Abstract
Background and Objectives
Advance care planning (ACP) conversations are important to provide goal-concordant care (i.e., the care that matches the patient’s previously stated goals) near end of life. While 31% of older adults presenting to the emergency department (ED) have dementia, only 39% have previously had ACP conversations. We refined and piloted an ED-based, motivational interview designed to stimulate ACP conversations (ED GOAL) for patients living with cognitive impairment and their caregivers.
Research Design and Methods
We systematically refined ED GOAL and then conducted an acceptability study in an urban, academic medical center. We prospectively enrolled adults aged 50+ with cognitive impairment and their caregivers. Trained clinicians conducted the intervention. We measured acceptability after the intervention and participants’ ACP engagement at baseline and 1-month follow-up.
Results
Specific statements to address both the patient and caregiver were added to the ED GOAL script. Of 60 eligible patient/caregiver dyads approached, 26 participated, and 20 (77%) completed follow-up assessments. Patient mean age was 79 years (SD 8.5); 65% were female, 92.3% were White, 96.2% were non-Hispanic, and 69% had moderate dementia. Most patients/caregivers reported feeling completely heard and understood by the study clinician about their future medical care preferences (58%, 15/26). They also reported that the study clinician was very respectful (96%, 25/26) when eliciting those preferences.
Discussion and Implications
Patients living with cognitive impairment and their caregivers found our refined ED GOAL acceptable and respectful. Future studies need to examine the effect of ED GOAL on ACP engagement among these dyads in the ED.
Keywords: Dementia, Physician–patient communication/relationships, Quality of life
Background and Objectives
Advance care planning (ACP) conversations, a key component of palliative care, can lead to well-informed, shared decision making and improved quality of life at the end of life (Wright et al., 2008). For seriously ill older adults, ACP conversations are associated with lower rates of in-hospital death, less aggressive medical care at the end of life, earlier hospice referrals, increased peacefulness, and a 56% greater likelihood to have end-of-life wishes known and followed (see, e.g., Khandelwal et al., 2016; Lakin et al., 2016; Shen et al., 2016; Wright et al., 2008). During the last 6 months of life, 75% of older adults (≥65 years) visit the emergency department (ED; Smith et al., 2012). ED visits are often inflection points in these patients’ illness trajectories, signaling a more rapid rate of decline (Deschodt et al., 2015; Nagurney et al., 2017; Wilber et al., 2010). More than 70% of these patients express priorities focused on comfort and quality of life rather than life extension (Steinhauser et al., 2000), yet a systematic review revealed that 56%–99% do not have advance directives at the time of an ED visit (Oulton et al., 2015) and are at risk of receiving care inconsistent with their goals and values (O’Connor et al., 2011).
Guided by the Social Cognitive Theory (Bandura, 1977) and modeled from previously successful ED behavioral interventions using the Transtheoretical Model (Bernstein et al., 2009; Bruguera et al., 2018; D’Onofrio et al., 2012; Miller, 1996; Prochaska & DiClemente, 1983; Sommers et al., 2013), we previously developed a practical approach to engaging seriously ill older adults to initiate or reintroduce ACP conversations in the ED. ED GOAL, our short, scripted, motivational interview-based intervention, prompts patients to articulate their values and preferences (patient-tested language in prior studies; Bernacki et al., 2014, 2015; Paladino et al., 2019) and communicate them in ACP conversations with their outpatient clinicians and avoids a time-consuming, sensitive conversation in the time-pressured ED environment. Seriously ill older adults (N = 50) found ED GOAL acceptable, and it motivated them to talk to their outpatient clinicians about their goals of care. However, our prior studies excluded patients living with mild cognitive impairment (MCI) or dementia despite the fact that 31% of older adults (aged 65 years and older) presenting to the ED have evidence of dementia (Carpenter et al., 2019) and only 39% of older adults with cognitive impairment and/or their caregivers had engaged in ACP conversations prior to presentation (Garand et al., 2011). Thus, we aimed to refine ED GOAL, using feedback from experts in dementia care, and pilot the refined ED GOAL for acceptability with cognitively impaired patients and their caregivers. To make it feasible to enroll patients with cognitive impairment in the ED, we developed a method to rapidly screen and estimate a patient’s cognitive impairment status as well (see Supplement A). The results of this acceptability study will inform what population is appropriate for a future randomized control trial (RCT) of ED GOAL.
Research Design and Methods
The development and previous testing of ED GOAL are reported elsewhere (Leiter et al., 2018; Ouchi et al., 2019; Pajka et al., 2021; Rubin et al., 2022). We sought to systematically refine ED GOAL so that it can be used with patients living with MCI/dementia and their caregivers. This study involved two steps: (1) refining ED GOAL for individuals with MCI/dementia and their caregivers (refined ED GOAL = ED GOALCG), and (2) testing the acceptability of ED GOALCG by patients with MCI/dementia and their caregivers. We focused on these outcomes because refining and demonstrating the acceptability of an intervention in a pilot test is the first stage of intervention development in NIH’s Stage Model for Behavioral Intervention Development (Onken et al., 2014). The study was approved by our institutional review board.
