Summary
Background
Although treatment for Hepatitis C Virus (HCV) is effective, individuals face access barriers. The utility of mobile health clinics (MHC), effective mechanisms for providing healthcare to underserved populations, is understudied for HCV-related interventions. We aimed to describe implementation of, and factors associated with, screening and treatment via MHCs.
Methods
Clemson Rural Health implemented a novel MHC program to reach and treat populations at-risk for HCV with a focus on care for uninsured individuals. We examined HCV screening and treatment initiation/completion indicators between May 2021 and January 2023.
Findings
Among 607 individuals screened across 31 locations, 94 (15.5%) tested positive via antibody and viral load testing. Treatment initiation and completion rates were 49.6% and 86.0%, respectively. Among those screened, the majority were male (57.5%), White (61.3%; Black/Hispanic: 28.2%/7.7%), and without personal vehicle as primary transportation mode (54.4%). Injection drug use (IDU) was 27.2% and uninsured rate was 42.8%. Compared to HCV-negative, those infected included more individuals aged 30–44 (52.1% vs. 36.4%, p = 0.023), male (70.2% vs. 55.2%, p = 0.009), White (78.5% vs. 60.2%, p < 0.0001), without personal vehicle (58.5% vs. 43.5%, p = 0.028), IDU (83.7% vs. 21.0%, p < 0.0001), and uninsured (61.2% vs. 48.8%, p = 0.050). Uninsured rates were higher among those initiating compared to not initiating treatment (74.5% vs. 45.3%, p = 0.004).
Interpretation
The MHC framework successfully reaching its target population: at-risk individuals with access barriers to healthcare. The high HCV screening and treatment initiation/completion rates demonstrate the utility of MHCs as effective and acceptable intervention settings among historically difficult-to-treat populations.
Funding
Gilead Sciences, Inc., and SC Center for Rural and Primary Healthcare.
Keywords: Mobile health clinics, Hepatitis C virus, Underserved populations, Uninsured populations, People who inject drugs
Research in context.
Evidence before this study
Hepatitis C virus (HCV) infection is a significant public health concern, with a surge in prevalence and associated mortality over the past decade. Despite the effectiveness of HCV treatments, most patients remain undiagnosed or untreated due to lack of awareness and inadequate access to healthcare services. Mobile Health Clinics (MHCs) offer potential solutions to these challenges, offering quality healthcare to vulnerable and underserved populations with access barriers to care. Previous research has shown the effectiveness of MHCs in providing a variety of healthcare services, reducing inequality in healthcare access, and improving health outcomes in underserved and rural populations. However, the potential of MHCs in screening and providing treatment for HCV has been underexplored. Furthermore, vulnerable populations, including individuals with low socioeconomic status, geographical burdens, minority populations, uninsured populations, and people who inject drugs (PWID), are at a heightened risk of HCV infection. Nevertheless, these populations face additional barriers to accessing HCV screening and treatment, including stigma, unstable housing, lack of transportation, and a lack of knowledge about HCV. While MHCs have shown success in serving these high-risk populations, HCV screening and treatment have not been widely integrated into MHC services. We reviewed literature for mobile health clinics/mobile medical clinics/mobile health units and HCV which resulted in four papers describing the integration of HCV services into MHCs. However, these studies described utilization of MHC services for HCV screening or treatment, but not both. Two papers (with one MHC program operating in Madrid, Spain and the other in Connecticut, United States) demonstrated the utility of MHCs for screening and testing for HCV but provided referral to external facilities for treatment rather than overseeing care. An additional paper examined a mobile hepatitis clinic’s feasibility for same-day treatment uptake among patients previously screened at external health centres in Rwanda. The final paper included HCV services through an MHC program, but HCV was not the focus of the program. We found no studies documenting implementation of MHCs for the spectrum of HCV care from screening through treatment initiation and completion and with focus on underserved, high-risk populations, particularly uninsured populations. This study aims to describe the implementation of, and factors associated with, HCV screening and treatment delivered via MHC in underserved populations.
Added value of this study
This study demonstrates the feasibility and effectiveness of a MHC framework for HCV screening and treatment among underserved, high-risk populations. The high rates of HCV screening, treatment initiation, and treatment completion demonstrate the utility of MHCs as an effective and acceptable intervention setting among historically difficult-to-treat populations. The MHCs successfully reached their target population of underserved individuals with access barriers to health care. Notably, the MHC did not only overcome physical barriers that may limit an individual’s access to care, but mitigated barriers from historically difficult-to-treat populations including injection drug users and uninsured individuals. Individuals without insurance, those without a personnel vehicle as their primary mode of transportation, and injection drug uses were substantially more likely to utilize MHC services for HCV screening and more likely to test positive for HCV. Uninsured individuals were also substantially more likely to initiative HCV treatment. These findings represent a significant step forward in understanding how to leverage MHCs to address the HCV epidemic, particularly among vulnerable populations.
Implications of all the available evidence
The demonstrated potential of MHCs to provide a comprehensive range of care services for HCV, from screening to treatment, suggests that these mobile clinics could be a key tool in reducing the health and economic impacts of HCV, particularly among underserved and vulnerable populations. Policymakers should consider investing in MHC programs as part of broader strategies to increase HCV diagnosis and treatment rates, achieve health equity, and progress towards HCV elimination as a public health threat. Additional research should assess the long-term health outcomes of HCV patients treated through MHCs and examine the cost-effectiveness of this approach to inform sustainable financing strategies. The findings of this study, combined with existing evidence, demonstrate the potential of MHCs to deliver screening and treatment not only for HCV but also other diseases or chronic conditions. Additionally, this research provides a valuable framework that can be applied by other health systems and expanded to other diseases, potentially enhancing early diagnosis, coordinated preventative care, and overall health outcomes. Future research should continue to explore and refine models of MHCs for disease screening and treatment, particularly for those conditions disproportionately affecting underserved populations.
