Abstract
Background
A recent study suggested that the protective effect of familial longevity becomes negligible for centenarians. However, the authors assessed the dependence on familial longevity in centenarians by comparing centenarians with 1 parent surviving to age 80+ to centenarians whose same-sexed parent did not survive to age 80. Here we test whether the protective effect of familial longevity persists after age 100 using more restrictive definitions of long-lived families.
Methods
Long-lived sibships were identified through 3 nationwide, consecutive studies in Denmark, including families with either at least 2 siblings aged 90+ or a Family Longevity Selection Score (FLoSS) above 7. Long-lived siblings enrolled in these studies and who reached age 100 were included. For each sibling, 5 controls matched on sex and year of birth were randomly selected among centenarians in the Danish population. Survival time from age 100 was described with Kaplan–Meier curves for siblings and controls separately. Survival analyses were performed using stratified Cox proportional hazards models.
Results
A total of 340 individuals from long-lived sibships who survived to age 100 and 1 700 controls were included. Among the long-lived siblings and controls, 1 650 (81%) were women. The results showed that long-lived siblings presented better overall survival after age 100 than sporadic long-livers (hazard ratio [HR] = 0.80, 95% confidence interval [CI] = 0.71–0.91), with even lower estimate (HR = 0.65, 95% CI = 0.50–0.85) if familial longevity was defined by FLoSS.
Conclusions
The present study, with virtually no loss to follow-up, demonstrated a persistence of protective effect of familial longevity after age 100.
Keywords: Aging, Centenarians, Familial longevity, Survival
Background
The protective effect of familial longevity has been demonstrated in many studies in high-income countries. Several studies reported better health in long-lived siblings than other “sporadic” long-livers, including a lower prevalence of Alzheimer’s disease and related disorders, diabetes, depression, heart failure, and osteoporosis (1–3). Other studies demonstrated better survival in long-lived siblings compared to sporadic long-livers (1,4), and, similarly, in their offspring and grandchildren: A study focusing on 3 generations of longevity-enriched Danish families found a lower incidence of virtually all disease categories (including cardiovascular diseases, cancer, respiratory diseases, mental disorders, etc.) as well as a lower all-cause and cause-specific mortality in offspring and grandchildren compared to controls (5). However, there are few studies of the protective effect of familial longevity in centenarians.
Some studies reported sustainable and life-long advantages of familial longevity for siblings of centenarians compared to control populations, supporting the finding of a significant familial component to exceptional longevity (6,7). Nevertheless, a much larger recent U.S. study by Gavrilova and Gavrilov suggested that the protective effect of familial longevity becomes negligible for centenarians (8). The authors reported survival advantages of siblings and children from long-lived families until age 95–100 only. They also showed no effect of paternal and maternal longevity on the survival of centenarians leading to the conclusion of vanishing mortality advantage. However, the paternal and maternal longevity only required 1 parent surviving to age 80+. Indeed, the authors assessed the dependence on familial longevity in centenarians by comparing centenarians with 1 parent surviving to age 80+ to centenarians whose same-sexed parent did not survive to age 80. In this way, the long-lived centenarians included in the study by Gavrilova and Gavrilov included sibships whose selection for longevity was based on only 1 long-lived individual.
In Denmark, 3 nationwide studies of long-lived siblings provide an excellent basis for testing whether the protective effect of familial longevity persists to extreme ages if more strict definitions of familial longevity are used, that is, the presence of at least 2 long-lived siblings in the sibship or a high Family Longevity Selection Score (FLoSS) (9). Using data from these studies and from the Danish national registers, we investigated survival from age 100 in individuals from long-lived sibships comparing it to the survival of sporadic 100-year-old individuals from the general Danish population.
