Summary
Background
Snakebite envenoming (SBE) affects nearly three million people yearly, causing up to 180,000 deaths and 400,000 cases of permanent disability. Brazil's state of Amazonas is a global hotspot for SBE, with one of the highest annual incidence rates per 100,000 people, worldwide. Despite this burden, snake antivenom remains inaccessible to a large proportion of SBE victims in Amazonas. This study estimates the costs, and health and economic benefits of scaling up antivenom to community health centers (CHCs) and hospitals in the state.
Methods
We built a decision tree model to simulate three different antivenom scale-up scenarios: (1) scale up to 95% of hospitals, (2) scale up to 95% of CHCs, and (3) scale up to 95% of hospitals and 95% of CHCs. We consider each scenario with and without a 10% increase in demand for antivenom among SBE victims. For each scenario, we model the treatment costs averted, deaths averted, and disability-adjusted life years (DALYs) averted from a societal, health system, and patient perspective relative to the status quo and over a time horizon of one year. For each scenario and perspective, we also calculate the incremental cost per DALY averted and per death averted. We use a willingness to pay threshold equal to the 2022 gross domestic product (GDP) per capita of Brazil.
Findings
Scaling up antivenom to 95% of hospitals averts up to 2022 DALYs, costs up to USD $460 per DALY averted from a health system perspective, but results in net economic benefits up to USD $4.42 million from a societal perspective. Scaling up antivenom to 95% of CHCs averts up to 3179 DALYs, costs up to USD $308 per DALY averted from a health system perspective, but results in net economic benefits up to USD $7.35 million from a societal perspective. Scaling up antivenom to 95% of hospitals and CHCs averts up to 3922 DALYs, costs up to USD $328 per DALY averted from a health system perspective, but results in net economic benefits up to USD $8.98 million from a societal perspective.
Interpretation
All three antivenom scale up scenarios – scale up to 95% of hospitals, scale up to 95% of CHCs, and scale up to 95% of hospitals and 95% of CHCs – avert a substantial proportion of the SBE burden in Amazonas and are cost-saving from a societal perspective and cost-effective from a health system perspective.
Funding
W.M. and J.S. were funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq productivity scholarships). W.M. was funded by Fundação de Amparo à Pesquisa do Estado do Amazonas (PRÓ-ESTADO, call n. 011/2021-PCGP/FAPEAM, call n. 010/2021-CT&I ÁREAS PRIORITÁRIAS, call n. 003/2022—PRODOC/FAPEAM, POSGRAD/FAPEAM) and by the Ministry of Health, Brazil (Proposal No. 733781/19-035). Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health under Award Number R21TW011944. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Keywords: Snakebite, Envenoming, Antivenom, Brazil, Amazon, Cost-effectiveness, Economic evaluation, LMIC
Research in context.
Evidence before this study
We searched the MEDLINE database for economic evaluations related to snake antivenom scale-up in low- and middle-income countries (LMICs). No language restrictions were applied and all studies published up to March 1, 2023 were considered. Key search terms included “snakebite”, “envenoming”, “antivenom”, “Latin America”, “Africa”, “Southeast Asia”, “LMIC”, “cost-effectiveness”, “cost-benefit”, and “economic evaluation”. Previous studies report the cost-effectiveness of scaling-up antivenom for snakebite envenoming (SBE) within various West African and Southeast Asian countries. However, no studies were found which estimate the health and economic benefits of scaling-up access to snake antivenom in Latin America.
Added value of this study
To our knowledge, this is the first study to model increased access to snake antivenom in Latin America. Our study quantifies the health and economic benefits of scaling-up snake antivenom to hospitals and community health centers (CHCs) in the Brazilian Amazon. Our results show that scale-up of snake antivenom to 95% of hospitals and 95% of CHCs in the Brazilian Amazon averts a substantial proportion of the region's SBE burden and is cost-effective from a patient, health system, and societal perspective.
Implications of all the available evidence
Results from our study can help inform future public health investments. Historically, the small global burden of SBE relative to other heavily funded diseases like HIV, tuberculosis, and malaria has disincentivized potential investors. However, our results, in combination with past research, show that expanding snake antivenom access to those communities with the greatest need, namely rural and indigenous communities, can generate substantial health and economic returns while remaining cost-effective. Policies that promote access to snake antivenom should be a priority to countries with an elevated SBE burden.
Introduction
Snakebite envenoming (SBE) is a disabling and deadly disease that affects 1.8–2.7 million people each year worldwide, resulting in up to 180,000 deaths and 400,000 cases of permanent disability including amputation, blindness, contractures, psychological impairment, restricted mobility, and extensive scarring.1 Regions with the highest incidence rates of SBE include Sub-Saharan Africa, Southeast Asia, and Tropical Latin America with 20.28, 18.82, and 14.97 annual cases per 100,000 people.2 For comparison, high income North America only has 0.79 annual cases per 100,000 people.2 Tropical Latin America includes Brazil's state of Amazonas which has one of the highest incidences of SBE in the world with a mean rate of 45.10 annual cases per 100,000 people, and localities reaching as high as 235.80 annual cases per 100,000 people.3 However, despite having this high burden of SBE, access to antivenom in Amazonas is limited.4,5
In 2018, the WHO established the Snakebite Envenoming Working Group to develop a roadmap for reducing the global burden of SBE. Central to this roadmap is the ambitious goal of reducing deaths and disability from SBE by 50% before the year 2030 through safe and effective treatments, community empowerment, and health system strengthening.6 Currently, antivenom is the only evidence-based therapy used to treat SBE and should be administered as soon as possible to reduce the risk of complications and death.7,8 In the state of Amazonas, antivenom is only available in urban hospitals.4 This is problematic because most SBE cases in the state occur within remote populations that lack timely access to urban-based care.3 For example, among populations in the state that are vulnerable to SBE, the average travel time to the nearest major city is greater than 24 h.2 Improving antivenom accessibility in Amazonas could improve health outcomes among SBE patients by reducing delays to care and increasing antivenom uptake. Increases in antivenom accessibility could also generate economic returns through productivity gains resulting from improved health.
