ad-22-108-s001.pdf.
References
- 1.Lin HK, Steckelbroeck S, Fung KM, Jones AN, Penning TM. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3alpha-hydroxysteroid dehydrogenase/type 5 17beta-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69:795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Zakharov V, Lin HK, Azzarello J, McMeekin S, Moore KN, Penning TM, et al. Suppressed expression of type 2 3alpha/type 5 17beta-hydroxysteroid dehydrogenase (AKR1C3) in endometrial hyperplasia and carcinoma. Int J Clin Exp Pathol. 2010;3:608–617. [PMC free article] [PubMed] [Google Scholar]
- 3.Miller VL, Lin HK, Murugan P, Fan M, Penning TM, Brame LS, et al. Aldo-keto reductase family 1 member C3 (AKR1C3) is expressed in adenocarcinoma and squamous cell carcinoma but not small cell carcinoma. Int J Clin Exp Pathol. 2012;5:278–289. [PMC free article] [PubMed] [Google Scholar]
- 4.Frycz BA, Murawa D, Borejsza-Wysocki M, Wichtowski M, Spychała A, Marciniak R, et al. Transcript level of AKR1C3 is downregulated in gastric cancer. Biochem Cell Biol. 2016;94:138–146. doi: 10.1139/bcb-2015-0096. [DOI] [PubMed] [Google Scholar]
- 5.Azzarello JT, Lin HK, Gherezghiher A, Zakharov V, Yu Z, Kropp BP, et al. Expression of AKR1C3 in renal cell carcinoma, papillary urothelial carcinoma, and Wilms’ tumor. Int J Clin Exp Pathol. 2009;3:147–155. [PMC free article] [PubMed] [Google Scholar]
- 6.de Melo FHM, Molognoni F, Galvonas M. In: Recent advances in the biology, therapy and management of melanoma. Davids LM, editor. InTech; 2013. The role of oxidative stress in melanoma development, progression and treatment; pp. 83–109. [Google Scholar]
- 7.Xiong W, Zhao J, Yu H, Li X, Sun S, Li Y, et al. Elevated expression of AKR1C3 increases resistance of cancer cells to ionizing radiation via modulation of oxidative stress. PLoS One. 2014;9:e111911. doi: 10.1371/journal.pone.0111911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Jang A, Seo SJ, Myung SC. Epigenetic regulation of filaggrin gene expression in human epidermal keratinocytes. Ann Dermatol. 2020;32:122–129. doi: 10.5021/ad.2020.32.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeon M, Kim Y, Jung HS, Jeoung D. Histone deacetylase inhibitors to overcome resistance to targeted and immuno therapy in metastatic melanoma. Front Cell Dev Biol. 2020;8:486. doi: 10.3389/fcell.2020.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salimi V, Shahsavari Z, Safizadeh B, Hosseini A, Khademian N, Tavakoli-Yaraki M. Sodium butyrate promotes apoptosis in breast cancer cells through reactive oxygen species (ROS) formation and mitochondrial impairment. Lipids Health Dis. 2017;16:208. doi: 10.1186/s12944-017-0593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.