Abstract
Mental health disorders are linked to systemic inflammation. Due to high inflammation and mental health disorders in COVID‐19 patients, we aimed to investigate the relationship between blood inflammatory markers such as red cell distribution width to platelet ratio (RPR), platelet‐lymphocyte ratio (PLR), neutrophil/lymphocyte ratio (NLR), red cell distribution width (RDW), white blood cell (WBC), and psychological function in COVID‐19 patients. In the current cross‐sectional study, neuro‐psychological function, and a complete blood count (CBC) were measured on 120 COVID‐19 patients aged >30 years from the Imam Reza Hospital in Mashhad, Iran. Our results showed that anxiety related to MCHC (mean ± SD: 32.71 ± 1.68, p < 0.05), WBC (mean ± SD: 12.23 ± 5.43, p < 0.05), and PLR (median (IQR): 28.72 (15.88–41.31), p < 0.05) significantly. In the stress subgroup, only RPR was associated with stress (p < 0.05). Linear regression between hematological parameters and psychological score indicated that RDW and PLR had a significantly positive association with depression (β = 0.086; p = 0.045 and β = 1.326; p = 0.016, respectively) and anxiety scores (β = 0.100; p = 0.038 and β = 1.356; p = 0.010, respectively). Moreover, a positive correlation was found between PLR and stress (β = 1.102; p = 0.012). This study showed a positive association between depression/anxiety/stress symptoms and levels of hematological inflammatory markers including PLR and RDW. The findings of this study provide novel insights into mental health and physiological markers, underscoring the potential influence of inflammation on mood disorders. Our findings offer exciting prospects for future research and may lead to innovative approaches in the management and treatment of depression, anxiety, and stress.
Keywords: anxiety, COVID‐19, depression, hematologic tests, psychological, stress
1. INTRODUCTION
The 2019 coronavirus (COVID‐19) pandemic, has affected more than 170 million individuals resulting in 3.5 million deaths globally by May 2021 (Fine et al., 2011). In addition to the impact on physical health, has taken a toll on mental health as a result of fear of illness and hospitalization, and social distancing efforts may have a unique and significant influence on mental health. Given the significance of stress in the etiology of anxiety and depressive disorders, the prevalence of these diseases may rise due to intensified stress related to COVID‐19. Previous studies have reported on the mental health of COVID‐19 hospitalized patients during the epidemic, while preliminary research suggests a potential link between these symptoms and disorders and a more severe progression of COVID‐19 (Dergaa et al., 2022; Varma et al., 2021). In this context, it is essential to investigate the prevalence and severity of anxiety and depression in COVID‐19‐infected patients (Khorasanchi et al., 2023; Reger et al., 2020).
In anxiety and related disorders, central and peripheral immune system cells release cytokines and cause inflammation in response to increased stress (Michopoulos et al., 2017). Inflammation and anxiety disorders are linked, and hematological parameters are also thought to be useful markers in the follow‐up of patients with anxiety disorders (Uzun & Akinci, 2021). Systemic inflammation has been linked to depression and anxiety, two common mood disorders (Bahrami et al., 2019; Duivis et al., 2013).
The white blood cell count (WBC) is a nonspecific inflammatory marker that is usually measured as part of a complete blood count (CBC) panel. The quantitative measure of anisocytosis, red cell distribution width (RDW), is a simple, low‐cost parameter that is routinely reported as part of the CBC test (McPherson et al., 2021). Previous research has found a significant association between RDW and inflammatory markers such as hs‐CRP and erythrocyte sedimentation rate. This indicates that increased RDW may result from an underlying inflammatory state associated with negative outcomes (Kalay et al., 2011; Rawi et al., 2023).
Previous research has found that personal experiences with COVID‐19 diagnosis, mortality in acquaintances, and COVID‐19‐related stress are related to a significantly increased risk of emotional disorder symptomatology and that the COVID‐19 pandemic may increase demand for mental health services (Gallagher et al., 2020).
In contrast to measuring peripheral cytokine levels, studies on this topic have found that measuring peripheral blood cells, which are the source of some of these cytokines, by CBC is a cheap, easy, and rapid procedure. The neutrophil/lymphocyte ratio (NLR), which is determined by the ratio of these two key cells participating in the inflammatory response, has been accepted as a measure of the systemic inflammatory response (Khorasanchi et al., 2022; Zahorec, 2001). NLR has been reported to be higher in several psychiatric diseases, such as mood disorders, anxiety disorders, and schizophrenia, than in healthy adults, and has already been considered a biomarker of increased inflammatory response (Bustan et al., 2018; Ekinci & Ekinci, 2017). Platelet/lymphocyte ratio (PLR) is a biomarker used as a diagnostic of chronic inflammation, similar to NLR, and is useful in several disease groups, especially autoimmune illnesses and cancer (Erre et al., 2019; Kumarasamy et al., 2019) and is often used to diagnose psychiatric diseases (Özdin et al., 2017).
In this study, the main goal was to analyze the relationship between various blood inflammatory markers and psychological well‐being indicators in individuals diagnosed with COVID‐19. The specific inflammatory markers that were assessed included WBC, RDW, NLR, and PLR. The reason behind conducting this investigation was the observation of elevated inflammation levels. The psychological well‐being indicators that were examined encompassed depression, anxiety, stress, and quality of life in COVID‐19 patients. The aim of this study was to obtain a comprehensive understanding of how inflammation levels could potentially impact the mental well‐being of COVID‐19 patients.