Introduction
Hepatitis C virus (HCV) infection is a debilitating condition whose prevalence has increased at an alarming rate and with significant consequences, including liver failure and high risk of mortality.1 A recent analysis from the Centers for Disease Control and Prevention found that HCV infections have increased three-fold since 2010 and deaths attributed to HCV have surpassed those from human immunodeficiency virus (HIV).2 Treatments can produce a virological cure (undetectable HCV infection 12 weeks after treatment completion), but few patients are aware of their diagnosis or available avenues to receive care and treatment.1,3 An estimated 81% of HCV-infected individuals are unaware of their status, with only 15% of those diagnosed having received treatment.1 Low screening and treatment rates for HCV are exacerbated by the fact that many of the hardest-hit populations have inadequate access to medical care and lifesaving medications.2,4 Mobile health clinics (MHC) offer one avenue to provide quality healthcare to vulnerable and underserved populations, and communities with geographical burdens to access.4 Key beneficiaries include those living in rural and low-income areas, homeless people, migrant and immigrant workers, minority groups, and those who are underinsured or unlinked to primary care.4, 5, 6, 7, 8, 9, 10 However, HCV is rarely addressed in MHCs. Effective protocols are needed for screening and understanding of the utility of MHCs for provision of treatment among populations with barriers to care.5,11, 12, 13
MHCs have documented success in providing a variety of healthcare services to underserved populations.4,5 Following heightened interest in MHCs due to the urgency for care for difficult-to-reach populations during the COVID-19 pandemic, MHCs are viewed as valuable tools that will remain a critical part of quality and accessible healthcare.11,14, 15, 16, 17 MHC services improve health outcomes through screenings, preventative care, management of chronic diseases, and enabling vulnerable populations to confidently manage their health. They reduce inequality in healthcare based on social determinants of health by providing care to populations that are generally underserved because of race, socioeconomic status, living conditions, or drug use.4 These achievements include the added advantage of reducing cost on the healthcare system, solidifying the evident benefits of MHCs across the healthcare spectrum.4,16,18
HCV presents barriers to the benefits of MHCs, even when MHCs are ideally positioned to serve high-risk populations for infection. Underserved populations are at heightened risk of HCV, with individuals with low socioeconomic status, homelessness, geographical burdens, minority populations, and uninsured populations disproportionally affected by HCV.19, 20, 21, 22, 23 In particular, uninsured patients are a primary population for MHCs, with a review of MHCs in the United States (U.S.) showing that 41% of patients were uninsured.5 Additionally, HCV is highly transmittable through injection drug use (IDU); thus, a large majority of HCV infections are among people who inject drugs (PWID).22,24, 25, 26 However, PWID have generally low treatment uptake.26,27 Barriers include fear of stigma if they sought treatment; impermanent housing and lack of transportation; lack of access to insurance, comprehensive healthcare, or treatment; and a lack of knowledge about HCV.20,24,25,27,28 That said, through their mobility and mission of delivering healthcare to underserved populations, MHCs may be optimal drivers of care among these populations. HCV screening and treatment is rarely a provided service in MHCs and when MHCs have been successful in screening patients for HCV and linking them to care, the MHC’s utility as a vehicle to follow-up or provide treatment is unexplored, particularly with primary focus on HCV and uninsured populations at-risk of HCV.12,13,29 In the face of challenges, the durability of MHCs relies on research and novel approaches to care to mitigate barriers and optimize their potential to serve the community.
The present study describes the implementation of a novel MHC program of interdisciplinary care coordination for underserved South Carolinians with HCV. Utilizing MHCs led by Nurse Practitioners, Clemson Rural Health developed an approach to screen and treat priority populations in the Upstate and Midlands regions of South Carolina. Through the mission-guided focus on improving health outcomes of underserved patients, the Clemson Rural Health framework was positioned to provide equitable access and address barriers to care. The framework also explored potential for an MHC program to be involved throughout the care process by delivering HCV treatment to un-stationary or homeless patients. The utility of an MHC program for facilitating treatment of HCV has not been explored in a U.S. setting and the program’s focus on care for uninsured individuals is an innovative approach to barriers to care in these populations. In this article, we describe the Clemson Rural Health framework and examine its reach in providing access to care and treatment for rural and uninsured populations with HCV.
Methods
Setting
Housed within a college of a major research university, Clemson Rural Health is the organising academic unit for Clemson University’s health service delivery across the state, including nine mobile health units. Delivering comprehensive clinical care through a multidisciplinary team in fixed and mobile facilities, Clemson Rural Health seeks to reduce premature mortality and preventable hospitalisations, while improving overall wellness.
The Clemson Rural Health MHCs have been working in rural and underserved communities in South Carolina since 1995 and are staffed by advanced practice registered nurses (APRNs) trained in HCV and addiction medicine, registered nurses, health educators, dietitians, social workers, and Spanish-language translators. The Clemson Rural Health MHC program began expansion in 2016 and has developed partnerships and programming in 31 of the 46 counties in South Carolina. Partnerships include local health systems, state agencies including the Department of Health and Environmental Control, community health events and free clinics, substance use treatment and rehabilitation facilities, faith-based organisations, and rural primary care practices. Migrant workers are specifically targeted for screening during annual outreach events. In the last fiscal year, from July 2021 to June 2022, the MHCs travelled more than 25,000 miles delivering care directly to patients in their own locale. Regular services include preventative screenings, women’s health, primary care, health education, and nutrition counselling. Most recently, the MHCs began screening for opioid use disorder, Hepatitis B Virus (HBV), HIV, and HCV through a Nurse Practitioner-led model. Given the proximity between MHC staff and patients, guidelines to mitigate the spread of COVID-19 were followed including use of personal protective equipment and maintenance of mandatory distances between patients.
Intervention
Clemson Rural Health began offering HCV screening and treatment in April 2021 to address the World Health Organization 2030 elimination goal. This best practice advisory involves screening all adults at least once in their lifetime, with additional screening recommended for those at high risk, including PWID, those with high-risk sexual behaviours, and those who share personal items with someone who is infected with HCV. Clemson Rural Health utilised existing relationships with community partners who serve populations who may be at higher risk to deploy screening services. HCV screening and treatment were integrated into the services provided by MHCs. Once HCV services were integrated, the program worked with communities to identify specific areas with gaps in HCV services and targeted these areas (e.g., methadone clinics, homeless shelters, food banks) in an effort to bring HCV care to those with limited access.
At the onset of the program, the framework was designed for success for insured individuals, with funding limiting care for HCV to insured individuals. Specifically, required lab work to confirm HCV infection with an RNA test for viral load (VL) was cost prohibitive without insurance. Clemson Rural Health extended the existing framework upon the discovery that many patients were uninsured, securing funding specifically for care for uninsured patients. This was done under the goal of ensuring that underserved patients have equitable access to care for HCV. The adjusted framework was proposed under the hypothesis that delivering care via MHCs would increase adherence and retention to treatment regimens and lead to better health outcomes for those with HCV.