Method
Study Population
The identification of long-lived siblings was undertaken in 3 nationwide, consecutive studies in Denmark, for which recruitment ran sequentially during the Years 2004–2009: the Danish Oldest Siblings (DOS) pilot study, the Genetics of Healthy Ageing (GeHA) study (10), and the Danish part of the Long Life Family Study (LLFS) (9,11). All individuals born before April 2, 1918, and alive in 2004 were identified in the Danish Civil Registration System (CRS). Long-lived siblings were defined in different ways depending on the study. Recruitment to DOS was conditional on at least 2 siblings being alive and 88 years or older (less than 5 sibships had not reached the age of 90+, but were close, at the time of interview); recruitment to GeHA required at least 2 siblings to be alive and above age 90, and the LLFS recruited only families with a FLoSS above 7 (9). The FLoSS is a metric developed to rank families for selection into a family study of longevity based on an estimated family longevity score built from birth-, gender-, and nation-specific cohort survival probabilities and with a bonus for older living siblings (9). A large FLoSS reflects either a large number of siblings reaching older ages or 2 siblings reaching extreme older ages (oldest percentile of a birth cohort). The present study comprises all sibships from DOS, GeHA, and LLFS combined and all siblings from these sibships were included in the analyses regardless of their time of death (ie, also siblings who died before the 3 surveys were conducted are included in the analyses). In all, 3 972 siblings from 659 families were enrolled in either DOS, GeHA, or LLFS, with 659 siblings from 114 families in DOS, 2 736 siblings from 469 families in GeHA, and 577 siblings from 76 families in the LLFS.
Long-lived siblings enrolled in these studies and who reached age 100 were included. For each sibling, 5 controls matched on sex and year of birth were randomly selected among individuals from the Danish population who had reached age 100. In the subanalysis of LLFS siblings, where the sample was markedly smaller, 20 controls per sibling were randomly selected with the same matching criteria as above.
Few long-lived siblings included in these 3 studies were interviewed after age 100 (n = 32). However, to avoid an immortal bias for these cases, 5 controls matched on sex and year of birth were randomly selected among individuals from the Danish population who had reached the age of the case at time of interview, and they were followed regarding mortality from that age.
A subanalysis was performed for the families from the LLFS who all fulfilled the requirement of FLoSS > 7. Thus, analysis of the subsample of LLFS siblings and their controls enabled us to study families with FLoSS > 7 as the definition of long-lived families (9).
The CRS
The CRS covers the entire population alive and residing in Denmark since April 2, 1968, and contains information on each resident’s vital status, sex, place, date of birth, and death (12,13). All persons registered in the CRS are assigned a unique personal identification number used in all national registers, enabling accurate linkage between all national registers. Once a person has been assigned a unique personal identification number, the same number will not be assigned to other persons, and this number follows the person afterwards.
Overall Survival
Survival time was calculated from age 100 to the date of death or last follow-up date (December 16, 2021), whichever came first. For the cases interviewed after age 100, survival time was calculated from the interview date onwards, and similarly for the matched controls.
All-cause mortality was defined as death from any cause. Individuals still alive were censored at the date of the last follow-up.
Statistical Analyses
The remaining life span from age 100 was described in terms of median 25th, 75th, and 90th percentiles. Survival time from age 100 was described with Kaplan–Meier curves for siblings and controls separately and compared using the log-rank test. Survival analyses were performed using stratified Cox proportional hazards models based on the matching strata. Proportional hazards assumption was assessed graphically using log–log plots.
Analyses were carried out for the entire population and subsequently for females and males separately.
The study has been approved by The Regional Scientific Ethical Committees for Southern Denmark (S-VF-20030227), The Danish Data Protection Agency (J.nr. 2008-41-1753), and University of Southern Denmark, Research & Innovation Organization (J.nr. 10 635).
Results
Population Characteristics
A total of 340 individuals from long-lived sibships who survived to age 100 and 1 700 controls were included. Among them, 1 650 (81%) were women. The median age at the end of follow-up was 101.7 years for women (interquartile range [IQR] = 100.7–103.1) and 101.3 years for men (IQR = 100.5–102.7).
Regarding the subanalysis, 69 long-lived siblings from the LLFS who survived to age 100 and 1 380 controls were included. The population was mainly female (n = 1 176, 81%). The median age at the end of follow-up was 101.4 years for women (IQR = 100.6–102.8) and 101.1 years for men (IQR = 100.4–102.6).
Remaining Life Span
Among long-lived siblings, the median remaining life span (ie, life span above age 100) was 25% longer than among controls: 1.9 years versus 1.5 years (Table 1). At higher percentiles, the relative differences were slightly attenuated while the absolute differences were slightly increased: at 75th percentile, remaining life spans of long-lived siblings and controls were 3.5 and 2.9 years (21% increase) and at 90th percentile they were 5.3 and 4.6 years (16% increase). While for males, there were not great differences except at the earliest ages, corresponding to the 25th percentile and somewhat at the median, these overall tendencies carried over to the females.