2. MATERIALS AND METHODS
2.1. Study design
The current cross‐sectional study was carried out in 2021 within a sample of COVID‐19 patients from the Imam Reza Hospital in Mashhad, Iran. The total subjects included 120 COVID‐19 patients aged >30 years. The exclusion criteria were: cancer, autoimmune diseases, hepatic or renal failure, metabolic bone disease, and without antidepressant drug treatment during the previous 6 months. A written consent form was filled out by the participants. Diagram of the study participants showed in Figure 1.
FIGURE 1.
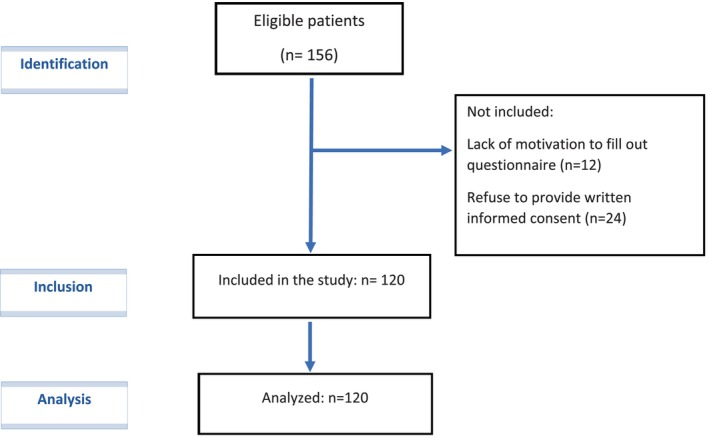
STROBE flow diagram of participants.
2.2. General and clinical characteristics
Demographics and clinical characteristics including age, smoking status, and comorbidity data were collected from each participant at the baseline by trained interviewers.
2.3. Blood collection and biochemical measurements
Blood samples have been taken once from each patient and all of them were taken in the morning and after 12 h of midnight fasting. A CBC including red blood cell (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC), platelet (PLT), platelet distribution width (PDW), mean platelet volume (MPV), RDW, and WBC were measured by using an auto hematology analyzer (Sysmex K‐800) for each individual.
2.4. Depression anxiety stress scales
The depression anxiety stress scale (DASS) is an accurate and valid tool to evaluate mood status (Henry & Crawford, 2005). This tool is a questionnaire that consists of three subscales including 7 questions, generally consists 21 items. Each question is ranged on a 4‐point (0–3) Likert scale to identify the severity of mood disorders including depression, anxiety, and stress. A score of each item must be doubled because DASS 21 is a brief version of DASS 42. In this tool, a higher score shows a higher degree of negative mood status and a lower score indicates a lower degree of negative emotion. The reliability and validity of DASS have been previously reported in the Iranian population (Sahebi et al., 2005). The scores for depression, anxiety, and stress were divided into two classifications: No or minimal scores, and some degree of mood disorder. Based on the scores obtained from each item were determined as follows: (≤9, No), (>9, some degree of depression), (≤7, no), (>7, some degree of anxiety), (≤14, no), (>4 some degree of stress).
2.5. Insomnia Severity Index (ISI)
The ISI is a seven‐item self‐report tool for determining insomnia symptoms and their consequences. The dimensions measured are the severity of sleep onset, sleep preservation, early morning awakening problems, sleep dissatisfaction, interference of sleep difficulties with daytime functioning, noticeability of sleep problems by others, and distress caused by sleep difficulties (Morin et al., 2011). Based on severity every item scored on a 0–4 scale. The total scale ranged from 0 to 28 and explained as follows: no insomnia (0–7), sub‐threshold insomnia (8–14), mild insomnia (15–21), and severe insomnia (22–28). The validity and reliability of the Persian version of this questionnaire have been confirmed in the Iranian population (Cronbach's a >0.8 and intra‐class correlation coefficient >0.7) (Yazdi et al., 2012).
2.6. Pittsburgh Sleep Quality Index (PSQI)
Sleep quality was evaluated using a 19‐item self‐reported PSQI questionnaire that assesses sleep quality over the last 30 days duration (Buysse et al., 1989). It consists of 19 items combined for 7 component scores, including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. The responses are scored on a 3‐point scale, ranging from 0 to 3. The overall score for sleep quality can be calculated by a combination of the 7 component scores, which range from 0 to 21. Patients were classified into two groups based on their PSQI score: the poor‐sleeper group (PSQI>5) and the good‐sleeper group (PSQI≤5). The Persian version of PSQI has also been validated in a previous study by Farrahi Moghaddam et al. in 2012 (Moghaddam et al., 2012).
2.7. Quality of life questionnaire
The Short Form Health Survey (SF‐36) validated questionnaire was used for evaluating the general quality of life. SF‐36 is categorized into eight subscales: Role Physical, Physical Functioning, General Health, Bodily Pain Social Functioning, Vitality, Role Emotional, and Mental Health. Scores of this questionnaire range from 0 to 100. The Persian version of SF‐36 was assessed in a previous study and indicated good reliability and construct validity (Montazeri et al., 2005).