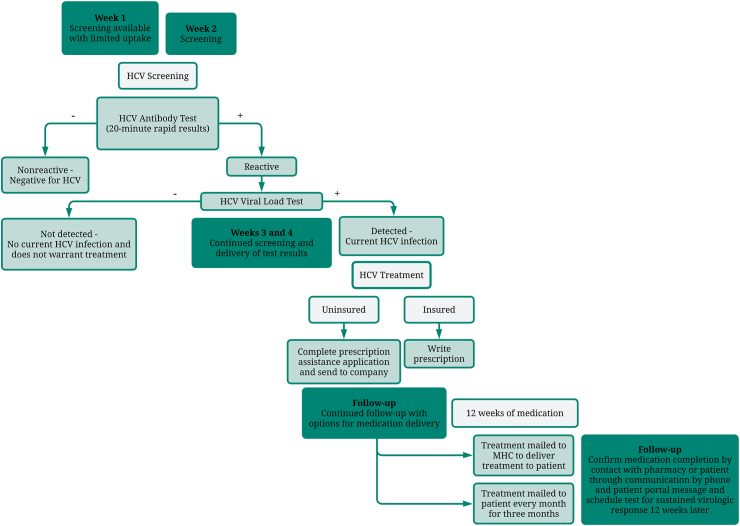
Clemson Rural Health MHCs began their enhanced care for uninsured HCV patients in July 2021. A diagram of the sequence of care for patients screened, tested, and treated for HCV is provided in Fig. 1. The program found that uptake was greatest when the MHC went to the same site once a week for four weeks in a row: in the first week, potential patients were unfamiliar with the MHC and uptake was limited; in the second week, patients allowed themselves to be screened; in the third and fourth weeks, the MHC continued screening and also followed-up with those who needed additional laboratory tests or treatment initiation due to a positive test. Any individual visiting the MHC who was of at least 18 years of age and consented to screening was eligible to be screened. Screening was offered at no cost to patients using rapid, point of care antibody testing that provided results in approximately 20 min. Basic demographic information was collected when screening patients, and those who tested positive were counselled by the MHC APRN on disease natural history and treatment options. This counselling included information about the risk of infection to a positive patient’s family or partners, and individuals were encouraged to bring these people to the MHC for screening as well. Next, MHC staff registered the patient within the electronic health record (EHR), including insurance information for billing purposes, where applicable. For uninsured patients, processes had been set up within the EHR to ensure that they were not billed for services. After completing consent for treatment and triage, as well as health history and social determinants of health screening (e.g., food security, transportation, social connections, housing status), the MHC APRN performed a physical exam and collected the individual’s labs to send out for processing for an HCV VL test. At the one-week follow-up, treatment was initiated through the Clemson Rural Health staff for those with a VL that warranted treatment, including a prescription for the treatment regimen. For patients without insurance, MHC staff completed prescription assistance applications and sent it to the drug company. Once approved, the company mailed the medication directly to the patient monthly for three months. As part of standard of care for HCV, those enrolled in treatment were also tested for HIV and HBV and were referred to local health departments for immunization and treatment if necessary.30
Fig. 1.
Diagram of the sequence of care at the MHCs for patients. Diagram shows patients screened, tested, and treated for HCV, including uptake of services by week of site visit, procedures for those testing positive, procedures for uninsured individuals, and MHC involvement in treatment and follow-up.
Treatment protocols followed the American Association for the Study of Liver Disease simplified treatment algorithm, with FIB-4 utilized to calculate cirrhosis risk.31 HCV genotyping was performed based on insurance requirements for treatment. MHC staff followed-up with patients throughout the course of treatment and delivered medication when necessary. When patients did not have safe or stable housing, the medications were shipped directly to the MHC for delivery to the patient. Additionally, if patients appeared to be at risk of dropping from treatment, MHC staff attempted contact at least three times through a variety of mechanisms such as phone, mail, messages through their patient portal, and emergency contacts. Phone and patient portal messages were also used to reach out to patients after 12 weeks of medication to schedule testing for sustained virologic response after an additional 12 weeks either at a local lab or through a visit with MHC staff.
Evaluation methods
We examined data from May 2021 to January 2023 to understand the reach and utility of the MHC for delivering care to underserved populations. Specifically, we used descriptive statistics, including number of patients who were screened for HCV, who had current infections, who initiated treatment, and who completed treatment to describe the program implementation. Current HCV infection was defined as a positive antibody test and detected HCV through a VL warranting treatment. Initiated treatment was defined as having picked up the first month’s bottle of medication at the pharmacy. Completed treatment was defined as having finished the medication regimen as confirmed by the patient through communication with MHC staff or having picked up their last medication bottle for their final month of treatment through confirmation with the patient’s pharmacy.32,33 Furthermore, we stratified these populations by age, sex, race, transportation type, IDU, and insurance. We performed chi-square tests to examine patient differences in categorical variables for VL test results (infected vs. not detected), treatment status (initiated vs. did not initiate), and treatment retention (completed/ongoing vs. incomplete). All analyses were conducted with R software version 4.2.2. The program was approved by the Prisma Health institutional review board (IRB Pro 00106348).
Role of the funding source
The program was supported by Gilead Sciences, Inc., (IN-US-987-5892) with additional funding specifically for uninsured patients from South Carolina Center for Rural and Primary Healthcare (2015593). The funders had no role in the design, conduct, reporting of the study, or decision to submit for publication.
Results
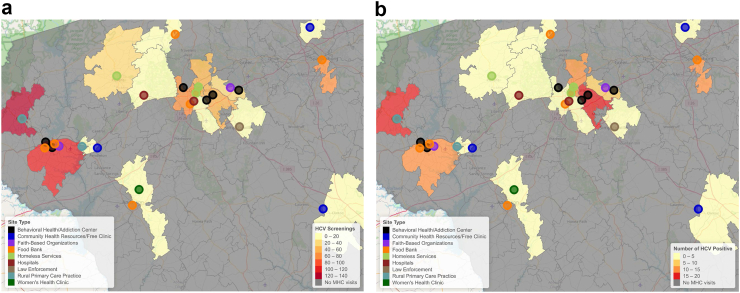
During the period from May 2021 to January 2023, the program conducted 118 site visits at 31 unique sites across 25 census tracts in the Upstate and Midlands regions of South Carolina. All but one of the MHC visits were conducted on weekdays. The location, and type, of site visit are shown in Fig. 2, with the number of people screened and with a current infection at each site type illustrated in Fig. 2 and listed in Table 1. A total of 607 individuals were screened for HCV. As shown in Fig. 2, the MHCs visited a variety of types of sites across the region, often with multiple types of sites within the same locale. Behavioural health or addiction clinics (N = 6), community health resources or free clinics (N = 5), food banks (N = 7), and homeless services (N = 4) were the most frequent types of sites visited by the MHCs. The highest number of people screened and detected cases occurred at behavioural health or addiction clinics (129 screened, 27 positive), food banks (210 screened, 23 positive), homeless services (80 screened, 20 positive), and law enforcement facilities (114 screened, 16 positive).
Fig. 2.
Maps of Upstate region MHC site visits. Maps showing number of people screened (a) and number of people with a detected current infection (b) in a visited zip-code. An additional two sites (Community Heath Resources/Free Clinics) were visited in the Midlands region with 13 people screened and 1 with detected HCV infection.
Table 1.
Number of locations of different MHC site types with individuals screened and testing positive by site type.
| Site type | Number of sites | Screened | HCV-infected (%) |
|---|---|---|---|
| Behavioral health/addiction centers | 6 | 129 | 27 (20.9) |
| Community health resources/free clinics | 5 | 20 | 1 (5.0) |
| Faith-based organizations | 2 | 16 | 3 (18.8) |
| Food banks | 7 | 210 | 23 (11.0) |
| Homeless services | 4 | 80 | 20 (25.0) |
| Hospitals | 2 | 3 | 0 (0.0) |
| Law enforcement | 2 | 114 | 16 (14.0) |
| Rural primary care practices | 2 | 27 | 2 (7.4) |
| Women’s health clinics | 1 | 5 | 0 (0.0) |
Descriptive statistics for individuals who were screened are given in the first column of Table 2, with continuous variables presented as median (inter-quartile range, IQR) and categorical variables presented as N (%). Median age in the screened population was 45 years of age (IQR: 35–58). The majority of people were 30–44 years of age (38.6%) or 45–64 years of age (38.1%). The majority were male (57.5%) and White (61.3%). An additional 28.2% identified as Black and 7.7% identified as Hispanic. IDU was 27.2%. Less than half of individuals used their own vehicle as the primary means of transportation (45.6%). Moreover, 25.1% had a primary mode of transportation through walking, biking, or scooter (36.2% among HCV-infected individuals). A total of 260 (42.8%) patients who participated in the program were uninsured, compared to 147 (24.2%) on Medicaid or Medicare and 104 (17.1%) with private insurance.