Table 1.
Descriptive Statistics of Life Span of Danish Centenarian Siblings Compared to Matched Centenarian Controls: Median, Lower and Upper Quartile, and 90th Percentile of the Subset of Siblings and Controls, Where Those Recruited After Age 100 (32 siblings; 160 controls) Were Removed
| N | Lower Quartile | 95% CI | Median | 95% CI | Upper Quartile | 95% CI | 90th Percentile | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|---|
| All siblings | LEF siblings | 340 | 101.0 | 100.8–101.1 | 101.9 | 101.6–102.1 | 103.5 | 103.2–103.8 | 105.3 | 104.6–105.8 |
| Matched controls | 1 700 | 100.6 | 100.6–100.7 | 101.5 | 101.4–101.6 | 102.9 | 102.7–103.1 | 104.6 | 104.3–104.8 | |
| Females | LEF siblings | 275 | 101.0 | 100.8–101.1 | 102.0 | 101.7–102.3 | 103.6 | 103.3–104.2 | 105.6 | 104.8–105.9 |
| Matched controls | 1 375 | 100.7 | 100.6–100.7 | 101.5 | 101.4–101.7 | 102.9 | 102.7–103.1 | 104.6 | 104.3–104.8 | |
| Males | LEF siblings | 65 | 100.9 | 100.6–101.2 | 101.6 | 101.2–101.9 | 102.9 | 102.1–103.4 | * | *–* |
| Matched controls | 325 | 100.5 | 100.4–100.6 | 101.2 | 101.1–101.6 | 102.8 | 102.4–103.1 | * | *–* | |
| LLFS siblings | LLFS siblings | 69 | 101.1 | 100.5–101.4 | 102.1 | 101.5–103.3 | 104.2 | 103.5–105.2 | 105.9 | 104.7–* |
| Matched controls | 1 380 | 100.6 | 100.6–100.7 | 101.4 | 101.3–101.6 | 102.8 | 102.6–103.0 | 104.6 | 104.4–104.9 |
Notes: CI = confidence interval; LEF = Longevity-enriched families; LLFS = Long Life Family Study.
*Not presented due to Danish rules regarding small samples.
The median remaining life span (Table 1) was 33% longer among LLFS siblings than among controls (2.0 years vs 1.5 years) and the relative differences were slightly attenuated at higher percentiles.
Survival After Age 100
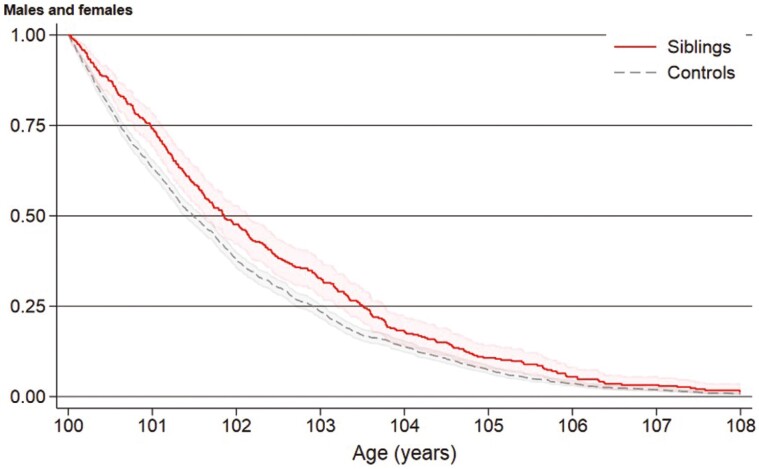
By the end of the follow-up, 1 963 (96%) individuals had died, comprising 325 (96%) siblings and 1 638 (96%) controls. Overall survival for siblings and controls was 87% and 80% at 6 months, 74% and 63% at 1 year, 48% and 38% at 2 years, and 11% and 7% at 5 years, respectively (Figure 1). Long-lived siblings presented better overall survival than sporadic long-livers (hazard ratio [HR] = 0.80, 95% confidence interval [CI] = 0.71–0.91).