2.8. Statistical analysis
SPSS Statistics for Windows v20 (SPSS, Inc., Chicago, IL) was used for statistical analysis. The normality of variables was analyzed using the Kolmogorov–Smirnov test. Descriptive statistics including mean and standard deviation (SD) were determined for all variables and expressed as mean ± SD for normally distributed variables and as the median and interquartile range (IQR) for non‐normally distributed variables. Moreover, categorical indices were demonstrated by number (%). Chi‐squared tests were used to compare the qualitative variables. Two independent sample t‐test and the Mann–Whitney test was used to evaluate the significant difference in normal and non‐normal variables between the two groups, respectively. Finally, multivariate linear regression was performed between hematological parameters and psychological scores. Five default assumptions were required to perform linear regression: (1) Linear relationship, (2) no or little multicollinearity (3) multivariate normality (4) homoscedasticity (5) no autocorrelation. These assumptions were checked, and then linear regression was performed. All analyses were considered bilateral, and a p‐Value of <0.05 was considered to be significant.
3. RESULTS
The demographic data of the patients are reported in Table 1, which shows the mean age, sex, smoking conditions, and underlying diseases of the participants. The number of patients participating in all subgroups of depression, anxiety, stress, sleep disorders, and quality of life questionnaire is listed separately.
TABLE 1.
Demographics features of study subjects.
| Depression, n = 78 | Anxiety, n = 90 | Stress, n = 93 | Sleep | Quality of life (score <55), n = 58 | ||
|---|---|---|---|---|---|---|
| Variable | Insomnia, n = 28 | Poor sleep quality, n = 85 | ||||
| Gender | ||||||
| Male | 44 (57.8%) | 49 (55.8%) | 51(55.7%) | 13 (45.5%) | 42 (49.4%) | 31 (53.4%) |
| Female | 34 (42.2%) | 41 (44.2%) | 42(44.3%) | 15 (54.5%) | 43 (50.6%) | 27 (46.6%) |
| Age (years) | 60.42 ± 14.85 | 59.71 ± 14.33 | 60.41 ± 14.30 | 57.59 ± 14.18 | 61.42 ± 13.56 | 60.77 ± 15.56 |
| Current smoking | 18 (28.5%) | 20 (26.3%)* | 21 (27%)* | 7 (25.0%) | 16 (18.8%) | 15 (25.8%) |
| Comorbidity | ||||||
| Cardiovascular disease | 14 (22.2%) | 17 (22.4%) | 17 (21.8%) | 4 (14.2%) | 14 (16.4%) | 9 (15.5%) |
| Hypertension | 25 (39.7%) | 31 (40.8%) | 32 (41.0%) | 10 (35.7%) | 30 (35.2%) | 20 (34.4%) |
| Diabetes | 25 (39.7%) | 33 (43.4%) | 35 (44.9%) | 10 (35.7%) | 32 (37.6%) | 19 (32.7%) |
Note: Data presented as mean ± SD or n (%). Obtained from t‐test for continuous variables and χ2 test for categorical variables.
p < 0.05.
Table 2 shows the relationship between hematological parameters and variables of depression, anxiety, stress, sleep disorders, and quality of life. No blood parameters were significantly associated with depression, sleep disorders, and quality of life (p > 0.05), but anxiety score showed a significant association with MCHC (mean ± SD: 32.71 ± 1.68, p < 0.05), WBC (mean ± SD: 12.23 ± 5.43, p < 0.05) and PLR concentration (median (IQR): 28.72 (15.88–41.31), p < 0.05). In the stress subgroup, only RPR with a level of 0.07 (0.05–0.11) was associated with stress (p < 0.05).
TABLE 2.
Hematological parameters in subjects with phycological disorders.
| Depression, n = 78 | Anxiety, n = 90 | Stress, n = 93 | Sleep | Quality of life (score < 55), n = 58 | ||
|---|---|---|---|---|---|---|
| Variable | Insomnia, n = 28 | Poor sleep quality, n = 85 | ||||
| RBC (1012/L) | 4.58 ± 0.86 | 4.60 ± 0.84 | 4.60 ± 0.82 | 4.52 ± 0.78 | 4.69 ± 0.78 | 4.61 ± 0.79 |
| Hemoglobin (g/dL) | 12.81 ± 2.58 | 12.95 ± 2.43 | 12.94 ± 2.41 | 12.39 ± 2.37 | 13.13 ± 2.30 | 12.85 ± 2.38 |
| Hematocrit | 39.35 ± 6.59 | 39.92 ± 6.59 | 39.92 ± 6.51 | 38.09 ± 6.61 | 40.23 ± 6.16 | 39.59 ± 6.52 |
| MCV (fL) | 86.46 ± 6.77 | 87.06 ± 6.55 | 87.06 ± 6.56 | 87.13 ± 7.40 | 86.69 ± 6.43 | 87.06 ± 7.01 |
| MCH (pg) | 28.53 ± 2.68 | 28.68 ± 2.52 | 28.96 ± 2.49 | 28.30 ± 2.98 | 28.58 ± 2.51 | 28.54 ± 2.67 |
| MCHC (g/dL) | 32.83 ± 1.89 | 32.71 ± 1.68* | 32.71 ± 1.65 | 32.54 ± 1.67 | 33.42 ± 6.72 | 33.54 ± 7.46 |
| RDW (%) | 14.75 ± 2.32 | 14.59 ± 1.99 | 14.67 ± 1.99 | 14.89 ± 2.05 | 14.48 ± 1.79 | 14.57 ± 1.82 |
| PDW (fL) | 14.02 ± 2.47 | 13.89 ± 2.41 | 13.88 ± 2.40 | 13.47 ± 2.80 | 13.83 ± 2.64 | 13.86 ± 2.48 |
| WBC (103/μL) | 9.83 ± 4.72 | 12.23 ± 5.43* | 11.25 ± 5.34 | 8.58 ± 3.55 | 9.26 ± 4.39 | 9.77 ± 4.76 |
| MPV (fL) | 9.70 ± 3.09 | 9.71 ± 2.77 | 9.72 ± 2.74 | 10.33 ± 0.87 | 9.78 ± 2.82 | 9.59 ± 3.41 |
| NLR | 8.15 (9.77) | 7.64 (9.26) | 7.86 (9.46) | 7.47 (9.16) | 7.75 (10.21) | 7.35 (9.76) |
| RPR | 0.07 (0.06) | 0.07 (0.05) | 0.07 (0.06)* | 0.06 (0.04) | 0.07 (0.05) | 0.07 (0.08) |
| PLR | 17.93 (18.39) | 28.72 (25.43)* | 23.25 (22.61) | 17.20 (16.63) | 16.1 (20.44) | 13.57 (17.44) |
Note: Data presented as mean ± SD or median (IQR). Obtained from independent sample t‐test for normal variable and Mann–Whitney test for the nonparametric variable.