Table 2.
Descriptive statistics for entire population, individuals who were HCV-infected through viral load test, individuals who were entered into treatment, and individuals who have ongoing treatment or have completed treatment.
| Characteristics | Screened N = 607 |
HCV-infected N = 94 |
Initiated treatment N = 57 |
Completed or ongoing treatment N = 50 |
|---|---|---|---|---|
| Median age (IQR) | 45 (35–58) | 41 (34–57) | 39 (33–53) | 40 (32–56) |
| Age group | ||||
| 18–29 | 59 (9.7%) | 5 (5.3%) | 7 (12.3%) | 7 (14.0%) |
| 30–44 | 234 (38.6%) | 49 (52.1%) | 29 (50.9%) | 23 (46.0%) |
| 45–64 | 231 (38.1%) | 32 (34%) | 19 (33.3%) | 18 (36.0%) |
| 65 and over | 78 (12.9%) | 8 (8.5%) | 2 (3.5%) | 2 (4.0%) |
| Unknown | 5 (0.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Sex | ||||
| Female | 258 (42.5%) | 28 (29.8%) | 19 (33.3%) | 16 (32.0%) |
| Male | 349 (57.5%) | 66 (70.2%) | 38 (66.7%) | 34 (68.0%) |
| Race/ethnicity | ||||
| Black | 171 (28.2%) | 20 (21.3%) | 9 (15.8%) | 9 (18.0%) |
| Hispanic | 47 (7.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| White | 372 (61.3%) | 73 (77.7%) | 47 (82.5%) | 40 (80.0%) |
| Other | 2 (0.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Unknown | 15 (2.5%) | 1 (1.1%) | 1 (1.7%) | 1 (2.0%) |
| Transportation | ||||
| Bike | 14 (2.3%) | 3 (3.2%) | 1 (1.8%) | 1 (2.0%) |
| Car | 277 (45.6%) | 39 (41.5%) | 27 (47.4%) | 22 (44.0%) |
| Other car | 41 (6.8%) | 11 (11.7%) | 7 (12.3%) | 6 (12.0%) |
| Public Transportation | 45 (7.4%) | 10 (10.6%) | 6 (10.5%) | 6 (12.0%) |
| Scooter | 7 (1.2%) | 2 (2.1%) | 2 (3.5%) | 2 (4.0%) |
| Walk | 131 (21.6%) | 29 (30.9%) | 13 (22.8%) | 12 (24.0%) |
| Unknown | 92 (15.2%) | 0 (0.0%) | 1 (1.8%) | 1 (2.0%) |
| Injection drug use | ||||
| Yes | 165 (27.2%) | 77 (81.9%) | 47 (82.5%) | 40 (80.0%) |
| No | 347 (57.2%) | 15 (16.0%) | 8 (14.0%) | 8 (16.0%) |
| Unknown | 95 (15.7%) | 2 (2.1%) | 2 (3.5%) | 2 (4.0%) |
| Insurance | ||||
| Medicaid/medicare | 147 (24.2%) | 22 (23.4%) | 7 (12.3%) | 6 (12.0%) |
| Private | 104 (17.1%) | 11 (11.7%) | 6 (10.5%) | 6 (12.0%) |
| Uninsured | 260 (42.8%) | 52 (55.3%) | 38 (66.7%) | 32 (64.0%) |
| Unknown | 96 (15.8%) | 9 (9.6%) | 6 (10.5%) | 6 (12.0%) |
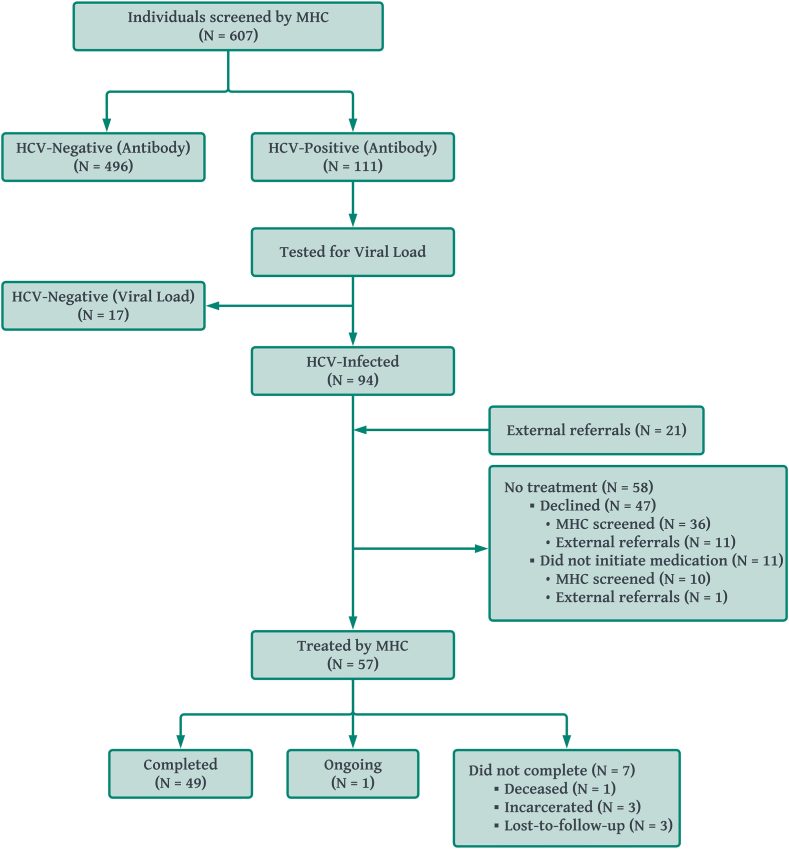
In addition to screening, the program tracked testing information and treatment progression. Descriptive statistics for individuals who had an HCV VL indicating current infection, individuals initiating treatment, and individuals who have completed or are actively undergoing treatment to date are shown in columns 2–4 of Table 2, respectively, with participation also illustrated in the diagram in Fig. 3. Among the 607 individuals who were screened, 111 (18.3%) tested positive with the rapid antibody test. Upon laboratory testing for HCV VL, HCV was not detected for 17 (15.3%) individuals, resulting in 94 (15.5%) of the original 607 with confirmed active HCV infection warranting treatment. Forty-eight of those 94 individuals (51.1%) initiated treatment. An additional 21 patients were screened elsewhere and referred to the MHC for treatment, nine of whom initiated treatment. This yielded a total of 57 individuals initiating treatment. Among those initiating treatment, 50 (87.7%) have either completed treatment or are undergoing treatment, with 7 (12.3%) no longer actively in treatment. Of those who did not complete treatment, one patient was deceased, three were incarcerated, and three were lost to follow-up. Compared to the population screened, the populations of HCV-infected, initiated treatment, and retained in treatment tended to have a high proportion of individuals who were 30–44 years of age (52.1%, 50.9%, and 46.0%, respectively, vs. 38.6%), identifying as male (70.2%, 66.7%, and 68.0% vs. 57.5%), White (77.7%, 82.5%, and 80.0% vs. 61.3%), with IDU (81.9%, 82.5%, and 80.0% vs. 27.2%), and uninsured (55.3%, 66.7%, and 64.0% vs. 42.8%).