Figure 1.
Survival from age 100 of Danish long-lived siblings (n = 340) and controls (n = 1 700), Kaplan–Meier curve.
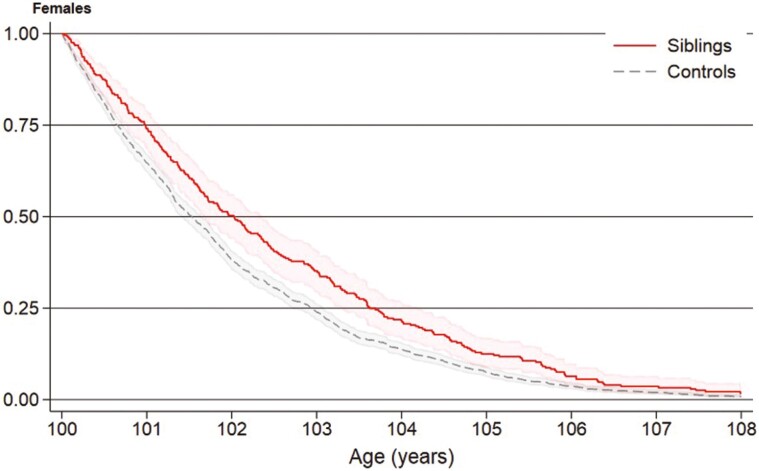
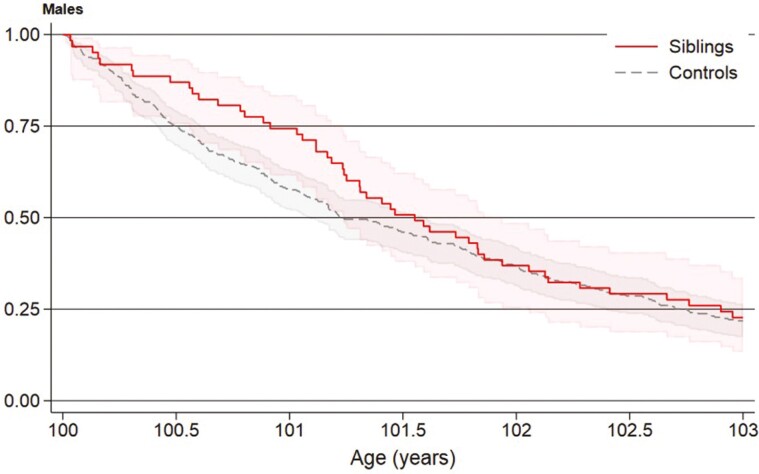
In women, overall survival for siblings and controls was 87% and 81% at 6 months, 74% and 65% at 1 year, 50% and 38% at 2 years, and 13% and 7% at 5 years, respectively (Figure 2). In men, overall survival for siblings and controls was 87% and 75% at 6 months, 74% and 58% at 1 year, and 37% and 37% at 2 years, respectively (Figure 3). Female long-lived siblings had better overall survival than controls (HR = 0.77, 95% CI = 0.77–0.88), but male long-lived siblings did not (HR = 0.99, 95% CI = 0.75–1.32). Nevertheless, male long-lived siblings had a lower, but nonsignificant, risk of death in the first 2 years after age 100 than controls (HR = 0.86, 95% CI = 0.61–1.21).
Figure 2.
Survival from age 100 of female Danish long-lived siblings (n = 275) and controls (n = 1 375), Kaplan–Meier curve.
Figure 3.
Survival from age 100 of male Danish long-lived siblings (n = 65) and controls (n = 325), Kaplan–Meier curve.