ED GOAL Refinement for Patients With MCI/Dementia by Experts
We used a comprehensive approach to refine our intervention for patients with MCI/dementia and their caregivers guided by an established framework (Stirman et al., 2013). We involved six experts in cognitive impairment assessments, dementia, and palliative care to review and provide feedback on the refined intervention script and study approach. These experts were not involved in the original development of ED GOAL. They rated the ED GOAL script on 4-point scale (e.g., 1 = irrelevant/not clear, 4 = essential/very clear) based on (1) each activity’s relevance to the intended theoretical element, (2) the likely effectiveness in the intervention goal, and (3) appropriateness for the population. The ratings were then used to compute a content validity index (0–1.0, ≥0.7 is considered adequate; Kassam-Adams et al., 2015).
Acceptability Testing of ED GOALCG
Setting and participants
The study took place in a tertiary care, academic medical center in the Northeast region of the United States. To ensure the acceptability testing of ED GOALCG in patients with well-defined cognitive statuses, we recruited patients with established diagnoses of MCI or dementia and their caregivers from behavioral neurology clinics. For patients with MCI or mild dementia, both the patient and caregiver were the participants of the study. For patients with moderate to advanced dementia, only the caregiver was the participant. Inclusion criteria were: (1) aged 50 and older; (2) have established diagnosis of MCI/dementia determined by extensive neurocognitive testings by behavioral neurologists; (3) have the capacity to consent (as noted above, for patients with moderate to advanced dementia, only the caregivers participated in the study, caregiver capacity was not assessed); and (4) English speaking. We excluded patients or caregivers who were determined by the treating clinician to be not fit for this study (e.g., patient lacked insight into dementia).
Procedures
Due to the coronavirus disease-pandemic restrictions on in-person research activities, all study procedures occurred remotely via Zoom. Trained research assistants (RAs) called to schedule enrollments within 10 days of clinic visits. ED GOALCG was primarily conducted by six trained research nurses or the Principal Investigator (an emergency physician). Briefly, the training involved a 1-hr didactic on the research methodologies, motivational interviewing, and serious illness communication skills followed by a 4-hr communication training with trained actors in the format described previously (Back et al., 2007; Bays et al., 2014). For patients with MCI or mild dementia, study clinicians assessed patient capacity using clinical judgment supplemented by the Mini-Mental State Examination (MMSE; total score ≥ 20) and enrolled both the patient and caregiver. While imperfect, the MMSE cutoff score was included as an additional safeguard for patients being enrolled. Verbal consent was obtained by the study clinician. A prespecified, intervention fidelity checklist was completed during the enrollment to ensure high fidelity (>70% components completed, see Supplement C). The utility and definition of high fidelity using intervention fidelity checklists for behavioral interventions were previously described (Borrelli, 2011). The trained RAs collected the postintervention data to assess acceptability, and pre-/postintervention data to assess the changes in participants’ self-reported ACP engagement. Similar to prior studies, participants were recruited until more than 65% found ED GOALCG acceptable and no new substantive changes were recommended to ED GOALCG (Ouchi et al., 2019). Participants were compensated $48 and received a written summary of the conversations, as did their outpatient clinicians. Follow-up outcome assessments were conducted over the telephone after 28 days (±1 week).
Outcomes
The primary outcome was acceptability of ED GOALCG measured immediately after the intervention. Acceptability was defined using a validated measure of “heard and understood” (Gramling et al., 2016) modified to the context of end-of-life care. The validated measure asks, “How much do you feel heard and understood by your doctors, nurses, and health care staff?” We modified this measure to focus on participants’ values and preferences toward end-of-life care (i.e., “How much have you felt heard and understood about what you would want in medical care if you were to get sicker?” from 1 = not at all to 5 = completely). Additionally, we examined patient-/caregiver-perceived respectfulness to autonomy as another measure to depict the acceptability.
The secondary outcomes included participant-reported ACP engagement before and 1 month after our intervention using the four-item validated measure (Sudore et al., 2013, 2017a). In addition, we examined participants’ self-reported completion of ACP conversations with their outpatient clinician one month after ED GOALCG. We measured ACP outcomes to demonstrate the feasibility of measuring these outcomes in patients with cognitive impairment and their caregivers to inform design of a larger study. Trained RAs also obtained information on documented ACP conversations from participants’ medical records at 1-month follow-up (Gilbert et al., 1996). The complete follow-up survey can be found in Supplements D and E.
Analysis
The chi-square test was used to evaluate whether one categorical response statistically predominates within the heard/understood measures. A one-sample binomial exact test of proportions for categorical outcomes was used to measure baseline and 1-month data for the ACP Engagement Survey. A p value of <.05 as the significance threshold was used. The analysis for this paper was generated using SAS software (SAS Institute Inc., Cary, North Carolina). Qualitative suggestions for improvement were reviewed by the study team (K. Ouchi and D. Rentz) twice during the study, and iterative refinement of the script was made until content saturation (e.g., adding a script to assess from the caregiver whether it is ok to use the word “dementia” in the presence of the patient), similar to a prior study of this nature (Ouchi et al., 2019).