Abbreviations: HCT, hematocrit; HGB, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; NLR, neutrophil to lymphocyte ratio; PLR, platelet lymphocyte ratio; RBC, red blood cell; RDW, red cell distribution width; RPR, RDW to platelet count ratio; WBC, white blood cell.
p < 0.05.
Multivariate linear regression analysis between hematological parameters and a score of mental health tests are indicated in Table 3. Linear regression indicated a positive relationship between RDW and PLR with depression (β = 0.086; p = 0.045 and β = 1.326; p = 0.016, respectively) and anxiety scores (β = 0.100; p = 0.038 and β = 1.356; p = 0.010, respectively). Moreover, a positive correlation was found between PLR and stress (β = 1.102; p = 0.012). Other hematological parameters were not significantly associated with depression, anxiety, stress, sleep disorders, and quality of life (p > 0.05) (Table 3).
TABLE 3.
Multivariate linear regression between hematological parameters and psychological scores.
| Hematological parameters | Depression | Anxiety | Stress | Insomnia | Sleep quality | Quality of life | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | β | p | β | p | |
| RBC (1012/L) | −0.001 | 0957 | −0.011 | 0.562 | 0.003 | 0.846 | −0.224 | 0.245 | 0.150 | 0.498 | 0.009 | 0.951 |
| Hemoglobin (g/dL) | −0.082 | 0.134 | −0.079 | 0.165 | −0.070 | 0.133 | −0.742 | 0.198 | 0.083 | 0.900 | 0.356 | 0.440 |
| Hematocrit | −0.136 | 0.355 | −0.154 | 0.315 | −0.105 | 0.397 | −2.066 | 0.192 | 0.481 | 0.786 | 0.607 | 0.632 |
| MCV (fL) | −0.197 | 0.147 | −0.114 | 0.421 | −0.207 | 0.070 | 0.646 | 0.658 | −1.246 | 0.463 | 0.241 | 0.836 |
| MCH (pg) | −0.100 | 0.051 | −0.092 | 0.086 | −0.104 | 0.016 | −0.096 | 0.867 | −0.264 | 0.687 | 0.198 | 0.664 |
| MCHC (g/dL) | −0.037 | 0.764 | −0.106 | 0.408 | −0.074 | 0.480 | −0.788 | 0.529 | 1.408 | 0.393 | −0.734 | 0.462 |
| RDW (%) | 0.086 | 0.045 | 0.100 | 0.038 | 0.064 | 0.103 | 0.374 | 0.454 | 0.500 | 0.331 | −0.144 | 0.717 |
| PDW (fl) | 0.084 | 0.121 | 0.014 | 0.798 | 0.046 | 0.299 | −0.311 | 0.594 | −0.118 | 0.873 | −0.193 | 0.682 |
| WBC (103/μL) | 0.021 | 0.841 | −0.158 | 0.143 | −0.093 | 0.296 | −1.414 | 0.204 | −0.865 | 0.475 | −0.398 | 0.657 |
| MPV (fL) | 0.097 | 0.207 | 0.007 | 0.926 | 0.006 | 0.923 | 0.739 | 0.269 | 0.079 | 0.926 | 0.344 | 0.542 |
| NLR | −0.375 | 0.058 | −0.242 | 0.207 | −0.219 | 0.168 | −1.156 | 0.553 | 1.282 | 0.601 | 1.133 | 0.472 |
| RPR | 0.003 | 0.241 | 0.004 | 0.107 | 0.003 | 0.185 | −0.017 | 0.535 | −0.005 | 0.892 | 0.010 | 0.649 |
| PLR | 1.326 | 0.016 | 1.356 | 0.010 | 1.102 | 0.012 | 4.146 | 0.445 | 1.078 | 0.876 | −3.372 | 0.440 |
Note: Adjusted for age, gender, and smoking status.
Abbreviations: HCT, hematocrit; HGB, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; NLR, neutrophil to lymphocyte ratio; PLR, platelet lymphocyte ratio; RBC, red blood cell; RDW, red cell distribution width; RPR, RDW to platelet count ratio; WBC, white blood cell.
Bold values indicate significant association.