Fig. 3.
Diagram of use of the mobile health clinics. In addition to the patients who were screened by the clinics and were found to have an HCV infection, external organizations without the capacity to provide treatment referred HCV-infected patients to the mobile health clinics (N = 21), nine of whom were treated by the MHC program.
Chi-square test results for HCV test result (infected or not detected) and treatment (initiated or did not initiate) are given in Table 3 (HCV test result) and Table 4 (HCV treatment). Statistically significant differences between HCV-infected and HCV-negative individuals showed that the infected population included a higher proportion of individuals 30–44 years of age (52.1% vs. 36.4%, p = 0.023), identifying as male (70.2% vs. 55.2%, p = 0.009), White (78.5% vs. 60.2%, p < 0.0001), with IDU (83.7% vs. 21.0%, p < 0.0001), and uninsured (61.2% vs. 48.8%, p = 0.0499). HCV-infected individuals also had higher likelihood of bike, walk, or scooter (32.2% vs. 28.0%) and lower likelihood of personal car (41.5% vs. 56.5%) as their primary mode of transportation (p = 0.028). Among those initiating or declining treatment, there was a statistical difference in uninsured rate, such that 74.5% of individuals initiating treatment were uninsured, compared to 45.3% of those who did not initiate treatment (p = 0.004). Statistically significant differences were not observed by treatment retention status (Appendix Table A1). A sensitivity analysis restricted to the 94 individuals who were screened by the MHC for individuals initiating (N = 48) or not initiating treatment (N = 46), as well as retained in treatment (N = 42) or incomplete (N = 6), did not show differences in terms of significance from the analysis including the external referrals (Appendix Tables A2 and A3).
Table 3.
Chi-square test for descriptive characteristics based on HCV test result.
| Characteristics | HCV test result |
p-value | |
|---|---|---|---|
| Infected N = 94 |
Not detected N = 513 |
||
| Age group | 0.023 | ||
| 18–29 | 5 (5.3%) | 54 (10.6%) | |
| 30–44 | 49 (52.1%) | 185 (36.4%) | |
| 45–64 | 32 (34.0%) | 199 (39.2%) | |
| 65 and over | 8 (8.5%) | 70 (13.8%) | |
| Sex | 0.009 | ||
| Female | 28 (29.8%) | 230 (44.8%) | |
| Male | 66 (70.2%) | 283 (55.2%) | |
| Race/ethnicity | <0.0001 | ||
| Black | 20 (21.5%) | 151 (30.4%) | |
| Hispanic | 0 (0.0%) | 47 (9.5%) | |
| White | 73 (78.5%) | 299 (60.2%) | |
| Transportation | 0.028 | ||
| Bike, scooter, or walk | 34 (36.2%) | 118 (28.0%) | |
| Personal car | 39 (41.5%) | 238 (56.5%) | |
| Other car or public transportation | 21 (22.3%) | 65 (15.5%) | |
| Injection drug use | <0.0001 | ||
| Yes | 77 (83.7%) | 88 (21.0%) | |
| No | 15 (16.3%) | 332 (79.0%) | |
| Insurance | 0.050 | ||
| Insured | 33 (38.8%) | 218 (51.2%) | |
| Uninsured | 52 (61.2%) | 208 (48.8%) | |
p-values <0.05 are bolded.
Table 4.
Chi-square test for descriptive characteristics based on treatment status.
| Characteristics | Initiated treatment |
p-value | |
|---|---|---|---|
| Yes N = 57 |
No N = 58 |
||
| Age group | 0.224 | ||
| 18–29 | 7 (12.3%) | 3 (5.2%) | |
| 30–44 | 29 (50.9%) | 29 (50.0%) | |
| 45–64 | 19 (33.3%) | 19 (32.8%) | |
| 65 and over | 2 (3.5%) | 7 (12.1%) | |
| Sex | 1.00 | ||
| Female | 19 (33.3%) | 20 (34.5%) | |
| Male | 38 (66.7%) | 38 (65.5%) | |
| Race/ethnicity | 0.401 | ||
| Black | 9 (16.1%) | 14 (24.1%) | |
| White | 47 (83.9%) | 44 (75.9%) | |
| Transportation | 0.250 | ||
| Bike, scooter, or walk | 16 (28.6%) | 24 (41.4%) | |
| Personal car | 27 (48.2%) | 26 (44.8%) | |
| Other car or public transportation | 13 (23.2%) | 8 (13.8%) | |
| Injection drug use | 0.514 | ||
| Yes | 47 (85.5%) | 45 (78.9%) | |
| No | 8 (14.5%) | 12 (21.1%) | |
| Insurance | 0.004 | ||
| Insured | 13 (25.5%) | 29 (54.7%) | |
| Uninsured | 38 (74.5%) | 24 (45.3%) | |
p-values <0.05 are bolded.
Discussion
The Clemson Rural Health MHC program demonstrated the feasibility of addressing disparities and mitigating barriers to care for patients with HCV. The program reached its target population of at-risk individuals with healthcare access barriers, while also demonstrating feasibility of MHCs to facilitate HCV screening, testing, and treatment among these underserved populations. The program’s ability to not only screen, but also facilitate the processing of laboratory tests, manage treatment costs, and deliver treatment to at-risk and uninsured populations allow for enhanced care novel to the current program. The framework implemented by Clemson Rural Health demonstrated that there is desire, opportunity, and ability to target underserved populations with MHCs and facilitate access for difficult-to-treat populations throughout the spectrum of care.