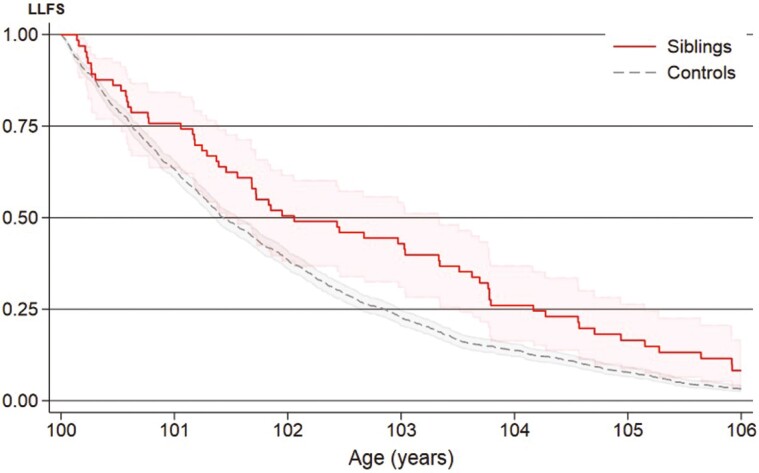
In the subanalysis of the LLFS siblings, 1 353 (93.4%) had died by the end of follow-up: 64 (93%) siblings and 1 289 (93%) controls. Overall survival for LLFS siblings and controls was 86% and 79% at 6 months, 76% and 63% at 1 year, 50% and 39% at 2 years, and 16% and 8% at 5 years, respectively (Figure 4). LLFS long-lived siblings had better overall survival than sporadic long-livers (HR = 0.65, 95% CI = 0.50–0.85).
Figure 4.
Survival from age 100 of Long Life Family Study (LLFS) long-lived siblings (n = 69) and controls (n = 1 380), Kaplan–Meier curve.
Discussion
Our results demonstrated the persistence of a protective effect of familial longevity after age 100. The long-lived siblings had substantially lower mortality risk after age 100 than sporadic centenarians.
Our study sample is mainly female (n = 81%), which aligns with previous reports. For example, 84% of European centenarians and nearly 85% of Danish centenarians were females in 2011 (14). A study published in 2017 reported that most centenarians die within the first 2 years after reaching age 100, with relatively few surviving much longer (15). In our study, at age 102, 60% of females and 63% of males were deceased.
Our results showed better overall survival from age 100 in long-lived siblings compared to controls. After stratifying the analyses by sex, only women had significantly better survival than controls. As mentioned in the introduction, Gavrilova and Gavrilov suggested that the protective effect of familial longevity becomes negligible for centenarians (8). However, in line with our study, another study reported a gap in survival between siblings of centenarians and controls at very old ages, including age 100+, but that study only demonstrated a survival advantage in women (7). Indeed, in this study, women had a relative death rate from age 100 of 0.66 (95% CI = 0.43–0.90), whereas the relative death rate in men was 1.15 (95% CI = 0.86–1.44). As women globally present higher life expectancy than men, male centenarians are scarce. Thus, our analyses may lack statistical power to demonstrate the protective effect of familial longevity in men. In addition, a Dutch study of longevity reported a marked survival advantage over sporadic long-livers at ages 89–99 years both for males and females (16). Although this Dutch study did not investigate survival advantages after age 100, long-lived families were selected according to criteria very similar to ours (2 long-lived siblings: males 89+ years, females 91+ years).
The importance of the role of the criteria for defining long-lived families has been underlined several studies focusing on optimizing family selection for longevity studies (17,18). In these studies, it was found that a large percentage of individuals in the family must be long-lived (30% of siblings and ancestors in upper 10th percentile). Using the familial longevity score threshold from the LLFS instead of 2 siblings above age 90+, we found similar results: the LLFS long-lived siblings had better survival after age 100 than sporadic centenarians. Because the LLFS typically selects families with many long-lived siblings, it might be expected that the survival advantage is particularly large in this subpopulation. In fact, in the present study, we found a 35% lower mortality among LLFS siblings as compared to a 20% lower mortality among all long-lived siblings.
The main explanation for the discrepancy between our results and those of Gavrilova and Gavrilov is likely to be the different definitions of long-lived sibships (8). In the study by Gavrilova and Gavrilov, long-lived sibships are defined by the presence of 1 centenarian and at least 1 parent living to age 80+, whereas in our study, they are defined more strictly, either by the presence of 2 long-lived siblings or a family FLoSS score above 7 (9). In addition, the use of national data made it possible to randomly select at least 5 controls per long-lived sibling. Finally, a strength of our study also lies in the fact that we had no missing data and virtually no loss to follow-up due to our registry-based design, which enabled a thorough study of the centenarians and provided high-quality data on death.