4. DISCUSSION
Our results suggest that higher depression and anxiety scores in COVID‐19 patients are associated with an enhanced inflammatory state, as assessed by higher hematological inflammatory markers including RDW and PLR. However, PLR was also associated with stress in participants.
Several theoretically viable hypotheses can be used to justify the potential prognostic role of anisocytosis in COVID‐19, including direct cytopathic injury disease associated with circulating erythrocytes or their bone marrow precursors, indirect erythrocyte damage caused by hemolytic anemia or intravascular coagulopathy, and profound iron metabolism disruption caused by the sustained inflammatory response (Henry et al., 2020). All of these factors would eventually lead to deranged erythrocyte biology and explain the wide range of erythrocyte sizes in circulation. The dramatic derangement of erythrocyte biology in patients with SARS‐CoV‐2 infection could also be explained by a coexisting indirect injury. First, cases of autoimmune hemolytic anemia have been linked to SARS‐CoV‐2 infection (Lazarian et al., 2020) a phenomenon that has been attributed to the high molecular similarity between the SARS‐CoV‐2 spike protein and the protein ankyrin 1 found on RBC surfaces (Angileri et al., 2020)In COVID‐19 patients who develop a severe or critical illness, intravascular coagulopathy, either localized to the lung parenchyma or disseminated, is also common (Lippi et al., 2020). The formation of micro‐ and microthrombi in various blood veins is a well‐known cause of erythrocyte injury, which would contribute to the existence of RBCs with numerous morphological defects in the circulation (Martinelli et al., 2020).
A higher WBC count has been identified in depressed and anxious people in several investigations (Abbaszadeh et al., 2021; Sunbul et al., 2016). One study showed a link between major depressive illness and leukocyte counts for males but not for women (Surtees et al., 2003). Also, WBC count was positively connected with anxiety score in women in another study (Pitsavos et al., 2006). In depressed patients, Darko et al. discovered leukocytosis, absolute neutrophilia, and relative lymphopenia. Also, the authors speculated that neutrophilia and leukocytosis could be side effects of taking specific medications (Darko et al., 1988). Several studies have demonstrated a link between depression and inflammation. Since WBC count is an independent predictor of cardiovascular disease, it is possible that higher WBC counts or the related inflammatory state could explain some of the elevated cardiac events shown in depressed people (Berk et al., 2013; Saharkhiz et al., 2021).
RDW has received a lot of attention in the last decade because of its ability to successfully predict the risk of death in the general population (Patel et al., 2010), patients with non‐cardiovascular critical illness, sepsis, pneumonia, and other respiratory tract infections (Hu et al., 2020; Luo et al., 2016). One study showed RDW to be a significant and independent predictor of disease severity and kidney injury in patients infected with SARS‐CoV‐2. In another study, patients with severe COVID‐19 had significantly higher RDW values than those with a milder form of the disease. Furthermore, both RDWs were found to be significant predictors of severe illness, with diagnostic accuracy ranging from 65% to 76% (Wang et al., 2020).
The PLR, a general inflammatory marker, represents a concurrent interaction of platelet and lymphocyte count, and it indicates aggregation as well as inflammatory pathways. It has been reported to be increased in response to a variety of acute and chronic proinflammatory conditions, and it has been linked to a poor prognosis in individuals with COPD and carcinomas (Erre et al., 2019; Li et al., 2018, 2020; Veronese et al., 2020). In COVID‐19 patients, the change in PLR from baseline appears to be linearly associated with the severity of illness and length of hospital stay (Qu et al., 2020). PLR appeared as an independent predictive factor for prolonged hospitalization in one study's multivariate analysis. A high PLR may indicate a more intense cytokine storm as a result of increased platelet activation (Fan, 2020). No major differences in PLR were seen in Tiwari's investigative process, as was observed in the current study. However, because platelets are a dynamic variable, the validity of PLR can only be determined by collecting follow‐up samples at different time points (Sahu et al., 2021). In spite of the strengths of this study, there are several limitations. The assessment of depression, anxiety, stress symptoms, sleep quality, insomnia, and quality of life was self‐administered based tools rather than more exact face‐to‐face interviews. Also, due to the condition of COVID‐19 wards in the first and second COVID‐19 peaks, researchers were instructed to limit their data collection time with the patients. Therefore, only necessary data were collected by the researchers. For instance, this study did not collect data on the patient's drug history. It will be worthwhile studying the relationship between hematological inflammatory markers and psychological function in COVID‐19 patients and also in COVID‐19 subjects that recovered from in a case–control study.
5. CONCLUSION
This study showed a positive association between depression/anxiety/stress symptoms and levels of hematological inflammatory markers including PLR and RDW in COVID‐19 patients, which persisted despite adjustment by potential confounders. Results of this study could help clinicians for patients with COVID‐19 who are affected by stress, anxiety, and depression.
AUTHOR CONTRIBUTIONS
Majid Ghayour Mobarhan and Zahra Khorasanchi initially conceptualized and designed the study, Mohammad Rashidmayvan and Elahe Hasanzadeh upgraded the design. The manuscript was written by Kimia Mohammadhasani, Mohammad Vahedi Fard, and Payam Sharifan. Parisa Asadiyan‐Sohan was responsible for the design optimization and statistical analysis. Naiemeh Varaste, Mohammad Reza Shadmand Foumani Moghadam, and Nafise Afkhami contribute to sampling. Gordon Ferns performed English editing. All authors read and approved the final manuscript.