Results
ED GOAL refinement
Based on Stirman’s framework, we identified several additions and refinements to the original ED GOAL: (1) rapid assessment of the stage of cognitive impairment during the anticipated recruitment in the ED; (2) confirmation of the capacity to provide informed consent; (3) solicitation of permission from the patient and caregiver to involve both in the conversation (as deemed clinically appropriate); and (4) verification of patient reporting by the caregivers to solicit their perspectives (as deemed clinically appropriate). The content validity indices for the above refinements were all >0.8. The refined script additions and rapid cognitive impairment assessment procedures are available in Supplement A and Supplement B, respectively.
Participant demographics
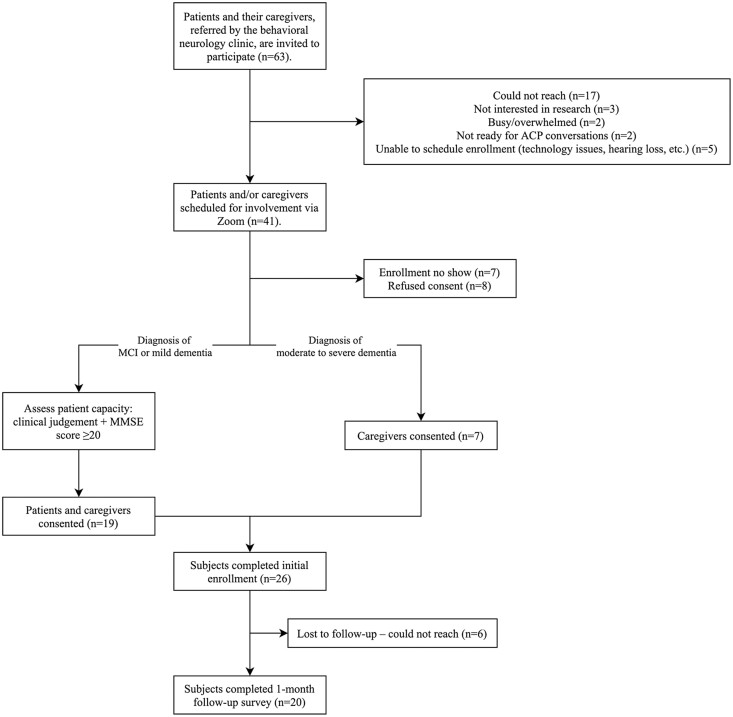
Of 63 eligible patients/eligible dyads approached, seven patients and 26 dyads participated; 20 (77%) patients/dyads completed the 1-month follow-up questionnaire (Table 1; Figure 1). Mean age of the patients was 79 years (SD 8.5), 65% were female, 92.3% were White, 96.2% were non-Hispanic, and 69% had moderate dementia. The seven clinicians that administered ED GOALCG spent a median of 30 min completing the intervention.
Table 1.
Patient demographics (N = 26)
| Variable | M (SD) | n (%) |
|---|---|---|
| Age in years | 79 (8.5) | |
| Sex | ||
| Female | 17 (65) | |
| Male | 9 (35) | |
| Race | ||
| White | 24 (92.3) | |
| Black/African American | 0 (0) | |
| Asian | 0 (0) | |
| Other | 0 (0) | |
| Unavailable | 2 (7.7) | |
| Ethnicity | ||
| Non-Hispanic/Latino | 25 (96.2) | |
| Hispanic/Latino | 0 (0) | |
| Unknown | 1 (3.8) | |
| Eligible serious illness | ||
| Mild cognitive impairment | 2 (7.7) | |
| Mild dementia | 5 (19.2) | |
| Moderate dementia | 18 (69.2) | |
| Advanced dementia | 1 (3.8) | |
| Caregiver relationship to patient | ||
| Spouse | 14 (53.9) | |
| Adult child | 11 (42.3) | |
| Sibling | 1 (3.8) | |
Note: SD = standard deviation.
Figure 1.
Modified CONSORT diagram. ACP = advance care planning; CONSORT = Consolidated standards of reporting trials; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination.
Acceptability outcomes following the intervention: heard and understood
After ED GOALCG, 23% (6/26) of participants reported being completely (5 out of 5 Likert scale rating, median = 3.5, interquartile range [IQR] = 1, p value = .461) heard and understood by their primary outpatient clinicians about what they would want in medical care if they got sicker, while 58% (15/26) of participants reported being completely (median = 5, IQR = 1, p value = .003) heard and understood by their study clinician about what they would want in medical care if they got sicker (Table 2).
Table 2.