However, our study also presents some limitations. Compared to previous studies including large centenarian population (6,8), we only included 340 individuals from long-lived sibships who survived to age 100. However, these individuals were matched to 5 sporadic long-livers from the general population, leading to a sizeable control group. In addition, this sample size enabled a demonstration of the protective effect of familial longevity. Nevertheless, the number of male centenarians was scarce, and our analyses lack statistical power for this group. This lack of power may partly explain the nonsignificant results in men. Moreover, socioeconomic status (including education, income, etc.) was not available, and we cannot rule out that the long-lived siblings had higher socioeconomic status than sporadic long-livers. However, in a previous study, we compared the siblings to control samples using the Danish 1916 Census, and we did not find statistically significant differences in income, wealth, or taxes between the long-lived families and the controls (5).
In conclusion, the protective effect of familial longevity persists after age 100, if “familial” is defined by the presence of 2 long-lived siblings (90+) or a FLoSS score above 7. This study suggests a stronger genetic component to longevity in families with more than 1 long-lived member, as compared to sporadic long-livers. While sporadic long-livers are likely to have gene variants that increase the chance of longevity, our results suggest that they are even more pronounced in long-lived families. This study supports the view that familial longevity cases could be much more informative for studying mechanisms of longevity than sporadic cases. Indeed, a recent study of the health of offspring and grandchildren of the long-lived siblings that provided the study base for the present study showed a health advantage for the grandchildren that started already at birth (5). Combined with the results from the present study, this suggests a protective effect of familial longevity from birth to age 100+.
Contributor Information
Angéline Galvin, Epidemiology, Biostatistics, and Biodemography Team, Department of Public Health, University of Southern Denmark, Odense, Denmark.
Jacob Krabbe Pedersen, Epidemiology, Biostatistics, and Biodemography Team, Department of Public Health, University of Southern Denmark, Odense, Denmark; The Danish Aging Research Center, Department of Public Health, University of Southern Denmark, Odense, Denmark.
Mary K Wojczynski, Division of Statistical Genomics, Department of Genetics, Washington University School of Medicine, St. Louis, Missouri, USA.
Svetlana Ukraintseva, Biodemography of Aging Research Unit, Social Science Research Institute, Duke University, Durham, North Carolina, USA.
Konstantin Arbeev, Biodemography of Aging Research Unit, Social Science Research Institute, Duke University, Durham, North Carolina, USA.
Mary Feitosa, Division of Statistical Genomics, Department of Genetics, Washington University School of Medicine, St. Louis, Missouri, USA.
Michael A Province, Division of Statistical Genomics, Department of Genetics, Washington University School of Medicine, St. Louis, Missouri, USA.
Kaare Christensen, Epidemiology, Biostatistics, and Biodemography Team, Department of Public Health, University of Southern Denmark, Odense, Denmark; The Danish Aging Research Center, Department of Public Health, University of Southern Denmark, Odense, Denmark.
Funding
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health (NIA/NIH) under award number U19AG063893. The work of K.A. and S.U. was partly supported by the NIA/NIH grant R01AG062623.
Conflict of Interest
None.
Author Contributions
Conceptualization: A.G., J.K.P., M.K.W., S.U., K.A., M.F., M.A.P., K.C. Data curation: A.G., J.K.P. Formal analysis: A.G., J.K.P., K.C. Funding acquisition: K.C. Methodology: A.G., J.K.P., M.K.W., S.U., K.A., M.F., M.A.P., K.C. Project administration: A.G., K.C. Supervision: K.C. Validation: A.G., J.K.P., M.K.W., S.U., K.A., M.F., M.A.P., K.C. Writing—original draft: A.G., K.C. Writing—review and editing: A.G., J.K.P., M.K.W., S.U., K.A., M.F., M.A.P., K.C.