Brazil is a self-sufficient manufacturer of snake antivenom.4 Under current policies, most antivenom is distributed to urban hospitals.4 Consequently, rural health facilities that serve those most vulnerable to SBE must refer patients to distant urban facilities if antivenom is needed, thus delaying appropriate treatment. To address this problem, we have previously proposed the decentralization of antivenom distribution leveraging Brazil's community health centers (CHCs) network which is more granular and accessible by rural populations.5,9 In this study, we estimate the health and economic benefits of scaling up antivenom in Brazil to CHCs across the Amazonas state. More specifically, we use a microsimulation model to show the disability-adjusted life years (DALYs), deaths, patient costs, health system costs, and societal costs that could be averted by the scale-up of antivenom to CHCs and additional hospitals.
Methods
Population and setting
Brazil's Amazonas state has a population of 3.9 million people distributed in an area of 1,571,000 km2. While a majority of the population resides in urban locations, most cases of SBE occur within rural and indigenous communities, specifically among individuals frequently engaged in farming, fishing, hunting, or forestry-related activities.3,5 Residents of these communities have access to a large network of CHCs that provide basic primary care but must travel longer distances to urban hospitals if advanced care or treatment (such as antivenom) is needed. Such travel often involves complex routes that require multiple modes of transportation (e.g., walking, motorcycle, car, bus, motorboat, canoe, airplane, or ambulance), which results in high costs and long delays in receiving antivenom.10 However, under Brazil's health system the provision of all public healthcare, including the administration of snake antivenom, is provided at no cost to the patient.4
Overview of approach
We built a decision tree model to estimate the health and economic burden of SBE in Amazonas under four antivenom scenarios – one status quo and three scale-up scenarios. In the baseline scenario, we model the status quo of antivenom availability by assuming that antivenom is only available in select urban hospitals. In the “Hospital scale-up” scenario, we assume that access to antivenom is scaled up to become available in 95% of hospitals and, consequently, 95% of SBE victims who seek care from a hospital receive antivenom. In the “CHC scale-up” scenario, we assume access to antivenom is scaled up to become available in 95% of CHCs; therefore, 95% of SBE victims who seek care from a CHC receive antivenom. In the “Hospital and CHC scale-up” scenario, we assume access to antivenom is scaled up to become available in 95% of hospitals and 95% of CHCs; therefore, 95% of SBE victims who seek care from a hospital or a CHC receive antivenom. Our assumption for the three scale-up scenarios – that scale-up to 95% of hospitals/CHCs means 95% of patients who seek care from a hospital/CHC will receive antivenom if needed – is based on the reasoning that antivenom vials would be distributed to hospitals and CHCs based on projected case numbers, and therefore SBE cases, which are already very low compared to other diseases, would not be sufficiently high enough to overwhelm available antivenom vials.
For each of the three scale-up scenarios, we also model two separate “demand shift” scenarios with different assumptions regarding patient preferences for seeking healthcare. Under the “No demand shift” assumption, patient preferences for their usual source of care do not change as more facilities acquire antivenom. So, a patient who prefers not to seek care for SBE from a health facility will not benefit from antivenom scale-up. Similarly, a person who prefers to seek care from a CHC will not benefit from a scale-up that involves only hospitals. Under the “Demand shift” assumption, there is a shift in patient demand for care as antivenom becomes available from more facilities. So, as antivenom becomes more available in any type of health facility, the likelihood of people's demand for SBE care from that type of facility increases. Table 1 provides further details of each scenario modeled.
Table 1.
Antivenom scale-up scenarios.
| Scenario | General definition | Operational definition (without demand shift) | Operational definition (with demand shift) |
|---|---|---|---|
| Status quo | 68% of hospitals carry antivenoma | 68% of SBE victims who seek hospital care receive antivenom | NA |
| Hospital scale-up | 95% of hospitals carry antivenom | 95% of SBE victims who seek hospital care receive antivenom | 95% of SBE victims who seek hospital care receive antivenom + 10%b increase in the number of SBE victims who seek hospital care |
| CHC scale-up | 95% of CHCs carry antivenom | 95% of SBE victims who seek CHC care receive antivenom | 95% of SBE victims who seek CHC care receive antivenom + 10%b increase in the number of SBE victims who seek CHC care |
| Hospital and CHC scale-up | 95% of hospitals and CHCs carry antivenom | 95% of SBE victims who seek hospital or CHC care receive antivenom | 95% of SBE victims who seek hospital or CHC care receive antivenom + 10%b increase in the number of SBE victims who seek CHC carec |
Under current antivenom distribution policies in Brazil, 68% of hospitals in the Amazonas carry antivenom.
The use of a 10% increase is an assumption. The appendix contains a probabilistic sensitivity analysis in which we vary this assumption by ±5%.
We assume that when antivenom is scaled up to both hospitals and CHCs, individuals will prioritize seeking care from a CHC over a hospital. Therefore, we only apply a 10% increase to the number of SBE victims who seek CHC care.
For all scenarios, we model a patient, health system, and societal perspective (Table 2). The patient perspective quantifies the collective health and economic returns to patients that result from antivenom scale up. Patient perspective costs include transportation to health facilities, non-prescription medications, and productivity loss due to death or disability. Health benefits modeled under the patient perspective include DALYs averted and deaths averted. The health system perspective quantifies health and economic returns to the health system that result from antivenom scale up. Health system costs include the provision of ambulatory care, CHC care, hospital care, and antivenom to SBE patients. Health benefits modeled under the health system perspective also include DALYs and deaths averted. The societal perspective quantifies the health and economic returns to society that result from antivenom scale up and includes all costs and health benefits from both the patient and health system perspective. An impact inventory detailing all costs and health benefits included and excluded from this analysis can be found in Supplementary Table 1.