FUNDING INFORMATION
The Mashhad University of Medical Sciences funded this study (grant nu: 981873).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict interests.
ETHICS STATEMENT
The Ethics Committee of MUMS (Mashhad University of Medical Sciences), Mashhad, Iran, approved the trial (Ethic no: IR.MUMS.REC.1399.237).
INFORMED CONSENT
Informed consent was obtained from all individual participant included in the study.
ACKNOWLEDGMENTS
We sincerely thank all patients that participated in this study in advance. We also thank our appreciation to those who helped us in this study.
Khorasanchi, Z. , Rashidmayvan, M. , Hasanzadeh, E. , Moghadam, M. R. S. F. , Afkhami, N. , Asadiyan‐Sohan, P. , Fard, M. V. , Mohammadhasani, K. , Varaste, N. , Sharifan, P. , Ferns, G. , & Mobarhan, M. G. (2023). The association of hematological inflammatory markers and psychological function in COVID‐19 patients: A cross‐sectional study. Physiological Reports, 11, e15889. 10.14814/phy2.15889
Zahra Khorasanchi and Mohammad Rashidmayvan equally first author.
DATA AVAILABILITY STATEMENT
The data are available on request.
REFERENCES
- Abbaszadeh, A. , Saharkhiz, M. , Khorasanchi, Z. , Karbasi, S. , Askari, M. , Hoseini, Z. S. , Ayadilord, M. , Mahmoudzadeh, S. , Rezapour, H. , & Enayati, H. (2021). Impact of a Nordic diet on psychological function in young students. Nutrition and Health, 27, 97–104. [DOI] [PubMed] [Google Scholar]
- Angileri, F. , Légaré, S. , Gammazza, A. M. , De Macario, E. C. , Macario, A. J. , & Cappello, F. (2020). Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID‐19? British Journal of Haematology., 190, e92–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami, A. , Bahrami‐Taghanaki, H. , Khorasanchi, Z. , Tayefi, M. , Ferns, G. A. , Sadeghnia, H. R. , & Ghayour‐Mobarhan, M. (2019). The association between neuropsychological function with serum vitamins A, D, and E and hs‐CRP concentrations. Journal of Molecular Neuroscience, 68, 243–250. [DOI] [PubMed] [Google Scholar]
- Berk, M. , Williams, L. J. , Jacka, F. N. , O'neil, A. , Pasco, J. A. , Moylan, S. , Allen, N. B. , Stuart, A. L. , Hayley, A. C. , & Byrne, M. L. (2013). So depression is an inflammatory disease, but where does the inflammation come from? BMC Medicine, 11, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustan, Y. , Drapisz, A. , Dor, D. H. B. , Avrahami, M. , Schwartz‐Lifshitz, M. , Weizman, A. , & Barzilay, R. (2018). Elevated neutrophil to lymphocyte ratio in non‐affective psychotic adolescent inpatients: Evidence for early association between inflammation and psychosis. Psychiatry Research, 262, 149–153. [DOI] [PubMed] [Google Scholar]
- Buysse, D. , Reynolds, C. , Monk, T. , Berman, S. R. C. F. , & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Darko, D. F. , Rose, J. , Gillin, J. C. , Golshan, S. , & Baird, S. M. (1988). Neutrophilia and lymphopenia in major mood disorders. Psychiatry Research, 25, 243–251. [DOI] [PubMed] [Google Scholar]
- Dergaa, I. , Abubakera, M. , Souissi, A. , Mohammed, A. R. , Varmaa, A. , Musa, S. , AL Naama, A. , Mkaouer, B. , & Ben Saad, H. (2022). Age and clinical signs as predictors of COVID‐19 symptoms and cycle threshold value. Libyan Journal of Medicine, 17.Sleep quality was evaluated using a 19‐item self‐reported PSQI questionnaire that assesses sleep quality over the last 30 days duration (Buysse et al.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duivis, H. E. , Kupper, N. , Penninx, B. W. , Na, B. , De Jonge, P. , & Whooley, M. A. (2013). Depressive symptoms and white blood cell count in coronary heart disease patients: Prospective findings from the heart and soul study. Psychoneuroendocrinology, 38, 479–487. [DOI] [PubMed] [Google Scholar]
- Ekinci, O. , & Ekinci, A. (2017). The connections among suicidal behavior, lipid profile and low‐grade inflammation in patients with major depressive disorder: A specific relationship with the neutrophil‐to‐lymphocyte ratio. Nordic Journal of Psychiatry, 71, 574–580. [DOI] [PubMed] [Google Scholar]
- Erre, G. L. , Paliogiannis, P. , Castagna, F. , Mangoni, A. A. , Carru, C. , Passiu, G. , & Zinellu, A. (2019). Meta‐analysis of neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratio in rheumatoid arthritis. European Journal of Clinical Investigation, 49, e13037. [DOI] [PubMed] [Google Scholar]
- Fan, B. E. (2020). Hematologic parameters in patients with COVID‐19 infection: A reply. American Journal of Hematology, 95, E215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine, P. , Eames, K. , & Heymann, D. L. (2011). “Herd immunity”: A rough guide. Clinical Infectious Diseases, 52, 911–916. [DOI] [PubMed] [Google Scholar]