References
- 1. Galvin A, Ukraintseva S, Arbeev K, Feitosa M, Christensen K.. Physical robustness and resilience among long-lived female siblings: a comparison with sporadic long-livers. Aging (Milano). 2020;12(14):15157–15168. doi: 10.18632/aging.103618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ash AS, Kroll-Desrosiers AR, Hoaglin DC, Christensen K, Fang H, Perls TT.. Are members of long-lived families healthier than their equally long-lived peers? Evidence from the Long Life Family Study. J Gerontol A Biol Sci Med Sci. 2015;70(8):971–976. doi: 10.1093/gerona/glv015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Newman AB, Glynn NW, Taylor CA, et al. . Health and function of participants in the Long Life Family Study: a comparison with other cohorts. Aging (Milano). 2011;3(1):63–76. doi: 10.18632/aging.100242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Westendorp RGJ, Van Heemst D, Rozing MP, et al. ; Leiden Longevity Study Group. Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: the Leiden Longevity Study: mortality risk and disease prevalence in familial longevity. J Am Geriatr Soc. 2009;57(9):1634–1637. doi: 10.1111/j.1532-5415.2009.02381.x [DOI] [PubMed] [Google Scholar]
- 5. Christensen K, Wojczynski MK, Pedersen JK, et al. . Mechanisms underlying familial aggregation of exceptional health and survival: a three‐generation cohort study. Aging Cell. 2020;19(10):e13228. doi: 10.1111/acel.13228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Willcox BJ, Willcox DC, He Q, Curb JD, Suzuki M.. Siblings of Okinawan centenarians share lifelong mortality advantages. J Gerontol A Biol Sci Med Sci. 2006;61(4):345–354. doi: 10.1093/gerona/61.4.345 [DOI] [PubMed] [Google Scholar]
- 7. Perls TT, Wilmoth J, Levenson R, et al. . Life-long sustained mortality advantage of siblings of centenarians. Proc Natl Acad Sci USA. 2002;99(12):8442–8447. doi: 10.1073/pnas.122587599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gavrilova NS, Gavrilov LA.. Protective effects of familial longevity decrease with age and become negligible for centenarians. J Gerontol A Biol Sci Med Sci. 2022;77(4):736–743. doi: 10.1093/gerona/glab380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sebastiani P, Hadley EC, Province M, et al. . A family longevity selection score: ranking sibships by their longevity, size, and availability for study. Am J Epidemiol. 2009;170(12):1555–1562. doi: 10.1093/aje/kwp309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skytthe A, Valensin S, Jeune B, et al. ; GEHA Consortium. Design, recruitment, logistics, and data management of the GEHA (Genetics of Healthy Ageing) project. Exp Gerontol. 2011;46(11):934–945. doi: 10.1016/j.exger.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wojczynski MK, Jiuan Lin S, Sebastiani P, et al. . NIA Long Life Family Study: objectives, design, and heritability of cross-sectional and longitudinal phenotypes. J Gerontol A Biol Sci Med Sci. 2022;77(4):717–727. doi: 10.1093/gerona/glab333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7_suppl):22–25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 13. Schmidt M, Pedersen L, Sørensen HT.. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 14. Teixeira L, Araújo L, Jopp D, Ribeiro O.. Centenarians in Europe. Maturitas. 2017;104:90–95. doi: 10.1016/j.maturitas.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 15. Modig K, Andersson T, Vaupel J, Rau R, Ahlbom A.. How long do centenarians survive? Life expectancy and maximum lifespan. J Intern Med. 2017;282(2):156–163. doi: 10.1111/joim.12627 [DOI] [PubMed] [Google Scholar]
- 16. van den Berg N, Rodríguez-Girondo M, de Craen AJM, Houwing-Duistermaat JJ, Beekman M, Slagboom PE.. Longevity around the turn of the 20th century: life-long sustained survival advantage for parents of today’s nonagenarians. J Gerontol A Biol Sci Med Sci. 2018;73(10):1295–1302. doi: 10.1093/gerona/gly049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van den Berg N, Rodríguez‐Girondo M, Mandemakers K, Janssens AAPO, Beekman M, Slagboom PE.. Longevity relatives count score identifies heritable longevity carriers and suggests case improvement in genetic studies. Aging Cell. 2020;19(6). doi: 10.1111/acel.13139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van den Berg N, Rodríguez-Girondo M, van Dijk IK, et al. . Longevity defined as top 10% survivors and beyond is transmitted as a quantitative genetic trait. Nat Commun. 2019;10(1):35. doi: 10.1038/s41467-018-07925-0 [DOI] [PMC free article] [PubMed] [Google Scholar]