Table 2.
Analysis perspectives.
| Perspective | Costs included | Health benefits included |
|---|---|---|
| Patient | Transportation to CHC, transportation to hospital, non-prescription medications, productivity losses | DALYs averted, deaths averted |
| Health system | Ambulatory care, outpatient care at CHC, outpatient care at hospital, inpatient care at hospital, administration of antivenom, discharge consultation at CHC, discharge consultation at hospital | DALYs averted, deaths averted |
| Societal | Transportation to CHC, transportation to hospital, non-prescription medications, productivity losses, ambulatory care, outpatient care at CHC, outpatient care at hospital, inpatient care at hospital, administration of antivenom, discharge consultation at CHC, discharge consultation at hospital | DALYs averted, deaths averted |
For this analysis we model all costs and health benefits over a 1-year time horizon, with the exception of patient productivity losses resulting from premature death which we model over the lifetime of SBE victims. All costs and health benefits are discounted at an annual rate of 0% and are reported in 2022 USD. Results in which the costs and health benefits are discounted at an annual rate of 3.0% can be found in Supplementary Tables 3 and 4. All results from this study are reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist11 which can be found in Supplementary Table 2.
Model description
We built a decision tree model to simulate the course (and associated costs and health outcomes) of SBE victims in Brazil's Amazonas state. The structure of our model was informed by literature reviews of SBE victim experiences in Amazonas as well as an expert panel composed of clinicians and researchers with previous experience treating and or interviewing SBE patients in Amazonas.9,10,12 In our model, SBE victims either seek hospital care, seek CHC care, use traditional home-based therapies, or self-medicate. Those who seek hospital or CHC care may either receive timely antivenom, delayed antivenom, or no antivenom (SBE victims who first seek CHC care but who do not receive antivenom may be transferred to a hospital via ambulance). Our model assumes that those who use traditional home-based therapies or who self-medicate do not seek and receive antivenom in the “no demand shift” scenarios. However, as described in Table 1, in the “demand shift” scenarios, 10% of these patients do seek formal care and therefore receive antivenom. Depending on whether timely antivenom, delayed antivenom, or no antivenom is received, an SBE victim may either recover with no complications, recover with complications, or die. A complete schematic of our decision tree model can be found in Supplementary Figure 1. Using this model, we conducted a microsimulation of a cohort of SBE victims under each of the antivenom scale-up scenarios described above. We used a cohort size equal to the mean annual incidence of SBE in the Amazonas over the last decade.8,10,13 In the microsimulation, each member of the cohort accrues costs, DALYs, and deaths that are dependent on the pathway traveled. We ran the microsimulation for each scenario 1000 times and averaged the total costs, DALYs, and deaths across simulations. All modeling was conducted in TreeAge Pro Healthcare Version 2022 R1.2 developed by TreeAge Software, Williamstown, MA, USA.14
Model input parameters
All model input parameters, and their sources, for the status quo scenario are shown in Table 3.
Table 3.
Status quo input parameters.
| Parameter | Value | Source |
|---|---|---|
| Cohort size | ||
| Average annual incidence of SBE in Amazonas | 13,229 | 8,10,13 |
| Probabilitiesa | ||
| P (seek formal care) | 0.700 | Expert opinion |
| P (do not seek formal care) | 0.300 | Expert opinion |
| P (seek hospital care | sought formal care) | 0.467 | 10 |
| P (seek CHC care | sought formal care) | 0.533 | 10 |
| P (use traditional home-based therapies | did not seek formal care) | 0.704 | 12 |
| P (self-medicate | did not seek formal care) | 0.296 | 12 |
| P (inpatient | sought hospital care) | 0.930 | 13 |
| P (outpatient | sought hospital care) | 0.070 | 13 |
| P (transfer from CHC to hospital) | 0.327 | 10 |
| P (receive timely antivenom | admitted to hospital) | 0.489 | SINAN |
| P (receive delayed antivenom | admitted to hospital) | 0.192 | SINAN |
| P (receive no antivenom | admitted to hospital) | 0.319 | SINAN |
| P (receive timely antivenom | admitted to CHC) | 0.000b | SINAN |
| P (receive delayed antivenom | admitted to CHC) | 0.000b | SINAN |
| P (receive no antivenom | admitted to CHC) | 1.000 | SINAN |
| P (recovery without complications | no antivenom) | 0.895 | SINAN |
| P (recovery with complications | no antivenom) | 0.076 | SINAN |
| P (death | no antivenom) | 0.029 | SINAN |
| P (recovery without complications | antivenom) | 0.924 | SINAN |
| P (recovery with complications | antivenom) | 0.073 | SINAN |
| P (death | antivenom) | 0.003 | SINAN |
| P (recovery without complications | delayed antivenom) | 0.860 | SINAN |
| P (recovery with complications | delayed antivenom) | 0.128 | SINAN |
| P (death | delayed antivenom) | 0.012 | SINAN |
| Costs (2022 USD) | ||
| Hospital care (inpatient) | $108.54 | 13 |
| Hospital care (outpatient) | $23.92 | 13 |
| CHC care (outpatient) | $9.40 | 13 |
| Ambulance care | $14.52 | 13 |
| Discharge consultation (hospital) | $3.87 | 13 |
| Discharge consultation (CHC) | $3.87 | 13 |
| Antivenom therapy | $281.43 | 13 |
| Transport to hospital | $44.77 | 13,15 |
| Transport to CHC | $28.03 | 13,15 |
| Non-prescription medication | $10.62 | 13 |
| Annual minimum wage | $2614.32 | 16 |
| Disease durations (years) | ||
| For cases that do not receive antivenom | 0.300 | 17 |
| For cases that do receive antivenom | 0.010 | 13 |
| Disability weights | ||
| For cases that do not develop complications | 0.107 | 18 |
| For cases that do develop complications | 0.198 | 18 |
| Life expectancy | ||
| Life expectancy at average age of death | 30.77 | 19,20 |
Regarding notation: P(A) means the probability of A. P(A|B) means the probability of A given B.