Patient-Reported Outcomes (N = 26)
| Outcome | n (%) | M (SD) | p Value |
|---|---|---|---|
| Heard and Understood | |||
| How much have you felt heard and understood by your PRIMARY DOCTORS about what you would want in medical care if you were to get sicker? | .461 | ||
| Not at all | 4 (15.4) | ||
| Slightly | 2 (7.7) | ||
| Moderately | 7 (26.9) | ||
| Quite a bit | 7 (26.9) | ||
| Completely | 6 (23.1) | ||
| After today’s study interview, how much do you feel heard and understood by the STUDY CLINICIAN about what you would want in medical care if you were to get sicker? | .003 | ||
| Not at all | 0 | ||
| Slightly | 0 | ||
| Moderately | 1 (3.8) | ||
| Quite a bit | 10 (38.5) | ||
| Completely | 15 (57.7) | ||
| After today’s study interview, how interested would you be in receiving additional information preparing you to engage with your doctor in talking about what you want in your care if you were to get sicker? | .003 | ||
| Not at all | 2 (7.7) | ||
| Slightly | 0 | ||
| Moderately | 2 (7.7) | ||
| Quite a bit | 5 (19.2) | ||
| Completely | 17 (65.4) | ||
| Respectfulness | |||
| How respectfully did the interview ask the patient for his/her input? | |||
| Very disrespectful | 0 | ||
| Somewhat disrespectful | 0 | ||
| Neutral | 0 | ||
| Somewhat respectful | 1 (3.8) | ||
| Very respectful | 25 (96.2) | ||
| How respectfully did the interview ask the caregiver(s) for his/her input? | |||
| Very disrespectful | 0 | ||
| Somewhat disrespectful | 0 | ||
| Neutral | 0 | ||
| Somewhat respectful | 0 | ||
| Very respectful | 26 (100.0) | ||
| Advance care planning engagement | |||
| Item 1: How ready are you to sign official papers naming a person or group of people to make medical decisions for you? | 4.7 (1.0) | .141 | |
| Before the intervention | 5.0 (0.2) | ||
| One month after the intervention | |||
| Item 2: How ready are you to talk to your decision maker about the kind of medical care you would want if you were very sick or near the end of life? | .872 | ||
| Before the intervention | 4.2 (1.3) | ||
| One month after the intervention | 4.6 (0.8) | ||
| Item 3: How ready are you to talk to your doctor about the kind of medical care you would want if you were very sick or near the end of life? | .364 | ||
| Before the intervention | 3.4 (1.5) | ||
| One month after the intervention | 4.2 (1.1) | ||
| Item 4: How ready are you to sign official papers putting your wishes in writing about the kind of medical care you would want if you were very sick or near the end of life? | .336 | ||
| Before the intervention | 3.8 (1.5) | ||
| One month after the intervention | 4.1 (1.5) | ||
Note: SD = standard deviation.
Respect to patient’s autonomy and caregivers’ input
Nearly all patients and caregivers (96%, 25/26) reported that the study clinician was very respectful (5 out of 5 Likert scale rating, median = 5, IQR = 0) to elicit what the patient would want in his/her future medical care. Hundred percent (26/26) of caregivers reported that the study clinician was very respectful (median = 5, IQR = 0) to elicit the caregivers’ inputs regarding what the patients may want.
ACP outcomes before and after the intervention
There was not a statistically significant change in overall ACP engagement as measured by the 4-point validated ACP engagement scale. However, the item “how ready are you to talk to your doctor about the type of care you would like to receive if you were very sick or near the end of life?” increased from 3.4 to 4.2 on the 5-point Likert scale.
Discussion and Implications
Using Stirman’s framework of refining interventions, we added specific phrases to address both the patient and caregivers (when clinically appropriate for patients with MCI and mild dementia) in ED GOAL (Supplement A). In addition, we have designed a rapid screening for cognitive impairment and capacity determination for the future implementation in mind (see Supplement B). Patients living with MCI/dementia and their caregivers found ED GOALCG acceptable. After ED GOALCG, most participants felt “completely” heard and understood about their wishes for medical care if they got sicker by the study clinicians. The findings suggest that when a structured, motivational interview-based intervention is tailored to patients living with MCI/dementia and their caregivers, clinically meaningful ACP conversations can occur so that patients and caregivers may feel more prepared to share their values and preferences for end-of-life care with their outpatient clinicians in the future.
Our findings are consistent with prior studies. With systematic and iterative refinement using an established framework (Stirman et al., 2013), similar ACP interventions have been successfully adapted to be used for patients living with MCI/dementia (Song et al., 2019). Such refinement and adaptation are shown to be possible with our intervention as well with distinctly unique features (see Supplements A and B): (1) rapid assessment of the stage of cognitive impairment during the anticipated recruitment in the ED; (2) confirmation of the capacity to provide informed consent; (3) solicitation of permission from the patient and caregiver to involve both in the conversation (as deemed clinically appropriate); and (4) verification of patient reporting by the caregivers to solicit their perspectives (as deemed clinically appropriate). Our acceptability outcomes suggest that patients living with MCI/dementia and their caregivers found ED GOALCG meaningful and potentially clinically useful. ED GOAL was originally designed to be used with seriously ill older adults in the ED. Successful adaptation demonstrated in this study suggests that ED GOALCG can now be tested in the ED setting to understand its impact. Prior ED GOAL studies excluded patients living with MCI/dementia, and this obstacle no longer exists. Given that a substantial proportion of ED populations may suffer from MCI/dementia (Carpenter, Bassett, et al., 2011; Carpenter, DesPain, et al., 2011; O’Sullivan et al., 2018), the potential for ED GOALCG to empower this especially vulnerable population with limited access to ACP is encouraging. This is especially true, as a recent scoping review found little research exploring communication the ED with patients living with dementia and that existing strategies do not adequately engage caregivers (Carpenter et al., 2022).