The framework implemented by Clemson Rural Health mitigated persistent barriers to care for underserved communities and priority populations for identifying HCV. While the majority of those screened at the MHC were White, there was a higher proportion of minority individuals than the median minority population among the MHC’s visited census tracts (Black: 28.2% vs. 16.7%; Hispanic: 7.7% vs. 6.1%). Additionally, consistent with previous research, the program also found that individuals screened at the MHC were largely uninsured,5 with the MHC screening close to four times more uninsured individuals than the median population among the visited census tracts (42.8% vs. 11.9%). These findings indicate that the MHCs were effective in reaching at-risk, vulnerable populations for HCV infection.19, 20, 21 Furthermore, the program identified the uninsured population as a priority for catching undetected cases. The rate of uninsured among HCV-infected individuals seen by the Clemson Rural Health MHCs (61.2%) is higher than those described in the literature for population-based data from the U.S. (ranging from 16.9% to 38.99%),20,34, 35, 36 suggesting that MHCs may be necessary tools for identifying infections among uninsured individuals. Based on discussions with on-site clinicians, the high majority of individuals with current infections were unaware of their status. Earlier diagnosis can lead to better health outcomes for individuals and the global population, including lower rates of liver disease and mortality, and progression towards HCV elimination.37, 38, 39
In addition to heightened screening and disease diagnosis, the Clemson Rural Health MHCs also demonstrated potential to mitigate barriers to treatment. Overall, the framework had a greater percent of patients initiate treatment (49.6%) than the average national and global treatment uptake among HCV-infected patients (9.9%–15.5%).1,3,40 Among MHC programs, a study which aimed to screen and link patients to external treatment reported that 63.0% (17/27) were linked, but the MHC did not directly prescribe medication, provide treatment, or follow-up; and a study prescribing medication reported that 32.8% (19/58) initiated treatment.12,29 There was particularly encouraging uptake among PWID and uninsured individuals. Lack of insurance and IDU are established barriers to downstream uptake of treatment following diagnosis.27,41,42 Our study found that a large majority of HCV-infected individuals initiating treatment had IDU (85.5%) and were uninsured (74.5%). Importantly, MHC staff were able to assure uninsured patients that they would not have to pay for services. Therefore, in the setting of a MHC, lack of insurance and IDU may be less of a barrier to initiation of care. By contrast, the results indicate that insurance may pose more of a barrier to initiation of care than lack of insurance when MHC resources, and financial assistance facilitated by the MHCs, are available. That is, as a result of the program targeting at-risk, uninsured individuals, it was easier to initiate treatment without insurance than proceed through the required steps to obtain treatment with the insurance company.43, 44, 45 The prescription assistance program allowed the APRNs to facilitate obtainment of prescriptions and medication, whereas many insurance companies require that a specialty physician write the prescription. In these cases, a collaborating physician was contacted who reviewed the patient’s case and wrote the prescription. These procedures would add at least two weeks to the patient’s timeline for initiating treatment, whereas uninsured patients were able to initiate treatment within the same week. Additionally, some insurances may not cover the cost of the medications. Out of pocket costs for the medication for those with insurance may have prevented individuals from initiating treatment and drive the finding that uninsured patients were more likely to initiate treatment than insured patients.45 As such, the results emphasize the feasibility of MHCs, especially when combined with financial assistance facilitation, as acceptable, and possibly necessary, treatment settings among historically difficult-to-treat populations.
A high proportion of patients completed treatment (86.0%), with an additional individual currently undergoing treatment (1.7%). Only three individuals (5.26%) were lost-to-follow-up as one patient died and three others were incarcerated after initiating treatment. Among those who did not complete treatment, there was not evidence that uninsured individuals or PWID were more likely to drop out of treatment than insured individuals or non-drug users. Furthermore, the MHCs’ potential and ambition to deliver treatment is a novel use of MHCs for HCV care and made the Clemson Rural Health framework a respected provider of treatment among the community. Notably, facilities without the capacity to treat HCV-infected patients referred their patients to the MHCs. The units would then be deployed specifically to treat these referred patients. In these ways, the MHC program can be viewed as a beneficial tool for screening availability and treatment uptake for HCV, with particular advantages for uninsured individuals at high risk of HCV.
The results of the Clemson Rural Health MHC program also showed success of multidisciplinary care teams in MHCs, as well as the necessity of community partnerships. Team-based models in community centres with health education and pharmaceutical expertise have evidenced accomplishments in treating HCV among uninsured populations.46 Furthermore, trust in Clemson Rural Health and the MHCs was achieved with underserved populations through community connections and dedication to revisiting communities. Through coordination with community partners, Clemson Rural Health identified locations within underserved regions that were best suited for allocation of the MHCs. The sites visited by the MHCs drew the targeted populations with a high proportion of the screenings and cases coming from resources frequented by high-risk and underserved populations (behavioural health or addition centres, food banks, and homeless services). Therefore, the partnerships facilitated effective allocation, as well as safe parking at sites, to bring about uptake of offered services through four weeks of repeat visits and additional follow-up visits.
The framework has clinical implications and applications for practice. The program showed the utility of MHCs for involvement in a range of care services, including screening, testing, and treatment, for individuals at high risk of HCV infection. Additionally, care can be expanded to difficult-to-treat populations, with opportunities to obtain and deliver resources for enhanced care for uninsured individuals. In these ways, MHCs may be a necessary avenue for decreasing the health and economic impact of HCV on the community through increased uptake of services and detection of HCV, as well as being an encouraging vehicle for treatment.
Limitations
The Clemson Rural Health MHC framework also has limitations and identified continued barriers to provide care that need to be addressed in future studies. Despite successes, MHCs face barriers to widespread use and operational sustainability, including financial issues and logistical challenges. Collectively, 58% of MHCs report financial issues as their primary operational barrier.4 There is inherit financial strain from purchasing and maintaining vehicles.47 Additionally, clinics are tasked with supporting necessary care to the high proportion of uninsured populations with their own resources or facing the inability to provide the highest quality of care. The Clemson Rural Health MHC framework mitigated these barriers with external funding specifically designed to provide enhanced care for uninsured populations. Given that MHCs have shown evidence of reducing financial strain on the healthcare system through preventative care,4 cost-effectiveness analyses for interventions with MHCs for HCV would be a meaningful future analysis.
While treatment initiation among the HCV-infected patients was higher in this study compared to documented uptake rates, approximately half of HCV-infected patients did not initiate treatment.1,3,12,29,40 A limitation of the current study is that reasoning for declining was not formally documented. Those with insurance may have been deterred from initiating treatment due to high out of pocket costs that the prescription assistance program would mitigate for uninsured patients.45 On-site clinicians indicated that many individuals preferred to follow-up with their primary care provider or were migrant workers with whom contact was lost due to the nature of their work.48 Program staff attempted to follow-up with all individuals at least three times, but not all patients were able to be reached despite these efforts. Future studies should qualitatively approach reasons for declining treatment with MHCs, such that future programs can address any additional barriers and focus on increasing HCV treatment initiation rates for at-risk populations. These factors, along with the high proportion of patients who do not have access to a personal vehicle as their primary mode of transportation, demonstrate the importance of MHC return visits to previous sites. However, given MHCs are a limited resource, this may come at the cost of providing care to additional communities. Modelling studies to evaluate the effectiveness of these approaches may be useful.
Finally, this study is an exploratory analysis designed to describe the implementation of a novel Nurse Practitioner-led HCV MHC program. The analyses reported here are likely underpowered and additional covariates may be necessary to detect further predictors of HCV infection, treatment initiation, and treatment completion. Patient-level details such as proportion of sex workers, comorbidities such as HIV and HBV, proportion of migrant workers, and behavioural data would be interesting factors to be considered in future analyses. Additionally, the patient population is restricted to underserved South Carolinians and therefore predictors of HCV infection, initiation, and treatment may be different for other populations. Follow-up analyses on larger populations and considering additional factors are necessary to confirm findings.