The probability is 0 because CHCs do not store antivenom under current antivenom distribution policies in the Amazonas state.
Cohort size
For all simulations, we used a cohort size equal to the mean annual incidence of SBE in Amazonas over the last decade.8,10,13
Probabilities
The probabilities of an SBE victim seeking hospital care, seeking CHC care, using traditional home-based therapies, and self-medicating were derived from expert opinions and peer-reviewed literature.10,12,13 The probabilities of an SBE victim receiving antivenom at a hospital and receiving antivenom at a CHC were defined as the proportion of hospitals and CHCs, respectively, in Amazonas that are equipped with antivenom. The probabilities of an SBE victim receiving timely antivenom and receiving delayed antivenom were based on data from Brazil's Notifiable Disease Information System (Sistema de Informações de Agravos de Notificação [SINAN]). Data from SINAN was made available by the Brazilian Ministry of Health. In Brazil, all health facilities are required to record cases of SBE in SINAN. The information recorded includes the time from SBE (as reported by the patient) to receipt of antivenom. We used SINAN data from 2016 to 2020 to calculate the proportion of all SBE patients in Amazonas during that time who received antivenom within 6 h of SBE and the proportion who received antivenom 6 or more hours after SBE. We considered the administration of antivenom timely if within 6 h of SBE and delayed if 6 or more hours after SBE. This threshold was based on evidence that suggests health outcomes are significantly worse among SBE patients who receive antivenom 6 or more hours after SBE in comparison to those who receive antivenom within 6 h of SBE.7,8 SINAN also records information on whether SBE patients recover, develop complications, and or die. Using this information, we derived probabilities for each of our health outcomes (recovery without complications, recovery with complications, and death) conditional on whether no antivenom, delayed antivenom, or timely antivenom was received. Complications recorded among SBE patients in SINAN include secondary infection, necrosis, compartment syndrome, functional deficit, amputation, renal insufficiency, respiratory failure, septicemia, and shock.
Costs
All costs were derived from peer-reviewed literature. From the patient perspective, all patients who sought hospital care or CHC care incurred the cost of transportation to a hospital or CHC. The cost of transportation to a hospital or CHC was calculated as the product of the average cost to travel one kilometer in Amazonas and the average distance (among residents of Amazonas) to the nearest hospital or CHC.13,15 SBE victims who used traditional home-based therapies incurred no direct costs, and those who self-medicated only incurred the cost of non-prescription medications.13 All patients incurred productivity losses due to death or disability. Productivity losses for the whole cohort was calculated as the product of total DALYs accrued by the cohort and the annual minimum wage in Brazil.16 From the health system perspective, hospitals incurred the costs of providing both inpatient and outpatient care while CHCs only incurred the costs of providing outpatient care. Both facility types also incurred the cost of providing antivenom to those patients who received antivenom and discharge consultations to those patients who did not die while receiving care.12 For patients who first sought care at a CHC and were then transferred to a hospital, the health system also incurred the cost of providing ambulatory care.13 Lastly, it is important to note that all health system unit costs used in this study represent the average cost per snakebite envenoming patient at a specific health facility. Thus, although these health system costs do not differ by level of injury severity within a health facility, they differ across health facilities (e.g., hospital, CHC).
Health outcomes
Total DALYs accrued by the cohort in each simulation were calculated as the sum of years of life lived with disability (YLDs) and years of life lost to death (YLLs). The number of YLDs accrued by any given SBE victim was based on their treatment status (received antivenom or did not receive antivenom) and their health outcome (recovered without complications or recovered with complications). For each combination of treatment status and health outcome, we assigned a disease duration and disability weight. An SBE victim's YLDs were then calculated as the product of their assigned disease duration and disability weight. All disease durations were derived from peer-reviewed literature,13,17 and all disability weights were derived from the 2013 Global Burden of Disease Study.18 For all non-complicated cases, we used the average of disability weights for poisoning, acute infection, hand/arm impairment, and walking impairment. For all complicated cases, we used the average of disability weights for amputation, motor and cognitive impairment, respiratory diseases, and blindness (use of these disability weights was informed by the type of complications recorded for snakebite patients in SINAN). All SBE victims who died accrued YLLs. The number of YLLs accrued per death was calculated as the average life expectancy across males and females at the average age of death among recorded SBE victims (i.e., 49 years old).19,20
Probabilistic sensitivity analysis
For each antivenom scale-up scenario, we conducted a probabilistic sensitivity analysis to account for uncertainty in our parameter estimates. We created probability distributions for all parameters for which appropriate data were available. Each simulation was run 1000 times and results were averaged across simulations. Details regarding the inputs for the probabilistic sensitivity analysis can be found in Supplementary Table 5.
Cost-effectiveness analysis
For all perspectives (societal, health system, and patient), we calculate the incremental cost per DALY averted and incremental cost per death averted for each antivenom scale-up scenario compared to the status quo. The incremental cost per DALY/death averted is defined as the difference in cost between two scenarios divided by the difference in DALYs/deaths accrued. For the societal perspective, we also calculate the benefit cost ratio of each antivenom scale-up scenario. We calculate the benefit cost ratio as productivity gains divided by net economic costs. Thus, the benefit cost ratio shows how much society benefits from productivity gains for each US dollar spent on antivenom scale-up. We use a willingness to pay threshold equal to the GDP per capita of Brazil in 2022 (USD $8860) to determine the cost-effectiveness of each antivenom scale up scenario.21
Role of the funding source
The funding source had no role in study design, data collection, data analysis, data interpretation, writing and editing of this report, or the decision to publish this report.