- Gallagher, M. W. , Zvolensky, M. J. , Long, L. J. , Rogers, A. H. , & Garey, L. (2020). The impact of Covid‐19 experiences and associated stress on anxiety, depression, and functional impairment in American adults. Cognitive Therapy and Research, 44, 1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, B. M. , Benoit, J. L. , Benoit, S. , Pulvino, C. , Berger, B. A. , Olivera, M. H. S. D. , Crutchfield, C. A. , & Lippi, G. (2020). Red blood cell distribution width (RDW) predicts COVID‐19 severity: A prospective, observational study from the cincinnati SARS‐CoV‐2 emergency department cohort. Diagnostics, 10, 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, J. D. , & Crawford, J. R. (2005). The short‐form version of the depression anxiety stress scales (DASS‐21): Construct validity and normative data in a large non‐clinical sample. British Journal of Clinical Psychology, 44, 227–239. [DOI] [PubMed] [Google Scholar]
- Hu, Z.‐D. , Lippi, G. , & Montagnana, M. (2020). Diagnostic and prognostic value of red blood cell distribution width in sepsis: A narrative review. Clinical Biochemistry, 77, 1–6. [DOI] [PubMed] [Google Scholar]
- Kalay, N. , Aytekin, M. , Kaya, M. G. , Ozbek, K. , Karayakali, M. , & Söğüt, E. (2011). The relationship between inflammation and slow coronary flow: Increased red cell distribution width and serum uric acid levels. Türk Kardiyoloji Derneği Arşivi, 39, 463–468. [DOI] [PubMed] [Google Scholar]
- Khorasanchi, Z. , Jafazadeh Esfehani, A. , Sharifan, P. , Hasanzadeh, E. , Shadmand Foumani Moghadam, M. R. , Ahmadi, O. , Ebrahimi, R. , Lotfi, S. Z. , Milani, N. , & Mozdourian, M. (2022). The effects of high dose vitamin D supplementation as a nutritional intervention strategy on biochemical and inflammatory factors in adults with COVID‐19: Study protocol for a randomized controlled trial. Nutrition and Health, 28, 311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorasanchi, Z. , Vahedifard, M. , Mohammadhasani, K. , Sharifan, Y. , Dehnavi, Z. , Naderian, R. , Jafarzadeh Esfehani, A. , Sharifan, P. , Zare‐Feyzabadi, R. , & Ferns, G. A. (2023). Psychological function and serum vitamin D concentration in COVID‐19 patients: A cross‐sectional study. Reviews in Clinical Medicine, 10, 6–12. [Google Scholar]
- Kumarasamy, C. , Sabarimurugan, S. , Madurantakam, R. M. , Lakhotiya, K. , Samiappan, S. , Baxi, S. , Nachimuthu, R. , Gothandam, K. M. , & Jayaraj, R. (2019). Prognostic significance of blood inflammatory biomarkers Nlr, Plr, and LMR in cancer—A protocol for systematic review and meta‐analysis. Medicine, 98, e14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarian, G. , Quinquenel, A. , Bellal, M. , Siavellis, J. , Jacquy, C. , Re, D. , Merabet, F. , Mekinian, A. , Braun, T. , & Damaj, G. (2020). Autoimmune haemolytic anaemia associated with COVID‐19 infection. British Journal of Haematology, 190, 29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, B. , Zhou, P. , Liu, Y. , Wei, H. , Yang, X. , Chen, T. , & Xiao, J. (2018). Platelet‐to‐lymphocyte ratio in advanced cancer: Review and meta‐analysis. Clinica Chimica Acta, 483, 48–56. [DOI] [PubMed] [Google Scholar]
- Lippi, G. , Sanchis‐Gomar, F. , & Henry, B. M. (2020). COVID‐19: Unravelling the clinical progression of nature's virtually perfect biological weapon. Annals of Translational Medicine, 8, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Shao, Z. , Yu, H. , Zhang, W. , Wang, H. , & Mei, Z. (2020). Is the platelet to lymphocyte ratio a promising biomarker to distinguish acute appendicitis? Evidence from a systematic review with meta‐analysis. PLoS One, 15, e0233470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, R. , Hu, J. , Jiang, L. , & Zhang, M. (2016). Prognostic value of red blood cell distribution width in non‐cardiovascular critically or acutely patients: A systematic review. PLoS One, 11, e0167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli, N. , Montagnana, M. , Pizzolo, F. , Friso, S. , Salvagno, G. L. , Forni, G. L. , Gianesin, B. , Morandi, M. , Lunardi, C. , & Lippi, G. (2020). A relative ADAMTS13 deficiency supports the presence of a secondary microangiopathy in COVID 19. Thrombosis Research, 193, 170–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcpherson, R. A. , Msc, M. , & Pincus, M. R. (2021). Henry's clinical diagnosis and management by laboratory methods E‐book. Elsevier Health Sciences. [Google Scholar]
- Michopoulos, V. , Powers, A. , Gillespie, C. F. , Ressler, K. J. , & Jovanovic, T. (2017). Inflammation in fear‐and anxiety‐based disorders: Ptsd, gad, and beyond. Neuropsychopharmacology, 42, 254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam, J. F. , Nakhaee, N. , Sheibani, V. , Garrusi, B. , & Amirkafi, A. (2012). Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI‐P). Sleep and Breathing, 16, 79–82. [DOI] [PubMed] [Google Scholar]
- Montazeri, A. , Goshtasebi, A. , Vahdaninia, M. , & Gandek, B. (2005). The short form health survey (SF‐36): Translation and validation study of the Iranian version. Quality of Life Research, 14, 875–882. [DOI] [PubMed] [Google Scholar]