In this acceptability study, ED GOALCG did not have a statistically significant impact on ACP behavior, as measured by the ACP Engagement Survey (Sudore et al., 2013, 2017b). Given that this study was designed to refine ED GOAL and measure acceptability, defined as Stage IA/B in the NIH Stage Model for Behavioral Intervention Development (Onken et al., 2014), it was not powered nor designed to detect the effects on the ACP outcomes. Previous studies of ED GOAL, which did detect significant changes in ACP behavior (Ouchi et al., 2019; Pajka et al., 2021), suggest that a larger sample size in this study would have similarly produced significant results. Furthermore, the seemingly small changes in ACP Engagement scores in this study (Table 2) do likely reflect clinically meaningful changes along the behavior change pathway (Shi et al., 2019).
Our study had several limitations. Given the nature of acceptability study, our sample size was small and limited to non-Hispanic Whites recruited from one academic medical center in the northeast region of the United States. Therefore, the acceptability outcomes for other populations are unknown at this time. Future studies to empirically test its acceptability in other populations are needed. Additionally, our participants were recruited with established diagnoses of MCI, mild/moderate/advanced dementia. Thus, ED GOALCG has never been administered to an undifferentiated patient with suspected MCI/dementia in the ED. However, with inputs from the expert consensus panel, we have established a method to administer rapid screening and estimation of patient’s cognitive impairment status in the ED (see Supplement B). We were only able to enroll two patients with MCI, and most participants had dementia. Therefore, the acceptability may be limited for patients with MCI and their caregivers. However, in the presence of patients with MCI, substantial and subtle scripts have been added to facilitate nuanced solicitation of caregivers’ input (suggested by our caregiver partners), which would maximize the chance of acceptability in this subpopulation (see Supplement A). Feasibility of participant screening in the ED and subsequent administration of ED GOALCG is currently under investigation.
To summarize, ED GOALCG, a brief, ACP intervention, was successfully adapted to be used for patients living with MCI/dementia and their caregivers. This adaptation enabled its administration to seriously ill older adults with MCI/dementia in the ED settings. The clinical effects of ED GOALCG, which are currently being testing as part of a larger RCT, remain to be seen.
Supplementary Material
Contributor Information
Kei Ouchi, Department of Emergency Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Department of Emergency Medicine, Harvard Medical School, Boston, Massachusetts, USA.
Christopher Joshi, Department of Emergency Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA; School of Medicine, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Jenson Kaithamattam, Department of Emergency Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Seth A Gale, Department of Neurology, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Center for Alzheimer Research and Treatment, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Gad A Marshall, Department of Neurology, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Center for Alzheimer Research and Treatment, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Alison Pietras, Department of Neurology, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Center for Alzheimer Research and Treatment, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Wei Wang, Division of Circadian and Sleep Disorders, Departments of Medicine and Neurology, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Edward W Boyer, Department of Emergency Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Department of Emergency Medicine, Harvard Medical School, Boston, Massachusetts, USA.
James A Tulsky, Department of Psychosocial Oncology and Palliative Care, Dana-Farber Cancer Institute, Boston, Massachusetts, USA; Division of Palliative Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Susan D Block, Department of Psychosocial Oncology and Palliative Care, Dana-Farber Cancer Institute, Boston, Massachusetts, USA; Division of Palliative Medicine, Department of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Dorene Rentz, Department of Neurology, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Center for Alzheimer Research and Treatment, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Mara A Schonberg, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA.
Funding
This work was supported by the National Institute on Aging (grant numbers K76AG064434 to K. Ouchi, T35AF038027 to C. Joshi) and Cambia Health Foundation.
Conflict of Interest
None declared.
Data Availability
By contacting our team to establish a data usage agreement, our data and analytic methods used in this study can be shared. This study was not preregistered given the main intent was to describe and demonstrate the intervention refinement and acceptability.