Ethics statement
This study was approved by the Universidade do Estado do Amazonas Research Ethics Committee (CAAE: 35855820.2.0000.5016) and the Duke Health Institutional Review Board (Pro00103272).
Results
Antivenom scale-up without demand shift
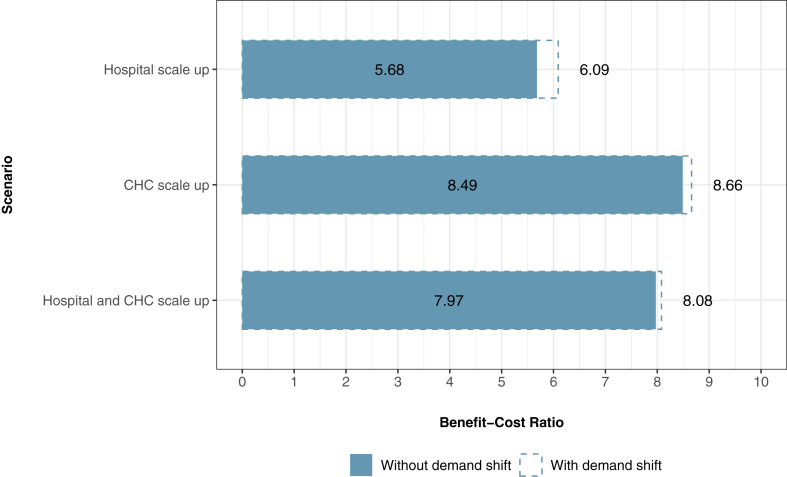
Figs. 1 and 2 show the total costs, DALYs, and deaths accrued in each antivenom scale-up scenario. The status quo scenario generates 9141 DALYs, 287 deaths, USD $24.24 million in patient costs, USD $1.84 million in health system costs, and USD $26.09 million in societal costs over a 1-year period. Scaling up antivenom to 95% of hospitals in Amazonas would avert 1006 DALYs and 31 deaths from SBE over a 1-year period with net economic benefits of USD $2.63 million from a patient perspective, an incremental cost of USD $463,346 from a health system perspective, and net economic benefits of USD $2.17 million from a societal perspective. Scaling up antivenom to 95% of CHCs would avert 2795 DALYs and 87 deaths over a 1-year period with net economic benefits of USD $7.31 million from a patient perspective, an incremental cost of USD $860,257 from a health system perspective, and net economic benefits of USD $6.45 million from a societal perspective. Scaling up antivenom to 95% of hospitals and CHCs would avert 3664 DALYs and 114 deaths over a one-year period with net economic benefits of USD $9.58 million from a patient perspective, an incremental cost of USD $1.20 million from a health system perspective, and net economic benefits of USD $8.38 million from a societal perspective. Table 4 shows the net economic benefits or incremental cost per DALY and death averted for each scenario and each perspective. Fig. 3 shows the societal perspective benefit cost ratios for each scenario.
Fig. 1.
Economic costs accrued in each scenario. Values in parentheses below bars represent the total societal costs. Patient direct medical costs only include the cost of non-prescription medications. Non-prescription medications are only purchased by those patients that self-medicate. Across all scenarios without demand shift the number of patients who self-medicate remains constant, so patient direct medical costs do not change. Similarly, across all scenarios with demand shift, the number of patients who self-medicate remains constant (albeit 10% lower than the no demand shift scenarios), so patient direct medical costs do not change. Patient direct non-medical costs only include the cost of transportation. Transportation costs are only incurred by patients who seek CHC or hospital care. Across all scenarios without demand shift, the number of people who seek CHC care and the number of people who seek hospital care remains constant, so patient direct non-medical costs do not change. Indirect costs only include productivity losses.
Fig. 2.
Deaths and DALYs lost in each antivenom scale-up scenario.
Table 4.
Incremental cost-effectiveness ratios for each scenario.
| Scenario | Societal perspectivea |
Health system perspective |
Patient perspectivea |
|||
|---|---|---|---|---|---|---|
| Incremental cost per DALY averted | Incremental cost per death averted | Incremental cost per DALY averted | Incremental cost per death averted | Incremental cost per DALY averted | Incremental cost per death averted | |
| Status quo | Reference | Reference | Reference | Reference | Reference | Reference |
| Hospital scale up (without demand shift) | −$2154 | −$69,932 | $460 | $14,947 | −$2614 | −$84,878 |
| CHC scale up (without demand shift) | −$2307 | −$74,101 | $308 | $9888 | −$2614 | −$83,989 |
| Hospital and CHC scale up (without demand shift) | −$2286 | −$73,492 | $328 | $10,544 | −$2614 | −$84,036 |
| Hospital scale up (with demand shift) | −$2185 | −$70,118 | $417 | $13,386 | −$2602 | −$83,504 |
| CHC scale up (with demand shift) | −$2312 | −$74,257 | $301 | $9674 | −$2614 | −$83,932 |
| Hospital and CHC scale up (with demand shift) | −$2291 | −$73,643 | $323 | $10,379 | −$2614 | −$84,022 |
A negative sign indicates that economic costs decreased as a result of antivenom scale-up. Negative values therefore reflect net economic benefits per DALY/death averted.
Fig. 3.
Societal perspective benefit cost ratios (BCRs) for each antivenom scale-up scenario. The BCR is calculated as productivity gains divided by net economic costs.
Antivenom scale-up with demand shift
Scaling up antivenom to 95% of hospitals in Amazonas would avert 2022 DALYs and 63 deaths from SBE over a one-year period with net economic benefits of USD $5.26 million from a patient perspective, an incremental cost of USD $843,348 from a health system perspective, and net economic benefits of USD $4.42 million from a societal perspective. Scaling up antivenom to 95% of CHCs would avert 3179 DALYs and 99 deaths over a 1-year period with net economic benefits of USD $8.31 million from a patient perspective, an incremental cost of USD $957,769 from a health system perspective, and net economic benefits of USD $7.35 million from a societal perspective. Scaling up antivenom to 95% of hospitals and CHCs would avert 3992 DALYs and 122 deaths over a 1-year period with net economic benefits of USD $10.25 million from a patient perspective, an incremental cost of USD $1.27 million from a health system perspective, and net economic benefits of USD $8.98 million from a societal perspective.