Conclusions
The Clemson Rural Health framework demonstrated the potential of MHC programs to mitigate barriers to care and treatment uptake for HCV in vulnerable populations. MHCs are ideally positioned to be involved in a range of care services, including HCV screening, testing, and treatment. The program showed an evidenced opportunity for MHCs as an avenue of equitable HCV care for underserved and uninsured populations. The high proportion of uninsured individuals, individuals with IDU, and individuals without reliable transportation among the HCV-infected population seen by the MHCs showed the necessity of similar programs for identifying HCV infections among vulnerable populations. The high screening, treatment initiation, and treatment retention rates demonstrate the utility of the MHC as an acceptable treatment setting among historically difficult-to-treat populations. The framework can be applied by other health systems and to additional diseases to promote early diagnosis, coordinated and comprehensive preventative care, and better health outcomes for individuals and the population.
Contributors
LR and KAH conceptualized this paper along with developing the methodology for the manuscript and original drafting of the manuscript. LR, KAH, and AHL performed project administration. CMK and AHL contributed to the original draft and, with AC, PR, KB, and RWG, performed the investigation described in this manuscript and acquired the data. FG performed data analysis. CMK, FG, and KB verified the data underlying this study. LR, KAH, CMK, FG, and AHL interpreted the data for the work. CMK and AHL acquired funding. All authors contributed to reviewing and editing the work, approve the final version, and agree to be accountable for all aspects.
Data sharing statement
Due to patient confidentiality, individual-level data cannot be shared. Aggregated data can be provided by the corresponding author upon reasonable request.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
The program was supported by Gilead Sciences, Inc., (IN-US-987-5892) with additional funding specifically for uninsured patients from South Carolina Center for Rural and Primary Healthcare (2015593). KB, RG, CMK, PR, and AHL received support from South Carolina Center for Rural and Primary Healthcare during this study. KB, CMK, and AC received support from Gilead Sciences, Inc., during this study. KB received support for leading a round table discussion at a conference from International Network on Health and Hepatitis in Substance Users, speaking on a panel at a conference from Gilead Sciences, Inc., and travel to a conference from International Network on Health and Hepatitis in Substance Users. LR and FG received support from the National Library of Medicine of the National Institutes of Health (R01 LM014193-01A1) during this study. The funders had no role in the design, conduct, reporting of the study, or decision to submit for publication. Payments were made to our institution. AHL has served on the advisory boards for Gilead Sciences, Inc., and AbbVie.
Acknowledgements
We would like to acknowledge Emily Kramer for her help with data verification. We appreciate the time and effort that you put into this project.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100648.
Contributor Information
Lior Rennert, Email: liorr@clemson.edu.
Alain H. Litwin, Email: Alain.Litwin@prismahealth.org.
Appendix A. Supplementary data
References
- 1.Stasi C., Silvestri C., Voller F. Update on hepatitis C epidemiology: unaware and untreated infected population could be the key to elimination. SN Compr Clin Med. 2020;2:2808–2815. doi: 10.1007/s42399-020-00588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havens P.L., Anderson J.R. Updated CDC recommendations for universal hepatitis C virus screening among adults and pregnant women: implications for clinical practice. JAMA. 2020;323:2258–2259. doi: 10.1001/jama.2020.3693. [DOI] [PubMed] [Google Scholar]
- 3.McGowan C.E., Fried M.W. Barriers to hepatitis C treatment. Liver Int. 2012;32:151–156. doi: 10.1111/j.1478-3231.2011.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu S.W.Y., Hill C., Ricks M.L., Bennet J., Oriol N.E. The scope and impact of mobile health clinics in the United States: a literature review. Int J Equity Health. 2017;16:178. doi: 10.1186/s12939-017-0671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malone N.C., Williams M.M., Smith Fawzi M.C., et al. Mobile health clinics in the United States. Int J Equity Health. 2020;19:40. doi: 10.1186/s12939-020-1135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwitters A., Lederer P., Zilversmit L., et al. Barriers to health care in rural Mozambique: a rapid ethnographic assessment of planned mobile health clinics for ART. Glob Health Sci Pract. 2015;3:109–116. doi: 10.9745/GHSP-D-14-00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvi R.A., Justason L., Liotta C., et al. The eagles eye mobile: assessing its ability to deliver eye care in a high-risk community. J Pediatr Ophthalmol Strabismus. 2015;52:98–105. doi: 10.3928/01913913-20150216-02. [DOI] [PubMed] [Google Scholar]
- 8.Nuttbrock L., Rosenblum A., Magura S., McQuistion H. Broadening perspectives on mobile medical outreach to homeless people. J Health Care Poor Underserved. 2003;14:5–16. doi: 10.1353/hpu.2010.0831. [DOI] [PubMed] [Google Scholar]
- 9.Thomas C.M., Liebman A.K., Galván A., Kirsch J.D., Stauffer W.M. Ensuring COVID-19 vaccines for migrant and immigrant farmworkers. Am J Trop Med Hyg. 2021;104:1963–1965. doi: 10.4269/ajtmh.21-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill C., Zurakowski D., Bennet J., et al. Knowledgeable neighbors: a mobile clinic model for disease prevention and screening in underserved communities. Am J Public Health. 2012;102:406–410. doi: 10.2105/AJPH.2011.300472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy P., McGlynn E., Hill A.B., et al. From pandemic response to portable population health: a formative evaluation of the Detroit mobile health unit program. PLoS One. 2021;16 doi: 10.1371/journal.pone.0256908. Telfair J, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morano J.P., Zelenev A., Lombard A., Marcus R., Gibson B.A., Altice F.L. Strategies for hepatitis C testing and linkage to care for vulnerable populations: point-of-care and standard HCV testing in a mobile medical clinic. J Community Health. 2014;39:922–934. doi: 10.1007/s10900-014-9932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazarus J.V., Øvrehus A., Demant J., Krohn-Dehli L., Weis N. The Copenhagen test and treat hepatitis C in a mobile clinic study: a protocol for an intervention study to enhance the HCV cascade of care for people who inject drugs (T’N’T HepC) BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alcendor D.J., Juarez P.D., Matthews-Juarez P., et al. Meharry Medical College Mobile Vaccination program: implications for increasing COVID-19 vaccine uptake among minority communities in middle Tennessee. Vaccines. 2022;10:211. doi: 10.3390/vaccines10020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assoumou S.A., Peterson A., Ginman E., et al. Addressing inequities in SARS-CoV-2 vaccine uptake: the Boston Medical Center Health System experience. Ann Intern Med. 2022;175:879–884. doi: 10.7326/M22-0028. [DOI] [PubMed] [Google Scholar]