References
- Back, A. L., Arnold, R. M., Baile, W. F., Fryer-Edwards, K. A., Alexander, S. C., Barley, G. E., Gooley, T. A., & Tulsky, J. A. (2007). Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Archives of Internal Medicine, 167(5), 453–460. doi: 10.1001/archinte.167.5.453 [DOI] [PubMed] [Google Scholar]
- Bandura, A. (1977). Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review, 84(2), 191–215. doi: 10.1037//0033-295x.84.2.191 [DOI] [PubMed] [Google Scholar]
- Bays, A. M., Engelberg, R. A., Back, A. L., Ford, D. W., Downey, L., Shannon, S. E., Doorenbos, A. Z., Edlund, B., Christianson, P., Arnold, R. W., O’Connor, K., Kross, E. K., Reinke, L. F., Cecere Feemster, L., Fryer-Edwards, K., Alexander, S. C., Tulsky, J. A., & Curtis, J. R. (2014). Interprofessional communication skills training for serious illness: Evaluation of a small-group, simulated patient intervention. Journal of Palliative Medicine, 17(2), 159–166. doi: 10.1089/jpm.2013.0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacki, R., Hutchings, M., Vick, J., Smith, G., Paladino, J., Lipsitz, S., Gawande, A. A., & Block, S. D. (2015). Development of the Serious Illness Care Program: A randomised controlled trial of a palliative care communication intervention. BMJ Open, 5(10), e009032. doi: 10.1136/bmjopen-2015-009032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacki, R. E., Block, S. D., & American College of Physicians High Value Care Task Force. (2014). Communication about serious illness care goals: A review and synthesis of best practices. JAMA Internal Medicine, 174(12), 1994–2003. doi: 10.1001/jamainternmed.2014.5271 [DOI] [PubMed] [Google Scholar]
- Bernstein, E., Edwards, E., Dorfman, D., Heeren, T., Bliss, C., & Bernstein, J. (2009). Screening and brief intervention to reduce marijuana use among youth and young adults in a pediatric emergency department. Academic Emergency Medicine, 16(11), 1174–1185. doi: 10.1111/j.1553-2712.2009.00490.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli, B. (2011). The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. Journal of Public Health Dentistry, 71(Suppl. 1), S52–S63. doi: 10.1111/j.1752-7325.2011.00233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruguera, P., Barrio, P., Oliveras, C., Braddick, F., Gavotti, C., Bruguera, C., López-Pelayo, H., Miquel, L., Segura, L., Colom, J., Ortega, L., Vieta, E., & Gual, A. (2018). Effectiveness of a specialized brief intervention for at-risk drinkers in an emergency department: Short-term results of a randomized controlled trial. Academic Emergency Medicine, 25(5), 517–525. doi: 10.1111/acem.13384 [DOI] [PubMed] [Google Scholar]
- Carpenter, C. R., Banerjee, J., Keyes, D., Eagles, D., Schnitker, L., Barbic, D., Fowler, S., & LaMantia, M. A. (2019). Accuracy of dementia screening instruments in emergency medicine: A diagnostic meta-analysis. Academic Emergency Medicine, 26(2), 226–245. doi: 10.1111/acem.13573 [DOI] [PubMed] [Google Scholar]
- Carpenter, C. R., Bassett, E. R., Fischer, G. M., Shirshekan, J., Galvin, J. E., & Morris, J. C. (2011). Four sensitive screening tools to detect cognitive dysfunction in geriatric emergency department patients: Brief Alzheimer’s Screen, Short Blessed Test, Ottawa 3DY, and the caregiver-completed AD8. Academic Emergency Medicine, 18(4), 374–384. doi: 10.1111/j.1553-2712.2011.01040.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, C. R., DesPain, B., Keeling, T. N., Shah, M., & Rothenberger, M. (2011). The six-item screener and AD8 for the detection of cognitive impairment in geriatric emergency department patients. Annals of Emergency Medicine, 57(6), 653–661. doi: 10.1016/j.annemergmed.2010.06.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, C. R., Leggett, J., Bellolio, F., Betz, M., Carnahan, R., Carr, D., Kelly, K., Morrow-Howell, N., Prusaczyk, B., Savage, B., Suyama, J., Vann, A. S., Rising, K. L., Hwang, U., & Shah, M. N. (2022). Emergency department communication in persons living with dementia and care partners: A scoping review. Journal of American Medical Director Association, 23(8), 1313.e15–1313.e46. doi: 10.1016/j.jamda.2022.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio, G., Fiellin, D. A., Pantalon, M. V., Chawarski, M. C., Owens, P. H., Degutis, L. C., Busch, S. H., Bernstein, S. L., & O’Connor, P. G. (2012). A brief intervention reduces hazardous and harmful drinking in emergency department patients. Annals of Emergency Medicine, 60(2), 181–192. doi: 10.1016/j.annemergmed.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschodt, M., Devriendt, E., Sabbe, M., Knockaert, D., Deboutte, P., Boonen, S., Flamaing, J., & Milisen, K. (2015). Characteristics of older adults admitted to the emergency department (ED) and their risk factors for ED readmission based on comprehensive geriatric assessment: A prospective cohort study. BMC Geriatrics, 15, 54. doi: 10.1186/s12877-015-0055-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garand, L., Dew, M. A., Lingler, J. H., & DeKosky, S. T. (2011). Incidence and predictors of advance care planning among persons with cognitive impairment. American Journal of Geriatric Psychiatry, 19(8), 712–720. doi: 10.1097/JGP.0b013e3181faebef [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert, E. H., Lowenstein, S. R., Koziol-McLain, J., Barta, D. C., & Steiner, J. (1996). Chart reviews in emergency medicine research: Where are the methods? Annals of Emergency Medicine, 27(3), 305–308. doi: 10.1016/s0196-0644(96)70264-0 [DOI] [PubMed] [Google Scholar]