Probabilistic sensitivity analysis
Our probabilistic sensitivity analysis suggests that our model is robust in the presence of uncertainty. Under the probabilistic sensitivity analysis, scaling up antivenom to 95% of hospitals and CHCs (without demand shift) generates net economic benefits of USD $2612 (95% CI: 2606–2619) per DALY averted from a patient perspective, costs USD $287 (95% CI: 278–295) per DALY averted from a health system perspective, and generates net economic benefits of USD $2326 (95% CI: 2315–2336) per DALY averted from a societal perspective (Fig. 4). Scaling up antivenom to 95% of hospitals and CHCs (with demand shift) generates net economic benefits of USD $2610 (95% CI: 2604–2617) per DALY averted from a patient perspective, costs USD $286 (95% CI: 277–294) per DALY averted from a health system perspective, and generates net economic benefits of USD $2325 (95% CI: 2315–2335) per DALY averted from a societal perspective (Fig. 4). Variation in the probability of death from SBE as well as the unit cost of antivenom therapy had the largest impact on our cost-effectiveness measures (see Supplementary Figure 2). Additional results from the probabilistic sensitivity analysis showing the total costs, DALYs, and deaths accrued in each of the other scale up scenarios can be found in Supplementary Table 6. Across all scenarios (with and without demand shift) antivenom scale-up remains cost-effective from a health system perspective, but does not at any point become cost-saving because the health system must incur additional costs as more patients are treated. Conversely, across all scenarios (with and without demand shift), antivenom scale-up remains cost-saving from a patient perspective because gains in productivity per additional patient treated outweigh the per patient out-of-pocket costs of transportation and non-prescription medications.
Fig. 4.
Scatterplots showing the incremental cost and incremental effectiveness of each antivenom scale-up scenario for each iteration of the probabilistic sensitivity analysis in reference to the status quo. Each color represents a different perspective. The dotted line represents a willingness to pay threshold of USD $8860 (the 2022 GDP per capita of Brazil). All scenarios are cost-effective from a health systems perspective and provide net economic benefits from a societal perspective.
Discussion
Our study is the first ever to provide evidence on the health and economic benefits of scaling up snake antivenom to hospitals and CHCs in Brazil's Amazonas state. Our results show that scaling up snake antivenom in Amazonas would have a significant impact on public health. Scaling up antivenom to 95% of hospitals in the region could avert 2022 DALYs and 63 deaths from SBE over a one-year period. Scaling up antivenom to 95% of CHCs could avert 3179 DALYs and 99 deaths from SBE over a one-year period. Lastly, expanding antivenom scale up to 95% of hospitals and 95% of CHCs could avert 3922 DALYs and 122 deaths from SBE over one year. Not only does scaling up antivenom avert a substantial proportion of the SBE burden in the region, but each scale up scenario is also cost-effective in comparison to standardized international thresholds, interventions for high burden diseases (ex. HIV, tuberculosis, malaria), and other antivenom evaluations.
Each antivenom scale up scenario modeled in this study is cost-effective from both a societal and health system perspective in comparison to standardized international thresholds. The World Health Organization (WHO) defines an intervention as cost-effective if the cost per DALY averted is less than three times the gross domestic product (GDP) per capita of the implementing country.22 The GDP per capita of Brazil in 2022 was USD $8860.21 The gross regional product (GRP) per capita of Amazonas in 2020 was USD $5578.23 From a societal perspective, scaling up antivenom generates net economic benefits of USD $2154 to USD $2312 per DALY averted depending on the scenario modeled. From a health system perspective scaling up antivenom costs USD $301 to USD $460 per DALY averted depending on the scenario modeled. Under both perspectives, and for all scenarios, the cost per DALY averted for antivenom scale up is well below the GDP per capita of Brazil and the GRP per capita of Amazonas making it a highly cost-effective strategy for reducing the burden of SBE in Amazonas.
Our snake antivenom scale up scenarios are also cost-effective in comparison to commonly implemented global health interventions. A review of intervention cost-effectiveness found the cost per DALY averted to be USD $350 to USD $500 for antiretroviral therapy, USD $132 to USD $2570 for oral rehydration therapy, USD $5 to USD $17 for insecticide treated bed nets, and USD $13 to USD $24 for complete immunization against tuberculosis, tetanus, pertussis, poliomyelitis, and measles.24,25 Our results show that antivenom scale up has a lower cost per DALY averted than each of these global health interventions when taking a societal perspective and a similar cost per DALY averted when taking a health system perspective. Neglected tropical diseases like SBE are often underfunded because the small burden of these diseases, relative to other heavily funded diseases like HIV, malaria, and tuberculosis, suggest a small return on investment.26 Our results, however, combat this notion and show that increasing access to antivenom for SBE would bring substantial health and economic returns to society while remaining cost-effective to health systems.