- Morin, C. M. , Belleville, G. , Bélanger, L. , & Ivers, H. (2011). The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34, 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdin, S. , Sarisoy, G. , & Böke, Ö. (2017). A comparison of the neutrophil‐lymphocyte, platelet‐lymphocyte and monocyte‐lymphocyte ratios in schizophrenia and bipolar disorder patients–a retrospective file review. Nordic Journal of Psychiatry, 71, 509–512. [DOI] [PubMed] [Google Scholar]
- Patel, K. V. , Semba, R. D. , Ferrucci, L. , Newman, A. B. , Fried, L. P. , Wallace, R. B. , Bandinelli, S. , Phillips, C. S. , Yu, B. , & Connelly, S. (2010). Red cell distribution width and mortality in older adults: A meta‐analysis. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 65, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitsavos, C. , Panagiotakos, D. B. , Papageorgiou, C. , Tsetsekou, E. , Soldatos, C. , & Stefanadis, C. (2006). Anxiety in relation to inflammation and coagulation markers, among healthy adults: The ATTICA study. Atherosclerosis, 185, 320–326. [DOI] [PubMed] [Google Scholar]
- Qu, R. , Ling, Y. , Zhang, Y. H. Z. , Wei, L. Y. , Chen, X. , Li, X. M. , Liu, X. Y. , Liu, H. M. , Guo, Z. , & Ren, H. (2020). Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. Journal of Medical Virology, 92, 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawi, O. , Zidan, M. , & AL Naama, A. (2023). Retrospective analysis of hematological abnormalities in older diabetic patients with SARS‐CoV‐2 infection: Retrospective analysis of hematological abnormalities in older diabetic patients with SARS‐CoV‐2 infection. New Asian Journal of Medicine, 1, 7–11. [Google Scholar]
- Reger, M. A. , Stanley, I. H. , & Joiner, T. E. (2020). Suicide mortality and coronavirus disease 2019—A perfect storm? JAMA Psychiatry, 77, 1093–1094. [DOI] [PubMed] [Google Scholar]
- Saharkhiz, M. , Khorasanchi, Z. , Karbasi, S. , Jafari‐Nozad, A. M. , Naseri, M. , Mohammadifard, M. , Siami Ali Abad, M. , Ayadilord, M. , Ferns, G. A. , & Bahrami, A. (2021). The association between adherence to a dietary approaches to stop hypertension (DASH) diet and neuro‐psychological function in young women. BMC Nutrition, 7, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebi, A. , Asghari, M. J. , & Salari, R. S. (2005). Validation of depression anxiety and stress scale (DASS‐21) for an Iranian population.
- Sahu, P. , Rai, A. , Sahu, S. , & Raut, K. (2021). Haematological findings and pattern analysis in patients with COVID‐19 infection. International Journal of Current Research and Review, 13, 36–41. [Google Scholar]
- Sunbul, E. A. , Sunbul, M. , Yanartas, O. , Cengiz, F. , Bozbay, M. , Sari, I. , & Gulec, H. (2016). Increased neutrophil/lymphocyte ratio in patients with depression is correlated with the severity of depression and cardiovascular risk factors. Psychiatry Investigation, 13, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees, P. , Wainwright, N. , Day, N. , Luben, R. , Brayne, C. , & Khaw, K.‐T. (2003). Association of depression with peripheral leukocyte counts in EPIC‐Norfolk—Role of sex and cigarette smoking. Journal of Psychosomatic Research, 54, 303–306. [DOI] [PubMed] [Google Scholar]
- Uzun, N. , & Akinci, M. A. (2021). Hemogram parameters in childhood anxiety disorders: Could anxiety disorders be related with inflammation? Medical Hypotheses, 146, 110440. [DOI] [PubMed] [Google Scholar]
- Varma, A. , Dergaa, I. , Mohammed, A. R. , Abubaker, M. , Al Naama, A. , Mohammed, S. , Rafique, M. A. , Manu, L. , Vedasalam, S. , & Parveze, P. (2021). COVID‐19 and diabetes in primary care–How do hematological parameters present in this cohort? Expert Review of Endocrinology & Metabolism, 16, 147–153. [DOI] [PubMed] [Google Scholar]
- Veronese, N. , Demurtas, J. , Yang, L. , Tonelli, R. , Barbagallo, M. , Lopalco, P. , Lagolio, E. , Celotto, S. , Pizzol, D. , & Zou, L. (2020). Use of corticosteroids in coronavirus disease 2019 pneumonia: A systematic review of the literature. Frontiers in Medicine, 7, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Deng, R. , Gou, L. , Fu, Z. , Zhang, X. , Shao, F. , Wang, G. , Fu, W. , Xiao, J. , & Ding, X. (2020). Preliminary study to identify severe from moderate cases of COVID‐19 using combined hematology parameters. Annals of Translational Medicine, 8, 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi, Z. , Sadeghniiat‐Haghighi, K. , Zohal, M. A. , & Elmizadeh, K. (2012). Validity and reliability of the Iranian version of the insomnia severity index. The Malaysian Journal of Medical Sciences: Mjms, 19, 31–36. [PMC free article] [PubMed] [Google Scholar]
- Zahorec, R. (2001). Ratio of neutrophil to lymphocyte counts‐rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske Lekarske Listy, 102, 5–14. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available on request.