- Gramling, R., Stanek, S., Ladwig, S., Gajary-Coots, E., Cimino, J., Anderson, W., Norton, S. A., Group, A. R. C. W., Aslakson, R. A., Ast, K., Elk, R., Garner, K. K., Gramling, R., Grudzen, C., Kamal, A. H., Lamba, S., LeBlanc, T. W., Rhodes, R. L., Roeland, E., & Unroe, K. T. (2016). Feeling heard and understood: A patient-reported quality measure for the inpatient palliative care setting. Journal of Pain and Symptom Management, 51(2), 150–154. doi: 10.1016/j.jpainsymman.2015.10.018 [DOI] [PubMed] [Google Scholar]
- Kassam-Adams, N., Marsac, M. L., Kohser, K. L., Kenardy, J. A., March, S., & Winston, F. K. (2015). A new method for assessing content validity in model-based creation and iteration of eHealth interventions. Journal of Medical Internet Research, 17(4), e95. doi: 10.2196/jmir.3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal, N., Benkeser, D. C., Coe, N. B., & Curtis, J. R. (2016). Potential influence of advance care planning and palliative care consultation on ICU costs for patients with chronic and serious illness. Critical Care Medicine, 44(8), 1474–1481. doi: 10.1097/CCM.0000000000001675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin, J. R., Block, S. D., Billings, J. A., Koritsanszky, L. A., Cunningham, R., Wichmann, L., Harvey, D., Lamey, J., & Bernacki, R. E. (2016). Improving communication about serious illness in primary care: A review. JAMA Internal Medicine, 176(9), 1380–1387. doi: 10.1001/jamainternmed.2016.3212 [DOI] [PubMed] [Google Scholar]
- Leiter, R. E., Yusufov, M., Hasdianda, M. A., Fellion, L. A., Reust, A. C., Block, S. D., Tulsky, J. A., & Ouchi, K. (2018). Fidelity and feasibility of a brief emergency department intervention to empower adults with serious illness to initiate advance care planning conversations. Journal of Pain and Symptom Management, 56(6), 878–885. doi: 10.1016/j.jpainsymman.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, W. R. (1996). Motivational interviewing: Research, practice, and puzzles. Addictive Behaviors, 21(6), 835–842. doi: 10.1016/0306-4603(96)00044-5 [DOI] [PubMed] [Google Scholar]
- Nagurney, J. M., Fleischman, W., Han, L., Leo-Summers, L., Allore, H. G., & Gill, T. M. (2017). Emergency department visits without hospitalization are associated with functional decline in older persons. Annals of Emergency Medicine, 69(4), 426–433. doi: 10.1016/j.annemergmed.2016.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor, A. E., Winch, S., Lukin, W., & Parker, M. (2011). Emergency medicine and futile care: Taking the road less travelled. Emergency Medicine Australas, 23(5), 640–643. doi: 10.1111/j.1742-6723.2011.01435.x [DOI] [PubMed] [Google Scholar]
- O’Sullivan, D., Brady, N., Manning, E., O’Shea, E., O’Grady, S., N, O. R., & Timmons, S. (2018). Validation of the 6-item Cognitive Impairment Test and the 4AT test for combined delirium and dementia screening in older emergency department attendees. Age and Ageing, 47(1), 61–68. doi: 10.1093/ageing/afx149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken, L. S., Carroll, K. M., Shoham, V., Cuthbert, B. N., & Riddle, M. (2014). Reenvisioning clinical science: Unifying the discipline to improve the public health. Clinical Psychological Science, 2(1), 22–34. doi: 10.1177/2167702613497932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi, K., George, N., Revette, A. C., Hasdianda, M. A., Fellion, L., Reust, A., Powell, L. H., Sudore, R., Schuur, J. D., Schonberg, M. A., Bernstein, E., Tulsky, J. A., & Block, S. D. (2019). Empower seriously ill older adults to formulate their goals for medical care in the emergency department. Journal of Palliative Medicine, 22(3), 267–273. doi: 10.1089/jpm.2018.0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulton, J., Rhodes, S. M., Howe, C., Fain, M. J., & Mohler, M. J. (2015). Advance directives for older adults in the emergency department: A systematic review. Journal of Palliative Medicine, 18(6), 500–505. doi: 10.1089/jpm.2014.0368 [DOI] [PubMed] [Google Scholar]
- Pajka, S. E., Hasdianda, M. A., George, N., Sudore, R., Schonberg, M. A., Bernstein, E., Tulsky, J. A., Block, S. D., & Ouchi, K. (2021). Feasibility of a brief intervention to facilitate advance care planning conversations for patients with life-limiting illness in the emergency department. Journal of Palliative Medicine, 24(1), 31–39. doi: 10.1089/jpm.2020.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladino, J., Bernacki, R., Neville, B. A., Kavanagh, J., Miranda, S. P., Palmor, M., Lakin, J., Desai, M., Lamas, D., Sanders, J. J., Gass, J., Henrich, N., Lipsitz, S., Fromme, E., Gawande, A. A., & Block, S. D. (2019). Evaluating an intervention to improve communication between oncology clinicians and patients with life-limiting cancer: A cluster randomized clinical trial of the Serious Illness Care Program. JAMA Oncology. doi: 10.1001/jamaoncol.2019.0292 [DOI] [PubMed] [Google Scholar]