- 16.Brown-Connolly N.E., Concha J.B., English J. Mobile health is worth it! Economic benefit and impact on health of a population-based mobile screening program in New Mexico. Telemed J E Health. 2014;20:18–23. doi: 10.1089/tmj.2013.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leibowitz A., Livaditis L., Daftary G., Pelton-Cairns L., Regis C., Taveras E. Using mobile clinics to deliver care to difficult-to-reach populations: a COVID-19 practice we should keep. Prev Med Rep. 2021;24 doi: 10.1016/j.pmedr.2021.101551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oriol N.E., Cote P.J., Vavasis A.P., et al. Calculating the return on investment of mobile healthcare. BMC Med. 2009;7:27. doi: 10.1186/1741-7015-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalili M., Wong R.J. Underserved does not mean undeserved: unfurling the HCV care in the safety net. Dig Dis Sci. 2018;63:3250–3252. doi: 10.1007/s10620-018-5316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong J.P., Collantes R., Pitts A., Martin L., Sheridan M., Younossi Z.M. High rates of uninsured among HCV-positive individuals. J Clin Gastroenterol. 2005;39:826–830. doi: 10.1097/01.mcg.0000177258.95562.43. [DOI] [PubMed] [Google Scholar]
- 21.Beck K.R., Kim N.J., Khalili M. Direct acting antivirals improve HCV treatment initiation and adherence among underserved African Americans. Ann Hepatol. 2018;17:413–418. doi: 10.5604/01.3001.0011.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin N.K., Vickerman P., Dore G.J., Hickman M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Curr Opin HIV AIDS. 2015;10:374–380. doi: 10.1097/COH.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arum C., Fraser H., Artenie A.A., et al. Homelessness, unstable housing, and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. Lancet Public Health. 2021;6:e309–e323. doi: 10.1016/S2468-2667(21)00013-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day E., Hellard M., Treloar C., et al. Hepatitis C elimination among people who inject drugs: challenges and recommendations for action within a health systems framework. Liver Int. 2019;39:20–30. doi: 10.1111/liv.13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treloar C., Rance J., Backmund M. Understanding barriers to hepatitis C virus care and stigmatization from a social perspective. Clin Infect Dis. 2013;57:S51–S55. doi: 10.1093/cid/cit263. [DOI] [PubMed] [Google Scholar]
- 26.Frankova S., Jandova Z., Jinochova G., Kreidlova M., Merta D., Sperl J. Therapy of chronic hepatitis C in people who inject drugs: focus on adherence. Harm Reduct J. 2021;18:69. doi: 10.1186/s12954-021-00519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta S.H., Genberg B.L., Astemborski J., et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2007;33:126. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donepudi I., Paredes A., Hubbard S., Awad C., Sterling R.K. Utility of evaluating HCV in an uninsured population. Dig Dis Sci. 2015;60:1092–1097. doi: 10.1007/s10620-014-3416-8. [DOI] [PubMed] [Google Scholar]
- 29.Rosecrans A., Harris R., Saxton R.E., et al. Mobile low-threshold buprenorphine integrated with infectious disease services. J Subst Abuse Treat. 2022;133 doi: 10.1016/j.jsat.2021.108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Association for the Study of Liver Disease . 2022. When and in whom to initiate HCV therapy.https://www.hcvguidelines.org/evaluate/when-whom [Google Scholar]
- 31.American Association for the Study of Liver Disease . 2022. HCV guidelines: recommendations for testing, managing, and treating hepatitis C.https://www.hcvguidelines.org/treatment-naive [Google Scholar]
- 32.Draper B.L., Pedrana A., Howell J., et al. Decentralized, community-based hepatitis C point-of-care testing and direct-acting antiviral treatment for people who inject drugs and the general population in Myanmar: protocol for a feasibility study. JMIR Res Protoc. 2020;9 doi: 10.2196/16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boeke C.E., Adesigbin C., Adisa O., et al. Patient outcomes in public sector hepatitis C treatment programmes: a retrospective cohort analysis across five low- and middle-income countries. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2022-062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galbraith J.W., Franco R.A., Donnelly J.P., et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology. 2015;61:776–782. doi: 10.1002/hep.27410. [DOI] [PubMed] [Google Scholar]
- 35.Bush H., Golabi P., de Avila L., Escheik C., Younossi Z. Impact of hepatitis C virus and insurance coverage on mortality. Am J Manage Care. 2019;25:61–67. [PubMed] [Google Scholar]
- 36.Stepanova M., Kanwal F., El-Serag H.B., Younossi Z.M. Insurance status and treatment candidacy of hepatitis C patients: analysis of population-based data from the United States. Hepatology. 2011;53:737–745. doi: 10.1002/hep.24131. [DOI] [PubMed] [Google Scholar]
- 37.Yousafzai M.T., Bajis S., Alavi M., Grebely J., Dore G.J., Hajarizadeh B. Global cascade of care for chronic hepatitis C virus infection: a systematic review and meta-analysis. J Viral Hepat. 2021;28:1340–1354. doi: 10.1111/jvh.13574. [DOI] [PubMed] [Google Scholar]
- 38.Gamkrelidze I., Pawlotsky J., Lazarus J.V., et al. Progress towards hepatitis C virus elimination in high-income countries: an updated analysis. Liver Int. 2021;41:456–463. doi: 10.1111/liv.14779. [DOI] [PubMed] [Google Scholar]
- 39.Fourati S., Feld J.J., Chevaliez S., Luhmann N. Approaches for simplified HCV diagnostic algorithms. J Int AIDS Soc. 2018;21 doi: 10.1002/jia2.25058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwo P.Y., Puenpatom A., Zhang Z., Hui S.L., Kelley A.A., Muschi D. Initial uptake, time to treatment, and real-world effectiveness of all-oral direct-acting antivirals for hepatitis C virus infection in the United States: a retrospective cohort analysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218759. Kanda T, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedman E. Health insurance, the uninsured, and hospitals: collision course. Front Health Serv Manag. 2005;21:3–15. [PubMed] [Google Scholar]
- 42.Ditah I., Al Bawardy B., Gonzalez H.C., et al. Lack of health insurance limits the benefits of hepatitis C virus screening: insights from the national health and nutrition examination hepatitis C follow-up study. Am J Gastroenterol. 2015;110:1126–1133. doi: 10.1038/ajg.2015.31. [DOI] [PubMed] [Google Scholar]
- 43.Wong R.J., Jain M.K., Therapondos G., et al. Race/ethnicity and insurance status disparities in access to direct acting antivirals for hepatitis C virus treatment. Am J Gastroenterol. 2018;113:1329–1338. doi: 10.1038/s41395-018-0033-8. [DOI] [PubMed] [Google Scholar]
- 44.Epstein R.L., Wang J., White L.F., et al. Medicaid hepatitis C virus treatment policies: impact on testing and treatment in the commercially insured. Am J Prev Med. 2022;63:e87–e98. doi: 10.1016/j.amepre.2022.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Federico C.A., Hsu P.C., Krajden M., et al. Patient time costs and out-of-pocket costs in hepatitis C. Liver Int. 2012;32:815–825. doi: 10.1111/j.1478-3231.2011.02722.x. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald L.S., Johnston B.E., Patel B., Stillwell T. Improving access to hepatitis C treatment using clinical pharmacist services in an uninsured, at-risk population at a community health center. J Health Care Poor Underserved. 2021;32:1433–1443. doi: 10.1353/hpu.2021.0142. [DOI] [PubMed] [Google Scholar]
- 47.DeBruhl N.D., Bassett L.W., Jessop N.W., Mason A.M. Mobile mammography: results of a national survey. Radiology. 1996;201:433–437. doi: 10.1148/radiology.201.2.8888236. [DOI] [PubMed] [Google Scholar]
- 48.Bechtel G.A., Davidhizar R., Spurlock W.R. Migrant farm workers and their families: cultural patterns and delivery of care in the United States. Int J Nurs Pract. 2000;6:300–306. doi: 10.1046/j.1440-172x.2000.00221.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.