Our findings are also similar to other evaluations of antivenom scale up. One study evaluated the cost-effectiveness of antivenom scale-up in sixteen West African countries using a health system perspective. The authors report a cost per DALY averted that ranges from USD $83 to USD $281 across the sixteen countries and conclude that antivenom scale up is highly cost-effective relative to the GDP per capita of each country analyzed.27 A similar study assessed the cost-effectiveness of scaling up antivenom in five Southeast Asian countries from a societal perspective. The authors report that in all countries scaling up antivenom is cost-saving.28 Our findings align with results from these studies and further highlight the value of increasing funding for antivenom scale up. Policy makers in Brazil and other regions with a high SBE burden should consider strategies to increase access to antivenom among their populations given the large health and economic returns that may result from doing so. Such strategies might initially focus on the establishment of safe, effective, and sustainable antivenom procurement and distribution systems that allow health facilities to receive and store antivenom, as well as the incorporation of SBE care into existing national health insurance schemes to make SBE treatment free of charge to patients. In countries like Brazil which already have a robust antivenom manufacturing and distribution network to expand on as well as a universal health care system that provides SBE treatment free of charge, additional strategies to increase access to antivenom might include community engagement to increase SBE awareness and prevention, and capacity building among SBE first responders to improve the quality of prehospital care available to SBE victims.1 Community engagement and capacity building strategies could be implemented independently or integrated into similar programs that address other areas of health (ex. vaccination or maternal and child health).
Our study provides a conservative estimate of the health and economic benefits of antivenom scale-up in Amazonas. We use the annual minimum wage to calculate productivity losses. Therefore, our estimate of productivity gains reflects the lower bound of potential gains from SBE. Furthermore, we model the health and economic benefits of antivenom scale up using an SBE cohort size that is equal to the mean annual number of SBE cases recorded in the SINAN database. However, this may be an underestimate as SINAN only records those cases that receive care from a CHC or hospital. Consequently, it is possible that a large number of SBE cases go unreported each year because these cases do not seek healthcare. One survey of 172 SBE victims in the western Brazilian Amazon, for example, found that 81 participants (47.1%) did not access healthcare following the occurrence of SBE.12 If the mean annual incidence of SBE in Amazonas is larger than what is simulated in this study, then the health and economic returns from antivenom scale up would be larger as well. We also assume that increasing antivenom accessibility increases demand for antivenom among SBE victims by no more than 10%. In reality, scaling up antivenom to 95% of hospitals and CHCs may increase the number SBE victims who seek care from these facilities by more than 10%. It is reasonable to assume, for example, that antivenom coverage would increase by 10 percentage points per year following scale up.29 Ultimately, higher antivenom coverage levels would create larger health and economic returns than what is seen in our simulations.
To our knowledge, this is the first study to estimate the health and economic benefits of increasing access to antivenom in Latin America. As our results may facilitate decision making by relevant policy makers in the region, there are important limitations to consider. First our models do not account for antivenom manufacturing and distribution costs. That being said, Brazil is a self-sufficient manufacturer of snake antivenom and is capable of producing and distributing up to 300,000 vials of five different types of antivenom per year.4 Given the antivenom supply chain, manufacturing, and distribution infrastructure that already exists within Brazil's universal health system, the costs incurred by the health system to scale up antivenom would likely be minimal in comparison to other countries with no antivenom manufacturing capacity. Second, a significant proportion of SBE victims in Amazonas use traditional therapies, but we do not model the effects of such therapies on health outcomes. Traditional therapies might worsen or improve SBE health outcomes, but we do not have sufficient data to consider such effects in our model. Finally, we use the annual minimum wage of Brazil to calculate productivity gains from DALYs averted. If the actual average annual income among SBE victims in Amazonas is lower or higher than Brazil's annual minimum wage, then our indirect patient cost calculations would be an under or overestimate, respectively.
Conclusion
Our study has shown that improving access to antivenom will reduce deaths and disability from SBE and provide substantial economic benefits. It also advances the goal of the WHO SBE working group in Brazil, with significant benefits to rural and indigenous communities of the Brazilian Amazon. Future studies should evaluate additional health and economic benefits that may be gained through other interventions in the WHO roadmap, including community empowerment and health system strengthening.
Contributors
Conceptualization: WM, JRNV, CJG, and OO. Methodology: AZ, WM, JRNV, ERS, TR, and OO. Formal analysis: AZ and OO. Investigation: AZ, WM, JRNV, ERS, TR, JS, FHW, CS, CJG, and OO. Data curation: AZ, WM, and TR. Writing (original draft): AZ. Writing (review and editing): AZ, WM, JRNV, ERS, TR, JS, FHW, CS, CJG, and OO. Visualization: AZ. Supervision: JRNV, CJG, and OO. Funding acquisition: WM, JRNV, JS, and CJG. OO is the guarantor of the article.
Data sharing statement
All data inputs used for the modeling in this study are available in the manuscript and Supplementary Material. For more specific data requests please contact the corresponding author.
Declaration of interests
CJG reports research funding from Ophirex Inc. for other studies.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100651.