- Prochaska, J. O., & DiClemente, C. C. (1983). Stages and processes of self-change of smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology, 51(3), 390–395. doi: 10.1037//0022-006x.51.3.390 [DOI] [PubMed] [Google Scholar]
- Rubin, B. R., Chung, M., Hasdianda, M. A., Gray, T. F., Aaronson, E. L., Dundin, A., Egorova, N. A., Revette, A. C., Berry, D., & Ouchi, K. (2022). Refinement of an emergency department-based, advance care planning intervention for nurses. Journal of Palliative Medicine, 25(4), 650–655. doi: 10.1089/jpm.2021.0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, M. J., Prigerson, H. G., Paulk, E., Trevino, K. M., Penedo, F. J., Tergas, A. I., Epstein, A. S., Neugut, A. I., & Maciejewski, P. K. (2016). Impact of end-of-life discussions on the reduction of Latino/non-Latino disparities in do-not-resuscitate order completion. Cancer, 122(11), 1749–1756. doi: 10.1002/cncr.29973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y., Barnes, D. E., Boscardin, J., You, J. J., Heyland, D. K., Volow, A. M., Howard, M., & Sudore, R. L. (2019). Brief English and Spanish survey detects change in response to advance care planning interventions. Journal of Pain and Symptom Management, 58(6), 1068–1074 e1065. doi: 10.1016/j.jpainsymman.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A. K., McCarthy, E., Weber, E., Cenzer, I. S., Boscardin, J., Fisher, J., & Covinsky, K. (2012). Half of older Americans seen in emergency department in last month of life; most admitted to hospital, and many die there. Health Affairs (Millwood), 31(6), 1277–1285. doi: 10.1377/hlthaff.2011.0922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers, M. S., Lyons, M. S., Fargo, J. D., Sommers, B. D., McDonald, C. C., Shope, J. T., & Fleming, M. F. (2013). Emergency department–based brief intervention to reduce risky driving and hazardous/harmful drinking in young adults: A randomized controlled trial. Alcoholism: Clinical and Experimental Research, 37(10), 1753–1762. doi: 10.1111/acer.12142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, M. K., Ward, S. E., Hepburn, K., Paul, S., Kim, H., Shah, R. C., Morhardt, D. J., Medders, L., Lah, J. J., & Clevenger, C. C. (2019). Can persons with dementia meaningfully participate in advance care planning discussions? A mixed-methods study of SPIRIT. Journal of Palliative Medicine, 22(11), 1410–1416. doi: 10.1089/jpm.2019.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauser, K. E., Christakis, N. A., Clipp, E. C., McNeilly, M., McIntyre, L., & Tulsky, J. A. (2000). Factors considered important at the end of life by patients, family, physicians, and other care providers. Journal of American Medical Association, 284(19), 2476–2482. https://www.ncbi.nlm.nih.gov/pubmed/11074777 [DOI] [PubMed] [Google Scholar]
- Stirman, S. W., Miller, C. J., Toder, K., & Calloway, A. (2013). Development of a framework and coding system for modifications and adaptations of evidence-based interventions. Implemention Science, 8, 65. doi: 10.1186/1748-5908-8-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudore, R. L., Heyland, D. K., Barnes, D. E., Howard, M., Fassbender, K., Robinson, C. A., Boscardin, J., & You, J. J. (2017a). Measuring advance care planning: Optimizing the advance care planning engagement survey. Journal of Pain and Symptom Management, 53(4), 669–681 e668. doi: 10.1016/j.jpainsymman.2016.10.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudore, R. L., Heyland, D. K., Barnes, D. E., Howard, M., Fassbender, K., Robinson, C. A., Boscardin, J., & You, J. J. (2017b). Measuring advance care planning: Optimizing the advance care planning engagement survey. Journal of Pain and Symptom Management, 53(4), 669–681. doi: 10.1016/j.jpainsymman.2016.10.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudore, R. L., Stewart, A. L., Knight, S. J., McMahan, R. D., Feuz, M., Miao, Y., & Barnes, D. E. (2013). Development and validation of a questionnaire to detect behavior change in multiple advance care planning behaviors. PLoS One, 8(9), e72465. doi: 10.1371/journal.pone.0072465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber, S. T., Blanda, M., Gerson, L. W., & Allen, K. R. (2010). Short-term functional decline and service use in older emergency department patients with blunt injuries. Academic Emergency Medicine, 17(7), 679–686. doi: 10.1111/j.1553-2712.2010.00799.x [DOI] [PubMed] [Google Scholar]
- Wright, A. A., Zhang, B., Ray, A., Mack, J. W., Trice, E., Balboni, T., Mitchell, S. L., Jackson, V. A., Block, S. D., Maciejewski, P. K., & Prigerson, H. G. (2008). Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. Journal of American Medical Association, 300(14), 1665–1673. doi: 10.1001/jama.300.14.1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
By contacting our team to establish a data usage agreement, our data and analytic methods used in this study can be shared. This study was not preregistered given the main intent was to describe and demonstrate the intervention refinement and acceptability.