Appendix A. Supplementary data
References
- 1.Snakebite envenoming -- a strategy for prevention and control [internet] https://www.who.int/publications-detail-redirect/9789241515641 Available from: [DOI] [PubMed]
- 2.Longbottom J., Shearer F.M., Devine M., et al. Vulnerability to snakebite envenoming: a global mapping of hotspots. Lancet. 2018;392(10148):673–684. doi: 10.1016/S0140-6736(18)31224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feitosa E.S., Sampaio V., Sachett J., et al. Snakebites as a largely neglected problem in the Brazilian Amazon: highlights of the epidemiological trends in the State of Amazonas. Rev Soc Bras Med Trop. 2015;48 Suppl 1:34–41. doi: 10.1590/0037-8682-0105-2013. [DOI] [PubMed] [Google Scholar]
- 4.Fan H.W., Monteiro W.M. History and perspectives on how to ensure antivenom accessibility in the most remote areas in Brazil. Toxicon. 2018;151:15–23. doi: 10.1016/j.toxicon.2018.06.070. [DOI] [PubMed] [Google Scholar]
- 5.Monteiro W.M., de Farias A.S., Val F., et al. Providing antivenom treatment access to all Brazilian Amazon indigenous areas: ‘every life has equal value’. Toxins. 2020;12(12):772. doi: 10.3390/toxins12120772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams D.J., Faiz M.A., Abela-Ridder B., et al. Strategy for a globally coordinated response to a priority neglected tropical disease: snakebite envenoming. PLoS Negl Trop Dis. 2019;13(2) doi: 10.1371/journal.pntd.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feitosa E.L., Sampaio V.S., Salinas J.L., et al. Older age and time to medical assistance are associated with severity and mortality of snakebites in the Brazilian Amazon: a case-control study. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Silva Souza A., de Almeida Gonçalves Sachett J., Alcântara J.A., et al. Snakebites as cause of deaths in the Western Brazilian Amazon: why and who dies? Deaths from snakebites in the Amazon. Toxicon. 2018;145:15–24. doi: 10.1016/j.toxicon.2018.02.041. [DOI] [PubMed] [Google Scholar]
- 9.Beck T.P., Tupetz A., Farias A.S., et al. Mapping of clinical management resources for snakebites and other animal envenomings in the Brazilian Amazon. Toxicon X. 2022;16 doi: 10.1016/j.toxcx.2022.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cristino J.S., Salazar G.M., Machado V.A., et al. A painful journey to antivenom: the therapeutic itinerary of snakebite patients in the Brazilian Amazon (the QUALISnake study) PLoS Negl Trop Dis. 2021;15(3) doi: 10.1371/journal.pntd.0009245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husereau D., Drummond M., Augustovski F., et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BJOG. 2022;129(3):336–344. doi: 10.1111/1471-0528.17012. [DOI] [PubMed] [Google Scholar]
- 12.Maciel Salazar G.K., Saturnino Cristino J., Vilhena Silva-Neto A., et al. Snakebites in “invisible populations”: a cross-sectional survey in riverine populations in the remote Western Brazilian Amazon. PLoS Negl Trop Dis. 2021;15(9) doi: 10.1371/journal.pntd.0009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magalhães S.F.V., Peixoto H.M., de Almeida Gonçalves Sachett J., et al. Snakebite envenomation in the Brazilian Amazon: a cost-of-illness study. Trans R Soc Trop Med Hyg. 2020;114(9):635–642. doi: 10.1093/trstmh/traa005. [DOI] [PubMed] [Google Scholar]
- 14.TreeAge Pro 2021, R1 [Internet]. TreeAge Software, Williamstown, MA. http://www.treeage.com Available from:
- 15.Isaacson J.E., Joiner A.P., Kozhumam A.S., et al. Emergency care sensitive conditions in Brazil: a geographic information system approach to timely hospital access. Lancet Reg Health Am. 2021;4 doi: 10.1016/j.lana.2021.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ILO Data Explorer [Internet] https://www.ilo.org/shinyapps/bulkexplorer2/ Available from:
- 17.Kasturiratne A., Pathmeswaran A., Wickremasinghe A.R., et al. The socio-economic burden of snakebite in Sri Lanka. PLoS Negl Trop Dis. 2017;11(7) doi: 10.1371/journal.pntd.0005647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salomon J.A., Haagsma J.A., Davis A., et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3(11):e712–e723. doi: 10.1016/S2214-109X(15)00069-8. [DOI] [PubMed] [Google Scholar]
- 19.Institute for Health Metrics and Evaluation [Internet]. GBD Results. https://vizhub.healthdata.org/gbd-results Available from:
- 20.World Population Prospects - Population Division - United Nations [Internet] https://population.un.org/wpp/ Available from:
- 21.World Economic Outlook (October 2022) - GDP per capita, current prices [Internet] https://www.imf.org/external/datamapper/NGDPDPC@WEO Available from:
- 22.Bertram M.Y., Lauer J.A., Stenberg K., Edejer T.T.T. Methods for the economic evaluation of health care interventions for priority setting in the health system: an update from WHO CHOICE. Int J Health Policy Manag. 2021;10(11):673–677. doi: 10.34172/ijhpm.2020.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regional Accounts of Brazil | IBGE [Internet] https://www.ibge.gov.br/en/statistics/economic/national-accounts/16855-regional-accounts-of-brazil.html Available from:
- 24.Laxminarayan R., Chow J., Shahid-Salles S.A. In: Disease control priorities in developing countries [internet] 2nd ed. Jamison D.T., Breman J.G., Measham A.R., et al., editors. The International Bank for Reconstruction and Development/The World Bank; Washington (DC): 2006. Intervention cost-effectiveness: overview of main messages.http://www.ncbi.nlm.nih.gov/books/NBK11784/ Available from: [PubMed] [Google Scholar]
- 25.Chao T.E., Sharma K., Mandigo M., et al. Cost-effectiveness of surgery and its policy implications for global health: a systematic review and analysis. Lancet Glob Health. 2014;2(6):e334–e345. doi: 10.1016/S2214-109X(14)70213-X. [DOI] [PubMed] [Google Scholar]
- 26.Beyeler N., Fewer S., Yotebieng M., Yamey G. Improving resource mobilisation for global health R&D: a role for coordination platforms? BMJ Glob Health. 2019;4(1) doi: 10.1136/bmjgh-2018-001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamza M., Idris M.A., Maiyaki M.B., et al. Cost-effectiveness of antivenoms for snakebite envenoming in 16 countries in West Africa. PLoS Negl Trop Dis. 2016;10(3) doi: 10.1371/journal.pntd.0004568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patikorn C., Ismail A.K., Zainal Abidin S.A., Othman I., Chaiyakunapruk N., Taychakhoonavudh S. Potential economic and clinical implications of improving access to snake antivenom in five ASEAN countries: a cost-effectiveness analysis. PLoS Negl Trop Dis. 2022;16(11) doi: 10.1371/journal.pntd.0010915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verguet S., Olson Z.D., Babigumira J.B., et al. Health gains and financial risk protection afforded by public financing of selected interventions in Ethiopia: an extended cost-effectiveness analysis. Lancet Glob Health. 2015;3(5):e288–e296. doi: 10.1016/S2214-109X(14)70346-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.